The High-Value Product, Bio-Waste, and Eco-Friendly Energy as the Tripod of the Microalgae Biorefinery: Connecting the Dots
Abstract
1. Introduction
2. Material and Methods
2.1. Life Cycle Assessment (LCA)
2.1.1. Goal and Scope Definition
Step 1: β-Carotene Production
Step 2: Bulk Oil Production
Step 3: Defatted Biomass Production
2.1.2. Life Cycle Inventory (LCI)
2.1.3. Life Cycle Impact Analysis (LCIA)
Global Warming (GWP)
Stratospheric Ozone Depletion (SOD)
Ionizing Radiation (IR)
Ozone Formation–Human Health (OFHH)
Fine Particulate Matter Formation (FPMF)
Ozone Formation–Terrestrial Ecosystems (OFTE)
Terrestrial Acidification (TA)
Freshwater Eutrophication (FEU)
Terrestrial Ecotoxicity (TE)
Freshwater Ecotoxicity (FEC)
Marine Ecotoxicity (ME)
Human Carcinogenic Toxicity (HCT)
Human Non-Carcinogenic Toxicity (HNCT)
Mineral Resource Scarcity (MRS)
Fossil Resource Scarcity (FRS)
Land Use (LU)
Water Consumption (WC)
2.1.4. Interpretation of Results
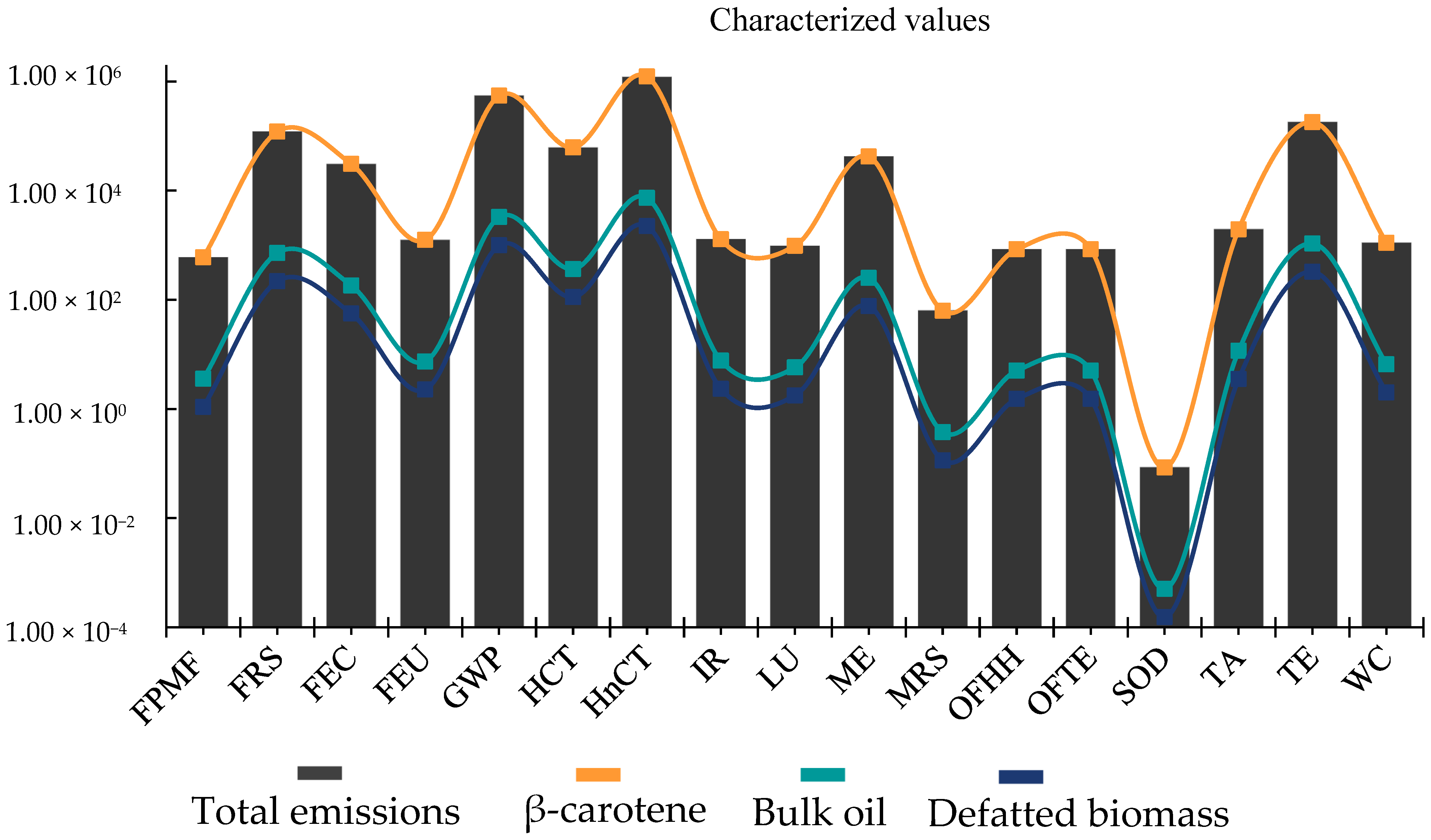
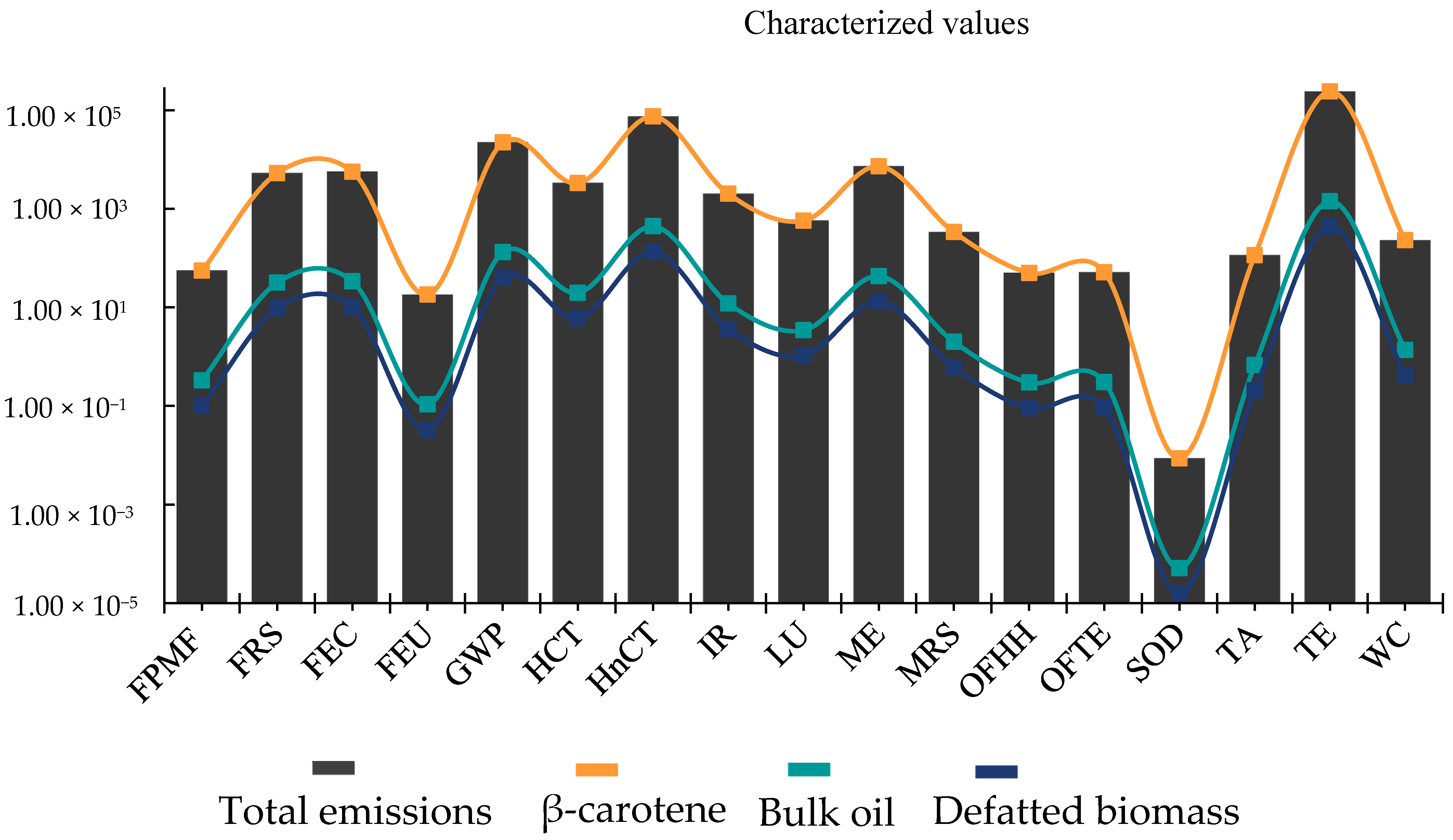
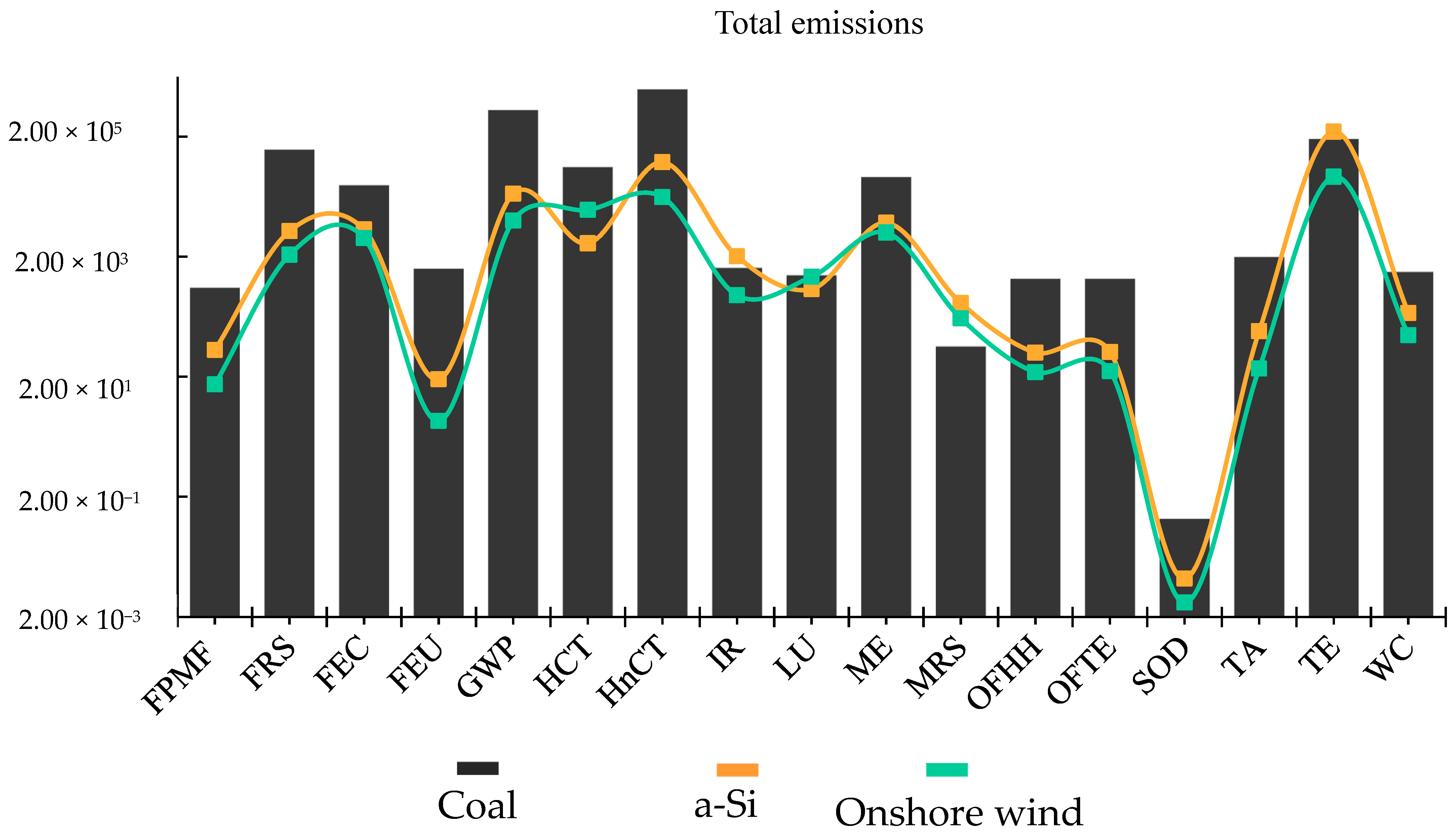
3. Results and Discussion
3.1. Environmental Sustainability Analysis of the Microalgae Biorefinery
3.2. Transposing the Environmental Deficit in Microalgae Biorefinery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Persson, L.; Carney Almroth, B.M.; Collins, C.D.; Cornell, S.; de Wit, C.A.; Diamond, M.L.; Fantke, P.; Hassellöv, M.; MacLeod, M.; Ryberg, M.W.; et al. Outside the safe operating space of the planetary boundary for novel entities. Environ. Sci. Technol. 2022, 56, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Palahi, M.; Adams, J. Why the world needs a ‘circular bioeconomy’—For jobs, biodiversity and prosperity. In World Economic Forum; World Economic Forum: Cologny, Switzerland, 2020. [Google Scholar]
- Tan, E.C.; Lamers, P. Circular bioeconomy concepts—A perspective. Front. Sustain. 2021, 2, 701509. [Google Scholar] [CrossRef]
- Premaratne, M.; Nishshanka, G.K.S.H.; Anthonio, R.A.D.P.; Liyanaarachchi, V.C.; Thevarajah, B.; Nimarshana, P.H.V.; Malik, A.; Ariyadasa, T.U. Resource recovery from waste streams for production of microalgae biomass: A sustainable approach towards high-value biorefineries. Bioresour. Technol. Rep. 2022, 18, 101070. [Google Scholar] [CrossRef]
- Deprá, M.C.; Severo, I.A.; dos Santos, A.M.; Zepka, L.Q.; Jacob-Lopes, E. Environmental impacts on commercial microalgae-based products: Sustainability metrics and indicators. Algal Res. 2020, 51, 102056. [Google Scholar] [CrossRef]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; de Paula Pereira, A.S.A.; Marangon, B.B.; de Assis, L.R.; Lorentz, J.F. Bioproducts from microalgae biomass: Technology, sustainability, challenges and opportunities. Chemosphere 2022, 305, 135508. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Two-stage cultivation of microalgae for production of high-value compounds and biofuels: A review. Algal Res. 2021, 57, 102353. [Google Scholar] [CrossRef]
- Ruiz, J.; Wijffels, R.H.; Dominguez, M.; Barbosa, M.J. Heterotrophic vs. autotrophic production of microalgae: Bringing some light into the everlasting cost controversy. Algal Res. 2022, 64, 102698. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Energy from microalgae: A short history. In Algae for Biofuels and Energy; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–15. [Google Scholar]
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]
- Dias, R.R.; Deprá, M.C.; Zepka, L.Q.; Jacob-Lopes, E. Roadmap to net-zero carbon emissions in commercial microalgae-based products: Environmental sustainability and carbon offset costs. J. Appl. Phycol. 2022, 34, 1255–1268. [Google Scholar] [CrossRef]
- ISO-14040; Environmental Management–Life Cycle Assessment–Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- Xi, Y.; Bian, J.; Luo, G.; Kong, F.; Chi, Z. Enhanced β-carotene production in Dunaliella salina under relative high flashing light. Algal Res. 2022, 67, 102857. [Google Scholar] [CrossRef]
- de Souza Celente, G.; Rizzetti, T.M.; Sui, Y.; de Souza Schneider, R.D.C. Potential use of microalga Dunaliella salina for bioproducts with industrial relevance. Biomass Bioenergy 2022, 167, 106647. [Google Scholar] [CrossRef]
- Milousi, M.; Souliotis, M.; Arampatzis, G.; Papaefthimiou, S. Evaluating the environmental performance of solar energy systems through a combined life cycle assessment and cost analysis. Sustainability 2019, 11, 2539. [Google Scholar] [CrossRef]
- dos Santos, A.M.; Deprá, M.C.; dos Santos, A.M.; Cichoski, A.J.; Zepka, L.Q.; Jacob-Lopes, E. Sustainability metrics on microalgae-based wastewater treatment system. Desalin. Water Treat. 2020, 185, 51–61. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Kanagaraj, N.; Sadeghi, S.; Yousefi, H. Midpoint and endpoint impacts of electricity generation by renewable and nonrenewable technologies: A case study of Alberta, Canada. Renew. Energy 2022, 197, 22–39. [Google Scholar] [CrossRef]
- Maroneze, M.M.; Dias, R.R.; Severo, I.A.; Queiroz, M.I. Microalgae-Based Processes for Pigments Production. In Pigments from Microalgae Handbook; Springer: Berlin/Heidelberg, Germany, 2020; pp. 241–264. [Google Scholar]
- Deprá, M.C.; dos Santos, A.M.; Jacob-Lopes, E. Sustainability metrics in the microalgae-based pigments production: A life cycle assessment approach. In Pigments from Microalgae Handbook; Springer: Berlin/Heidelberg, Germany, 2020; pp. 363–390. [Google Scholar]
- Peralta-Ruiz, Y.; González-Delgado, A.D.; Kafarov, V. Evaluation of alternatives for microalgae oil extraction based on exergy analysis. Appl. Energy 2013, 101, 226–236. [Google Scholar] [CrossRef]
- Lardon, L.; Hélias, A.; Sialve, B.; Steyer, J.P.; Bernard, O. Life-cycle assessment of biodiesel production from microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef]
- Martinez-Guerra, E.; Gude, V.G. Energy aspects of microalgal biodiesel production. Aims Energy 2016, 4, 347–362. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Morais Júnior, W.G.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae biomolecules: Extraction, separation and purification methods. Processes 2020, 9, 10. [Google Scholar] [CrossRef]
- Patel, S.; Kannan, D.C. A method of wet algal lipid recovery for biofuel production. Algal Res. 2021, 55, 102237. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Bravo-Sánchez, M.G.; Olán-Acosta, M.D.L.Á.; Barajas-Fernández, J.; Guzmán-López, A.; Petriz-Prieto, M.A. Technoeconomic Evaluation of Microalgae Oil Production: Effect of Cell Disruption Method. Fermentation 2022, 8, 301. [Google Scholar] [CrossRef]
- Chalermthai, B.; Giwa, A.; Moheimani, N.; Taher, H. Techno-economic strategies for improving economic viability of β-carotene extraction using natural oil and supercritical solvent: A comparative assessment. Algal Res. 2022, 68, 102875. [Google Scholar] [CrossRef]
- Huijbregts, M.A.; Steinmann, Z.J.; Elshout, P.M.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; Van Zelm, R. ReCiPe2016: A harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Zepka, L.Q.; Deprá, M.C. Sustainability Indicators and Metrics of Environmental Impact: Industrial and Agricultural Life Cycle Assessment, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Ye, C.; Mu, D.; Horowitz, N.; Xue, Z.; Chen, J.; Xue, M.; Zhou, W. Life cycle assessment of industrial scale production of spirulina tablets. Algal Res. 2018, 34, 154–163. [Google Scholar] [CrossRef]
- Chen, R.; Qin, Z.; Han, J.; Wang, M.; Taheripour, F.; Tyner, W.; O’Connor, D.; Duffield, J. Life cycle energy and greenhouse gas emission effects of biodiesel in the United States with induced land use change impacts. Bioresour. Technol. 2018, 251, 249–258. [Google Scholar] [CrossRef]
- Roser, M. Why Did Renewables Become So Cheap So Fast? and What Can We Do to Use This Global Opportunity for Green Growth; Our World in Data: Oxford, UK, 2020. [Google Scholar]
- Mordor Intelligence. Algae Products Market Size & Share Analysis—Growth Trends & Forecasts (2023–2028). 2023. Available online: https://www.mordorintelligence.com/ (accessed on 1 July 2023).
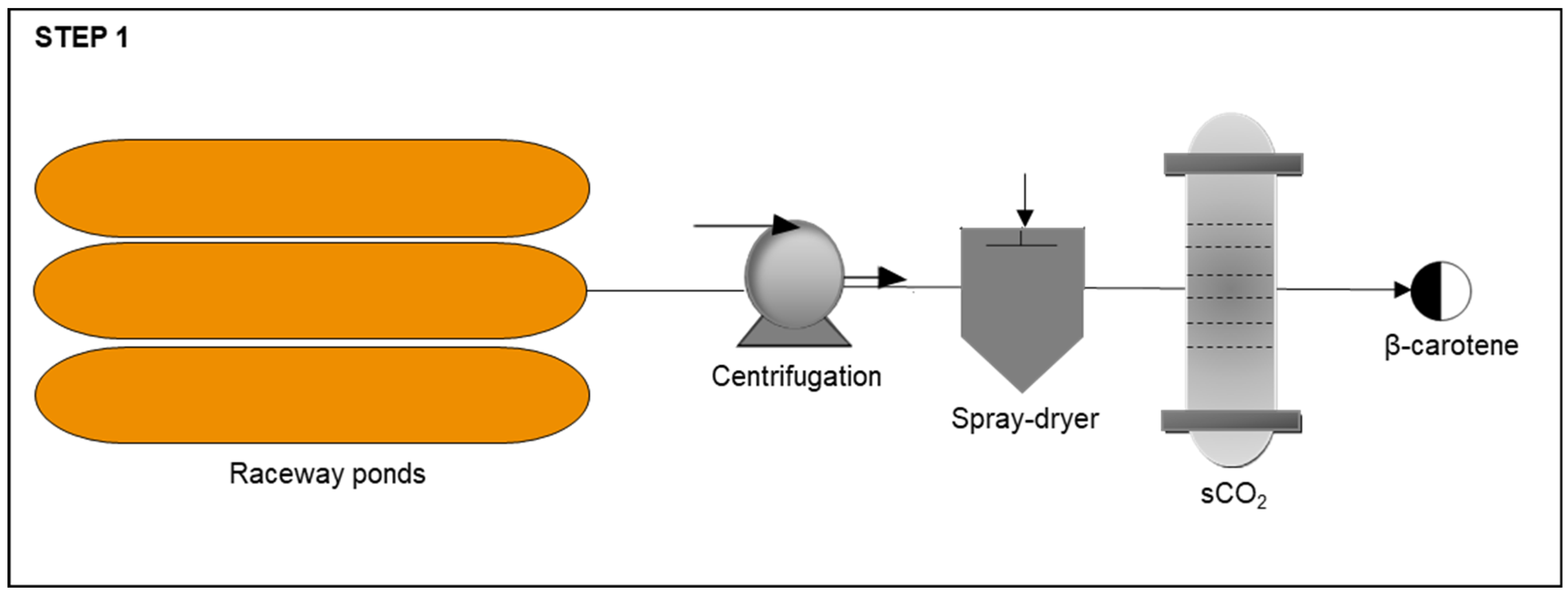

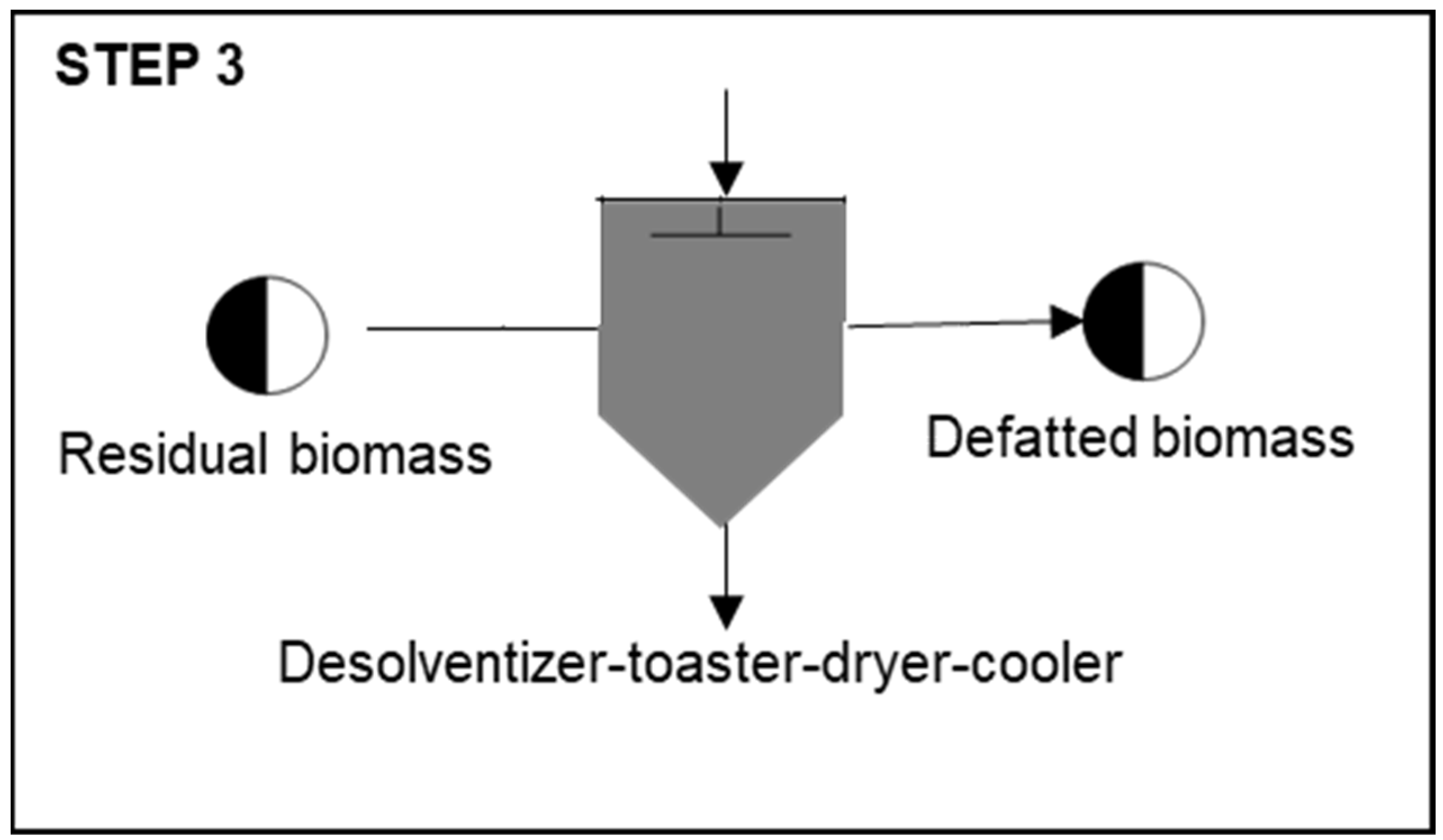

| Process | Unit | Base Case |
|---|---|---|
| Cultivation | ||
| Raceway pond | m3 | 63.16 |
| Electric energy for paddle wheel | kWh | 270,122.68 |
| Electric energy for water pumping | kWh | 50,022.72 |
| Electric energy for CO2 injection | kWh | 165,074.97 |
| Water evaporation | m3 | 8.21 |
| Biomass productivity | ton/m3/year | 0.15 |
| Output | ||
| Algae liquid | ton | 50.03 |
| Harvest | ||
| Input | ||
| Energy consumption centrifugation | kWh | 1894.80 |
| Drying | ||
| Input | ||
| Wet biomass | ton | 12.50 |
| Spray-dryer | kWh | 13,632.25 |
| Output | ||
| Dry biomass | ton | 9.38 |
| Pigment extraction | ||
| Input | ||
| Dry biomass | ton | 9.38 |
| sCO2 | kWh | 7504.0 |
| Output | ||
| β-carotene pigment | ton | 1.0 |
| Bulk oil production | ||
| Input | ||
| Residual biomass | ton | 8.38 |
| Extractor | kWh | 594.51 |
| Energy consumption centrifugation | kWh | 875.24 |
| Solvent recuperation | kWh | 1037.72 |
| Evaporation/Stripper | kWh | 194.13 |
| Desolventizer | kWh | 328.21 |
| Output | ||
| Bulk oil | ton | 1.51 |
| Defatted biomass production | ||
| Residual biomass | ton | 6.71 |
| Desolventizer-toaster-dryer-cooler | kWh | 922.73 |
| Defatted biomass | ton | 2.34 |
| Total electric energy requirement | kWh/year | 512,203.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Dias, R.; Deprá, M.C.; Ragagnin de Menezes, C.; Queiroz Zepka, L.; Jacob-Lopes, E. The High-Value Product, Bio-Waste, and Eco-Friendly Energy as the Tripod of the Microalgae Biorefinery: Connecting the Dots. Sustainability 2023, 15, 11494. https://doi.org/10.3390/su151511494
Rodrigues Dias R, Deprá MC, Ragagnin de Menezes C, Queiroz Zepka L, Jacob-Lopes E. The High-Value Product, Bio-Waste, and Eco-Friendly Energy as the Tripod of the Microalgae Biorefinery: Connecting the Dots. Sustainability. 2023; 15(15):11494. https://doi.org/10.3390/su151511494
Chicago/Turabian StyleRodrigues Dias, Rosangela, Mariany Costa Deprá, Cristiano Ragagnin de Menezes, Leila Queiroz Zepka, and Eduardo Jacob-Lopes. 2023. "The High-Value Product, Bio-Waste, and Eco-Friendly Energy as the Tripod of the Microalgae Biorefinery: Connecting the Dots" Sustainability 15, no. 15: 11494. https://doi.org/10.3390/su151511494
APA StyleRodrigues Dias, R., Deprá, M. C., Ragagnin de Menezes, C., Queiroz Zepka, L., & Jacob-Lopes, E. (2023). The High-Value Product, Bio-Waste, and Eco-Friendly Energy as the Tripod of the Microalgae Biorefinery: Connecting the Dots. Sustainability, 15(15), 11494. https://doi.org/10.3390/su151511494









