Synthesis and Characterization of a Quercetin-Based Nanocomposite and Its Ameliorating Impacts on the Growth, Physiological, and Biochemical Parameters of Ocimum basilicum L. under Salinity Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Quercetin-Based Nanocomposites
2.2. Materials
2.3. Growth Parameters
2.4. Photosynthetic and Gas Exchange Parameters
2.5. Stress Markers (Electrolyte Leakage, Malondialdehyde, and Hydrogen Peroxide)
2.6. Compatible Solutes (Proline and Glycine Betaine)
2.7. Biochemical Parameters (Antioxidant Enzymes, Soluble Protein, Free Amino Acids and Soluble Sugar)
2.8. Phytochemical Parameters (Flavonoid, Anthocyanin, Tannin and Phenol Content)
2.9. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of Quercetin-Based Nanocomposites
3.2. Effect of Qu and QNCs on Growth Parameters and RWC
3.3. Effect of Qu and QNCs on Photosynthetic Pigments and Gas Exchange Parameters
3.4. Effect of Qu and QNCs on Stress Markers and Compatible Solutes
3.5. Effect of Qu and QNCs on Antioxidant Enzymes Activity
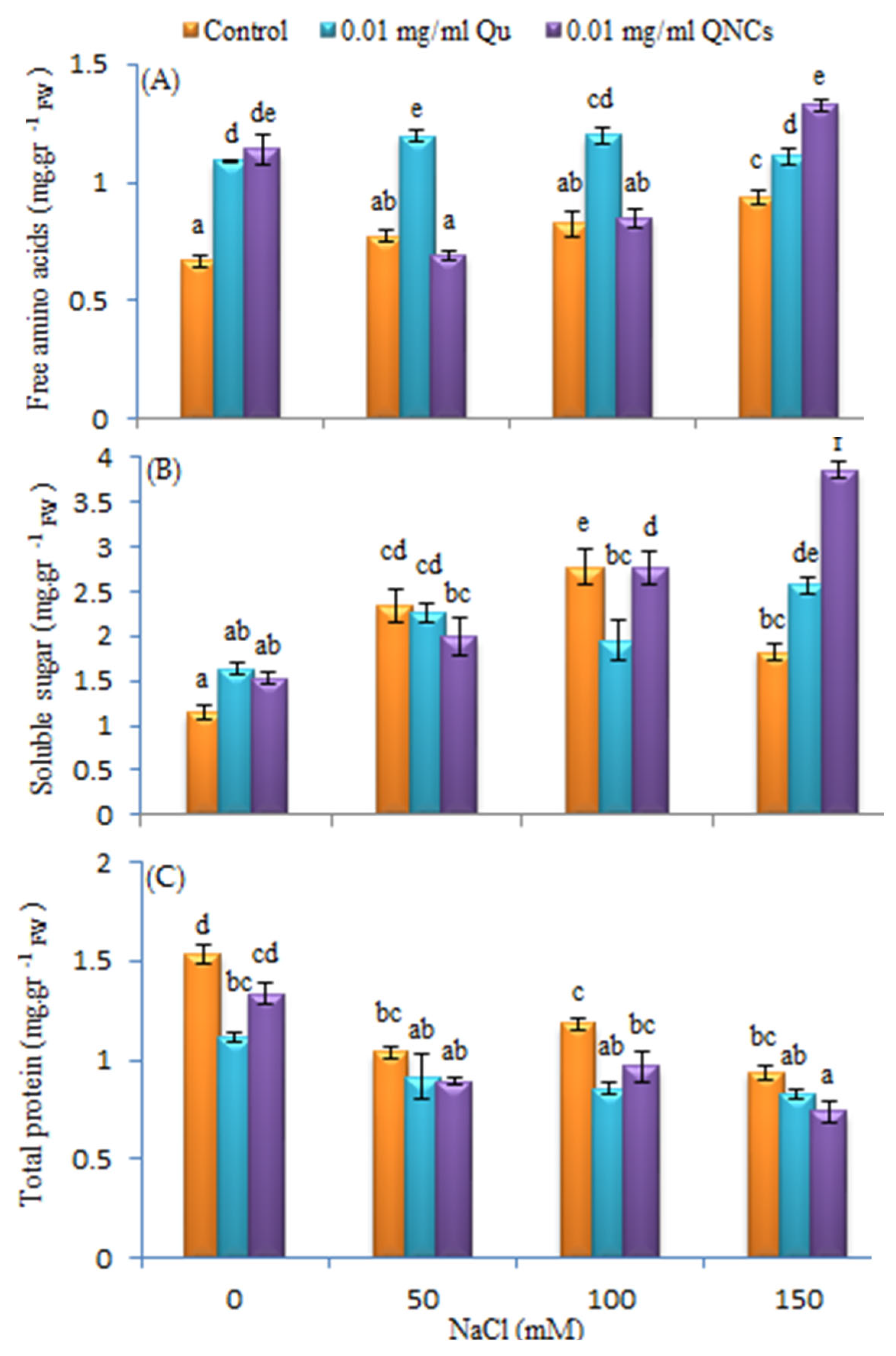
3.6. Effect of Qu and QNCs on Free Amino Acids, Soluble Sugar and Total Protein
3.7. Effect of Qu and QNCs on Flavonoids, Anthocyanins, Tannins, and Phenols Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maghsodi, M.; Najafi, N. Effects of Nanomaterial Overdose in Plant Nutrition. J. Land Manag. 2019, 6, 179–195. [Google Scholar]
- Ashfaq, M.; Talreja, N.; Chuahan, D.; Srituravanich, W. Polymeric Nanocomposite-Based Agriculture Delivery System: Emerging Technology for Agriculture. In Genetic Engineering: A Glimpse of Techniques and Applications; Intechopen: London, UK, 2020; pp. 1–16. [Google Scholar]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Kumar, R.; Ashfaq, M.; Verma, N. Synthesis of novel PVA–starch formulation-supported Cu–Zn nanoparticle carrying carbon nanofibers as a nanofertilizer: Controlled release of micronutrients. J. Mater. Sci. 2018, 53, 7150–7164. [Google Scholar] [CrossRef]
- Jarosiewicz, A.; Tomaszewska, M. Controlled-release NPK fertilizer encapsulated by polymeric membranes. J. Agric. Food Chem. 2003, 51, 413–417. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. Int. J. Biol. Macromol. 2018, 114, 426–433. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Singh, S.K.; Bajpai, J.; Bajpai, A.K. Controlled pesticide release from biodegradable polymers. Cent. Eur. J. Chem. 2014, 12, 453–469. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Nanocomposite of carboxymethyl cellulose and attapulgite as a novel pH-sensitive superabsorbent: Synthesis, characterization and properties. Carbohydr. Polym. 2010, 82, 83–91. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, X.Y.; Gong, W.; Li, X.; Huang, H.B.; Zhu, X.M. Nanoparticles based on carboxymethylcellulose-modified rice protein for efficient delivery of lutein. Food Funct. 2020, 11, 2380–2394. [Google Scholar] [CrossRef]
- Heiba, H.F.; Taha, A.A.; Mostafa, A.R.; Mohamed, L.A.; Fahmy, M.A. Synthesis and characterization of CMC/MMT nanocomposite for Cu2+ sequestration in wastewater treatment. Korean J. Chem. Eng. 2018, 35, 1844–1853. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Heydaripour, S.; Abdouss, M. Novel carboxymethyl cellulose based nanocomposite membrane: Synthesis, characterization and application in water treatment. J. Environ. Manag. 2016, 166, 457–465. [Google Scholar] [CrossRef]
- Migut, D.; Jańczak-Pieniążek, M.; Piechowiak, T.; Buczek, J.; Balawejder, M. Physiological response of maize plants (Zea mays l.) to the use of the potassium quercetin derivative. Int. J. Mol. Sceinces 2021, 22, 7384. [Google Scholar] [CrossRef] [PubMed]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.; Mohsin, S.M.; Fujita, M. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef] [Green Version]
- Pralhad, T.; Rajendrakumar, K. Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J. Pharm. Biomed. Anal. 2004, 34, 333–339. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Cadena, P.G.; Pereira, M.A.; Cordeiro, R.B.; Cavalcanti, I.M.; Neto, B.B.; Maria do Carmo, C.B.; Santos-Magalhães, N.S. Nanoencapsulation of quercetin and resveratrol into elastic liposomes. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2013, 1828, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghani-Samani, J.; Ghasemi Pirbalouti, A.; Malekpoor, F.; Rajabzadeh, F. Chemical composition and yield of essential oil from two sweet basil species (Ocimum ciliatum L. and O. basilicum L.) under different fertilizers. J. Med. Herbs 2021, 12, 27–33. [Google Scholar]
- Tolay, I. The impact of different Zinc (Zn) levels on growth and nutrient uptake of Basil (Ocimum basilicum L.) grown under salinity stress. PLoS ONE 2021, 16, e0246493. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Menezes, R.V.; Gheyi, H.R.; Costa Conceicao Silva, P.; Cova, A.M.W.; Ribas, R.F.; de Oliveira Ribeiro, M. Salt-induced changes in solutes, pigments and essential oil of two basil (Ocimum basilicum L.) genotypes under hydroponic cultivation. Aust. J. Crop Sci. 2019, 13, 1856–1864. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhuyan, M.B.; Oku, H.; Fujita, M. Exogenous nitric oxide pretreatment protects Brassica napus L. seedlings from paraquat toxicity through the modulation of antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2018, 126, 173–186. [Google Scholar] [CrossRef]
- Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Toan, N.P.; Minh, T.N.; Xuan, T.D. Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Joshi, P.N.; Wangnoo, S.; Louis, M. Carboxymethyl cellulose based multifunctional targeted drug delivery platform for pancreatic cancer: Nanotheranostic potential and biocompatibility analysis. World J. Pharm. Sci. 2015, 3, 1347–1359. [Google Scholar]
- ISTA. International Rules for Seed Testing; International Seed Testing Association (ISTA): Wallisellen, Switzerland, 2010. [Google Scholar]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates Incorporated: Sunderland, CT, USA, 2015. [Google Scholar]
- Ritchie, S.W.; Nguyen, H.T.; Holaday, A.S. Leaf water content and gas-exchange parameters of two wheat genotypes differing in drought resistance. Crop Sci. 1990, 30, 105–111. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ALKahtani, M.D.; Attia, K.A.; Hafez, Y.M.; Khan, N.; Eid, A.M.; Ali, M.A.; Abdelaal, K.A. Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with chitosan and plant growth promoting rhizobacteria. Agronomy 2020, 10, 1180. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Stewart, R.R.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.; Grattan, S. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; pp. 673–684. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Raymond, J.; Rakariyatham, N.; Azanza, J.L. Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochemistry 1993, 34, 927–931. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.N.; Ederer, G.M. Rapid hippurate hydrolysis method for presumptive identification of group B streptococci. J. Clin. Microbiol. 1975, 1, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Roe, H.; Joseph, H.R. The determination of sugar in blood and spinal fluid with anthrone reagent. J. Biol. Chem. 1955, 212, 335–343. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Martinez, A.E.; Favret, E.A. Anthocyanin synthesis and lengthening in the first leaf of barley isogenic lines. Plant Sci. 1990, 71, 35–43. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Bluemmel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Kayacetin, F.; Efeoglu, B.; Alizadeh, B. Effect of NaCl and PEG-induced osmotic stress on germination and seedling growth properties in wild mustard (Sinapis arvensis L.). Anadolu Ege Tarımsal Araştırma Enstitüsü Derg. 2018, 28, 62–68. [Google Scholar]
- Zhang, M.; He, S.; Zhan, Y.; Qin, B.; Jin, X.; Wang, M.; Wu, Y. Exogenous melatonin reduces the inhibitory effect of osmotic stress on photosynthesis in soybean. PLoS ONE 2019, 14, e0226542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, T.I.D.; Silva, J.D.S.; Dias, M.G.; Martins, J.V.D.S.; Ribeiro, W.S.; Dias, T.J. Salicylic acid attenuates the harmful effects of salt stress on basil. Rev. Bras. Eng. Agrícola E Ambient. 2022, 26, 399–406. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Maksimović, J.D.; Maksimović, V.; Shabala, L.; Živanović, B.D.; Tian, Y.; Shabala, S. Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct. Plant Biol. 2015, 43, 75–86. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Jiang, L.; Zhan, Y.G.; Fan, G.Z. Quercetin alleviates seed germination and growth inhibition in Apocynum venetum and Apocynum pictum under mannitol-induced osmotic stress. Plant Physiol. Biochem. 2021, 159, 268–276. [Google Scholar] [CrossRef]
- Hmidi, D.; Abdelly, C.; Athar, H.U.R.; Ashraf, M.; Messedi, D. Effect of salinity on osmotic adjustment, proline accumulation and possible role of ornithine-δ-aminotransferase in proline biosynthesis in Cakile maritima. Physiol. Mol. Biol. Plants 2018, 24, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Hameed, A.; Azooz, M.M.; Siddiqi, T.O.; Ahmad, P. Salt Stress: Causes, Types and Responses of Plants. In Ecophysiology and Responses of Plants Under Salt Stress; Springer: New York, NY, USA, 2013; pp. 1–24. [Google Scholar]
- Amist, N.; Singh, N.B. Responses of enzymes involved in proline biosynthesis and degradation in wheat seedlings under stress. Allelopath. J. 2017, 42, 195–206. [Google Scholar] [CrossRef]
- De la Torre-González, A.; Montesinos-Pereira, D.; Blasco, B.; Ruiz, J.M. Influence of the proline metabolism and glycine betaine on tolerance to salt stress in tomato (Solanum lycopersicum L.) commercial genotypes. J. Plant Physiol. 2018, 231, 329–336. [Google Scholar] [CrossRef]
- Sarkar, M.M.; Pradhan, N.; Subba, R.; Saha, P.; Roy, S. Sugar-terminated carbon-nanodots stimulate osmolyte accumulation and ROS detoxification for the alleviation of salinity stress in Vigna radiata. Sci. Rep. 2022, 12, 17567. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 2012, 27, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Alp, F.N.; Kucukoduk, M.; Turkan, I. Naringenin induces tolerance to salt/osmotic stress through the regulation of nitrogen metabolism, cellular redox and ROS scavenging capacity in bean plants. Plant Physiol. Biochem. 2020, 157, 264–275. [Google Scholar] [CrossRef]
- Hatamipoor, S.; Shabani, L.; Farhadian, S. Supportive effect of naringenin on NaCl-induced toxicity in Carthamus tinctorius seedlings. Int. J. Phytoremediat. 2023, 25, 889–899. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Mohsin, S.M.; Nahar, K.; Fujita, M. Coumarin improves tomato plant tolerance to salinity by enhancing antioxidant defence, glyoxalase system and ion homeostasis. Plant Biol. 2021, 23, 181–192. [Google Scholar] [CrossRef]
- Alharbi, K.; Al-Osaimi, A.A.; Alghamdi, B.A. Sodium chloride (NaCl)-induced physiological alteration and oxidative stress generation in Pisum sativum (L.): A toxicity assessment. ACS Omega 2022, 7, 20819–20832. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Li, C. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef] [Green Version]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (Antioxidants Systems) in Higher Plants and Their Response to Stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Cham, Switzerland, 2018; pp. 253–268. [Google Scholar]
- Aslam, M.A.; Ahmed, S.; Saleem, M.; Shah, A.A.; Shah, A.N.; Tanveer, M.; Khan, J. Quercetin ameliorates chromium toxicity through improvement in photosynthetic activity, antioxidative defense system; and suppressed oxidative stress in Trigonella corniculata L. Front. Plant Sci. 2022, 13, 956249. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Skalicky, M. Chitosan–Selenium Nanoparticle (Cs–Se NP) Foliar Spray Alleviates Salt Stress in Bitter Melon. Nanomaterials 2021, 11, 684. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Migut, D.; Piechowiak, T.; Buczek, J.; Balawejder, M. The Effect of Exogenous Application of Quercetin Derivative Solutions on the Course of Physiological and Biochemical Processes in Wheat Seedlings. Int. J. Mol. Sci. 2021, 22, 6882. [Google Scholar] [CrossRef]
- Sultana, R.; Wang, X.; Azeem, M.; Hussain, T.; Mahmood, A.; Fiaz, S.; Qasim, M. Coumarin-mediated growth regulations, antioxidant enzyme activities, and photosynthetic efficiency of Sorghum bicolor under saline conditions. Front. Plant Sci. 2022, 13, 799404. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.M.; Hechler, P.J.; Muday, G.K. Ethylene-induced avonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 2014, 164, 1707–1717. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.I.; El-Hamahmy, M.A.M.; Rafudeen, M.S.; Ebrahim, M.K.H. Exogenous spermidine enhances expression of Calvin cycle genes and photosynthetic efficiency in sweet sorghum seedlings under salt stress. Biol. Plant. 2019, 63, 511–518. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatma, M.; Asgher, M.; Masood, A.; Khan, N.A. Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ. Exp. Bot. 2014, 107, 55–63. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Martinkova, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil Environ. 2017, 63, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2023, 10, 1627. [Google Scholar] [CrossRef]
- Dobrikova, A.G.; Apostolova, E.L. Damage and protection of the photosynthetic apparatus from UV-B radiation. II. Effect of quercetin at different pH. J. Plant Physiol. 2015, 184, 98–105. [Google Scholar] [CrossRef]
- Chichiriccò, G.; Poma, A. Penetration and Toxicity of Nanomaterials in Higher Plants. Nanomaterials 2015, 5, 851–873. [Google Scholar] [CrossRef]
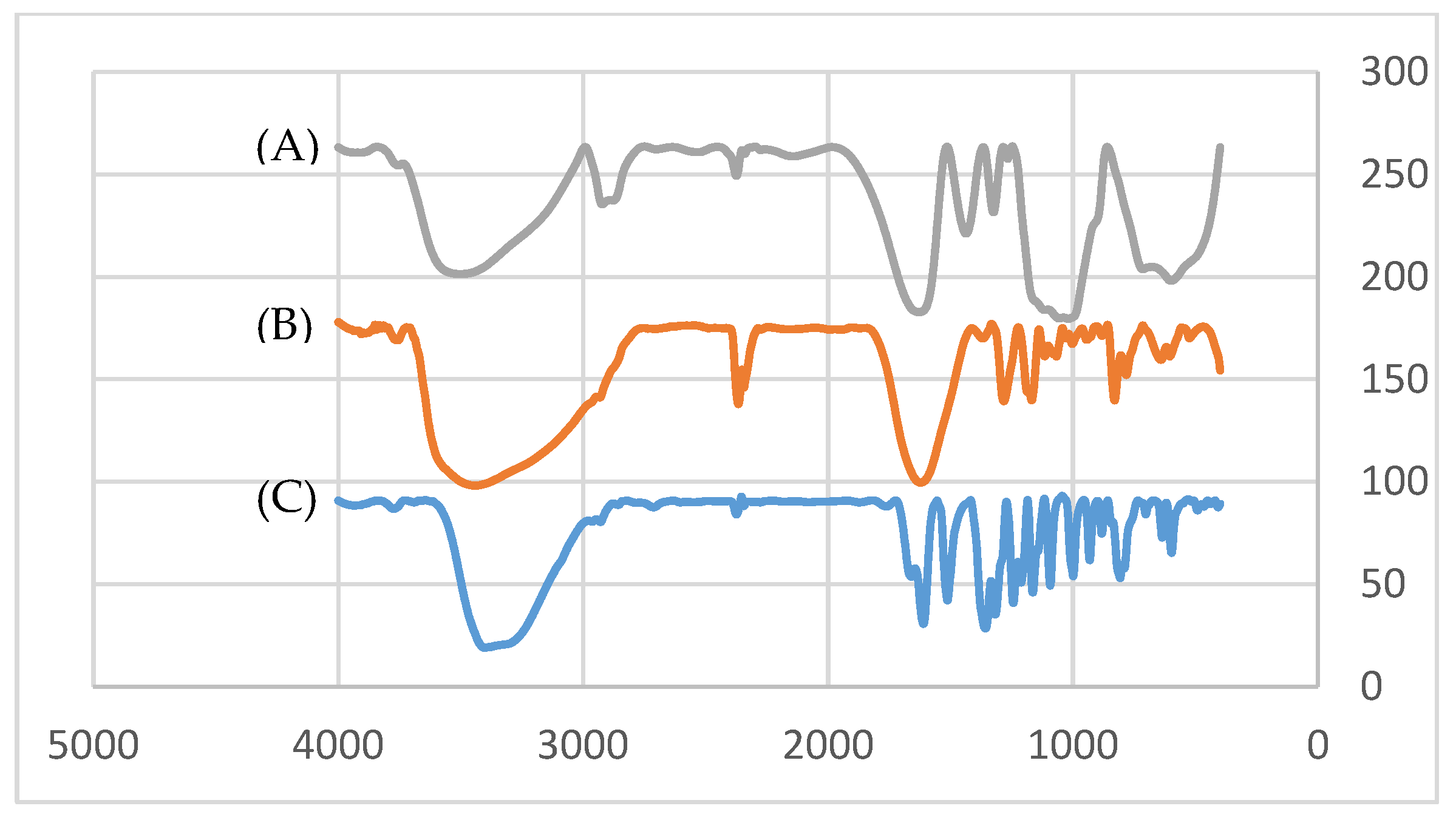

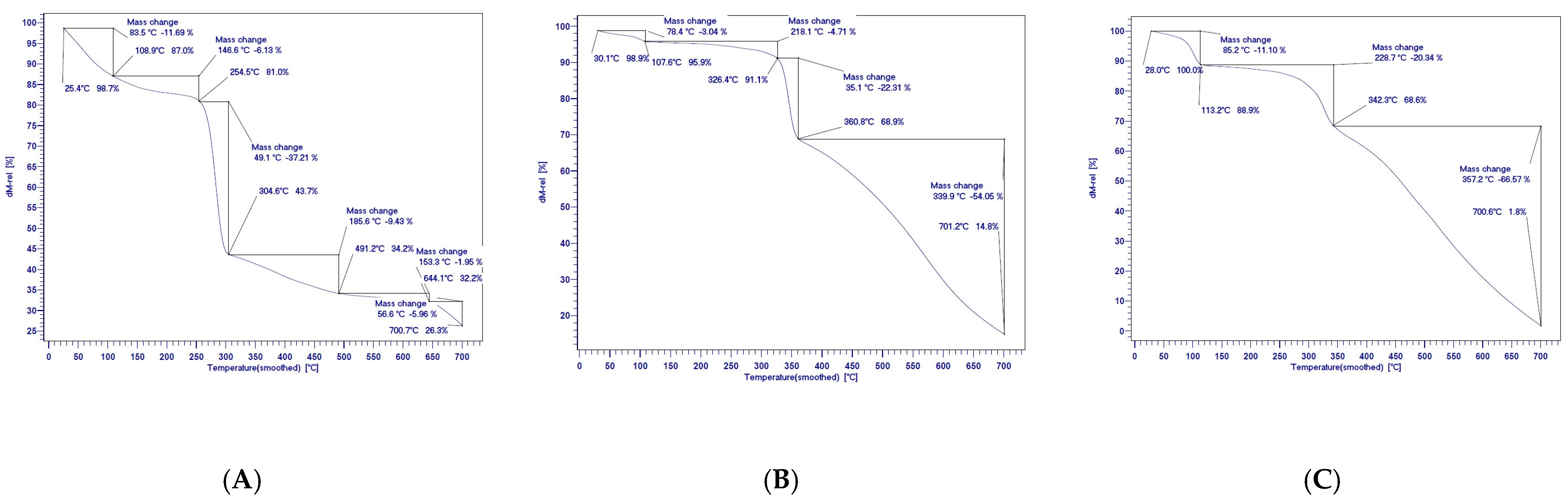





| NaCl (mM) | Qu | QNCs | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | GP (%) | Shoot Length (cm) | Root Length (cm) | Treatments | GP (%) | Shoot Length (cm) | Root Length (cm) | |
| 0 | Control | 90 ± 5.77 e | 14.3 ± 2.01 f | 40.36 ± 5.12 e | Control | 90 ± 5.77 ef | 14.3 ± 2.01 f | 40.36 ± 5.12 e |
| 0.001 mg/mL | 86.6 ± 8.81 d | 14 ± 0.82 f | 37 ± 2.33 de | 0.001 mg/mL | 90 ± 3.33 ef | 14.8 ± 0.93 f | 38 ± 2.63 de | |
| 0.01 mg/mL | 93.3 ± 3.33 d | 16.1 ± 0.72 g | 38.1 ± 1 de | 0.01 mg/mL | 96.6 ± 3.33 f | 16.3 ± 0.59 g | 45.8 ± 2.1 f | |
| 1 mg/mL | 63.3 ± 6.66 bc | 12.4 ± 0.54 ef | 20.33 ± 1.25 bc | 1 mg/mL | 63.3 ± 3.33 c | 12 ± 0.44 de | 30 ± 1.7 ef | |
| 50 | Control | 76.6 ± 6.66 cd | 11.8 ± 0.45 e | 28.1 ± 4.1 cde | Control | 76.6 ± 6.6 de | 11.8 ± 0.45 de | 33.8 ± 2.36 cde |
| 0.001 mg/mL | 76.6 ± 8.81 cd | 12 ± 0.96 e | 31.2 ± 2.24 cd | 0.001 mg/mL | 80 ± 5.77 e | 12.5 ± 0.84 de | 39.8 ± 1.87 cd | |
| 0.01 mg/mL | 83.3 ± 6.66 d | 14 ± 0.64 f | 34.5 ± 0.94 de | 0.01 mg/mL | 90 ± 5.77 ef | 15.4 ± 0.74 e | 35.5 ± 3.5 cd | |
| 1 mg/mL | 60 ± 5.77 b | 9.6 ± 0.49 d | 3.8 ± 0.9 a | 1 mg/mL | 60 ± 5.77 bc | 10.6 ± 0.55 d | 15.7 ± 0.49 bc | |
| 100 | Control | 66.6 ± 8.81 c | 6.8 ± 1.1 cd | 6.3 ± 1.07 a | Control | 66.6 ± 8/81 cd | 6.8 ± 1.1 bc | 8.5 ± 1.91 b |
| 0.001 mg/mL | 70 ± 8.81 cd | 8 ± 0.29 d | 6.36 ± 1.13 ab | 0.001 mg/mL | 76.6 ± 3.3 cd | 7.3 ± 0.32 cd | 16.3 ± 0.92 ab | |
| 0.01 mg/mL | 76.6 ± 3.33 cd | 9.8 ± 0.64 d | 11.53 ± 2.31 ab | 0.01 mg/mL | 83.3 ± 3.33 e | 9.9 ± 0.71 d | 16.5 ± 2 ab | |
| 1 mg/mL | 56.6 ± 5.77 b | 4 ± 0.29 bc | 1.7 ± 0.7 a | 1 mg/mL | 56.6 ± 3.33 bc | 4.7 ± 0.37 bc | 4.7 ± 0.27 b | |
| 150 | Control | 53.3 ± 3.33 a | 2.1 ± 0.79 a | 1.3 ± 0.4 a | Control | 53.3 ± 3.33 b | 2.1 ± 0.79 a | 1.3 ± 0.33 a |
| 0.001 mg/mL | 63.3 ± 3.33 bc | 3 ± 0.57 b | 3.2 ± 0.57 a | 0.001 mg/mL | 53.3 ± 3.33 b | 3.2 ± 0.62 b | 3.2 ± 0.17 a | |
| 0.01 mg/mL | 66.6 ± 6.66 c | 4.8 ± 0.21 c | 1.7 ± 0.16 a | 0.01 mg/mL | 73.3 ± 3.33 d | 5.9 ± 0.29 c | 2.3 ± 0.27 a | |
| 1 mg/mL | 50 ± 3.33 a | 2.3 ± 0.1 a | 1.1 ± 0.25 a | 1 mg/mL | 46.6 ± 3.33 a | 2.5 ± 0.18 a | 1.8 ± 0.03 a |
| NaCl (mM) | Treatments | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Total Chlorophyll (mg g−1 FW) | Carotenoids (µg g−1 FW) | Minimum Fluorescence | Maximum Fluorescence | Fv/Fm | SPAD | Photosynthesis Rate (µmolm−2s−1) | Stomatal Conductance (mmolm−2s−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Control | 0.411 ± 0.003 g | 0.159 ± 0.003 de | 0.564 ± 0.007 d | 4. 09 ± 0.039 de | 402.75 ± 39.5 c | 2031 ± 182.35 ab | 0.851 ± 0.016 d | 5.31 ± 0.44 d | 12.11 ± 0.32 hi | 301.6 ± 31.9 bc |
| 0.01 mg/mL Qu | 0.415 ± 0.004 g | 0.154 ± 0.004 de | 0.569 ± 0.004 d | 4.46 ± 0.023 e | 355.5 ± 25.72 bc | 2083.7 ± 86.04 ab | 0.829 ± 0.01 bc | 5.55 ± 0.23 de | 12.35 ± 0.23 i | 301.3 ± 47.38 bc | |
| 0.01 mg/mL QNCs | 0.436 ± 0.006 g | 0.176 ± 0.005 e | 0.613 ± 0.005 e | 4.59 ± 0.026 e | 327.2 ± 13.2 ab | 1998.7 ± 133.9 a | 0.84 ± 0.037 c | 6.66 ± 0.2 e | 13.32 ± 0.32 j | 355.3 ± 23.95 d | |
| 50 | Control | 0.339 ± 0.007 e | 0.155 ± 0.001 de | 0.49 ± 0.005 cd | 3.72 ± 0.038 cd | 462 ± 44 e | 2381.5 ± 27.57 bcd | 0/8 ± 0.014 bc | 4.7 ± 0.23 bc | 10.33 ± 0.12 f | 265.3 ± 58.89 b |
| 0.01 mg/mL Qu | 0.359 ± 0.026 ef | 0.165 ± 0.006 de | 0.514 ± 0.01 cd | 3.95 ± 0.034 cde | 281.2 ± 85.74 a | 2186.2 ± 80.22 abc | 0/801 ± 0.017 a | 5.05 ± 0.56 c | 10.8 ± 0.3 fg | 324.3 ± 12.73 cd | |
| 0.01 mg/mL QNCs | 0.381 ± 0.013 f | 0.159 ± 0.02 de | 0.56 ± 0.013 d | 4.03 ± 0.04 cde | 344.7 ± 21.7 abc | 2168.7 ± 33.87 abc | 0.826 ± 0.06 bc | 5.18 ± 0.69 cd | 11.4 ± 0.36 gh | 329 ± 7.76 bc | |
| 100 | Control | 0.211 ± 0.001 b | 0.188 ± 0.001 b | 0.33 ± 0.001 b | 2.76 ± 0.021 b | 446.2 ± 50 de | 2484 ± 103.89 d | 0.788 ± 0.04 a | 4.79 ± 0.74 bc | 7.74 ± 0.25 d | 228.6 ± 30.38 ab |
| 0.01 mg/mlQu | 0.304 ± 0.002 d | 0.145 ± 0.004 cd | 0.448 ± 0.002 c | 3.5 ± 0.32 bc | 346.7 ± 43.4 abc | 2151.5 ± 171.4 abc | 0.804 ± 0.019 b | 5.38 ± 0.55 d | 8.44 ± 0.17 de | 378 ± 16.92 e | |
| 0.01 mg/mL QNCs | 0.309 ± 0.001 d | 0.148 ± 0.004 d | 0.458 ± 0.002 c | 3.66 ± 0.087 bc | 339 ± 24.18 abc | 2211.5 ± 48.5 bc | 0.822 ± 0.026 bc | 5 ± 0.56 c | 8.8 ± 0.22 e | 268.6 ± 3.84 bc | |
| 150 | Control | 0.15 ± 0.001 a | 0.08 ± 0.001 a | 0.23 ± 0.003 a | 2.01 ± 0.086 a | 430.75 ± 34 d | 2449.2 ± 38.09 cd | 0.759 ± 0.023 a | 3.53 ± 0.33 a | 3.45 ± 0.34 a | 194.3 ± 61.19 a |
| 0.01 mg/mlQu | 0.234 ± 0.001 b | 0.101 ± 0.008 b | 0.335 ± 0.06 b | 2.75 ± 0.028 b | 367.7 ± 23.6 bc | 2249.7 ± 29.13 bc | 0.804 ± 0.031 b | 4.36 ± 0.47 b | 4.29 ± 0.23 b | 239 ± 48.52 ab | |
| 0.01 mg/mL QNCs | 0.274 ± 0.002 c | 0.122 ± 0.01 bc | 0.294 ± 0.08 b | 3.75 ± 0.099 cd | 315.7 ± 10.82 ab | 2207 ± 41.5 bc | 0.848 ± 0.012 c | 4.5 ± 0.4 b | 5.46 ± 0.35 c | 289.6 ± 2.33 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshneshin, H.; Salimi, A.; Razavi, S.M.; Khoshkam, M. Synthesis and Characterization of a Quercetin-Based Nanocomposite and Its Ameliorating Impacts on the Growth, Physiological, and Biochemical Parameters of Ocimum basilicum L. under Salinity Stress. Sustainability 2023, 15, 12059. https://doi.org/10.3390/su151512059
Arshneshin H, Salimi A, Razavi SM, Khoshkam M. Synthesis and Characterization of a Quercetin-Based Nanocomposite and Its Ameliorating Impacts on the Growth, Physiological, and Biochemical Parameters of Ocimum basilicum L. under Salinity Stress. Sustainability. 2023; 15(15):12059. https://doi.org/10.3390/su151512059
Chicago/Turabian StyleArshneshin, Homa, Azam Salimi, Seyed Mehdi Razavi, and Maryam Khoshkam. 2023. "Synthesis and Characterization of a Quercetin-Based Nanocomposite and Its Ameliorating Impacts on the Growth, Physiological, and Biochemical Parameters of Ocimum basilicum L. under Salinity Stress" Sustainability 15, no. 15: 12059. https://doi.org/10.3390/su151512059






