Microbial–Plant Collaborative Remediation of Cd-Contaminated Wastewater and Soil in the Surrounding Area of Nuclear Power Plants and Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Cadmium-Resistant Strains and Other Materials and Equipment
2.1.1. Basic Culture Medium Preparation
2.1.2. Experimental Equipment and Instruments
2.1.3. Material Handling Methods
2.2. Wastewater Source and Data Analysis
2.2.1. Wastewater Source
2.2.2. Data Analysis and Processing Methods
2.2.3. Adsorption Kinetics
2.3. Polluted Soil Selection and Data Analysis
2.3.1. Tested Soil Selection and Physicochemical Properties
2.3.2. Data Analysis and Processing
2.4. Risk Assessment Methods for Microbial–Plant Collaborative Remediation of Cd-Contaminated Environment
2.4.1. Pollution Environmental Assessment Model and Risk Assessment Procedure
2.4.2. Risk Assessment Calculation Model
3. Microorganisms and Plants in the Treatment Analysis and Risk Assessment of Cadmium-Contaminated Wastewater and Soil Remediation
3.1. Growth Analysis of Cadmium-Resistant Strains in Different Concentrations of Cd2+ Solution
3.2. Analysis of the Effect of Flat Bamboo Flower with Cadmium-Resistant Strain in the Treatment of Cadmium-Contaminated Wastewater from Nuclear Discharge
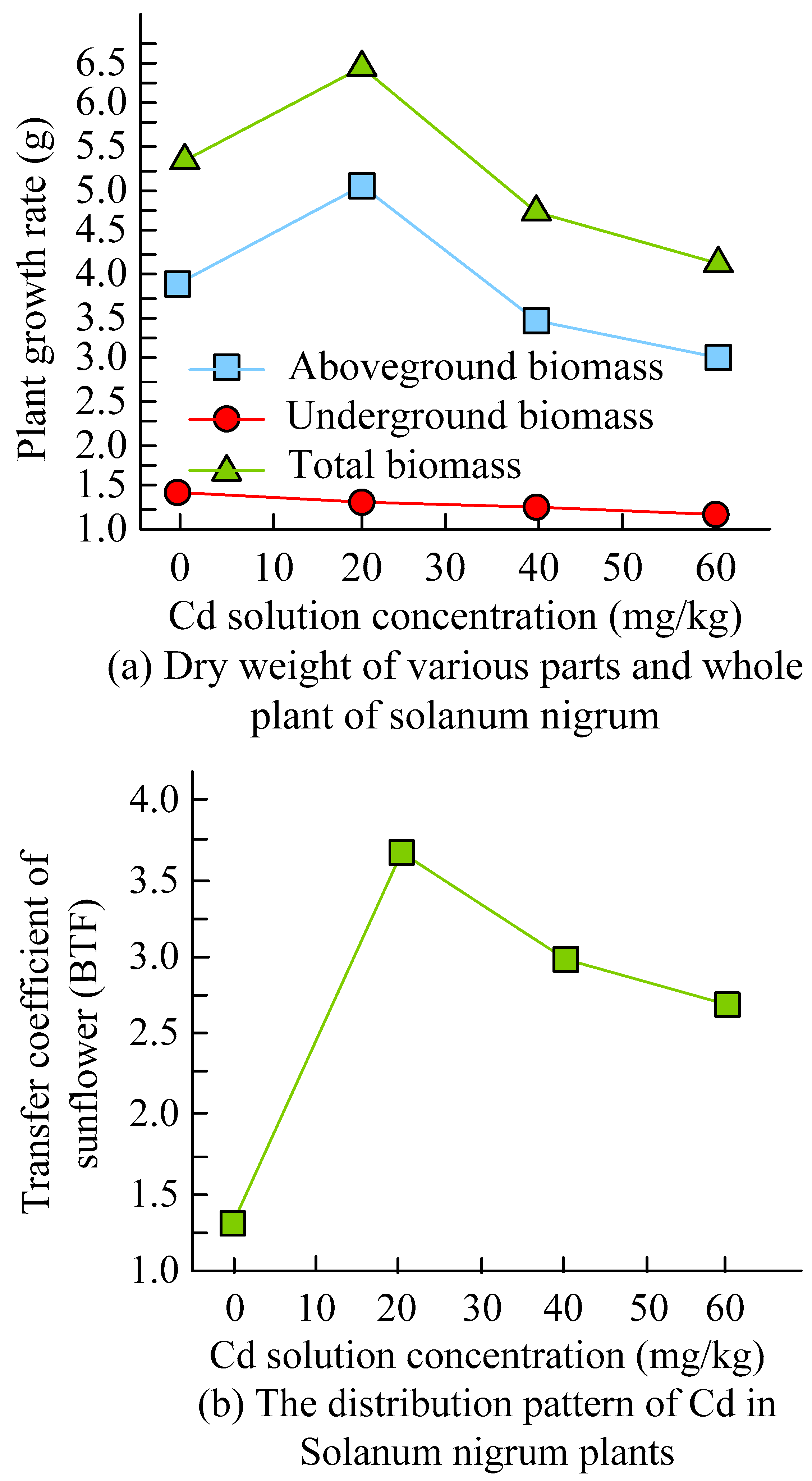
3.3. Analysis of the Effect of Solanum nigrum–Cadmium-Resistant Strain in Heavy Metal Soil Remediation
3.4. Risk Assessment Results of Microbial Plant Collaborative Remediation of Contaminated Areas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kour, D.; Kaur, T.; Devi, R.; Yadav, A.; Singh, M.; Joshi, D.; Saxena, A.K. Beneficial microbiomes for bioremediation of diverse contaminated environments for environmental sustainability: Present status and future challenges. Environ. Sci. Pollut. Res. 2021, 28, 24917–24939. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Lin, D.; Li, J.; Zeng, J.; Wang, D.; Yang, F. Ecological treatment technology for agricultural non-point source pollution in remote rural areas of China. Environ. Sci. Pollut. Res. 2021, 28, 40075–40087. [Google Scholar] [CrossRef] [PubMed]
- Shijie, L.; Chunchun, W.; Yanping, L.; Yazi, L.; Mingjie, C.; Wei, Z.; Xiaoguang, D. S-scheme MIL-101(Fe) octahedrons modified Bi2WO6 microspheres for photocatalytic decontamination of Cr(VI) and tetracycline hydrochloride: Synergistic insights, reaction pathways, and toxicity analysis. Chem. Eng. J. 2023, 455, 140943. [Google Scholar] [CrossRef]
- Smarandache, F. Plithogeny, plithogenic set, logic, probability and statistics: A short review. J. Comput. Cogn. Eng. 2022, 1, 47–50. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of biochar and modified biochar in heavy metal contaminated soil: A descriptive review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Ghosh, D.; Maiti, S.K. Biochar assisted phytoremediation and biomass disposal in heavy metal contaminated mine soils: A review. Int. J. Phytoremediat 2021, 23, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Mazarji, M.; Bayero, M.T.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Tereshchenko, A.; Keswani, C. Realizing united nations sustainable development goals for greener remediation of heavy metals-contaminated soils by biochar: Emerging trends and future directions. Sustainability 2021, 13, 13825. [Google Scholar] [CrossRef]
- Raheem, A.; He, Q.; Mangi, F.H.; Areeprasert, C.; Ding, L.; Yu, G. Roles of heavy metals during pyrolysis and gasification of metal-contaminated waste biomass: A review. Energy Fuels 2022, 36, 2351–2368. [Google Scholar] [CrossRef]
- Shuzhen, Z.; Hao, D.; Lingxuan, Y.; Meng, T.; Ningyi, L.; Yangjie, F.; Derek, H.; Qi, W. PDINH bridged NH2-UiO-66(Zr) Z-scheme heterojunction for promoted photocatalytic Cr(VI) reduction and antibacterial activity. J. Hazard. Mater. 2023, 447, 130–849. [Google Scholar] [CrossRef]
- Yangjie, F.; Youran, X.; Yanjie, M.; Meng, T.; Qin, H.; Huixiu, M.; Hao, D.; Derek, H.; Qi, W. Multi-functional Ag/Ag3PO4/AgPMo with S-scheme heterojunction for boosted photocatalytic performance. Sep. Purif. Technol. 2023, 317, 123922. [Google Scholar] [CrossRef]
- Wang, L.; Rinklebe, J.; Tack, F.M.; Hou, D. A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Li, F.; Ji, W.; Chen, Y.; Gui, X.; Li, J.; Zhao, J.; Zhou, C. Effect of temperature on the properties of liquid product from hydrothermal carbonization of animal manure and function as a heavy metal leaching agent in soil. Water Air Soil Pollut. 2021, 232, 189. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Crini, G. Removal of emerging contaminants from wastewater using advanced treatments. A review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Hao, Y.; Wu, X.; Guo, Y. Study on test and detection method of mechanical properties of heavy metal contaminated soil. Soil Sediment Contam. 2020, 29, 929–939. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, W.; Zhao, W.; Ge, L.; Niu, Y. Wood vinegar enhances humic acid-based remediation material to solidify pb (ii) for metal-contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 12648–12658. [Google Scholar] [CrossRef] [PubMed]
- Kosiorek, M.; Wyszkowski, M. Macroelement content in plants after amendment application to cobalt-contaminated soil. J. Soils Sediments 2021, 21, 1769–1784. [Google Scholar] [CrossRef]
- Wei, F.; Shahid, M.J.; Alnusairi, G.S.H.; Afzal, M.; Khan, A.; El-Esawi, M.A.; Ali, S. Implementation of floating treatment wetlands for textile wastewater management: A review. Sustainability 2020, 12, 5801. [Google Scholar] [CrossRef]
- Moragaspitiya, C.; Rajapakse, J.; Millar, G.J. Effect of struvite and organic acids on immobilization of copper and zinc in contaminated bio-retention filter media. J. Environ. Sci. 2020, 97, 35–44. [Google Scholar] [CrossRef]
- Imam, A.; Kanaujia, P.K.; Ray, A.; Suman, S.K. Removal of petroleum contaminants through bioremediation with integrated concepts of resource recovery: A review. Indian J. Microbiol. 2021, 61, 250–261. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Wang, M.; Han, Y.; Ge, J. Remediation of heavy-metal-contaminated soil by egta washing enhanced with reduction solubilization. Huan Jing Ke Xue Huanjing Kexue 2020, 41, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xiao, C.; Zhou, N.; Chi, R. Speciation, toxicity, microbial remediation and phytoremediation of soil chromium contamination. Environ. Chem. Lett. 2021, 19, 1413–1431. [Google Scholar] [CrossRef]
- Mahfooz, Y.; Yasar, A.; Islam, Q.U.; Rasheed, R.; Naeem, U.; Mukhtar, S. Field testing phytoremediation of organic and inorganic pollutants of sewage drain by bacteria assisted water hyacinth. Int. J. Phytoremediat 2021, 23, 139–150. [Google Scholar] [CrossRef]
- Fang, X.; Peng, B.; Song, Z.; Wu, S.; Tu, X. Geochemistry of heavy metal-contaminated sediments from the four river inlets of dongting lake, China. Environ. Sci. Pollut. Res. 2021, 10, 27593–27613. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Sindhu, M.; Kumar, A.; Dhaka, R.K.; Chahar, M.; Singh, S.; Nain, L. Restoration of heavy metal-contaminated soil and water through biosorbents: A review of current understanding and future challenges. Physiol. Plant. 2021, 173, 394–417. [Google Scholar] [CrossRef] [PubMed]
- Khatawkar, D.S.; James, P.S.; Dhalin, D.R. Energy self-sufficient farmstead: Design analysis. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3006–3025. [Google Scholar] [CrossRef]
- Jlc, A.; Gvpm, A.; Jtv, A.; Ej, B.; Dcre, A.; Jast, A. Kinetic investigation of self-reduction basic oxygen furnace dust briquettes using charcoals from different biomass. J. Mater. Res. Technol. 2020, 9, 13282–13293. [Google Scholar] [CrossRef]
- Katiyar, P.; Pandey, N.; Sahu, K.K. Biological approaches of fluoride remediation: Potential for environmental clean-up. Environ. Sci. Pollut. Res. 2020, 27, 13044–13055. [Google Scholar] [CrossRef]
- Thakare, M.; Sarma, H.; Datar, S.; Roy, A.; Pawar, P.; Gupta, K.; Prasad, R. Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr. Res. Biotechnol. 2021, 3, 84–98. [Google Scholar] [CrossRef]
- Ghanbary, E.; Kouchaksaraei, M.T.; Zarafshar, M.; Bader, K.; Mirabolfathy, M.; Ziaei, M. Differential physiological and biochemical responses of quercus infectoria and q. libani to drought and charcoal disease. Physiol. Plant. 2020, 168, 876–892. [Google Scholar] [CrossRef]
- Chan, N.I.; Rittmann, B.E.; Elser, J. Suitability of an algal biofuel species, scenedesmus acutus, as a fertilizer for growth of conventional and genetically modified lettuce. HortSci. A Publ. Am. Soc. Hortic. Sci. 2021, 56, 589–594. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Tarigan, N.B.; Hidayat, A.; Manurung, R. Valorization of rice straw and tofu residue using hermetia illucens to produce protein rich biomass and organic fertilizer. Res. J. Appl. Sci. Eng. Technol. 2021, 18, 26–32. [Google Scholar] [CrossRef]
- Basta, A.H.; Lotfy, V.F.; Salem, A. Valorization of biomass pulping waste as effective additive for enhancing the performance of liquid crystal hydroxypropyl cellulose nanocomposite film. Waste Biomass Valorization 2021, 13, 2217–2231. [Google Scholar] [CrossRef]
- Ejesieme, V.O.; Riaza, J.; Vorster, N.; Dugmore, G.; Zeelie, B. Reclamation of ultra-fine coal with scenedesmus microalgae and comprehensive combustion property of the coalgae composite. J. Energy S. Afr. 2020, 31, 14–27. [Google Scholar] [CrossRef]
- Bapat, S.; Jaspal, D.; Malviya, A. Efficacy of parthenium hysterophorus waste biomass compared with activated charcoal for the removal of ci reactive red 239 textile dye from wastewater. Color. Technol. 2021, 137, 234–250. [Google Scholar] [CrossRef]
- You, M.; Wang, L.; Zhou, G.; Wang, Y.; Wang, K.; Zou, R.; Cao, W.; Fan, H. Effects of microbial agents on cadmium uptake in Solanum nigrum L. and rhizosphere microbial communities in cadmium-contaminated soil. Front. Microbiol. 2022, 13, 1106254. [Google Scholar] [CrossRef]
- Mohanta, S.; Sahu, M.K.; Mishra, P.C.; Giri, A.K. Removal of cr (vi) from aqueous solution by activated charcoal derived from Sapindus trifoliate L fruit biomass using continuous fixed bed column studies. Water Sci. Technol. 2021, 84, 55–65. [Google Scholar] [CrossRef]
- Cai, J.; Rahman, M.M.; Zhang, S.; Sarker, M.; Zhang, X.; Zhang, Y.; Fini, E.H. Review on aging of bio-oil from biomass pyrolysis and strategy to slowing aging. Energy Fuels 2021, 35, 11665–11692. [Google Scholar] [CrossRef]
- Kumar, L.; Chugh, M.; Kumar, S.; Kumar, K.; Sharma, J.; Bharadvaja, N. Remediation of petrorefinery wastewater contaminants: A review on physicochemical and bioremediation strategies. Process Saf. Environ. Prot. 2022, 159, 362–375. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, C.; Zhang, Y.Z.; Xing, H.W.; Zhao, K. Flue gas circulating sintering based on biomass fuel on reduction of nox and SO2 emission. ISIJ Int. 2020, 60, 1633–1640. [Google Scholar] [CrossRef]









| Beef Paste (g) | Peptone (g) | Sodium Chloride (g) | Agar (g) | pH | Deionized Water (L) |
|---|---|---|---|---|---|
| 3.00 | 10.00 | 10.00 | 15.00 | 7 | 1 |
| Instrument | Model | Manufacturer |
|---|---|---|
| Electronic analytical balance | AL204 | Mettler toledo instruments (Shanghai) Co., Ltd. |
| Box-type resistance furnace | SX2-4-10Z | Shanghai youyi instrument Co., Ltd. |
| Vertical double-layer double-door large-capacity shaking table | SPH-2 102 | Shanghai shiping experimental equipment Co., Ltd. |
| Electric blast constant-temperature drying oven | DGG-9070B | Shanghai senxin experimental instrument Co., Ltd. |
| Biochemical incubator | SHX70III | Shanghai jianzhu instrument Co., Ltd. |
| Atomic fluorescence spectrophotometer | AFS-230E | Beijing haiguang instrument Co., Ltd. |
| pH | Total P(g/kg) | Effective P (g/kg) | Total N (%) | Total Cd(%) | Effective Cd (g/kg) | Effective K(g/kg) | Quick-Acting k(g/kg) |
|---|---|---|---|---|---|---|---|
| 5.90 | 0.115 | 14 | 0.19 | 0.07 | 0.32 | 15 | 27.5 |
| Source | Pollutant Exposure Pathways |
|---|---|
| Surface soil | Direct intake |
| Human skin contact | |
| Inhaling volatile vapors from surface soil | |
| Inhaling volatile vapors from surface soil | |
| Subsoil | Inhaling vapor that migrates from the lower soil layer into the room |
| Concentration (mg/L) | Cd2+ Internal Diffusion Equation | |||||||
|---|---|---|---|---|---|---|---|---|
| Flat Bamboo Flower | Flat Bamboo Flower–Cadmium Resistant Strain | Processed Flat Bamboo Flowers | Processed Flat Bamboo Flower–Cadmium-Resistant Strain | |||||
| - | ||||||||
| 50 | 2.7370 | 0.8553 | 10.9196 | 0.8879 | 8.7105 | 0.9523 | 9.8011 | 0.9060 |
| 100 | 7.0526 | 0.8804 | 7.8883 | 0.6176 | 10.9112 | 0.9528 | 11.5366 | 0.9639 |
| 200 | 5.0730 | 0.9145 | 6.5347 | 0.4279 | 5.2296 | 0.9704 | 5.7189 | 0.9805 |
| 300 | 4.2136 | 0.9251 | 0.5645 | 0.3548 | 4.2518 | 0.9701 | 6.2571 | 0.9701 |
| / | Cd2+ Quasi-Second-Order Kinetic Equation | ||||
|---|---|---|---|---|---|
| Concentration | (mg/L) | (mg/L) | |||
| Flat bamboo flower | 50 | 36.8 | 0.0182 | 34.53 | 0.9917 |
| 100 | 65.66. | 0.0106 | 67.58 | 0.9908 | |
| 200 | 99.84 | 0.0022 | 91.83 | 0.9945 | |
| 300 | 99.98 | 00.0021 | 90.28 | 0.9932 | |
| Flat bamboo flower–cadmium-resistant strain | 50 | 45.5 | 0.054 | 43.2 | 0.9968 |
| 100 | 73.86 | 0.031 | 74.43 | 0.9985 | |
| 200 | 54.08. | 0.0019 | 54.42 | 0.9989 | |
| 300 | 49.79 | 0.0015 | 62.35 | 0.9981 | |
| Alkali-treated flat bamboo flowers | 50 | 37.12 | 0.0048 | 38 | 0.9911 |
| 100 | 84.35. | 0.0032 | 85.62. | 0.9963 | |
| 200 | 89.44. | 0.0018 | 93.68 | 0.9941 | |
| 300 | 89.22 | 0.0011 | 92.81 | 0.9924 | |
| Alkali-treated flat bamboo flowers–cadmium-resistant strain | 50 | 39.01 | 0.0054 | 38.48 | 0.9973 |
| 100 | 89.74 | 0.0018 | 88.12 | 0.9906 | |
| 200 | 100.48 | 0.0017 | 104.64 | 0.9939 | |
| 300 | 100.06 | 0.0014 | 100.05 | 0.9937 | |
| Cd2+ Concentration(mg/kg) | Blank Control | Solanum nigrum | ||
|---|---|---|---|---|
| Cadmium-Resistant Strain | Non-Cadmium-Resistant Strain | Cadmium-Resistant Strain | Non-Cadmium-Resistant Strain | |
| 0 | 18.64 | 20.01 | 10.64 | 10.01 |
| 20 | 48.24 | 46.66 | 22.92 | 20.66 |
| 40 | 104.30 | 98.19 | 80.32 | 79.03 |
| 60 | 135.91 | 136.52 | 108.73 | 100.62 |
| Cd2+ Concentration (mg/kg) | Solanum nigrum +Cd | Solanum nigrum + Cadmium-Resistant STRAIN + Cd | ||||
|---|---|---|---|---|---|---|
| Aboveground Part | Underground Part | Whole Plant | Aboveground Part | Underground Part | Whole Plant | |
| 0 | 48.24 | 36.43 | 84.67 | 56.78 | 40.99 | 97.77 |
| 20 | 153.51 | 68.17 | 221.67 | 185.95 | 76.18 | 262.13 |
| 40 | 164.32 | 89.45 | 253.77 | 196.07 | 105.72 | 301.79 |
| 60 | 165.42 | 116.28 | 281.7 | 203.61 | 141.6 | 345.21 |
| Cd2+ Concentration (mg/kg) | Solanum nigrum | Solanum nigrum + Cadmium-Resistant Strain | ||
|---|---|---|---|---|
| BCF | BTF | BCF | BTF | |
| 0 | 1.07 | 1.32 | 1.21 | 1.39 |
| 20 | 1.82 | 2.25 | 1.97 | 2.45 |
| 40 | 1.46 | 2.21 | 1.57 | 2.32 |
| 60 | 1.03 | 1.42 | 1.14 | 2.24 |
| Contaminants | Super Screening Value Point | Risk Value | |||
|---|---|---|---|---|---|
| Cancer Risk Index | Exceeding Acceptable Level Multiple | Non-Carcinogenic Hazard Quotient | Exceeding Acceptable Level Multiple | ||
| Cd | 1 | 9.17E−05 | 91.7 | 8.93E+00 | 8.93 |
| 2 | 1.41E−04 | 141 | 1.38E+01 | 13.8 | |
| 3 | 2.39E−04 | 238 | 2.33E+01 | 23.3 | |
| 4 | 6.61E−05 | 66.1 | 6.43E+00 | 6.43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Song, Y. Microbial–Plant Collaborative Remediation of Cd-Contaminated Wastewater and Soil in the Surrounding Area of Nuclear Power Plants and Risk Assessment. Sustainability 2023, 15, 11757. https://doi.org/10.3390/su151511757
Wei W, Song Y. Microbial–Plant Collaborative Remediation of Cd-Contaminated Wastewater and Soil in the Surrounding Area of Nuclear Power Plants and Risk Assessment. Sustainability. 2023; 15(15):11757. https://doi.org/10.3390/su151511757
Chicago/Turabian StyleWei, Wei, and Yan Song. 2023. "Microbial–Plant Collaborative Remediation of Cd-Contaminated Wastewater and Soil in the Surrounding Area of Nuclear Power Plants and Risk Assessment" Sustainability 15, no. 15: 11757. https://doi.org/10.3390/su151511757
APA StyleWei, W., & Song, Y. (2023). Microbial–Plant Collaborative Remediation of Cd-Contaminated Wastewater and Soil in the Surrounding Area of Nuclear Power Plants and Risk Assessment. Sustainability, 15(15), 11757. https://doi.org/10.3390/su151511757







