Linking Nematode Communities and Soil Health under Climate Change
Abstract
:1. Introduction
2. Nematode Community Dynamics under Climate Change
2.1. Temperature
2.2. Water Stress
2.3. Land Use
2.4. Nutrient Enrichment
2.5. Combined Stressors
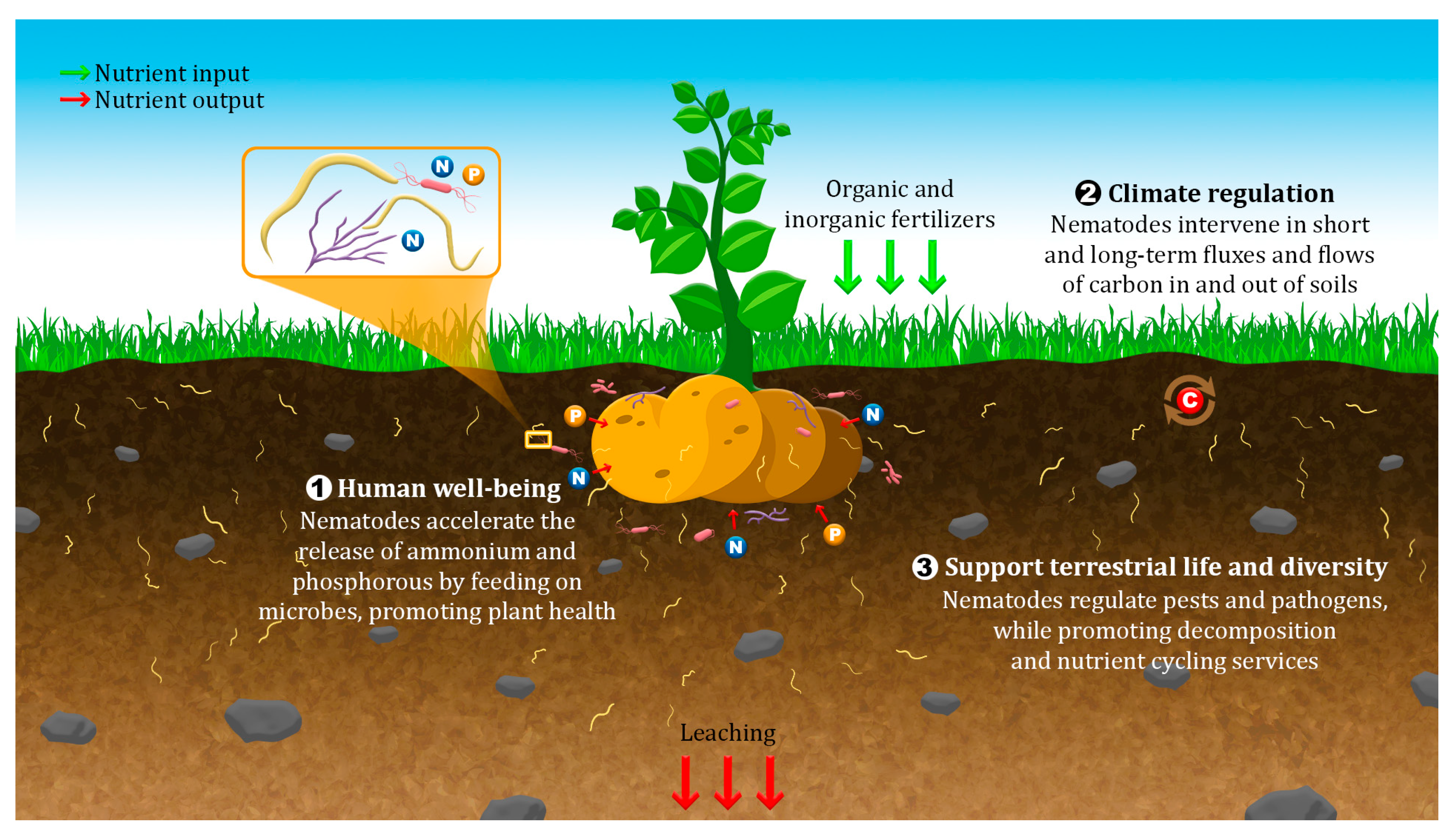
3. Nematode Contributions to Soil Health
4. How Nematodes Promote Soil Resilience
5. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brevik, E.C.; Cerdà, A.; Mataix-Solera, J.; Pereg, L.; Quinton, J.N.; Six, J.; Van Oost, K. The Interdisciplinary Nature of SOIL. SOIL 2015, 1, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Doran, J.W.; Zeiss, M.R. Soil Health and Sustainability: Managing the Biotic Component of Soil Quality. Appl. Soil. Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Soil Health and Climate Change: An Overview. In Soil Health and Climate Change; Singh, B.P., Cowie, A.L., Chan, K.Y., Eds.; Springer: Berlin, Heidelberg, 2011; Volume 29, pp. 3–24. ISBN 978-3-642-20256-8. [Google Scholar]
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Alcamo, J. Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: A Framework for Assessment; Island Press: Washington, DC, USA, 2005; pp. 49–70. [Google Scholar]
- Bardgett, R.D.; van der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological Linkages Between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- US Department of Agriculture Soil Health. Available online: https://www.nrcs.usda.gov/conservation-basics/natural-resource-concerns/soils/soil-health (accessed on 17 February 2023).
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The Concept and Future Prospects of Soil Health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Girija Veni, V.; Srinivasarao, C.; Sammi Reddy, K.; Sharma, K.L.; Rai, A. Soil Health and Climate Change. In Climate Change and Soil Interactions; Prasad, M.N.V., Pietrzykowski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 751–767. ISBN 978-0-12-818032-7. [Google Scholar]
- Tripathi, A.; Pandey, V.; Ranjan, M.R. Climate Change and Its Impact on Soil Properties. In Climate Change and the Microbiome; Choudhary, D.K., Mishra, A., Varma, A., Eds.; Springer: Cham, Switzerland, 2021; Volume 63, pp. 139–153. ISBN 978-3-030-76863-8. [Google Scholar]
- Rillig, M.C.; van der Heijden, M.G.A.; Berdugo, M.; Liu, Y.-R.; Riedo, J.; Sanz-Lazaro, C.; Moreno-Jiménez, E.; Romero, F.; Tedersoo, L.; Delgado-Baquerizo, M. Increasing the Number of Stressors Reduces Soil Ecosystem Services Worldwide. Nat. Clim. Chang. 2023, 13, 478–483. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M.; et al. Temperature and Soil Organic Matter Decomposition Rates - Synthesis of Current Knowledge and a Way Forward. Glob. Chang. Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Hopkins, F.M.; Torn, M.S.; Trumbore, S.E. Warming Accelerates Decomposition of Decades-Old Carbon in Forest Soils. Proc. Natl. Acad. Sci. USA 2012, 109, E1753–E1761. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, S. The Phylogenetic Systematics of Freeliving Nematodes; Platt, H.M., Ed.; Ray Society: London, UK, 1994. [Google Scholar]
- Kergunteuil, A.; Campos-Herrera, R.; Sánchez-Moreno, S.; Vittoz, P.; Rasmann, S. The Abundance, Diversity, and Metabolic Footprint of Soil Nematodes Is Highest in High Elevation Alpine Grasslands. Front. Ecol. Evol. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Holterman, M.; Schratzberger, M.; Helder, J. Nematodes as Evolutionary Commuters between Marine, Freshwater and Terrestrial Habitats. Biol. J. Linn. Soc. 2019, 128, 756–767. [Google Scholar] [CrossRef] [Green Version]
- Sapir, A. Why Are Nematodes so Successful Extremophiles? Commun. Integr. Biol. 2021, 14, 24–26. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S.S. Feeding Habits in Soil Nematode Families and Genera-an Outline for Soil Ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar] [PubMed]
- Bongers, T. The Maturity Index: An Ecological Measure of Environmental Disturbance Based on Nematode Species Composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yeates, G.W.; Bongers, T. Nematode Diversity in Agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 113–135. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes as Soil Indicators: Functional and Biodiversity Aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode Community Structure as a Bioindicator in Environmental Monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A Framework for Soil Food Web Diagnostics: Extension of the Nematode Faunal Analysis Concept. Appl. Soil. Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Ferris, H. Contribution of Nematodes to the Structure and Function of the Soil Food Web. J. Nematol. 2010, 42, 63–67. [Google Scholar]
- Ferris, H.; Venette, R.C.; Scow, K.M. Soil Management to Enhance Bacterivore and Fungivore Nematode Populations and Their Nitrogen Mineralisation Function. Appl. Soil. Ecol. 2004, 25, 19–35. [Google Scholar] [CrossRef]
- Paul, E.A. Soil Microbiology, Ecology and Biochemistry, 4th ed.; Paul, E.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780125468077. [Google Scholar]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil Nematode Abundance and Functional Group Composition at a Global Scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, E.M.; Ramirez, K.S.; Fraser, T.D.; Wall, D.H. Soil Biodiversity Integrates Solutions for a Sustainable Future. Sustainability 2020, 12, 2662. [Google Scholar] [CrossRef] [Green Version]
- Neher, D.A. Role of Nematodes in Soil Health and Their Use as Indicators. J. Nematol. 2001, 33, 161–168. [Google Scholar] [PubMed]
- Martin, T.; Wade, J.; Singh, P.; Sprunger, C.D. The Integration of Nematode Communities into the Soil Biological Health Framework by Factor Analysis. Ecol. Indic. 2022, 136, 108676. [Google Scholar] [CrossRef]
- Sánchez-Moreno, S.; Ferris, H. Nematode Ecology and Soil Health. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CAB International: Wallingford, UK, 2018; pp. 62–86. [Google Scholar]
- Heděnec, P.; Jiménez, J.J.; Moradi, J.; Domene, X.; Hackenberger, D.; Barot, S.; Frossard, A.; Oktaba, L.; Filser, J.; Kindlmann, P.; et al. Global Distribution of Soil Fauna Functional Groups and Their Estimated Litter Consumption across Biomes. Sci. Rep. 2022, 12, 17362. [Google Scholar] [CrossRef]
- Wilschut, R.A.; Geisen, S. Nematodes as Drivers of Plant Performance in Natural Systems. Trends Plant. Sci. 2021, 26, 237–247. [Google Scholar] [CrossRef]
- Topalović, O.; Hussain, M.; Heuer, H. Plants and Associated Soil Microbiota Cooperatively Suppress Plant-Parasitic Nematodes. Front. Microbiol. 2020, 11, 313. [Google Scholar] [CrossRef] [Green Version]
- Tu, C.; Koenning, S.R.; Hu, S. Root-Parasitic Nematodes Enhance Soil Microbial Activities and Nitrogen Mineralization. Microb. Ecol. 2003, 46, 134–144. [Google Scholar] [CrossRef]
- Topalović, O.; Geisen, S. Nematodes as Suppressors and Facilitators of Plant Performance. New. Phytol. 2023, 238, 2305–2312. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Li, G.; Bardgett, R.D.; Wu, T.; Garey, J.R. Global-Scale Patterns of Assemblage Structure of Soil Nematodes in Relation to Climate and Ecosystem Properties. Glob. Ecol. Biogeogr. 2014, 23, 968–978. [Google Scholar] [CrossRef]
- Yan, D.; Yan, D.; Song, X.; Yu, Z.; Peng, D.; Ting, X.; Weng, B. Community Structure of Soil Nematodes under Different Drought Conditions. Geoderma 2018, 325, 110–116. [Google Scholar] [CrossRef]
- Stevnbak, K.; Maraldo, K.; Georgieva, S.; Bjørnlund, L.; Beier, C.; Schmidt, I.K.; Christensen, S. Suppression of Soil Decomposers and Promotion of Long-Lived, Root Herbivorous Nematodes by Climate Change. Eur. J. Soil. Biol. 2012, 52, 1–7. [Google Scholar] [CrossRef]
- Ferris, H.; Griffiths, B.S.; Porazinska, D.L.; Powers, T.O.; Wang, K.-H.; Tenuta, M. Reflections on Plant and Soil Nematode Ecology: Past, Present and Future. J. Nematol. 2012, 44, 115–126. [Google Scholar]
- A’Bear, A.D.; Jones, T.H.; Boddy, L. Potential Impacts of Climate Change on Interactions among Saprotrophic Cord-Forming Fungal Mycelia and Grazing Soil Invertebrates. Fungal Ecol. 2014, 10, 34–43. [Google Scholar] [CrossRef]
- Ayres, E.; Wall, D.; Simmons, B.; Field, C.; Milchunas, D.; Morgan, J.; Roy, J. Belowground Nematode Herbivores Are Resistant to Elevated Atmospheric CO2 Concentrations in Grassland Ecosystems. Soil. Biol. Biochem. 2008, 40, 978–985. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Niklaus, P.A.; Hungate, B.A. A Meta-Analysis of Responses of Soil Biota to Global Change. Oecologia 2011, 165, 553–565. [Google Scholar] [CrossRef]
- Cesarz, S.; Reich, P.B.; Scheu, S.; Ruess, L.; Schaefer, M.; Eisenhauer, N. Nematode Functional Guilds, Not Trophic Groups, Reflect Shifts in Soil Food Webs and Processes in Response to Interacting Global Change Factors. Pedobiologia 2015, 58, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Shevliakova, E.; Artaxo, P.; de Noblet-Ducoudré, N.; Houghton, R.; House, J.; Kitajima, K.; Lennard, C.; Popp, A.; Sirin, A.; et al. Land–Climate Interactions. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendía, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; IPCC: Geneva, Switzerland, 2019; pp. 131–247. [Google Scholar]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil Biodiversity and Human Health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Zhang, X.; Liu, X.; Li, L.; Shi, X.; Hu, H.; Pan, G. Changes in Soil Nematode Abundance and Composition under Elevated [CO2] and Canopy Warming in a Rice Paddy Field. Plant. Soil. 2019, 445, 425–437. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Liu, P.; Zhou, H.; Chen, Z.; Suonan, J. Plant-Soil Mediated Effects of Long-Term Warming on Soil Nematodes of Alpine Meadows on the Qinghai–Tibetan Plateau. Biology 2022, 11, 1596. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Raaijmakers, C.E.; van Ruijven, J.; Berendse, F.; van der Putten, W.H. Plant Species Identity and Diversity Effects on Different Trophic Levels of Nematodes in the Soil Food Web. Oikos 2004, 106, 576–586. [Google Scholar] [CrossRef]
- Franco, A.L.C.; Gherardi, L.A.; de Tomasel, C.M.; Andriuzzi, W.S.; Ankrom, K.E.; Shaw, E.A.; Bach, E.M.; Sala, O.E.; Wall, D.H. Drought Suppresses Soil Predators and Promotes Root Herbivores in Mesic, but Not in Xeric Grasslands. Proc. Natl. Acad. Sci. USA 2019, 116, 12883–12888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, A.L.C.; Gherardi, L.A.; de Tomasel, C.M.; Andriuzzi, W.S.; Ankrom, K.E.; Bach, E.M.; Guan, P.; Sala, O.E.; Wall, D.H. Root Herbivory Controls the Effects of Water Availability on the Partitioning between Above- and Below-ground Grass Biomass. Funct. Ecol. 2020, 34, 2403–2410. [Google Scholar] [CrossRef]
- Franco, A.L.C.; Guan, P.; Cui, S.; Tomasel, C.M.; Gherardi, L.A.; Sala, O.E.; Wall, D.H. Precipitation Effects on Nematode Diversity and Carbon Footprint across Grasslands. Glob. Chang. Biol. 2022, 28, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Veen, G.F.; Wagenaar, R.; Manrubia, M.; ten Hooven, F.C.; van der Putten, W.H. Temporal Dynamics of Range Expander and Congeneric Native Plant Responses during and after Extreme Drought Events. Ecol. Monogr. 2022, 92, e1529. [Google Scholar] [CrossRef] [PubMed]
- Homet, P.; Ourcival, J.-M.; Gutiérrez, E.; Domínguez-Begines, J.; Matías, L.; Godoy, O.; Gómez-Aparicio, L. Short- and Long-Term Responses of Nematode Communities to Predicted Rainfall Reduction in Mediterranean Forests. Soil. Biol. Biochem. 2023, 179, 108974. [Google Scholar] [CrossRef]
- Siebert, J.; Ciobanu, M.; Schädler, M.; Eisenhauer, N. Climate Change and Land Use Induce Functional Shifts in Soil Nematode Communities. Oecologia 2020, 192, 281–294. [Google Scholar] [CrossRef]
- Renčo, M.; Gömöryová, E.; Čerevková, A. The Effect of Soil Type and Ecosystems on the Soil Nematode and Microbial Communities. Helminthologia 2020, 57, 129–144. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Geisen, S.; Bellard, C.; Hu, F.; Li, H.; Chen, X.; Liu, M. Agriculture Erases Climate Constraints on Soil Nematode Communities across Large Spatial Scales. Glob. Chang. Biol. 2020, 26, 919–930. [Google Scholar] [CrossRef]
- Krashevska, V.; Kudrin, A.A.; Widyastuti, R.; Scheu, S. Changes in Nematode Communities and Functional Diversity with the Conversion of Rainforest into Rubber and Oil Palm Plantations. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Yogaswara, D.A.; Kasmara, H.; Hermawan, W. Using Nematode Community to Evaluate Banana Soil Food Web in Mekargalih, Cianjur, West Java. Pertanika J. Trop. Agric. Sci. 2021, 44, 465–483. [Google Scholar] [CrossRef]
- Gao, D.; Wang, F.; Li, J.; Yu, S.; Li, Z.; Zhao, J. Soil Nematode Communities as Indicators of Soil Health in Different Land Use Types in Tropical Area. Nematology 2020, 22, 595–610. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, D.; Wu, Y.; Hu, S.; Li, L.; Bai, Y. Grazing Simplifies Soil Micro-food Webs and Decouples Their Relationships with Ecosystem Functions in Grasslands. Glob. Chang. Biol. 2020, 26, 960–970. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.; Du, X.; Li, Y.; Wang, Z.; Jiang, S.; Li, Q. Effect of Grassland Degradation on Soil Quality and Soil Biotic Community in a Semi-Arid Temperate Steppe. Ecol. Process. 2020, 9, 63. [Google Scholar] [CrossRef]
- Vazquez, C.; Goede, R.G.M.; Korthals, G.W.; Rutgers, M.; Schouten, A.J.; Creamer, R. The Effects of Increasing Land Use Intensity on Soil Nematodes: A Turn towards Specialism. Funct. Ecol. 2019, 33, 2003–2016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, J.; Ren, Z.; Hu, Z.; Tian, S.; Fan, W.; Chen, X.; Griffiths, B.S.; Hu, F.; Liu, M. Root Traits Mediate Functional Guilds of Soil Nematodes in an Ex-Arable Field. Soil. Biol. Biochem. 2020, 151, 108038. [Google Scholar] [CrossRef]
- Puissant, J.; Villenave, C.; Chauvin, C.; Plassard, C.; Blanchart, E.; Trap, J. Quantification of the Global Impact of Agricultural Practices on Soil Nematodes: A Meta-Analysis. Soil. Biol. Biochem. 2021, 161, 108383. [Google Scholar] [CrossRef]
- Xiong, D.; Wei, C.; Wang, X.; Lü, X.; Fang, S.; Li, Y.; Wang, X.; Liang, W.; Han, X.; Bezemer, T.M.; et al. Spatial Patterns and Ecological Drivers of Soil Nematode Β-diversity in Natural Grasslands Vary among Vegetation Types and Trophic Position. J. Anim. Ecol. 2021, 90, 1367–1378. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, Y.; Chen, X.; Chen, D.; Wu, Y.; Wu, L.; Liu, S.; Yue, L.; Wang, Y.; Bai, Y. Even Short-term Revegetation Complicates Soil Food Webs and Strengthens Their Links with Ecosystem Functions. J. Appl. Ecol. 2022, 59, 1721–1733. [Google Scholar] [CrossRef]
- Yin, H.; Su, Y.; Liu, S.; Li, X.; Li, X.; Fan, C.; Guan, P.; Xie, Z.; Wang, S.; Scheu, S.; et al. Consistent Response of Nematode Communities to Management of Coniferous Plantations. For. Ecosyst. 2022, 9, 100045. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Liu, X.; Yang, R.; Xiao, D.; He, X.; Wang, K. Grass Harvesting Eliminates the Beneficial Effects of Legume Addition on Soil Nematode Communities in a Tall Grass Pasture. Agric. Ecosyst. Environ. 2023, 349, 108468. [Google Scholar] [CrossRef]
- Trap, J.; Ranoarisoa, M.P.; Raharijaona, S.; Rabeharisoa, L.; Plassard, C.; Mayad, E.H.; Bernard, L.; Becquer, T.; Blanchart, E. Agricultural Practices Modulate the Beneficial Activity of Bacterial-Feeding Nematodes for Plant Growth and Nutrition: Evidence from an Original Intact Soil Core Technique. Sustainability 2021, 13, 7181. [Google Scholar] [CrossRef]
- Chen, D.; Xing, W.; Lan, Z.; Saleem, M.; Wu, Y.; Hu, S.; Bai, Y. Direct and Indirect Effects of Nitrogen Enrichment on Soil Organisms and Carbon and Nitrogen Mineralization in a Semi-arid Grassland. Funct. Ecol. 2019, 33, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Shaw, E.A.; Boot, C.M.; Moore, J.C.; Wall, D.H.; Baron, J.S. Long-Term Nitrogen Addition Shifts the Soil Nematode Community to Bacterivore-Dominated and Reduces Its Ecological Maturity in a Subalpine Forest. Soil. Biol. Biochem. 2019, 130, 177–184. [Google Scholar] [CrossRef]
- Liu, T.; Mao, P.; Shi, L.; Eisenhauer, N.; Liu, S.; Wang, X.; He, X.; Wang, Z.; Zhang, W.; Liu, Z.; et al. Forest Canopy Maintains the Soil Community Composition under Elevated Nitrogen Deposition. Soil. Biol. Biochem. 2020, 143, 107733. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, B.; Lu, S.; Chen, D.; Wu, Y.; Zhu, Y.; Hu, S.; Bai, Y. Soil Acidification Reduces the Effects of Short-term Nutrient Enrichment on Plant and Soil Biota and Their Interactions in Grasslands. Glob. Chang. Biol. 2020, 26, 4626–4637. [Google Scholar] [CrossRef]
- Wan, B.; Hu, Z.; Liu, T.; Yang, Q.; Li, D.; Zhang, C.; Chen, X.; Hu, F.; Kardol, P.; Griffiths, B.S.; et al. Organic Amendments Increase the Flow Uniformity of Energy across Nematode Food Webs. Soil. Biol. Biochem. 2022, 170, 108695. [Google Scholar] [CrossRef]
- Varga, I.; Benković-Lačić, T.; Lončarić, Z.; Popović, B.; Brmež, M. Liming, Phosphorus and Zinc Influence on Soil Nematode Community Structure at Hot Pepper. Hortic. Sci. 2019, 46, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Olatunji, O.A.; Gong, S.; Tariq, A.; Pan, K.; Sun, X.; Chen, W.; Zhang, L.; Dakhil, M.A.; Huang, D.; Tan, X. The Effect of Phosphorus Addition, Soil Moisture, and Plant Type on Soil Nematode Abundance and Community Composition. J. Soils Sediments 2019, 19, 1139–1150. [Google Scholar] [CrossRef]
- Caruso, T.; Hogg, I.D.; Nielsen, U.N.; Bottos, E.M.; Lee, C.K.; Hopkins, D.W.; Cary, S.C.; Barrett, J.E.; Green, T.G.A.; Storey, B.C.; et al. Nematodes in a Polar Desert Reveal the Relative Role of Biotic Interactions in the Coexistence of Soil Animals. Commun. Biol. 2019, 2, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chen, X.; Zhu, H.; Ren, Z.; Jiao, J.; Hu, F.; Liu, M. Effects of Historical Legacies on Soil Nematode Communities Are Mediated by Contemporary Environmental Conditions. Ecol. Evol. 2020, 10, 6732–6740. [Google Scholar] [CrossRef] [PubMed]
- Neilson, R.; Caul, S.; Fraser, F.C.; King, D.; Mitchell, S.M.; Roberts, D.M.; Giles, M.E. Microbial Community Size Is a Potential Predictor of Nematode Functional Group in Limed Grasslands. Appl. Soil. Ecol. 2020, 156, 103702. [Google Scholar] [CrossRef]
- Xiong, D.; Wei, C.; Wubs, E.R.J.; Veen, G.F.; Liang, W.; Wang, X.; Li, Q.; Putten, W.H.; Han, X. Nonlinear Responses of Soil Nematode Community Composition to Increasing Aridity. Glob. Ecol. Biogeogr. 2020, 29, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Siebert, J.; Sünnemann, M.; Auge, H.; Berger, S.; Cesarz, S.; Ciobanu, M.; Guerrero-Ramírez, N.R.; Eisenhauer, N. The Effects of Drought and Nutrient Addition on Soil Organisms Vary across Taxonomic Groups, but Are Constant across Seasons. Sci. Rep. 2019, 9, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, M.P.; Del Real, I.M.; Cesarz, S.; Steinauer, K.; Reich, P.B.; Hobbie, S.; Ciobanu, M.; Rich, R.; Worm, K.; Eisenhauer, N. Soil Microbial, Nematode, and Enzymatic Responses to Elevated CO2, N Fertilization, Warming, and Reduced Precipitation. Soil. Biol. Biochem. 2019, 135, 184–193. [Google Scholar] [CrossRef]
- Zhang, G.; Sui, X.; Li, Y.; Jia, M.; Wang, Z.; Han, G.; Wang, L. The Response of Soil Nematode Fauna to Climate Drying and Warming in Stipa Breviflora Desert Steppe in Inner Mongolia, China. J. Soils Sediments 2020, 20, 2166–2180. [Google Scholar] [CrossRef]
- Nisa, R.U.; Tantray, A.Y.; Kouser, N.; Allie, K.A.; Wani, S.M.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L.; Shah, A.A. Influence of Ecological and Edaphic Factors on Biodiversity of Soil Nematodes. Saudi J. Biol. Sci. 2021, 28, 3049–3059. [Google Scholar] [CrossRef]
- Wang, H.; Liu, G.; Huang, B.; Wang, X.; Xing, Y.; Wang, Q. Long-Term Nitrogen Addition and Precipitation Reduction Decrease Soil Nematode Community Diversity in a Temperate Forest. Appl. Soil. Ecol. 2021, 162, 103895. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Liao, X.; Yi, Q.; Zhang, W.; Lin, H.; Liu, K.; Peng, P.; Wang, K. Long-Term Returning Agricultural Residues Increases Soil Microbe-Nematode Network Complexity and Ecosystem Multifunctionality. Geoderma 2023, 430, 116340. [Google Scholar] [CrossRef]
- Ferris, H. Form and Function: Metabolic Footprints of Nematodes in the Soil Food Web. Eur. J. Soil. Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Zhang, X.; Ferris, H.; Mitchell, J.; Liang, W. Ecosystem Services of the Soil Food Web after Long-Term Application of Agricultural Management Practices. Soil. Biol. Biochem. 2017, 111, 36–43. [Google Scholar] [CrossRef]
- Wood, J.R.; Holdaway, R.J.; Orwin, K.H.; Morse, C.; Bonner, K.I.; Davis, C.; Bolstridge, N.; Dickie, I.A. No Single Driver of Biodiversity: Divergent Responses of Multiple Taxa across Land Use Types. Ecosphere 2017, 8, e01997. [Google Scholar] [CrossRef] [Green Version]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, J.; Geisen, S.; Wall, D.H.; Wardle, D.A.; Traunspurger, W.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Ferris, H.; Bardgett, R.D.; et al. A Global Database of Soil Nematode Abundance and Functional Group Composition. Sci. Data 2020, 7, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karuri, H. Nematode Community Structure and Functional Guilds Differ in Tea Fields and Tropical Forest. Geoderma 2021, 392, 115006. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Liu, Y.; Han, Y.; Wang, Y.; Wang, Q.; Liu, T. Nematodes and Their Bacterial Prey Improve Phosphorus Acquisition by Wheat. New Phytol. 2023, 237, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Dini-Andreote, F.; Luan, L.; Geisen, S.; Xue, J.; Li, H.; Sun, B.; Jiang, Y. Nematode Predation and Competitive Interactions Affect Microbe-Mediated Phosphorus Dynamics. mBio 2022, 13, 3. [Google Scholar] [CrossRef]
- Chen, X.; Xue, W.; Xue, J.; Griffiths, B.S.; Liu, M. Contribution of Bacterivorous Nematodes to Soil Resistance and Resilience under Copper or Heat Stress. Soil. Ecol. Lett. 2020, 2, 220–229. [Google Scholar] [CrossRef]
- da Silva, J.V.C.d.L.; Hirschfeld, M.N.C.; Cares, J.E.; Esteves, A.M. Land Use, Soil Properties and Climate Variables Influence the Nematode Communities in the Caatinga Dry Forest. Appl. Soil. Ecol. 2020, 150, 103474. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Gallardo, A.; García-Velázquez, L.; García, C.; Hart, S.C.; Pérez, C.A.; Santos, F.; et al. Climatic Vulnerabilities and Ecological Preferences of Soil Invertebrates across Biomes. Mol. Ecol. 2020, 29, 752–761. [Google Scholar] [CrossRef]
- Majdi, N.; Traunspurger, W.; Fueser, H.; Gansfort, B.; Laffaille, P.; Maire, A. Effects of a Broad Range of Experimental Temperatures on the Population Growth and Body-Size of Five Species of Free-Living Nematodes. J. Biol. 2019, 80, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Andriuzzi, W.S.; Franco, A.L.C.; Ankrom, K.E.; Cui, S.; de Tomasel, C.M.; Guan, P.; Gherardi, L.A.; Sala, O.E.; Wall, D.H. Body Size Structure of Soil Fauna along Geographic and Temporal Gradients of Precipitation in Grasslands. Soil. Biol. Biochem. 2020, 140, 107638. [Google Scholar] [CrossRef]
- Wilschut, R.A.; Geisen, S.; Martens, H.; Kostenko, O.; Hollander, M.; Hooven, F.C.; Weser, C.; Snoek, L.B.; Bloem, J.; Caković, D.; et al. Latitudinal Variation in Soil Nematode Communities under Climate Warming-related Range-expanding and Native Plants. Glob. Chang. Biol. 2019, 25, 2714–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xiao, S.; Yang, X.; Liu, Z.; Zhou, X.; Du, G.; Zhang, L.; Guo, A.; Chen, S.; Nielsen, U.N. Dominant Plant Species Influence Nematode Richness by Moderating Understory Diversity and Microbial Assemblages. Soil. Biol. Biochem. 2019, 137, 107566. [Google Scholar] [CrossRef]
- Bennett, J.A.; Koch, A.M.; Forsythe, J.; Johnson, N.C.; Tilman, D.; Klironomos, J. Resistance of Soil Biota and Plant Growth to Disturbance Increases with Plant Diversity. Ecol. Lett. 2020, 23, 119–128. [Google Scholar] [CrossRef]
- Wu, L.; Chen, H.; Chen, D.; Wang, S.; Wu, Y.; Wang, B.; Liu, S.; Yue, L.; Yu, J.; Bai, Y. Soil Biota Diversity and Plant Diversity Both Contributed to Ecosystem Stability in Grasslands. Ecol. Lett. 2023, 26, 858–868. [Google Scholar] [CrossRef]
- Belkhir, L.; Elmeligi, A. Assessing ICT Global Emissions Footprint: Trends to 2040 & Recommendations. J. Clean. Prod. 2018, 177, 448–463. [Google Scholar] [CrossRef]
- Dwivedi, Y.K.; Hughes, L.; Kar, A.K.; Baabdullah, A.M.; Grover, P.; Abbas, R.; Andreini, D.; Abumoghli, I.; Barlette, Y.; Bunker, D.; et al. Climate Change and COP26: Are Digital Technologies and Information Management Part of the Problem or the Solution? An Editorial Reflection and Call to Action. Int. J. Inf. Manag. 2022, 63, 102456. [Google Scholar] [CrossRef]
- Heeb, L.; Jenner, E.; Cock, M.J.W. Climate-Smart Pest Management: Building Resilience of Farms and Landscapes to Changing Pest Threats. J. Pest. Sci. 2019, 92, 951–969. [Google Scholar] [CrossRef]
- Dutta, T.K.; Phani, V. The Pervasive Impact of Global Climate Change on Plant-Nematode Interaction Continuum. Front. Plant. Sci. 2023, 14, 1143889. [Google Scholar] [CrossRef] [PubMed]
- Henneron, L.; Bernard, L.; Hedde, M.; Pelosi, C.; Villenave, C.; Chenu, C.; Bertrand, M.; Girardin, C.; Blanchart, E. Fourteen Years of Evidence for Positive Effects of Conservation Agriculture and Organic Farming on Soil Life. Agron. Sustain. Dev. 2015, 35, 169–181. [Google Scholar] [CrossRef] [Green Version]
- de Ruiter, P.C.; Neutel, A.-M.; Moore, J.C. Energetics, Patterns of Interaction Strengths, and Stability in Real Ecosystems. Science 1995, 269, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological Intensification: Harnessing Ecosystem Services for Food Security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Ingham, R.E.; Trofymow, J.A.; Ingham, E.R.; Coleman, D.C. Interactions of Bacteria, Fungi, and Their Nematode Grazers: Effects on Nutrient Cycling and Plant Growth. Ecol. Monogr. 1985, 55, 119–140. [Google Scholar] [CrossRef]
- Forero, L.E.; Kulmatiski, A.; Grenzer, J.; Norton, J.M. Plant-Soil Feedbacks Help Explain Biodiversity-Productivity Relationships. Commun. Biol. 2021, 4, 789. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adelekan, I.; Adler, C.; Adrian, R.; Aldunce, P.; Ali, E.; Begum, R.A.; Friedl, B.B.; et al. Climate Change 2022: Impacts, Adaptation and Vulnerability; Technical Summary; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; ISBN 9781009325844. [Google Scholar]
- Sprunger, C.D.; Lindsey, A.; Lightcap, A. Above- and Belowground Linkages during Extreme Moisture Excess: Leveraging Knowledge from Natural Ecosystems to Better Understand Implications for Row-Crop Agroecosystems. J. Exp. Bot. 2023, 74, 2845–2859. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y.; Wei, G. Soil Multitrophic Network Complexity Enhances the Link between Biodiversity and Multifunctionality in Agricultural Systems. Glob. Chang. Biol. 2022, 28, 140–153. [Google Scholar] [CrossRef]
| Factor | Effects on Soil Nematodes | Reference |
|---|---|---|
| High temperature | Reduction in nematode diversity and genera richness | [48] |
| Reduction in herbivores, with a short-term decrease in bacterivores and fungivores who recover over time, and relative tolerance of omnivores–predators | [49] |
| Factor(s) | Effects on Soil Nematodes | Reference |
|---|---|---|
| Long-term increase in mean annual precipitation | Decline in total nematode abundance due to predation pressure, and increase in nematode abundance | [51] |
| High proportion of plant-parasitic nematodes | [52] | |
| Decrease in free-living nematode diversity and evenness | [53] | |
| Water scarcity | Reduction in total nematode abundance, and decrease in relative abundance of bacterivores, fungivores, and predators; relative abundance of herbivores unaffected | [54] |
| Lower bacterivore and fungivore abundance, marginal increase in omnivores and decrease in fungivores, higher maturity and structure index, and lower prey:predator ratio | [55] |
| Factor(s) | Effects on Soil Nematodes | Reference |
|---|---|---|
| Intensive land use | Increase in bacterivores, fungivores, and herbivores | [56] |
| Soil type | Lower biodiversity and C/N ratios in cultivated soils, resulting in a reduction in nematode abundance and diversity; increase in abundance of trophic groups, total abundance, and diversity of nematodes in soils with higher pH and C and N contents | [57] |
| Land use conversion | Negative impacts on soil taxa across a large spatial scale, but agronomic practices limit the climatic constraints on belowground biodiversity | [58] |
| Monoculture | Increase in fungivores, and decrease in bacterivores, herbivores, and omnivores–predators | [59] |
| Monoculture, tillage, and manure | Increase in bacterivores and fungivores, and decrease in omnivores–predators | [60] |
| Vegetation succession | Positive effect on nematode abundance, diversity, complexity of community structure, and diversity-weighted abundance | [61] |
| Livestock grazing | Decline in bacterivores, herbivores, and omnivores–predators abundance, lower total nematode abundance, and no detrimental effect on fungivore abundance | [62] |
| Land degradation | Lower nematode trophic diversity | [63] |
| Intensive land use | Generalists equally abundant in all ecosystems, with specialists dominating agricultural landscapes and less abundant in low disturbed soils; highest richness and diversity in grasslands and dairy farms, with low abundances in shrubland–woodland habitats | [64] |
| Plant resource-use strategies | Plants with acquisitive strategies promoted nematode abundance, but fewer opportunistic nematodes in the rhizosphere of acquisitive plants compared to conservative plants | [65] |
| Agricultural practices | (i) Conventional practices decrease abundance, trophic structure, and taxonomic richness of nematode communities; (ii) agroecological practices enhance the functional and taxonomic diversity of nematodes: total nematode abundance and absolute abundance of fungivores, herbivores, and omnivores–predators; reduction in herbivore abundance in crop rotation; increase in omnivores–predators in cover crops; increase in bacterivores and fungivores in organic fertilization; reduction in nematode abundance and food web structure in monoculture and pesticide application, while copiotrophic nematodes are favored | [66] |
| Spatial distance and environmental filtering | Plant type altered β-diversity of nematodes; spatial turnover was the primary process driving total β-diversity of the nematode community | [67] |
| Vegetation restoration with varying degrees of degradation | Strong effects on soil nematodes observed under low degradation | [68] |
| Crop-tree thinning | Increase in abundance of soil nematodes, along with the relative abundance of herbivores in all systems; increase in proportion of stress-tolerant enrichment and general opportunists | [69] |
| Harvest frequency and legume density | Legume addition and density were drivers of total nematode abundance, especially bacterivores, while improving metabolic activities of total nematodes, bacterivores, and omnivores–predators; positive effects of legume addition subsided after increased harvesting frequency | [70] |
| Agricultural practices | Plant diversity enhanced the activity of beneficial nematodes; positive effects of nematodes on plant growth and function associated with higher values in soil pH and cation contents | [71] |
| Factor(s) | Effects on Soil Nematodes | Reference |
|---|---|---|
| Long-term N enrichment | Plant removal dwindled nematode taxon richness and abundance of bacterivores and herbivores; the abundance of fungivores and omnivores–predators increased under the same conditions | [72] |
| Long-term N fertilization | Greater nematode abundance in fertilized plots, while richness, diversity, and ecological maturity were lower; enriched food web mostly driven by bacterivores and herbivores, with persisting effects overtime | [73] |
| High N deposition | Decrease in most nematode trophic groups and community diversity under understory addition of N compared to canopy addition of N | [74] |
| Short-term N and P enrichment under soil acidification | Nematode variables, including community structure, were largely unaffected by short-term nutrient enrichment under soil acidification | [75] |
| Long-term organic amendments and mineral fertilization | Positive effect on the abundance of most functional guilds by organic amendments, which enhanced the energy transfer among nematode communities, while increasing the relative allocation of energy flux to bacterivores and fungivores and decreasing the relative allocation to herbivores | [76] |
| Liming, P, and zinc inputs | P input significantly increased nematode diversity and genera; bacterivores and herbivores were the most abundant trophic groups, and predators the least common; nematode biodiversity was unaffected by liming, and nematode diversity and maturity were reduced in the absence of liming | [77] |
| Factors | Effects on Soil Nematodes | Reference |
|---|---|---|
| Soil moisture, P addition, and aboveground vegetation | Plant type and water availability had a greater impact on nematode abundance and community composition; drought was detrimental to the total density of nematodes and functional guilds; bacterivores, herbivores, and omnivores were significantly more abundant in soils with legumes | [78] |
| Biotic (microbial biomass and competition) and abiotic variables (moisture, salinity, and elevation) | Spatial segregation between two competing bacterivore species, with contrasting responses to abiotic factors: one best adapted to high salinity, lower temperatures, and low moisture environments, while the other thrives at higher temperatures, higher soil moisture, and lower salinity | [79] |
| Climatic, soil, and historical factors | Current factors, particularly climate, are more influential than historical factors in shaping nematode diversity patterns on a broader scale | [80] |
| Liming treatments | Interacting nematode and microbial communities minimally impacted by liming, with an increase in omnivores and predators, who keep bacterivores under control; stronger interaction in the presence of an abundant microbial community | [81] |
| Increasing aridity across a large spatial scale | Decline in total and relative nematode abundance of each functional guild under increasing aridity; taxonomic richness of total nematode community and functional guilds decreased under moisture scarcity; at the dry end of the aridity gradient, richness of bacterivores was higher, while herbivores declined steadily; richness of fungivores and omnivores–predators remained relatively stable up to a certain point, before dropping steeply | [82] |
| Drought and fertilization | Drought favored bacterivores and fungivores, and likely had detrimental effects on higher trophic levels; fertilization caused a prominent increase in bacterivores and an equally significant drop in fungivores | [83] |
| Elevated CO2 and N, warming, and drought | Increase in nematode density at elevated N and ambient CO2, and ambient N and elevated CO2 | [84] |
| Warming and precipitation | Decrease in nematode abundance, especially of bacterivores and herbivores (with minor effects on fungivores), under artificial warming, but the nematode community diversity and functions remained stable; decrease in nematode abundance, especially of bacterivores and omnivores–predators, under reduced precipitation, with fungivores and herbivores relatively insensitive to water stress; increase in nematode abundance and community diversity with water availability | [85] |
| Ecological and edaphic factors | Reduced nematode abundance and diversity with increasing altitude, with bacterivores consistently the dominant group; nematode diversity was mostly influenced by temperature and moisture; decrease in nematode abundance with increasing soil acidity; nematode diversity and richness were directly proportional to nutrient (N and P) levels | [86] |
| N deposition under reduced water availability | Reduced nematode abundance and diversity under N addition and reduced water input; synergistic effects of N addition and reduced water input on soil nematode communities at higher trophic levels; sole addition of N was more detrimental to the nematode community | [87] |
| Returning agricultural residues | Nematode diversity was lower in treatments with conventional chemical NPK fertilizers; positive correlation between omnivore–predator abundance and ecosystem multifunctionality and soil fertility | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, D.; Orlando, V.; Collett, R.L.; Moreira, D.; Costa, S.R.; Inácio, M.L. Linking Nematode Communities and Soil Health under Climate Change. Sustainability 2023, 15, 11747. https://doi.org/10.3390/su151511747
Pires D, Orlando V, Collett RL, Moreira D, Costa SR, Inácio ML. Linking Nematode Communities and Soil Health under Climate Change. Sustainability. 2023; 15(15):11747. https://doi.org/10.3390/su151511747
Chicago/Turabian StylePires, David, Valeria Orlando, Raymond L. Collett, David Moreira, Sofia R. Costa, and Maria L. Inácio. 2023. "Linking Nematode Communities and Soil Health under Climate Change" Sustainability 15, no. 15: 11747. https://doi.org/10.3390/su151511747
APA StylePires, D., Orlando, V., Collett, R. L., Moreira, D., Costa, S. R., & Inácio, M. L. (2023). Linking Nematode Communities and Soil Health under Climate Change. Sustainability, 15(15), 11747. https://doi.org/10.3390/su151511747








