1. Introduction
The term “geopolymer” was coined by Professor Joseph Davidovits in 1979 [
1]. Geopolymers exhibit a structure similar to organic polymers, characterized by long chains and interconnected networks of mineral molecules. However, in geopolymers, these molecules are of a mineral nature and are connected through covalent bonds. It is worth mentioning that geopolymers differ from conventional ceramics, as they possess a polymer-like structure and form at low temperatures below 100° Celsius [
2]. Nowadays, geopolymers have a wide range of potential applications, including prefabricated structural and non-structural components, concrete pavements, products for hazardous materials containment, radioactive and toxic waste management, refractory ceramics, fire-resistant composites, the automotive industry, and power plants [
3,
4].
The geopolymerization process involves several stages that typically occur simultaneously [
1,
5]: (1) dissolution of silicon (Si) and aluminium (Al) ions present in solid aluminosilicate materials in a strong alkaline solution; (2) formation of Si-O-Si and Si-O-Al bonds; (3) formation of a three-dimensional aluminosilicate structure known as the geopolymer structure; and (4) incorporation of solid particles and filler materials into the geopolymer structure, leading to the overall hardening of the composite and formation of the final geopolymer structure. Geopolymers can exhibit comparable performance to conventional cementitious materials within their respective applications. Additionally, they offer the advantage of reducing greenhouse gas emissions [
6]. The key distinction between geopolymers and Portland cement lies in their hardening mechanisms. Geopolymers undergo polymerization, which typically occurs within a short period, whereas cement hydration takes place over a longer duration, lasting several years [
7].
Several studies have explored the effects of incorporating different materials into geopolymer mixtures. Allahverdi et al. [
8] investigated the setting time and strength of geopolymer cements utilizing a combination of pumice and GGBFS. They observed that incorporating GGBFS in pumice specimens, ranging from 5% to 25%, resulted in a reduction in strength at 28 days of curing at room temperature. Moreover, an increase in GGBFS percentage decreased the initial setting time while increasing the final setting time. Nedoushan et al. [
9] examined the impact of activator solutions on the compressive strength and workability of geopolymer mortars containing GGBFS and pumice. They found that increasing the substitution of GGBFS with pumice from 0% to 100% led to an increase in strength at 3 days, followed by a decrease in strength with 100% substitution at 28 and 91 days. Additionally, an increase in pumice level resulted in decreased mortar workability. Partha et al. [
10] investigated the strength and permeability of geopolymer specimens incorporating blended fly ash and GGBFS. They observed that substituting 10% and 20% of fly ash with GGBFS at various ages and curing at room temperature could increase the strength characteristics and has a reduction effect on the permeability. Bellum et al. [
11] examined the mechanical properties of geopolymer specimens with the combined use of fly ash and GGBFS. They observed that increasing the substitution level of GGBFS from 30% to 70% in specimens with fly ash resulted in increased compressive, flexural, and tensile strength.
It must be mentioned that there are several uncertainties when determining the characteristics of materials in real conditions. For instance, Li et al. [
12] revealed that the sample size could significantly influence the permeability of anisotropic geomaterials. In some papers such as [
13], methods for determining the microstructure of porous materials to estimate some properties were proposed. Using numerical methods to estimate the mechanical characteristics is also suggested [
14]. However, these methods need limited conditions and specifications to be applicable. Wang et al. [
15] investigated the optimal water-to-cement ratio for cement-based soils. The results showed that increasing the water-to-cement ratio leads to a decrease in compressive strength. Additionally, with an increase in the water-to-cement ratio, the water absorption of cement-based specimens increases.
Madani et al. [
16] investigated the characteristics of geopolymer composites produced from the waste soil of aggregate production plants. In this study, the influence of variations in the alkali concentration, calcium hydroxide content, and curing regimes was scrutinized. This was a practical and innovative study, and the results were utilizable to produce construction materials. However, as it was shown in [
16], the waste soil is not a highly active material to produce geopolymer binders. Subsequently, the current study was conducted to produce a stronger geopolymer with enhanced properties. For this purpose, GGBFS, as a highly active material, was introduced in the mixes to substitute for a part of the waste soil. Moreover, pumice, which has an activity level between the waste soil and GGBFS, was also investigated to verify that the hybrid use of GGBFS with a less active material could be an appropriate solution to produce a more sustainable geopolymer mixture. In this regard, plain mixtures were prepared with 100% GGBFS, 100% pumice, and 100% waste soil. Additional composites were also created by varying the ratios of GGBFS/soil waste and GGBFS/pumice (75/25, 50/50, and 25/75). The mechanical properties and durability of these mixtures were investigated.
In comparison to other studies conducted on geopolymer materials, the focus of the present research is on the simultaneous use of low-activity base materials and high-activity industrial by-products. Based on this approach, the results of this study can provide the possibility of economically producing and developing geopolymer products in the future by utilizing a wide range of industrial and natural waste materials.
2. Materials and Methods
2.1. Materials
The raw materials employed for geopolymerization in this study comprise waste soil from aggregate production plants, GGBFS from iron smelting furnaces, and pumice.
Figure 1 represents the pictures of the raw materials. The waste soil was obtained from aggregate production plants and had a SiO
2-to-Al
2O
3 ratio of 4.32. The utilized pumice possesses a SiO
2-to-Al
2O
3 ratio of 3.34, and GGBFS possesses a SiO
2-to-Al
2O
3 ratio of 3.7.
Table 1 presents the XRF analysis results of the base materials, including GGBFS, pumice, and the waste soil. The specifications of the hydrated lime are outlined in
Table 2.
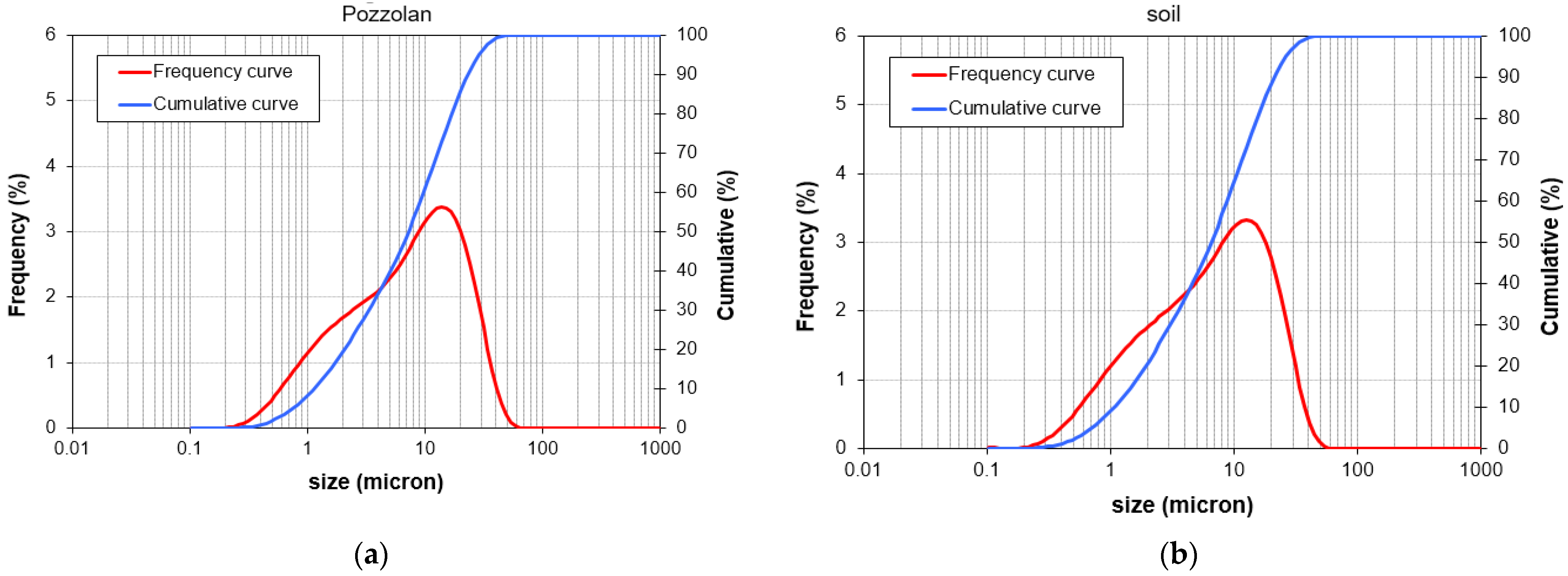
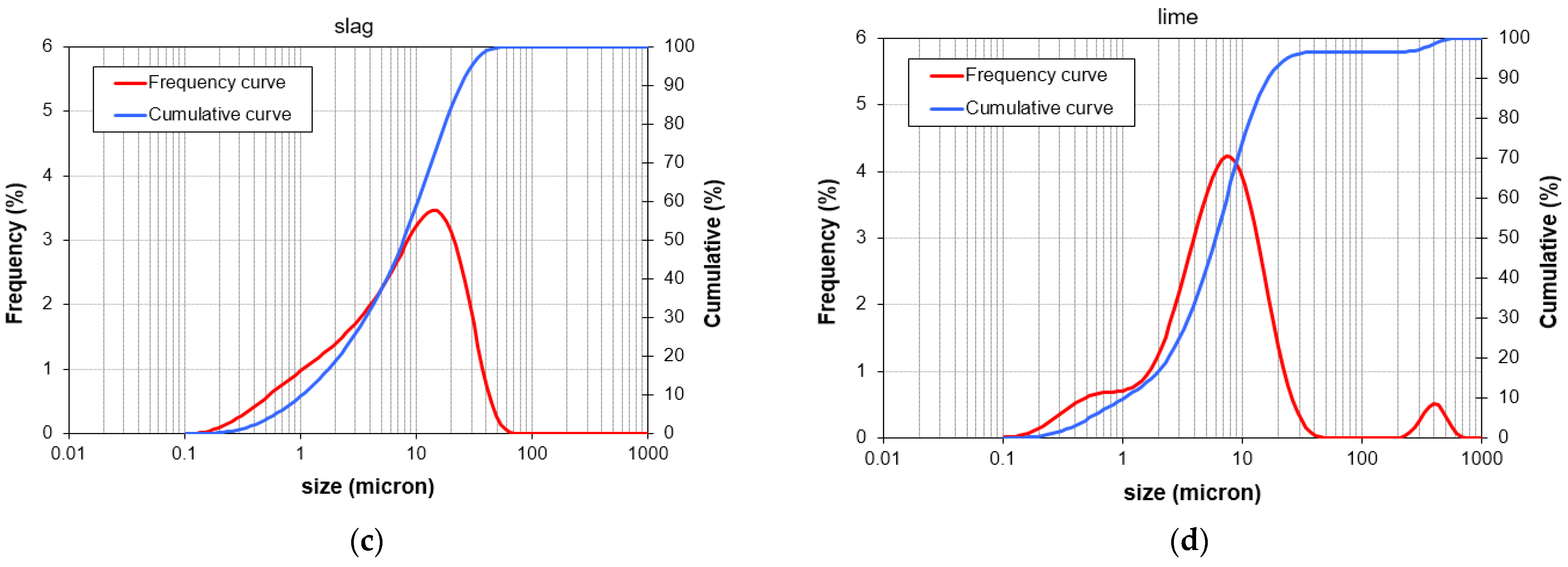
Figure 2 represents the particle size distributions of GGBFS, pumice, waste soil, and hydrated lime. The alkaline activating solutions employed in this research consist of liquid sodium silicate with a SiO
2-to-Na
2O ratio of 2.5 and powdered sodium hydroxide with a purity level exceeding 99%. The chemical analysis of these two alkaline materials is presented in
Table 3. Two different sizes of sand were incorporated in the mixtures: a medium size (with nominal dimension of 0.6 mm) and a smaller one with sizes that pass the sieve No#40. The physical characteristics of the sands are presented in
Table 4.
The raw materials used for geopolymerization include pumice, iron blast furnace slag, and municipal solid waste; these materials were obtained, respectively, from the Khash cement factory, the Sepahan cement factory, and aggregate production plants in the eastern region of Tehran, Iran. Hydrated lime was sourced from the Spandar factories in the Tehran province. Liquid sodium silicate was supplied by Nafis Silicate Sepahan Company in the Isfahan province, Iran. Sodium hydroxide powder was acquired from Kian Kaveh Azma Company in the Tehran province, Iran. The chemical characteristics of the materials such as sodium silicate, sodium hydroxide, and calcium hydroxide, as shown in
Table 2 and
Table 3, were obtained from the technical data provided by the suppliers.
2.2. Specimens Preparation
In this study, three plain mixtures were produced, of which the raw materials were GGBFS, pumice, and the waste soil, respectively. In the six other mixtures, the raw materials were used in the hybrid compositions. The mix proportions of the geopolymers are presented in
Table 5. It indicates that three mixtures were prepared using only one base material (GGBFS, pumice, and soil waste), while the other six mixtures had different combinations of GGBFS with two other materials.
The specimens were prepared in the following dimensions: cubic specimens with dimensions of 5 cm, cylindrical specimens with a diameter of 10 cm and a height of 20 cm, and prismatic specimens with dimensions of 16 × 4 × 4 cm3. These specimens were subjected to the tests of compressive strength, flexural strength, sorptivity, water absorption, and chloride ion migration. The day before each mixture preparation, an 8 molar NaOH solution was prepared to reach the equilibrium temperature. On the day of the mix production, the dry materials (base material, hydrated lime, and sand) were mixed in a mixer for one minute. Then, the alkaline activating solution, including the NaOH solution and the sodium silicate solution, was added, and subsequent mixing was carried out for 4 min. The molded specimens were placed on a vibrating table for 30 s.
The selection of specimen geometry is crucial in determining the mechanical properties of materials, so it is necessary to use different specimen geometries to assess their mechanical properties and durability. The choice of geometry depends on the type of test conducted and the desired properties for evaluation [
17]. In this study, cubic specimens were used to determine compressive strength. The main reason for using these specimens was the ability to create smaller-sized samples and facilitate the manufacturing process. For flexural specimens, truncated cone molds were utilized, enabling the four-point bending test to be performed. In durability tests such as RCMT and sorptivity, cylindrical specimens were employed in accordance with the test method.
To achieve optimal economic and satisfactory results, this study aimed to use an appropriate molarity and ratio of sodium hydroxide and water glass. Higher molarities and ratios of these components lead to additional costs for geopolymer production without economic benefits.
The water content used in geopolymer mixtures has a significant impact on their strength and durability. The mechanical properties and durability of geopolymer mortars are improved when the water content in the mixture is low. On the other hand, adding more water can enhance the workability of the mixture but may reduce other properties of geopolymer concrete. Therefore, maintaining a low water content while ensuring adequate workability is crucial for optimizing the geopolymerization process and achieving desirable performance [
18].
2.3. The Curing Conditions and Tests
The specimens were subjected to specific curing conditions, which are described as follows:
- -
Curing regime 1 (C.24H): The specimens were placed in an oven at a temperature of 70 °C for duration of 24 h. During this period, the specimens were surrounded by wet cloth and enclosed in plastic containers. To prevent sticking, a paper was placed between the plastic containers and the tray surface of the oven.
- -
Curing regime 2 (C.72H): Initially, the specimens were subjected to the same conditions as curing condition 1 for 24 h at 70 °C. Subsequently, after the specified time, the temperature was increased to 100 °C for an additional 48 h. In this condition, the wet cloth was removed, but the specimens remained in the plastic containers. The total curing time for this condition was 72 h. To prevent sticking, a paper was used between the plastic containers and the tray surface of the oven.
- -
Curing regime 3 (C.24H+7d): This condition was similar to curing condition 1, with the only difference being that after removal from the oven, the specimens were kept at room temperature for 7 days.
- -
Curing regime 4 (C.72H+7d): Similar to curing condition 2, the specimens were initially subjected to the same conditions but were then kept at room temperature for 7 days after removal from the oven.
The cubic specimens were divided into two groups for testing. One group was subjected to curing regimes 1 (C.24H) and 2 (C.72H) for compressive strength tests, while the second group was subjected to curing regimes 3 (C.24H+7d) and 4 (C.72H+7d) for water absorption tests. The cylindrical specimens were subjected to curing regime 3 (C.24H+7d) for chloride ion penetration and sorptivity tests. The prismatic specimens underwent curing conditions 1 (C.24H) and 3 (C.24H+7d) for flexural tests.
These specific curing conditions were employed to prevent initial cracking of the specimens and to preserve the water content necessary for geopolymerization processes. After the initial 24 h and subsequent 48 h, the specimens did not exhibit further cracking, allowing the removal of the wet cloth. However, they were still kept inside the plastic containers to achieve maximum strength.
Several tests, including compressive strength, flexural strength, water absorption, sorptivity, and chloride ion penetration, were conducted on the specimens to assess their mechanical properties and durability. For the chloride ion migration test, the specimens were taken from the middle section of the cylindrical specimens, while samples from the top and bottom sections were used for sorptivity and water absorption tests, respectively.
3. Results
3.1. Compressive Strength
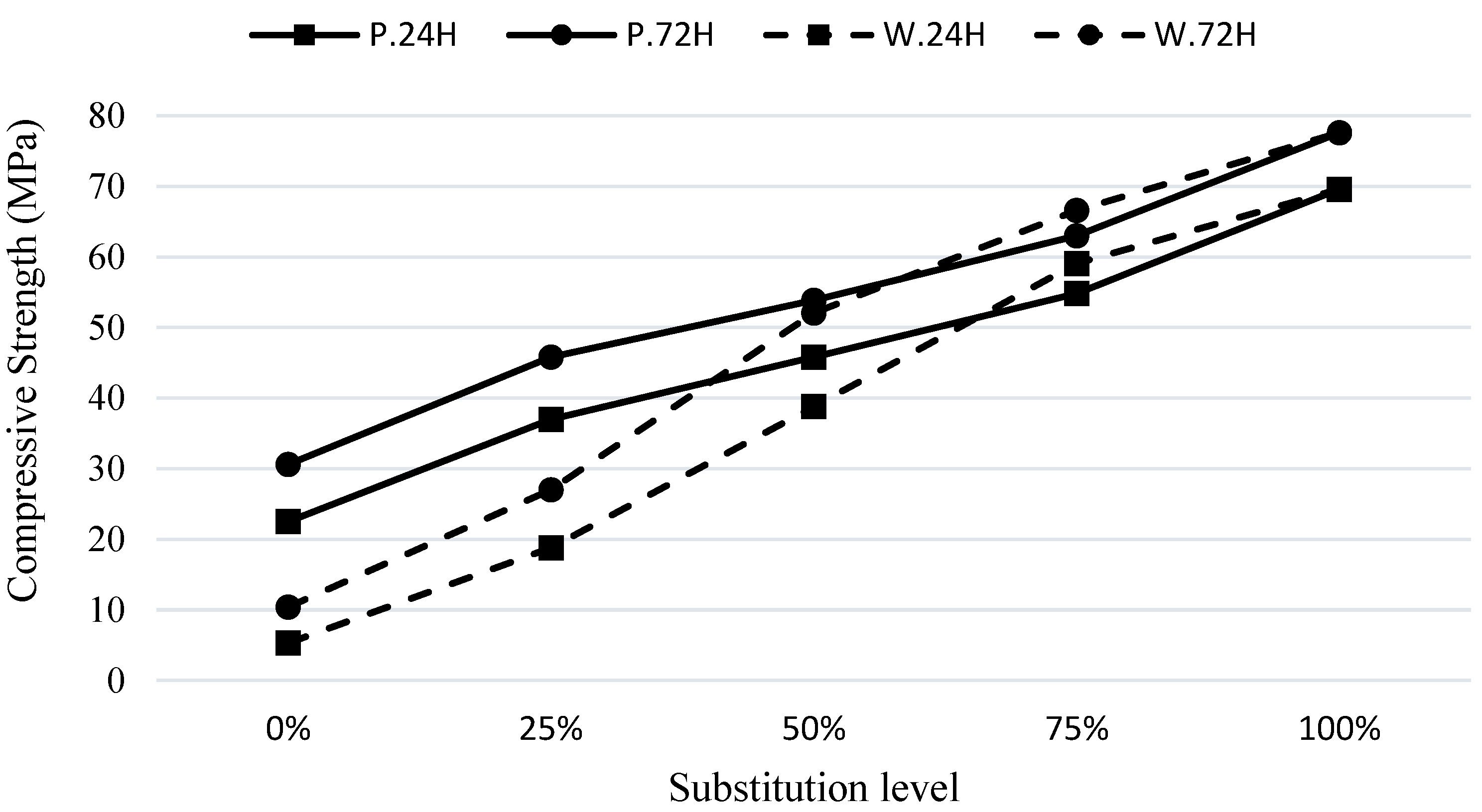
The compressive strengths of the S100 mixture, containing only GGBFS, were 70 MPa and 78 MPa for the C.24H and C.72H curing methods, respectively. These values were 23 MPa and 30 MPa for the mixture containing pumice, and 5 MPa and 10 MPa for the mixture containing the waste. These results indicate the enhanced reactivity of GGBFS compared to the other two materials.
In
Figure 3, the legends P24.H, P72.H, W24.H, and W72.H are used. In these abbreviations, 24.H and 72.H refer to the curing conditions mentioned in
Section 2.3. Additionally, the letters P and W represent mix designs containing pumice and waste soil, respectively. As seen on the horizontal axis of the figure, varying the substitution percentage of these materials with iron blast furnace slag from 0% to 100% allows for investigating changes in the mechanical properties. Similar preparations were made for other figures.
As shown in
Figure 3, the introduction of GGBFS as a partial replacement for pumice and soil waste in different proportions significantly enhances the compressive strength of the composites. Specifically, in specimens cured under the C.72H method, the mixtures incorporating 25% GGBFS replacement exhibited an increase in compressive strength by 49% and 160%, respectively. This shows the substantial influence of GGBFS as a participating component in the geopolymerization process, which aligns with the findings reported by Partha et al. [
10] and Bellum et al. [
11] in their studies on GGBFS utilization.
In the production of geopolymer specimens, water is not directly introduced into the mixture, as the bonding process relies on the use of solution-based materials (activators and sodium silicate). These solutions have a constant water-to-dry material ratio during the geopolymerization process. Therefore, manipulating the water-to-dry material ratio in geopolymer mixes is not feasible. Consequently, the mixtures have been carefully formulated to ensure that geopolymer pastes have low water content while maintaining fluidity and suitable workability. This approach minimizes the negative impact on the strength and durability of the samples.
As shown in
Figure 3, after exposure to the C.24H curing method, W100 exhibited the lowest compressive strength, while S100 had the highest compressive strength. It is worth mentioning that by incorporating GGBFS at waste soil substitution proportions of 25%, 50%, and 75% (W75S25, W50S50, and W25S75), significant strength enhancements of 250%, 630%, and 1000% were obtained, respectively. In comparison, replacing pumice with GGBFS in the mixtures of P75S25, P50S50, and P25S75 led to strength improvements of 65%, 100%, and 140%, respectively. The pronounced strength improvements observed in the waste soil mixtures were notably superior to those achieved in pumice mixes.
Moreover, transitioning the curing regime from C.24H to C.72H resulted in enhanced compressive strength across all cases, signifying the improved structural characteristics of the mixes due to increased alumino-silicate bonding.
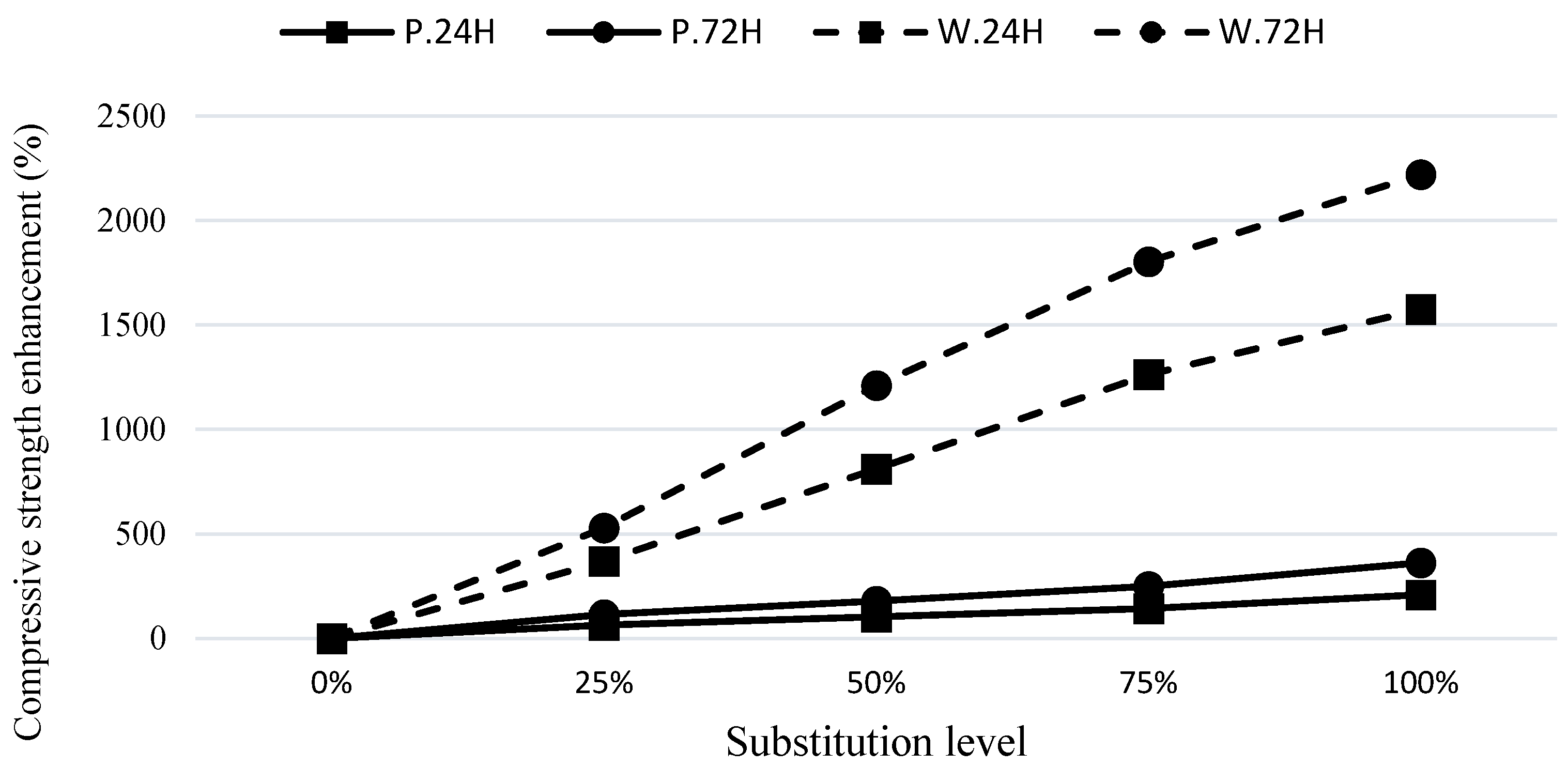
Figure 4 provides a visual representation of the improvement in strength for the various curing conditions.
The C.24H curing method demonstrates remarkable structural enhancements and significant strength gains, even at early ages, in the specimens incorporating GGBFS and the waste soil. This confirms the significant influence of GGBFS on enhancing the structural properties and strength of the mixtures, especially during the early stages of the microstructure formation.
3.2. Modulus of Rupture
In order to perform the modulus of rupture test according to the EN 196 [
19] standard, the specimens were subjected to a four-point bending test. The loading rate applied to the specimens was 0.04 in/min.
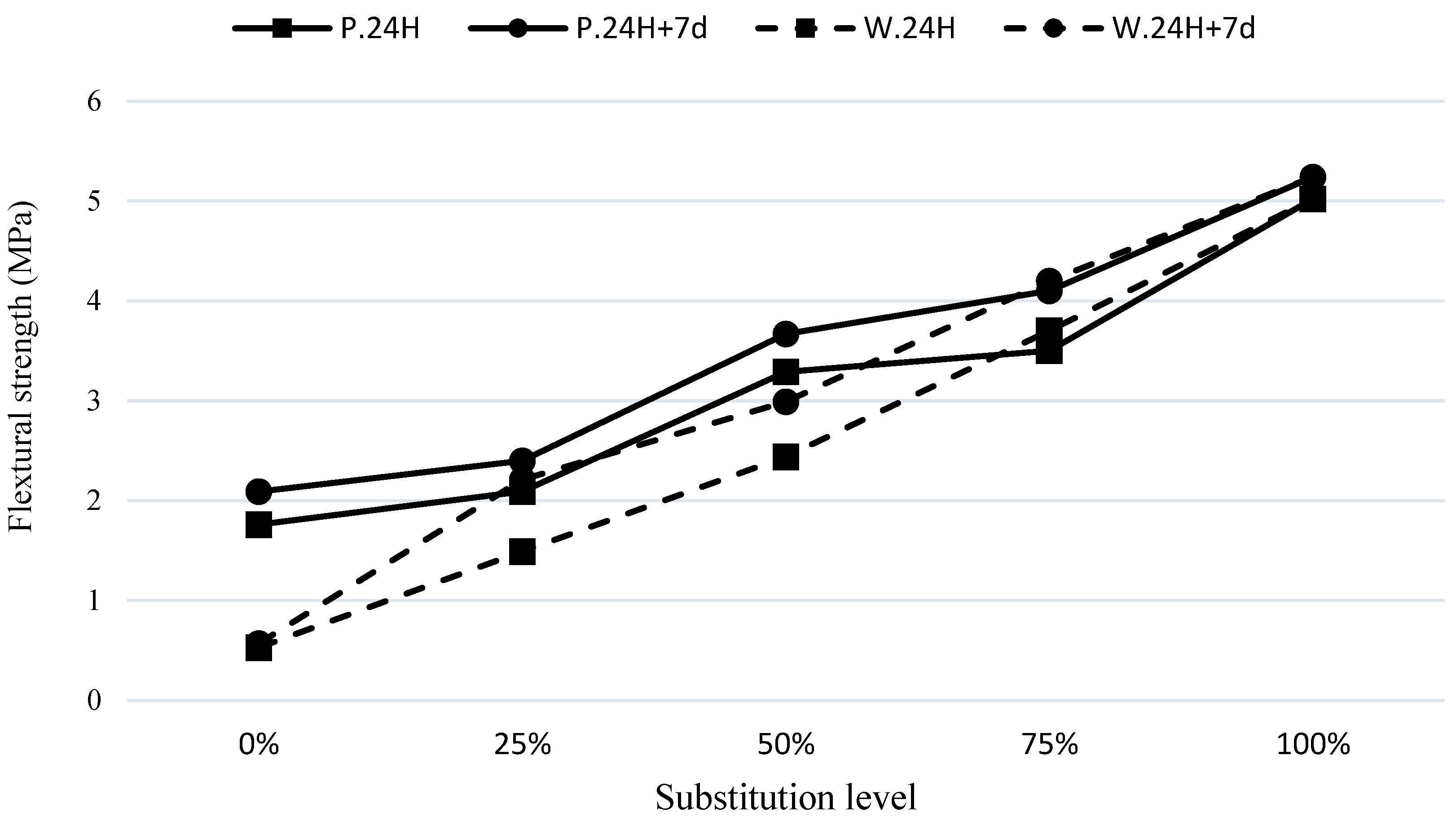
According to
Figure 5, it can be observed that the S100 mixture exhibited the highest strength of 5.02 MPa, while the W100 mixture had the lowest strength of 0.53 MPa in the curing condition of C.24H. It is evident that the increase in the modulus of rupture of the waste soil mix with 25% GGBFS (W75S25) was much higher compared to the increase in flexural strength of the pumice with 25% GGBFS (P75S25). This indicates that GGBFS has a superior influence on the waste soil compared to its effect on the pumice. In the C.24H+7d curing condition, the specimens had a slight increase in strength, indicating that 7 days curing at room temperature could contribute to the improvement of the microstructure. The reason for this phenomenon can be attributed to the continuous reactions taking place in the geopolymer compositions containing waste and pozzolanic materials after accelerated curing. It appears that the initial activity of the base materials has had a greater impact on their final performance, and the synergistic effect of slag on materials with lower activity (waste soil and pozzolan) has been more significant.
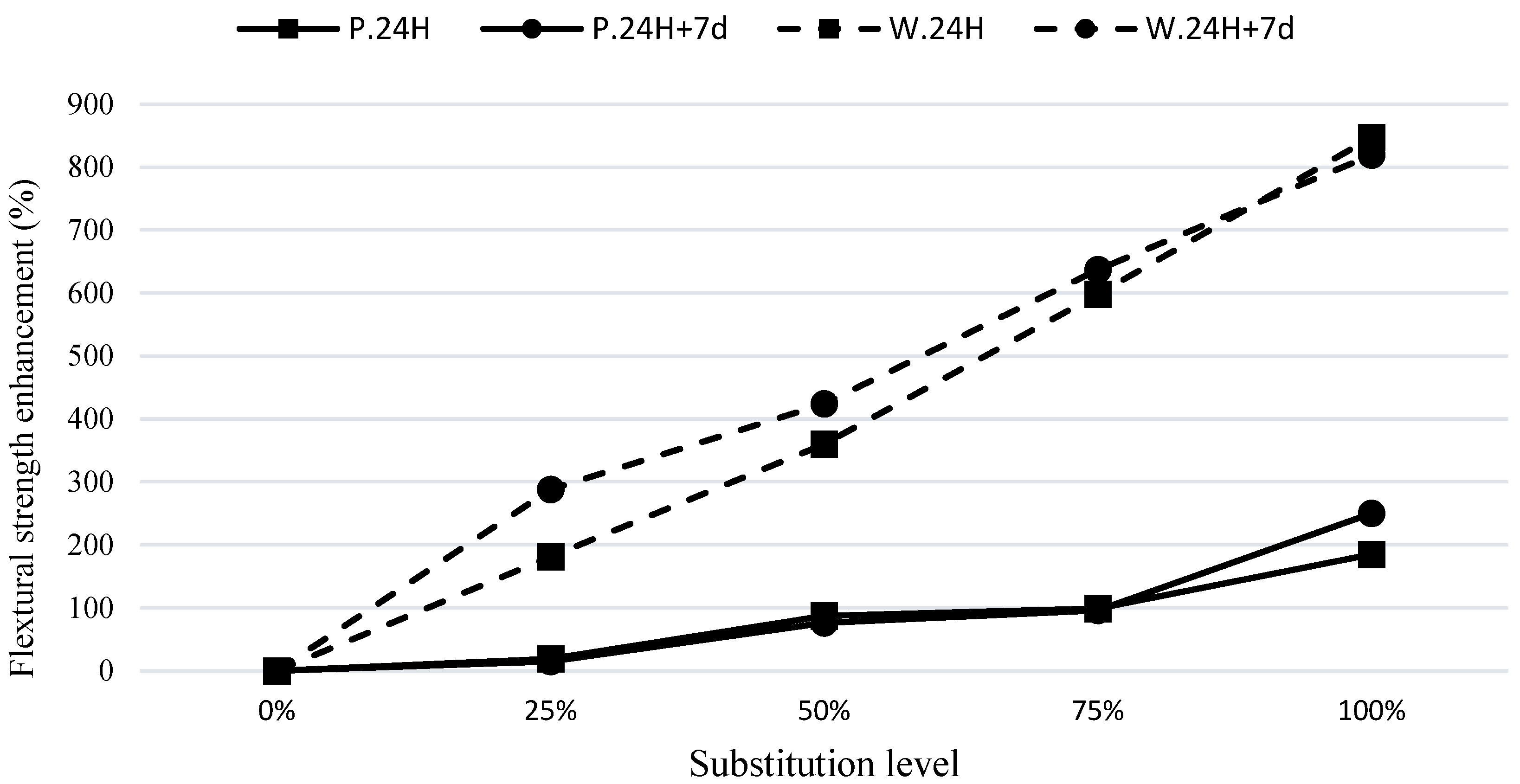
As shown in
Figure 6, for the modified waste soil mixes, the increase in the modulus of rupture with curing conditions of C.24H+7d was higher than the increase in curing conditions C.24H. This indicates that the 100% waste soil sample (W100), with the addition of GGBFS, experienced a significant improvement in flexural strength at a higher curing time. This can be attributed to the continuation of reactions in the geopolymer compounds containing waste soil after a conventional curing of 7 days. It appears that the initial activity of the base materials had a greater impact on their final performance, and the synergistic effect of GGBFS on the material with lower activity (waste soil) was more noticeable.
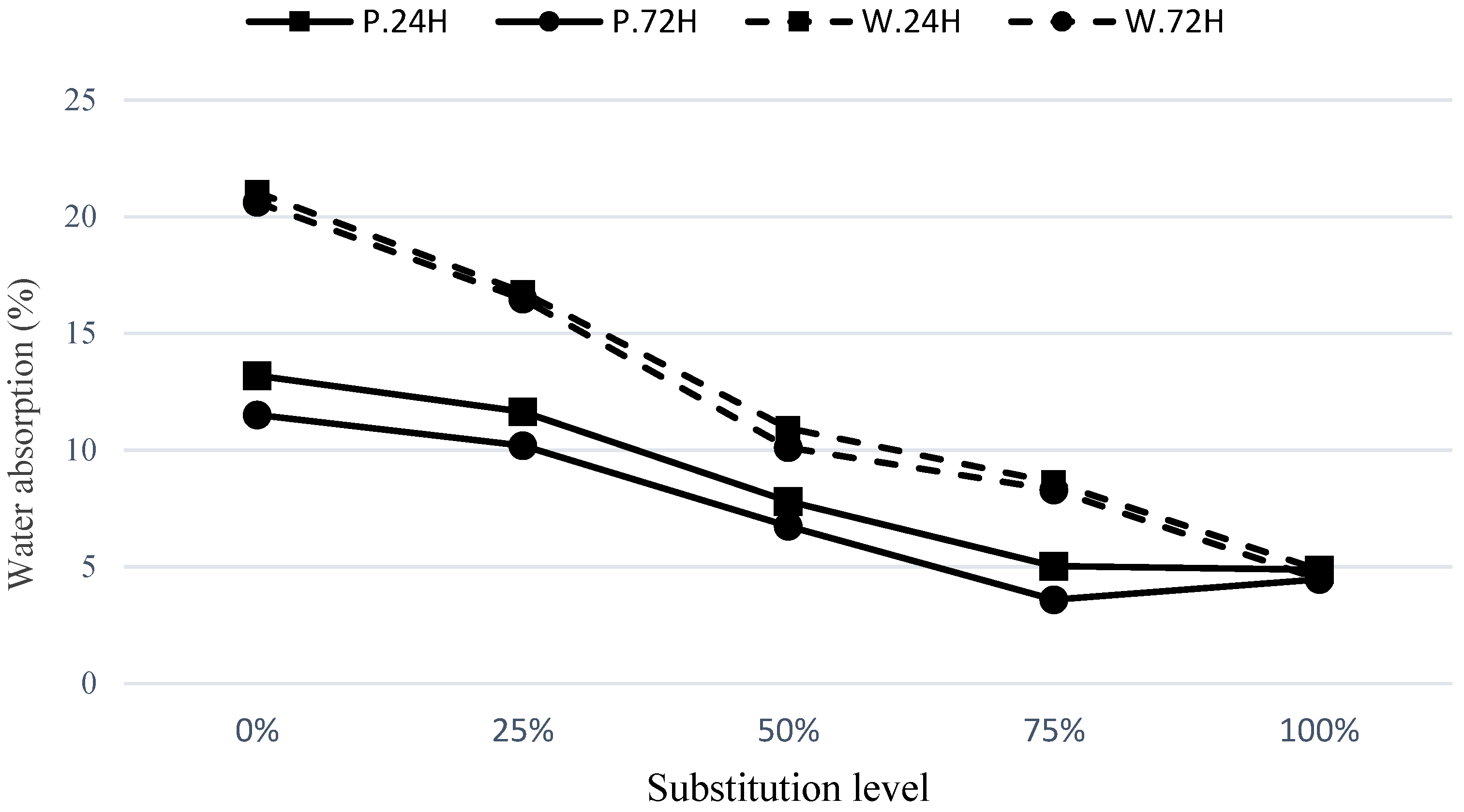
3.3. Water Absorption
The water absorption test was performed on cubic specimens with dimensions of 5 cm, as per ASTM C 642–06 [
20]. After a 7-day period, the cubic specimens were placed inside an oven at a temperature of 100 °C, and their weights were monitored at the first 30 min and every 24 h until the weight difference compared to the average dry weight of the specimens was less than 1%. The specimens were then removed from the oven, and their weights were considered as the dry weights. Subsequently, the specimens were immersed in water, and their weights were measured every 24 h until the weight difference compared to the previous day was less than 1%. In this state, the weights of the samples were noted as the saturated weight.
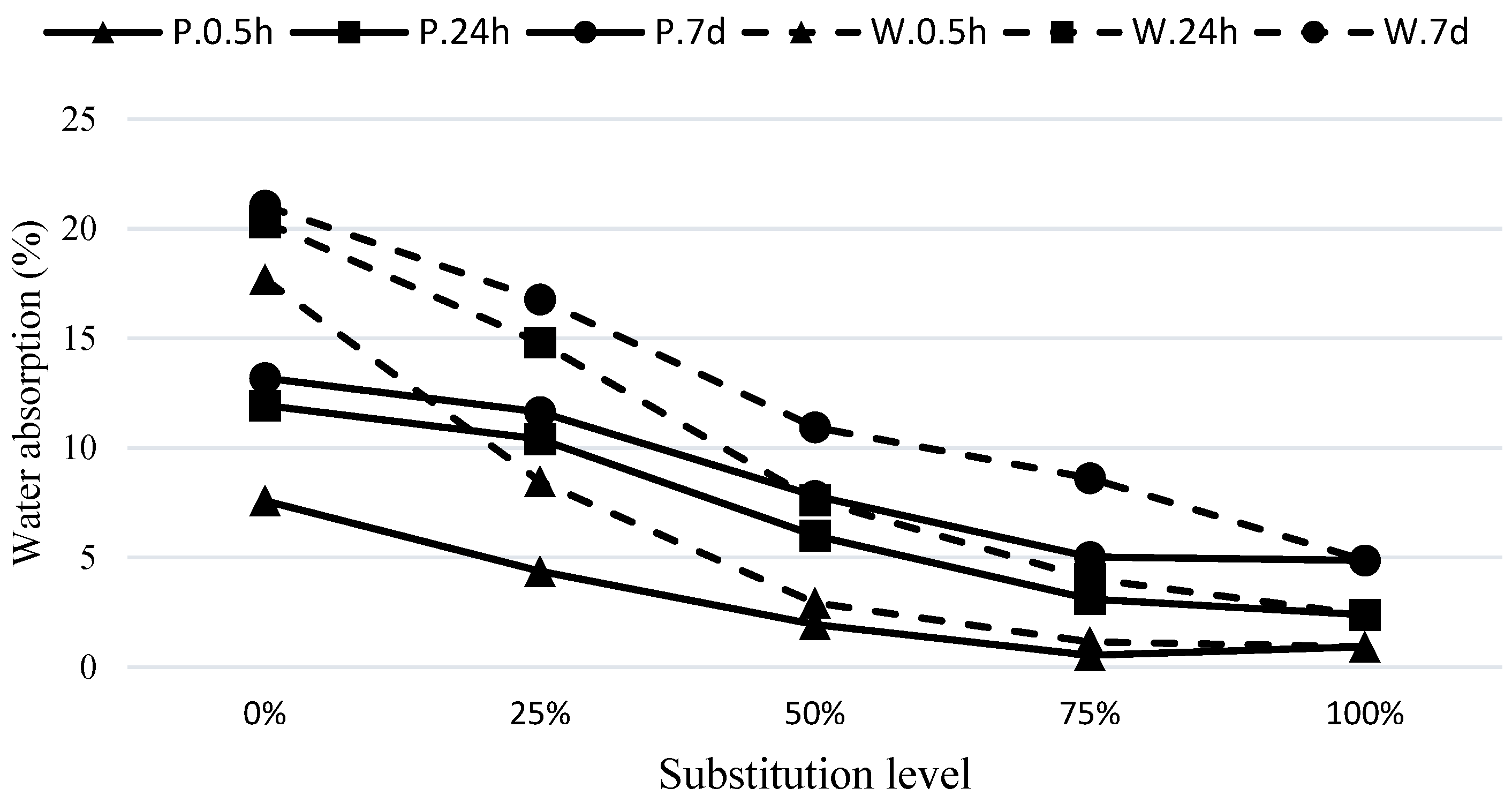
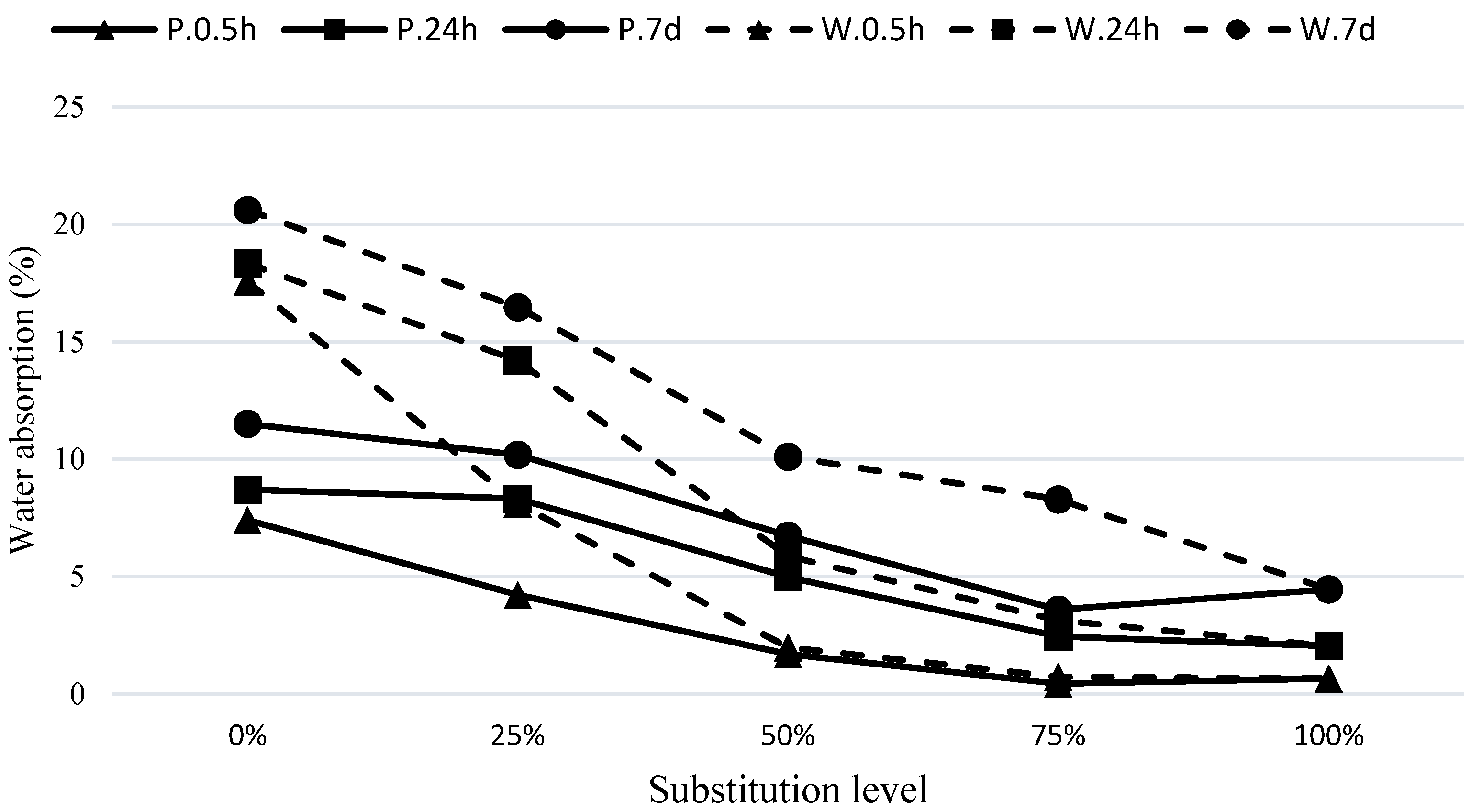
The water absorption values at 30 min, 24 h, and 7 days for the concrete mixtures under C.24H and C.72H curing conditions are presented in
Figure 7 and
Figure 8, respectively. A comparison between the final water absorption contents of the mixes under different curing regimes has been made in
Figure 9.
As observed in
Figure 7, under the C.24H curing method, the mixes containing pumice have lower water absorption compared to the waste soil mixtures. As the substitution level of GGBFS in the hybrid mixes increases, the permeability decreases. This is evident in the mixtures with 25% and 50% GGBFS, which show a significant reduction in water absorption. For example, in the W75S25 mixture compared to W100, the water absorption contents at 30 min and 24 h and the final absorption were reduced by about 52%, 27%, and 20%, respectively. Similarly, compared to P100, the mixture of P75S25 had reduced water absorptions of 42%, 13%, and 12%, respectively. It can be noticed that the effect of GGBFS replacement on improving the absorption within the first 30 min is much higher compared to its effect on 24 h and final water absorptions, indicating a significant improvement in the microstructure in terms of fineness and blockage of pores in these mixtures. The reduction in 24 h and final water absorptions also indicates a decrease in overall porosity of these mixtures. As shown in
Figure 8, similar results have been obtained using the curing method of C.72H.
In
Figure 9, a comparison is made between the final water absorption of the mixtures cured for 72 h (C.72H) and 24 h (C.24H). A certain reduction is observed in the 72 h curing method compared to the other one. Furthermore, as the replacement level of GGBFS relative to the waste soil and pumice increases, the water absorption of the specimens decreases. When comparing the waste soil and pumice at the same incorporation levels, the mixtures containing pumice have lower water absorption compared to those with the waste soil. However, with an increase in the curing period, a greater reduction in water absorption of the waste soil mixes was obtained compared to the pumice incorporated ones. These results indicate that due to the higher reactivity of pumice compared to the waste soil, better results were achieved for all the mixes of this material. Due to less reactivity of the waste soil, GGBFS has a higher enhancing effect on mixtures containing this material.
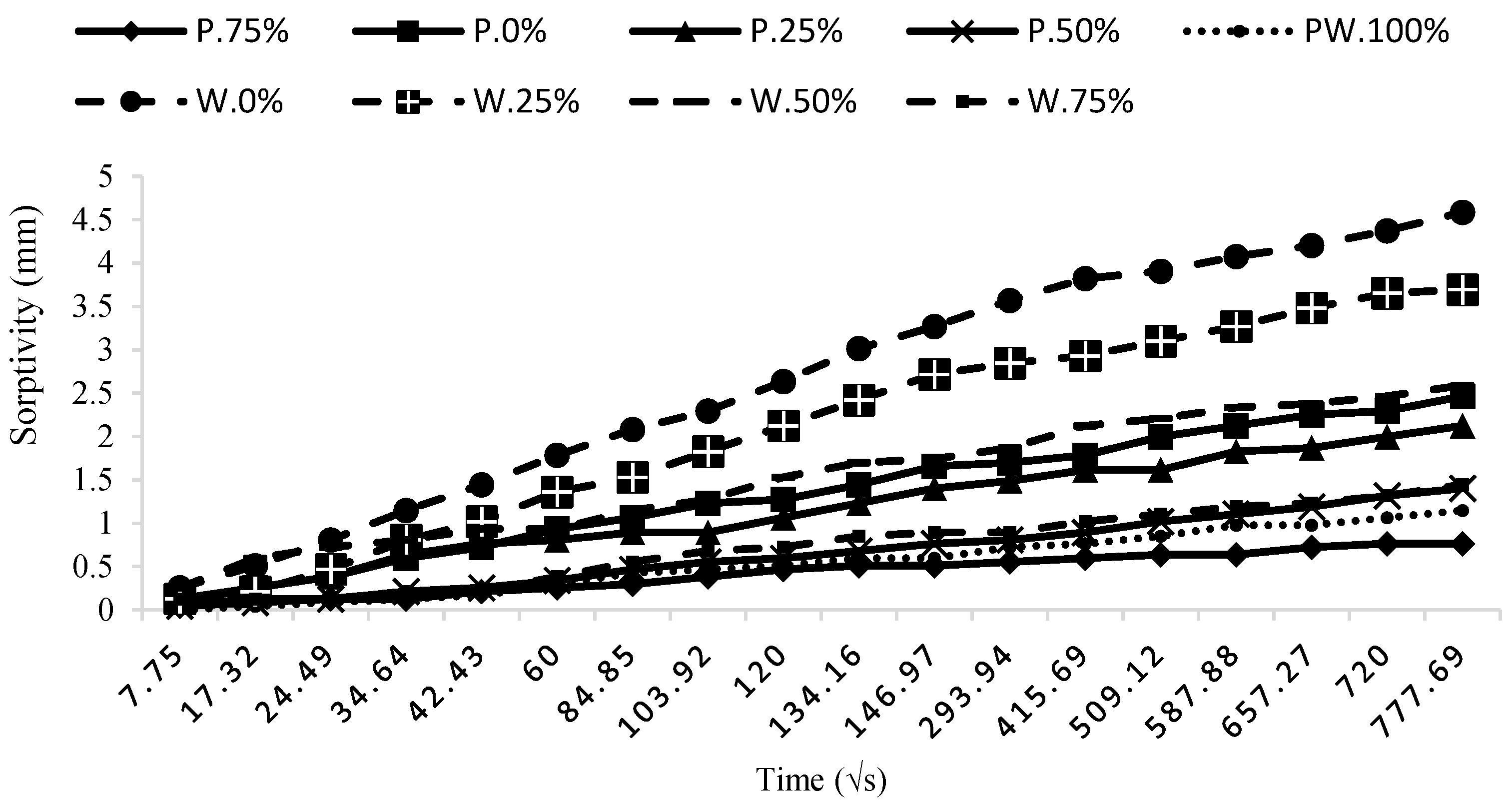
3.4. Sorptivity
According to the ASTM C1585-04 [
21] standard, the sorptivity test was conducted on cylindrical specimens. In this regard, the specimens were subjected to a drying period of one week at a temperature of 70 °C to remove water from the voids in the concrete microstructure. This test was carried out on cylindrical specimens using the C.24H+7d curing procedure. The results in
Figure 10 demonstrate that the use of GGBFS, even at a replacement level of 25%, could significantly improve the microstructure of the specimens. The mixture of W100 exhibited the highest sorptivity compared to the other specimens, while P25S75 had the best performance. The variations observed with the GGBFS replacement in mixtures containing waste soil are significantly greater than the variations in mixtures containing pumice. This result suggests a more active role of pumice compared to the waste soil. However, the mixture with 25% waste soil and 75% GGBFS (W25S75) had an acceptable performance among the investigated mixes.
According to
Figure 10, it can be observed that an increase in the percentage of GGBFS in the specimens leads to a decrease in the slope of the sorptivity curve, indicating a reduction in the rate of water penetration through the pores and defects of the concrete. The replacement of 25% GGBFS in mixtures containing pumice and the waste soil resulted in a reduction of 18% and 19% in the specimens, respectively. Additionally, the mixes with 75% GGBFS (P25S75 and W25S75) had a decrease of 53% and 68%, respectively, in the final water penetration compared to the plain mixes (P100 and W100).
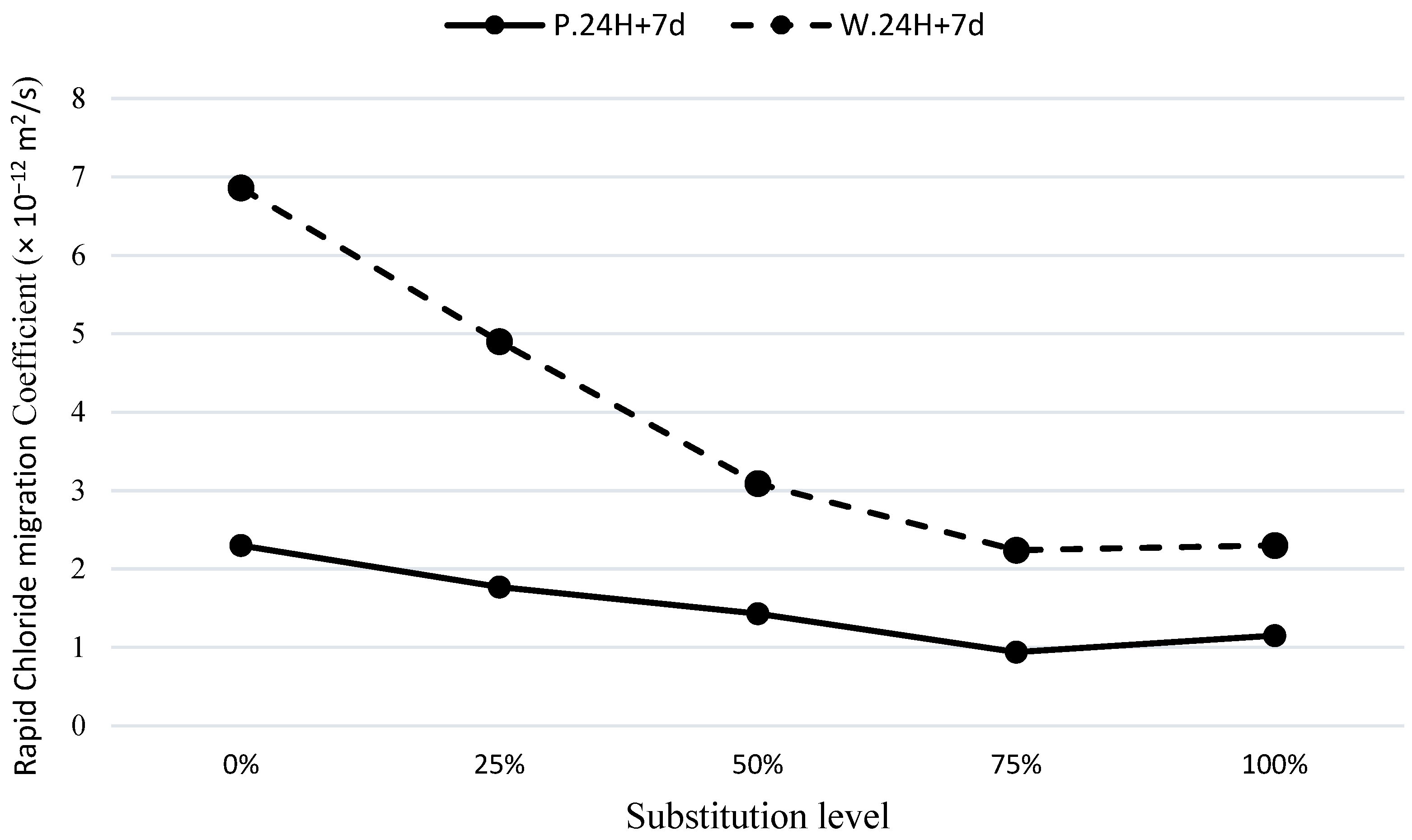
3.5. Rapid Chloride Migration Test (RCMT)
The Rapid Chloride Migration Test (RCMT) is one of the most reliable tests for assessing the permeability of concrete. This test was conducted according to the NT BUILD 492 standard [
22]. The results of RCMT for the mixtures in this study are presented in
Figure 11. The test was performed on cylindrical specimens subjected to the C.24H+7d curing regime. By comparing the plain mixtures P100 and W100 with the modified mixes, it was found that the use of GGBFS for modifying the base material of the geopolymer mixture reduces the chloride ion migration coefficient. Compared to the P100 mixture, the reductions in the RCMT coefficients of the geopolymers with GGBFS replacement levels of 25%, 50%, and 75%, were approximately 20%, 35%, and 60%, respectively. The enhancements in chloride permeability, compared to the W100 mixture, with the mentioned substitution levels were approximately 30%, 63%, and 72%, respectively.
An important observation from the graph is the effect of pumice on GGBFS in the P25S75, which improves the structure of the modified GGBFS samples and reduces chloride ion penetration by approximately 20% compared to S100. It is worth mentioning that this result is in good conformance with the findings obtained from the sorptivity and water absorption test results. Therefore, the combination of 25% pumice and 75% GGBFS can be considered as an optimal composition in terms of permeability characteristics in the durability aspects. The highest impact on the permeability reduction of the samples is attributed to the two mixes of W50S50 and W25S75, where a significant reduction in permeability is observed in the waste soil mixtures containing 50% and 75% GGBFS.
4. Discussion
In the geopolymerization process, the raw material is decomposed in a highly alkaline or acidic medium, and, subsequently, the geopolymer network is formed. However, the raw material may be less active, and creation of a strong network of geopolymer compounds could not be achieved. For instance, in a study by Madani et al. [
16], it was shown that in order to activate the waste soil of the aggregate production plants, particular conditions are required. The main hypothesis of the current study is that it is not necessary to decompose all the raw material to produce a geopolymer binder. In other words, if an active aluminosilicate material is used along with a less active one, the active material could play the main role in producing the geopolymer binder, and the less active one acts as a filler. However, the less active material could also participate in the geopolymerization process. The less active materials investigated in this study are pumice and the waste soil of the aggregate production plants. The active raw material is also GGBFS.
The results reveal that GGBFS has synergistic effects on pumice and the waste soil. In this regard, the mixture of P75S25 outperforms even the plain mixture of GGBFS in the most mechanical and durability characteristics. This may be due to several reasons. First, GGBFS has synergistic effects on pumice performance; second, the chemical composition of pumice leads to an improved structure of the geopolymer binder, and third, due to the filler effect of pumice, enhanced microstructure of the geopolymer composite was obtained.
The influence of GGBFS on enhancement of the characteristics of the waste soil incorporated composites is more pronounced compared to the pumice incorporated mixes. Thus, it is highly recommended to include an active material in the geopolymer composites with a less active one. In other words, hybridization of these materials could provide enough geopolymer gel and filler to bind the composite particles, leading to creation of a better formed microstructure. However, based on the application, the ratio of the materials may be changed.
Prolonging the curing period from 24 h to 72 h leads to enhanced mechanical properties and lower water absorption. This effect is more pronounced in the mixes with the waste soil. Increasing the curing period leads to a higher rate of geopolymerization, leading to a denser microstructure in the composites. In other words, at a prolonged curing time, the raw materials, especially the less active ones, could have greater participation in the geopolymerization process.
In the present study, various raw materials and their combinations have been utilized in the production of geopolymer materials. These materials differ from each other in terms of their chemical composition and mineralogy, resulting in variations in the properties of the final geopolymer composite [
3]. For example, the SiO
2/Al
2O
3 ratio in pumice, GGBFS, and waste soil is 3.3, 3.7, and 4.3, respectively. This ratio plays a crucial role in the geopolymerization process and the final properties of the product [
23]. It has been shown in [
23] that the SiO
2/Al
2O
3 ratio can affect the setting time, development of mechanical properties, and microstructure of geopolymer materials. Materials with higher SiO
2/Al
2O
3 ratios are richer in silica, but it should also be noted that the solubility of silica present in the material is an important factor [
24]. In the present study, the SiO
2/Al
2O
3 ratio in the waste soil is higher than that in GGBFS, but this material has a lower reactivity compared to GGBFS. Therefore, it is possible to achieve a geopolymer composite with desired mechanical properties and durability by combining these two materials. The lower reactivity in materials such as waste soil and pumice is attributed to their natural structure, which consists mainly of crystalline minerals [
16,
25]. It is worth mentioning that the particle sizes of GGBFS, pumice, and waste soil are almost similar. The median sizes of these materials are approximately 8.0 μm, 7.6 μm, and 6.8 μm, respectively. Therefore, it does not appear that the particle size distribution of these materials has a direct impact on the results.
To sum up, it is highly recommended to include a hybrid of less active and active raw materials to produce sustainable geopolymer mixtures with enhanced characteristics.
5. Conclusions
The substitution of the waste soil and pumice with GGBFS at various replacement levels ranging from 25% to 75% has resulted in a substantial enhancement in the mechanical properties, including the compressive strength and modulus of rupture. Remarkably, certain mixtures containing 75% GGBFS have even exhibited higher strengths than those comprising 100% GGBFS.
Moreover, transitioning from C.24H to C.72H curing conditions has consistently led to increased compressive and flexural strength across all the scenarios. This observed improvement may be attributed to the improved microstructure of the specimens, owing to the development of alumino-silicate bonds, potentially promoted by the extended curing duration.
In comparison to the mixtures containing the waste soil, the pumice-incorporated composites, at similar substitution levels, had superior mechanical properties and durability. Notably, due to the relatively lower initial reactivity of the waste soil, when compared to pumice, replacing it with GGBFS has yielded a more pronounced positive effect on enhancing the properties, strongly supporting its recommended usage.
Considering the sustainability aspects, the waste soil is a by-product of aggregate production plants, which is produced in large amounts per year. Thus, several applications for its usage should be proposed. In this research, production of improved geopolymer composites from this material has been suggested.
The P25S75 mixture had the most favorable performance in terms of durability characteristics. This particular mixture exhibits lower chloride ion penetration, water absorption, and sorptivity in comparison to all other compositions, even outperforming the GGBFS plain mixture. Moreover, it has consistently exhibited highly desirable strength properties. Consequently, considering the overall findings, this mixture can be deemed the optimal design in the present study.
It is important to note that the research has solely examined the effect of altering the curing time on water absorption content. In the water absorption test, prolonging the curing time from 24 h to 72 h resulted in reduced water absorption values at 30 min and 24 h and at the final measurement (7 days). However, with an increased curing time under the C.72H regime, higher decrease in the water absorption was noticed in the P25S75 mixture compared to S100, indicating enhanced pumice reactivity at this specific ratio under prolonged curing time.
This research has been a study on the influence of GGBFS on the characteristics of less active geopolymer binders. However, several complementary tests such as microstructural analyses of geopolymer composites have not been investigated here and are recommended for future studies.
Author Contributions
Conceptualization, H.M.; methodology, H.M. and M.N.N.; software, J.H.; validation, J.H., H.M. and M.N.N.; formal analysis, J.H. and H.M.; investigation, J.H. and H.M.; resources, J.H. and H.M.; data curation, J.H. and M.N.N.; writing—original draft preparation, J.H. and H.M.; writing—review and editing, J.H., H.M. and M.N.N.; visualization, J.H. and H.M.; supervision, H.M.; project administration, J.H. and H.M.; funding acquisition, J.H. and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. However, please note that the data cannot be made publicly available due to a confidentiality agreement with the participants.
Acknowledgments
The preparation, processing, and all experiments conducted in this study were carried out at the Graduate University of Advanced Technology, Kerman, Iran. The authors would like to express their gratitude to the university for the support and facilities provided for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davidovits, J. The Need to Create a New Technical Language for the Transfer of Basic Scientific Information. In Proceedings of the Transfer and Exploitation of Scientific and Technical Information, Luxembourg, 10–12 June 1981; Commission of the European Communities: Luxembourg, 1982; pp. 19–23. [Google Scholar]
- Davidovits, J. Geopolymers: Inorganic Polymeric New Materials. J. Therm. Anal. Calorim. 2005, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Institut Géopolymère: Saint-Quentin, France, 2008. [Google Scholar]
- Davidovits, J. Environmentally Driven Geopolymer Cement Applications. 1 January 2002. Available online: https://www.geopolymer.org/wp-content/uploads/ENVIRONMENT.pdf (accessed on 20 July 2023).
- Thakur, R.N.; Ghosh, S. Effect of mix composition on compressive strength and microstructure of fly ash based geopolymer composites. J. Eng. Appl. Sci. 2009, 4, 68–74. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Neville, A.M. Properties of Concrete, 4th ed.; Pearson Education Limited: London, UK, 2004. [Google Scholar]
- Allahverdi, A.; Yazdanipour, M.; Hashemi, M. Investigation the set and strength behaviors of Blast-furnace GGBFS Blended Geopolymer Cement Based on Pumice. In Proceedings of the 11th Iranian Chemical Engineering Congress (ICHEC11), Tehran, Iran, 28–30 November 2006. [Google Scholar]
- Nadoushan, M.J.; Ramezanianpour, A.A. The Effect of Type and Concentration of Activators on Flowability and Compressive Strength of Natural Pozzolan and Slag-Based Geopolymers. Constr. Build. Mater. 2016, 111, 337–347. [Google Scholar] [CrossRef]
- Partha, S.D.; Pradip, N.; Prabir, K.S. Strength and Permeation Properties of Slag Blended Fly Ash Based Geopolymer Concrete. Adv. Mater. Res. 2013, 651, 168–173. [Google Scholar] [CrossRef]
- Bellum, R.R.; Nerella, R.; Madduru, S.R.C.; Indukuri, C.S.R. Mix Design and Mechanical Properties of Fly Ash and GGBFS-Synthesized Alkali-Activated Concrete (AAC). Infrastructures 2019, 4, 20. [Google Scholar] [CrossRef]
- Li, K.-Q.; Chen, G.; Liu, Y.; Yin, Z.-Y. Scale Effect on the Apparent Anisotropic Hydraulic Conductivity of Geomaterials. ASME J. Risk Uncertain. Eng. Syst. Part A Civ. Eng. 2023, 9, 04023020. [Google Scholar] [CrossRef]
- Li, K.-Q.; Liu, Y.; Yin, Z.-Y. An Improved 3D Microstructure Reconstruction Approach for Porous Media. Acta Mater. 2023, 242, 118472. [Google Scholar] [CrossRef]
- Li, K.-Q.; Li, D.-Q.; Li, P.-T.; Liu, Y. Meso-Mechanical Investigations on the Overall Elastic Properties of Multi-Phase Construction Materials Using Finite Element Method. Constr. Build. Mater. 2019, 228, 116727. [Google Scholar] [CrossRef]
- Wang, F.; Li, K.; Liu, Y. Optimal Water-Cement Ratio of Cement-Stabilized Soil. Constr. Build. Mater. 2022, 320, 126211. [Google Scholar] [CrossRef]
- Madani, H.; Ramezanianpour, A.A.; Shahbazinia, M.; Ahmadi, E. Geopolymer bricks made from less active waste materials. Constr. Build. Mater. 2020, 247, 118441. [Google Scholar] [CrossRef]
- Yi, S.-T.; Yang, E.-I.; Choi, J.-C. Effect of Specimen Sizes, Specimen Shapes, and Placement Directions on Compressive Strength of Concrete. Nucl. Eng. Des. 2006, 236, 115–127. [Google Scholar] [CrossRef]
- Wei, X.; Ming, F.; Li, D.; Chen, L.; Liu, Y. Influence of Water Content on Mechanical Strength and Microstructure of Alkali-Activated Fly Ash/GGBFS Mortars Cured at Cold and Polar Regions. Materials 2020, 13, 138. [Google Scholar] [CrossRef]
- BS EN 196-1:2016; Methods of Testing Cement—Determination of Strength. British Standards: London, UK, 2005.
- ASTM C 642-06; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C1585-20; Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes. ASTM International: West Conshohocken, PA, USA, 2020.
- Nordtest Method. NT BUILD 492, Chloride Migration Coefficient From Non-Steady-State Migration Experiments; Nordtest Method: Oslo, Norway, 1999. [Google Scholar]
- Słomka-Słupik, B.; Wiśniewska, P.; Bargieł, W. Multicomponent Low Initial Molar Ratio of SiO2/Al2O3 Geopolymer Mortars: Pilot Research. Materials 2022, 15, 5943. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Madani, H.; Norouzifar, M.N.; Rostami, J. The Synergistic Effect of Pumice and Silica Fume on the Durability and Mechanical Characteristics of Eco-Friendly Concrete. Constr. Build. Mater. 2018, 174, 356–368. [Google Scholar] [CrossRef]
Figure 1.
The raw materials: (a) Waste Soil, (b) GGBFS, (c) Pumice.
Figure 1.
The raw materials: (a) Waste Soil, (b) GGBFS, (c) Pumice.
Figure 2.
The particle size distributions of (a) pumice, (b) waste soil, (c) GGBFS, and (d) hydrated lime.
Figure 2.
The particle size distributions of (a) pumice, (b) waste soil, (c) GGBFS, and (d) hydrated lime.
Figure 3.
The effect of GGBFS on the compressive strength of the mixes with the waste soil and pumice.
Figure 3.
The effect of GGBFS on the compressive strength of the mixes with the waste soil and pumice.
Figure 4.
The increase in compressive strength of the composites with GGBFS compared to the plain mixtures.
Figure 4.
The increase in compressive strength of the composites with GGBFS compared to the plain mixtures.
Figure 5.
The influence of GGBFS on the modulus of rupture of the mixes with the waste soil and pumice.
Figure 5.
The influence of GGBFS on the modulus of rupture of the mixes with the waste soil and pumice.
Figure 6.
Increase in the modulus of rupture of the geopolymer hybrid mixes containing GGBFS compared to the plain mixtures.
Figure 6.
Increase in the modulus of rupture of the geopolymer hybrid mixes containing GGBFS compared to the plain mixtures.
Figure 7.
Water absorption at 30 min and 24 h and the final absorption of the mixes subjected to the C.24H curing condition.
Figure 7.
Water absorption at 30 min and 24 h and the final absorption of the mixes subjected to the C.24H curing condition.
Figure 8.
Water absorption at 30 min and 24 h and the final absorption of the mixes subjected to the C.72H curing condition.
Figure 8.
Water absorption at 30 min and 24 h and the final absorption of the mixes subjected to the C.72H curing condition.
Figure 9.
The effect of GGBFS on the final water absorption content of the mixes made with waste soil and pumice.
Figure 9.
The effect of GGBFS on the final water absorption content of the mixes made with waste soil and pumice.
Figure 10.
The sorptivity of the modified mixes with GGBFS under the C.24H treatment.
Figure 10.
The sorptivity of the modified mixes with GGBFS under the C.24H treatment.
Figure 11.
The rapid chloride ion migration coefficient under C.24H+7d curing conditions.
Figure 11.
The rapid chloride ion migration coefficient under C.24H+7d curing conditions.
Table 1.
XRF analyses of GGBFS, pumice, and the waste soil.
Table 1.
XRF analyses of GGBFS, pumice, and the waste soil.
| | Chemical Analysis | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | Cl | SO3 | L.O.I |
|---|
| GGBFS | Result (%) | 32.5 | 8.8 | 44 | 1 | 6.8 | 0.025 | 2.5 | 0.3 |
| Pumice | Result (%) | 60.5 | 18.4 | 7.8 | 5 | 2.2 | 0.03 | 0.3 | 2.5 |
| Waste Soil | Result (%) | 52.3 | 12.11 | 16.96 | 5.61 | 2.38 | – | 0.24 | – |
Table 2.
Properties of the hydrated lime.
Table 2.
Properties of the hydrated lime.
| Purity Grade (CaO) | Specific Weight (kg/m3) |
|---|
| 0.98 | 2200 |
Table 3.
Chemical analysis of the sodium hydroxide and the sodium silicate solution.
Table 3.
Chemical analysis of the sodium hydroxide and the sodium silicate solution.
| NaOH | Na2SiO3 Solution |
|---|
| Chemical Analysis | Result | Unit | Chemical Analysis | Result | Unit |
|---|
| NaOH | >99 | % | SiO2
Na2O
Water | 20.95
8.38
70.67 | %
%
% |
| Na2CO3 | <1 | % |
| Pb | <0.05 | % |
| Cl | <0.05 | % |
| SO4 | <0.05 | % |
| Density | 2.13 g/cm3 | Density | 1.230 g/cm3 |
Table 4.
Physical characteristics of the used aggregate.
Table 4.
Physical characteristics of the used aggregate.
| | Size (mm) | Moisture %SSD | Specific Gravity (g/cm3) |
|---|
| Fine Sand | <0.425 | 0.8 | 2.6 |
| Coarse Sand | 0.15–1.81 | 0.8 | 2.6 |
Table 5.
Proportions used in the mixes of the present study (relative values according to the base material weight (total weight of pumice, GGBFS, and waste soil)) 1,2,3,4.
Table 5.
Proportions used in the mixes of the present study (relative values according to the base material weight (total weight of pumice, GGBFS, and waste soil)) 1,2,3,4.
| Mix Designs | Base Material | Calcium Hydroxide | Sodium Hydroxide Solution | Sodium Silicate Solution |
|---|
| Waste | Pumice | GGBFS |
|---|
| S100 | - | - | 1 | 0.11 | 0.14 | 0.35 |
| P100 | - | 1 | - | 0.11 | 0.14 | 0.35 |
| W100 | 1 | - | - | 0.11 | 0.14 | 0.35 |
| P75S25 | - | 0.75 | 0.25 | 0.11 | 0.14 | 0.35 |
| P50S50 | - | 0.5 | 0.5 | 0.11 | 0.14 | 0.35 |
| P25S75 | - | 0.25 | 0.75 | 0.11 | 0.14 | 0.35 |
| W75S25 | 0.75 | - | 0.25 | 0.11 | 0.14 | 0.35 |
| W50S50 | 0.5 | - | 0.5 | 0.11 | 0.14 | 0.35 |
| W25S75 | 0.25 | - | 0.75 | 0.11 | 0.14 | 0.35 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).