A Comparison of Multiple Macroalgae Cultivation Systems and End-Use Strategies of Saccharina latissima and Gracilaria tikvahiae Based on Techno-Economic Analysis and Life Cycle Assessment
Abstract
1. Introduction
2. Methods
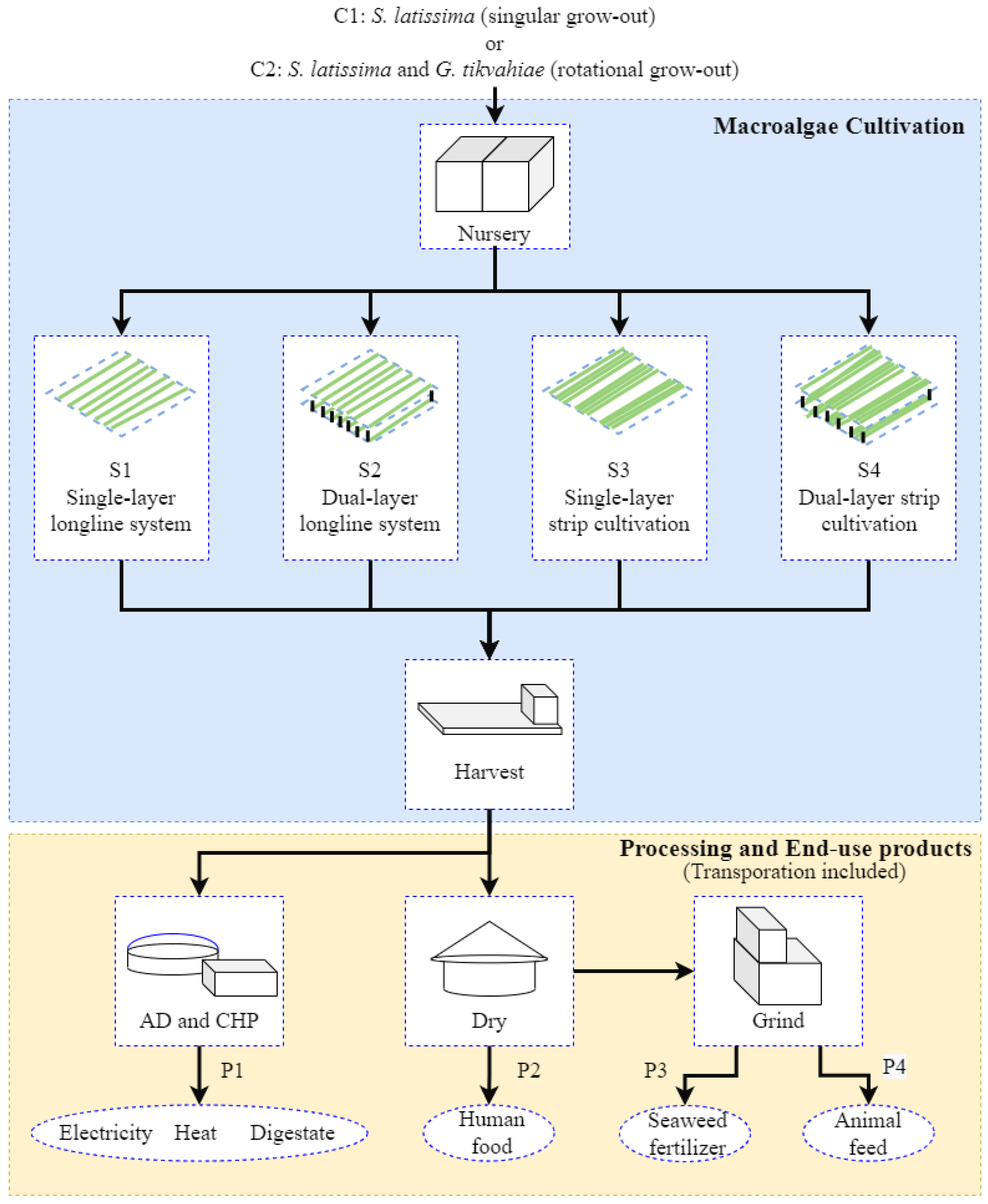
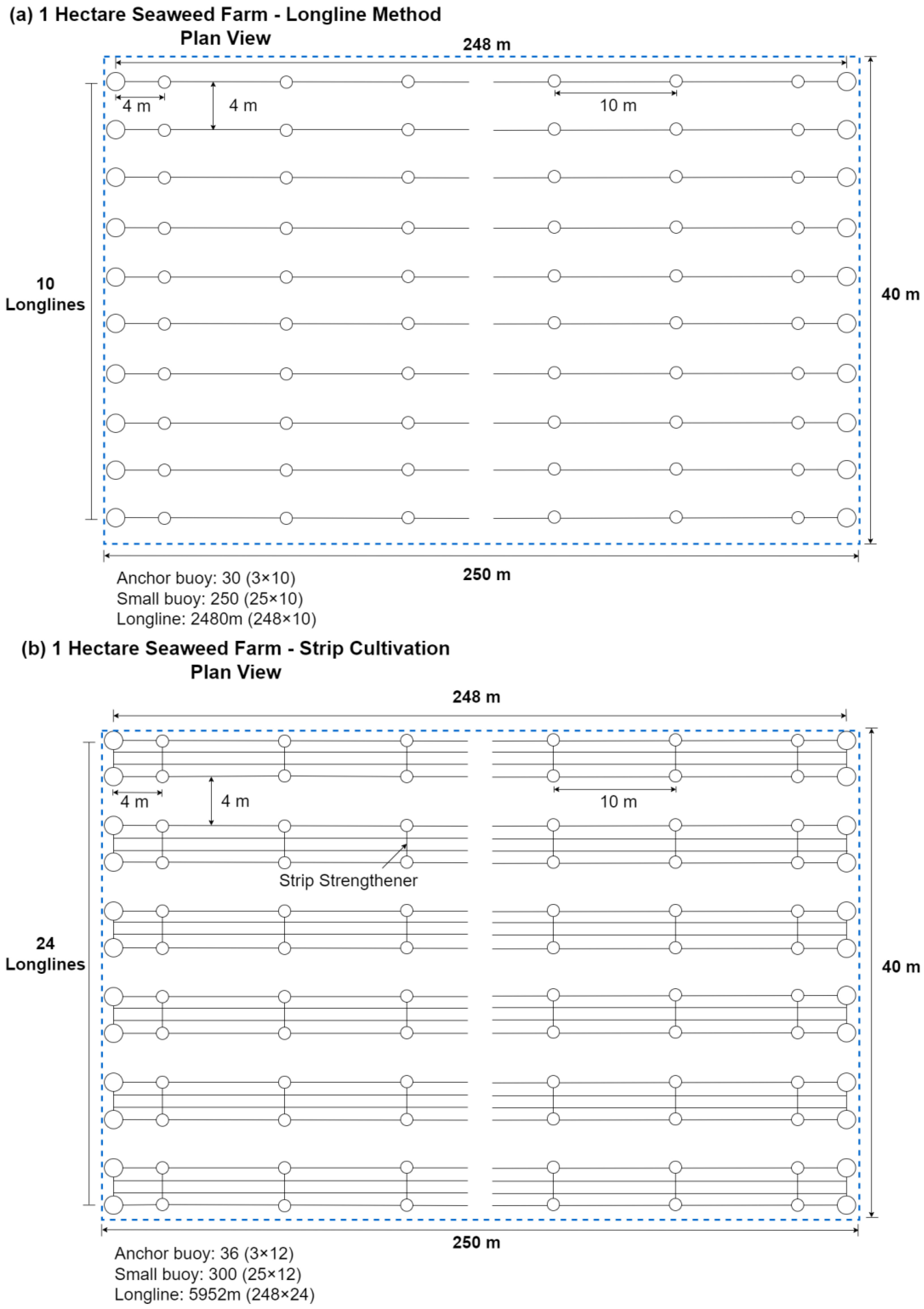
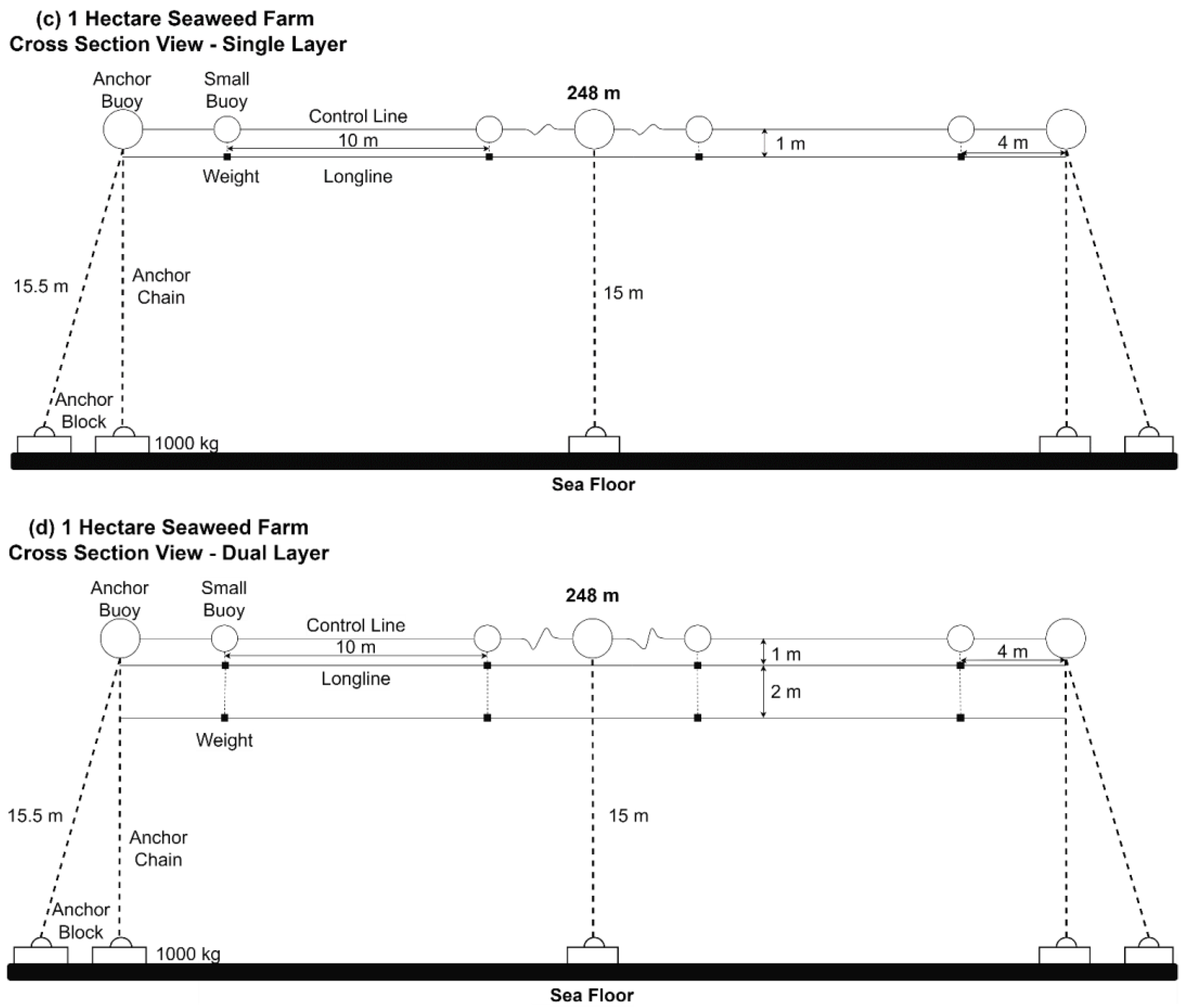
2.1. Seaweed Cultivation Platforms
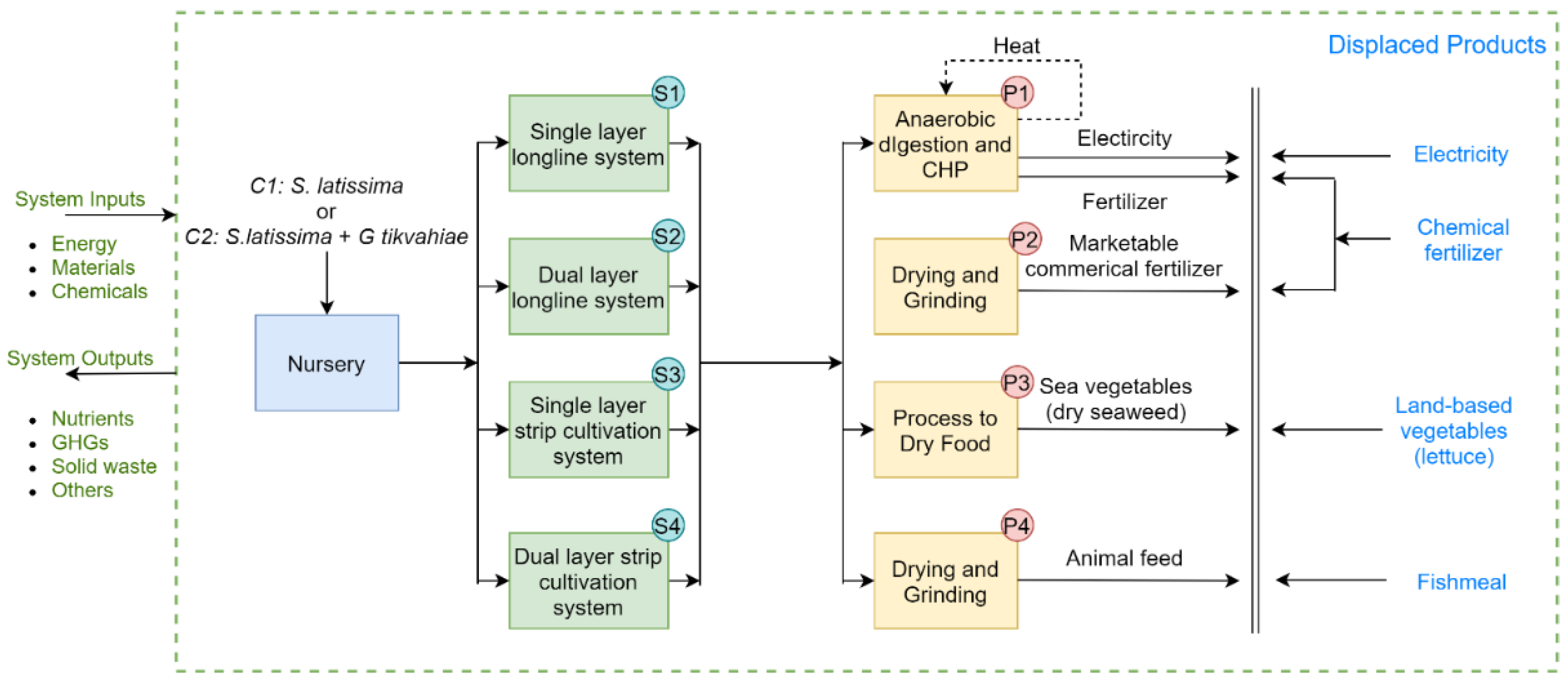
2.2. Seaweed Biomass Production and Utilization
2.3. Techno-Economic Analysis
2.4. Life Cycle Assessment
3. Results
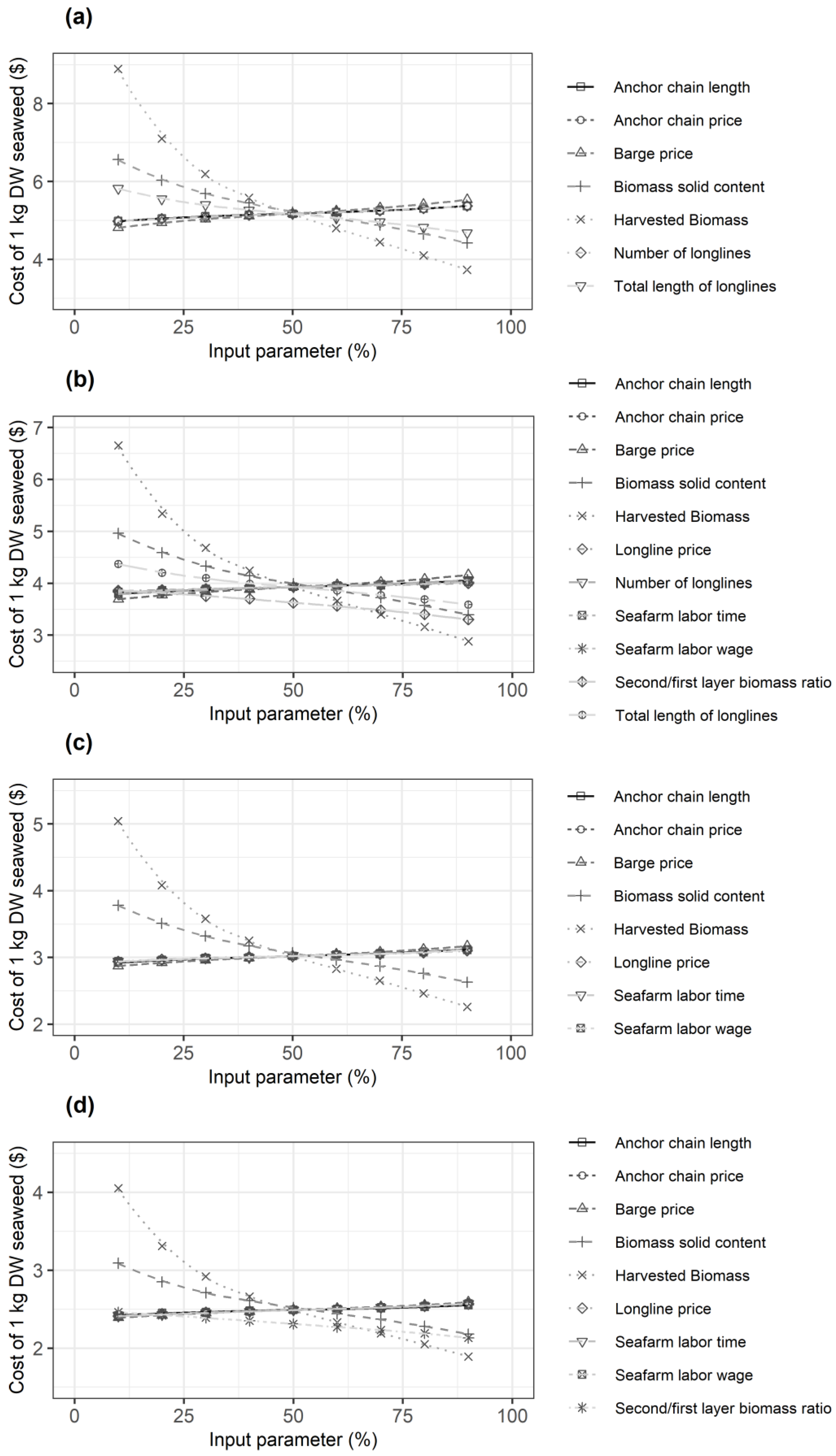
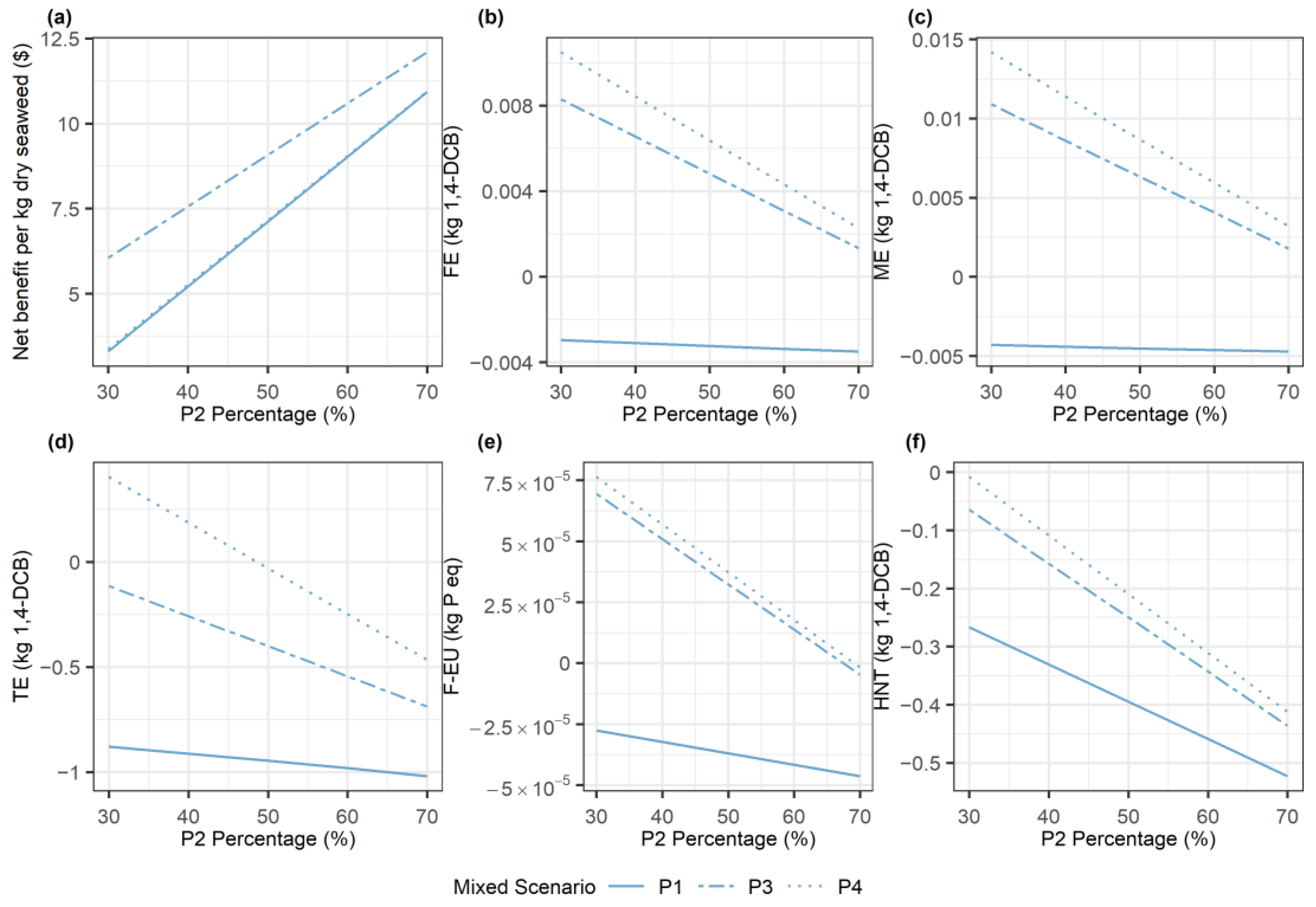
3.1. Sensitivity Analysis
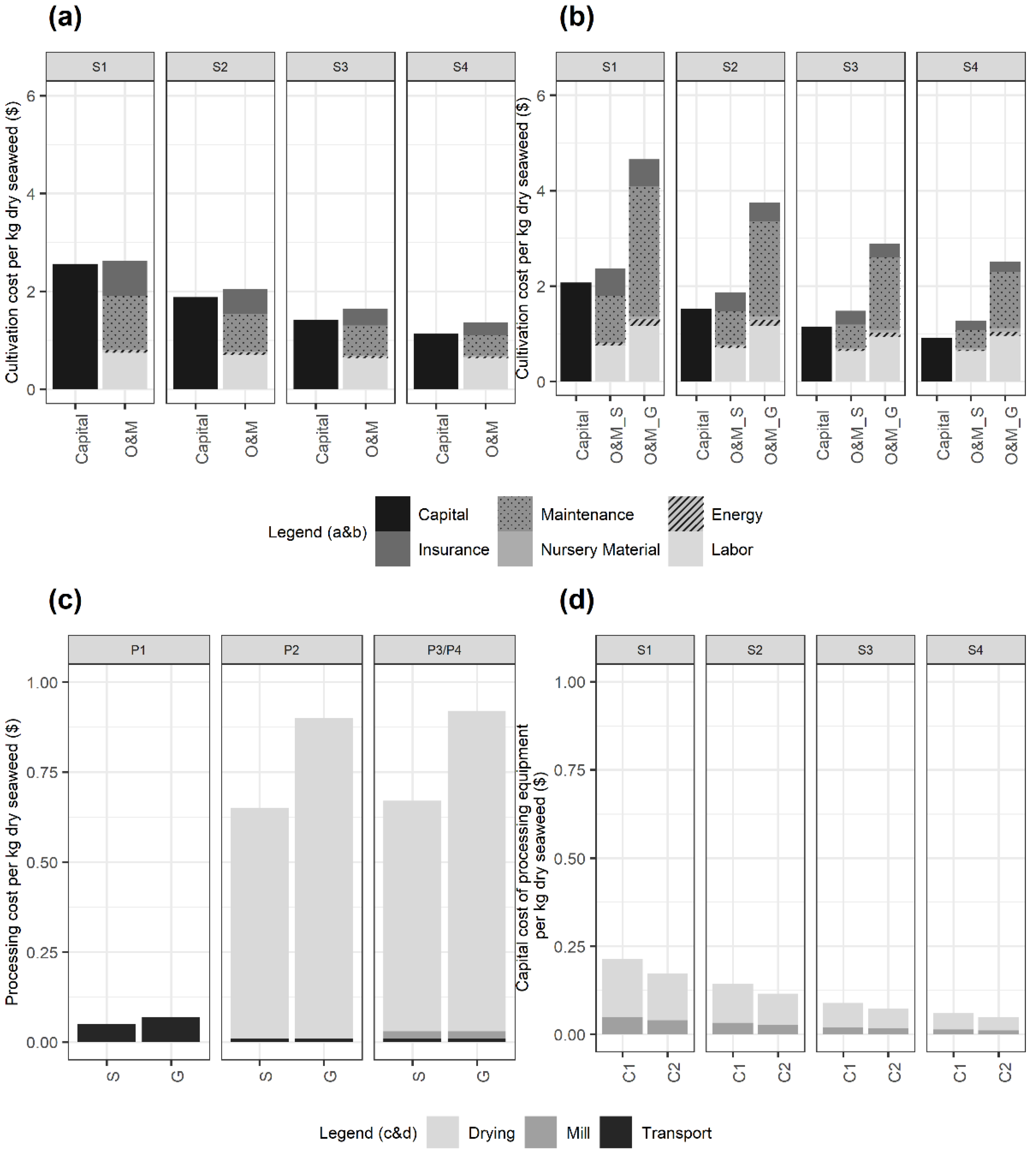
3.2. Techno-Economic Analysis
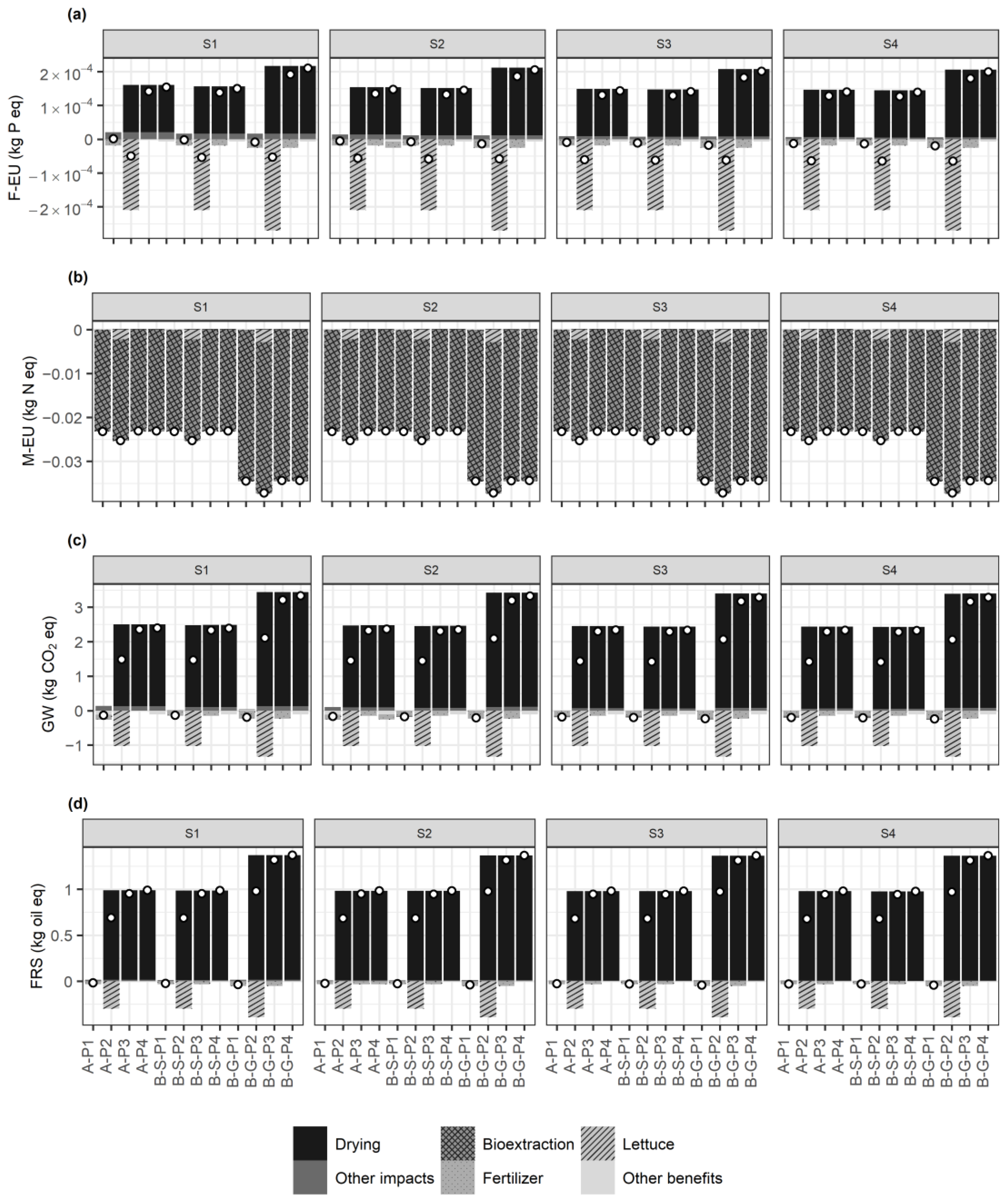
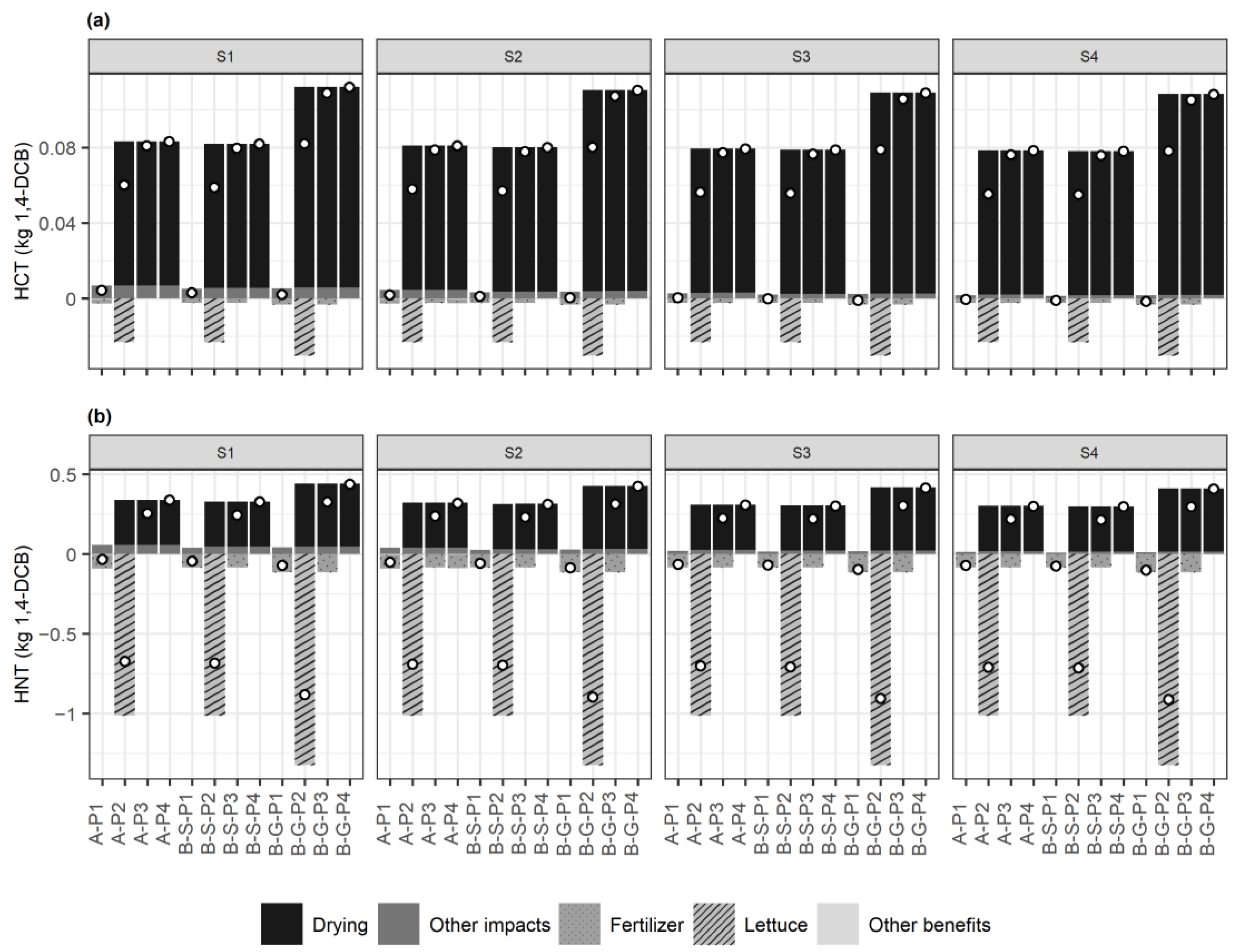
3.3. Life Cycle Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waughray, D. Water Security: The Water-Energy-Food-Climate Nexus; World Economic Forum: Washington, DC, USA, 2011. [Google Scholar]
- Bazilian, M.; Nussbaumer, P.; Rogner, H.H.; Brew-Hammond, A.; Foster, V.; Pachauri, S.; Williams, E.; Howells, M.; Niyongabo, P.; Musaba, L.; et al. Energy Access Scenarios to 2030 for the Power Sector in Sub-Saharan Africa. Util Policy 2012, 20, 1–16. [Google Scholar] [CrossRef]
- Smil, V. Feeding the World: How Much More Rice Do We Need? Rice is life: Scientific perspectives for the 21st century. In Proceedings of the World Rice Research Conference, Tsukuba, Japan, 4–7 November 2004; pp. 21–23. [Google Scholar]
- Hoff, H. Understanding the Nexus—Background Paper for the Bonn2011 Nexus Conference. In Proceedings of the Bonn2011 Conference The Water, Energy and Food Security Nexus Solutions for the Green Economy, Bonn, Germany, 16–18 November 2011; Stockholm Environment Institute: Stockholm, Sweden, 2011; pp. 1–52. [Google Scholar]
- Thirlwell, G.M.; Madramootoo, C.A.; Heathcote, I.W. Energy-Water Nexus: Energy Use in the Municipal, Industrial, and Agricultural Water Sectors. In Proceedings of the Canada–US Water Conference, Orlando, FL, USA, 21–25 October 2007; pp. 1–16. [Google Scholar]
- Van Vuuren, D.P.; Riahi, K.; Moss, R.; Edmonds, J.; Thomson, A.; Nakicenovic, N.; Kram, T.; Berkhout, F.; Swart, R.; Janetos, A.; et al. A Proposal for a New Scenario Framework to Support Research and Assessment in Different Climate Research Communities. Glob. Environ. Chang. 2012, 22, 21–35. [Google Scholar] [CrossRef]
- United Nations. United Nations World Population Prospects: The 2015 Revision; United Nations: New York, NY, USA, 2015; Volume XXXIII. [Google Scholar]
- Spagnuolo, D.; Russo, V.; Manghisi, A.; Di Martino, A.; Morabito, M.; Genovese, G.; Trifilò, P. Screening on the Presence of Plant Growth Regulators in High Biomass Forming Seaweeds from the Ionian Sea (Mediterranean Sea). Sustainability 2022, 14, 3914. [Google Scholar] [CrossRef]
- Khan, N.; Sudhakar, K.; Mamat, R. Thermogravimetric Analysis of Marine Macroalgae Waste Biomass as Bio-Renewable Fuel. J. Chem. 2022, 2022, 6417326. [Google Scholar] [CrossRef]
- Filote, C.; Santos, S.C.R.; Popa, V.I.; Botelho, C.M.S.; Volf, I. Biorefinery of Marine Macroalgae into High-Tech Bioproducts: A Review. Environ. Chem. Lett. 2020, 19, 969–1000. [Google Scholar] [CrossRef]
- da Rosa, M.D.H.; Alves, C.J.; dos Santos, F.N.; de Souza, A.O.; da Zavareze, E.R.; Pinto, E.; Noseda, M.D.; Ramos, D.; de Pereira, C.M.P. Macroalgae and Microalgae Biomass as Feedstock for Products Applied to Bioenergy and Food Industry: A Brief Review. Energies 2023, 16, 1820. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Piconi, P.; Veidenheimer, R.; Chase, B. Edible Seaweed Market Analysis; Island Institute: Rockland, ME, USA, 2020. [Google Scholar]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. Seaweed Aquaculture: Cultivation Technologies, Challenges and Its Ecosystem Services. Algae 2017, 32, 1–13. [Google Scholar] [CrossRef]
- Jevne, L.S.; Forbord, S.; Olsen, Y. The Effect of Nutrient Availability and Light Conditions on the Growth and Intracellular Nitrogen Components of Land-Based Cultivated Saccharina latissima (Phaeophyta). Front. Mar. Sci. 2020, 7, 914. [Google Scholar] [CrossRef]
- Boderskov, T.; Nielsen, M.M.; Rasmussen, M.B.; Balsby, T.J.S.; Macleod, A.; Holdt, S.L.; Sloth, J.J.; Bruhn, A. Effects of Seeding Method, Timing and Site Selection on the Production and Quality of Sugar Kelp, Saccharina latissima: A Danish Case Study. Algal Res. 2021, 53, 102160. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020—Sustainability in Action; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Handå, A.; Forbord, S.; Wang, X.; Broch, O.J.; Dahle, S.W.; Størseth, T.R.; Reitan, K.I.; Olsen, Y.; Skjermo, J. Seasonal-and Depth-Dependent Growth of Cultivated Kelp (Saccharina latissima) in Close Proximity to Salmon (Salmo salar) Aquaculture in Norway. Aquaculture 2013, 414, 191–201. [Google Scholar] [CrossRef]
- Augyte, S.; Yarish, C.; Redmond, S.; Kim, J.K. Cultivation of a Morphologically Distinct Strain of the Sugar Kelp, Saccharina latissima Forma Angustissima, from Coastal Maine, USA, with Implications for Ecosystem Services. J. Appl. Phycol. 2017, 29, 1967–1976. [Google Scholar] [CrossRef]
- Samocha, T.M.; Fricker, J.; Ali, A.M.; Shpigel, M.; Neori, A. Growth and Nutrient Uptake of the Macroalga Gracilaria tikvahiae Cultured with the Shrimp Litopenaeus Vannamei in an Integrated Multi-Trophic Aquaculture (IMTA) System. Aquaculture 2015, 446, 263–271. [Google Scholar] [CrossRef]
- Johnson, R.B.; Kim, J.K.; Armbruster, L.C.; Yarish, C. Nitrogen Allocation of Gracilaria tikvahiae Grown in Urbanized Estuaries of Long Island Sound and New York City, USA: A Preliminary Evaluation of Ocean Farmed Gracilaria for Alternative Fish Feeds. Algae 2014, 29, 227–235. [Google Scholar] [CrossRef]
- Kim, J.K.; Kraemer, G.P.; Yarish, C. Field Scale Evaluation of Seaweed Aquaculture as a Nutrient Bioextraction Strategy in Long Island Sound and the Bronx River Estuary. Aquaculture 2014, 433, 148–156. [Google Scholar] [CrossRef]
- Kim, J.K.; Kraemer, G.P.; Yarish, C. Use of Sugar Kelp Aquaculture in Long Island Sound and the Bronx River Estuary for Nutrient Extraction. Mar. Ecol. Prog. Ser. 2015, 531, 155–166. [Google Scholar] [CrossRef]
- Radulovich, R.; Neori, A.; Valderrama, D.; Reddy, C.R.K.; Cronin, H.; Forster, J. Farming of Seaweeds. In Seaweed Sustainability: Food and Non-Food Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 27–59. ISBN 9780124199583. [Google Scholar]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An Overview of Marine Macroalgae as Bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Fernand, F.; Israel, A.; Skjermo, J.; Wichard, T.; Timmermans, K.R.; Golberg, A. Offshore Macroalgae Biomass for Bioenergy Production: Environmental Aspects, Technological Achievements and Challenges. Renew. Sustain. Energy Rev. 2017, 75, 35–45. [Google Scholar] [CrossRef]
- Sharma, S.; Neves, L.; Funderud, J.; Mydland, L.T.; Øverland, M.; Horn, S.J. Seasonal and Depth Variations in the Chemical Composition of Cultivated Saccharina latissima. Algal Res. 2018, 32, 107–112. [Google Scholar] [CrossRef]
- Van Oirschot, R.; Thomas, J.B.E.; Gröndahl, F.; Fortuin, K.P.J.; Brandenburg, W.; Potting, J. Explorative Environmental Life Cycle Assessment for System Design of Seaweed Cultivation and Drying. Algal Res. 2017, 27, 43–54. [Google Scholar] [CrossRef]
- Druehl, L.D.; Baird, R.; Lindwall, A.; Lloyd, K.E.; Pakula, S. Longline Cultivation of Some Laminariaceae in British Columbia, Canada. Aquac. Res. 1988, 19, 253–263. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweeds as Food. In Seaweed in Health and Disease Prevention; Academic Press: San Diego, CA, USA, 2016; pp. 149–167. ISBN 9780128027936. [Google Scholar]
- Cottier-Cook, E.J.; Nagabhatla, N.; Badis, Y.; Campbell, M.L.; Chopin, T.; Fang, J.; He, P.; Hewitt, C.L.; Kim, G.H.; Huo, Y.; et al. Safeguarding the Future of the Global Seaweed Aquaculture Industry. In United Nations University and Scottish Association for Marine Science Policy Brief; United Nations University (INWEH) and Scottish Association for Marine Science: Hamilton, ON, Canada, 2016; Volume 12. [Google Scholar]
- Rocha, C.M.R.; Sousa, A.M.M.; Kim, J.K.; Magalhães, J.M.C.S.; Yarish, C.; do Pilar Gonçalves, M. Characterization of Agar from Gracilaria tikvahiae Cultivated for Nutrient Bioextraction in Open Water Farms. Food Hydrocoll. 2019, 89, 260–271. [Google Scholar] [CrossRef]
- Palmieri, N.; Forleo, M.B. The Potential of Edible Seaweed within the Western Diet. A Segmentation of Italian Consumers. Int. J. Gastron. Food Sci. 2020, 20, 100202. [Google Scholar] [CrossRef]
- Engle, C.; Cygler, A.; Kotowicz, D.; McCann, J. Potential Supply Chains for Seaweed Produced for Food in the Northeastern United States. In Final Report USDA FSMIP Award No. 16FSMIPR10004; The University of Rhode Island: Kingston, RI, USA, 2018. [Google Scholar]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a Sustainable Aquafeed Ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Qiu, X.; Neori, A.; Kim, J.K.; Yarish, C.; Shpigel, M.; Guttman, L.; Ben Ezra, D.; Odintsov, V.; Davis, D.A. Evaluation of Green Seaweed Ulva Sp. as a Replacement of Fish Meal in Plant-Based Practical Diets for Pacific White Shrimp, Litopenaeus Vannamei. J. Appl. Phycol. 2018, 30, 1305–1316. [Google Scholar] [CrossRef]
- Langlois, J.; Sassi, J.F.; Jard, G.; Steyer, J.P.; Delgenes, J.P.; Hélias, A. Life Cycle Assessment of Biomethane from Offshore-Cultivated Seaweed. Biofuels Bioprod. Biorefining 2012, 6, 387–404. [Google Scholar] [CrossRef]
- Alvarado-Morales, M.; Boldrin, A.; Karakashev, D.B.; Holdt, S.L.; Angelidaki, I.; Astrup, T. Life Cycle Assessment of Biofuel Production from Brown Seaweed in Nordic Conditions. Bioresour. Technol. 2013, 129, 92–99. [Google Scholar] [CrossRef]
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. A Review of Macroalgae Production, with Potential Applications in Biofuels and Bioenergy. Renew. Sustain. Energy Rev. 2016, 54, 473–481. [Google Scholar] [CrossRef]
- Vivekanand, V.; Eijsink, V.G.H.; Horn, S.J. Biogas Production from the Brown Seaweed Saccharina latissima: Thermal Pretreatment and Codigestion with Wheat Straw. J. Appl. Phycol. 2012, 24, 1295–1301. [Google Scholar] [CrossRef]
- Seghetta, M.; Romeo, D.; D’Este, M.; Alvarado-Morales, M.; Angelidaki, I.; Bastianoni, S.; Thomsen, M. Seaweed as Innovative Feedstock for Energy and Feed—Evaluating the Impacts through a Life Cycle Assessment. J. Clean. Prod. 2017, 150, 1–15. [Google Scholar] [CrossRef]
- Aitken, D.; Bulboa, C.; Godoy-Faundez, A.; Turrion-Gomez, J.L.; Antizar-Ladislao, B. Life Cycle Assessment of Macroalgae Cultivation and Processing for Biofuel Production. J. Clean. Prod. 2014, 75, 45–56. [Google Scholar] [CrossRef]
- Kerrison, P.D.; Stanley, M.S.; Edwards, M.D.; Black, K.D.; Hughes, A.D. The Cultivation of European Kelp for Bioenergy: Site and Species Selection. Biomass Bioenergy 2015, 80, 229–242. [Google Scholar] [CrossRef]
- Soleymani, M.; Rosentrater, K.A. Techno-Economic Analysis of Biofuel Production from Macroalgae (Seaweed). Bioengineering 2017, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Seghetta, M.; Tørring, D.; Bruhn, A.; Thomsen, M. Bioextraction Potential of Seaweed in Denmark—An Instrument for Circular Nutrient Management. Sci. Total Environ. 2016, 563, 513–529. [Google Scholar] [CrossRef]
- Niero, M.; Pizzol, M.; Bruun, H.G.; Thomsen, M. Comparative Life Cycle Assessment of Wastewater Treatment in Denmark Including Sensitivity and Uncertainty Analysis. J. Clean. Prod. 2014, 68, 25–35. [Google Scholar] [CrossRef]
- Greene, J.M. Techno-Economic and Life Cycle Assessment of a Novel Offshore Macroalgae Biorefinery; Colorado State University: Fort Collins, CO, USA, 2019. [Google Scholar]
- Vijay Anand, K.G.; Eswaran, K.; Ghosh, A. Life Cycle Impact Assessment of a Seaweed Product Obtained from Gracilaria Edulis—A Potent Plant Biostimulant. J. Clean. Prod. 2018, 170, 1621–1627. [Google Scholar] [CrossRef]
- Wu, J.; Rogers, S.W.; Schaummann, R.; Higgins, C.; Price, N. Bioextractive Aquaculture as an Alternative Nutrient Management Strategy for Water Resource Recovery Facilities. Water Res. 2022, 212, 118092. [Google Scholar] [CrossRef]
- Nilsson, J.; Martin, M. Exploratory Environmental Assessment of Large-Scale Cultivation of Seaweed Used to Reduce Enteric Methane Emissions. Sustain. Prod. Consum. 2022, 30, 413–423. [Google Scholar] [CrossRef]
- Flavin, K.; Flavin, N.; Flahive, B. Kelp Farming Manual. A Guide to the Processes, Tecniques, and Equipment for Farming Kelp in New England Waters; Ocean Approved LLC: Saco, ME, USA, 2013; p. 130. [Google Scholar]
- Peteiro, C.; Freire, Ó. Effect of Outplanting Time on Commercial Cultivation of Kelp Laminaria Saccharina at the Southern Limit in the Atlantic Coast, N.W. Spain. Chin. J. Oceanol. Limnol. 2009, 27, 54–60. [Google Scholar] [CrossRef]
- Watson, L.; Matthew, D.; Dring, M. Business Plan for the Establishment of a Seaweed Hatchery & Grow-Out Farm; Bord Iascaigh Mhara: Dublin, Ireland, 2013. [Google Scholar]
- Riley, D.M.; Tian, J.; Güngör-Demirci, G.; Phelan, P.; Rene Villalobos, J.; Milcarek, R.J. Techno-Economic Assessment of Chp Systems in Wastewater Treatment Plants. Environments 2020, 7, 74. [Google Scholar] [CrossRef]
- Yarish, C.; Kim, J.K.; Lindell, S. Developing an Environmentally and Economically Sustainable Sugar Kelp Aquaculture Industry in Southern New England: From Seed to Market; USDA/National Institute of Food and Agriculture (NIFA): Washington, DC, USA, 2017.
- Van Dijk, W.; Rinze Van Der Schoot, J.; Ur, A.-W. An Economic Model for Offshore Cultivation of Macroalgae Energetic Algae; EnAlgae: Swansea, UK, 2015; Volume 21. [Google Scholar]
- Zhang, X.; Boderskov, T.; Bruhn, A.; Thomsen, M. Blue Growth and Bioextraction Potentials of Danish Saccharina latissima Aquaculture—A Model of Eco-Industrial Production Systems Mitigating Marine Eutrophication and Climate Change. Algal. Res. 2022, 64, 102686. [Google Scholar] [CrossRef]
- Taelman, S.E.; Champenois, J.; Edwards, M.D.; De Meester, S.; Dewulf, J. Comparative Environmental Life Cycle Assessment of Two Seaweed Cultivation Systems in North West Europe with a Focus on Quantifying Sea Surface Occupation. Algal. Res. 2015, 11, 173–183. [Google Scholar] [CrossRef]
- Seghetta, M.; Hou, X.; Bastianoni, S.; Bjerre, A.B.; Thomsen, M. Life Cycle Assessment of Macroalgal Biorefinery for the Production of Ethanol, Proteins and Fertilizers—A Step towards a Regenerative Bioeconomy. J. Clean. Prod. 2016, 137, 1158–1169. [Google Scholar] [CrossRef]
- Alexis, J.; Diaz, V. Opportunities for Offshore Large-Scale Macro-Algae Production in the Dutch North Sea; University of Groningen: Groningen, The Netherlands, 2021. [Google Scholar]
- Grebe, G.S.; Byron, C.J.; Brady, D.C.; Geisser, A.H.; Brennan, K.D. The Nitrogen Bioextraction Potential of Nearshore Saccharina latissima Cultivation and Harvest in the Western Gulf of Maine. J. Appl. Phycol. 2021, 33, 1741–1757. [Google Scholar] [CrossRef]
- Broch, O.J.; Ellingsen, I.H.; Forbord, S.; Wang, X.; Volent, Z.; Alver, M.O.; Handå, A.; Andresen, K.; Slagstad, D.; Reitan, K.I.; et al. Modelling the Cultivation and Bioremediation Potential of the Kelp Saccharina latissima in Close Proximity to an Exposed Salmon Farm in Norway. Aquac. Environ. Interact. 2013, 4, 187–206. [Google Scholar] [CrossRef]
- Chen, Y.; Lan, L.; Zhang, J.; Wang, Q.; Liu, Y.; Li, H.; Gong, Q.; Gao, X. Physiological Impacts of Nitrogen Starvation and Subsequent Recovery on the Red Seaweed Grateloupia Turuturu (Halymeniaceae, Rhodophyta). Sustainability 2023, 15, 7032. [Google Scholar] [CrossRef]
- Zhang, X.; Thomsen, M. Techno-Economic and Environmental Assessment of Novel Biorefinery Designs for Sequential Extraction of High-Value Biomolecules from Brown Macroalgae Laminaria Digitata, Fucus Vesiculosus, and Saccharina latissima. Algal. Res. 2021, 60, 102499. [Google Scholar] [CrossRef]
- Nilsson, A.E.; Bergman, K.; Gomez Barrio, L.P.; Cabral, E.M.; Tiwari, B.K. Life Cycle Assessment of a Seaweed-Based Biorefinery Concept for Production of Food, Materials, and Energy. Algal. Res. 2022, 65, 102725. [Google Scholar] [CrossRef]
- Thomas, J.-B.E.; Sodré Ribeiro, M.; Potting, J.; Cervin, G.; Nylund, G.M.; Olsson, J.; Albers, E.; Undeland, I.; Pavia, H.; Gröndahl, F. A Comparative Environmental Life Cycle Assessment of Hatchery, Cultivation, and Preservation of the Kelp Saccharina latissima. ICES J. Mar. Sci. 2020, 78, 451–467. [Google Scholar] [CrossRef]
- Duarte, C.M.; Delgado-Huertas, A.; Marti, E.; Gasser, B.; Martin, I.S.; Cousteau, A.; Neumeyer, F.; Reilly-Cayten, M.; Boyce, J.; Kuwae, T.; et al. Carbon Burial in Sediments below Seaweed Farms. bioRxiv 2023, 26. [Google Scholar] [CrossRef]
- Fujita, R.; Augyte, S.; Bender, J.; Brittingham, P.; Buschmann, A.H.; Chalfin, M.; Collins, J.; Davis, K.A.; Gallagher, J.B.; Gentry, R.; et al. Seaweed Blue Carbon: Ready? Or Not? Mar. Policy 2023, 155, 105747. [Google Scholar] [CrossRef]
- Ricart, A.M.; Krause-Jensen, D.; Hancke, K.; Price, N.N.; Masqué, P.; Duarte, C.M. Sinking Seaweed in the Deep Ocean for Carbon Neutrality Is Ahead of Science and beyond the Ethics. Environ. Res. Lett. 2022, 17, 081003. [Google Scholar] [CrossRef]
- Allen, E.; Wall, D.M.; Herrmann, C.; Xia, A.; Murphy, J.D. What Is the Gross Energy Yield of Third Generation Gaseous Biofuel Sourced from Seaweed? Energy 2015, 81, 352–360. [Google Scholar] [CrossRef]
- Bak, U.G.; Nielsen, C.W.; Marinho, G.S.; Gregersen, Ó.; Jónsdóttir, R.; Holdt, S.L. The Seasonal Variation in Nitrogen, Amino Acid, Protein and Nitrogen-to-Protein Conversion Factors of Commercially Cultivated Faroese Saccharina latissima. Algal Res. 2019, 42, 101576. [Google Scholar] [CrossRef]
- Freitas, J.R.C.; Salinas Morrondo, J.M.; Cremades Ugarte, J. Saccharina latissima (Laminariales, Ochrophyta) Farming in an Industrial IMTA System in Galicia (Spain). J. Appl. Phycol. 2016, 28, 377–385. [Google Scholar] [CrossRef]
- Gorman, L.; Kraemer, G.P.; Yarish, C.; Boo, S.M.; Kim, J.K. The Effects of Temperature on the Growth Rate and Nitrogen Content of Invasive Gracilaria Vermiculophylla and Native Gracilaria tikvahiae from Long Island Sound, USA. Algae 2017, 32, 57–66. [Google Scholar] [CrossRef]
- Habig, C.; DeBusk, T.A.; Ryther, J.H. The Effect of Nitrogen Content on Methane Production by the Marine Algae Gracilaria tikvahiae and Ulva sp. Biomass 1984, 4, 239–251. [Google Scholar] [CrossRef]
- Habig, C.; Andrews, D.A.; Ryther, J.H. Nitrogen Recycling and Methane Production Using Gracilaria tikvahiae: A Closed System Approach. Resour. Conserv. 1984, 10, 303–313. [Google Scholar] [CrossRef]
- Horrocks, J.L.; Stewart, G.R.; Dennison, W.C. Tissue Nutrient Content of Gracilaria spp. (Rhodophyta) and Water Quality along an Estuarine Gradient. Mar. Freshw. Res. 1995, 46, 975–983. [Google Scholar] [CrossRef]
- Jard, G.; Jackowiak, D.; Carrère, H.; Delgenes, J.P.; Torrijos, M.; Steyer, J.P.; Dumas, C. Batch and Semi-Continuous Anaerobic Digestion of Palmaria Palmata: Comparison with Saccharina latissima and Inhibition Studies. Chem. Eng. J. 2012, 209, 513–519. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Ryther, J.H. Some Aspects of the Growth and Yield of Gracilaria tikvahiae in Culture. Aquaculture 1978, 15, 185–193. [Google Scholar] [CrossRef]
- Pechsiri, J.S.; Thomas, J.B.E.; Risén, E.; Ribeiro, M.S.; Malmström, M.E.; Nylund, G.M.; Jansson, A.; Welander, U.; Pavia, H.; Gröndahl, F. Energy Performance and Greenhouse Gas Emissions of Kelp Cultivation for Biogas and Fertilizer Recovery in Sweden. Sci. Total Environ. 2016, 573, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Peteiro, C.; Freire, Ó. Biomass Yield and Morphological Features of the Seaweed Saccharina latissima Cultivated at Two Different Sites in a Coastal Bay in the Atlantic Coast of Spain. J. Appl. Phycol. 2013, 25, 205–213. [Google Scholar] [CrossRef]
- Reid, G.K.; Chopin, T.; Robinson, S.M.C.; Azevedo, P.; Quinton, M.; Belyea, E. Weight Ratios of the Kelps, Alaria Esculenta and Saccharina latissima, Required to Sequester Dissolved Inorganic Nutrients and Supply Oxygen for Atlantic Salmon, Salmo Salar, in Integrated Multi-Trophic Aquaculture Systems. Aquaculture 2013, 408–409, 34–46. [Google Scholar] [CrossRef]
- Schiener, P.; Atack, T.; Wareing, R.A.; Kelly, M.S.; Hughes, A.D. The By-Products from Marine Biofuels as a Feed Source for the Aquaculture Industry: A Novel Example of the Biorefinery Approach. Biomass Convers. Biorefin. 2016, 6, 281–287. [Google Scholar] [CrossRef]
- Scoggan, J.; Zhimeng, Z. Culture of Kelp (Laminaria Japonica) in China. In Training Manual; FAO: Rome, Italy, 1989. [Google Scholar]
- Wang, X.; Broch, O.J.; Forbord, S.; Handå, A.; Skjermo, J.; Reitan, K.I.; Vadstein, O.; Olsen, Y. Assimilation of Inorganic Nutrients from Salmon (Salmo Salar) Farming by the Macroalgae (Saccharina latissima) in an Exposed Coastal Environment: Implications for Integrated Multi-Trophic Aquaculture. J. Appl. Phycol. 2014, 26, 1869–1878. [Google Scholar] [CrossRef]

| Scenario 1 | Seaweed | Mean | SD | Kurtosis | 50% Cost | 5% to 95% Cost |
|---|---|---|---|---|---|---|
| S1 (C1) | S. latissima | $6.17 | 2.93 | 9.26 | $5.35 | $3.28–$11.77 |
| S1 (C2) | S. latissima | $5.00 | 1.79 | 3.50 | $4.57 | $2.98–$8.49 |
| G. tikvahiae | $7.30 | 1.70 | 1.88 | $7.01 | $5.09–$10.51 | |
| S2 (C1) | S. latissima | $4.29 | 1.95 | 10.22 | $3.77 | $2.36–$8.08 |
| S2 (C2) | S. latissima | $3.53 | 1.21 | 4.07 | $3.25 | $2.16–$5.93 |
| G. tikvahiae | $5.24 | 1.16 | 1.74 | $5.07 | $3.67–$7.41 | |
| S3 (C1) | S. latissima | $3.60 | 1.56 | 7.65 | $3.17 | $2.07–$6.68 |
| S3 (C2) | S. latissima | $2.98 | 0.96 | 3.80 | $2.75 | $1.91–$4.92 |
| G. tikvahiae | $4.39 | 0.85 | 1.77 | $4.25 | $3.28–$6.00 | |
| S4 (C1) | S. latissima | $2.72 | 1.12 | 6.81 | $2.41 | $1.60–$4.89 |
| S4 (C2) | S. latissima | $2.28 | 0.70 | 3.34 | $2.12 | $1.49–$3.65 |
| G. tikvahiae | $3.37 | 0.63 | 1.33 | $3.28 | $2.51–$4.56 | |
| P1 | S. latissima | $0.05 | 0.01 | 0.40 | $0.05 | $0.03–$0.08 |
| G. tikvahiae | $0.07 | 0.02 | −0.32 | $0.07 | $0.04–$0.10 | |
| P2 | S. latissima | $0.68 | 0.14 | 0.44 | $0.66 | $0.48–$0.94 |
| G. tikvahiae | $0.87 | 0.12 | −0.20 | $0.86 | $0.70–$1.08 | |
| P3/P4 | S. latissima | $0.69 | 0.14 | 0.44 | $0.67 | $0.49–$0.95 |
| G. tikvahiae | $0.88 | 0.11 | −0.20 | $0.87 | $0.71–$1.09 |
| Aquaculture Platform | Cultivation Strategy and Seaweed Species | Processing and End-Use Product | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | |||
| S1 | C1: | S. latissima | −$5.09 | $13.72 | −$1.48 | −$5.31 |
| C2: | S. latissima | −$4.36 | $14.50 | −$0.68 | −$4.52 | |
| G. tikvahiae | −$6.66 | $11.96 | −$3.22 | −$7.05 | ||
| S2 | C1: | S. latissima | −$3.85 | $15.05 | −$0.13 | −$3.96 |
| C2: | S. latissima | −$3.32 | $15.61 | $0.44 | −$3.39 | |
| G. tikvahiae | −$5.21 | $13.48 | −$1.68 | −$5.52 | ||
| S3 | C1: | S. latissima | −$2.99 | $15.96 | $0.81 | −$3.02 |
| C2: | S. latissima | −$2.55 | $16.42 | $1.27 | −$2.53 | |
| G. tikvahiae | −$3.97 | $14.76 | −$0.38 | −$4.22 | ||
| S4 | C1: | S. latissima | −$2.41 | $16.57 | $1.43 | −$2.41 |
| C2: | S. latissima | −$2.11 | $16.89 | $1.75 | −$2.09 | |
| G. tikvahiae | −$3.36 | $15.40 | $0.26 | −$3.58 | ||
| Aquaculture Platform | Cultivation Strategy | Seaweed Species | F-EU | HNT | FE | ME | TE |
|---|---|---|---|---|---|---|---|
| S1 | C1 | S. latissima | 78.48% | 33.54% | 90.94% | 91.75% | 56.06% |
| S1 | C2 | S. latissima | 76.61% | 32.48% | 88.46% | 89.21% | 54.33% |
| G. tikvahiae | 78.92% | 33.33% | 91.19% | 91.90% | 55.91% | ||
| S2 | C1 | S. latissima | 75.31% | 31.76% | 86.68% | 87.40% | 53.23% |
| S2 | C2 | S. latissima | 74.06% | 31.05% | 84.98% | 85.66% | 52.05% |
| G. tikvahiae | 76.97% | 32.26% | 88.55% | 89.22% | 54.25% | ||
| S3 | C1 | S. latissima | 73.15% | 30.58% | 83.70% | 84.37% | 51.47% |
| S3 | C2 | S. latissima | 72.31% | 30.09% | 82.58% | 83.24% | 50.62% |
| G. tikvahiae | 75.57% | 31.50% | 86.69% | 87.34% | 53.07% | ||
| S4 | C1 | S. latissima | 71.76% | 29.79% | 81.82% | 82.46% | 50.16% |
| S4 | C2 | S. latissima | 71.19% | 29.45% | 81.09% | 81.69% | 49.56% |
| G. tikvahiae | 74.74% | 31.04% | 85.58% | 86.18% | 52.33% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Rogers, S.W.; Schaummann, R.; Price, N.N. A Comparison of Multiple Macroalgae Cultivation Systems and End-Use Strategies of Saccharina latissima and Gracilaria tikvahiae Based on Techno-Economic Analysis and Life Cycle Assessment. Sustainability 2023, 15, 12072. https://doi.org/10.3390/su151512072
Wu J, Rogers SW, Schaummann R, Price NN. A Comparison of Multiple Macroalgae Cultivation Systems and End-Use Strategies of Saccharina latissima and Gracilaria tikvahiae Based on Techno-Economic Analysis and Life Cycle Assessment. Sustainability. 2023; 15(15):12072. https://doi.org/10.3390/su151512072
Chicago/Turabian StyleWu, Jingjing, Shane W. Rogers, Rebekah Schaummann, and Nichole N. Price. 2023. "A Comparison of Multiple Macroalgae Cultivation Systems and End-Use Strategies of Saccharina latissima and Gracilaria tikvahiae Based on Techno-Economic Analysis and Life Cycle Assessment" Sustainability 15, no. 15: 12072. https://doi.org/10.3390/su151512072
APA StyleWu, J., Rogers, S. W., Schaummann, R., & Price, N. N. (2023). A Comparison of Multiple Macroalgae Cultivation Systems and End-Use Strategies of Saccharina latissima and Gracilaria tikvahiae Based on Techno-Economic Analysis and Life Cycle Assessment. Sustainability, 15(15), 12072. https://doi.org/10.3390/su151512072








