Performance of a Full-Scale Vermifilter for Sewage Treatment in Removing Organic Matter, Nutrients, and Antibiotic-Resistant Bacteria
Abstract
1. Introduction
2. Materials and Methods
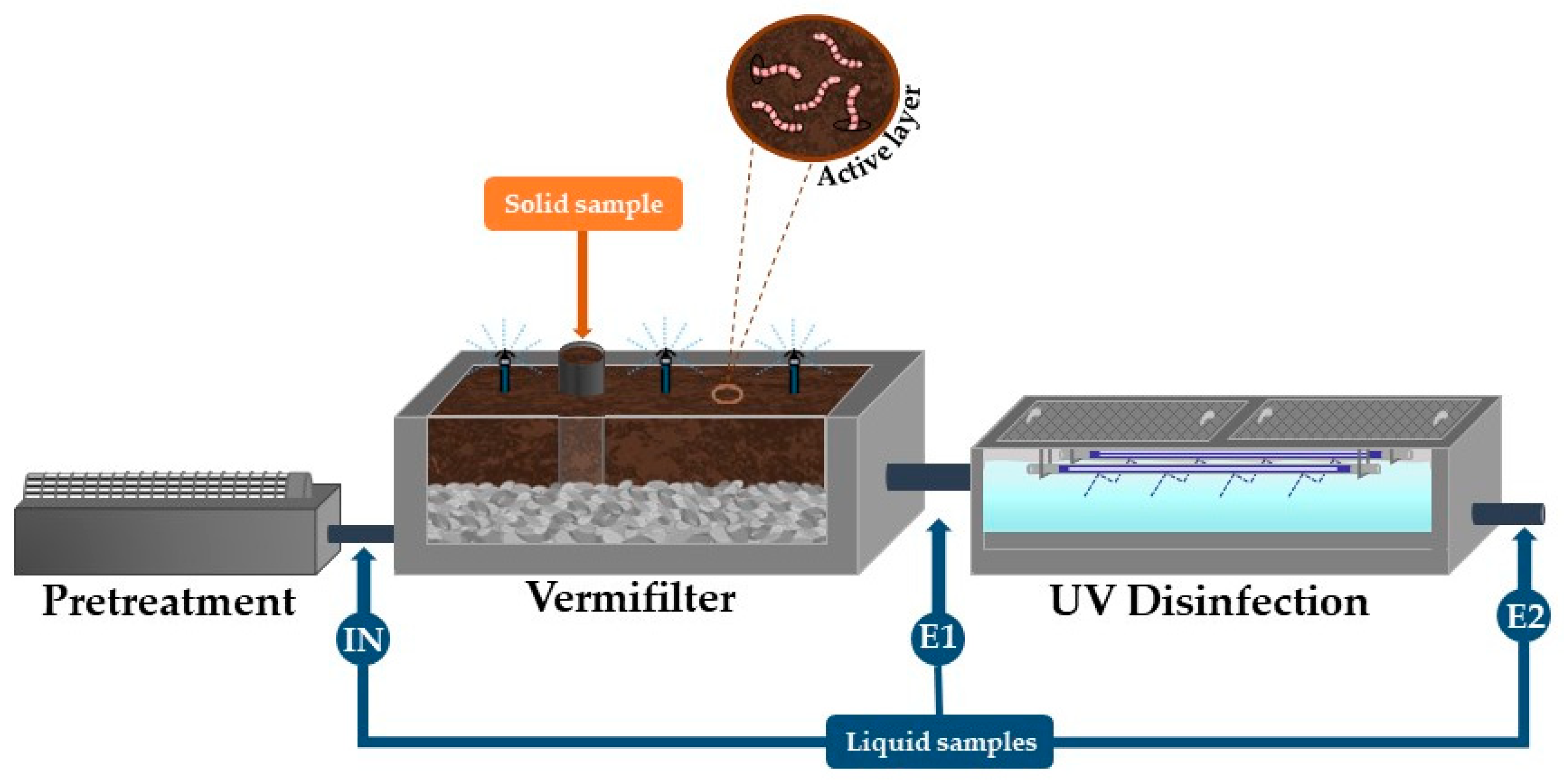
2.1. Design and Operating Conditions
2.2. Monitoring Strategy
2.3. Physicochemical Analysis
2.3.1. Liquid Samples
2.3.2. Solid Samples
2.4. Molecular Weight Distribution Analysis of Liquid Samples
2.5. Microbiological Analysis of Liquid Samples
2.6. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Vermifilter Performance in Organic Matter and Nutrient Removal
3.1.1. Organic Matter
| Scale | Volume (m3) | Active Layer | Density (worm/m3) | HLR (m3/m2d) | OLR (kg COD /m2d) | Organic Matter (% Removal) | Nutrients (% Removal) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| COD | BOD5 | TN | TP | |||||||
| Pilot | 0.50 | Woodchips | 13,263 | 1.30 | 1.20 | 91 | 91 | 3 | 15 | [39] |
| Pilot | 1.50 | Compost/soil | 5000–6000 | 2.00 | 0.37 | 60 | - | - | - | [45] |
| Pilot | - | Woodchips | - | 0.48 | 1.70–2.93 | 84 | - | 65 | 70 | [25] |
| Pilot | 0.10 | Sand | 285 | 0.08 | 0.07 | 68 | - | 60 | 45 | [47] |
| Pilot | 0.30 | Organic fraction/vermigratings | 7143 | 1.00 | 0.24 | 78 | 88 | - | - | [27] |
| Pilot | 0.02 | Vermigratings | 30,000 | 1.00 | 0.45 | 74 | 90 | - | - | [11] |
| Pilot | 0.10 | Soil/woodchips | 8929 | 1.05 | 0.11–0.43 | - | 61 | 62 | [48] | |
| Pilot | 0.45 | Soil/woodchips | 3061 | 0.06 | 0.01–0.02 | 81 | - | 80 | 81 | [35] |
| Full-scale | 4.20 | Ceramsite | 16,000 | 4.20 | 0.39 | 68 | 78 | - | - | [24] |
| Full-scale | 433 | Woodchips | A:1100 B: 7000 | 0.50 | 0.60 | 77 | 84 | 53 | 36 | Present study |
3.1.2. Nutrients
3.1.3. Molecular Weight Distribution Analysis of Organic Matter and Nutrients
3.2. Effect of Seasonality on the Removal of Organic Matter and Nutrients by a Vermifilter
3.2.1. Organic Matter
3.2.2. Nutrients
3.3. Removal of Microbiological Compounds by the Vermifilter
3.3.1. Coliforms
3.3.2. Antibiotic-Resistant Bacteria
4. Conclusions
- Generates statistically significant removals (p < 0.05) of COD (77%), BOD5 (84%), TN (53%), and TP (36%). Seasonality is a factor that significantly influenced COD, BOD5, and TN removal. COD and BOD5 are eliminated at 9% and 11% higher rates in spring–summer, respectively, while TN is eliminated at a 21% higher rate in fall–winter. This is because the temperature is influential in the growth and activity of earthworms and microorganisms causing changes in removal efficiencies.
- The molecular weight distribution indicates that the organic matter (COD and TOC) percentage decreases by an average of 6% in the <1000 Da fraction after the VF, while it increases by an average of 16% in the >10,000 Da fraction; therefore, the VF does not generate considerable changes in the molecular weight distribution of organic matter and NH4+-N due to processes of degradation, adsorption, and the formation of aggregates with high molecular weights.
- Coliform removal by the VF was statistically significant (p < 0.05) at 99.4% and 98.9% for FC and TC, respectively, although the concentrations in the effluents were 6.2 and 5.8 log10(MPN/100 mL), respectively; therefore, the WWTP does not reduce coliforms to safe levels. In addition, ARB removal was not significant (p > 0.05); thus, ARB selection and ARG transfer processes are occurring within the system. The effluents discharged into the receiving river could lead to the dissemination of antibiotic resistance in the environment.
- In consideration of the results obtained, the projections of this research are focused on optimizing full-scale VFs in the elimination of organic matter, nutrients, and pathogens. This includes evaluating different operating and design parameters, determining the efficiency of VFs synchronized to other technologies, and specifying an adequate cost-effective disinfection method for the efficient removal of ARB and ARG.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Technical Brief on Water, Sanitation, Hygiene and Wastewater Management to Prevent Infections and Reduce the Spread of Antimicrobial Resistance; World Health Organization (WHO): Geneva, Switzerland, 2020; 32p.
- Geng, M.; Zhang, W.; Hu, T.; Wang, R.; Cheng, X.; Wang, J. Eutrophication causes microbial community homogenization via modulating generalist species. Water Res. 2022, 210, 118003. [Google Scholar] [CrossRef] [PubMed]
- Massoud, M.A.; Tarhini, A.; Nasr, J.A. Decentralized approaches to wastewater treatment and management: Applicability in developing countries. J. Environ. Manag. 2009, 90, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Milobedzka, A.; Ferreira, C.; Vaz-Moreira, I.; Calderón-Franco, D.; Gorecki, A.; Purkrtova, S.; Bartacek, J.; Dziewit, L.; Singleton, C.M.; Nielsen, P.H.; et al. Monitoring antibiotic resistance genes in wastewater environments: The challenges of filling a gap in the One-Health cycle. J. Hazard. Mater. 2022, 424, 127407. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2016–2059. [Google Scholar] [CrossRef]
- De Oliveira, D.; Forde, B.; Kidd, T.; Harris, P.; Schembri, M.; Beatson, S.; Paterson, D.; Walker, M. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.S.; Su, H.C.; Ying, G.G.; Liu, F.; Liu, S.S.; He, L.Y.; Chen, Z.F.; Yang, Y.Q.; Chen, F.R. Removal of antibiotics and antibiotic resistance genes in rural wastewater by an integrated constructed wetland. Environ. Sci. Pollut. Res. 2014, 22, 1794–1803. [Google Scholar] [CrossRef]
- Adugna, A.T.; Andrianisa, H.A.; Konate, Y.; Maiga, A.H. Fate of filter materials and microbial communities during vermifiltration process. J. Environ. Manag. 2019, 242, 98–105. [Google Scholar] [CrossRef]
- Arora, S.; Saraswat, S.; Mishra, R.; Rajvanshi, J.; Sethi, J.; Verma, A.; Nag, A.; Saxena, S. Design, performance evaluation and investigation of the dynamic mechanisms of earthworm-microorganisms interactions for wastewater treatment through vermifiltration technology. Bioresour. Technol. 2020, 12, 100603. [Google Scholar] [CrossRef]
- Tahar, A.; Feighan, J.; Hannon, L.; Clifford, E. Optimization of operational conditions and performances of pilot scale lumbrifiltration for real raw municipal wastewater treatment. Environ. Sci. Pollut. Res. 2022, 29, 32717–32731. [Google Scholar] [CrossRef]
- Huang, L.; He, J.; Jiang, C.; Weng, S.; Zhao, F.; Zhong, H.; Chen, Y. Strategies to alleviate clogging in constructed wetlands: What can be learned from the microbial fuel cell coupled membrane bioreactor? J. Clean. Prod. 2023, 405, 136973. [Google Scholar] [CrossRef]
- Zhou, X.; Fernández-Palacios, E.; Dorado, A.; Lafuente, J.; Gamisans, X.; Gabriel, D. The effect of slime accumulated in a long-term operating UASB using crude glycerol to treat S-rich wastewater. J. Environ. Sci. 2024, 135, 353–366. [Google Scholar] [CrossRef]
- Luan, Y.-N.; Yin, Y.; An, Y.; Zhang, F.; Wang, X.; Zhao, F.; Xiao, Y.; Liu, C. Investigation of an intermittently-aerated moving bed biofilm reactor in rural wastewater treatment under low dissolved oxygen and C/N condition. Bioresour. Technol. 2022, 358, 127405. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Y.; Hu, X.; Zeng, G.; Wang, H.; Zhou, L.; Tan, X.; Huang, B.; Liu, S. The use of microbial-earthworm ecofilters for wastewater treatment with special attention to influencing factors in performance: A review. Bioresour. Technol. 2016, 200, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bhunia, P.; Dash, R.R. A mechanistic review on vermifiltration of wastewater: Design, operation and performance. J. Environ. Manag. 2017, 197, 656–672. [Google Scholar] [CrossRef]
- Arora, S.; Kazmi, A.A. The effect of seasonal temperature on pathogen removal efficacy of vermifilter for wastewater treatment. Water Res. 2015, 74, 88–99. [Google Scholar] [CrossRef]
- Gutiérrez, V.; Gómez, G.; Rodríguez, D.; Vidal, G. Critical analysis of wastewater treatment using vermifilters: Operating parameters, wastewater quality, and greenhouse gas emissions. J. Environ. Chem. Eng. 2023, 11, 109683. [Google Scholar] [CrossRef]
- Muga, H.E.; Mihelcic, J.R. Sustainability of wastewater treatment technologies. J. Environ. Manag. 2008, 88, 437–447. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.Y.; Yang, J.; Lu, B. Addressing the role of earthworms in treating domestic wastewater by analyzing biofilm modification through chemical and spectroscopic methods. Environ. Sci. Pollut. Res. 2016, 23, 4768–4777. [Google Scholar] [CrossRef]
- Samal, K.; Dash, R.R.; Bhunia, P. Effect of hydraulic loading rate and pollutants degradation kinetics in two stage hybrid macrophyte assisted vermifiltration system. Biochem. Eng. J. 2018, 132, 47–59. [Google Scholar] [CrossRef]
- Wang, J.; Tang, X.; Liu, Y.; Xie, B.; Li, G.; Liang, H. Self-sustained ultrafiltration coupling vermifiltration for decentralized domestic wastewater treatment: Microbial community and mechanism. Resour. Conserv. Recycl. 2022, 177, 106008. [Google Scholar] [CrossRef]
- Kumar, T.; Bhargava, R.; Prasad, K.H.; Pruthi, V. Evaluation of vermifiltration process using natural ingredients for effective wastewater treatment. Ecol. Eng. 2015, 75, 370–377. [Google Scholar] [CrossRef]
- Arora, S.; Saraswat, S.; Rajpal, A.; Shringi, H.; Mishra, R.; Sethi, J.; Rajvanshi, J.; Nag, A.; Saxena, S.; Kazmi, A.A. Effect of earthworms in reduction and fate of antibiotic resistant bacteria (ARB) and antibiotic resistant genes (ARGs) during clinical laboratory wastewater treatment by vermifiltration. Sci. Total Environ. 2021, 773, 145152. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Z.; Zhang, J.; Xing, M.; Yang, J. Phylogenetic characterization of microbial communities in a full-scale vermifilter treating rural domestic sewage. Ecol. Eng. 2013, 61, 100–109. [Google Scholar] [CrossRef]
- Tompkins, D.; Bumbac, C.; Clifford, E.; Dussaussois, J.B.; Hannon, L.; Salvadó, V.; Schellenberg, T. EU Horizon 2020 research for a sustainable future: INNOQUA—A Nature-Based Sanitation Solution. Water 2019, 11, 2461. [Google Scholar] [CrossRef]
- 1010; Standard Methods for the Examination of Water and Wastewater (APHA). American Public Health Association: Washington, DC, USA, 2005.
- Arora, S.; Rajpal, A.; Kazmi, A.A. Antimicrobial activity of bacterial community for removal of pathogens during vermifiltration. J. Environ. Eng. 2016, 142, 04016012. [Google Scholar] [CrossRef]
- Caselles-Osorioa, A.; Puigaguta, P.; Segu, E.; Vaelloa, N.; Granés, F.; García, D.; García, J. Solids accumulation in six full-scale subsurface flow constructed wetlands. Water Res. 2007, 41, 1388–1398. [Google Scholar] [CrossRef]
- Vidal, G.; Videla, S.; Diez, M.C. Molecular weight distribution of Pinus radiata kraft mill wastewater treated by anaerobic digestion. Bioresour. Technol. 2001, 77, 183–191. [Google Scholar] [CrossRef]
- Gómez, G.; Salinas, M.; Ruiz-Tagle, N.; Sossa, K.; Vidal, G. Molecular weight distribution of the recalcitrant organic matter contained in kraft mill effluents and the identification of microbial consortia responsible for an anaerobic biodegradable fraction. Environ. Sci. Health A 2020, 55, 281–291. [Google Scholar] [CrossRef]
- Wayne, P.A. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-First Informational Supplement; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2014; pp. 100–122. [Google Scholar]
- Monsalves, N.; Leiva, A.M.; Gómez, G.; Vidal, G. Organic compounds and antibiotic-resistance bacteria behavior in greywater treated by a constructed wetland. Int. J. Environ. Res. Public Health 2023, 20, 2305. [Google Scholar] [CrossRef]
- Lourenço, N.; Nunes, L.M. Optimization of a vermifiltration process for treating urban wastewater. Ecol. Eng. 2017, 100, 138–146. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Z.; Che, Y.; Yang, F.; Chao, J.; Gao, Y.; Zhang, Y. The effect of vermifiltration height and wet: Dry time ratio on nutrient removal performance and biological features, and their influence on nutrient removal efficiencies. Ecol. Eng. 2014, 71, 165–172. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Ge, Z.; Hu, C.; Zhang, H. Effects of influent C/N ratios on wastewater nutrient removal and simultaneous greenhouse gas emission from the combinations of vertical subsurface flow constructed wetlands and earthworm eco-filters for treating synthetic wastewater. Environ. Sci. Process. Impacts. 2014, 16, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Karla, M.R.; Alejandra, V.A.C.; Lenys, F.; Patricio, E.M. Operational performance of corncobs/sawdust biofilters coupled to microbial fuel cells treating domestic wastewater. Sci. Total Environ. 2022, 809, 151115. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.S.; Wang, L.; Jia, J.W.; Fu, X.H.; Le, Y.Q.; Chen, X.Z.; Sun, Y. Response of soil microbial community in Jiuduansha wetland to different successional stages and its implications for soil microbial respiration and carbon turnover. Soil Biol. Biochem. 2011, 43, 638–646. [Google Scholar] [CrossRef]
- Xing, M.; Li, X.; Yang, J.; Lv, B.; Lu, Y. Performance and mechanism of vermifiltration system for liquid-state sewage sludge treatment using molecular and stable isotopic techniques. Chem. Eng. J. 2012, 197, 143–150. [Google Scholar] [CrossRef]
- Pous, N.; Barcelona, A.; Sbardella, L.; Gili, O.; Hidalgo, M.; Colomer, J.; Serra, T.; Salvadó, V. Vermifilter and zooplankton-based reactor integration as a nature-based system for wastewater treatment and reuse. Case Stud. Chem. Environ. Eng. 2021, 4, 100153. [Google Scholar] [CrossRef]
- Tejedor, J.; Cóndor, V.; Almeida-Naranjo, C.E.; Guerrero, V.H.; Villamar, C.A. Performance of wood chips/peanut shells biofilters used to remove organic matter from domestic wastewater. Sci. Total Environ. 2020, 738, 139589. [Google Scholar] [CrossRef]
- Shukla, A.; Zhang, Y.H.; Dubey, P.; Margrave, J.; Shukla, S.S. The role of sawdust in the removal of unwanted materials from water. J. Hazard. Mater. 2022, 95, 137–152. [Google Scholar] [CrossRef]
- Singh, R.; D’Alessio, M.; Meneses, Y.; Bartelt-Hunt, S.; Ray, C. Nitrogen removal in vermifiltration: Mechanisms, influencing factors, and future research needs. J. Environ. Manag. 2021, 281, 111868. [Google Scholar] [CrossRef]
- Samal, K.; Dash, R.R.; Bhunia, P. Treatment of wastewater by vermifiltration integrated with macrophyte filter: A review. J. Environ. Chem. Eng. 2017, 5, 2274–2289. [Google Scholar] [CrossRef]
- Hughes, R.J.; Nair, J.; Mathew, K.; Ho, G. Toxicity of domestic wastewater pH to key species within an innovative decentralized vermifiltration system. Water Sci. Technol. 2007, 55, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Mirzaie, M.; Hasan-Zadeh, A.; Ashrafnejad, M.; Hashemian, S.J.; Shahnemati, S.R. Design, operation, performance evaluation and mathematical optimization of a vermifiltration pilot plan for domestic wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 103587. [Google Scholar] [CrossRef]
- Chowdhury, S.D.; Bhunia, P. Simultaneous carbon and nitrogen removal from domestic wastewater using high rate vermifilter. Indian J. Microbiol. 2021, 61, 218–228. [Google Scholar] [CrossRef]
- Lavrnić, S.; Cristino, S.; Zapater-Pereyra, M.; Vymazal, J.; Cupido, D.; Lucchese, G.; Mancini, B.; Mancini, M.L. Effect of earthworms and plants on the efficiency of vertical flow systems treating university wastewater. Environ. Sci. Pollut. 2019, 26, 10354–10362. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, Y.; Wu, J.; Zhang, J.; Zheng, Z. Effects of different influent C/N ratios on the performance of various earthworm eco-filter systems: Nutrient removal and greenhouse gas emission. World J. Microbiol. Biotechnol. 2014, 30, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Rajpal, A.; Arora, S.; Bhargava, R.; Hari Prasad, K.S.; Kazmi, A.A. A comparative study on vermifiltration using epigeic earthworm Eisenia foetida and Eudrilus eugeniae. Desalin. Water Treat. 2016, 57, 6347–6354. [Google Scholar] [CrossRef]
- Imfeld, G.; Braeckevelt, M.; Kuschk, P.; Richnow, H.H. Monitoring and assessing processes of organic chemicals removal in constructed wetlands. Chemosphere 2009, 74, 349–362. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Lou, S.; Yang, J. Wastewater treatment performance of a vermifilter enhancement by a converter slag-coal cinder filter. Ecol. Eng. 2010, 36, 489–494. [Google Scholar] [CrossRef]
- Li, X.; Xing, M.; Yang, J.; Zhao, L.; Dai, X. Organic matter humification in vermifiltration process for domestic sewage sludge treatment by excitation-emission matrix fluorescence and Furier transform infrared spectroscopy. J. Hazard. Mater. 2013, 261, 491–499. [Google Scholar] [CrossRef]
- De Boer, M.A.; Hammerton, M.; Slootweg, J.C. Uptake of pharmaceuticals by sorbent-amended struvite fertilisers recovered from human urine and their bioaccumulation in tomato fruit. Water Res. 2018, 133, 19–26. [Google Scholar] [CrossRef]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Saraswat, S. Vermifiltration as a natural, sustainable, and green technology for environmental remediation: A new paradigm for wastewater treatment process. J. Environ. Manag. 2021, 4, 100061. [Google Scholar] [CrossRef]
- Furlong, C.; Gibson, W.T.; Oak, A.; Thakar, G.; Kodgire, M.; Patankar, R. Technical and user evaluation of a novel worm-based, on-site sanitation system in rural India. Waterlines 2016, 35, 148–162. [Google Scholar] [CrossRef]
- Yin, Z.G.; Xing, M.Y.; Zhang, J.; Yang, J.; Huang, Z.D. Effect of filter bed temperature on municipal wastewater treatment efficiency by vermifiltration. Energy Procedia 2011, 11, 3446–3453. [Google Scholar]
- Edwards, C.A.; Fletcher, K.E. Interactions between earthworms and microorganisms in organic-matter breakdown. Agric. Ecosyst. Environ. 1988, 24, 235–247. [Google Scholar] [CrossRef]
- Arora, S.; Rajpal, A.; Bhargava, R.; Pruthi, V.; Bhatia, A.; Kazmi, A.A. Antibacterial and enzymatic activity of microbial community during wastewater treatment by pilot scale vermifiltration system. Bioresour. Technol. 2014, 166, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cho, J.S.; Park, J.H.; Heo, J.S.; Ok, Y.S.; Delaune, R.D.; Seo, D.C. Long-term performance of vertical-flow and horizontal-flow constructed wetlands as affected by season, N load, and operating stage for treating nitrogen from domestic sewage. Environ. Sci. Pollut. Res. 2016, 23, 1108–1119. [Google Scholar] [CrossRef]
- Wand, H.; Vacca, G.; Kuschk, P.; Krüger, M.; Kästner, M. Removal of bacteria by filtration in planted and non-planted sand columns. Water Res. 2007, 41, 159–167. [Google Scholar] [CrossRef]
- Das, P.; Paul, K. A review on integrated vermifiltration as a sustainable treatment method for wastewater. J. Environ. Manag. 2023, 328, 116974. [Google Scholar]
- Hussain, M.; Liaqat, L.; Ali, N.M.; Arshad, N.; Hanif, U.; Sajjad, S.; Sardar, A.A.; Awan, U.F.; Khan, F.S.; Slahuddin. Antibacterial and bacteriostatic potential of coelomic fluid and body paste of Pheretima posthuman (Vaillant, 1868) (Clitellata, Megascolecidae) against ampicillin resistant clinical bacterial isolates. Braz. J. Biol. 2023, 83, e247016. [Google Scholar] [CrossRef]
- Chauhan, P.; Tomar, J.; Prasad, G.B.K.S.; Agrawal, O.P. Antimicrobial activity of earthworm Eudrilus eugeniae tissue extract. J. Chem. Pharm. Res. 2014, 6, 28–38. [Google Scholar]
- González, Y.; Salgado, P.; Vidal, G. Disinfection behavior of a UV-treated wastewater system using constructed wetlands and the rate of reactivation of pathogenic microorganisms. Water Sci. Technol. 2019, 80, 1870–1879. [Google Scholar] [CrossRef]
- Xu, G.; Wang, T.; Wei, Y.; Zhang, Y.; Chen, J. Fecal coliform distribution and health risk assessment in surface water in an urban-intensive catchment. J. Hydrol. 2022, 604, 127204. [Google Scholar] [CrossRef]
- Tyagi, V.; Sahoo, B.; Khursheed, A.; Kazmi, A.; Ahmad, Z.; Chopra, A. Fate of coliforms and pathogenic parasite in four full-scale sewage treatment systems in India. Environ. Monit. Assess. 2010, 181, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, Q.; Nie, X.; Chen, B.; Xiao, Y.; Zhou, Q.; Liang, X. Ocurrence and elimination of antibiotic resistance genes in a long-term operation integrated surface flow constructed wetland. Chemosphere 2017, 173, 99–106. [Google Scholar] [CrossRef]
- Abou-Kandil, A.; Shibli, A.; Azaizeh, H.; Wolff, D.; Wick, A.; Jadoun, J. Fate and removal of bacteria and antibiotic resistance genes in horizontal subsurface constructed wetlands: Effect of mixed vegetation and substrate type. Sci. Total. Environ. 2021, 59, 144193. [Google Scholar] [CrossRef]
- Cerqueira, F.; Matamoros, V.; Bayona, J.; Elsinga, G.; Hornstra, L.; Piña, B. Distribution of antibiotic resistance genes in soils and crops. A field study in legume plants (Vicia faba L.) grown under different watering regimes. Environ. Res. 2019, 170, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Leiva, A.M.; Piña, B.; Vidal, G. Antibiotic resistance dissemination in wastewater treatment plants: A challenge for the reuse of treated wastewater in agriculture. Rev. Environ. Sci. Bio. 2021, 20, 1043–1072. [Google Scholar] [CrossRef]
- Badul, S.; Abia, A.; Amoako, D.; Perrett, K.; Bester, L.; Essack, S. From the farms to the dining table: The distribution and molecular characteristics of antibiotic-resistant Enterococcus spp. in intensive pig farming in South Africa. Microorganisms 2021, 9, 882. [Google Scholar] [PubMed]
- García, J.; García-Galán, M.; Day, J.; Boopathy, R.; White, J.; Wallace, S.; Hunter, R. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123–228. [Google Scholar] [CrossRef]
- Song, H.L.; Zhang, S.; Guo, J.; Yang, Y.L.; Zhang, L.M.; Li, H.; Yang, X.L.; Liu, X. Vertical up-flow constructed wetlands exhibited efficient antibiotic removal but induced antibiotic resistance genes in effluent. Chemosphere 2018, 203, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Monsalves, N.; Leiva, A.M.; Gómez, G.; Vidal, G. Antibiotic-resistance gene behavior in constructed wetlands treating sewage: A critical review. Sustainability 2022, 14, 8524. [Google Scholar] [CrossRef]
- Craddock, H.A.; Chattopadhyay, S.; Rjoub, Y.; Rosen, D.; Greif, J.; Lipchin, C.; Mongodin, E.F.; Sapkota, A.R. Antibiotic-resistant Escherichia coli and Klebsiella spp. in greywater reuse systems and pond water used for agricultural irrigation in the West Bank, Palestinian Territories. Environ. Res. 2020, 188, 109777. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Nurul, S.; Schmitt, H.; Sutton, N.B.; Murk, T.A.; Blokland, M.H.; Rijnaarts, H.H.; Langenhoff, A.A. Evaluation of attenuation of pharmaceuticals, toxic potency, and antibiotic resistance genes in constructed wetlands treating wastewater effluents. Sci. Total. Environ. 2018, 631–632, 1572–1581. [Google Scholar] [CrossRef]
- Ma, L.; Yang, H.; Guan, L.; Liu, X.; Zhang, T. Risk of antibiotic resistance genes and antimicrobial resistance under chlorination disinfection with public health concerns. Environ. Int. 2022, 158, 106978. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, Y.; Dong, H.; Li, J.; Zhang, W.; Shao, Y.; Shao, Y. Effects of heavy metals and antibiotic on antibiotic resistance genes and microbial communities in soil. Environ. Prot. 2023, 169, 418–427. [Google Scholar] [CrossRef]
- Manaia, C.M.; Rocha, J.; Scaccia, N.; Marano, R.; Radu, E.; Biancullo, F.; Cerqueira, F.; Fortunato, G.; Iakovides, I.C.; Zammit, I.; et al. Antibiotic resistance in wastewater treatment plants: Tackling the black box. Environ. Int. 2018, 115, 312–324. [Google Scholar] [CrossRef]
- Zhang, W.; Sturm, B.S.M.; Knapp, C.W.; Graham, D.W. Accumulation of tetracycline resistance genes in aquatic biofilms due to periodic waste loadings from swine lagoons. Environ. Sci. Technol. 2009, 43, 7643–7650. [Google Scholar] [CrossRef]
- Engemann, C.A.; Keen, P.L.; Knapp, C.W.; Hall, K.J.; Graham, D.W. Fate of tetracycline resistance genes in aquatic systems: Migration from the water column to peripheral biofilms. Environ. Sci. Technol. 2008, 42, 5131–5136. [Google Scholar] [CrossRef]
- Stange, C.; Sidhu, J.P.S.; Toze, S.; Tiehm, A. Comparative removal of antibiotic resistance genes during chlorination, ozonation, and UV treatment. Int. J. Hyg. Environ. Health 2019, 222, 541–548. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ren, H.; Geng, J.; Zhang, Y.; Zhang, Y.; Ding, L.; Xu, K. Inactivation of antibiotic resistance genes in municipal wastewater by chlorination, ultraviolet, and ozonation disinfection. Environ. Sci. Pollut. Res. 2015, 22, 7037–7044. [Google Scholar] [CrossRef] [PubMed]
- Bueno, I.; Verdugo, C.; Jimenez-Lopez, O.; Alvarez, P.P.; Gonzalez-Rocha, G.; Lima, C.A.; Travis, D.A.; Wass, B.; Zhang, Q.; Ishii, S.; et al. Role of wastewater treatment plants on environmental abundance of antimicrobial resistance genes in chilean rivers. Int. J. Hyg. Environ. Health 2020, 223, 56–64. [Google Scholar] [CrossRef]
- Proia, L.; Anzil, A.; Subirats, J.; Borrego, C.; Farrè, M.; Llorca, M.; Balcázar, J.L.; Servais, P. Antibiotic resistance along an urban river impacted by treated wastewaters. Sci. Total Environ. 2018, 628–629, 453–466. [Google Scholar] [CrossRef]
- Barancheshme, F.; Munir, M. Strategies to combat antibiotic resistance in the wastewater treatment plants. Front. Microbiol. 2018, 8, 2603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuang, Y.; Geng, J.; Ren, H.; Zhang, Y.; Ding, L.; Xu, K. Inactivation of antibiotic resistance genes in municipal wastewater effluent by chlorination and sequential UV/chlorination disinfection. Sci. Total. Environ. 2015, 512–513, 125–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, Y.; Geng, J.; Ren, H.; Xu, K.; Ding, L. Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes. Sci. Total Environ. 2016, 550, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.; et al. Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters. Appl. Catal. B 2018, 224, 810–824. [Google Scholar] [CrossRef]

 = <1000 Da;
= <1000 Da;  = 1000–5000 Da;
= 1000–5000 Da;  = 5000–10,000 Da;
= 5000–10,000 Da;  = >10,000 Da.
= >10,000 Da.
 = <1000 Da;
= <1000 Da;  = 1000–5000 Da;
= 1000–5000 Da;  = 5000–10,000 Da;
= 5000–10,000 Da;  = >10,000 Da.
= >10,000 Da.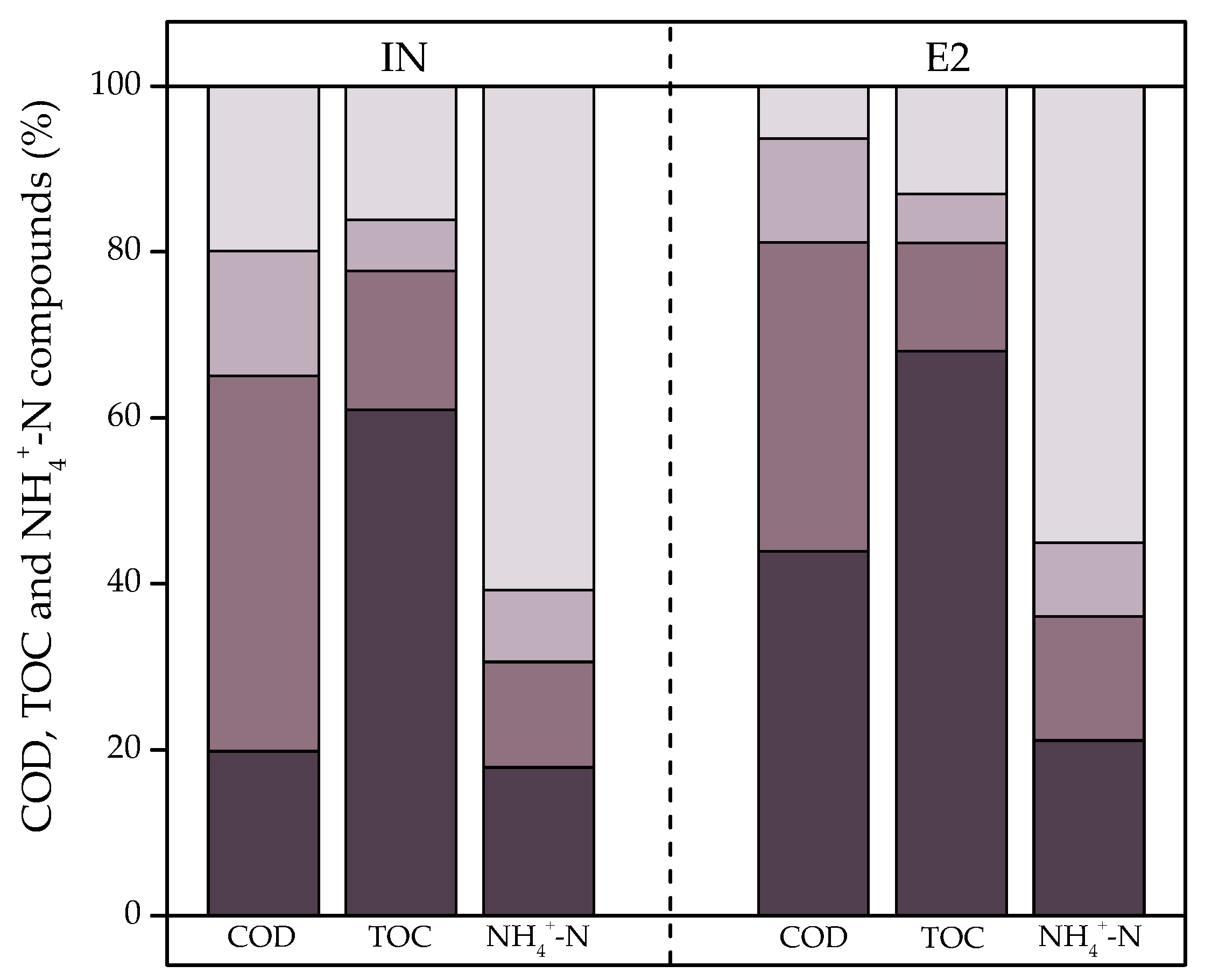
 ), E2 (
), E2 ( ), and removal efficiencies (%) in fall–winter and spring–summer.
), and removal efficiencies (%) in fall–winter and spring–summer.
 ), E2 (
), E2 ( ), and removal efficiencies (%) in fall–winter and spring–summer.
), and removal efficiencies (%) in fall–winter and spring–summer.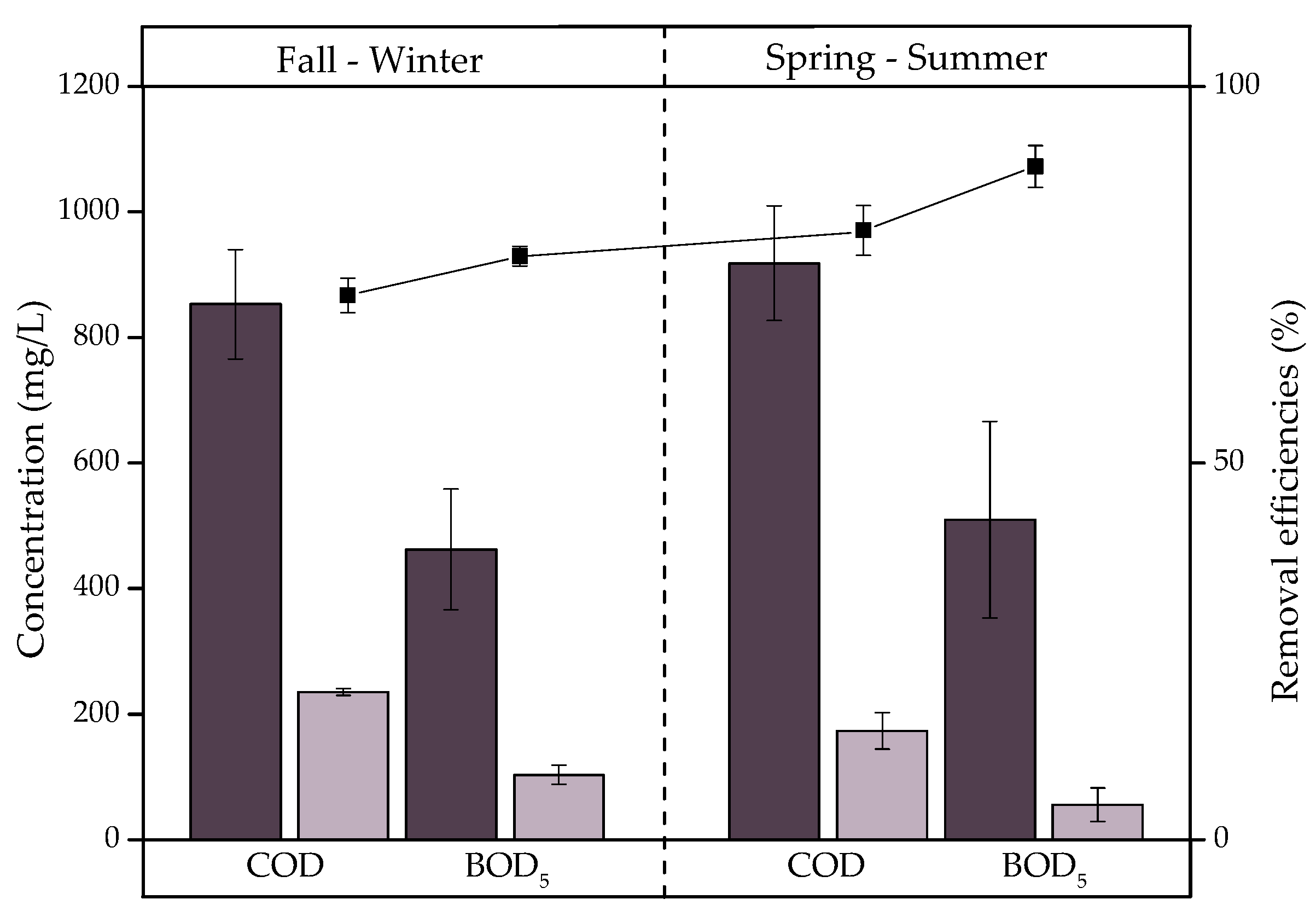
 ) and E2 (
) and E2 ( ) in fall–winter and spring–summer.
) in fall–winter and spring–summer.
 ) and E2 (
) and E2 ( ) in fall–winter and spring–summer.
) in fall–winter and spring–summer.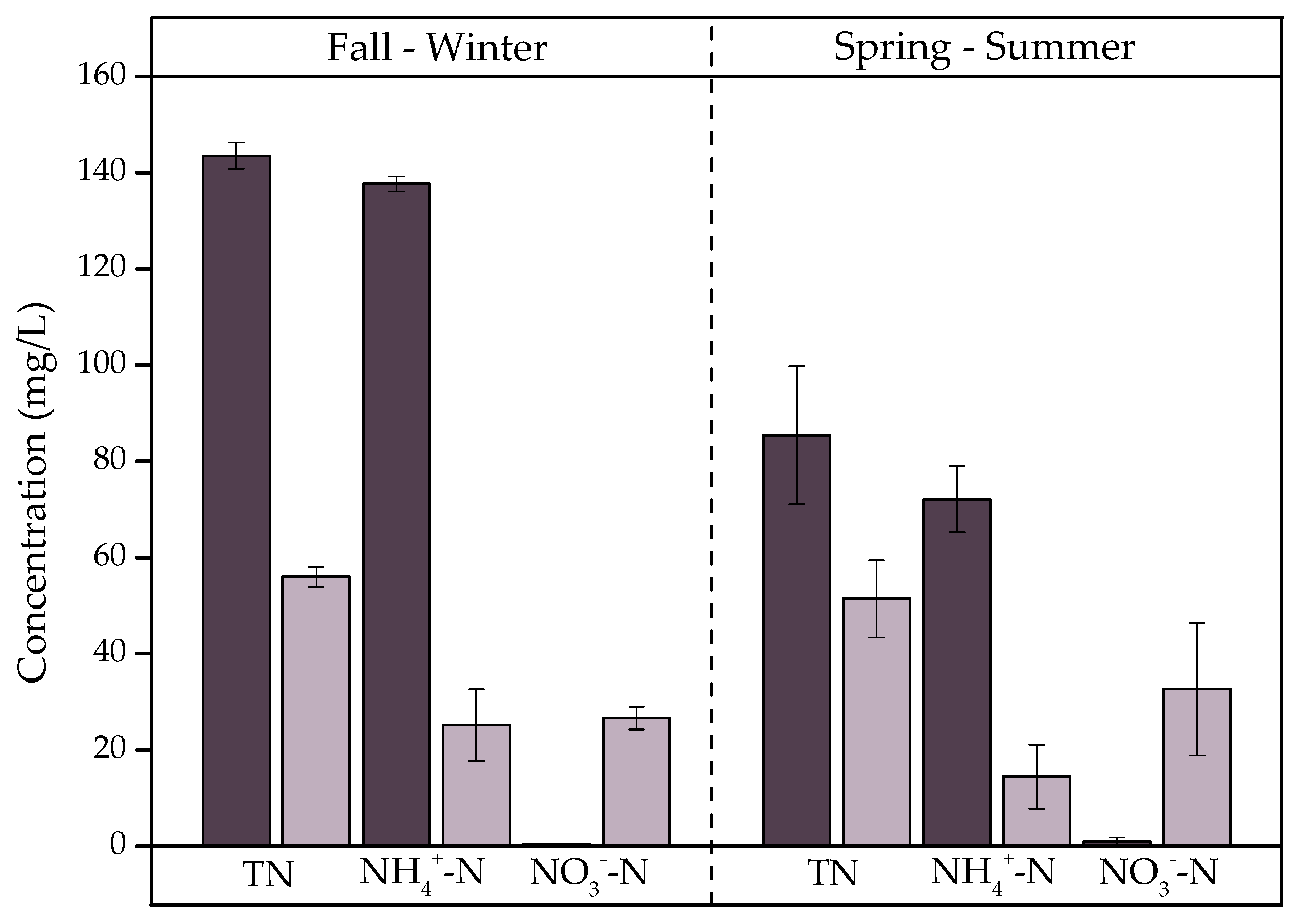
 ), E1 (
), E1 ( ), and E2 (
), and E2 ( ) and removal efficiencies (%).
) and removal efficiencies (%).
 ), E1 (
), E1 ( ), and E2 (
), and E2 ( ) and removal efficiencies (%).
) and removal efficiencies (%).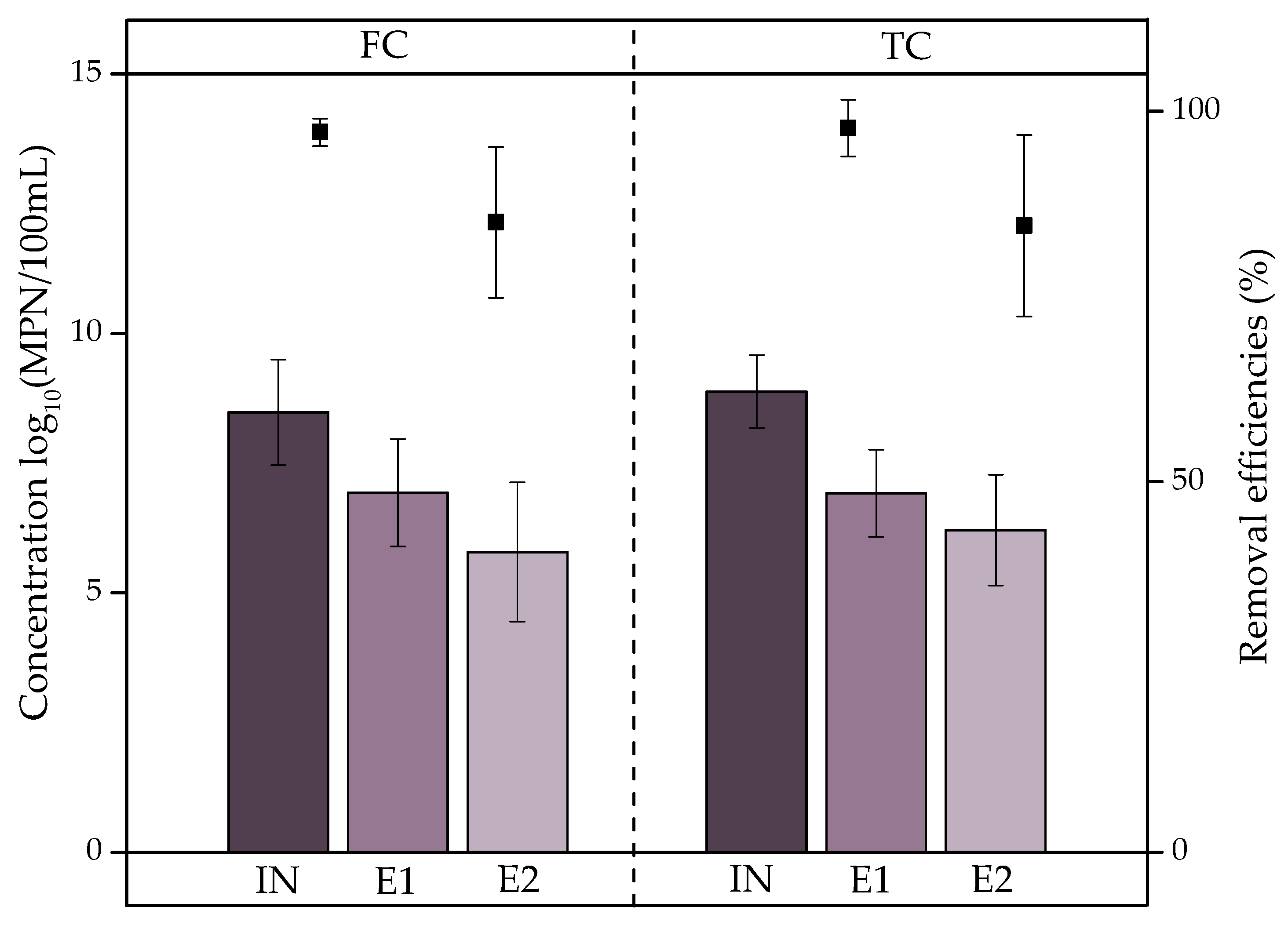
 ), E1 (
), E1 ( ), and E2 (
), and E2 ( ), and ARB removal efficiencies (%).
), and ARB removal efficiencies (%).
 ), E1 (
), E1 ( ), and E2 (
), and E2 ( ), and ARB removal efficiencies (%).
), and ARB removal efficiencies (%).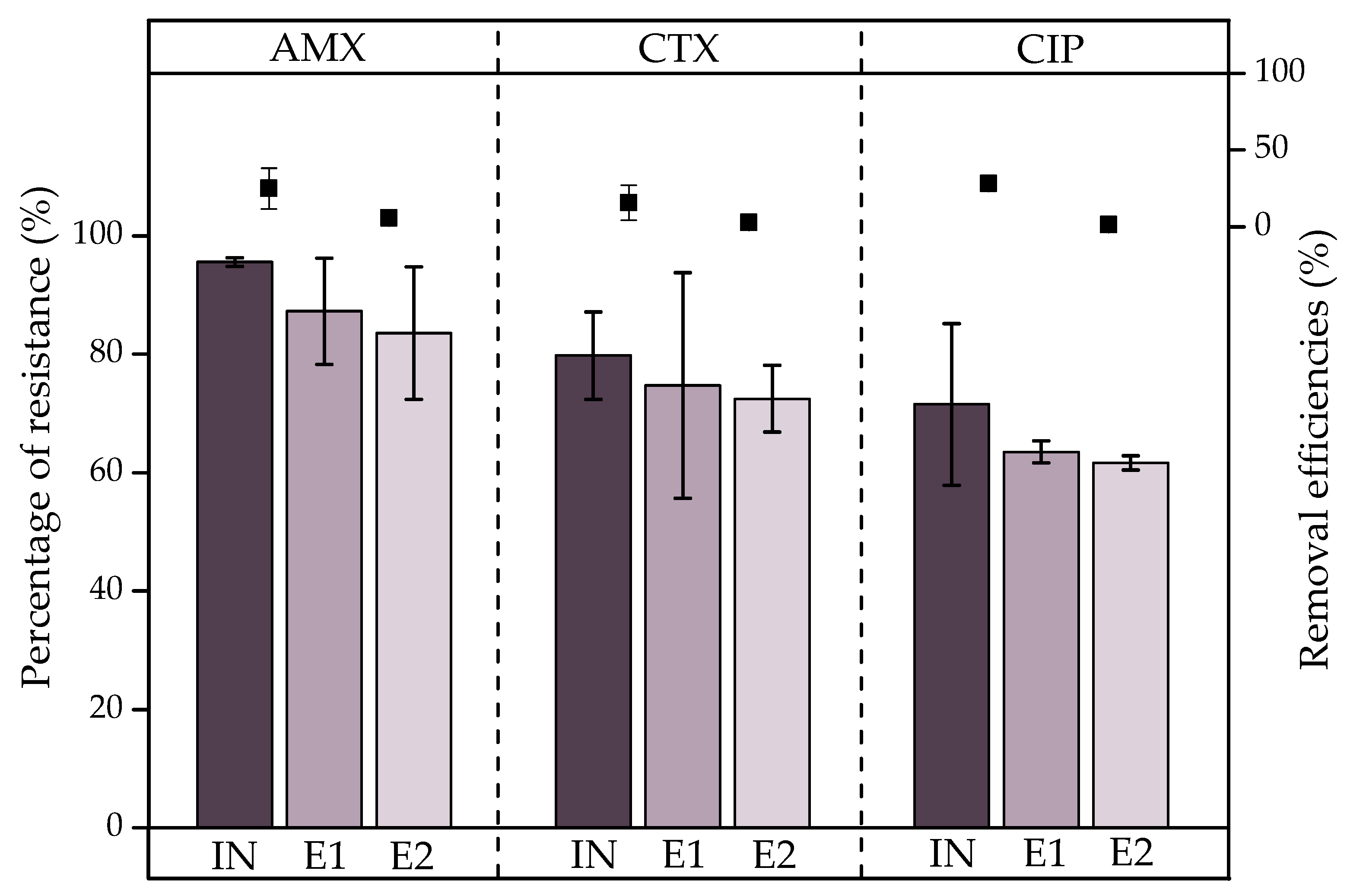
| Parameter | Unit | Value |
|---|---|---|
| Vermifilter volume | m3 | 442 |
| Vermifilter area | m2 | 276 |
| Vermifilter height | m | 1.60 |
| Influent flow | m3/d | 160 |
| HLR | m3/m2d | 0.60 |
| OLR | kg COD/m2d | 0.50 |
| Carbon/Nitrogen ratio | - | 9 |
| Temperature | °C | 21 |
| Active layer | - | woodchip |
| Active layer height | m | 0.90 ± 0.12 |
| Species | - | Eisenia foetida |
| Earthworm density zone A | worm/m3 | ~1100 |
| Earthworm density zone B | worm/m3 | ~7000 |
| Parameter | Unit | IN (Mean ± SD) | Effluent (Mean ± SD) | ||
|---|---|---|---|---|---|
| E1 | E2 | ||||
| In situ | T | °C | 17.1 ± 3.7 | 15.9 ± 3.6 | 15.8 ± 3.4 |
| pH | - | 7.8 ± 0.4 | 6.9 ± 0.2 | 6.8 * ± 0.3 | |
| ORP | mV | −25.4 ± 141.7 | 181.8 ± 77.3 | 245.9 * ± 127.5 | |
| EC | µS/cm | 1.6 ± 0.2 | 1.0 ± 0.4 | 1.2 ± 0.1 | |
| DO | mg/L | 1.2 ± 0.5 | 5.1 ± 0.6 | 5.0 * ± 0.8 | |
| Turbidity | NTU | 210.8 ± 59.4 | 61.2 ± 22.6 | 66.2 * ± 30.3 | |
| Nutrients | NH4+-N | mg/L | 104.9 ± 35.3 | 23.0 ± 10.7 | 19.8 * ± 8.7 |
| TN | mg/L | 114.4 ± 32.5 | 57.5 ± 8.1 | 53.7 * ± 6.0 | |
| PO43−-P | mg/L | 10.3 ± 2.6 | 6.5 ± 2.4 | 6.8 * ± 1.0 | |
| TP | mg/L | 12.5 ± 2.0 | 7.9 ± 2.9 | 8.0 * ± 1.5 | |
| Organic matter | COD | mg/L | 885.8 ± 89.9 | 286.0 ± 60.7 | 204.2 * ± 38.4 |
| BOD5 | mg/L | 486.3 ± 123.1 | 109.6 ± 27.5 | 79.4 * ± 32.6 | |
| TSS | mg/L | 239.1 ± 101.4 | 67.3 ± 16.5 | 53.4 * ± 17.9 | |
| VSS | mg/L | 186.6 ± 68.1 | 50.8 ± 18.9 | 39.5 * ± 14.1 | |
| Microbiological | TC | log10(MPN/100 mL) | 8.7 ± 0.7 | 6.5 ± 0.9 | 5.2 * ± 1.0 |
| FC | log10(MPN/100 mL) | 7.8 ± 1.0 | 5.9 ± 1.0 | 4.4 * ± 1.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, V.; Monsalves, N.; Gómez, G.; Vidal, G. Performance of a Full-Scale Vermifilter for Sewage Treatment in Removing Organic Matter, Nutrients, and Antibiotic-Resistant Bacteria. Sustainability 2023, 15, 6842. https://doi.org/10.3390/su15086842
Gutiérrez V, Monsalves N, Gómez G, Vidal G. Performance of a Full-Scale Vermifilter for Sewage Treatment in Removing Organic Matter, Nutrients, and Antibiotic-Resistant Bacteria. Sustainability. 2023; 15(8):6842. https://doi.org/10.3390/su15086842
Chicago/Turabian StyleGutiérrez, Victor, Naomi Monsalves, Gloria Gómez, and Gladys Vidal. 2023. "Performance of a Full-Scale Vermifilter for Sewage Treatment in Removing Organic Matter, Nutrients, and Antibiotic-Resistant Bacteria" Sustainability 15, no. 8: 6842. https://doi.org/10.3390/su15086842
APA StyleGutiérrez, V., Monsalves, N., Gómez, G., & Vidal, G. (2023). Performance of a Full-Scale Vermifilter for Sewage Treatment in Removing Organic Matter, Nutrients, and Antibiotic-Resistant Bacteria. Sustainability, 15(8), 6842. https://doi.org/10.3390/su15086842






