Increasing Output Power of a Microfluidic Fuel Cell Using Fuzzy Modeling and Jellyfish Search Optimization
Abstract
1. Introduction
- A reliable fuzzy model is constructed to model the microfluidic FC.
- A new application of the jellyfish search optimizer is finding the optimal operating parameters of microfluidic FC operated with environmental glycerol.
- The power density of microfluidic FC is increased.
- The results are compared with those obtained by Ref. [21].
2. Methodology
2.1. Dataset
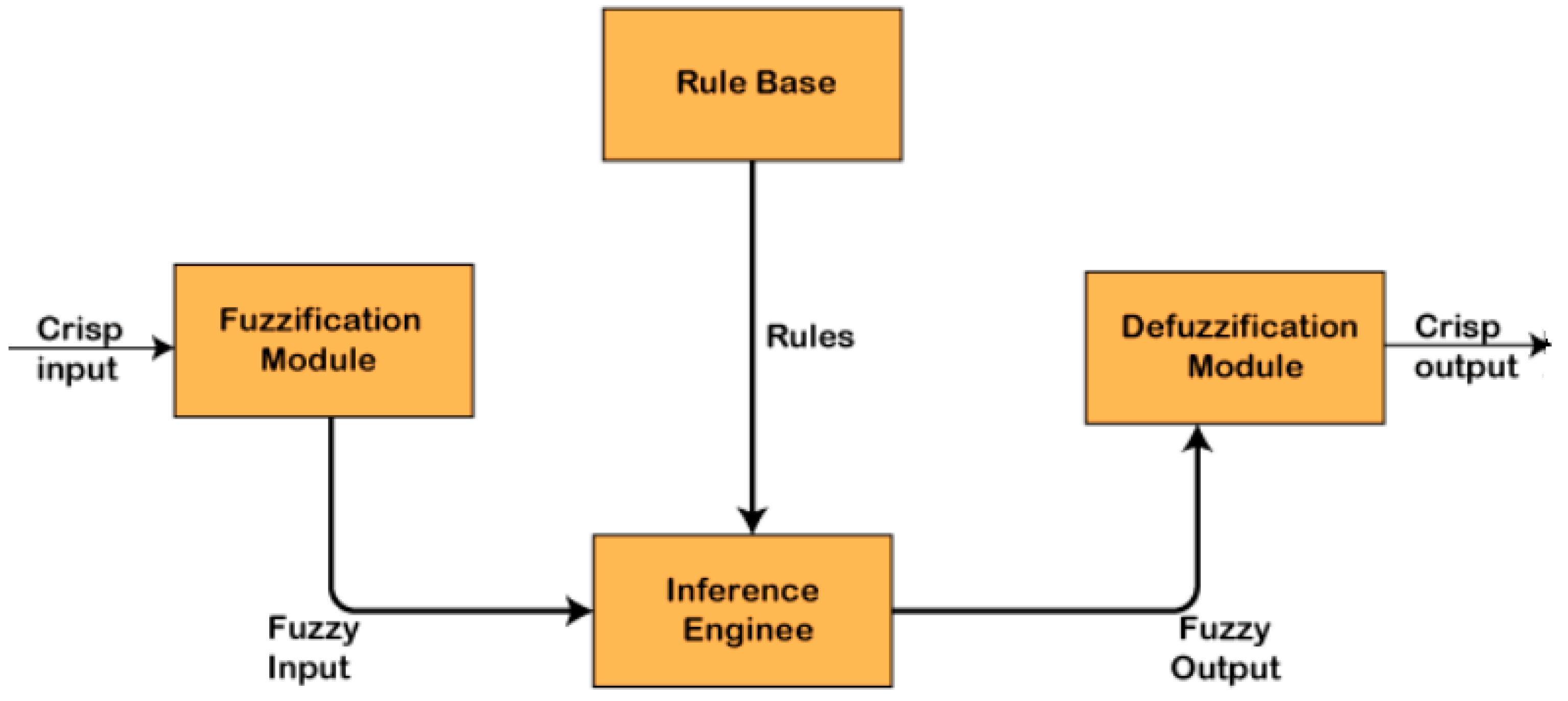
2.2. Fuzzy Model of MFC
2.3. Jellyfish Search Optimizer
3. Results and Discussion
Parameter Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olabi, A.G.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 2022, 153, 111710. [Google Scholar] [CrossRef]
- Raza, S.; Zhang, J.; Ali, I.; Li, X.; Liu, C. Recent trends in the development of biomass-based polymers from renewable resources and their environmental applications. J. Taiwan Inst. Chem. Eng. 2020, 115, 293–303. [Google Scholar] [CrossRef]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste heat recovery technologies and applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Jouhara, H.; Almahmoud, S.; Chauhan, A.; Delpech, B.; Bianchi, G.; Tassou, S.A.; Llera, R.; Lago, F.; Arribas, J.J. Experimental and theoretical investigation of a flat heat pipe heat exchanger for waste heat recovery in the steel industry. Energy 2017, 141, 1928–1939. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; He, W.; Zhao, Y.; Wang, X. Lattice Boltzmann simulation of the structural degradation of a gas diffusion layer for a proton exchange membrane fuel cell. J. Power Sources 2023, 556, 232452. [Google Scholar] [CrossRef]
- Bahaa, A.; Abdelkareem, M.A.; Al Naqbi, H.; Mohamed, A.Y.; Shinde, P.A.; Yousef, B.A.A.; Sayed, E.T.; Alawadhi, H.; Chae, K.-J.; Al-Asheh, S.; et al. High energy storage quasi-solid-state supercapacitor enabled by metal chalcogenide nanowires and iron-based nitrogen-doped graphene nanostructures. J. Colloid Interface Sci. 2022, 608, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-J.; Zhang, Q.; Li, Y.-N.; Chen, Y.; Yang, L.; He, H.-Y.; Xu, X.-T.; Huang, H.-J. Nanosized Rh grown on single-walled carbon nanohorns for efficient methanol oxidation reaction. Rare Met. 2022, 41, 2108–2117. [Google Scholar] [CrossRef]
- Abdallah, M.; Feroz, S.; Alani, S.; Sayed, E.T.; Shanableh, A. Continuous and scalable applications of microbial fuel cells: A critical review. Rev. Environ. Sci. Bio/Technol. 2019, 18, 543–578. [Google Scholar] [CrossRef]
- Shen, L.-L.; Zhang, G.-R.; Venter, T.; Biesalski, M.; Etzold, B.J. Towards best practices for improving paper-based microfluidic fuel cells. Electrochim. Acta 2019, 298, 389–399. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, B.; Zhu, X.; Ye, D.-D.; Chen, R.; Zhang, T.; Gong, X.-L.; Liao, Q. Enhancing fuel transport in air-breathing microfluidic fuel cells by immersed fuel micro-jet. J. Power Sources 2020, 445, 227326. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.; Kwok, H.Y.; Pan, W.; Zhang, Y.; Zhao, X.; Leung, D.Y. Microfluidic fuel cells with different types of fuels: A prospective review. Renew. Sustain. Energy Rev. 2021, 141, 110806. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zhang, Z.; Li, H.; Wang, X. Lattice Boltzmann simulation of a gas diffusion layer with a gradient polytetrafluoroethylene distribution for a proton exchange membrane fuel cell. Appl. Energy 2022, 320, 119248. [Google Scholar] [CrossRef]
- Ardi, M.; Aroua, M.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation: A review. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Schouten, K.J.P.; Koper, M.T. Mechanism of the catalytic oxidation of glycerol on polycrystalline gold and platinum electrodes. ChemCatChem 2011, 3, 1176–1185. [Google Scholar] [CrossRef]
- Erable, B.; Oliot, M.; Lacroix, R.; Bergel, A.; Serov, A.; Kodali, M.; Santoro, C.; Atanassov, P. Iron-Nicarbazin derived platinum group metal-free electrocatalyst in scalable-size air-breathing cathodes for microbial fuel cells. Electrochim. Acta 2018, 277, 127–135. [Google Scholar] [CrossRef]
- Ficca, V.C.; Santoro, C.; Placidi, E.; Arciprete, F.; Serov, A.; Atanassov, P.; Mecheri, B. Exchange current density as an effective descriptor of poisoning of active sites in platinum group metal-free electrocatalysts for oxygen reduction reaction. ACS Catal. 2023, 13, 2162–2175. [Google Scholar] [CrossRef]
- Dector, A.; Cuevas-Muñiz, F.; Guerra-Balcázar, M.; Godínez, L.A.; Ledesma-García, J.; Arriaga, L. Glycerol oxidation in a microfluidic fuel cell using Pd/C and Pd/MWCNT anodes electrodes. Int. J. Hydrogen Energy 2013, 38, 12617–12622. [Google Scholar] [CrossRef]
- Panjiara, D.; Pramanik, H. Synthesis of Pd and Pt Based Low Cost Bimetallic Anode Electrocatalyst for Glycerol Electrooxidation in Membraneless Air Breathing Microfluidic Fuel Cell. J. Electrochem. Sci. Technol. 2021, 12, 38–57. [Google Scholar] [CrossRef]
- Maya-Cornejo, J.; Guerra-Balcázar, M.; Arjona, N.; Álvarez-Contreras, L.; Valadez, F.J.R.; Gurrola, M.; Ledesma-García, J.; Arriaga, L. Electrooxidation of crude glycerol as waste from biodiesel in a nanofluidic fuel cell using Cu@ Pd/C and Cu@ Pt/C. Fuel 2016, 183, 195–205. [Google Scholar] [CrossRef]
- Panjiara, D.; Pramanik, H. Optimization of process parameters using response surface methodology (RSM) for power generation via electrooxidation of glycerol in T-Shaped air breathing microfluidic fuel cell (MFC). Int. J. Hydrogen Energy 2020, 45, 33968–33979. [Google Scholar] [CrossRef]
- Abrego-Martínez, J.; Moreno-Zuria, A.; Wang, Y.; Cuevas-Muñiz, F.; Arriaga, L.; Sun, S.; Mohamedi, M. Fabrication and evaluation of passive alkaline membraneless microfluidic DMFC. Int. J. Hydrogen Energy 2017, 42, 21969–21975. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, R.; Chang, H.; Wang, H.; Yu, Y. Machine Learning to approximate free-surface Green’s function and its application in wave-body interactions. Eng. Anal. Bound. Elem. 2022, 134, 35–48. [Google Scholar] [CrossRef]
- Li, Y.; Mao, W.; Wang, G.; Liu, J.; Wang, S. A general-purpose machine learning framework for predicting singular integrals in boundary element method. Eng. Anal. Bound. Elem. 2020, 117, 41–56. [Google Scholar] [CrossRef]
- Abidou, D.; Yusoff, N.; Nazri, N.; Awang, M.O.; Hassan, M.A.; Sarhan, A.A. Numerical simulation of metal removal in laser drilling using radial point interpolation method. Eng. Anal. Bound. Elem. 2017, 77, 89–96. [Google Scholar] [CrossRef]
- Abdellatief, T.M.M.; Ershov, M.A.; Kapustin, V.M.; Chernysheva, E.A.; Savelenko, V.D.; Salameh, T.; Abdelkareem, M.A.; Olabi, A.G. Novel promising octane hyperboosting using isoolefinic gasoline additives and its application on fuzzy modelling. Int. J. Hydrogen Energy 2022, 47, 4932–4942. [Google Scholar] [CrossRef]
- Rathoure, A.K.; Pramanik, H. Electrooxidation study of methanol using H2O2 and air as mixed oxidant at cathode in air breathing microfluidic fuel cell. Int. J. Hydrogen Energy 2016, 41, 15287–15294. [Google Scholar] [CrossRef]
- Bensaber, B.A.; Diaz, C.G.P.; Lahrouni, Y. Design and modeling an Adaptive Neuro-Fuzzy Inference System (ANFIS) for the prediction of a security index in VANET. J. Comput. Sci. 2020, 47, 101234. [Google Scholar] [CrossRef]
- Chou, J.-S.; Asmare, M. Recent advances in use of bio-inspired jellyfish search algorithm for solving optimization problems. Sci. Rep. 2022, 12, 19157. [Google Scholar] [CrossRef]
- Chou, J.-S.; Truong, D.-N. A novel metaheuristic optimizer inspired by behavior of jellyfish in ocean. Appl. Math. Comput. 2021, 389, 125535. [Google Scholar] [CrossRef]
- Antolini, E. Glycerol electro-oxidation in alkaline media and alkaline direct glycerol fuel cells. Catalysts 2019, 9, 980. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Xu, H.-B.; Fen, G.; Zheng, S.-L.; Zhang, Y. Density and viscosity of aqueous solution of K2CrO4/KOH mixed electrolytes. Trans. Nonferrous Met. Soc. China 2010, 20, s32–s36. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Sayed, E.T.; Alawadhi, H.; Alami, A.H. Synthesis and testing of cobalt leaf-like nanomaterials as an active catalyst for ethanol oxidation. Int. J. Hydrogen Energy 2020, 45, 17311–17319. [Google Scholar] [CrossRef]








| Run | Catalyst Load at Anode/mg cm−2 | Glycerol Conc./M | Conc. Anodic Electrolyte/M | Conc. Cathodic Electrolyte/M | Power Output/mW cm−2 |
|---|---|---|---|---|---|
| 1 | 0.5 | 0.5 | 1.5 | 0.5 | 1 |
| 2 | 0.5 | 1 | 2 | 0.5 | 1.1 |
| 3 | 0.5 | 1 | 1.5 | 0.25 | 2.2 |
| 4 | 0.5 | 1 | 1.5 | 0.75 | 1.1 |
| 5 | 0.5 | 1 | 1 | 0.5 | 0.95 |
| 6 | 0.5 | 1.5 | 1.5 | 0.5 | 0.96 |
| 7 | 1 | 0.5 | 1.5 | 0.25 | 1.95 |
| 8 | 1 | 0.5 | 1 | 0.5 | 1.48 |
| 9 | 1 | 0.5 | 2 | 0.5 | 1.65 |
| 10 | 1 | 1 | 1 | 0.75 | 1.9 |
| 11 | 1 | 1 | 2 | 0.25 | 2.44 |
| 12 | 1 | 1 | 1.5 | 0.5 | 2.65 |
| 13 | 1 | 1 | 1.5 | 0.5 | 2.6 |
| 14 | 1 | 1 | 2 | 0.75 | 2.43 |
| 15 | 1 | 1 | 1.5 | 0.5 | 2.77 |
| 16 | 1 | 1 | 1.5 | 0.5 | 2.77 |
| 17 | 1 | 1 | 1 | 0.25 | 2.1 |
| 18 | 1 | 1 | 1.5 | 0.5 | 2.77 |
| 19 | 1 | 1.5 | 2 | 0.5 | 2.1 |
| 20 | 1 | 1.5 | 1.5 | 0.75 | 2.32 |
| 21 | 1 | 1.5 | 1 | 0.5 | 1.4 |
| 22 | 1 | 1.5 | 1.5 | 0.25 | 1.64 |
| 23 | 1.5 | 0.5 | 1.5 | 0.5 | 0.8 |
| 24 | 1.5 | 1 | 1 | 0.5 | 1.38 |
| 25 | 1.5 | 1 | 1.5 | 0.75 | 2.2 |
| 26 | 1.5 | 1 | 1.5 | 0.25 | 1.4 |
| 27 | 1.5 | 1 | 2 | 0.5 | 1.3 |
| 28 | 1.5 | 1.5 | 1.5 | 0.5 | 1.7 |
| MSE | RMSE | R-Squared | ||||||
|---|---|---|---|---|---|---|---|---|
| Training | Testing | All | Training | Testing | All | Training | Testing | All |
| 4.8 × 10−4 | 0.0260 | 0.0084 | 0.0219 | 0.1613 | 0.0917 | 0.9988 | 0.8706 | 0.9798 |
| Strategy | Concentration of Glycerol Fuel (M) | Concentration of Anode Electrolyte (M) | Loading of Anodic Electrocatalyst (mg/cm2) | Concentration of Cathode Electrolyte (M) | Power Density (mW/m2) |
|---|---|---|---|---|---|
| Experimental [21] | 1.0 | 1.5 | 1.0 | 0.5 | 2.77 |
| RSM [21] | 1.07 | 1.62 | 1.12 | 0.69 | 2.79 |
| JSO and Fuzzy | 1.4 | 1.63 | 1.16 | 0.59 | 3.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhumade, H.; Moujdin, I.A.; Al-Shahrani, S. Increasing Output Power of a Microfluidic Fuel Cell Using Fuzzy Modeling and Jellyfish Search Optimization. Sustainability 2023, 15, 11279. https://doi.org/10.3390/su151411279
Alhumade H, Moujdin IA, Al-Shahrani S. Increasing Output Power of a Microfluidic Fuel Cell Using Fuzzy Modeling and Jellyfish Search Optimization. Sustainability. 2023; 15(14):11279. https://doi.org/10.3390/su151411279
Chicago/Turabian StyleAlhumade, Hesham, Iqbal Ahmed Moujdin, and Saad Al-Shahrani. 2023. "Increasing Output Power of a Microfluidic Fuel Cell Using Fuzzy Modeling and Jellyfish Search Optimization" Sustainability 15, no. 14: 11279. https://doi.org/10.3390/su151411279
APA StyleAlhumade, H., Moujdin, I. A., & Al-Shahrani, S. (2023). Increasing Output Power of a Microfluidic Fuel Cell Using Fuzzy Modeling and Jellyfish Search Optimization. Sustainability, 15(14), 11279. https://doi.org/10.3390/su151411279







