Abstract
The cultivation of microalgae using urban wastewater as a nutrient substrate represents a promising bio-refinery concept that can serve multiple purposes; indeed, it allows for the generation of biomass, which can be used for various applications while meanwhile removing nutrients from wastewater. In this study, the potential of urban wastewater collected at two different time periods in a farmhouse as a nutrient substrate for microalgal growth was assessed. Wastewater samples were treated on a laboratory scale, inoculating reactors with two common species, Chlorella vulgaris (CV) and Scenedesmus quadricauda (SQ), and with an autochthonous strain of Klebsormidium sp. K39 (Kleb), directly isolated from effluents of the same system. The main aim of the study was to compare the microalgae’s performances in terms of wastewater re-mediation and biomass productivity. In the first case study, which involved an effluent with a lower pollutant level, microalgal cultivation showed removal efficiencies in the range of 57–63% for total nitrogen, 65–92% for total phosphorous, 94–95% for COD, and 100% for E. coli. In the second case study, involving an effluent with a higher pollutant level, the remediation performances of the three microalgae strains ranged from 93 to 96% for total nitrogen, from 62 to 74% for total phosphorous, from 96 to 97% for COD, and 100% for E. coli. At the end of the experimental trials, treated waters showed values of pollutants suitable for irrigation use, in accordance with environmental and national legislation, which established specific thresholds for irrigation purposes.
1. Introduction
In the last few years, the rapidly expanding population, coupled with global climate changes, has represented a considerable pressure on Earth’s resources. Indeed, climate change negatively impacts agricultural productivity and affects the water cycle, leading to altered precipitation patterns and increasing water scarcity in some regions, as well as the increase in population putting a strain on freshwater resources [].
A further important issue is related to the release of municipal wastewaters and, in turn, the environmental challenges they pose to receiving water bodies [,]. The high concentration of pollutants, such as excess nitrogen and phosphorus, may cause an important alteration in the health of the water system [,]. Furthermore, conventional treatment methods, such as activated sludge systems or chemical coagulation, are still very expensive and often unable to completely eliminate microcompounds or inorganic nutrients [,].
The use of reclaimed water (RW), a suitable strategy in agriculture for irrigation purposes, may represent a risk for plants, soils, and humans [,] for the accumulation and propagation of biological (animal and human pathogens, phytopathogens), xenobiotic contaminants (drugs and metals), and antibiotic-resistant genes [,,,]. The World Health Organisation guidelines established safety criteria for irrigation purposes, for which RW must comply with standard criteria. In the EU, the use of RW is under Regulation (EU) 2020/741 on minimum requirements for water reuse, which establishes a threshold of 10 CFU 100 mL−1 (<1 Log 100 mL−1) of Escherichia coli for RW classifying as class “A”, useful for irrigation of food crops [].
In this context, the exploitation of microalgae is emerging as an interesting alternative green source with a low carbon dioxide (CO2) footprint [,]. Microalgae are also attracting the interest of worldwide researchers, mainly due to their multipurpose applications as raw materials for the development of new agricultural products [,,]. Moreover, microalgae are taken into account as important sustainable sources of valuable chemicals, pharmaceuticals, and other products [,,].
The microalgae-based wastewater treatment process is a sustainable, eco-friendly process with no secondary pollution [], able to recover wastewater from various organic and inorganic contaminants, ranging from aromatic hydrocarbons, food residues, solvents, plasticisers, antioxidants, washing and cleaning-related compounds, to high nutrient loads such as nitrogen and phosphorous []. Furthermore, previous studies have shown that microalgae-based wastewater treatment has a rate of coliform removal of up to 99% [,]. Microalgae may be adapted to a wide range of types of wastewater, providing a tertiary biotreatment coupled with the production of valuable biomass, a potential feedstock for the development of added-value products for the agricultural sector [].
Among microalgae species suitable for wastewater treatment, the genera Chlorella and Scenedesmus are the most largely used []. However, a limitation in applying such a strategy is related to the difficulties of maintaining monoalgal cultures with constant biomass composition []. The remediation abilities of these two genera are largely reported [,]. For instance, Wang et al. [] demonstrated that Chlorella sp., employed for urban wastewater treatment, was able to remove high contents of nitrogen, ranging from 62.5 to 82.4%; phosphorus, from 83.2 to 90.6%; and heavy metals. In the same way, Wong et al. [] investigated the lipid production and nutrient removal capabilities of S. quadricauda using different types of wastewater from a sewage treatment plant. The results showed interesting performances for both evaluated properties, indicating that the microalga is a viable candidate for wastewater treatment and lipid production. It is relevant to point out that the major pollutants in urban wastewater are nutrients and heavy metals; therefore, a relevant trait for the selection of microalgae strains to be used for this purpose is to detect these abilities [].
Moreover, microalgae cultivation can provide an opportunity to produce valuable biomass, which can be utilized to obtain bioproducts for multipurpose applications. It is worth noting that research in this field is ongoing, and further studies are needed to optimize the processes, explore different microalgae species, and assess the scalability and economic feasibility of using microalgae for wastewater treatment and resource recovery.
To achieve a ‘win-win’ solution by linking wastewater remediation and microalgae biomass accumulation, different types of wastewater could be used as a culture medium for the cultivation of different microalgae species. Based on the above perspectives, this study is aimed at evaluating the phycoremediation performance and biomass accumulation of an indigenous strain of filamentous microalga, previously identified as Klebsormidium sp. K39, in urban wastewater treatment, compared to Chlorella vulgaris (CV) and S. quadricauda (SQ). These performances were evaluated for two different magnitudes of pollutants in wastewater from a farmhouse.
2. Materials and Methods
2.1. Raw Wastewaters
Wastewater samples were collected from a constructed wetland active on a farm holiday in Sicily (Italy) in two different periods, as the different host affluence levels (due to the COVID emergency) caused significant differences in their composition. The collected raw wastewaters were preliminary analysed (see detailed methods below in Section 2.2) and used as growth substrates for microalgae.
In Figure 1, a scheme of the phytodepuration system acting in the farm holiday is reported. The wastewater samples used for the experimental trials were collected directly from the Imhoff tank.
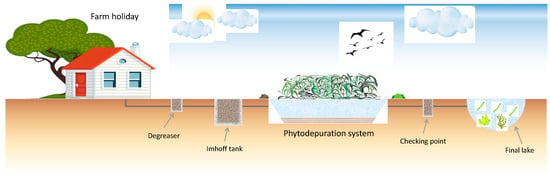
Figure 1.
Phytodepuration system scheme at the farmhouse.
The characteristics of the raw wastewaters used in this study are reported in Table 1 (analyses are described in Section 2.2).

Table 1.
Composition of raw wastewaters: Total Kjeldahl Nitrogen (TKN), Total Phosphorous (TP), Chemical Oxygen Demand (COD), Biochemical Oxygen Demand (BOD5), and Escherichia coli.
2.2. Chemical Analyses and Nutrient Removal Rate Determination
The wastewater samples were first centrifuged at 4000× g for 5 min, and the supernatants were collected []. Measurements of EC and pH values were performed using an XS Cond 7 and an XS pH 80+ DHS, respectively. In order to evaluate the preliminary composition of wastewaters and the nutrient removal ability of microalgae, chemical characterization by monitoring several parameters, including TKN, TP, heavy metals, COD, and BOD5, was performed following the standard methods recommended by the American Public Health Association [].
TKN was performed by the Kjeldahl method in 50 mL of sample. In a test tube, 2 catalyst tablets were added, each containing 3.5 g of K2SO4 and 3.5 mg of Se, and 10 mL of concentrated sulphuric acid. The tubes were placed in the digestor and treated for 60 min at 200 °C and 120 min at 370 °C. After digestion, samples were treated with an acid solution and boiled in concentrated sulfuric acid. The samples were then distilled according to a pre-defined method of the instrument (Method n° 26, VELP UDK 130 A). The distillation of the samples was performed by adding an excess of 35% NaOH to the acid digestion mixture to convert NH4+ to NH3, followed by boiling and condensation of the ammonia (NH3) gas in a receiving solution (4% H3BO3). Finally, to quantify the amount of ammonia in the receiving solution, the water samples were titrated. For the titration, to each sample were added 10 drops of Tashiro’s indicator (0.75 g L−1 methyl red sodium salt + 0.375 g L−1 methylene blue in ethanol 50% (v/v), denatured) and 0.2 N HCl until the endpoint of the titration.
Analysis to determine TP contents was based on the persulfate oxidation under acidic conditions of the samples [], converting the various forms of phosphate and phosphorus to the orthophosphate form. The phosphorus contents were determined by putting 50 mL of sample, or a diluted amount of 50 mL, into an Erlenmeyer flask, adding 1 drop of phenolphthalein indicator, and 5 M sulphuric acid or 2 M sodium hydroxide until the samples developed a red colour. The next steps were the addition of 1 mL of 10 M sulphuric acid and 0.4 g of potassium persulphate, followed by the transfer of the samples into an incubator at 95–100 °C for 2 h. After cooling, the samples were added to 1 drop of phenolphthalein and neutralized to a faint pink colour with 2 M sodium hydroxide, made up to 100 mL with distilled water. Then, at each sample, 10 mL of a mixed reagent was added, composed of 100 mL of 30 g L−1 ammonium molybdate solution, 250 mL of diluted sulphuric acid (1:6.4, H2SO4:H2O), 100 mL of 54 g L−1 ascorbic acid solution, and 50 mL of 1.36 g L−1 potassium antimony tartrate solution. We allowed at least 10 min for colour development and measured the absorbance at 880 nm using a reagent blank to zero the spectrophotometer. The reagent blank was made using 50 mL of distilled water carried through the digestion and subsequent steps. Finally, the samples’ absorbances were checked against the calibration curve phosphate standard, and the concentrations were determined.
The determination of heavy metals was performed by Standard Methods for Examination of Water and Wastewater []. The metal analyses (Zn, Cu, Cd, Pb, Ni, and Hg) were carried out by means of atomic absorption spectrophotometry (Perkin Elmer 3110, Waltham, MA, USA). Each wastewater sample was filtered through a 0.45-micron nylon filter and acidified to a pH of 4–5 with HCl. Afterwards, 35 mL of Methyl isobutyl ketone (MIBK) and 7 mL of 1% (w/v) ammonium pyrrolidine dithiocarbamate (APDC) were added to 750 mL of the filtered solution, and each sample was equilibrated for 30 min on a mechanical shaker, and the organic layer was separated in a separatory funnel. The concentration of the heavy metals (Zn, Cu, Cd, Pb, Ni, and Hg) was determined by reading the concentrations of the elements of interest directly versus appropriate standards and a reagent blank. Wastewater was analysed for heavy metals only at the beginning of removal experiments because, in both cases (MW1 and MW2), the contents were below the detectable limits.
COD analysis was performed using specific test kits (Nanocolor CSB 40 and Nanocolor CSB 1500), and BOD5 was monitored using the Velp Respirometric Sensor BOD5 (Monza-Brianza, Italy). For BOD5 analysis, all samples were saturated with oxygen using an air pump, and after 5 days of incubation in the dark, the final dissolved oxygen level was taken directly from the sensor, and the difference between the final and initial levels was recorded.
Each analysis was replicated in triplicate.
To evaluate the nutrient removal ability by microalgae, Total Kjeldahl Nitrogen (TKN), Total Phosphorus (TP), Chemical Oxygen Demand (COD), Biological Oxygen Demand (BOD5), pH, and Electrical Conductivity (EC) were determined according to the standard methods recommended by the American Public Health Association []. For these parameters, removal quantity (RQ, mg·L−1) and removal efficiency (RE, %) were calculated using the following equations []:
where and are defined as the mean values of nutrient concentrations at initial time t0 and final time ti, respectively.
2.3. Microalgae Strains and Cultivation Conditions
The microalgae tested in the present study were Chlorella vulgaris ACUF863 and Scenedesmus quadricauda ACUF581, which were kindly provided by the Algal Collection Federico II of Naples (Italy). In addition, a strain of Klebsormidium sp. K39, belonging to the Di3A microbial culture collection and previously isolated from the same phytoremediation pond [], was used. All strains were cultured in sterilized standard Bold Basal Medium (BBM) or BBM agar medium.
Microalgae cultivation was carried out in axenic conditions in 2 L Erlenmeyer flasks maintained at 25 ± 1 °C in a climate chamber under a light intensity of 100 µmol photons·m−2 s−1 with a light source (PHILIPS SON-T AGRO 400, Eindhoven, The Netherlands), and a photoperiod of 16 h on/off, according to the best microalgae growth conditions. The cultures were bubbled with air with immersion water pumps [].
The microalgae species used in the described experiments were inoculated at their logarithmic growth phase.
2.4. Evaluation of Bacterial Removal Efficiency
In order to evaluate the Escherichia coli removal efficiency of the tested microalgal treatments, microbiological analyses were performed following the membrane filtration method []. In detail, 100 mL of sample were treated on membrane filters (0.45 μm pores, Cellulose, Merck, Darmstadt, Germany), and the filters were then poured into RAPID’ E. coli 2 Agar plates (Bio-Rad, Milan, Italy). Plates were incubated at 37 °C for 24 h. The analyses were performed in triplicate, and results were expressed as mean log10 colony-forming units (CFU) per unit of volume.
2.5. Experimental Set-Up
The experimental set-up consisted of eight lab-scale open photobioreactors (Table 2), each with a 4 L capacity, illuminated for a 12 h photoperiod by an LED lamp (100 µmol photons·m−2 s−1), in order to simulate the nearest natural environmental conditions. Each reactor was filled with 3 L of wastewater [Wastewater 1 (MW 1) and Wastewater 2 (MW 2)] collected from the Imhoff tank of the phytoremediation system at the farmhouse, as above described (Figure 1).

Table 2.
Design criteria and conditions adopted in each photobioreactor used in the experimental trials.
Each microalga, grown in BBM, was collected by centrifugation at 4000 rpm for 10 min when it reached the logarithmic growth phase. Pellets were washed with deionized water and centrifuged a second time at the same conditions, then were suspended in a small quantity of wastewater, and, finally, inoculated in the reactors [].
The photobioreactors were inoculated with C. vulgaris, S. quadricauda, and the autochthonous Klebsormidium sp. K39 strains at an initial cell concentration, as determined by cell count in the Burker counting chamber (Blaubrand), of 100 mg·L−1, equal to 1.6, 2.2, and 1.8 × 109 cells·L−1, respectively. For each microalga, the cell dry weight and the size of the inoculums were found to be 0.42, 0.44, and 0.45 g (fresh weight), respectively. The wastewater samples that were not inoculated were routinely used as controls. The microalgae were thus fed in the reactors exclusively with the wastewaters as they are, without nutrient addition or dilution, considering that the effluents can supply all inorganic nutrients required for microalgae growth [].
Samples of 50 mL were then collected after 2, 5, 10, 30, 45, and 60 days from each photobioreactor in order to evaluate the remediation ability of the tested microalgae, determining the concentrations of Total Kjeldahl Nitrogen (TKN), Total Phosphorus (TP), Chemical Oxygen Demand (COD), Biological Oxygen Demad (BOD5), pH, and Electrical Conductivity (EC) (as previously described). In order to monitor microbiological parameters, samples were collected at 0, 2, 5, 7, 9, 15, 30, 45, and 60 days after inoculum and immediately processed for E. coli detection and microalgae counting (as previously described). All experiments were carried out in triplicate.
2.6. Determination of Microalgal Growth
The microalgal growth was determined as cell number by Burker’s counting chamber (Blaubrand), as fresh weight, measuring the weight (mg) of fresh biomass per litre and as dry weight, measuring the weight (mg) of dry biomass per litre, obtained oven-dried at 60 °C until a constant weight was reached.
The daily productivity (g L−1·d) was calculated according to the following formula []:
where and are the final and initial concentrations of cell dry weight and and are the final and initial time.
Moreover, at the end of the experimental test, the samples containing the microalgae were centrifuged at 2500 rpm for 10 min, and the pellet was oven-dried at 60 °C until constant weight and weighed to measure the total biomass [].
2.7. Statistical Analysis
The collected data were subjected to a two-way analysis of variance (ANOVA) based on a factorial combination (specie × time). Since the laboratory assays were performed in triplicate, F and p values were calculated to evaluate whether the effects of single factors such as as specie, time, and the interaction specie × time were significant. In post-hoc analyses, the means were compared using Fischer’s protected least significant difference (LSD) test (p ≤ 0.05). The calculations were carried out on Excel version 2019 (Microsoft Corporation, Redmond, WA, USA) and Minitab (version 16.1.1, Minitab Inc., State College, PA, USA).
3. Results
3.1. Dynamics of Microalgae Population
The microalgae strains were cultivated in wastewater for 60 days, and the growth performances, in terms of cell density, are reported in Figure 2. The lag phase, or time necessary for their adaptation to wastewater conditions, was found to be quite short in both case studies (48 h), and in this period the main parameters monitored were not significantly reduced.
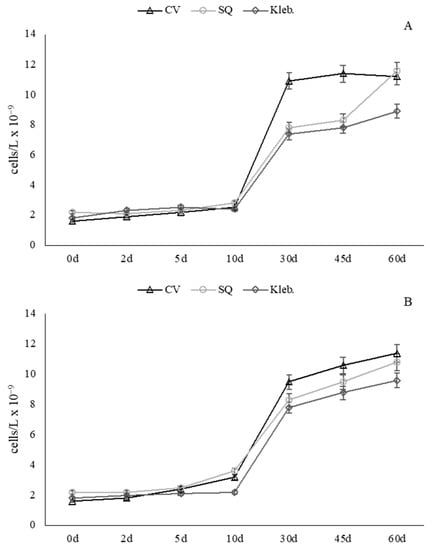
Figure 2.
Microalgal growth performance in (A) Wastewater 1 (MW 1) and (B) Wastewater 2 (MW 2). CV: Chlorella vulgaris, SQ: Scenedesmus quadricauda, Kleb: Klebsormidium sp. K39.
However, the effect of a single factor (species) was found to be not significant for any of the parameters monitored in both trials (Table 3 and Table 4).

Table 3.
Effects of single factors in ANOVA relative to the daily productivity, the fresh weight of biomass collected, and the dry weight of biomass collected in MW1.

Table 4.
Effects of single factors in ANOVA relative to the daily productivity, the fresh weight of biomass collected, and the dry weight of biomass collected in MW2.
In the first case study (MW 1), microalgae quickly adapted to the conditions, as shown by the growth curves (Figure 2A). In details, the C. vulgaris strain reached the stationary phase earlier (30 days) compared to the other species, whereas at the end of the trial (60 days), a similar number of cells to those obtained using S. quadricauda were counted. As regards Klebsormidium sp. K39, a cell number always lower than other species was recorded, although daily productivity and microalgae biomasses collected were similar to those of C. vulgaris and S. quadricauda (Table 3 and Table 5). Furthermore, in Table 5, in which the daily productivity and the microalgae biomasses collected at the end of the trials are reported, it is relevant to point out that no differences in terms of cell density growth or daily productivity were observed.

Table 5.
Microalgae daily productivity and biomasses collected at the end of the trial (60 days).
In the second case study (MW 2), although water samples exhibited a higher nutrient concentration, the three microalgae showed a similar behaviour of adapting to the culturing conditions, as shown by the growth curves reported in Figure 2B. However, the differences in cell numbers among species were less evident, and no significant differences in microalgae growth were detected (Table 4). The daily productivity of the strains was 0.017, 0.015, and 0.018 g L−1·d−1 for C. vulgaris, S. quadricauda, and Klebsormidium sp. K39, respectively (Table 5).
3.2. Nutrient Removal
Removal pollutant indices were calculated to evaluate the performance of microalgae treatments. As regards the pH values of the wastewaters, they continued to increase from the lag phase through the microalgae growth phase, as shown in Figure 3A (MW1) and Figure 4A (MW2), while EC values showed a decreasing tendency (Figure 3B and Figure 4B), according to nutrient consumption.
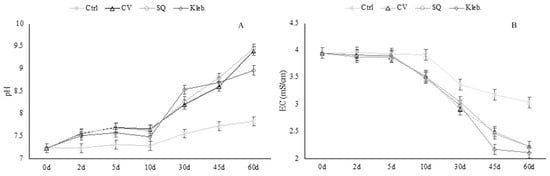
Figure 3.
pH (A) and EC (mS·cm−1) (B) values measured at each sampling (MW1).
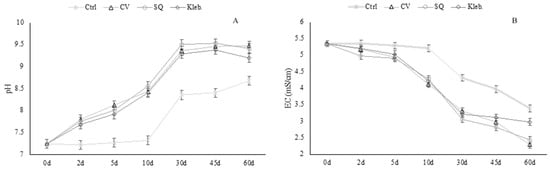
Figure 4.
pH (A) and EC (mS·cm−1) (B) values measured at each sampling (MW2).
In the first case study, the effects of single factors, species, and time were always significant on all the parameters monitored, as was the interaction between them on TKN, TP, and COD parameters, except for the BOD5 parameter (Table 6).

Table 6.
Effects of single factors and their interaction in ANOVA—MW1.
The variations in total nitrogen, total phosphorous, chemical oxygen demand, and biological oxygen demand contents during the two experiments are depicted in Figure 5.
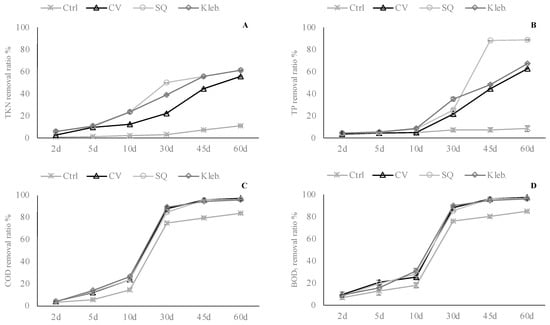
Figure 5.
Removal percentage of monitored parameters at each sampling—MW 1 ((A)—TKN; (B)—TP; (C)—COD; (D)—BOD5).
Post-hoc analyses to establish the ranking of effectiveness at each sampling are shown in Supplementary Materials Table S1. Based on these data, at each sampling, the microalgae significantly reduced all the parameters monitored with respect to the control in MW1. The pollutant concentration in all the tested wastewaters showed a different decrease during the first 2 days. The removal of pollutants gradually levelled off until the end of the experimental trial. At the end of the treatment, the maximum removal efficiency of C. vulgaris, S. quadricauda, and Klebsormidium sp. K39 was 55.5, 61.0, and 61.2% for total nitrogen, 62.7, 88.7, and 67.2% for total phosphorous, and 97.3, 96.6, and 96.2% for COD, respectively. The maximum total nitrogen, total phosphorous, and COD removal efficiency from wastewater control were 11.1%, 8.5%, and 83.8%, respectively.
As regards the second case study, the performance evaluation of microalgae in contaminants degradation showed that the effects of species, time, and species × time were always significant versus all pollutant parameters monitored (Table 7).

Table 7.
Effects of single factors and their interaction in ANOVA—MW2.
The variations in total nitrogen, total phosphorous, COD, and BOD5 contents during the two experiments are shown in Figure 6.
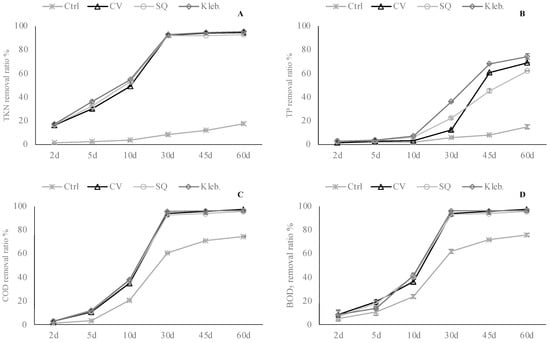
Figure 6.
Removal percentage of monitored parameters at each sampling—MW 2 ((A)—TKN; (B)—TP; (C)—COD; (D)—BOD5).
Post-hoc analyses to establish the ranking of effectiveness at each sampling are shown in Supplementary Materials Table S2. Post-hoc analysis of the data revealed a similar ranking of efficacy among the three tested microalgae, which gradually levelled off until the end of the experimental trial for all parameters monitored (Figure 6). In detail, at this sampling, each microalga significantly reduced the TKN variable with values between 92.7 and 95.5%. As well, concerning the removal of TP, COD, and BOD5, C. vulgaris, S. quadricauda, and Klebsormidium sp. K39 significantly reduced from 62.0 up to 74.3%, from 95.6 up to 97.3%, and from 95.4 up to 97.4% compared to the starting values.
As already seen in the above-mentioned trial, a decrease of the same parameters in the control (not-inoculate wastewater) was observed, and the maximum total nitrogen, total phosphorous, COD, and BOD5 degradation were 16.9, 14.7, 74.5, and 75.0%, respectively.
3.3. E. coli Removal Efficiency
The cell density of E. coli detected in MW1 (panel A) and MW2 (panel B) water samples, un-inoculated (control) and inoculated with different microalgal cultures (C. vulgaris ACUF863, S. quadricauda ACUF581, Klebsormidium sp. K39) after 0, 2, 5, 7, 9, 15, 30, 45, and 60 days from the inoculum is reported in Figure 7. Overall, a significant decrease in cell density was observed in all tested samples except the controls. In particular, regarding MW1 samples (Figure 7, panel A), no significant difference was detected in the removal efficiency of the tested microalgae. In detail, 5 days after the inoculum, S. quadricauda ACUF581 and C. vulgaris ACUF863 induced a decrease of 3.14 and 3.28 unit Log in E. coli cell densities, whereas Klebsormidium sp. K39 induced a decrease of 2.74 unit Log. After 7 days, higher reductions were registered in microalgal treatments as 1.43 unit Log by S. quadricauda ACUF581 and C. vulgaris ACUF863 and 1.75 unit Log by Klebsormidium sp. K39, while E. coli in the control sample was at 6.1 Log CFU mL−1. After 9 days, E. coli showed a cell density of 6.2 Log CFU mL−1 while in treated samples higher decreases, as 0.45, 0.50, and 0.55, were observed for C. vulgaris ACUF863, S. quadricauda ACUF581, and Klebsormidium sp. K39, respectively.
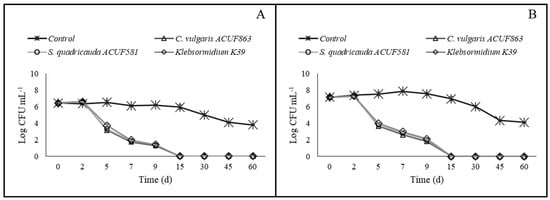
Figure 7.
E. coli load detected (as Log cells mL−1) detected in MW1 (A) and MW2 (B) samples, un-inoculated (control) and inoculated with different microalgal cultures (C. vulgaris ACUF863, S. quadricauda ACUF581, or Klebsormidium sp. K39) after 0, 2, 5, 7, 9, 15, 30, 45, and 60 days from the inoculum.
In the same samples, no E. coli was detected after 15, 30, 45, and 60 days from the microalgal inoculum. A different trend was observed in controls, where E. coli was constantly increasing, reaching, at the end of the trial (60 days), a cell density of 3.80 Log CFU mL−1. The bacterial removal results on MW2 samples were significant (Figure 7, panel B). In details, after 5 days from inoculum, in samples treated with S. quadricauda ACUF581 and C. vulgaris ACUF863, the target bacteria were reduced by 3.34 and 3.49 unit Log, respectively, whereas in samples treated with Klebsormidium sp. K39, the target bacteria were reduced by 3.15 unit Log. The reduction values were significantly different compared to the control sample, where the E. coli density was found to be 7.53 Log CFU mL−1, while no significant differences were found among the treatments. After 7 days, more than 0.97, 1.03, and 1.06 unit Log CFU mL−1 of reduction were observed for S. quadricauda ACUF581, Klebsormidium sp. K39, and C. vulgaris ACUF863, respectively, when the target bacteria cell density in control samples showed a load of 7.85 Log CFU mL−1. After 9 days, the target bacteria showed a cell density of 7.54 Log CFU mL−1, while the treated samples registered a higher reduction, as 0.80, 0.84, and 0.85 for C. vulgaris ACUF863, S. quadricauda ACUF581, and Klebsormidium sp. K39, respectively. After days 15, 30, 45, and 60 days, E. coli was never detected in any treated samples, while its density was found at a mean value of 4.1 Log CFU mL−1 in untreated samples at the end of the trial (60 days).
4. Discussion
Discharge of wastewater into water bodies represents a serious issue because the high concentrations of contaminants may pose a serious threat to ecosystem health. In this frame, one of the main reasons for removing nutrients from wastewater is to control eutrophication, which is due to the uncontrolled growth of algae or higher hydrophytes triggered by the addition of a nutrient surplus in the ecosystem [,]. In the present study, a sustainable and eco-friendly wastewater treatment was tested in order to support a circular system in which the microalgae play a key role, representing both the agent of the remediation and the final product of the process, which leads to a useful biomass suitable for several further purposes. The importance of low-cost biomass production is crucial because the economic and environmental drawbacks could be partly overcome using urban wastewater as a microalgae growth substrate [,]. Because of their ability to perform photoautotrophic, mixotrophic, or heterotrophic metabolism, microalgae represent a promising biological system for a variety of wastewaters. To achieve this aim, employing species able to remediate wastewater is crucial and guarantees a successful sustainable process, and the best candidate is represented by autochthonous microalgae, which are able to naturally grow in a specific wastewater. Furthermore, microalgal systems are designed mainly to achieve high biomass productivity with minimum energy inputs because essential nutrients and a carbon source, required for an efficient cultivation process, are largely available in the effluent [,].
The identification process of several isolates recently affiliated with the genus Klebsormidum revealed that Klebsormidum sp. K39 lacks a proper grouping at the species level due to unclear species boundaries []. For this genus, the morphological traits as well as some features considered taxonomically relevant (showing variations depending on the age and the physiological conditions) result in a taxonomically and systematically complex taxon in which phylogenetic relationships are still poorly understood [,]. Despite Klebsormidum sp. K39 being subjected to molecular analyses for phylogenetic study, further studies are required to cluster this strain into a species, as Novis [] had already shown, with the description of the Klebsormidium acidophilum species. It is relevant to highlight that the Klebsormidum sp. K39 strain used in the present study has been recently tested to evaluate its dynamic within an autochthonous microalgal pool in terms of E. coli removal efficiency [].
Zooming in on microalgal yields obtained during the phycoremediation process, they were quite different from data reported in the literature due to the different composition of treated effluents [,]. In particular, Li et al. [], cultivating five microalgae species, among them C. vulgaris and S. quadricauda, in post hydrothermal liquefaction wastewater, obtained a daily productivity of 0.031 and 0.0071 g L−1·d−1, respectively. Regarding Klebsormidium sp., available data indicate a biomass production that may vary from about 0.010 g L−1·d−1 in horticultural wastewater to about 0.035 g L−1·d−1 in synthetic wastewater []. Although, the yields are quite different than optimal conditions, at the end of the present experimental tests, all the microalgae demonstrated a good growth aptitude in urban wastewaters with different pollutant contents, and this could be mainly related to their physiochemical and biochemical characteristics. Indeed, many studies report the remediation ability and biomass production of C. vulgaris and S. quadricauda using wastewater from various sources; they have proven abilities of removing nitrogen, phosphorus, and COD and shown their potentiality as a tertiary biotreatment step in the remediation process []. For instance, Baglieri et al. [] investigated the feasibility of cultivating C. vulgaris and S. quadricauda in agricultural wastewater for inorganic nutrient removal, and the two species showed similar behavior, determining comparable remediation performance in terms of nitrogen (both about 99%) and phosporous (88 and 94%, respectively).
On the contrary, limited studies on the cultivation of Klebsormidium sp. K39 in wastewater are still reported. Among Klebsormidium species, Klebsormidium flaccidum showed good feasibility for nutrient removal from municipal wastewater, being able to provide a complete removal of nitrogen and phosphorous []. Similarly, Liu and Vyverman [] evaluated differences in the uptake of nutrients of Klebsormidium sp. from wastewater under varying nitrogen and phosphorous contents. The authors observed that the microalgae achieved an approximately 99% phosphorous removal rate and a consistent nitrogen removal rate (about 99%) under almost any tested conditions. However, with a N/P ratio of 20, Klebsormidium sp. exhibited a lower nitrogen removal efficiency (76.4%).
Overall, the daily productivity and the growth results confirm the suitability of urban wastewater as a substrate for cultivation of Klebsormidium sp. K39 and the absence of negative effects. Similarly, the three species showed quite comparable increases in terms of fresh and dry biomass produced. A good adaptability of Klebsormidium sp. K39 was also observed in a study under consideration []. In particular, Klebsormidium sp. K39, during a lab-scale wastewater treatment at lab scale using a microalgae pool, was the dominant microalgae at the end of the treatment.
Results clearly showed that the initial concentrations of both tested wastewaters did not affect the final biomass accumulation or the daily productivity of the three microalgae species. This may be mainly due to the characteristics of the tested urban wastewater, a kind of effluent usually rich in nutrient compounds and characterized by low concentrations of toxic substances that may inhibit microalgae growth.
In detail, in the first case study using MW 1, S. quadricauda showed the highest phosphorous removal rate (91.9%), followed by Klebsormidium sp. K39 (69.6%) and C. vulgaris (64.7%) of total phosphorous. In terms of nitrogen removal, no significant differences were detected between S. quadricauda and Klebsormidium sp. K39, which showed the highest removal efficiency (62.8 and 63.1%, respectively), while for C. vulgaris, a lower degradation rate was observed at each sampling time. In the control, the decrease of total nitrogen and total phosphorous due to naturally occurring abiotic degradation, was very low. Regarding the removal of COD and BOD5, slight differences were observed among the tested strains, and both of these parameters always significantly decreased at any time in all treated samples.
In the second case study, using MW 2, C. vulgaris, S. quadricauda, and Klebsormidium sp. K39 induced a progressive reduction of measured parameters with increasing treatment time in total nitrogen, total phosphorous, COD, and BOD5 to values below the reuse for irrigation in agriculture, according to law limits (Italian Ministerial Decree n. 185/2003) for irrigation use. A comparable bioremediation performance, in terms of total nitrogen, COD, and BOD5, was recorded regardless of the microalgae species. Instead, the highest phosphorous removal rate was achieved by S. quadricauda.
The highest amount of nutrient removal matched the biomass production; in fact, it is well known that the nutrient reduction is mainly related to the metabolic activity of microalgae cells []. In both case studies, the E. coli removal rates achieved with C. vulgaris, S. quadricauda, and Klebsormidium sp. K39 were in line with the values previously reported. Although pathogen removal mechanisms of microalgae have been related to different phenomena such as competition for nutrients, pH increases, and higher dissolved oxygen levels, for E. coli removal, adherence to the microalgal surface [] is reported as the most likely mechanism [,]. In a study conducted in photobioreactors, Chlorella sorokiniana performed a E. coli removal rate of 99.8% in anaerobically treated black water in photobioreactors []. Overall, as reported in a recent review, the E. coli removal rate is on average higher than 98% [].
The results of the present study indicate that the two different levels of contaminants did not negatively affect the nutrient removal ratio or cell growth, in accordance with findings reported in several studies [,,]. In these studies, the authors, starting from effluents with various nutrient concentrations, observed that the microalgae screened, including C. vulgaris and S. quadricauda, were able to reproduce similar performances in terms of both cell growth and nutrient uptake capacity. In Table 8, a summary of nutrient removal rates reported in various recent studies is provided, supporting and confirming the remediation capacity of the microalgae species tested in the current study.

Table 8.
Removal rates by C. vulgaris, S. quadricauda and Klebsormidium sp. K39 in wastewaters.
5. Conclusions
The use of microalgae as wastewater remediation agents is becoming an interesting alternative to conventional treatments, offering two undeniable benefits, i.e., the wastewater remediation and the production of valuable biomass for multipurpose applications. Overall, our findings confirm that microalgae-based treatment offers potential for sustainable, eco-friendly, and resource-efficient solutions for wastewater remediation that may also be used for irrigation in agriculture, contributing to a more environmentally friendly approach to water management.
Furthermore, it is noteworthy that this study represents the first investigation into the use of Klebsormidium sp. K39, according to the promising performances of other species of this genus for wastewater remediation treatment. Our findings demonstrate that this species exhibits high adaptability to various wastewater conditions and displays efficient nutrient removal capabilities. These results are promising because they suggest that indigenous species like Klebsormidium sp. K39 exhibit the potential to deliver similar decontamination performances as the extensively studied microalgae species. However, further studies, as well as a full-scale demonstration, are necessary to verify the practicality, efficiency, and cost-effectiveness of microalgae-based treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su151511644/s1. Table S1: Wastewater parameters (mg L−1) along the experimental period in MW 1; Table S2: Wastewater parameters (mg L−1) along the experimental period in MW 2.
Author Contributions
Conceptualization, I.P. and A.B.; methodology, E.L.B. and P.S.O.; software, E.L.B.; validation, C.L.R., C.C., I.P. and A.B.; formal analysis, E.L.B., P.S.O., F.F., N.R. and R.S.; data curation, E.L.B.; writing—original draft preparation, E.L.B. and P.S.O.; writing—review and editing C.C., I.P. and A.B.; supervision, C.C., I.P., A.B. and C.L.R.; funding acquisition, C.C., I.P., A.B. and C.L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been partially supported by PON “RICERCA E INNOVAZIONE” 2014–2020, Azione II—Obiettivo Specifico 1b—Progetto “Miglioramento delle produzioni agroalimentari mediterranee in condizioni di carenza di risorse idriche”—WATER4AGRIFOOD and partially funded by the European Union (NextGeneration EU) through the MUR-PNRR project Sustainable management of natural resources in agriculture: SAMOTHRACE (ECS00000022) and the Agritech National Research Center (PNRR—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions; neither the European Union nor the European Commission can be considered responsible for them.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was conducted within a Ph.D. research program in Agricultural, Food, and Environmental Science (cycle XXXVI) by Emanuele la Bella (Scientific Tutor: Ivana Puglisi and Andrea Baglieri) and Biotechnology (cycle XXXVI) by Paride Salvatore Occhipinti (Scientific Tutor: Cinzia Caggia and Cinzia Lucia Randazzo), who received a grant from the University of Catania. The authors sincerely thank Antonino Pollio, from the University of Naples, for kindly providing the strains Chlorella vulgaris ACUF863 and Scenedesmus quadricauda ACUF581.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schewe, J.; Heinke, J.; Gerten, D.; Haddeland, I.; Arnell, N.W.; Clark, D.B.; Dankers, R.; Eisner, S.; Fekete, B.M.; Colón-González, F.J.; et al. Multimodel Assessment of Water Scarcity under Climate Change. Proc. Natl. Acad. Sci. USA 2014, 111, 3245–3250. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Saxena, S. Cultivation of Azolla Microphylla Biomass on Secondary-Treated Delhi Municipal Effluents. Biomass Bioenergy 2005, 29, 60–64. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Immobilized Microalgae for Removing Pollutants: Review of Practical Aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef]
- Chai, W.S.; Tan, W.G.; Halimatul Munawaroh, H.S.; Gupta, V.K.; Ho, S.-H.; Show, P.L. Multifaceted Roles of Microalgae in the Application of Wastewater Biotreatment: A Review. Environ. Pollut. 2021, 269, 116236. [Google Scholar] [CrossRef]
- Olguín, E.J. Phycoremediation: Key Issues for Cost-Effective Nutrient Removal Processes. Biotechnol. Adv. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs New Advanced Treatment Methods for the Removal of Contaminants of Emerging Concern from Urban Wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- La Bella, E.; Baglieri, A.; Fragalà, F.; Puglisi, I. Multipurpose Agricultural Reuse of Microalgae Biomasses Employed for the Treatment of Urban Wastewater. Agronomy 2022, 12, 234. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Safe Use of Wasterwater Excreta and Greywater; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Ofori, S.; Puškáčová, A.; Růžičková, I.; Wanner, J. Treated Wastewater Reuse for Irrigation: Pros and Cons. Sci. Total Environ. 2021, 760, 144026. [Google Scholar] [CrossRef]
- Łuczkiewicz, A.; Jankowska, K.; Fudala-Ksiazek, S.; Olańczuk-Neyman, K. Antimicrobial Resistance of Fecal Indicators in Municipal Wastewater Treatment Plant. Water Res. 2010, 44, 5089–5097. [Google Scholar] [CrossRef]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and Fate of Antibiotic Resistant Bacteria in Wastewater Treatment Plants: A Review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef]
- Novo, A.; André, S.; Viana, P.; Nunes, O.C.; Manaia, C.M. Antibiotic Resistance, Antimicrobial Residues and Bacterial Community Composition in Urban Wastewater. Water Res. 2013, 47, 1875–1887. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Ventura, D.; Consoli, S.; Barbagallo, S.; Marzo, A.; Vanella, D.; Licciardello, F.; Cirelli, G.L. How to Overcome Barriers for Wastewater Agricultural Reuse in Sicily (Italy)? Water 2019, 11, 335. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.-P. Impact of Microalgae Characteristics on Their Conversion to Biofuel. Part II: Focus on Biomethane Production. Biofuels Bioprod. Biorefin. 2012, 6, 205–218. [Google Scholar] [CrossRef]
- Puglisi, I.; Barone, V.; Fragalà, F.; Stevanato, P.; Baglieri, A.; Vitale, A. Effect of Microalgal Extracts from Chlorella vulgaris and Scenedesmus quadricauda on Germination of Beta vulgaris Seeds. Plants 2020, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- La Bella, E.; Baglieri, A.; Rovetto, E.I.; Stevanato, P.; Puglisi, I. Foliar Spray Application of Chlorella vulgaris Extract: Effect on the Growth of Lettuce Seedlings. Agronomy 2021, 11, 308. [Google Scholar] [CrossRef]
- Puglisi, I.; La Bella, E.; Rovetto, E.I.; Stevanato, P.; Fascella, G.; Baglieri, A. Morpho-Biometric and Biochemical Responses in Lettuce Seedlings Treated by Different Application Methods of Chlorella vulgaris Extract: Foliar Spray or Root Drench? J. Appl. Phycol. 2022, 34, 889–901. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation Into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Vaz, B.D.S.; Moreira, J.B.; Morais, M.G.D.; Costa, J.A.V. Microalgae as a New Source of Bioactive Compounds in Food Supplements. Curr. Opin. Food. Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Vanni, A.; Anfossi, L.; Cignetti, A.; Baglieri, A.; Gennari, M. Degradation of Pyrimethanil in Soil: Influence of Light, Oxygen, and Microbial Activity. J. Environ. Sci. Health B 2006, 41, 67–80. [Google Scholar] [CrossRef]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual Role of Microalgae: Phycoremediation of Domestic Wastewater and Biomass Production for Sustainable Biofuels Production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient Recovery from Wastewater Streams by Microalgae: Status and Prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Colak, O.; Kaya, Z. A Study on the Possibilities of Biological Wastewater Treatment Using Algae. Doga Biyolji Serisi 1988, 12, 18–29. [Google Scholar]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and Wastewater Treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Zurano, A.; Lafarga, T.; Morales-Amaral, M.M.; Gómez-Serrano, C.; Fernández-Sevilla, J.M.; Acién-Fernández, F.G.; Molina-Grima, E. Wastewater Treatment Using Scenedesmus almeriensis: Effect of Operational Conditions on the Composition of the Microalgae-Bacteria Consortia. J. Appl. Phycol. 2021, 33, 3885–3897. [Google Scholar] [CrossRef]
- García, D.; Posadas, E.; Blanco, S.; Acién, G.; García-Encina, P.; Bolado, S.; Muñoz, R. Evaluation of the Dynamics of Microalgae Population Structure and Process Performance during Piggery Wastewater Treatment in Algal-Bacterial Photobioreactors. Bioresour. Technol. 2018, 248, 120–126. [Google Scholar] [CrossRef]
- Law, X.N.; Cheah, W.Y.; Chew, K.W.; Ibrahim, M.F.; Park, Y.-K.; Ho, S.-H.; Show, P.L. Microalgal-Based Biochar in Wastewater Remediation: Its Synthesis, Characterization and Applications. Environ. Res. 2022, 204, 111966. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of Green Algae Chlorella sp. in Different Wastewaters from Municipal Wastewater Treatment Plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.K.; Yung, K.K.L.; Tsang, Y.F.; Xia, Y.; Wang, L.; Ho, K.C. Scenedesmus quadricauda for Nutrient Removal and Lipid Production in Wastewater. Water Environ. Res. 2015, 87, 2037–2044. [Google Scholar] [CrossRef]
- Baglieri, A.; Sidella, S.; Barone, V.; Fragalà, F.; Silkina, A.; Nègre, M.; Gennari, M. Cultivating Chlorella vulgaris and Scenedesmus quadricauda Microalgae to Degrade Inorganic Compounds and Pesticides in Water. Environ. Sci. Pollut. Res. 2016, 23, 18165–18174. [Google Scholar] [CrossRef]
- Ren, H.; Tuo, J.; Addy, M.M.; Zhang, R.; Lu, Q.; Anderson, E.; Chen, P.; Ruan, R. Cultivation of Chlorella vulgaris in a Pilot-Scale Photobioreactor Using Real Centrate Wastewater with Waste Glycerol for Improving Microalgae Biomass Production and Wastewater Nutrients Removal. Bioresour. Technol. 2017, 245, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Li, Z.; Haifeng, L.; Zhang, Y.; Shanshan, M.; Baoming, L.; Zhidan, L.; Na, D.; Minsheng, L.; Buchun, S.; Jianwen, L. Microalgae Production for Bioenergy from Post Hydrothermal Liquefaction Wastewater: Effects of Strain, Nutrients Concentration and Inoculum Size on Microalgae Culture. Int. J. Agric. Biol. Eng. 2017, 10, 194–204. [Google Scholar]
- Occhipinti, P.S.; Russo, N.; Foti, P.; Pino, A.; Randazzo, C.L.; Pollio, A.; Caggia, C. An indigenous microalgal pool from a constructed wetland as an alternative strategy for Esch-erichia coli removal in urban wastewater. J. Environ. Manag. 2023. submitted. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Liu, J.; Danneels, B.; Vanormelingen, P.; Vyverman, W. Nutrient Removal from Horticultural Wastewater by Benthic Filamentous Algae Klebsormidium sp., Stigeoclonium spp. and Their Communities: From Laboratory Flask to Outdoor Algal Turf Scrubber (ATS). Water Res. 2016, 92, 61–68. [Google Scholar] [CrossRef]
- Ruiz-Martinez, A.; Garcia, N.M.; Romero, I.; Seco, A.; Ferrer, J. Microalgae cultivation in wastewater: Nutrient removal from anaerobic membrane bioreactor effluent. Bioresour. Technol. 2012, 126, 247–253. [Google Scholar] [CrossRef]
- Pham, M.; Schideman, L.; Scott, J.; Rajagopalan, N.; Plewa, M.J. Chemical and Biological Characterization of Wastewater Generated from Hydrothermal Liquefaction of Spirulina. Environ. Sci. Technol. 2013, 47, 2131–2138. [Google Scholar] [CrossRef]
- Baglieri, A.; Nègre, M.; Trotta, F.; Bracco, P.; Gennari, M. Organo-Clays and Nanosponges for Acquifer Bioremediation: Adsorption and Degradation of Triclopyr. J. Environ. Sci. Health B 2013, 48, 784–792. [Google Scholar] [CrossRef]
- Hammouda, O.; Gaber, A.; Abdel-Raouf, N. Microalgae and Wastewater Treatment. Ecotoxicol. Environ. Saf. 1995, 31, 205–210. [Google Scholar] [CrossRef]
- Delrue, F.; Álvarez-Díaz, P.D.; Fon-Sing, S.; Fleury, G.; Sassi, J.-F. The Environmental Biorefinery: Using Microalgae to Remediate Wastewater, a Win-Win Paradigm. Energies 2016, 9, 132. [Google Scholar] [CrossRef]
- Nasr, M. Design considerations of algal systems for wastewater treatment. In Application of Microalgae in Wastewater Treatment: Volume 1: Domestic and Industrial Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 411–426. [Google Scholar]
- Nasr, M. Aquatic pollution and wastewater treatment system. In Algae and Aquatic Macrophytes in Cities; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–37. [Google Scholar]
- Škaloud, P.; Rindi, F. Ecological Differentiation of Cryptic Species within an Asexual Protist Morphospecies: A Case Study of Filamentous Green Alga Klebsormidium (Streptophyta). J. Eukaryot. Microbiol. 2013, 60, 350–362. [Google Scholar] [CrossRef]
- Rindi, F.; Mikhailyuk, T.I.; Sluiman, H.J.; Friedl, T.; López-Bautista, J.M. Phylogenetic Relationships in Interfilum and Klebsormidium (Klebsormidiophyceae, Streptophyta). Mol. Phylogenet. Evol. 2011, 58, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Novis, P.M. Taxonomy of Klebsormidium (Klebsormidiales, Charophyceae) in New Zealand Streams and the Significance of Low-PH Habitats. Phycologia 2006, 45, 293–301. [Google Scholar] [CrossRef]
- Umetani, I.; Sposób, M.; Tiron, O. Indigenous Green Microalgae for Wastewater Treatment: Nutrient Removal and Resource Recovery for Biofuels and Bioproducts. Bioenergy Res. 2023, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Vyverman, W. Differences in Nutrient Uptake Capacity of the Benthic Filamentous Algae Cladophora sp., Klebsormidium sp. and Pseudanabaena sp. under Varying N/P Conditions. Bioresour. Technol. 2015, 179, 234–242. [Google Scholar] [CrossRef]
- Markou, G.; Wang, L.; Ye, J.; Unc, A. Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns. Biotechnol. Adv. 2018, 36, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Ansa, E.D.O.; Lubberding, H.J.; Ampofo, J.A.; Gijzen, H.J. The role of algae in the removal of Escherichia coli in a tropical eutrophic lake. Ecol. Eng. 2011, 37, 317–324. [Google Scholar] [CrossRef]
- Cho, K.H.; Wolny, J.; Kase, J.A.; Unno, T.; Pachepsky, Y. Interactions of E. coli with algae and aquatic vegetation in natural waters. Water Res. 2022, 209, 117952. [Google Scholar] [CrossRef]
- Slompo, N.D.M.; Quartaroli, L.; Fernandes, T.V.; Silva, G.H.R.D.; Daniel, L.A. Nutrient and Pathogen Removal from Anaerobically Treated Black Water by Microalgae. J. Environ. Manag. 2020, 268, 110693. [Google Scholar] [CrossRef]
- Amaro, H.M.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.M.; Esteves, A.F. Microalgae Systems-Environmental Agents for Wastewater Treatment and Further Potential Biomass Valorisation. J. Environ. Manag. 2023, 337, 117678. [Google Scholar] [CrossRef]
- Kube, M.; Mohseni, A.; Fan, L.; Roddick, F. Impact of Alginate Selection for Wastewater Treatment by Immobilised Chlorella vulgaris. Chem. Eng. J. 2019, 358, 1601–1609. [Google Scholar] [CrossRef]
- Singh, D.V.; Upadhyay, A.K.; Singh, R.; Singh, D.P. Implication of Municipal Wastewater on Growth Kinetics, Biochemical Profile, and Defense System of Chlorella vulgaris and Scenedesmus vacuolatus. Environ. Technol. Innov. 2022, 26, 102334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).