Role of Reductive Soil Disinfestation and Chemical Soil Fumigation on the Fusarium Wilt of Dioscorea batatas Decne Suppression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Area and Materials Description
2.2. Experimental Design
2.3. Measurement of the Soil Physicochemical Properties
2.4. Measurement of the Soil Microbial Population
2.5. Measurement of the Soil Bacterial Community and Function
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Parameters
3.2. Soil Bacteria, Fungi, and Fusarium oxysporum Quantification
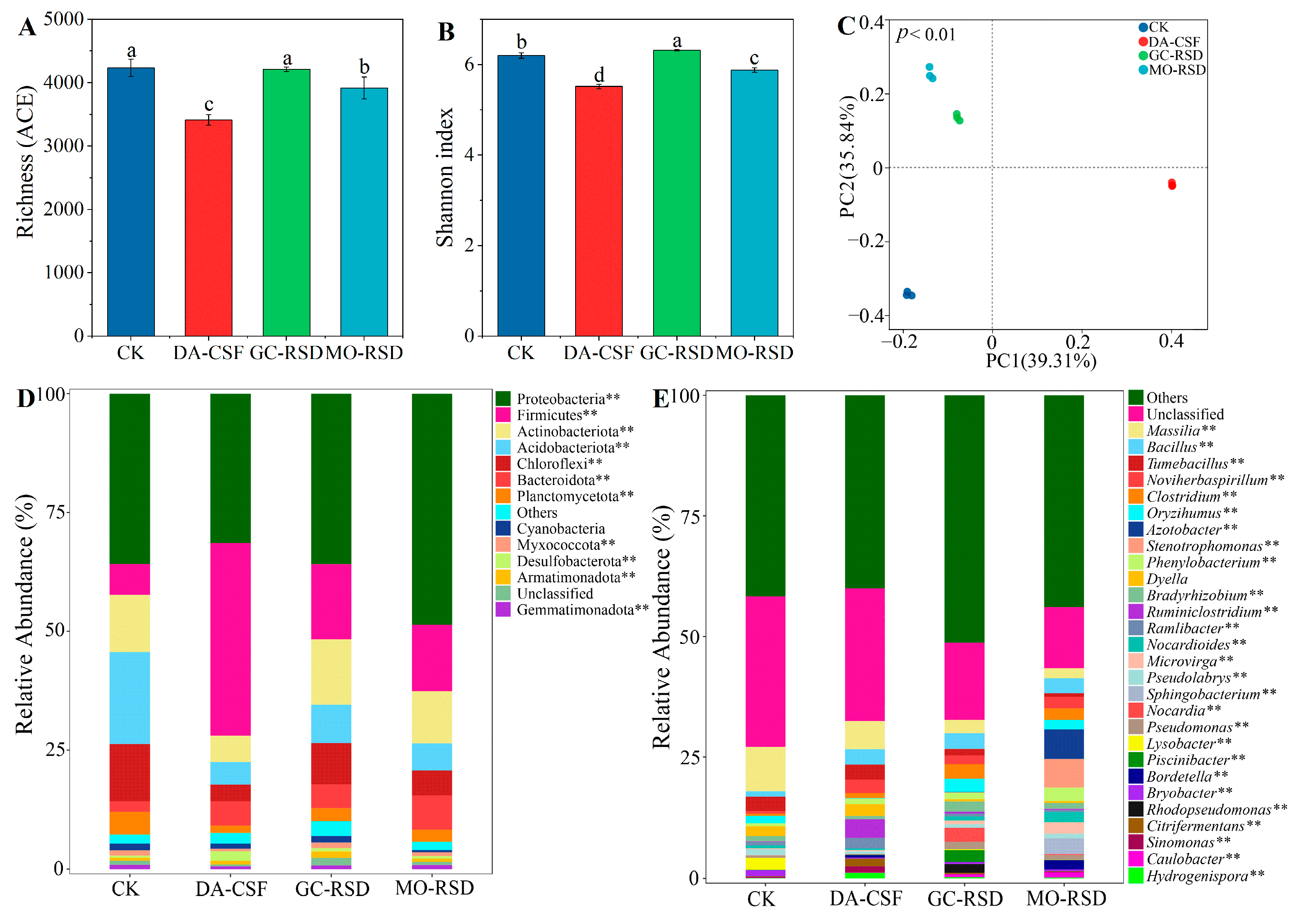
3.3. Bacterial Community Diversity and Composition
3.3.1. Bacterial Community Diversity
3.3.2. Bacterial Community Composition
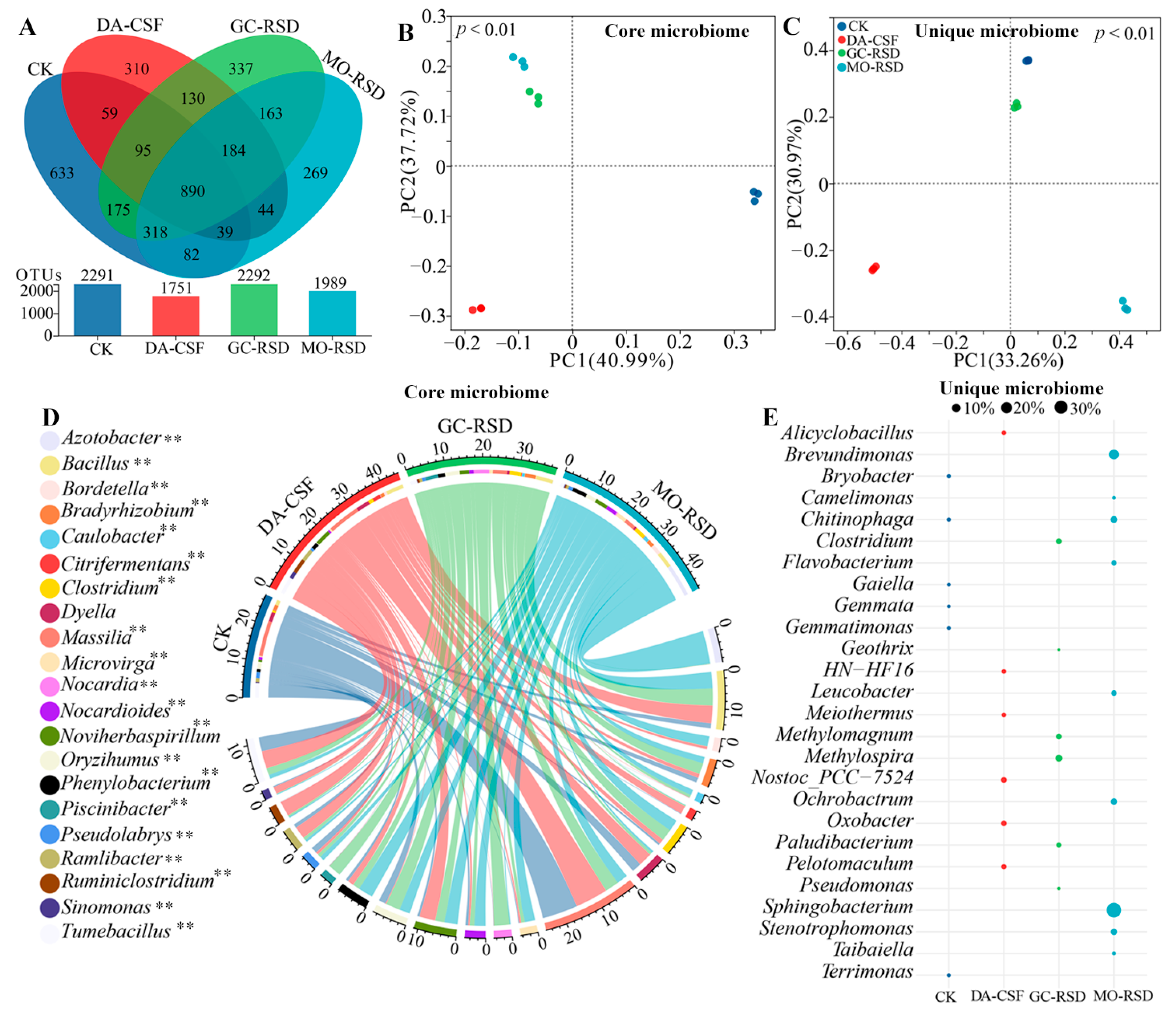
3.4. Soil Core and Unique Soil Microbiome Compositions
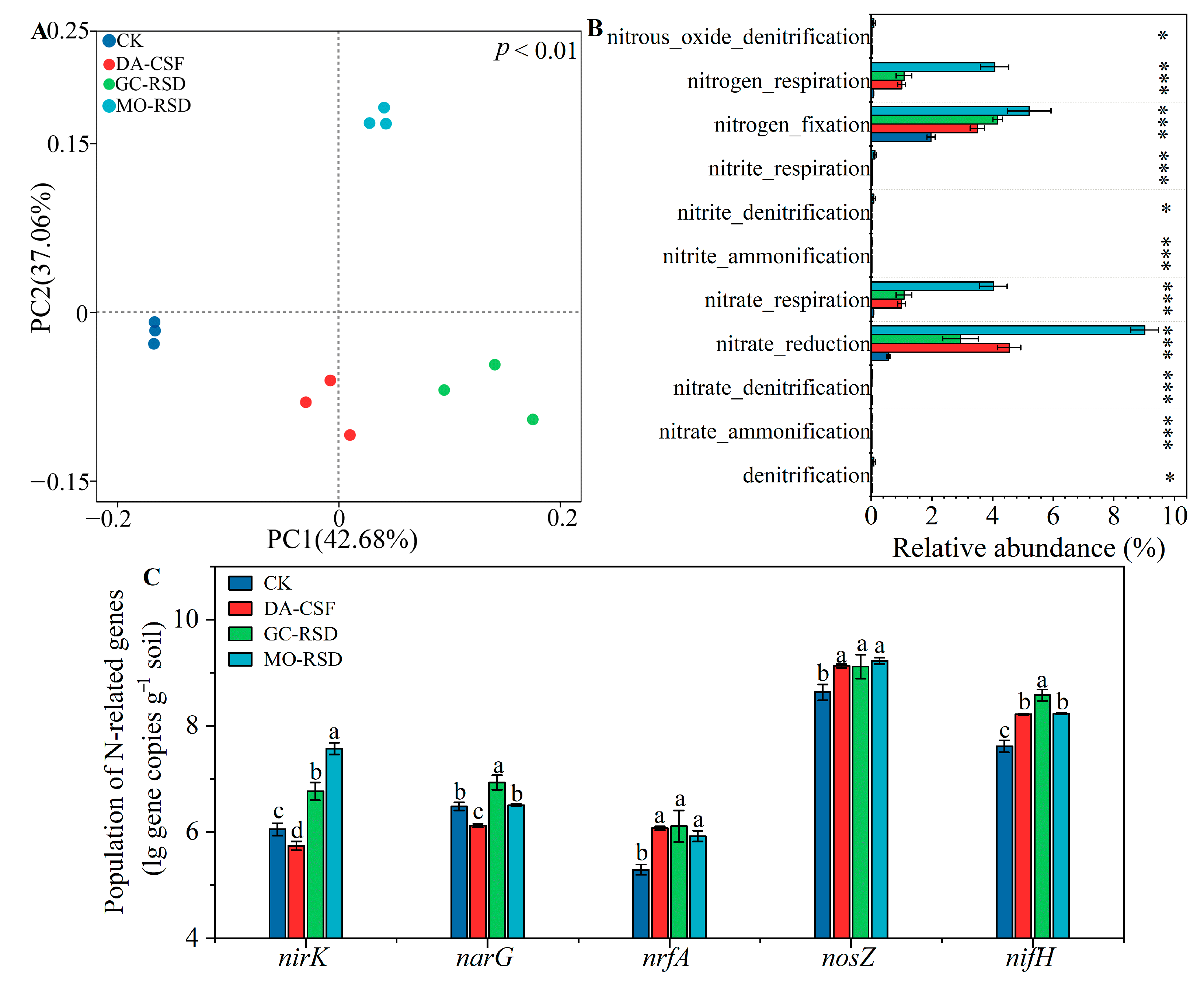
3.5. Bacterial Functional and Nitrogen-Related Gene Compositions
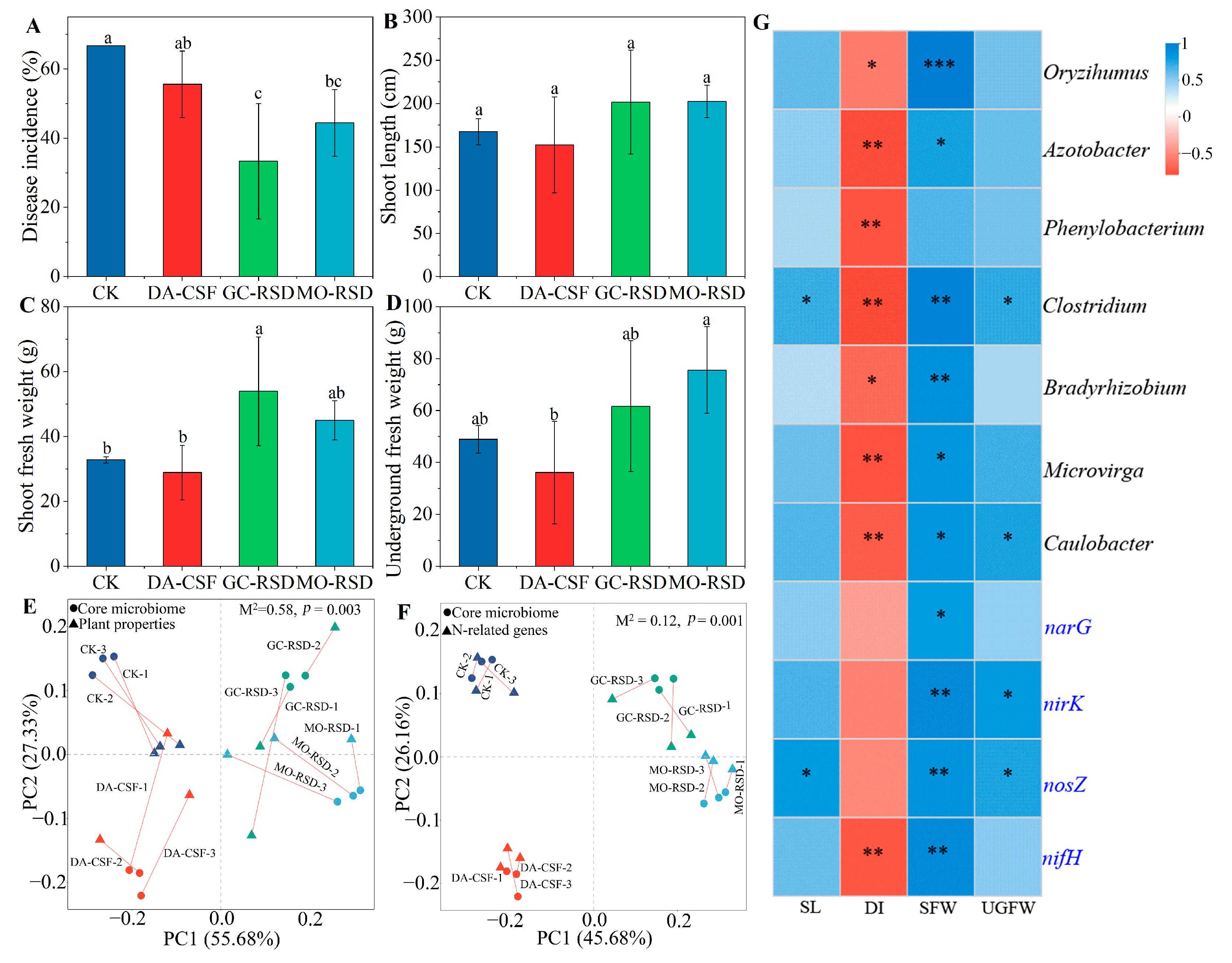
3.6. Relationships among Plant Properties, Core Microbiome, and N-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, M.; Suh, S.J.; Yang, J.H.; Lu, Y.; Kim, S.J.; Kwon, S.; Jo, T.H.; Kim, J.W.; Park, Y.I.; Ahn, G.W.; et al. Anti-inflammatory activity of bark of Dioscorea batatas Decne through the inhibition of iNOS and COX-2 expressions in RAW264.7 cells via NF-κB and ERK1/2 inactivation. Food Chem. Toxicol. 2010, 48, 3073–3079. [Google Scholar] [CrossRef]
- Choi, E.M.; Koo, S.J.; Hwang, J.K. Immune cell stimulating activity of mucopolysaccharide isolated from yam (Dioscorea batatas). J. Ethnopharmacol. 2004, 91, 1–6. [Google Scholar] [CrossRef]
- Kim, S.; Jwa, H.; Yanagawa, Y.; Park, T. Extract from Dioscorea batatas ameliorates insulin resistance in mice fed a high-fat diet. J. Med. Food 2012, 15, 527–534. [Google Scholar] [CrossRef]
- Zhao, J.; Mei, Z.; Zhang, X.; Xue, C.; Zhang, C.; Ma, T.; Zhang, S. Suppression of Fusarium wilt of cucumber by ammonia gas fumigation via reduction of Fusarium population in the field. Sci. Rep. 2017, 7, 43103. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.Z.; Ren, G.D.; Wang, G.F.; Ma, Y. Impacts on soil microbial characteristics and their restorability with different soil disinfestation approaches in intensively cropped greenhouse soils. Appl. Microbiol. Biot. 2019, 103, 6369–6383. [Google Scholar] [CrossRef]
- Montalvo, A.E.; Meléndez, P.L. Histopathology of interrelations between Fusarium oxysporum f. sp. dioscoreae and two nematode species on yam. J. Agric. Univ. Puerto Rico 1969, 70, 245–254. [Google Scholar]
- Nwankiti, A.O.; Gwa, V.I. Evaluation of antagonistic effect of Trichoderma harzianum against Fusarium oxysporum causal agent of white yam (Dioscorearotundata poir) tuber rot. Trends Tech. Sci. Res. 2018, 1, 555554. [Google Scholar]
- Ohr, H.D.; Sims, J.J.; Grech, N.M.; Becker, J.O.; McGiffen Jr, M.E. Methyl iodide, an ozone-safe alternative to methyl bromide as a soil fumigant. Plant Dis. 1996, 80, 731–735. [Google Scholar] [CrossRef]
- Cebolla, V.; Busto, J.; Ferrer, A.; Miguel, A.; Maroto, V.; Gullino, M.L.; Katan, J.; Matta, A. Methyl bromide alternatives on horticultural crops. Acta Hortic. 2000, 532, 237–242. [Google Scholar] [CrossRef]
- Di Primo, P.; Gamliel, A.; Austerweil, M.; Steiner, B.; Beniches, M.; Peretz-Alon, I.; Katan, J. Accelerated degradation of metam-sodium and dazomet in soil: Characterization and consequences for pathogen control. Crop Prot. 2003, 22, 635–646. [Google Scholar] [CrossRef]
- Huang, X.Q.; Zhao, J.; Zhou, X.; Han, Y.S.; Zhang, J.B.; Cai, Z.C. How green alternatives to chemical pesticides are environmentally friendly and more efficient. Eur. J. Soil Sci. 2019, 70, 518–529. [Google Scholar] [CrossRef]
- Mao, L.G.; Wang, Q.X.; Yan, D.D.; Xie, H.W.; Li, Y.; Guo, M.X.; Cao, A.C. Evaluation of the combination of 1, 3-dichloropropene and dazomet as an efficient alternative to methyl bromide for cucumber production in China. Pest Manag. Sci. 2012, 68, 602–609. [Google Scholar] [CrossRef]
- Ślusarski, C.; Pietr, S.J. Combined application of dazomet and Trichoderma asperellum as an efficient alternative to methyl bromide in controlling the soil-borne disease complex of bell pepper. Crop Prot. 2009, 28, 668–674. [Google Scholar] [CrossRef]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Shinmura, A. Causal agent and control of root rot of welsh onion. PSJ Soil-borne Dis. Workshop Rep. 2000, 20, 133–143. (In Japanese) [Google Scholar]
- Momma, N.; Kobara, Y.; Uematsu, S.; Kita, N.; Shinmura, A. Development of biological soil disinfestation in Japan. Appl. Microbiol. Biotechnol. 2013, 97, 3801–3809. [Google Scholar] [CrossRef]
- Huang, X.Q.; Wen, T.; Zhang, J.B.; Meng, L.; Zhu, T.B.; Cai, Z.C. Toxic organic acids produced in biological soil disinfestation mainly caused the suppression of Fusarium oxysporum f. sp. cubense. BioControl 2015, 60, 113–124. [Google Scholar] [CrossRef]
- Momma, N.; Kobara, Y.; Momma, M. Fe2+ and Mn2+, potential agents to induce suppression of Fusarium oxysporum for biological soil disinfestation. J. Gen. Plant Pathol. 2011, 77, 331–335. [Google Scholar] [CrossRef]
- Shrestha, U.; Ownley, B.H.; Bruce, A.; Rosskopf, E.N.; Butler, D.M. Anaerobic soil disinfestation efficacy against Fusarium oxysporum is affected by soil temperature, amendment type, rate, and C: N ratio. Phytopathology 2021, 111, 1380–1392. [Google Scholar] [CrossRef]
- van Agtmaal, M.; van Os, G.J.; Hol, W.H.G.; Hundscheid, M.P.J.; Runia, W.T.; Hordijk, C.A.; de Boer, W. Legacy effects of anaerobic soil disinfestation on soil bacterial community composition and production of pathogen suppressing volatiles. Front. Microbiol. 2015, 6, 701. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.L.; Huang, X.Q.; Zhao, J.; Zhang, J.B.; Cai, Z.C. Characterizing the key agents in a disease-suppressed soil managed by reductive soil disinfestation. Appl. Environ. Microb. 2019, 85, e02992-18. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.Z.; Yang, Y.J.; Cai, Z.C.; Ma, Y. The control of Fusarium oxysporum in soil treated with organic material under anaerobic condition is affected by liming and sulfate content. Biol. Fertil. Soils 2018, 54, 295–307. [Google Scholar] [CrossRef]
- Zúñiga-Silgado, D.; Rivera-Leyva, J.C.; Coleman, J.J.; Sánchez-Reyes, A.; Valencia-Díaz, S.; Serrano, M.; de-Bashan, L.E.; Folch-Mallol, J.L. Soil Type Affects Organic Acid Production and Phosphorus Solubilization Efficiency Mediated by Several Native Fungal Strains from Mexico. Microorganisms 2020, 8, 1337. [Google Scholar] [CrossRef]
- Zúñiga-Silgado, D.; Sánchez-Reyes, A.; Ortiz-Hernández, M.L.; Otero, M.; Balcázar-López, E.; Valencia-Díaz, S.; Serrano, M.; Coleman, J.J.; Sarmiento-López, L.; De-Bashan, L.E.; et al. Soil Type Influences Novel “Milpa” Isolates of Trichoderma virens and Aspergillus tubingensis That Promote Solubilization, Mineralization, and Phytoabsorption of Phosphorus in Capsicum annuum L. J. Fungi 2022, 8, 1178. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Klarer, E.; Reed, A.J.; Leisso, R.; Poirier, B.; Honaas, L.; Mazzola, M. Temporal dynamics of the soil metabolome and microbiome during simulated anaerobic soil disinfestation. Front. Microbiol. 2019, 10, 2365. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.L.; Xie, Y.; Zhong, X.; Deng, Q.Q.; Shao, Q.; Cai, Z.C.; Huang, X.Q. Facilitating effects of the reductive soil disinfestation process combined with Paenibacillus sp. amendment on soil health and physiological properties of Momordica charantia. Front. Plant Sci. 2023, 13, 1095656. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Xie, Y.; Zhang, J.Q.; Li, R.M.; Ali, A.; Cai, Z.C.; Huang, X.Q.; Liu, L.L. Effects of reductive soil disinfestation combined with Liquid−Readily decomposable compounds and solid plant residues on the bacterial community and functional composition. Microb. Ecol. 2023, 86, 1132–1144. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics, 2nd ed.; Stackenbrandt, E., Goodfellow, M., Eds.; John Wiley and Sons, Inc.: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microb. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Lievens, B.; Brouwer, M.; Vanachter, A.C.R.C.; Cammue, B.P.A.; Thomma, B.P.H.J. Real-time PCR for detection and quantification of fungal and oomycete tomato pathogens in plant and soil samples. Plant Sci. 2006, 171, 155–165. [Google Scholar] [CrossRef]
- Lievens, B.; Brouwer, M.; Vanachter, A.C.R.C.; Lévesque, C.A.; Cammue, B.P.A.; Thomma, B.P.H.J. Quantitative assessment of phytopathogenic fungi in various substrates using a DNA macroarray. Environ. Microbiol. 2005, 7, 1698–1710. [Google Scholar] [CrossRef]
- Lopez-Gutierrez, J.C.; Henry, S.; Hallet, S.; Martin-Laurent, F.; Catroux, G.; Philippot, L. Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J. Microbiol. Methods 2004, 57, 399–407. [Google Scholar] [CrossRef]
- Baraker, G.; Fesefeldt, A.; Witzel, K.P. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 1998, 64, 3769–3775. [Google Scholar] [CrossRef] [Green Version]
- Rösch, C.; Bothe, H. Improved assessment of denitrifying, N2-fixing, and total-community bacteria by terminal restriction fragment length polymorphism analysis using multiple restriction enzymes. Appl. Environ. Microb. 2005, 71, 2026–2035. [Google Scholar] [CrossRef] [Green Version]
- Poly, F.; Ranjard, L.; Nazaret, S.; Gourbière, F.; Monrozier, L.J. Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl. Environ. Microb. 2001, 67, 2255–2262. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Lane, D.J.; Pace, B.; Olsen, G.J.; Stahl, D.A.; Sogin, M.L.; Pace, N.R. Rapid determination of 16s ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 1985, 82, 6955–6959. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic. Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Shade, A.; Handelsman, J. Beyond the Venn diagram: The hunt for a core microbiome. Environ. Microbiol. 2012, 14, 4–12. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, S.Z.; Zhou, X.; Xia, Q.; Liu, X.; Zhang, S.R.; Zhang, J.B.; Cai, Z.C.; Huang, X.Q. Reductive soil disinfestation incorporated with organic residue combination significantly improves soil microbial activity and functional diversity than sole residue incorporation. Appl. Microbiol. Biot. 2020, 104, 7573–7588. [Google Scholar] [CrossRef]
- Gamliel, A.; Austerweil, M.; Kritzman, G. Non-chemical approach to soilborne pest management-organic amendments. Crop Prot. 2000, 19, 847–853. [Google Scholar] [CrossRef]
- Frick, A.; Zebarth, B.J.; Szeto, S.Y. Behavior of the soil fumigant methyl isothiocyanate in repacked soil columns. J. Environ. Qua. 1998, 27, 1158–1169. [Google Scholar] [CrossRef]
- Duniway, J.M. Status of chemical alternatives to methyl bromide for pre-plant fumigation of soil. Phytopathology 2002, 92, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.L.; Chen, S.H.; Zhao, J.; Zhou, X.; Wang, B.Y.; Li, Y.L.; Zheng, G.Q.; Zhang, J.B.; Cai, Z.C.; Huang, X.Q. Watermelon planting is capable to restructure the soil microbiome that regulated by reductive soil disinfestation. Appl. Soil Ecol. 2018, 129, 52–60. [Google Scholar] [CrossRef]
- Bever, J.D.; Broadhurst, L.M.; Thrall, P.H. Microbial phylotype composition and diversity predicts plant productivity and plant-soil feedbacks. Ecol. Lett. 2012, 16, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Garbeva, P.; van Veen, J.A.; van Elsas, J.D. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for Disease suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef]
- Franche, C.; Lindstrm, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Fan, K.K.; Delgado-Baquerizo, M.; Guo, X.S.; Wang, D.Z.; Zhu, Y.G.; Chu, H.Y. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment. ISME J. 2021, 15, 550–561. [Google Scholar] [CrossRef]
- Abbass, Z.; Okon, Y. Plant growth promotion by Azotobacter paspali in the rhizosphere. Soil Biol. Biochem. 1993, 25, 1075–1083. [Google Scholar] [CrossRef]
- Cueva-Yesquén, L.C.; Goulart, M.C.; de Angelis, D.A.; Alves, M.N.; Fantinatti-Garboggini, F. Multiple plant growth-promotion traits in endophytic bacteria retrieved in the vegetative stage from passionflower. Front. Plant Sci. 2021, 11, 621740. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Fu, Y.P.; Zhao, H.Z.; Cheng, J.S.; Xie, J.T.; Jiang, D.H. Enrichment of bacteria involved in the nitrogen cycle and plant growth promotion in soil by sclerotia of rice sheath blight fungus. Stress Biol. 2022, 2, 13. [Google Scholar] [CrossRef]
- Zhang, C.S.; Lin, Y.; Tian, X.Y.; Xu, Q.; Chen, Z.H.; Lin, W. Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria abundance. Appl. Soil Ecol. 2017, 112, 90–96. [Google Scholar] [CrossRef]
- Kyselková, M.; Kopecký, J.; Frapolli, M.; Défago, G.; Ságová-Marečková, M.; Grundmann, G.L.; Moënne-Loccoz, Y. Comparison of rhizobacterial community composition in soil suppressive or conducive to tobacco black root rot disease. ISME J. 2009, 3, 1127–1138. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wei, Z.; Weidner, S.; Friman, V.P.; Xu, Y.C.; Shen, Q.R.; Jousset, A. Probiotic Pseudomonas communities enhance plant growth and nutrient assimilation via diversity-mediated ecosystem functioning. Soil Biol. Biochem. 2017, 113, 122–129. [Google Scholar] [CrossRef]
- Xue, J.; Rahman, M.K.U.; Ma, C.; Zheng, X.; Wu, F.; Zhou, X. Silicon modification improves biochar’s ability to mitigate cadmium toxicity in tomato by enhancing root colonization of plant-beneficial bacteria. Ecotoxicol. Environ. Saf. 2023, 249, 114407. [Google Scholar]
- Mowlick, S.; Takehara, T.; Kaku, N.; Ueki, K.; Ueki, A. Proliferation of diversified Clostridial species during biological soil disinfestation incorporated with plant biomass under various conditions. Appl. Microbiol. Biot. 2013, 97, 8365–8379. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe (III) and Mn (IV) reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar]
- Li, B.; Zheng, Y.Y.; Cai, Y.; Liu, J.X.; Wang, R.T.; Cui, G.W.; Li, Y.G.; Meng, L. Identification and assessment of a biocontrol agent, Ochrobactrum intermediumi-5, for management of alfalfa root rot caused by Fusarium tricinctum. Phytopathology 2021, 111, 1927–1934. [Google Scholar] [CrossRef]
- Liu, H.W.; Li, J.Y.; Carvalhais, L.C.; Percy, C.D.; Prakash, V.J.; Schenk, P.M.; Singh, B.K. Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 2021, 229, 2873–2885. [Google Scholar] [CrossRef]
- Nishioka, T.; Marian, M.; Kobayashi, I.; Kobayashi, Y.; Yamamoto, K.; Tamaki, H.; Suga, H.; Shimizu, M. Microbial basis of Fusarium wilt suppression by Allium cultivation. Sci. Rep. 2019, 9, 1715. [Google Scholar] [CrossRef] [PubMed]
- Saimmai, A.; Sobhon, V.; Maneerat, S. Production of biosurfactant from a new and promising strain of Leucobacter komagatae 183. Ann. Microbiol. 2012, 62, 391–402. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.M.; Zhang, L.X.; Han, M. An investigation of panax ginseng meyer growth promotion and the biocontrol potential of antagonistic bacteria against ginseng black spot. J. Ginseng Res. 2018, 42, 304–311. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primers | Sequence (5′-3′) | Reference |
|---|---|---|---|
| bacteria | Eub338-F | CCTACGGGAGGCAGCAG | [28] |
| Eub518-R | ATTACCGCGGCTGCTGG | [29] | |
| fungi | ITS1-F | CTTGGTCATTTAGAGGAAGTAA | [30] |
| ITS2-R | GCTGCGTTCTTCATCGATGC | [31] | |
| F. oxysporum | ITS1-F | CTTGGTCATTTAGAGGAAGTAA | [30] |
| AFP308-R | CGAATTAACGCGAGTCCCAAC | [32] | |
| narG | 1960m2-F | TAYGTSGGGCAGGARAAACTG | [33] |
| 2050m2-R | CGTAGAAGAAGCTGGTGCTGTT | ||
| nirK | nirK-F | GGMATGGTKCCSTGGCA | [34] |
| nirK-R | GCCTCGATCAGRTTRTGG | ||
| nosZ | NosZ-F | AACGCCTAYACSACSCTGTTC | [35] |
| NosZ-R | TCCATGTGCAGNGCRTGGCAGAA | ||
| nifH | Pol-F | TGCGAYCCSAARGCBGACTC | [36] |
| Pol-R | ATSGCCATCATYTCRCCGGA | ||
| 16S rRNA | 515-F | GTGCCAGCMGCCGCGG | [37] |
| 907-R | CCGTCAATTCMTTTRAGTTT | [38] |
| Treatment | pH | NH4+–N (mg kg−1) | Available Potassium (mg kg−1) |
|---|---|---|---|
| CK | 5.91 ± 0.06 c | 18.56 ± 1.06 d | 643.3 ± 19.0 b |
| DA-CSF | 5.83 ± 0.02 c | 66.92 ± 0.58 b | 518.0 ± 115.9 b |
| GC-RSD | 6.11 ± 0.02 b | 62.61 ± 0.68 c | 878.0 ± 143.7 a |
| MO-RSD | 6.37 ± 0.06 a | 157.17 ± 0.82 a | 508.0 ± 129.9 b |
| Treatment | Bacteria (lg 16S Copies g−1 Soil) | Fungi (lg ITS Copies g−1 Soil) | F. oxysporum (lg ITS Copies g−1 Soil) |
|---|---|---|---|
| CK | 10.42 ± 0.05 b | 8.06 ± 0.06 b | 6.06 ± 131.2 a |
| DA-CSF | 10.35 ± 0.02 c | 7.47 ± 0.04 c | 4.38 ± 70.6 c |
| GC-RSD | 10.62 ± 0.03 a | 8.15 ± 0.15 b | 5.01 ± 137.5 b |
| MO-RSD | 10.56 ± 0.02 a | 8.54 ± 0.11 a | 4.88 ± 69.9 bc |
| Treatment | Core (%) | Unique (%) | ||
|---|---|---|---|---|
| OTUs | Sequences | OTUs | Sequences | |
| CK | 38.85 | 77.76 | 27.63 | 4.53 |
| DA-CSF | 50.83 | 66.11 | 17.70 | 5.98 |
| GC-RSD | 38.83 | 70.58 | 14.70 | 4.16 |
| MO-RSD | 44.75 | 67.87 | 13.52 | 8.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Q.; Li, X.; Zhao, T.; Wu, Y.; Xiang, L.; Pan, S.; Guo, Z.; Liu, L. Role of Reductive Soil Disinfestation and Chemical Soil Fumigation on the Fusarium Wilt of Dioscorea batatas Decne Suppression. Sustainability 2023, 15, 11991. https://doi.org/10.3390/su151511991
Shao Q, Li X, Zhao T, Wu Y, Xiang L, Pan S, Guo Z, Liu L. Role of Reductive Soil Disinfestation and Chemical Soil Fumigation on the Fusarium Wilt of Dioscorea batatas Decne Suppression. Sustainability. 2023; 15(15):11991. https://doi.org/10.3390/su151511991
Chicago/Turabian StyleShao, Qin, Xiaopeng Li, Tian Zhao, Yiyang Wu, Liqin Xiang, Shengfu Pan, Zihan Guo, and Liangliang Liu. 2023. "Role of Reductive Soil Disinfestation and Chemical Soil Fumigation on the Fusarium Wilt of Dioscorea batatas Decne Suppression" Sustainability 15, no. 15: 11991. https://doi.org/10.3390/su151511991
APA StyleShao, Q., Li, X., Zhao, T., Wu, Y., Xiang, L., Pan, S., Guo, Z., & Liu, L. (2023). Role of Reductive Soil Disinfestation and Chemical Soil Fumigation on the Fusarium Wilt of Dioscorea batatas Decne Suppression. Sustainability, 15(15), 11991. https://doi.org/10.3390/su151511991





