Essential Oil Volatiles as Sustainable Antagonists for the Root-Knot Nematode Meloidogyne ethiopica
Abstract
1. Introduction
2. Materials and Methods
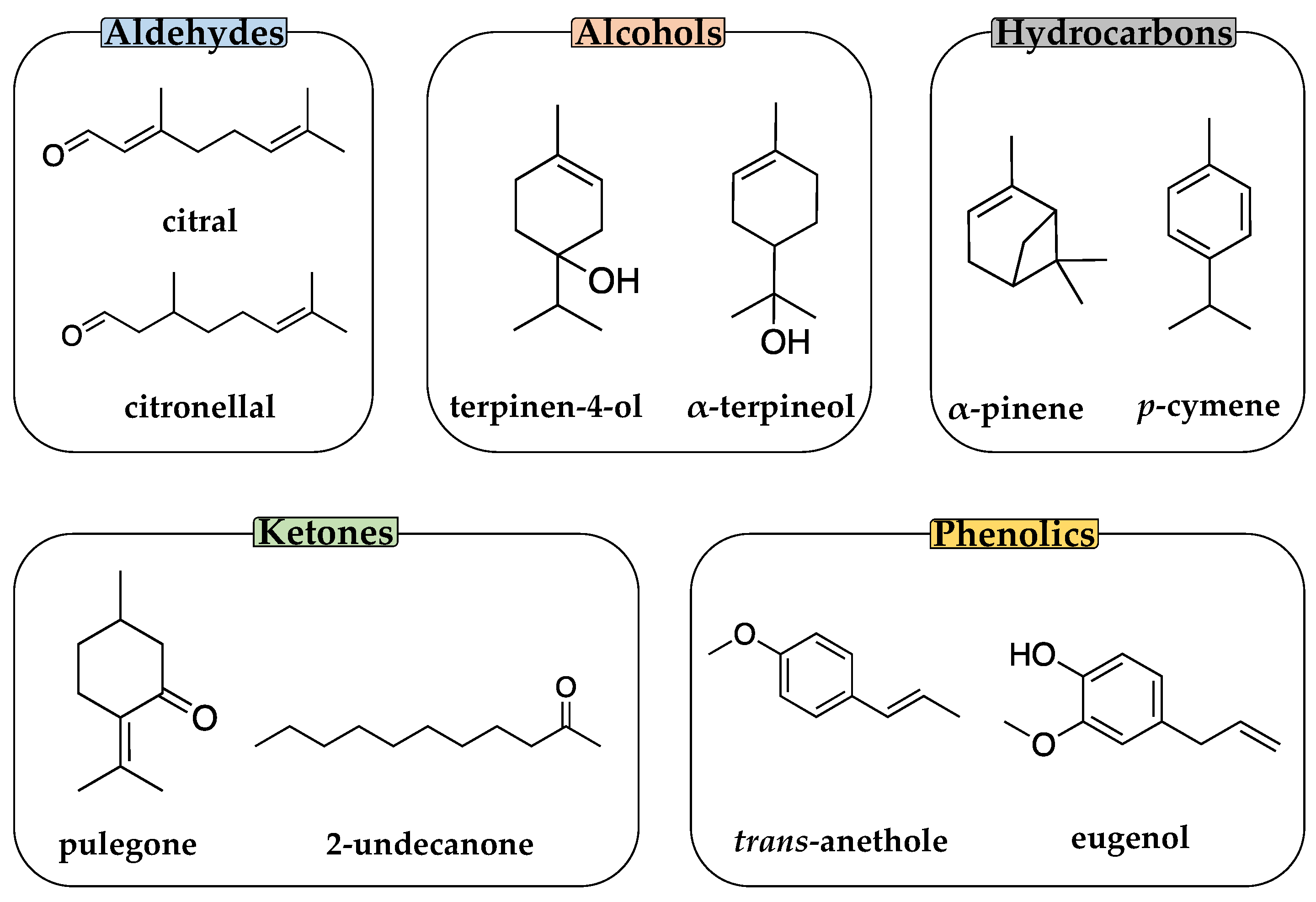
2.1. Chemicals
2.2. Meloidogyne ethiopica Inoculum
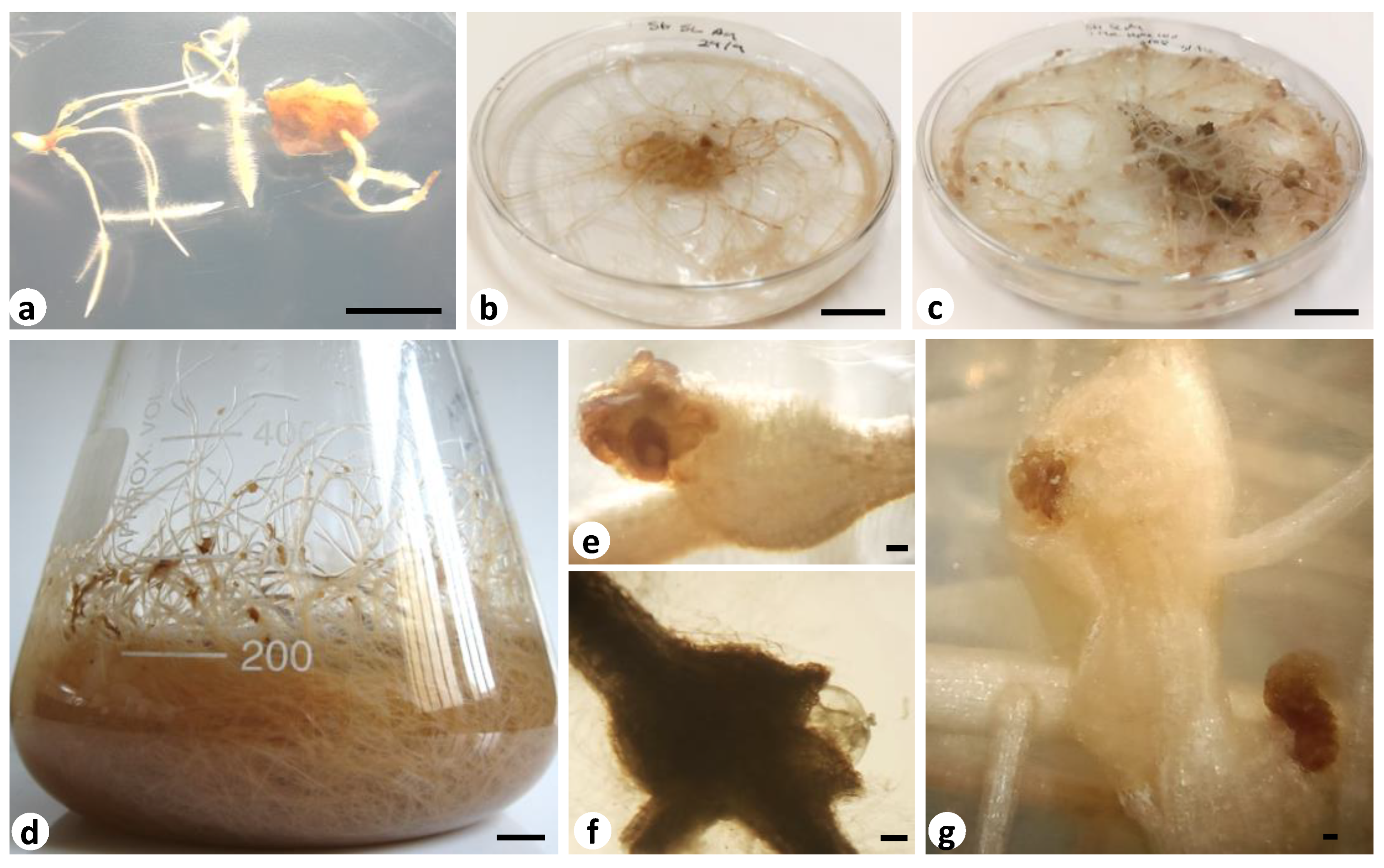
2.3. In Vitro Tomato Hairy Root Cultures
2.4. Co-Cultures of Solanum lycopersicum Hairy Roots with Meloidogyne ethiopica
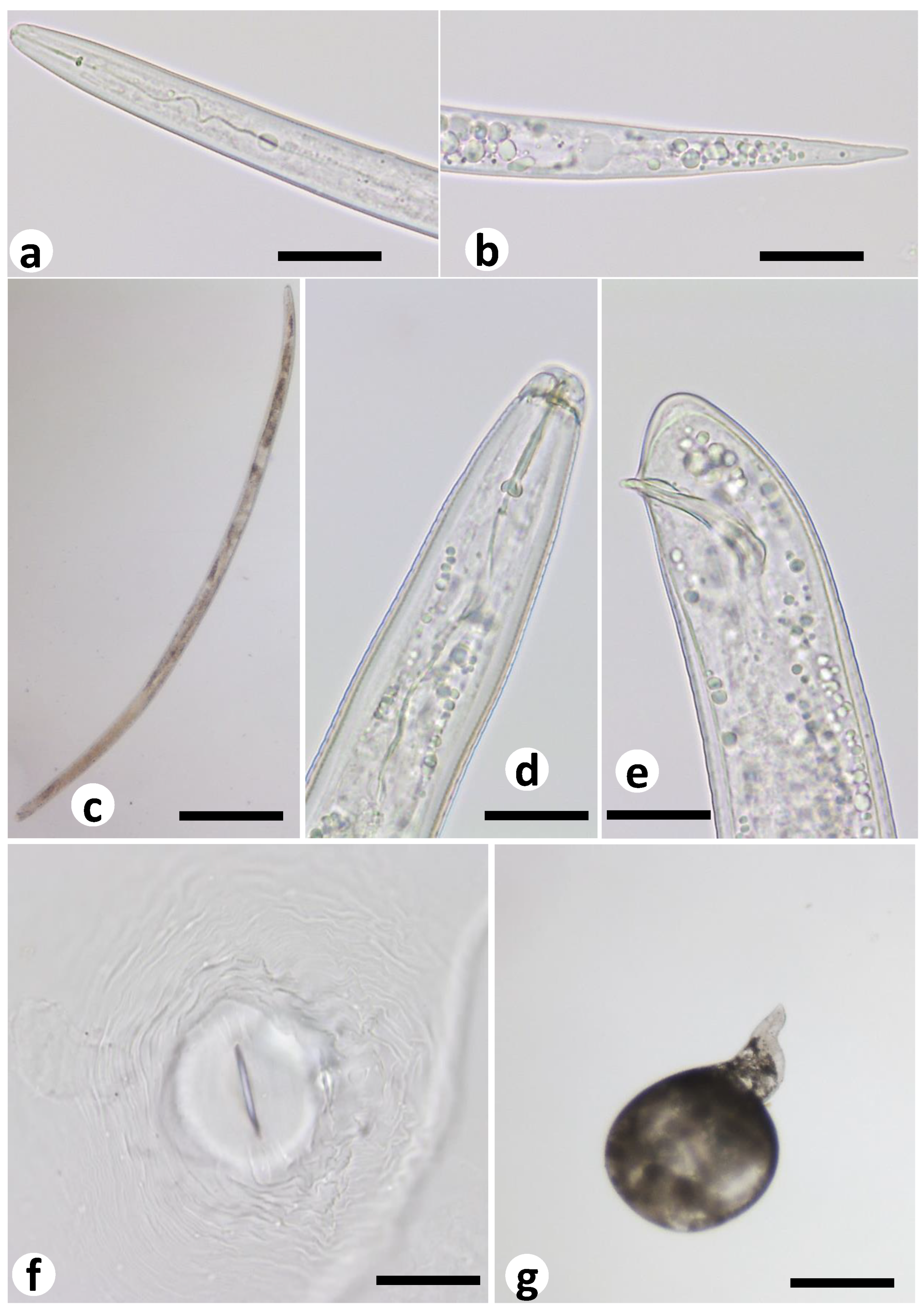
2.5. Characterization of M. ethiopica Life Stages in the Co-Culture
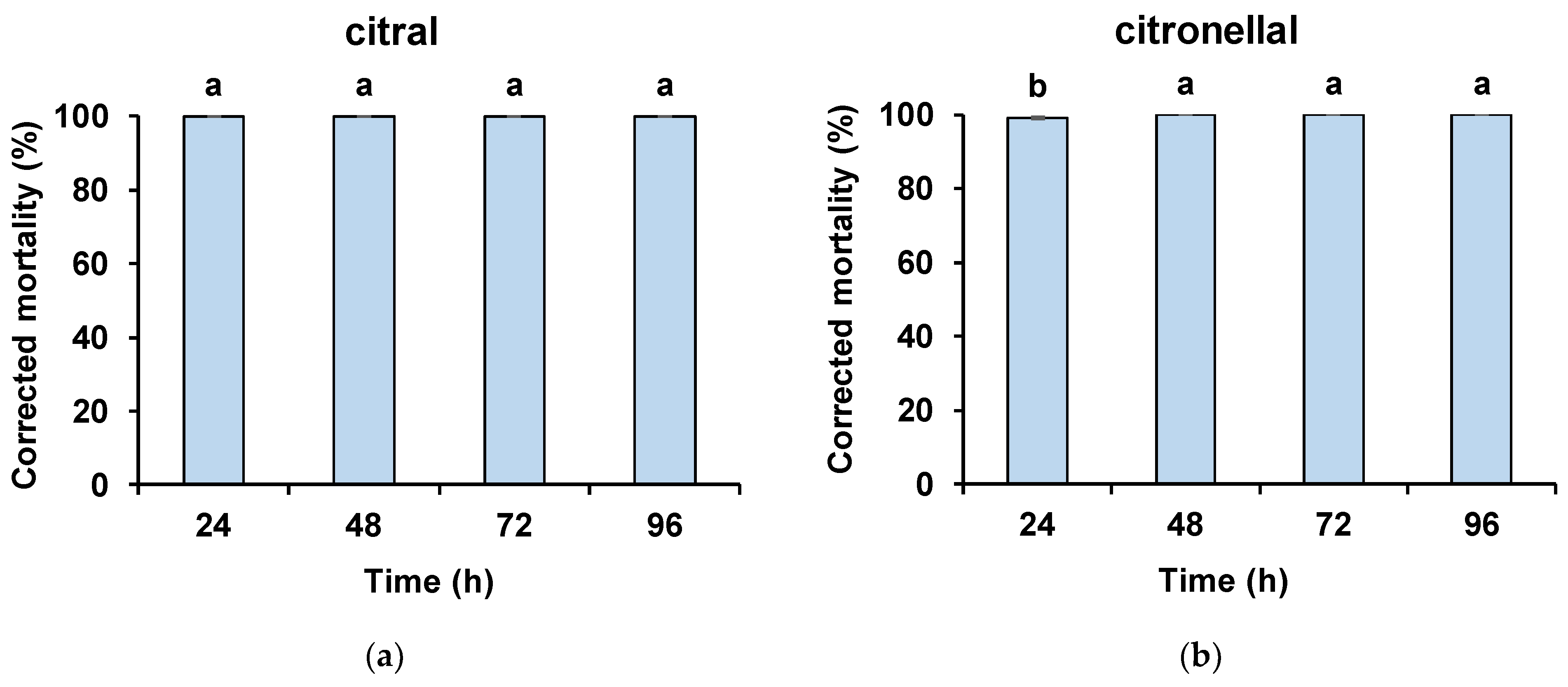
2.6. Direct Contact Bioassays with Volatiles
2.7. Predicted Environmental Fate of the Nematicidal Volatiles
2.8. Acute Toxicity Thresholds of the Nematicidal Volatiles
2.9. Data Treatment and Statistical Analysis
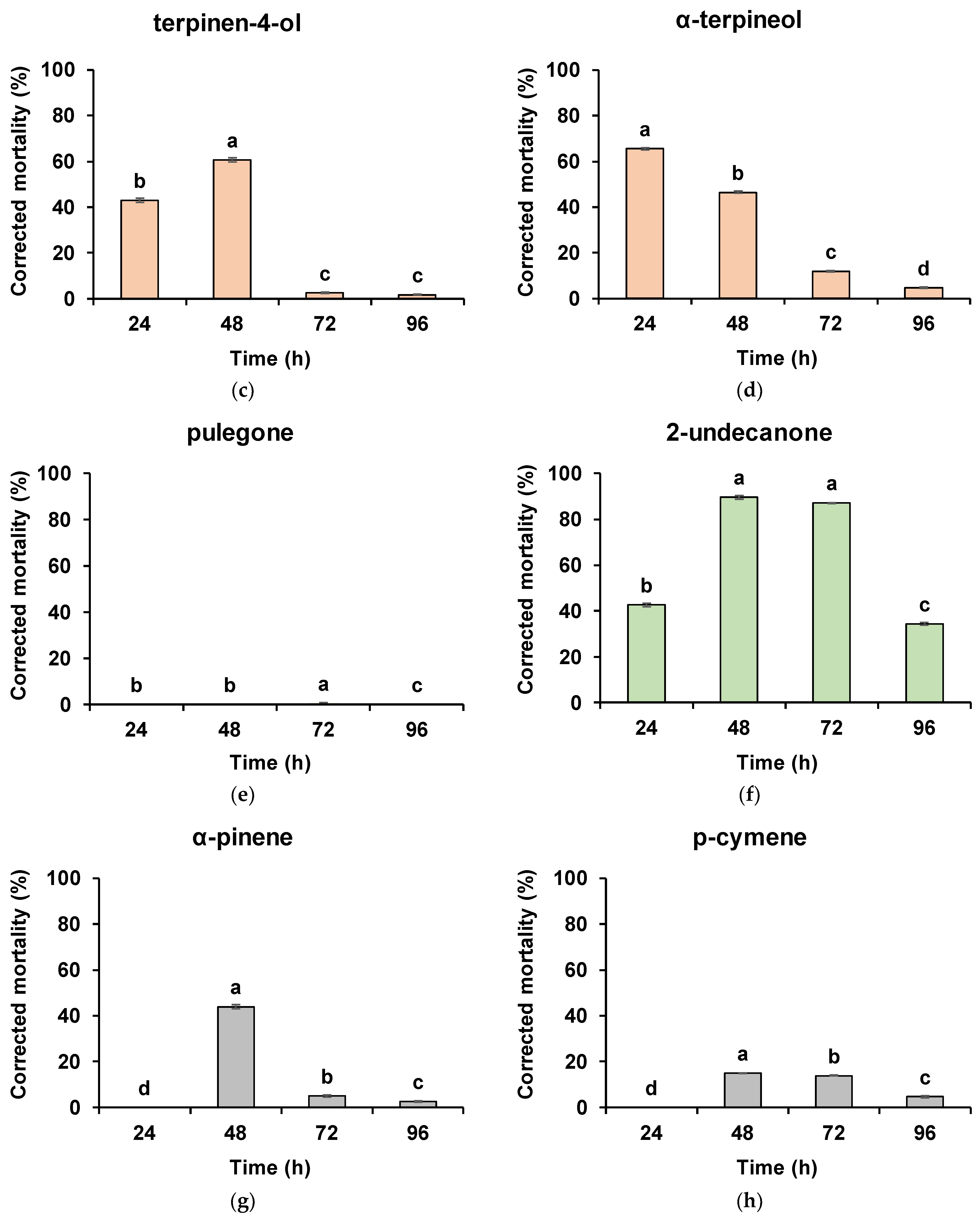
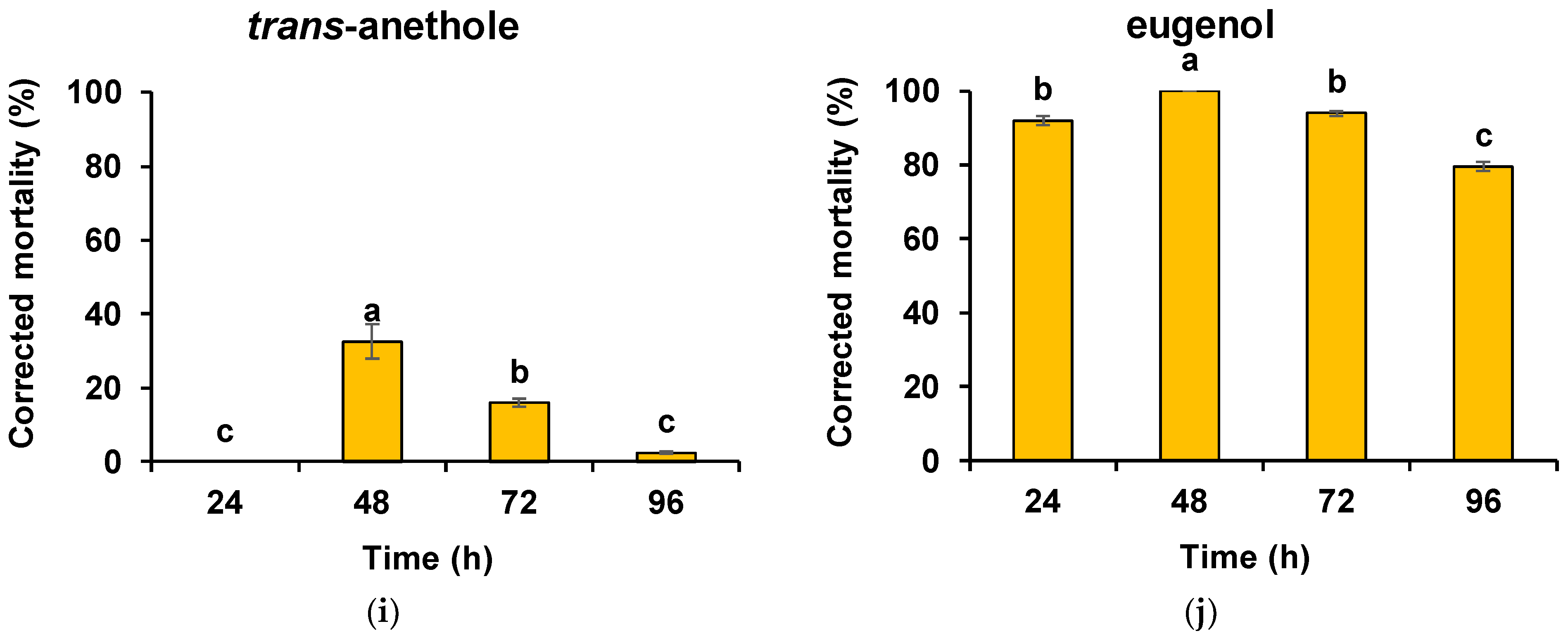
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Pavela, R. History, Presence and Perspective of Using Plant Extracts as Commercial Botanical Insecticides and Farm Products for Protection against Insects—A Review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential Oils. In Essential Oil Research; Malik, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar]
- León-Méndez, G.; Pájaro-Castro, N.; Pájaro-Castro, E.; Torrenegra-Alarcón, M.; Herrera-Barros, A. Essential Oils as a Source of Bioactive Molecules. Rev. Colomb. Cienc. Químico-Farm. 2019, 48, 80–93. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Vieira, P.; Vicente, C.S.L.; Figueiredo, A.C.; Mota, M. Phytochemicals as Biopesticides against the Pinewood Nematode Bursaphelenchus Xylophilus: A Review on Essential Oils and Their Volatiles. Plants 2021, 10, 2614. [Google Scholar] [CrossRef]
- El-Habashy, D.E.; Abdel Rasoul, M.A.; Abdelgaleil, S.A.M. Nematicidal Activity of Phytochemicals and Their Potential Use for the Control of Meloidogyne javanica Infected Eggplant in the Greenhouse. Eur. J. Plant Pathol. 2020, 158, 381–390. [Google Scholar] [CrossRef]
- Yang, T.; Xin, Y.; Liu, T.; Li, Z.; Liu, X.; Wu, Y.; Wang, M.; Xiang, M. Bacterial Volatile-Mediated Suppression of Root-Knot Nematode (Meloidogyne incognita). Plant Dis. 2022, 106, 1358–1365. [Google Scholar] [CrossRef]
- Disi, J.O.; Mohammad, H.K.; Lawrence, K.; Kloepper, J.; Fadamiro, H. A Soil Bacterium Can Shape Belowground Interactions between Maize, Herbivores and Entomopathogenic Nematodes. Plant Soil 2019, 437, 83–92. [Google Scholar] [CrossRef]
- Pires, D.; Vicente, C.S.L.; Menéndez, E.; Faria, J.M.S.; Rusinque, L.; Camacho, M.J.; Inácio, M.L. The Fight against Plant-Parasitic Nematodes: Current Status of Bacterial and Fungal Biocontrol Agents. Pathogens 2022, 11, 1178. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 Plant-Parasitic Nematodes in Molecular Plant Pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Whitehead, A.G. Taxonomy of Meloidogyne (Nematodea: Heteroderidae) with Descriptions of Four New Species. Trans. Zool. Soc. Lond. 1968, 31, 263–401. [Google Scholar] [CrossRef]
- Carneiro, R.; Randig, O.; Almeida, M.R.; Gomes, A.C. Additional Information on Meloidogyne ethiopica Whitehead, 1968 (Tylenchida: Meloidogynidae), a Root-Knot Nematode Parasitising Kiwi Fruit and Grape-Vine from Brazil and Chile. Nematology 2004, 6, 109–123. [Google Scholar] [CrossRef]
- Organization European and Mediterranean Plant Protection (EPPO) EPPO Alert List: Addition of Meloidogyne luci Together with M. ethiopica. Available online: https://gd.eppo.int/reporting/article-6186 (accessed on 29 March 2023).
- Whitehead, A.G. The Distribution of Root-Knot Nematodes (Meloidogyne spp.) in Tropical Africa. Nematologica 1969, 15, 315–333. [Google Scholar] [CrossRef]
- Carneiro, R.; Gomes, C.; Almeida, M.; Gomes, A.; Martins, I. First Record of Meloidogyne ethiopica Whitehead, 1968 on Kiwi in Brazil and Reaction of Different Plant Species. Nematol. Bras. 2003, 27, 151–159. [Google Scholar]
- Širca, S.; Urek, G.; Karssen, G. First Report of the Root-Knot Nematode Meloidogyne ethiopica on Tomato in Slovenia. Plant Dis. 2004, 88, 680. [Google Scholar] [CrossRef]
- Conceição, I.L.; Tzortzakakis, E.A.; Gomes, P.; Abrantes, I.; da Cunha, M.J. Detection of the Root-Knot Nematode Meloidogyne ethiopica in Greece. Eur. J. Plant Pathol. 2012, 134, 451–457. [Google Scholar] [CrossRef]
- Aydınlı, G.; Mennan, S.; Devran, Z.; Širca, S.; Urek, G. First Report of the Root-Knot Nematode Meloidogyne ethiopica on Tomato and Cucumber in Turkey. Plant Dis. 2013, 97, 1262. [Google Scholar] [CrossRef]
- Gerič Stare, B.; Strajnar, P.; Susič, N.; Urek, G.; Širca, S. Reported Populations of Meloidogyne ethiopica in Europe Identified as Meloidogyne luci. Plant Dis. 2017, 101, 1627–1632. [Google Scholar] [CrossRef]
- Lima, E.A.; Mattos, J.K.; Moita, A.W.; Carneiro, R.G.; Carneiro, R.M. Host Status of Different Crops for Meloidogyne ethiopica Control. Trop. Plant Pathol. 2009, 34, 152–157. [Google Scholar] [CrossRef][Green Version]
- Strajnar, P.; Širca, S.; Stare, B.G.; Urek, G. Characterization of the Root-Knot Nematode, Meloidogyne ethiopica Whitehead, 1968, from Slovenia. Russ. J. Nematol. 2009, 17, 135–142. [Google Scholar]
- Carneiro, R.M.D.G.; Almeida, M.R.A.; Cofcewicz, E.T.; Magunacelaya, J.C.; Aballay, E. Meloidogyne ethiopica, a Major Root-Knot Nematode Parasitising Vitis Vinifera and Other Crops in Chile. Nematology 2007, 9, 633–639. [Google Scholar] [CrossRef]
- Aballay, E.; Persson, P.; Mårtensson, A. Plant-Parasitic Nematodes in Chilean Vineyards. Nematropica 2009, 39, 85–97. [Google Scholar]
- Faria, J.M.S.; Sena, I.; Maleita, C.M.; Vieira da Silva, I.; Ascensão, L.; Abrantes, I.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. In Vitro Co-Culture of Solanum tuberosum Hairy Roots with Meloidogyne chitwoodi: Structure, Growth and Production of Volatiles. Plant Cell Tissue Organ Cult. 2014, 118, 519–530. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Rodrigues, A.M.; Sena, I.; Moiteiro, C.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity of Ruta Graveolens and Satureja Montana Essential Oils on Solanum tuberosum Hairy Roots and Solanum tuberosum Hairy Roots with Meloidogyne chitwoodi Co-Cultures. J. Agric. Food Chem. 2016, 64, 7452–7458. [Google Scholar] [CrossRef] [PubMed]
- Rusinque, L.; Nóbrega, F.; Cordeiro, L.; Lima, A.; Andrade, S.; Inácio, M.L. Root-Knot Nematode Species Associated with Horticultural Crops in the Island of Azores, Portugal. Horticulturae 2022, 8, 101. [Google Scholar] [CrossRef]
- Boisson-Dernier, A.; Chabaud, M.; Garcia, F.; Bécard, G.; Rosenberg, C.; Barker, D.G. Agrobacterium Rhizogenes -Transformed Roots of Medicago Truncatula for the Study of Nitrogen-Fixing and Endomycorrhizal Symbiotic Associations. Mol. Plant-Microbe Interact. 2001, 14, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Schenk, R.U.; Hildebrandt, A.C. Medium and Techniques for Induction and Growth of Monocotyledonous and Dicotyledonous Plant Cell Cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Sena, I.; Ribeiro, B.; Rodrigues, A.M.; Maleita, C.M.N.; Abrantes, I.; Bennett, R.; Mota, M.; Figueiredo, A.C. da S. First Report on Meloidogyne chitwoodi Hatching Inhibition Activity of Essential Oils and Essential Oils Fractions. J. Pest Sci. 2016, 89, 207–217. [Google Scholar] [CrossRef]
- Bybd Jr, D.W.; Kirkpatrick, T.; Barker, K.R. An Improved Technique for Clearing and Staining Plant Tissues for Detection of Nematodes. J. Nematol. 1983, 15, 142. [Google Scholar]
- Mackay, D.; Di Guardo, A.; Paterson, S.; Cowan, C.E. Evaluating the Environmental Fate of a Variety of Types of Chemicals Using the EQC Model. Environ. Toxicol. Chem. 1996, 15, 1627–1637. [Google Scholar] [CrossRef]
- Trent University Level I Model. Available online: https://www.trentu.ca/cemc/resources-and-models/level-i-model (accessed on 29 March 2023).
- National Library of Medicine PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 29 March 2023).
- University of Hertfordshire PPDB: Pesticide Properties DataBase. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/ (accessed on 29 March 2023).
- ECHA ECHA European Chemicals Agency. Available online: https://echa.europa.eu/pt/home (accessed on 29 March 2023).
- Faria, J.M.S.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus Xylophilus: Nematotoxics from Essential Oils, Essential Oils Fractions and Decoction Waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef]
- Kong, J.O.; Lee, S.M.; Moon, Y.S.; Lee, S.G.; Ahn, Y.J. Nematicidal Activity of Plant Essential Oils against Bursaphelenchus Xylophilus (Nematoda: Aphelenchoididae). J. Asia. Pac. Entomol. 2006, 9, 173–178. [Google Scholar] [CrossRef]
- Sanadhya, P.; Kumar, A.; Bucki, P.; Fitoussi, N.; Carmeli-Weissberg, M.; Borenstein, M.; Brown-Miyara, S. Tomato Divinyl Ether-Biosynthesis Pathway Is Implicated in Modulating of Root-Knot Nematode Meloidogyne javanica’s Parasitic Ability. Front. Plant Sci. 2021, 12, 670772. [Google Scholar] [CrossRef] [PubMed]
- Bosselut, N.; Van Ghelder, C.; Claverie, M.; Voisin, R.; Onesto, J.-P.; Rosso, M.-N.; Esmenjaud, D. Agrobacterium Rhizogenes-Mediated Transformation of Prunus as an Alternative for Gene Functional Analysis in Hairy-Roots and Composite Plants. Plant Cell Rep. 2011, 30, 1313–1326. [Google Scholar] [CrossRef]
- Guimaraes, L.A.; Pereira, B.M.; Araujo, A.C.G.; Guimaraes, P.M.; Brasileiro, A.C.M. Ex Vitro Hairy Root Induction in Detached Peanut Leaves for Plant–Nematode Interaction Studies. Plant Methods 2017, 13, 25. [Google Scholar] [CrossRef]
- Xu, C.-L.; Jiao, C.-W.; Yu, L.; Xie, H.; Wang, D.-W.; Li, Y.; Cheng, X. Establishment of New Monoxenic Culture Systems for Root-Knot Nematodes, Meloidogyne spp., on Axenic Water Spinach Roots. Nematology 2015, 17, 725–732. [Google Scholar] [CrossRef]
- Rechenmacher, C.; Wiebke-Strohm, B.; de Oliveira-Busatto, L.A.; Weber, R.L.M.; Corso, M.C.M.; Lopes-Caitar, V.S.; Silva, S.M.H.; Dias, W.P.; Marcelino-Guimarães, F.C.; Carlini, C.R.; et al. Endogenous Soybean Peptide Overexpression: An Alternative to Protect Plants against Root-Knot Nematodes. Biotechnol. Res. Innov. 2019, 3, 10–18. [Google Scholar] [CrossRef]
- Ohara, A.; Daimon, H.; Momota, Y.; Chin, D.P.; Mii, M. Plant Regeneration from Crotalaria Spectabilis Hairy Roots Which Showed Inhibited Growth of Root-Knot Nematodes (Meloidogyne hapla and M. incognita) in vitro. Plant Biotechnol. 2012, 29, 425–430. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Li, X.; Song, M.; Ma, S.; Tian, Y.; Gao, L. Peat-Based Hairy Root Transformation Using Rhizobium Rhizogenes as a Rapid and Efficient Tool for Easily Exploring Potential Genes Related to Root-Knot Nematode Parasitism and Host Response. Plant Methods 2023, 19, 22. [Google Scholar] [CrossRef]
- Wubben, M.J.; Callahan, F.E.; Triplett, B.A.; Jenkins, J.N. Phenotypic and Molecular Evaluation of Cotton Hairy Roots as a Model System for Studying Nematode Resistance. Plant Cell Rep. 2009, 28, 1399–1409. [Google Scholar] [CrossRef]
- Pak, H.-K.; Sim, J.-S.; Rhee, Y.; Ko, H.-R.; Ha, S.-H.; Yoon, M.-S.; Kang, C.-H.; Lee, S.; Kim, Y.-H.; Hahn, B.-S. Hairy Root Induction in Oriental Melon (Cucumis melo) by Agrobacterium Rhizogenes and Reproduction of the Root-Knot Nematode (Meloidogyne incognita). Plant Cell Tissue Organ Cult. 2009, 98, 219–228. [Google Scholar] [CrossRef]
- Verdejo, S.; Jaffee, B.A.; Mankau, R. Reproduction of Meloidogyne javanica on Plant Roots Genetically Transformed by Agrobacterium Rhizogenes. J. Nematol. 1988, 20, 599–604. [Google Scholar] [PubMed]
- de Freitas Silva, M.; Paulo Campos, V.; Barros, A.F.; Pereira da Silva, J.C.; Pedroso, M.P.; Silva, F.d.J.; Gomes, V.A.; Justino, J.C. Medicinal Plant Volatiles Applied against the Root-Knot Nematode Meloidogyne incognita. Crop Prot. 2020, 130, 105057. [Google Scholar] [CrossRef]
- Kundu, A.; Dutta, A.; Mandal, A.; Negi, L.; Malik, M.; Puramchatwad, R.; Antil, J.; Singh, A.; Rao, U.; Saha, S.; et al. A Comprehensive in Vitro and in Silico Analysis of Nematicidal Action of Essential Oils. Front. Plant Sci. 2021, 11, 614143. [Google Scholar] [CrossRef] [PubMed]
- Echeverrigaray, S.; Zacaria, J.; Beltrão, R. Nematicidal Activity of Monoterpenoids against the Root-Knot Nematode Meloidogyne incognita. Phytopathology 2010, 100, 199–203. [Google Scholar] [CrossRef]
- Mukherjee, A.; SinhaBabu, S.P. Potential of Citral and Menthol for Suppression of Meloidogyne incognita Infection of Okra Plants. J. Essent. Oil Bear. Plants 2014, 17, 359–365. [Google Scholar] [CrossRef]
- Kokalis-Burelle, N.; Martinez-Ochoa, N.; Rodríguez-Kábana, R.; Kloepper, J. Development of Multi-Component Transplant Mixes for Suppression of Meloidogyne incognita on Tomato (Lycopersicon esculentum). J. Nematol. 2002, 34, 362–369. [Google Scholar] [PubMed]
- Ajith, M.; Pankaj; Shakil, N.A.; Kaushik, P.; Rana, V.S. Chemical Composition and Nematicidal Activity of Essential Oils and Their Major Compounds against Meloidogyne graminicola (Rice Root-Knot Nematode). J. Essent. Oil Res. 2020, 32, 526–535. [Google Scholar] [CrossRef]
- Chahal, K.K.; Singh, B.; Kataria, D.; Dhillon, N.K. Nematotoxicity of Lemongrass Oil, Citral and Its Derivatives against Meloidogyne incognita. Allelopath. J. 2016, 39, 217–230. [Google Scholar]
- Barros, A.F.; Campos, V.P.; de Paula, L.L.; de Oliveira, D.F.; Silva, G.H. Synergism of Citral from Cymbopogon Citratus Essential Oil and Undecan-2-One against Meloidogyne incognita. Nematology 2020, 22, 1101–1110. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Manconi, F.; Leonti, M.; Maxia, A.; Caboni, P. Aliphatic Ketones from Ruta chalepensis (Rutaceae) Induce Paralysis on Root Knot Nematodes. J. Agric. Food Chem. 2011, 59, 7098–7103. [Google Scholar] [CrossRef]
- Zhai, Y.; Shao, Z.; Cai, M.; Zheng, L.; Li, G.; Huang, D.; Cheng, W.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Multiple Modes of Nematode Control by Volatiles of Pseudomonas putida 1A00316 from Antarctic Soil against Meloidogyne incognita. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Diyapoglu, A.; Chang, T.-H.; Chang, P.-F.L.; Yen, J.-H.; Chiang, H.-I.; Meng, M. Fumigant Activity of Bacterial Volatile Organic Compounds against the Nematodes Caenorhabditis elegans and Meloidogyne incognita. Molecules 2022, 27, 4714. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Argentieri, M.P.; Laquale, S.; Candido, V.; Avato, P. Relationship between Chemical Composition and Nematicidal Activity of Different Essential Oils. Plants 2020, 9, 1546. [Google Scholar] [CrossRef] [PubMed]
- Sobkowiak, R.; Bojarska, N.; Krzyżaniak, E.; Wągiel, K.; Ntalli, N. Chemoreception of Botanical Nematicides by Meloidogyne incognita and Caenorhabditis elegans. J. Environ. Sci. Health Part B 2018, 53, 493–502. [Google Scholar] [CrossRef]
- Ntalli, N.; Ratajczak, M.; Oplos, C.; Menkissoglu-Spiroudi, U.; Adamski, Z. Acetic Acid, 2-Undecanone, and (E)-2-Decenal Ultrastructural Malformations on Meloidogyne incognita. J. Nematol. 2016, 48, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Nasiou, E.; Giannakou, I.O. The Potential of Eugenol as a Nematicidal Agent against Meloidogyne javanica (Treub) Chitwood. J. Nematol. 2020, 52, 1–10. [Google Scholar] [CrossRef]
- Li, H.; Liu, Q.; Liu, Z.; Du, S.; Deng, Z. Chemical Composition and Nematicidal Activity of Essential Oil of Agastache rugosa against Meloidogyne incognita. Molecules 2013, 18, 4170–4180. [Google Scholar] [CrossRef]
- Walker, J.T.; Melin, J.B. Mentha × piperita, Mentha spicata and Effects of Their Essential Oils on Meloidogyne in Soils. J. Nematol. 1996, 28, 629–635. [Google Scholar]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and Antagonistic Interactions of Terpenes against Meloidogyne incognita and the Nematicidal Activity of Essential Oils from Seven Plants Indigenous to Greece. Pest Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef]
| Volatiles | Nematicides | ||||
|---|---|---|---|---|---|
| Citronellal | Citral | Oxamyl | Metham Sodium | Fluopyram | |
| CAS number | 106-23-0 | 5392-40-5 | 23135-22-0 | 137-42-8 | 658066-35-4 |
| Molecular mass (g/mol) | 154.25 | 152.23 | 219.26 | 129.19 | 396.76 |
| Melting point (°C) | −16.0 | −10.0 | 98.5 | 88.5 | 117.5 |
| Vapor pressure (Pa) | 33.331 | 12.172 | 1.800 × 10−5 | 0.058 | 1.200 × 10−6 |
| Solubility in H2O (mg/L) | 70.2 | 1340.0 | 148,100.0 | 578,290.0 | 16.0 |
| Henry’s law constant (Pa.m3/mol) | 73.235 | 1.383 | 2.670 × 10−7 | 1.280 × 10−5 | 2.980 × 10−5 |
| logKOW (unitless) | 3.53 | 2.76 | −0.44 | −2.91 | 3.30 |
| KOC (unitless) | 650 | 83 | 15 | 18 | 279 |
| J2 | Males | |||
|---|---|---|---|---|
| In Vitro | In Vivo | In Vitro | In Vivo | |
| Body length (µm) | 412.6 ± 10.5 (372.1–461.6) | 468.0 ± 3.0 (326.0–510.0) | 1849.3 ± 130.8 (1172.4–2313.9) | 1171.0 ± 3.0 (890.0–1500.0) |
| a 1 | 24.4 ± 0.3 (22.8–26.4) | 24.0 ± 0.3 (21.3–28.2) | 42.4 ± 1.2 (39.2–52.7) | 27.7 ± 0.8 (24.8–31.0) |
| c 2 | 7.7 ± 0.4 (5.8–9.3) | 4.8 ± 0.1 (3.9–6.4) | 155.9 ± 10.3 (99.9–184.7) | 114.0 ± 12.2 (69.5–147.2) |
| Greatest body diam. (µm) | 16.9 ± 0.5 (14.1–19.0) | 20.0 ± 0.3 (15.0–22.0) | 42.9 ± 2.0 (30.2–52.7) | 48.0 ± 0.8 (32.0–59.0) |
| Tail length (µm) | 54.1 ± 2.1 (43.9–64.1) | 62.0 ± 0.6 (52.0–72.0) | 11.7 ± 0.3 (9.4–12.8) | 13.4 ± 0.5 (10.2–17.0) |
| Stylet length (µm) | 14.9 ± 0.2 (14.0–15.5) | 12.2 ± 0.1 (11.0–14.0) | 25.3 ± 0.4 (22.7–26.9) | 24.8 ± 0.6 (23.0–27.0) |
| DGO 3 (µm) | 2.8 ± 0.1 (2.1–3.3) | 2.6 ± 0.1 (2.0–3.0) | 4.3 ± 0.1 (3.8–4.9) | 2.5 ± 0.1 (2.0–3.5) |
| Hyaline tail terminus (µm) | 10.8 ± 0.6 (7.3–12.6) | 13.5 ± 0.2 (12.0–15.0) | - | - |
| Volatiles | Nematicides | ||||
|---|---|---|---|---|---|
| Citronellal | Citral 1 | Oxamyl | Metham Sodium | Fluopyram | |
| PED (%) | |||||
| Air | 85.84 | 19.07 | 5.20 × 10−6 | 2.49 × 10−4 | 3.71 × 10−4 |
| Sediments | 0.18 | 0.27 | 0.07 | 0.08 | 0.83 |
| Soil | 8.16 | 12.26 | 3.14 | 3.74 | 37.28 |
| Water | 5.81 | 68.38 | 96.79 | 96.17 | 61.86 |
| Ecotoxicology (mg/L) | |||||
| Fish | 22.00 2 | 6.10 3 | 3.13 3 | >0.18 4 | >0.98 5 |
| Daphnia 6 | 8.68 | 10.00 | 0.32 | 0.99 | >100.00 |
| Algae | 13.33 7 | 5.00 7 | 0.93 8 | 1.08 8 | >1.13 9 |
| Toxicology (mg/kg) | |||||
| Oral 10 | >5000 | 6800 | 2.5 | 896 | >2000 |
| Dermal 10 | >2500 | >1000 | 5000 | 2000 | 2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S.; Rusinque, L.; Cavaco, T.; Nunes, J.C.; Inácio, M.L. Essential Oil Volatiles as Sustainable Antagonists for the Root-Knot Nematode Meloidogyne ethiopica. Sustainability 2023, 15, 11421. https://doi.org/10.3390/su151411421
Faria JMS, Rusinque L, Cavaco T, Nunes JC, Inácio ML. Essential Oil Volatiles as Sustainable Antagonists for the Root-Knot Nematode Meloidogyne ethiopica. Sustainability. 2023; 15(14):11421. https://doi.org/10.3390/su151411421
Chicago/Turabian StyleFaria, Jorge M. S., Leidy Rusinque, Tomás Cavaco, João C. Nunes, and Maria L. Inácio. 2023. "Essential Oil Volatiles as Sustainable Antagonists for the Root-Knot Nematode Meloidogyne ethiopica" Sustainability 15, no. 14: 11421. https://doi.org/10.3390/su151411421
APA StyleFaria, J. M. S., Rusinque, L., Cavaco, T., Nunes, J. C., & Inácio, M. L. (2023). Essential Oil Volatiles as Sustainable Antagonists for the Root-Knot Nematode Meloidogyne ethiopica. Sustainability, 15(14), 11421. https://doi.org/10.3390/su151411421









