UV Disinfection Systems for Wastewater Treatment: Emphasis on Reactivation of Microorganisms
Abstract
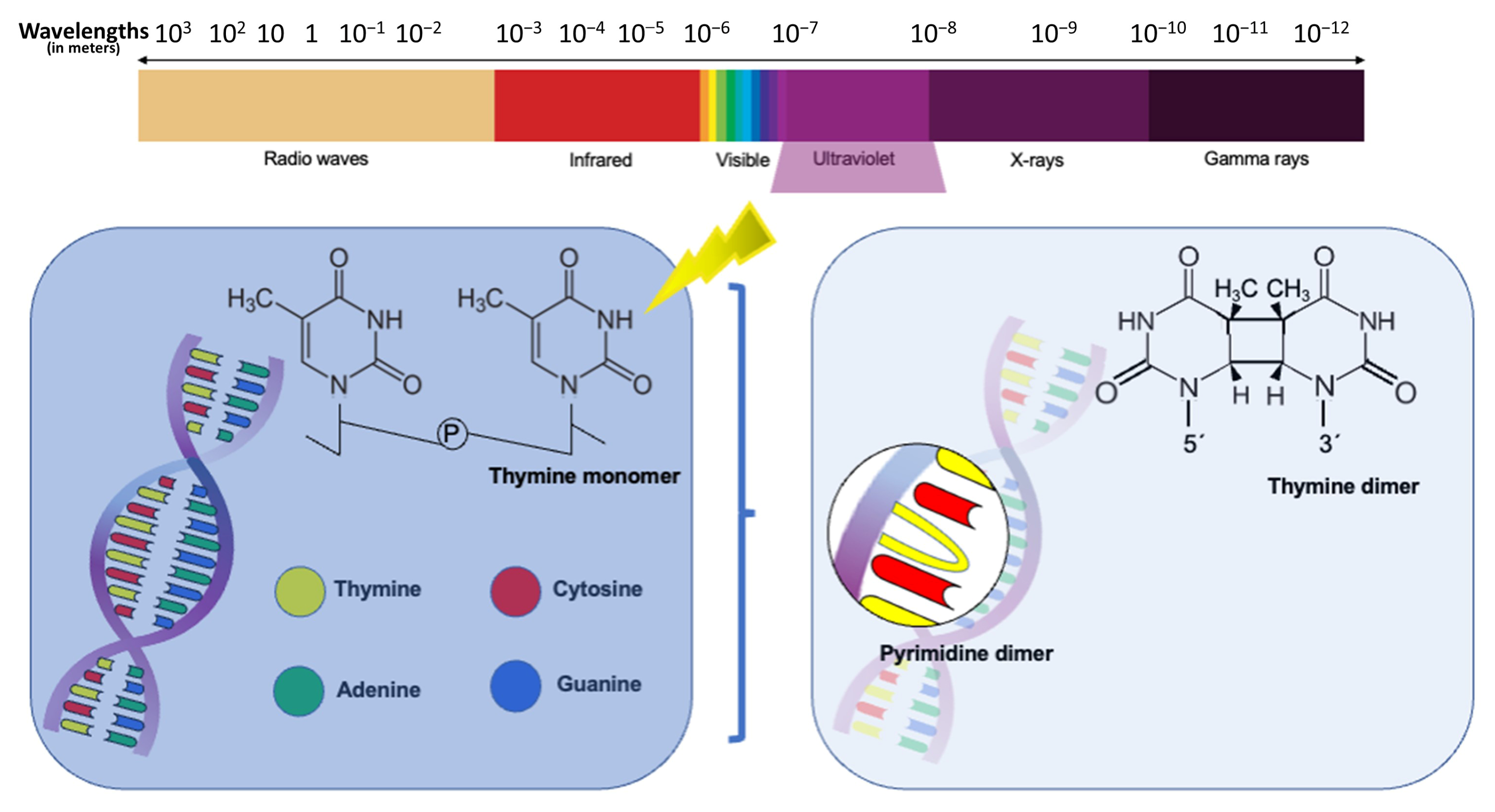
1. Introduction
2. Methods
- All search fields are included.
- Only scientific articles are included, excluding reviews, books, book chapters, proceedings, and others.
- Includes articles published from 2003 to 2022.
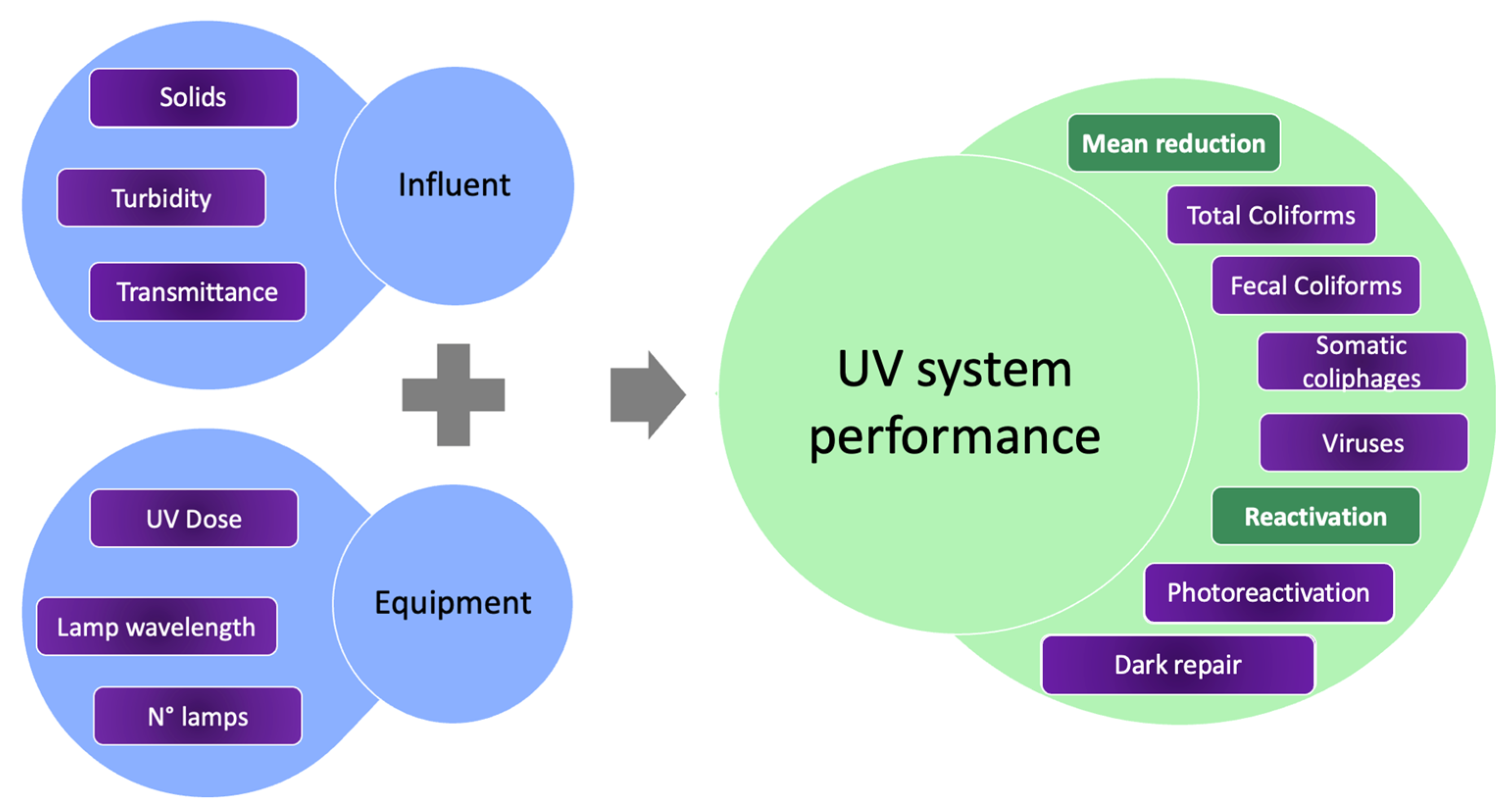
3. UV Systems Implemented in WWTPs
- Hydraulic properties of the reactor. There should be a uniform flow with sufficient axial movement, preventing dead zones that can decrease the contact time and divert the trajectory of the organism;
- UV radiation intensity. Lamp age, fouling, and placement inside the reactor must be considered;
- Wastewater characteristics. Suspended and colloidal solids, flow rate, and bacterial density are parameters to consider for UV system implantation. The higher these parameters are, the less radiation the organisms will absorb, affecting final disinfection.
3.1. UV System Disinfection Method
3.2. Analysis of Factors Affecting UV Disinfection
3.2.1. UV Dose
3.2.2. Total Suspended Solids
- Transmittance
- Particle size
- Zeta potential
4. Reactivation of Pathogenic Microorganisms
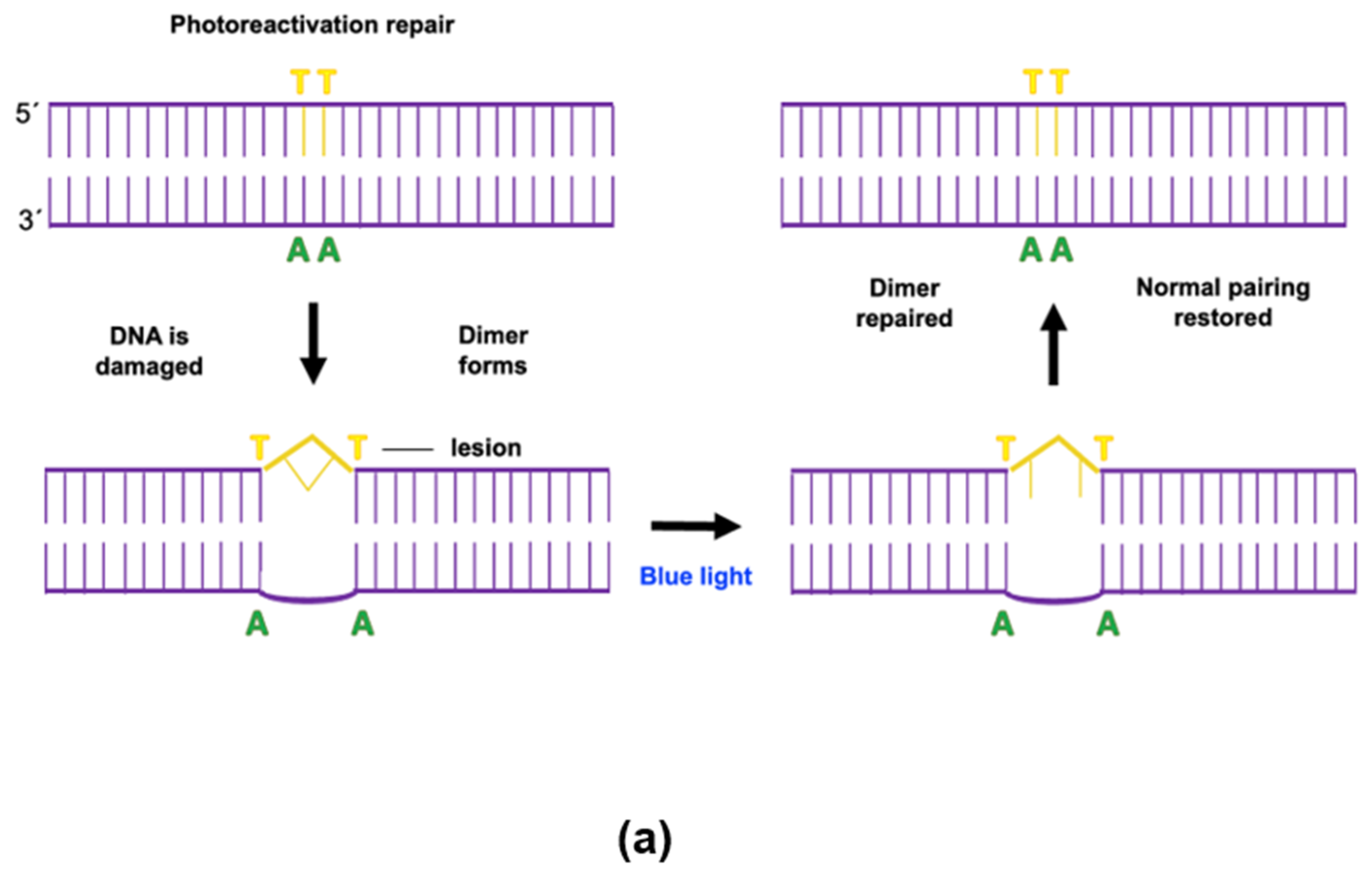
4.1. Photoreactivation
- Formation of PRE-dimer complex. The organisms present photoreactivating enzymes (PREs). Their quantity can vary by organism. In the presence or absence of light, a PRE binds with a pyrimidine dimer, forming a complex. This is a reversible step, but formation kinetics are heavily favored. Factors such as temperature, pH, and ionic strength affect the speed of complex formation;
- Release of repaired DNA and PRE. Photoreactivation results in the monomerization of the dimer and subsequent release of the PRE. The reaction takes place in under a millisecond and the repair is perfect. The restoration of the dimer depends on the reaction kinetics and light energy intensity.
| UV System Operating Parameters | Operating Parameters for Photoreactivation | References | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wavelength (nm) | Lamp Type | Inactivating Dose (mJ/cm2) | Irradiance (mW/cm2) | N° Lamps | Power (W) | Model Lamps | Lamp Wavelength (nm) | Temperature (°C) | |
| 254 | UV-C | 50–200 | 0.10 | 1 | 3.7 | Philips TLD | 360 | 5,10,15,20,25,30 | [62] |
| 222–282 | UV-C/UV-LED/LP UV | 1–200 | 0.10–0.25 | 1–2 | 3.7–15 | Philips TLD | 360–365 | 4–37 | [62,63,64,65] |
| 254–310 | UV-C/UV-LED | 50–200 | 0.10–0.384 | 1 | 3.7 | Philips TLD | 360 | 5,10,15,20,25,30 | [62,66] |
| 222–282 | UV-C/UV-LED/LP UV | 5–200 | 0.10–0.25 | 1–2 | 3.7–15 | Philips TLD | 360–365 | 4–30 | [62,63,64,67] |
| 254 | UV-C | 50–200 | 0.10 | 1 | 3.7 | Philips TLD | 360 | 5,10,15,20,25,30 | [62] |
| 222–310 | UV- C/UV–LED/LP UV | 1–200 | 0.10–0.25 | 1–2 | 3.7–15 | Philips TLD | 360–365 | 4–37 | [62,63,64,65] |
| 254 | UV-C | 50–200 | 0.10 | 1 | 3.7 | Philips TLD | 360 | 5–30 | [62] |
| 222–282 | UV-C/LP UV | 5–200 | 0.10–0.25 | 1–2 | 3.7–15 | Philips TLD | 360–365 | 5–37 | [62,63,64,67] |
| 267–310 | UV-LED | - | - | - | - | - | - | - | [66] |
| 222–282 | UV-LED/LP UV | - | 0.25 | 2 | 8–15 | Philips, Holland | 365 | 4–37 | [63,64] |
| 222–310 | UV-LED/LP UV | - | 0.25 | 2 | 8–15 | Philips, Holland | 365 | 4–37 | [63,64,67] |
| 254–280 | UV-LED/LP UV | - | 0.25 | 2 | 8–15 | Philips, Holland | 365 | 25 | [64] |
| 267–310 | UV-LED | - | - | - | - | - | - | - | [66] |
| 254–280 | UV-LED/LP UV | 1–5 | 0.25 | 2 | 8–15 | Philips, Holland | 365 | 25 | [64,65,67] |
4.2. Dark Repair
- Association of UvrA and UvrB in solution, which searches for possible lesions in the DNA. UvrA first searches for anomalies and, upon finding them, provides the DNA to UvrB, which will attempt to bind the DNA;
- When a lesion is present, it results in a tight complex of UvrB and DNA, from which the UvrA protein dissociates;
- UvrC joins this complex by making an incision at the fourth or fifth phosphodiester bond 3′ to the damage, and then makes an incision at the eighth phosphodiester bond 5′ to the damage;
- Following the incisions, UvrD, also called helicase II, eliminates the damaged oligo and then polymerase I and ligase to restore the DNA strand.
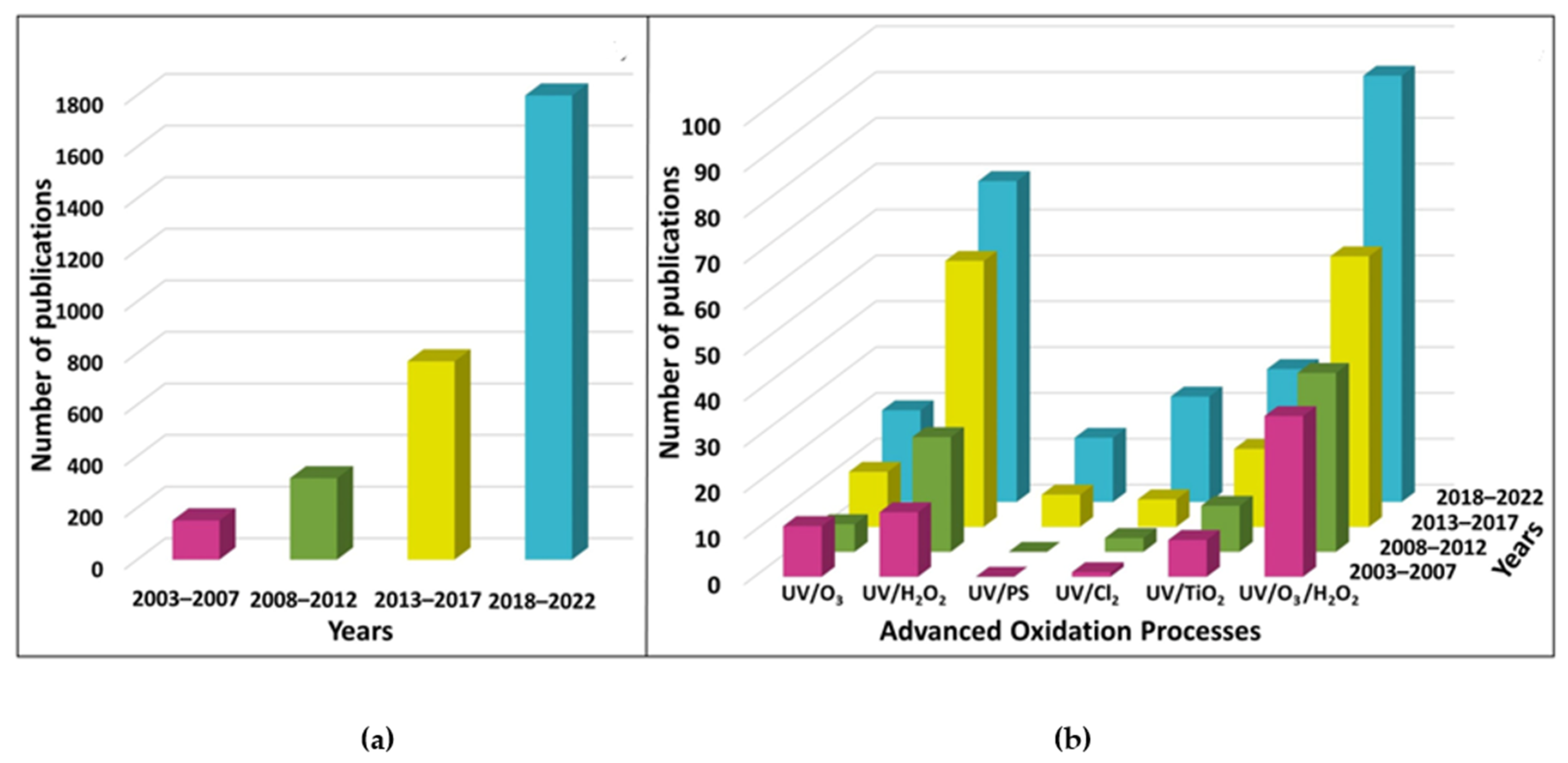
5. Advanced Disinfection Processes: Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.W.; Sheng, G.P.; Zeng, R.J.; Liu, X.W.; Yu, H.Q. China’s wastewater discharge standards in urbanization: Evolution, challenges and implications. Environ. Sci. Pollut. Res. 2012, 19, 1422–1431. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zeng, L.; Wang, P.; Wu, X.; Feng, T. Water cost for water purification: Renewability assessment of a typical wastewater treatment plant in China. J. Clean Prod. 2022, 349, 131474. [Google Scholar] [CrossRef]
- Salgado, P.; Melín, V.; Albornoz, M.; Mansilla, H.; Vidal, G.; Contreras, D. Effect of pH and substituted 1,2-dihydroxybenzenes on reaction pathway of Fenton-like systems. Appl. Catal. B 2018, 226, 93–102. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Suwan, P.; Koottatep, T.; Beck, S.E. Application of a novel, continuous-feeding ultraviolet light emitting diode (UV-LED) system to disinfect domestic wastewater for discharge or agricultural reuse. Water Res. 2019, 153, 53–62. [Google Scholar] [CrossRef]
- Anastasi, E.M.; Wohlsen, T.D.; Stratton, H.M.; Katouli, M. Survival of Escherichia coli in two sewage treatment plants using UV irradiation and chlorination for disinfection. Water Res. 2013, 47, 6670–6679. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, M.; Khiadani, M.; Kakhki, S.; Kholghi, V.; Naderi, K.; Yektay, S. Effect of ozonation-based disinfection methods on the removal of antibiotic resistant bacteria and resistance genes (ARB/ARGs) in water and wastewater treatment: A systematic review. Sci. Total Environ. 2022, 811, 151404. [Google Scholar] [CrossRef]
- Vidal, G.; Becerra, J.; Hernández, V.; Decap, J.; Xavier, C.R. Anaerobic biodegradation of sterols contained in kraft mill effluents. J. Biosci. Bioeng. 2007, 104, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.M.; Kim, H.Y.; Park, W.; Kim, T.H.; Yu, S. A comparative study of disinfection efficiency and regrowth control of microorganism in secondary wastewater effluent using UV, ozone, and ionizing irradiation process. J. Hazard. Mater. 2015, 295, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Postigo, C. Drinking water disinfection by-products. In Emerging Organic Contaminants and Human Health, 1st ed.; Barceló, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 20, pp. 93–137. [Google Scholar] [CrossRef]
- Feng, H.; Ruan, Y.; Wu, R.; Zhang, H.; Lam, P.K.S. Occurrence of disinfection by-products in sewage treatment plants and the marine environment in Hong Kong. Ecotoxicol. Environ. Saf. 2019, 181, 404–411. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, W.; Chen, S.; Zhou, W.; Chen, J. Problems of conventional disinfection and new sterilization methods for antibiotic resistance control. Chemosphere 2020, 254, 126831. [Google Scholar] [CrossRef]
- WWAP (United Nations World Water Assessment Programme). United Nations World Water Development Report 2017. Wastewater: The Untapped Resource; UNESCO: Paris, France, 2017; pp. 1–202. [Google Scholar]
- EPA (Environmental Protection Agency). Overview of Watershed Monitoring. Available online: https://cfpub.epa.gov/watertrain/pdf/modules/monitoring.pdf (accessed on 10 May 2023).
- Beard, V.A.; Satterthwaite, D.; Mitlin, D.; Du, J. Out of sight, out of mind: Understanding the sanitation crisis in global south cities. J. Environ. Manag. 2022, 306, 114285. [Google Scholar] [CrossRef]
- Alahdal, H.M.; AlYahya, S.; Ameen, F.; Sonbol, H.; Alomary, M.N. A review on Saudi Arabian wastewater treatment facilities and available disinfection methods: Implications to SARS-CoV-2 control. J. King Saud. Univ. Sci. 2021, 33, 101574. [Google Scholar] [CrossRef]
- Li, X.; Cai, M.; Wang, L.; Niu, F.; Yang, D.; Zhang, G. Evaluation survey of microbial disinfection methods in UV-LED water treatment systems. Sci. Total Environ. 2019, 659, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- EPA (Environmental Protection Agency). Wastewater Technology Fact Sheet: Ultraviolet Disinfection; EP832-f-99-064; EPA: Washington, DC, USA, 1999; pp. 1–7. [Google Scholar]
- Zhou, H.; Smith, D.W. Advanced technologies in water and wastewater treatment. J. Environ. Eng. Sci. 2002, 1, 247–264. [Google Scholar] [CrossRef]
- Pousty, D.; Hofmann, R.; Gerchman, Y.; Mamane, H. Wavelength-dependent time–dose reciprocity and stress mechanism for UV-LED disinfection of Escherichia coli. J. Photochem. Photobiol. B 2021, 217, 112129. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.F.; Zhang, R.J.; Huang, S.B.; Shao, J.H.; Cui, B.; Du, Z.L.; Xue, L.; Zhou, N.; Hou, B.; Lin, C. UV dose effects on the revival characteristics of microorganisms in darkness after UV disinfection: Evidence from a pilot study. Sci. Total Environ. 2020, 713, 136582. [Google Scholar] [CrossRef]
- Sinton, L.W.; Davies-Colley, R.J.; Bell, R.G. Inactivation of enterococci and fecal coliforms from sewage and meatworks effluents in seawater chambers. Appl. Environ. Microbiol. 1994, 60, 2040–2048. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.T.; Yuan, Q.B.; Yang, J. Microbial selectivity of UV treatment on antibiotic-resistant heterotrophic bacteria in secondary effluents of a municipal wastewater treatment plant. Water Res. 2013, 47, 6388–6394. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, Y.; Geng, J.; Ren, H.; Zhang, Y.; Ding, L.; Xu, K. Inactivation of antibiotic resistance genes in municipal wastewater effluent by chlorination and sequential UV/chlorination disinfection. Sci. Total Environ. 2015, 512–513, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Pai, C.W.; Wang, G.S. Treatment of PPCPs and disinfection by-product formation in drinking water through advanced oxidation processes: Comparison of UV, UV/chlorine, and UV/H2O2. Chemosphere 2022, 287, 132171. [Google Scholar] [CrossRef] [PubMed]
- Chick, H. An investigation of the laws of disinfection. J. Hyg. 1908, 8, 92–158. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.E. A note on the variation of the rate of disinfection with change in the concentration of the disinfectant. J. Hyg. 1908, 8, 536–542. [Google Scholar] [CrossRef]
- Sheriff, M.; Gehr, R. Laboratory investigation of inorganic fouling of low pressure UV disinfection lamps. Water Qual. Res. J. Can. 2001, 36, 71–92. [Google Scholar] [CrossRef]
- Nessim, Y.; Gehr, R. Fouling mechanisms in a laboratory-scale UV disinfection system. Water Environ. Res. 2006, 78, 2311–2323. [Google Scholar] [CrossRef]
- Artichowicz, W.; Luczkiewicz, A.; Sawicki, J.M. Analysis of the radiation dose in UV-disinfection flow reactors. Water 2020, 12, 231. [Google Scholar] [CrossRef]
- Cabaj, A.; Sommer, R.; Pribil, W.; Haider, T. What means “dose” in UV-disinfection with medium pressure lamps? Ozone Sci. Eng. 2007, 23, 239–244. [Google Scholar] [CrossRef]
- Yan, R.; Yun, J.; Gurtler, J.; Fan, X. Radiochromic film dosimetry for UV-C treatments of apple fruit. Postharvest. Biol. Technol. 2017, 127, 14–20. [Google Scholar] [CrossRef]
- Salcedo Dávila, I.; Andrade Balao, J.A.; Quiroga Alonso, J.M.; Nebot Sanz, E. Pilot Plan protocol for optimization of UV dose required to obtain an appropriate municipal wastewater disinfection. J. Water Supply Res. Technol. 2008, 57, 57–63. [Google Scholar] [CrossRef]
- De la Cruz, N.; Esquius, L.; Grandjean, D.; Magnet, A.; Tungler, A.; de Alencastro, L.F.; Pulgarín, C. Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res. 2013, 47, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Tacão, M.; Tavares, R.D.S.; Miranda, R.; Araújo, S.; Manaia, C.M.; Henriques, I. Fate of cefotaxime-resistant Enterobacteriaceae and ESBL-producers over a full-scale wastewater treatment process with UV disinfection. Sci. Total Environ. 2018, 639, 1028–1037. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Laski, E.; García-Cañibano, C.; Martín de Vidales, M.J.; Encinas, Á.; Kuch, B.; Marugán, J. Micropollutants removal by full-scale UV-C/sulfate radical based Advanced Oxidation Processes. Sci. Total Environ. 2018, 630, 1216–1225. [Google Scholar] [CrossRef]
- Caretti, C.; Lubello, C. Wastewater disinfection with PAA and UV combined treatment: A pilot plant study. Water Res. 2003, 37, 2365–2371. [Google Scholar] [CrossRef]
- Rauch, K.D.; MacIsaac, S.A.; Stoddart, A.K.; Gagnon, G.A. UV disinfection audit of water resource recovery facilities identifies system and matrix limitations. J. Water Process. Eng. 2022, 50, 103167. [Google Scholar] [CrossRef]
- Farrell, C.; Hassard, F.; Jefferson, B.; Leziart, T.; Nocker, A.; Jarvis, P. Turbidity composition and the relationship with microbial attachment and UV inactivation efficacy. Sci. Total Environ. 2018, 624, 638–647. [Google Scholar] [CrossRef]
- Cadena-Aizaga, M.I.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Occurrence and environmental hazard of organic UV filters in seawater and wastewater from Gran Canaria Island (Canary Islands, Spain). Environ. Pollut. 2022, 300, 118843. [Google Scholar] [CrossRef]
- Chahal, C.; van den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and particle associations in wastewater: Significance and implications for treatment and disinfection processes. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Kandasamy, J.; Cho, J. Characteristics of effluent organic matter in wastewater. In Water and Wastewater Treatment Technologies; Eolss Publisher Co., Ltd.: Oxford, UK, 2007; pp. 52–101. [Google Scholar]
- Hipsey, M.R.; Brookes, J.D.; Regel, R.H.; Antenucci, J.P.; Burch, M.D. In situ evidence for the association of total coliforms and Escherichia coli with suspended inorganic particles in an Australian reservoir. Water Air Soil Pollut. 2006, 170, 191–209. [Google Scholar] [CrossRef]
- Mattle, M.J.; Kohn, T. Inactivation and tailing during UV254 disinfection of viruses: Contributions of viral aggregation, light shielding within viral aggregates, and recombination. Environ. Sci. Technol. 2012, 46, 10022–10030. [Google Scholar] [CrossRef] [PubMed]
- Carré, E.; Pérot, J.; Jauzein, V.; Lopez-Ferber, M. Impact of suspended particles on UV disinfection of activated-sludge effluent with the aim of reclamation. J. Water Process. Eng. 2018, 22, 87–93. [Google Scholar] [CrossRef]
- Madge, B.A.; Jensen, J.N. Ultraviolet disinfection of fecal coliform in municipal wastewater: Effects of particle size. Water Environ. Res. 2006, 78, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Peperzak, L.; Stuut, J.B.W.; van der Woerd, H.J. Suspended matter filtration causes a counterintuitive increase in UV-absorption. Mar. Pollut Bull. 2022, 183, 114012. [Google Scholar] [CrossRef] [PubMed]
- Mantilla, C.; Pedraza, J.; Laverde Cataño, D.A. Use of zeta potential studies in the development of an alternative feldspar mineral flotation process. Dyna 2008, 75, 65–71. [Google Scholar]
- Ferreyra Maillard, A.P.V.; Espeche, J.C.; Maturana, P.; Cutro, A.C.; Hollmann, A. Zeta potential beyond materials science: Applications to bacterial systems and to the development of novel antimicrobials. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183597. [Google Scholar] [CrossRef]
- Feijoo, G.; Vidal, G.; Moreira, M.T.; Méndez, R.; Lema, J.M. Degradation of high molecular weigh compounds of Kraft pulp mill effluents by a combined treatment with fungi and bacteria. Biotech. Lett. 1995, 17, 1261–1268. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- González, Y.; Salgado, P.; Vidal, G. Disinfection behavior of a UV-treated wastewater system using constructed wetlands and the rate of reactivation of pathogenic microorganisms. Water Sci. Technol. 2019, 80, 1870–1879. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, C.; Lin, H.; Lv, L.; Yu, X. UV disinfection induces a VBNC state in Escherichia coli and Pseudomonas aeruginosa. Environ. Sci. Technol. 2015, 49, 1721–1728. [Google Scholar] [CrossRef]
- Moreno, Y.; Piqueres, P.; Alonso, J.L.; Jiménez, A.; González, A.; Ferrús, M.A. Survival and viability of Helicobacter pylori after inoculation into chlorinated drinking water. Water Res. 2007, 41, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shuai, X.; Xu, L.; Sun, Y.; Lin, Z.; Zhou, Z.; Meng, L.; Chen, H. Mechanisms underlying the effect of chlorination and UV disinfection on VBNC state Escherichia coli isolated from hospital wastewater. J. Hazard. Mater. 2022, 423, 127228. [Google Scholar] [CrossRef]
- Lindenauer, K.G.; Darby, J.L. Ultraviolet disinfection of wastewater: Effect of dose on subsequent photoreactivation. Water Res. 1994, 28, 805–817. [Google Scholar] [CrossRef]
- Hallmich, C.; Gehr, R. Effect of pre- and post-UV disinfection conditions on photoreactivation of fecal coliforms in wastewater effluents. Water Res. 2010, 44, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Locas, A.; Demers, J.; Payment, P. Evaluation of photoreactivation of Escherichia coli and Enterococci after UV disinfection of municipal wastewater. Can. J. Microbiol. 2008, 54, 971–975. [Google Scholar] [CrossRef]
- Nebot Sanz, E.; Salcedo Dávila, I.; Andrade Balao, J.A.; Quiroga Alonso, J.M. Modelling of reactivation after UV disinfection: Effect of UV-C dose on subsequent photoreactivation and dark repair. Water Res. 2007, 41, 3141–3151. [Google Scholar] [CrossRef]
- Yin, F.; Zhu, Y.; Koutchma, T.; Gong, J. Inactivation and potential reactivation of pathogenic Escherichia coli O157:H7 in bovine milk exposed to three monochromatic ultraviolet UVC lights. Food Microbiol. 2015, 49, 74–81. [Google Scholar] [CrossRef]
- Wan, Q.; Wen, G.; Cao, R.; Xu, X.; Zhao, H.; Li, K.; Wang, J.; Huang, T. Comparison of UV-LEDs and LPUV on inactivation and subsequent reactivation of waterborne fungal spores. Water Res. 2020, 173, 115553. [Google Scholar] [CrossRef]
- Guo, M.T.; Kong, C. Antibiotic resistant bacteria survived from UV disinfection: Safety concerns on genes dissemination. Chemosphere 2019, 224, 827–832. [Google Scholar] [CrossRef]
- Nyangaresi, P.O.; Qin, Y.; Chen, G.; Zhang, B.; Lu, Y.; Shen, L. Effects of single and combined UV-LEDs on inactivation and subsequent reactivation of E. coli in water disinfection. Water Res. 2018, 147, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Huang, J.; Hu, H.; Liu, W. Growth and repair potential of three species of bacteria in reclaimed wastewater after UV disinfection. Biomed. Environ. Sci. 2011, 24, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Cao, R.; Wen, G.; Xu, X.; Xia, Y.; Wu, G.; Li, Y.; Wang, J.; Xu, H.; Lin, Y.; et al. Efficacy of UV-LED based advanced disinfection processes in the inactivation of waterborne fungal spores: Kinetics, photoreactivation, mechanism and energy requirements. Sci. Total Environ. 2022, 803, 150107. [Google Scholar] [CrossRef]
- Goosen, N.; Moolenaar, G.F. Repair of UV Damage in Bacteria. DNA Repair 2008, 7, 353–379. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Wang, W.L.; Huo, Z.Y.; Lu, Y.; Hu, H.Y. Comparison of UV-LED and low pressure UV for water disinfection: Photoreactivation and dark repair of Escherichia coli. Water Res. 2017, 126, 134–143. [Google Scholar] [CrossRef]
- Fitzhenry, K.; Clifford, E.; Rowan, N.; Val del Rio, A. Bacterial inactivation, photoreactivation and dark repair post flow-through pulsed UV disinfection. J. Water Process. Eng. 2021, 41, 102070. [Google Scholar] [CrossRef]
- Kalisvaart, B.F. Re-use of wastewater: Preventing the recovery of pathogens by using medium-pressure UV lamp technology. Water Sci. Technol. 2004, 50, 337–344. [Google Scholar] [CrossRef]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced oxidation processes for water and wastewater viral disinfection. A systematic review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef]
- Marjanovic, M.; Giannakis, S.; Grandjean, D.; de Alencastro, L.F.; Pulgarin, C. Effect of μM Fe addition, mild heat and solar UV on sulfate radical-mediated inactivation of bacteria, viruses, and micropollutant degradation in water. Water Res. 2018, 140, 220–231. [Google Scholar] [CrossRef]
- Giannakis, S.; Rtimi, S.; Pulgarin, C. Light-assisted advanced oxidation processes for the elimination of chemical and microbiological pollution of wastewaters in developed and developing countries. Molecules 2017, 22, 1070. [Google Scholar] [CrossRef]
- Galeano, L.A.; Guerrero-Flórez, M.; Sánchez, C.A.; Gil, A.; Vicente, M.Á. Disinfection by chemical oxidation methods. In Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment, 1st ed.; Gil, A., Galeano, L., Vicente, M., Eds.; Springer: Cham, Switzerland, 2017; Volume 67, pp. 257–295. [Google Scholar] [CrossRef]
- Kaswan, V.; Kaur, H. A Comparative study of advanced oxidation processes for wastewater treatment. Water Pract. Technol. 2023, 18, 1233–1254. [Google Scholar] [CrossRef]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: A review. Ozone Sci. Eng. 2007, 28, 353–414. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef] [PubMed]
- Woo, O.T.; Chung, W.K.; Wong, K.H.; Chow, A.T.; Wong, P.K. Photocatalytic oxidation of polycyclic aromatic hydrocarbons: Intermediates identification and toxicity testing. J. Hazard. Mater. 2009, 168, 1192–1199. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, W.; Yuan, M.; Feng, C.; Ren, Y.; Wei, C. Degradation of polycyclic aromatic hydrocarbons in a coking wastewater treatment plant residual by an O3/ultraviolet fluidized bed reactor. Environ. Sci. Pollut. Res. Int. 2014, 21, 10329–10338. [Google Scholar] [CrossRef]
- Dudziak, M.; Burdzik, E. Oxidation of bisphenol A from simulated and real urban wastewater effluents by UV, O3 and UV/O3. Desalin. Water Treat. 2014, 57, 1075–1083. [Google Scholar] [CrossRef]
- Gong, J.; Liu, Y.; Sun, X. O3 and UV/O3 oxidation of organic constituents of biotreated municipal wastewater. Water Res. 2008, 42, 1238–1244. [Google Scholar] [CrossRef]
- Bairagi, M.; Adak, A.; Islam, M. Bacterial inactivation in wastewater using UV/H2O2 advanced oxidation. J. Indian Chem. Soc. 2020, 97, 621–627. [Google Scholar]
- Yonar, T.; Kestioglu, K.; Azbar, N. Treatability studies on domestic wastewater using UV/H2O2 process. Appl. Catal. B 2006, 67, 223–228. [Google Scholar] [CrossRef]
- Malayeri, A.H.; Mohseni, M.; Cairns, B.; Bolton, J.R.; Chevrefils, G.; Caron, E.; Barbeau, B.; Wright, H.; Linden, K.G. Fluence (UV dose) required to achieve incremental log inactivation of bacteria, protozoa, viruses and algae. IUVA News 2016, 18, 4–6. [Google Scholar]
- Demeersseman, N.; Saegeman, V.; Cossey, V.; Schuermans, A. Shedding a light on UV-C technologies in the hospital environment. J. Hosp. Infect. 2022, 132, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chevremont, A.C.; Farnet, A.M.; Coulomb, B.; Boudenne, J.L. Effect of coupled UV-A and UV-C LEDs on both microbiological and chemical pollution of urban wastewaters. Sci. Total Environ. 2012, 426, 304–310. [Google Scholar] [CrossRef] [PubMed]

| Reactivation Time (h) | Indicator | Photoreactivation | Dark Repair | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage of Reactivation (%) | Survival Ratio (%) | First-Order Kinetic Parameters | Percentage of Reactivation (%) | Survival Ratio (%) | Sm (%) | First-Order Kinetic Parameters | |||||||
| K1 (L/min) | K2 (%min) | Sm (%) | R2 | K2 (%min) | R2 | ||||||||
| 0.5 | Total Coliforms/Fecal Coliforms | - | 0.01–0.12 | - | 0.039–1.301 | 0.012–0.945 | 0.932–0.997 | - | 0.004–0.062 | 0.004–0.073 | 0.341–4.737 | 0.821–0.987 | [62] |
| 1 | Aspergillus niger/Total Coliforms/Fecal Coliforms/Escherichia coli O157:H7/Escherichia coli HB 102 | 4.41–8.44 | 0.01–2.27 | 0.0041–0.0091 | 0.031–1.301 | 0.012–69.89 | 0.91–0.997 | 0.94–14.13 | 0.004–6.17 | 0.004–0.073 | 0.341–4.737 | 0.821–0.987 | [62,63,64,65] |
| 1.5 | Total Coliforms/Fecal Coliforms/Escherichia Coli | 1.00–1.18 | 0.01–0.48 | - | 0.031–1.301 | 0.012–0.945 | 0.932–0.997 | 0.12–0.41 | 0.004–0.061 | 0.004–0.073 | 0.341–4.737 | 0.821–0.987 | [62,66] |
| 2 | Aspergillus niger/Total Coliforms/Fecal Coliforms/Escherichia coli O157:H7 | 5. 87–12.28 | 0.01–2.20 | 0.0041–0.0091 | 0.031–1.301 | 0.012–69.89 | 0.91–0.997 | 1.53–26.59 | 0.004–10.84 | 0.004–0.073 | 0.341–4.737 | 0.821–0.987 | [62,63,64,67] |
| 2.5 | Total Coliforms/Fecal Coliforms | - | 0.01–0.64 | - | 0.031–1.301 | 0.012–0.945 | 0.932–0.997 | - | 0.004–0.058 | 0.004–0.073 | 0.341–4.737 | 0.821–0.987 | [62] |
| 3 | Aspergillus niger/Total Coliforms/Fecal Coliforms/Escherichia coli O157:H7/Escherichia coli HB 102/Escherichia coli | 2.18–32.87 | 0.01–2.05 | 0.0041–0.0091 | 0.031–1.301 | 0.012–69.89 | 0.91–0.997 | 0.43–38.32 | 0.003–17.20 | 0.004–0.073 | 0.341–4.737 | 0.821–0.987 | [62,63,64,65,66] |
| 3.5 | Total Coliforms/Fecal Coliforms | - | 0.01–0.94 | - | 0.031–1.301 | 0.012–0.945 | 0.932–0.997 | - | 0.003–0.049 | 0.004–0.073 | 0.341–4,737 | 0.821–0.987 | [62] |
| 4 | Aspergillus niger/Total Coliforms/Fecal Coliforms/Escherichia coli O157:H7/Escherichia coli | 12.05–12.28 | 0.01–2.80 | 0.0041–0.0091 | 0.031–1.301 | 0.012–69.89 | 0.91–0.997 | 2.56–51.50 | 0.002–33.08 | 0.004–0.073 | 0.341–4.737 | 0.821–0.987 | [62,63,64,67] |
| 4.5 | Escherichia coli | 6.96–18.11 | - | - | - | - | - | 0.66–4.95 | - | - | - | - | [66] |
| 5 | Aspergillus niger/Escherichia coli O157:H7 | - | 0.83–2.12 | 0.0041–0.0091 | - | 28.12–69.89 | 0.91–0.98 | 3.24–61.56 | 18.88–37.00 | - | - | - | [63,64] |
| 6 | Aspergillus niger/Escherichia coli/Escherichia coli O157:H7 | 12.58–30.36 | 0.83–2.20 | 0.0041–0.0091 | - | 28.12–69.89 | 0.91–0.98 | 2.05–66.59 | 23.36–47.48 | - | - | - | [63,64,66] |
| 7 | Aspergillus niger | - | 0.91–2.12 | 0.0041–0.0091 | - | 28.12–69.89 | 0.91–0.98 | - | 26.36–55.14 | - | - | - | [64] |
| 7.5 | Escherichia coli | 15.93–31.19 | - | - | - | - | - | 2.40–7.66 | - | - | - | - | [66] |
| 8 | Aspergillus niger/Fecal coliforms/Escherichia coli HB 102/Escherichia coli | 9.70–43.45 | 0.91–2.42 | 0.0041–0.0091 | - | 28.12–69.89 | 0.91–0.98 | - | 28.79–65.98 | - | - | - | [64,65,67] |
| 9 | Escherichia coli | 15.93–31.19 | - | - | - | - | - | 2.40–7.85 | - | - | - | - | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, Y.; Gómez, G.; Moeller-Chávez, G.E.; Vidal, G. UV Disinfection Systems for Wastewater Treatment: Emphasis on Reactivation of Microorganisms. Sustainability 2023, 15, 11262. https://doi.org/10.3390/su151411262
González Y, Gómez G, Moeller-Chávez GE, Vidal G. UV Disinfection Systems for Wastewater Treatment: Emphasis on Reactivation of Microorganisms. Sustainability. 2023; 15(14):11262. https://doi.org/10.3390/su151411262
Chicago/Turabian StyleGonzález, Yenifer, Gloria Gómez, Gabriela E. Moeller-Chávez, and Gladys Vidal. 2023. "UV Disinfection Systems for Wastewater Treatment: Emphasis on Reactivation of Microorganisms" Sustainability 15, no. 14: 11262. https://doi.org/10.3390/su151411262
APA StyleGonzález, Y., Gómez, G., Moeller-Chávez, G. E., & Vidal, G. (2023). UV Disinfection Systems for Wastewater Treatment: Emphasis on Reactivation of Microorganisms. Sustainability, 15(14), 11262. https://doi.org/10.3390/su151411262






