Abstract
In this study, the effect of 14 years of GE exclusion in a desert grassland on soil microbial community diversity and metabolites was examined. GE changed the bacterial community structure, the alpha diversity of the bacterial community, and the total phosphorus (TP) and total potassium (TK) content in the soil. More specifically, the relative abundance of Actinobacteria, Proteobacteria, and Chloroflexi increased with GE. In contrast, the relative abundance of Acidobacteria was higher during grazing (G), so it is believed that soil bacteria adapt to environmental changes. Both amino acid and carbohydrate metabolism were enhanced, while lipid metabolism was decreased under GE. It was concluded that GE could trigger changes in both bacterial diversity and soil metabolites, increase the energy supply, and regulate ecosystem function. Consequently, GE would have positive effects on the restoration of desert grasslands by altering the soil microbial community. This work provides new insights into the response of soil microbes to GE.
1. Introduction
Different varieties of grasslands cover 6 billion acres in China. Grasslands are an important ecosystem and natural resource in China, as they have a crucial and vital role in preserving national ecological security, fostering sustainable economic and social development, and other factors. However, China’s grassland ecosystem as a whole is still fragile, protection and restoration efforts are insufficient, and more than 90% have different degrees of degradation [1]. Additionally, the majority of the severely degraded grassland types are desert grasslands. Previous studies have shown that desert grasslands have the function of conserving biodiversity and maintaining ecological balance [2]. The damage to these grassland ecosystems has been constantly increasing due to global climate change and the impact of anthropogenic grazing, which has intensified grassland degradation. Grassland degradation has also led to a reduction in microbial diversity, decreased ecosystem function, and stability, as well as decreased soil nutrient content. The inadequate access of microorganisms to nutrients in subsurface ecosystems may lead to significant changes in subsurface ecosystem functions [3,4], posing a significant challenge to sustainable development [5]. Therefore, measures to effectively restore grassland ecosystem functions are urgently needed, of which GE is one of the common approaches [6,7,8]. GE reduces animal trampling, enabling grasslands to be undisturbed by animals, ensuring normal plant growth and development, and increasing the productivity of grassland plants, thus contributing to the recovery of grasslands. Currently, GE is widely used in grasslands with various degrees of degradation, which is important for the sustainable development of grassland ecosystems, especially desert grasslands [9,10].
Soil nutrients are a key indicator of soil fertility, as they directly provide essential substances for plant growth [11,12]. Short-term GE has been shown to significantly increase soil fast-acting phosphorus content [13,14], suggesting that GE is beneficial to the restoration of grassland vegetation and soil physicochemical properties. However, fewer studies have been conducted on long-term GE. Plants have a significant role in the process of restoring ecosystems, and animal activities like G that decrease the input of apoplastic biomass can result in degraded lands and plant communities, which can make the ecosystem homogeneous and less resilient. GE will reduce the ecological pressure on grasslands and provide favorable conditions for vegetation growth so that grassland ecosystems can carry out their restoration [15]. Some studies have shown highly significant increases in average vegetation height, cover, and above-ground biomass after short-term GE. However, there is limited research on long-term GE.
Soil microorganisms are regarded as an important component of soil ecosystems [16,17], they play an important role in ecosystem structure and function [18,19,20], and they drive nutrient transport and cycling in soils [21]. Grassland degradation often disrupts the soil microbial community structure and function. Therefore, to restore degraded grasslands, the rebuilding of the microbial community is particularly important [9]. It has also been reported that GE affects typical grasslands, meadows, and semi-arid grasslands. GE increases the relative abundance of Bacillus immobilis and Bacillus thuringiensis by 7.40% and 10.37%, respectively, in the 0–5 cm layer of desert soils in northwestern China [7]. This indicates that to adapt to changes in grasslands, microorganisms may modify the diversity and composition of their communities in response to environmental changes [1,22,23]. Previous research has shown that changes in soil microbial communities are effective indicators to evaluate the effectiveness of GE [24]. However, there have been few reports on the response of microorganisms in desert grasslands to GE. Accordingly, to more accurately determine whether GE is effective in desert grasslands, microbial community composition and diversity changes need to be deeply understood [9].
The balance between microbial nutrient requirements and nutrient availability is regulated by the metabolic activity of the soil ecosystem [25]. Therefore, elucidating soil metabolism is of great importance for understanding the response of microbial communities to GE in desert grasslands. Metabolomics is a relatively recent technique to characterize the properties of metabolic networks via the expression of small metabolites and their trends under the influence of various factors [26]. The application of metabolomics to soil microbial communities [24,27] would directly reflect detectable biological responses under different conditions [28,29]. In addition, metabolomics offers new tools for characterizing the metabolism of soils and exploring microbial communities [30,31]. Soil metabolomics can also be applied to microbial communities to distinguish microbial community functions. For example, metabolomics techniques have been reported to reflect differences in the microbial community structure between inter- and root-perimeter soils, when the metabolism and bacterial diversity of inter-root soils of pepper cultivated in greenhouses were explored [32]. Inter-root bacterial communities can enhance their salt tolerance by regulating soil metabolites, further defining the relationship between inter-root microbiota and soil metabolites [33]. There is limited research on the effects of GE on soil microbial metabolism. However, the combination of metabolomics with microbial diversity yields a more comprehensive understanding of the role of microorganisms in the ecosystem [32] and provides a different perspective on the impact of GE on desert grasslands.
In the present study, a desert grassland in Yushugou, Urumqi, Xinjiang Uygur Autonomous Region that had been excluded from grazing for 14 years was selected to systematically investigate the influence of GE on soil microbial diversity and metabolism. The two primary objectives of this study were to (1) analyze the reaction of soil bacterial populations and metabolism under GE, as well as (2) identify the association between various metabolites and microbial communities under GE settings.
2. Materials and Methods
2.1. Experimental Design and Sampling
The study area was in the desert grassland of Yushugou, Urumqi, Xinjiang Uygur Autonomous Region (43°46.617′ N, 087°42.999′ E at an altitude of 1058 m and 43°46.555′ N, 087°43.593′ E at an altitude of 1049 m). We chose desert grasslands with 14 years of grazing exclusion and nearby free-grazing regions as our test location, and soil samples were taken in November 2021. Each sampling area was randomly set up with five 1 × 1 m standard survey plots. The surface debris was removed from the sample plots and soil was collected using the five-point sampling method. The soil samples were collected from depths of 0–20 cm and filtered through a 2 mm sieve to remove various impurities, such as roots, stones, and foliage. The five soil samples from each sample plot were mixed and divided into three parts. Two parts were put into sterile aluminum boxes and transported in a vehicle refrigerator at 4 °C. At the laboratory, they were stored in an ultra-low-temperature refrigerator at −80 °C for high-throughput sequencing and detection of soil metabolic activities. The other soil samples were air-dried in the laboratory and used for the determination of the soil’s physical and chemical properties. Additionally, five 1 × 1 m and 10 × 10 m plots were randomly established in each sampling area for measuring vegetation cover, and above-ground and below-ground biomass. Above-ground biomass was dried at 60 °C for 36 h. Roots were washed in distilled water and then dried at 60 °C for 36 h to measure belowground biomass.
2.2. Soil Physical and Chemical Property Index Measurement Methods
Total phosphorus (TP) was determined using the Mo–Sb colorimetric method. Total nitrogen (TN) was determined by Kjeldahl digestion and analysis using a continuous-flow analyzer type AA3 (SEAL flow analyzer Auto Analyzer3, SEAL, Norderstedt, Germany). Total potassium (TK) was determined by NaOH melting and flame photometry. Soil organic matter (SOM) was determined using the potassium dichromate method (Titrette titration). Soil organic carbon (SOC) = SOM/1.742. The pH was measured using a DELTA 320 pH meter. Available phosphorus (AP) was measured by 0.5 mol·L−1 of NaHCO3 leaching, using the Mo–Sb colorimetric method, while available nitrogen (AN) was determined by the alkali diffusion method (Titrette titrator, Brand, Wertheim, Germany). Available potassium (AK) was measured by flame spectrometry. Statistical analyses of soil physicochemical data and plant biomass data for the GE and G samples were performed by SPSS (v26, Chicago, IL, USA). Analysis of soil physical and chemical indicators was performed using the independent samples T-test method. The significance was calculated by Tukey’s test (p < 0.05).
2.3. Soil DNA Sequence Extraction
Total genomic DNA extraction from microbial communities was performed using the E.Z.N.A.® soil DNA kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer’s instructions. The quality of the extracted genomic DNA was determined by 1% agarose gel electrophoresis, whereas DNA concentration and purity were determined using a NanoDrop2000 (Thermo Scientific, Wilmington, NC, USA). Primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’were used for PCR amplification of the V3–V4 variable region of the 16S rRNA gene. Sequencing was performed using Illumina’s Miseq PE300/NovaSeq PE250 platform. Quality control (QC) of double-ended raw sequenced sequences was performed using fastp v0.19.6 [30] (https://github.com/OpenGene/fastp (accessed on 5 January 2022)) and splicing was performed using FLASH v1.2.11 [34] (http://www.cbcb.umd.edu/software/flash (accessed on 5 January 2022)). The quality-controlled spliced sequences were clustered and chimeras were removed by using UPARSE v7.1 (http://drive5.com/uparse (accessed on 13 January 2022)) based on 97% similarity to the operational taxonomic unit (OTU).
To minimize the impact of sequencing depth on the subsequent alpha diversity and beta diversity data analysis, all sample sequence numbers were drawn flat to 60,619. Additionally, the RDP classifier (http://rdp.cme.msu.edu/ (accessed on 20 January 2022), v2.11) was used to annotate the Silva 16S rRNA gene database (v138) for OTU species taxonomy with a confidence threshold of 70% and the community composition of each sample was counted at different species taxonomic levels.
2.4. Microbial Diversity Analysis
The Chao, Shannon, Ace, Sobs, and Simpson alpha diversity indices were calculated using the Mothur [35] software package (http://www.mothur.org/wiki/Calculators (accessed on 25 January 2022)), and the Wilcoxon rank sum test was performed to analyze intergroup differences in alpha diversity. The similarity of the microbial community structure between samples was examined by principal coordinate analysis (PCoA) based on the Bray–Curtis distance algorithm. This was combined with the ANOSIM method to determine whether the differences in microbial community structure between sample groups were significant. Bar and heat maps were built using the R software package (v3.2.4) for language tool mapping. Linear discriminant analysis effect size (LEfSe) analysis (http://huttenhower.sph.harvard.edu/LEfSe (accessed on 25 January 2022)) (LDA > 4, p < 0.05) was conducted to identify bacterial taxa that differed significantly in abundance between groups from the phylum to the genus level. RDA analysis was performed using the R language (v3.2.4) vegan package with the RDA or CCA analysis and graphing functions.
2.5. Soil Metabolites Analysis
Fifty milligrams of solid sample was placed in a 1.5 mL centrifuge tube and 400 μL of extraction solution (acetonitrile: methanol = 1:1) was added. The mixture was vortexed to mix for 30 s and then low-temperature ultrasonic extraction was performed for 30 min (5 °C, 40 kHz). The sample was then cooled at −20 °C for 30 min and then centrifuged at 4 °C and 13,000× g for 15 min. The supernatant was removed and blow-dried under nitrogen. Next, 120 µL of reagent solution (acetonitrile: water = 1:1) was added at low temperature for 5 min (5 °C, 40 kHz) to re-solubilize the residue. The sample was centrifuged at 4 °C and 13,000× g for 5 min and the supernatant was removed to a feeding vial with an insert tube. Low-temperature ultrasonic extraction was performed for 5 min (5 °C, 40 kHz), followed by centrifugation at 4 °C and 13,000× g for 5 min. The supernatant was then placed in an injection vial with an internal insertion tube for machine analysis.
After the machine analysis was completed, the raw LC-MS data were imported into the metabolomics processing software package Progenesis QI (Waters Corporation, Milford, MA, USA) for baseline filtering, peak identification, integration, retention time correction, and peak alignment. Finally, a data matrix of retention time, mass-to-charge ratio, and peak intensity was calculated. The data matrix used the 80% rule to remove missing values; that is, at least one set of variables with non-zero values above 80% was retained and then the vacant values were filled (the smallest value in the original matrix was used to fill in the vacant values). To reduce the errors caused by the sample preparation process and instrument instability, the response intensities of the sample mass spectrometry peaks were normalized by using the sum normalization method, and the normalized data matrix was obtained. The variables with relative standard deviation (RSD) > 30% for QC samples were also removed and log10 logarithmic processing was performed to obtain the final data matrix for subsequent analysis. The MS and MSMS mass spectrometry information was also matched with the metabolic public databases HMDB (http://www.hmdb.ca/ (accessed on 15 February 2022)) and Metlin (https://metlin.scripps.edu/ (accessed on 15 February 2022)) to obtain metabolite information.
The pre-processed data were uploaded on the Megabio cloud platform (https://cloud.majorbio.com (accessed on 21 February 2022)) to perform data analysis. The R software package (v3.2.4) was used for principal component analysis (PCA) and orthogonal least squares discriminant analysis (OPLS-DA), while seven rounds of interaction validation were performed to assess the stability of the model. Additionally, Student’s t-test and difference multiple analyses were also conducted. The selection of significantly different metabolites was based on the variable weight value (VIP) obtained from the OPLS-DA model and the Student’s t-test, p value. Metabolites with VIP > 1 and p < 0.05 were significantly different metabolites. Furthermore, the pathways in which the differential metabolites were involved were obtained by metabolic pathway annotation of the screened differential metabolites using the KEGG database (https://www.kegg.jp/kegg/pathway.html (accessed on 28 February 2022)). The Python package scipy. stats were also used for pathway enrichment analysis and Fisher’s exact test was employed to obtain the experimental treatment with the most relevant biological pathways. The KEGG compound classification bar chart compared the identified metabolites to the KEGG Compound database (https://www.kegg.jp/kegg/compound/ (accessed on 28 February 2022)) to obtain a metabolite classification profile and statistical plotting. The PLS-DA score plot from the R software package (v3.2.4) was used to visualize the classification effect of the model, while volcano and bubble plots, also from the R software package (v3.2.4), were also constructed. The analysis and plotting of the differential metabolite bar graphs were performed using the GraphPad Prism software package v8.0.1 (Microsoft Windows, Los Angeles, CA, USA). Tricarboxylic acid (TCA) cycle diagrams were drawn with Visio v2021. Heat maps were plotted using R software (v3.2.4). Based on the Spearman correlation |r| > 0.5 and p < 0.05, the top 30 microbial bacteria were selected for correlation network graph analysis with differential metabolites [36].
3. Results
3.1. Changes in Soil Physicochemical Properties in Grazing Exclusion
GE significantly increased the plant cover, aboveground biomass, and belowground biomass (p < 0.05) (Table 1). Significant changes in the physicochemical properties of soils in the GE group were detected, compared with the G group. More specifically, the changes in the GE and G groups were the following: TP (p < 0.001), AP (p < 0.05), and TN (p < 0.05) were significantly higher in the GE group compared with the G group, while TK (p < 0.01) and C/N (p > 0.05) were significantly lower. SOC was also higher in the GE than in the G group, while AK, AN, and pH decreased; however, these results were not significantly different (Table 2).

Table 1.
Characteristics of sampling areas in the sealing process.

Table 2.
Changes in soil physicochemical properties during grazing exclusion.
3.2. Influence of Grazing Exclusion on Soil Bacterial Diversity
Alpha diversity refers to the diversity within a specific region or ecosystem, and the commonly used metrics are Chao, Ace, Sobs, Shannon, and Simpson, where Chao, Ace, and Sobs reflect community richness and Shannon and Simpson reflect community diversity. Our experimental results showed that GE significantly increased the values of Chao, Ace, and Sobs indices (p < 0.05), indicating that the richness of GE was significantly higher than that of G. The Shannon and Simpson indices did not change significantly (Table 3), A highly significant difference in bacterial community beta diversity between GE and G (p < 0.01) was also observed, indicating that the soil bacterial community changed significantly between the GE conditions and that bacterial diversity increased after GE (Figure 1A).

Table 3.
Alpha diversity indices during grazing exclusion.
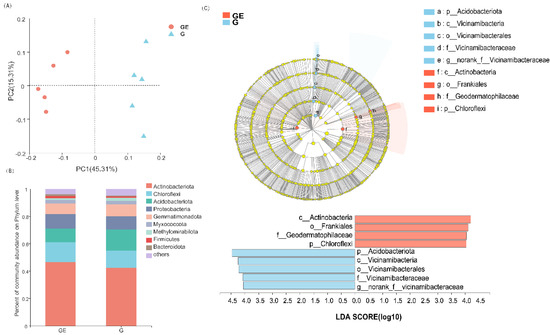
Figure 1.
Principal component analysis plot of soil bacterial community diversity (A), histogram of sample community structure at the bacterial phylum level (B), branch and score plots annotated with different species bands (C). GE: grazing exclusion, G: grazing.
3.3. Influence of Grazing Exclusion on Bacterial Community Composition
The difference in flora between GE and G was mirrored in the shift in the relative abundance of bacterial populations. The PCoA diagram showed that PC1 was 45.31 percent and PC2 was 15.31 percent, both falling within the 95% confidence range. Figure 1A showed that there was a definite division between the bacterial populations in the GE group and the G group. The abundance of bacterial communities under the GE treatment was accounted for as follows: Actinobacteria predominated, making up 46.6% of all bacteria. Chloroflexi made up 14.5% of all bacteria, while Acidobacteriota, Proteobacteria, Gemmatimonadota, Myxococcota, Methylomirabilota, Firmicutes, and Bacteroidota made up the remaining percentages (Figure 1B). To accurately determine the specific bacterial communities under GE versus G conditions, statistical analyses were performed using the LEfSe tool from the phylum level to the genus level with an LDA score of >4 (Figure 1C). Chloroflexi at the phylum level, Actinobacteria at the phylum level, Frankiales at the order level, and Geodermatophilaceae at the family level were enriched in the GE group. Acidobacteriota and Vicinamibacteria at the phylum level, Vicinamibacterales at the order level, Vicinamibacteraceae at the family level, and norank_f__Vicinamibacteraceae at the genus level were enriched in group G. The functional genes prediction was conducted by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt). Among them, metabolomic analysis allows us to observe different metabolic pathways, especially carbohydrate, amino acid, and lipid metabolism. The PICRUSt results showed that GE resulted in a relative upregulation of carbohydrate, amino acid, and lipid metabolism functions.
3.4. Impact of Grazing Exclusion on Soil Metabolites
The LC-MS untargeted metabolomics analysis identified 291 compounds, of which 232 were identified in the positive ion mode and 59 were identified in the negative ion mode. The PLS-DA results also revealed a clear separation between soil metabolite groups in the GE and G groups, indicating that GE did have a significant influence on metabolite accumulation in the soil (Figure 2A). The types of differential metabolites were mainly concentrated in four major groups: lipids and lipid-like molecules, phenylpropanoids and polyketides, organic acids and derivatives, and organic oxygen compounds (Figure 2B). A total of 60 differential metabolites were detected, of which 41 were upregulated and 19 were downregulated (Figure 2C). Further enrichment analysis of the signaling pathways in which the differential metabolites are directly involved was also performed, identifying the ABC transport, carbohydrate digestion, and absorption, as well as cutin, suberin, and wax biosynthesis (Figure 2D) pathways.
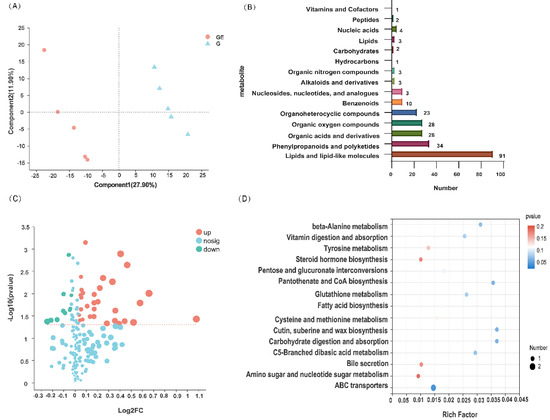
Figure 2.
PLS-DA plots of metabolites during grazing exclusion and grazing (A), categorical statistics of detected metabolites (B), volcano plots (C), and metabolic pathway enrichment bubble plots (top 20) (D). GE: grazing exclusion, G: grazing.
Twelve major differential metabolites were involved in the pathway of enrichment. Hexadecanedioic acid, myristic acid, and 4-methylene-L-glutamine were significantly lower under the GE measure, whereas 15-hydroxyandrosta-3,17-dione glucuronide, 2-(4-hydroxyphenyl) ethanol, pantothenic acid, S-adenosylhomocysteine, lactosamine, pyroglutamic acid, choline sulfate, estriol-16-glucuronide, and maltotriose were significantly higher under the GE measure (Figure 3).
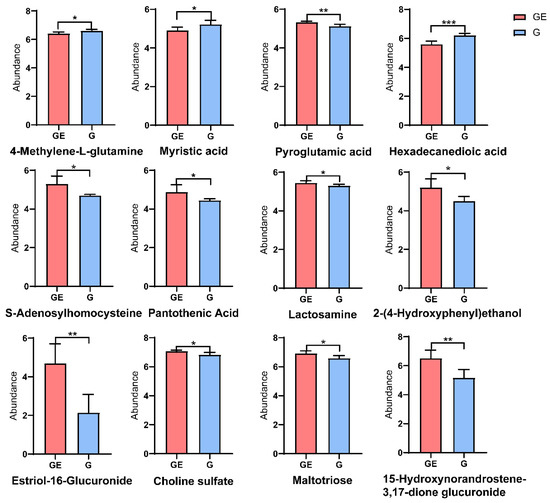
Figure 3.
Microbial differential metabolites in grazing-excluded versus grazing-treated soils (where p < 0.001 is marked as ***, p < 0.01 is marked as **, and p < 0.05 is marked as *). GE: grazing exclusion, G: grazing.
The differential metabolites between GE and G were mainly carbohydrates, lipids, and amino acids. Based on the metabolic pathways in the KEGG database, combined with microbial functional genes and metabolites, the relationship between soil microbial communities and their metabolism could be described. PICRUSt, which was used to couple soil metabolites with predicted functional genes in the corresponding metabolic pathways, enabled the TCA cycle metabolic map to be constructed [37]. As can be observed from Figure 4, the increase in S-adenosylhomocysteine and 2-(4-hydroxyphenyl) ethanol in the GE group indicated that sequestration significantly promoted the synthesis and metabolism of amino acids and, at the same time, facilitated the synthesis of succinate, fumarate, and oxaloacetate in the TCA cycle. GE also increased estriol-16-glucuronide synthesis in the lipid metabolic network, and cis-aconitate inhibited 4-methyleneL-glutamine synthesis in the carbohydrate metabolic network. However, both lactosamine and 15-hydroxynorandrostene-3,17-dione glucuronide were increased in the carbohydrate metabolic network, which promoted the synthesis of 2-oxoglutarate. During the TCA cycle, acetyl-CoA inhibited the biosynthesis of the lipid compounds hexadecanedioic acid and myristic acid in the GE group.
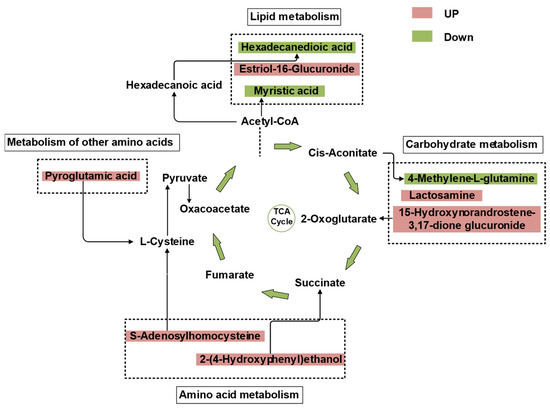
Figure 4.
Metabolic network of soil carbohydrates, amino acids, and lipids. Red represents upregulation and green represents downregulation.
Based on the heat map analysis, significant correlations were also found between the soil physicochemical properties and both bacterial communities and differential metabolites (Figure 5). For the bacterial communities, TK was significantly positively correlated with Acidobacteria, while TP, SOC, and TN were significantly positively correlated with Chloroflexi. Interestingly, Gemmatimonadota was most significantly and negatively correlated with physicochemical factors (Figure 5A). TP, TK, and AP had a highly significant positive correlation with differential metabolites. TP and AP were significantly positively correlated with pyroglutamic acid and choline sulfate, and TK was highly significantly positively correlated with hexadecanedioic acid and significantly negatively correlated with 15-hydroxynorandrostene-3,17-dione glucuronide and estriol-16-glucuronide (Figure 5B). The RDA plots showed that TP, TK, AP, and AN were the main factors affecting the bacterial community, and TK and C/N were the main factors affecting the soil metabolites (Figure 6).
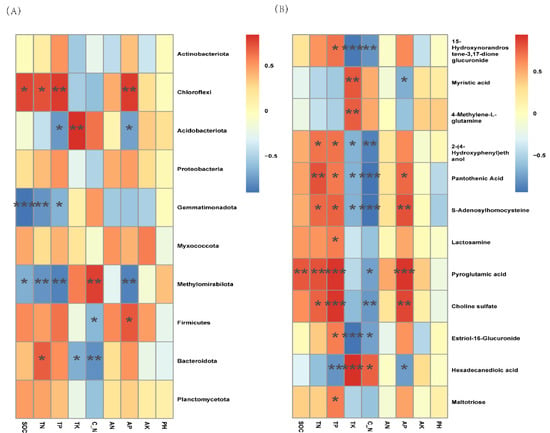
Figure 5.
Correlations between top 10 abundances of bacteria and physicochemical factors (A) and between differential metabolites and soil physicochemical factors (B) (where p < 0.001 is marked ***, p < 0.01 is marked **, and p < 0.05 is marked *).
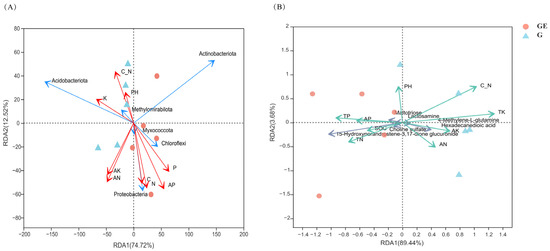
Figure 6.
The plot of redundancy analysis of bacterial community at gate level with physicochemical factors during sequestration (A), and redundancy analysis of differential metabolites with physicochemical factors (B). GE: grazing exclusion, G: grazing.
3.5. Metabolic Differentials and Bacterial Communities for Joint Analysis
The bacterial association network analysis graph of the differential metabolites and the top 30 bacteria consisted of 192 nodes, with dominant bacteria closely associated with metabolites at the genus level. Among the differential metabolites, those with a high number of nodes connected to bacterial genera were selected to illustrate the relationship with the bacteria; these were choline sulfate, pyroglutamic acid, S-adenosylhomocysteine, and lactosamine, as displayed in Figure 7. Choline sulfate had the greatest correlation with soil bacteria, being correlated with 17 bacterial genera. Choline sulfate was positively correlated with two genera under Acidobacteriota, and negatively correlated with genera under Gemmatimonadota. Choline sulfate was mostly positively correlated with genera under the Chloroflexi branch. Pyroglutamic acid was correlated with 15 bacterial genera, mostly negatively. The genera of the Actinobacteriota phylum had the highest number of correlation nodes but with equal numbers of positive and negative correlations. Pyroglutamic acid was mostly positively correlated with the genera of the Chloroflexi phylum, negatively correlated with the genera under Gemmatimonadota, and negatively correlated with the genera under Acidobacteriota. S-Adenosylhomocysteine was correlated with 10 bacterial genera, mostly positively. The genera of the Actinobacteriota phylum had the highest number of correlation nodes, and most of the correlations with genera under the Chloroflexi phylum were positive. Furthermore, S-adenosylhomocysteine was positively correlated with the genera of the Gemmatimonadota phylum. Lactosamine was correlated with nine bacterial genera, mostly negatively. The genera of the Actinobacteriota family exhibited the highest number of correlated nodes. Most of the correlations with genera of the Chloroflexi family were negatively correlated with lactosamine, while most of the correlations with genera of the Gemmatimonadota branch were positive.
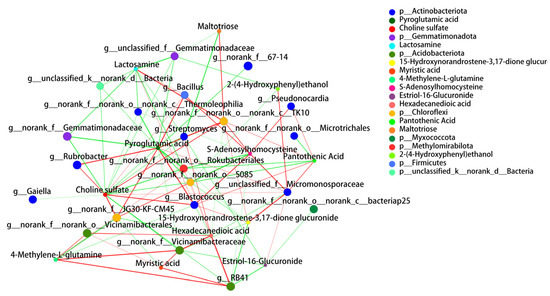
Figure 7.
The taxa correlation network diagram mainly reflects the correlation between bacteria and differential metabolites at taxonomic levels under grazing exclusion conditions. The size of the nodes in the diagram indicates the species abundance, and different colors indicate different species; the colors of the connecting lines indicate positive and negative correlations; red indicates positive correlation and green indicates negative correlation.
4. Discussion
4.1. Impact of Grazing Exclusion on Soil Physicochemical Factors
The cover, above-ground biomass of shrubs, above-ground biomass of herbs, litter biomass, and below-ground biomass were significantly higher in the GE group than in the G group. This indicated that the removal of grazing was beneficial to the recovery of grassland and was an effective measure for grassland restoration. Compared to the G group, a significant improvement in the physicochemical properties of the soil during GE was also observed. The effective increase in AN content [38], as well as TN, TP, and SOC content under GE [39], as demonstrated by previous studies [40], would increase the availability of resources, while the variation in C, N, and P content during nutrient cycling is considered to be an important factor for ecological stability [41]. In the present study, SOC increased under GE conditions, while previous studies have shown that GE is an effective method for carbon sequestration in soils [42]. The observed decrease in C/N in the present study [43] may have been due to the accumulation of animal manure and urine in the grazing area and the increase in AN [44]. These results indicated that the decomposition of soil microorganisms was faster during GE.
4.2. Impact of Grazing Exclusion on Bacterial Communities and Their Drivers
During the restoration of grassland ecosystems, microorganisms’ community structure and function showed adaptation to the GE conditions [45]. It was also found that the dominant bacterial communities in the GE and G areas were similar but differed in relative abundance. The most valuable bacteria phylum in the entire GE was Actinobacteria, which are widely distributed terrestrial Gram-positive bacteria that are crucial for the upkeep of metabolism and the transformation of organic matter [46,47]. Geodermatophilaceae is a family of Actinobacteria. In addition, bacteria belonging to this family is regarded as one of the pioneer organisms of extreme environments and is generally more tolerant to harsh environments, such as drought and low nutrients [22]. This experiment’s findings are in line with those of earlier research [48]. The most abundant genus in both the GE and G group was Actinomycetes, indicating that this bacterial group had adapted to drought conditions [46]. An increase in the abundance of Actinomycetes was detected in the GE group compared to the G group and GE was conducive to its survival. The abundance of Chloroflexi in the GE area was higher than that in the G area, indicating that GE promotes the growth of Chloroflexi. Chlorflexi can participate in microbially driven geochemical cycles by oxidizing low concentrations of atmospheric CO to CO2 into the biosphere and Chloroflexi also participates in biogeochemical cycles of elements such as N and S [49,50]. Chloroflexi were usually affected by TP, SOC, and TN, as shown by the significant positive correlations with these parameters. The environmental factors SOC, TN, and TP had a positive effect on the abundance of Chloroflexi. Furthermore, the GE area had more Proteobacteria than the G area. Various literature reports have shown that Proteobacteria may be beneficial to soil restoration: when nutrients are available, they gradually enrich [51,52]. Thus, the dominant abundance of Proteobacteria in the GE area demonstrated GE to be an effective measure to restore grassland. The abundance of Acidobacteria communities in area G was higher than that in area GE, which is consistent with previous findings [53]. Acidobacteria abundance was higher in the G region, suggesting that Acidobacteria are oligotrophic and prefer nutrient-poor environments [54]. This phylum was significantly positively correlated with TK, indicating that TK had a positive effect on Acidobacteria abundance, whereas it was negatively correlated with TP and AP, indicating that TP and AP had a negative effect on Acidobacteria abundance. The dominance of Gemmatimonades in the G area compared to the GE area could be attributed to their stronger oligotrophic properties, as this phylum was negatively correlated with TP, TN, and SOC. Additionally, the RDA analysis plots showed that TP, TK, AP, and AN were the main factors affecting the bacterial community structure.
4.3. Impact of Grazing Exclusion on Soil Metabolism and Its Drivers
Soil metabolomics can reveal changes in soil and material cycles [55]. The results of this study showed that GE measures caused significant changes in soil metabolites, and the KEGG pathway enrichment of soil differential metabolites revealed that GE altered lipid, amino acid, and carbohydrate contents. This is similar to the previous results on inter-root metabolites of maize under different treatments [56]. Amino acids and fatty acids are important primary metabolites that promote metabolism and biosynthesis [57], with the metabolic pathways of both being related to carbon and nitrogen metabolism [58]. From the 12 differential metabolites that were found to be enriched via the KEGG analysis, GE was also found to promote the synthesis of amino acid metabolites and the inhibition of lipid metabolite synthesis [59]. Under the GE conditions, the content of many amino acid metabolites was increased, for example, it promoted the synthesis of S-adenosine homocysteine and 2-(4-hydroxyphenyl)ethanol compounds. S-adenosylhomocysteine and 2-(4-hydroxyphenyl) ethanol had positive significant correlations with TN and significant negative correlations with TK and C/N. Pyroglutamic acid had a significant positive correlation with SOC, TN, TP, and AP, and a significant negative correlation with C/N. However, G increased the content of lipid metabolites, such as myristic acid and hexadecanedioic acid, which has been shown to increase under unfavorable conditions as an adaptation to environmental changes [60]. Both myristic acid and hexadecanedioic acid were significantly positively correlated with TK and negatively correlated with AP. Carbohydrates are important energy supply substances in the metabolism of biological growth. GE promoted the synthesis of carbohydrates such as lactosamine and 15-hydroxynorandrostene-3,17-dione glucuronide, both of which were significantly positively correlated with TP, and 15-hydroxynorandrostene-3,17-dione glucuronide was significantly negatively correlated with TK and C/N. Furthermore, the RDA analysis plot showed that TK and C/N were the main factors affecting the differential metabolites.
4.4. Joint Analysis of Bacterial Communities and Differential Metabolites
The construction of an interaction network between the microbial bacterial communities and differential metabolites revealed that microbial communities were closely linked to differential metabolites [61]. Like the results of previous studies, there was a large correlation between soil microorganisms and soil metabolites in the GE and G areas [58]. For example, under GE conditions, choline sulfate was mainly negatively correlated with soil bacteria abundance and an increase in choline sulfate may have been associated with a decrease in the abundance of negatively correlated bacterial communities. The next-most correlated metabolite with bacteria was pyroglutamic acid, which was detected in enhanced quantities under GE conditions compared to G. It can therefore be deduced that if microorganisms are negatively correlated with metabolites, then these metabolites are detrimental to the growth of microorganisms. A third metabolite that was closely associated with bacterial abundance was S-adenosylhomocysteine, a metabolite that was often positively correlated with microorganisms. The positive correlation between microorganisms and metabolites leads to the inference that these metabolites are favorable for microbial growth [62].
5. Conclusions
In this study, we systematically analyzed how soil physicochemical properties, microbial diversity, and soil metabolism changed under GE measures. GE exhibited significant positive effects on TP, TN, and AP, while the structure of the soil microbial community was greatly altered, and bacterial diversity and community composition were affected. The metabolomics analysis showed that GE significantly and positively affected amino acid and carbohydrate metabolism. Overall, GE had a positive impact on bacterial diversity and metabolism. This work provides a sound scientific approach to investigating the influence of GE on desert grasslands.
Author Contributions
Conceptualization, M.G., X.W. and X.L.; methodology, M.G., X.W. and X.L.; software, M.G.; writing—original draft preparation, M.G.; investigation, M.G., X.W., X.L. and P.L.; writing—review and editing, X.W. and X.L.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Xinjiang Uygur Autonomous Region, project number (2022D01C397).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cheng, J.M.; Jing, G.H.; Wei, L.; Jing, Z.B. Long-term grazing exclusion effects on vegetation characteristics, soil properties, and bacterial communities in the semi-arid grasslands of China. Ecol. Eng. 2016, 97, 170–178. [Google Scholar] [CrossRef]
- Kang, B.T.; Bowatte, S.; Hou, F.J. Soil microbial communities and their relationships to soil properties at different depths in an alpine meadow and desert grassland in the Qilian mountain range of China. J. Arid. Environ. 2021, 184, 104316. [Google Scholar] [CrossRef]
- Farrell, H.L.; Barberan, A.; Danielson, R.E.; Fehmi, J.S.; Gornish, E.S. Disturbance is more important than seeding or grazing in determining soil microbial communities in a semiarid grassland. Restor. Ecol. 2020, 28, S335–S343. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.S.; Shao, X.Q.; Li, H.; He, Y.X.; Sirimuji; Wang, B.J. Microbes require a relatively long time to recover in natural succession restoration of degraded grassland ecosystems. Ecol. Indic. 2021, 129, 107881. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.K.; Gao, Q.Z.; Fan, C.; Fayiah, M.; Ganjurjav, H.; Hu, G.Z.; Wang, X.X.; Yan, Y.L.; Gao, X.X.; et al. Grazing changed plant community composition and reduced stochasticity of soil microbial community assembly of alpine grasslands on the Qinghai-Tibetan plateau. Front. Plant Sci. 2022, 13, 864085. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Z.W.; Yan, Q.Y.; Yang, S.; Van Nostrand, J.D.; Wang, Z.R.; He, Z.L.; Zhou, J.Z.; Jiang, Y.; Deng, Y. Soil microbial beta-diversity is linked with compositional variation in aboveground plant biomass in a semi-arid grassland. Plant Soil 2018, 423, 465–480. [Google Scholar] [CrossRef]
- Cui, Y.X.; Dong, Y.Q.; Liu, H.X.; Sun, Z.J. Short-term grazing exclusions reduced soil organic carbon but not bacterial diversity in the sagebrush desert, Northwest China. Glob. Ecol. Conserv. 2021, 31, e01872. [Google Scholar] [CrossRef]
- Wang, H.H.; Li, J.Y.; Zhang, Q.C.; Liu, J.; Yi, B.; Li, Y.; Wang, J.W.; Di, H.J. Grazing and enclosure alter the vertical distribution of organic nitrogen pools and bacterial communities in semiarid grassland soils. Plant Soil 2019, 439, 525–539. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, W.; Cao, W.X.; Abalori, T.A.; Liu, Y.Z.; Xin, Y.Q.; Wang, S.L.; Zhang, D.G. Soil bacterial community responses to short-term grazing exclusion in a degraded alpine shrubland–grassland ecotone. Ecol. Indic. 2021, 130, 108043. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Y.; Jin, K.; Struik, P.C.; Sun, S.X.; Ji, B.M.; Zhang, Y.J.; Li, X.L. Soil bacterial and fungal communities are linked with plant functional types and soil properties under different grazing intensities. Eur. J. Soil Sci. 2022, 73, e13195. [Google Scholar] [CrossRef]
- Ng, C.W.W.; Tasnim, R.; Capobianco, V.; Coo, J.L. Influence of soil nutrients on plant characteristics and soil hydrological responses. Géotechnique Lett. 2018, 8, 19–24. [Google Scholar] [CrossRef]
- Wu, J.Q. Effects of Vegetation Degradation on Soil Physicochemical Properties and Enzyme Activities of Gahai Wet Meadow. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2021. (In Chinese). [Google Scholar]
- Nian, Y.P.; Lui, L.X.; Yi, L.C.; Wei, D.C. Response of soil microbial diversity to long-term enclosure in degraded patches of alpine meadow in the source zone of the yellow river. Environ. Sci. 2023, 44, 2293–2303. [Google Scholar]
- Gao, F.; Wang, B.; Shi, Y.X.; Zhang, G.X.; Wang, J.; Si, G.C.; Han, C.H.; Yuan, Y.L.; Hu, A. The response of alpine grasslands ecosystem in the north Tibet to short-term enclosure. Acta Ecol. Sin. 2017, 37, 4366–4374. [Google Scholar]
- Gao, Y.N. Effects of Grazing Prohibition on Grassland Soil Microbial Community Structure and Function in Yunwu Mountain. Master’s Thesis, North West Agriculture and Forestry University, Xianyang, China, 2022. (In Chinese). [Google Scholar]
- Yin, Y.L.; Wang, Y.Q.; Li, S.X.; Liu, Y.; Zhao, W.; Ma, Y.S.; Bao, G.S. Soil microbial character response to plant community variation after grazing prohibition for 10 years in a Qinghai-Tibetan alpine meadow. Plant Soil 2021, 458, 175–189. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Travers, S.K.; Val, J.; Wang, J.T.; Liu, H.W.; Singh, B.K.; Delgado-Baquerizo, M. Grazing regulates the spatial heterogeneity of soil microbial communities within ecological networks. Ecosystems 2020, 23, 932–942. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.B.; Song, Z.L.; Wang, J.; Guo, L. Interactions of soil bacteria and fungi with plants during long-term grazing exclusion in semiarid grasslands. Soil Biol. Biochem. 2018, 124, 47–58. [Google Scholar] [CrossRef]
- Goenster-Jordan, S.; Ingold, M.; Jannoura, R.; Buerkert, A.; Joergensen, R.G. Soil microbial properties of subalpine steppe soils at different grazing intensities in the Chinese Altai Mountains. Sci. Rep. 2021, 11, 1653. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Liang, C.; Chen, L.J.; Wang, H.T.; Xu, Q.S.; Jiang, Y.J.; Sun, B. Coupling bacterial community assembly to microbial metabolism across soil profiles. Msystems 2020, 5, e00298-20. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Staley, C.; Gao, H.L.; Ishii, S.; Wei, X.R.; Liu, J.; Cheng, J.M.; Hao, M.D.; Sadowsky, M.J. Impact of long-term grazing exclusion on soil microbial community composition and nutrient availability. Biol. Fertil. Soils 2019, 55, 121–134. [Google Scholar] [CrossRef]
- Pereira, A.P.D.; Lima, L.A.L.; Bezerra, W.M.; Pereira, M.L.; Normando, L.R.O.; Mendes, L.W.; de Oliveira, J.G.B.; Araujo, A.S.F.; Melo, V.M.M. Grazing exclusion regulates bacterial community in highly degraded semiarid soils from the Brazilian Caatinga biome. Land Degrad. Dev. 2021, 32, 2210–2225. [Google Scholar] [CrossRef]
- Guo, Y.J.; Du, Q.F.; Li, G.D.; Ni, Y.; Zhang, Z.; Ren, W.B.; Hou, X.Y. Soil phosphorus fractions and arbuscular mycorrhizal fungi diversity following long-term grazing exclusion on semi-arid steppes in Inner Mongolia. Geoderma 2016, 269, 79–90. [Google Scholar] [CrossRef]
- Li, Y.X.; Laborda, P.; Xie, X.L.; Zhou, R.; Chen, Y.; Li, T.; Pu, Z.H.; Wang, Y.L.; Deng, Z.F. Spartina alterniflora invasion alters soil microbial metabolism in coastal wetland of China. Estuar. Coast. Shelf Sci. 2020, 245, 106982. [Google Scholar] [CrossRef]
- Yu, J.L.; Bing, H.J.; Chang, R.Y.; Cui, Y.X.; Shen, G.T.; Wang, X.X.; Zhang, S.P.; Fang, L.C. Microbial metabolic limitation response to experimental warming along an altitudinal gradient in alpine grasslands eastern Tibetan Plateau. Catena 2022, 214, 106243. [Google Scholar] [CrossRef]
- Bi, B.Y.; Wang, K.; Zhang, H.; Wang, Y.; Fei, H.Y.; Pan, R.P.; Han, F.P. Plants use rhizosphere metabolites to regulate soil microbial diversity. Land Degrad. Dev. 2021, 32, 5267–5280. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Sdepanian, S.; Lofts, S.; Svendsen, C.; Spurgeon, D.J.; Maguire, M.L.; Griffin, J.L. Metabolomic analysis of soil communities can be used for pollution assessment. Environ. Toxicol. Chem. 2014, 33, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.P.; Li, W.J.; Wang, J.Y.; Zhu, Y.F.; Feng, Y.Z.; Yang, G.H.; Zhang, W.; Han, X.H. Soil eco-enzymatic stoichiometry reveals microbial phosphorus limitation after vegetation restoration on the Loess Plateau, China. Sci. Total Environ. 2022, 815, 152918. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.X.; Zhang, X.C.; Ju, W.L.; Duan, C.J.; Wang, Y.Q.; Fang, L.C. A novel extracellular enzyme stoichiometry method to evaluate soil heavy metal contamination: Evidence derived from microbial metabolic limitation. Sci. Total Environ. 2020, 738, 139709. [Google Scholar] [CrossRef]
- Withers, E.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Use of untargeted metabolomics for assessing soil quality and microbial function. Soil Biol. Biochem. 2020, 143, 107758. [Google Scholar] [CrossRef]
- Abram, F. Systems-based approaches to unravel multi-species microbial community functioning. Comput. Struct. Biotechnol. J. 2015, 13, 24–32. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.N.; Yao, S.; Yang, X.L.; Jiang, X. Correlations between soil metabolomics and bacterial community structures in the pepper rhizosphere under plastic greenhouse cultivation. Sci. Total Environ. 2020, 728, 138439. [Google Scholar] [CrossRef]
- Lian, T.X.; Huang, Y.Y.; Xie, X.N.; Huo, X.; Shahid, M.Q.; Tian, L.; Lan, T.; Jin, J. Rice SST variation shapes the rhizosphere bacterial community, conferring tolerance to salt stress through regulating soil metabolites. MSystems 2021, 5, e00721-20. [Google Scholar] [CrossRef] [PubMed]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537. [Google Scholar] [CrossRef] [PubMed]
- Barberan, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Tejero Rioseras, A.; Singh, K.D.; Nowak, N.; Gaugg, M.T.; Bruderer, T.; Zenobi, R.; Sinues, P.M.L. Real-time monitoring of tricarboxylic acid metabolites in exhaled breath. Anal. Chem. 2018, 90, 6453–6460. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, D.; Li, Q.; Wang, Q. Effects of Long-Term Enclosing on Vertical Distributions of Soil Physical Properties and Nutrient Stocks in Grassland of Inner Mongolia. Agronomy 2021, 11, 1832. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhao, H.; Zhao, X.Y.; Zhang, T.H.; Li, Y.L.; Cui, J.Y. Effects of grazing and livestock exclusion on soil physical and chemical properties in desertified sandy grassland, Inner Mongolia, northern China. Environ. Earth Sci. 2011, 63, 771–783. [Google Scholar] [CrossRef]
- Yang, F.; Niu, K.C.; Collins, C.G.; Yan, X.B.; Ji, Y.G.; Ling, N.; Zhou, X.H.; Du, G.Z.; Guo, H.; Hu, S.J. Grazing practices affect the soil microbial community composition in a Tibetan alpine meadow. Land Degrad. Dev. 2019, 30, 49–59. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Shi, L.L.; Zhou, Y.; Yang, M.; Cao, J.X.; Wu, S.H.; Lei, G.C. Effects of different grazing intensities on soil C, N, and P in an alpine meadow on the Qinghai—Tibetan Plateau, China. Int. J. Environ. Res. Public Health 2018, 15, 2584. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.S.; Fu, B.J.; Zhou, W.M.; Liu, H.F.; Liu, G.H. Restoration of ecosystem carbon and nitrogen storage and microbial biomass after grazing exclusion in semi-arid grasslands of Inner Mongolia. Ecol. Eng. 2014, 73, 395–403. [Google Scholar] [CrossRef]
- Bagchi, S.; Roy, S.; Maitra, A.; Sran, R.S. Herbivores suppress soil microbes to influence carbon sequestration in the grazing ecosystem of the Trans-Himalaya. Agric. Ecosyst. Environ. 2017, 239, 199–206. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.J.; Chang, S.J.; Kan, H.M.; Lin, L.J. Impact of grazing on soil carbon and microbial biomass in typical steppe and desert steppe of Inner Mongolia. PLoS ONE 2012, 7, e36434. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, Y.J.; Chang, S.J.; Kan, H.M.; Lin, L.J. Phylogenetic and functional changes in the microbial community of long-term restored soils under semiarid climate. Soil Biol. Biochem. 2013, 65, 12–21. [Google Scholar]
- Campbell, B.J.; Polson, S.W.; Hanson, T.E.; Mack, M.C.; Schuur, E.A.G. The effect of nutrient deposition on bacterial communities in Arctic tundra soil. Environ. Microbiol. 2010, 12, 1842–1854. [Google Scholar] [CrossRef]
- Stach, J.E.M.; Maldonado, L.A.; Ward, A.C.; Goodfellow, M.; Bull, A.T. New primers for the class Actinobacteria: Application to marine and terrestrial environments. Environ. Microbiol. 2003, 5, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Y.; Zhang, X.F.; Adamowski, J.F.; Biswas, A.; Holden, N.M.; Hu, Z.Y. Grassland grazing management altered soil properties and microbial β-diversity but not α-diversity on the Qinghai-Tibetan Plateau. Appl. Soil Ecol. 2021, 167, 104032. [Google Scholar] [CrossRef]
- Hug, L.A.; Castelle, C.J.; Wrighton, K.C.; Thomas, B.C.; Sharon, I.; Frischkorn, K.R.; Williams, K.H.; Tringe, S.G.; Banfield, J.F. Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 2013, 1, 22. [Google Scholar] [CrossRef]
- Jing, C.L.; Xu, Z.C.; Zou, P.; Tang, Q.; Li, Y.Q.; You, X.W.; Zhang, C.S. Coastal halophytes alter properties and microbial community structure of the saline soils in the yellow river delta, china. Appl. Soil Ecol. 2019, 134, 1–7. [Google Scholar] [CrossRef]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Ling, N.; Chen, D.M.; Guo, H.; Wei, J.X.; Bai, Y.F.; Shen, Q.R.; Hu, S.J. Differential responses of soil bacterial communities to long-term n and p inputs in a semi-arid steppe. Geoderma 2017, 292, 25–33. [Google Scholar] [CrossRef]
- Wang, Z.H.; Bai, Y.; Hou, J.F.; Li, F.; Li, X.Q.; Cao, R.; Deng, Y.Y.; Wang, H.B.; Jiang, Y.R.; Yang, W.Q. The changes in soil microbial communities across a subalpine forest successional series. Forests 2022, 13, 289. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.L.; Ji, B.M.; Struik, P.C.; Jin, K.; Tang, S.M. Coupling between the responses of plants, soil, and microorganisms following grazing exclusion in an overgrazed grassland. Front. Plant Sci. 2021, 12, 640789. [Google Scholar] [CrossRef]
- Yang, X.; Lai, J.L.; Zhang, Y.; Luo, X.G.; Han, M.W.; Zhao, S.P. Microbial community structure and metabolome profiling characteristics of soil contaminated by TNT, RDX, and HMX. Environ. Pollut. 2021, 285, 117478. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Long, F.Y.; Liu, J.B.; Liu, H.M.; Wang, H.Y. Effects of organic planting on metabolites in maize rhizosphere soil. J. Northeast. Agric. Univ. 2022, 53, 30–41. (In Chinese) [Google Scholar]
- Lin, R.; Liu, W.T.; Piao, M.Y.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, H.W.; Liang, A.P.; Liu, J.; Song, L.H.; Lv, M.; Zhu, D. Testosterone amendment alters metabolite profiles of the soil microbial community. Environ. Pollut. 2021, 272, 115928. [Google Scholar] [CrossRef]
- Li, Y.X.; Zou, J.; Zhu, H.H.; He, J.Q.; Setter, T.L.; Wang, Y.H.; Meng, Y.L.; Chen, B.L.; Zhao, W.Q.; Wang, S.S. Drought deteriorated the nutritional quality of cottonseed by altering fatty acids and amino acids compositions in cultivars with contrasting drought sensitivity. Environ. Exp. Bot. 2022, 194, 104747. [Google Scholar] [CrossRef]
- Li, X.N.; Qu, C.S.; Bian, Y.R.; Gu, C.G.; Jiang, X.; Song, Y. New insights into the responses of soil microorganisms to polycyclic aromatic hydrocarbon stress by combining enzyme activity and sequencing analysis with metabolomics. Environ. Pollut. 2019, 255, 113312. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: A review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef]
- Devi, S.; Rehman, S.A.A.; Tarique, K.F.; Gourinath, S. Structural characterization and functional analysis of cystathionine β-synthase: An enzyme involved in the reverse transsulfuration pathway of Bacillus anthracis. FEBS J. 2017, 284, 3862–3880. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).