Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices
Abstract
1. Introduction
2. Classification of Nanomaterials
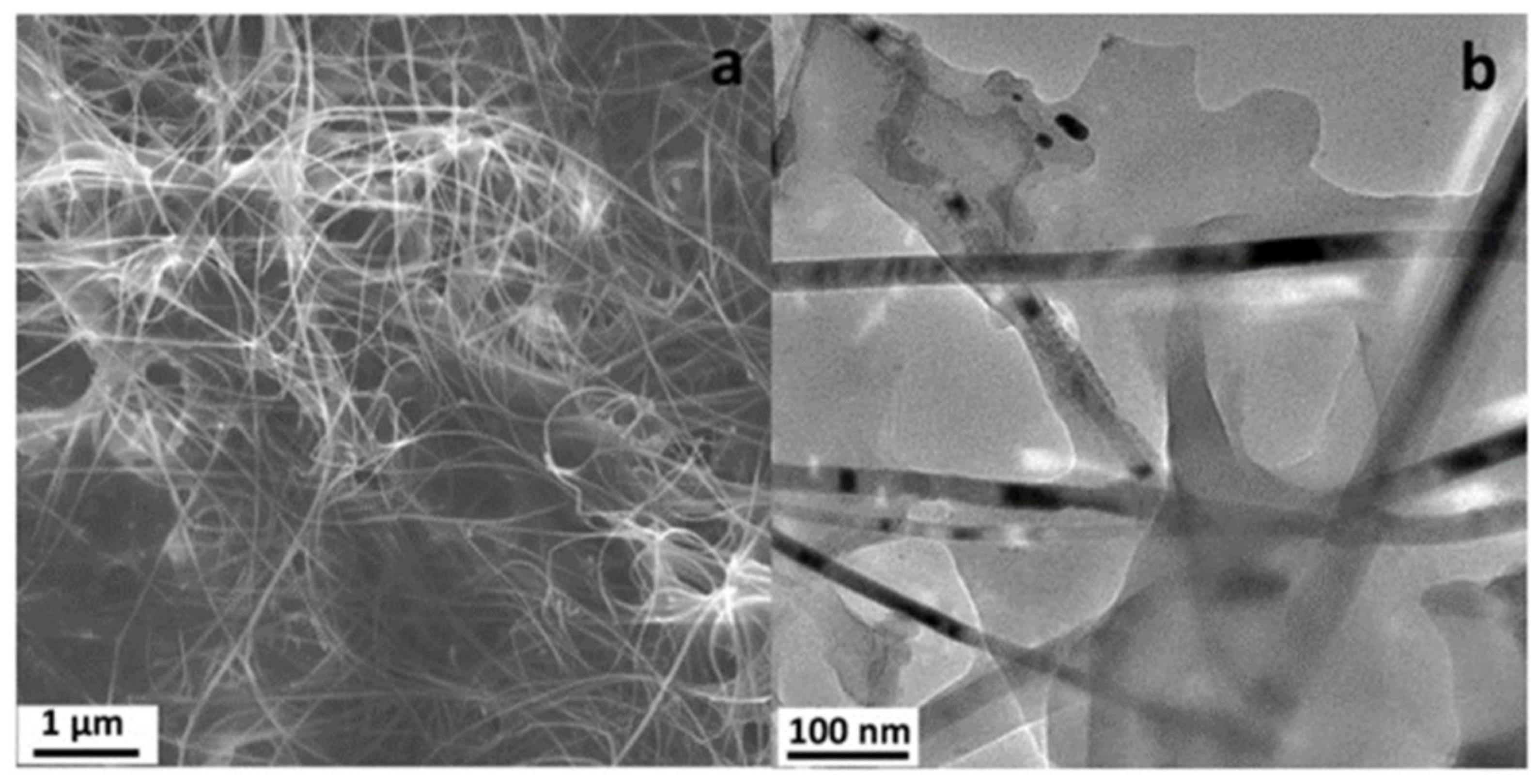
3. Applications of Nanomaterials in Electrochemical Devices
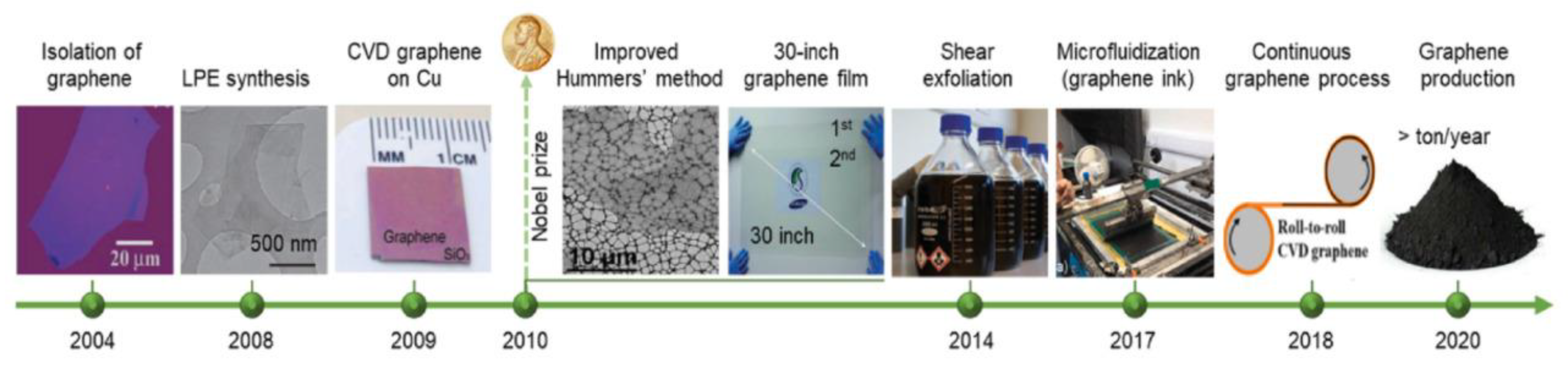
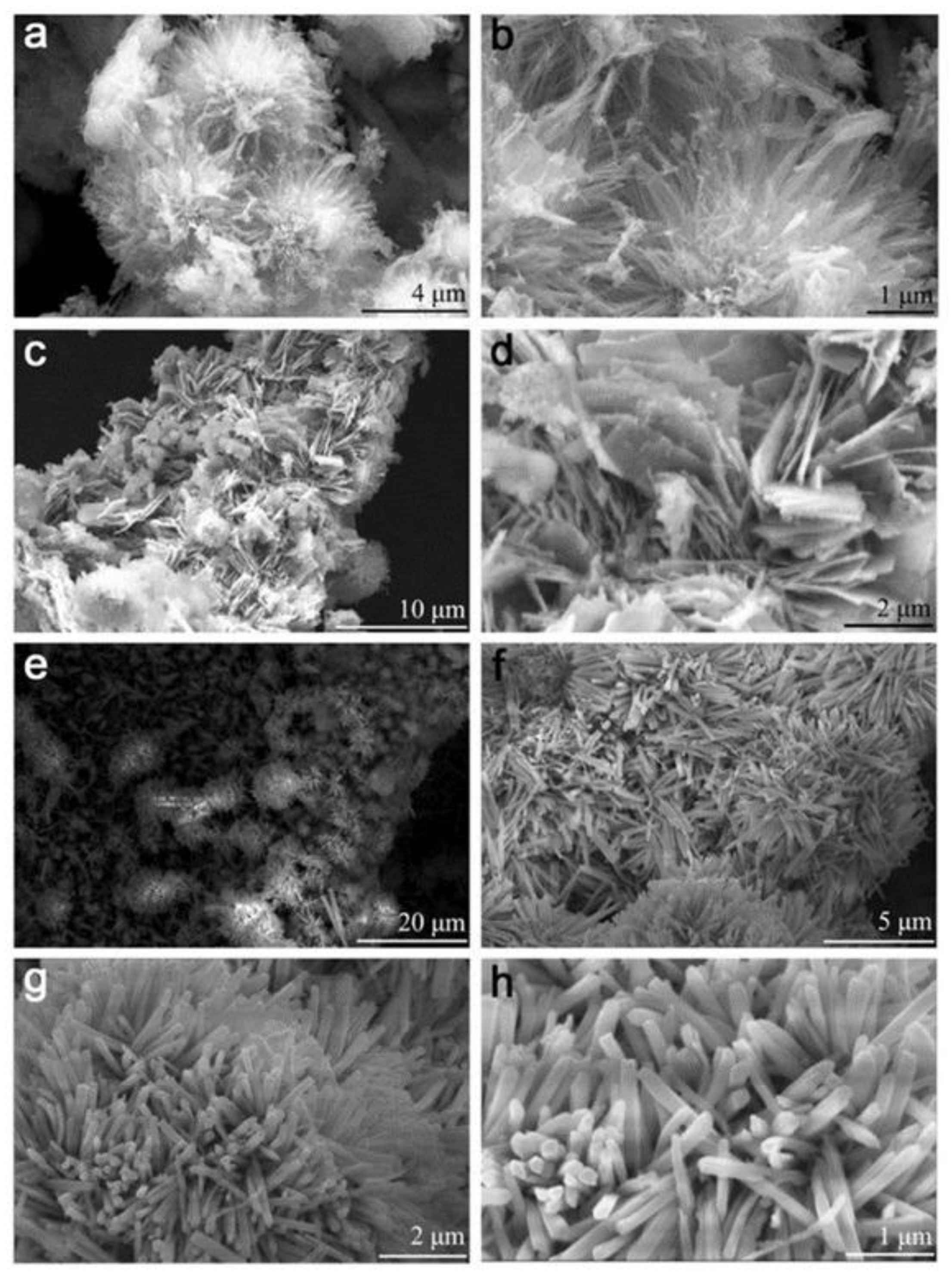
4. Morphology and Properties of Nanomaterials Prepared by Different Synthesis Methods

5. Nanotechnology-Based Electrochemical Sensors
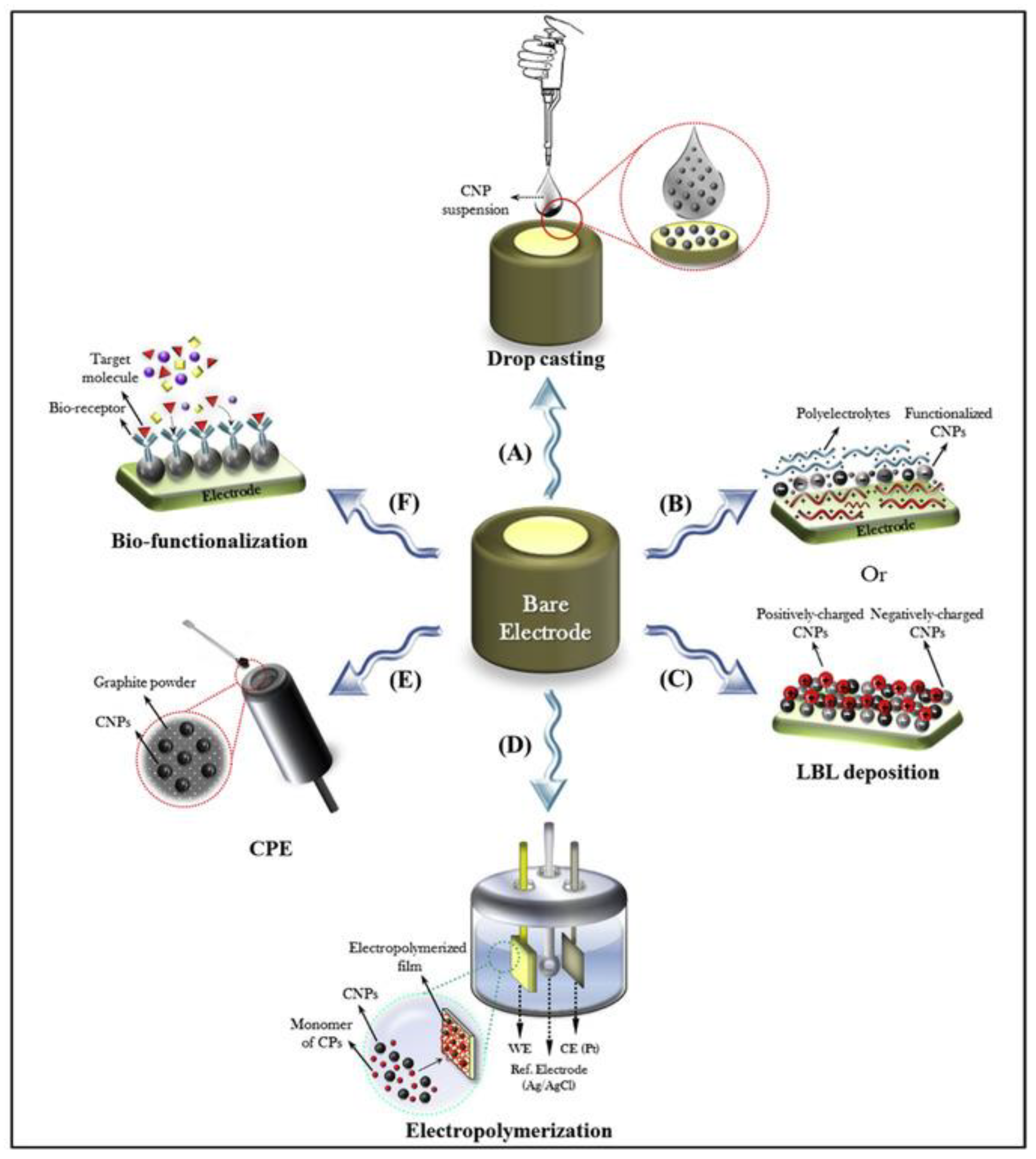
6. Nanostructured Materials for Enhanced Electrochemical Performance in Energy Storage Devices
7. New Types of Nanomaterials for Electrochemical Devices
8. Nanotechnology through Electrochemistry in Water Purification
9. Green Nanoscale and Electrochemical Methods in High-Precision Economical Products
10. A Fusion of Nanostructures with the Electrochemical System in Applications of Economic Importance
11. Future and Challenges
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1D | One-dimensional |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| AgNPs | Silver nanoparticles |
| Au | Gold |
| AuPt | Gold–platinum |
| BET | Brunauer–Emmett–Teller |
| BF | Basic fuchsin |
| BTD-COF | Boronate ester-linked COF |
| CNTs | Carbon nanotubes |
| CNTs | Carbon nanotubes |
| Co | Cobalt |
| CO3O4 | Cobalt tetraoxide |
| CoFe2O | Cobalt ferrite |
| COFs | Covalent organic frameworks |
| Cu | Copper |
| CuO | Copper oxide |
| CV | Cyclic voltammetry |
| EB | Erythrosine B |
| EDX | Energy dispersive X-ray |
| ES | Electrical sensors |
| GCE | Glassy carbon electrode |
| GO | Graphene oxide |
| GOX | Glucose oxidase |
| HPLC | High-performance liquid chromatography |
| Iso-AgNPs | Isoimperatorin-mediated silver nanoparticles |
| LC/MS | Liquid chromatography–mass spectrometry |
| Li | Lithium |
| LiFePO4 | lithium ferrophosphate |
| MA | Methyl-ammonium |
| MB | Methylene Blue |
| MOFs | Metal–organic frameworks (MOFs) |
| MoP-NC | Molybdenum phosphide nanoparticles and nitrogen-doped carbon |
| MS | Mechanochemical synthesis |
| NaOH | Sodium hydroxide |
| NF | New Fuchsine |
| NFs | Nanoflowers |
| Ni (OH)2 | Nickel(II) hydroxide |
| Ni | Nickle |
| NiONPs | Nickel oxide nanoparticles |
| NiP | Nickel phosphide |
| NPS | Nanoparticles |
| POFs | First covalent organic frameworks |
| Pt | Platinum |
| RGO | Reduced graphene oxide |
| SEM | Scanning electron microscope |
| Si | Silicon |
| SiCNPs | Silicon carbide nanoparticles |
| SiO2NPs | Silicon Dioxide Nanoparticles |
| TEM | Transmission electron microscope |
| TiO2 | Titanium dioxide |
| XO | Xanthone |
| XT | Xanthene |
| ZnO | Zinc oxide |
References
- Xu, H.; Ci, S.; Ding, Y.; Wang, G.; Wen, Z. Recent Advances in Precious Metal-Free Bifunctional Catalysts for Electrochemical Conversion Systems. J. Mater. Chem. A 2019, 7, 8006–8029. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Grohens, Y.; Kokol, V.; Kalarikkal, N.; Thomas, S. Synthesis and Processing of Emerging Two-Dimensional Nanomaterials. In Nanomaterials Synthesis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–25. [Google Scholar]
- Ouyang, D.; Huang, Z.; Choy, W.C.H. Solution-processed Metal Oxide Nanocrystals as Carrier Transport Layers in Organic and Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1804660. [Google Scholar] [CrossRef]
- Devasahayam, S.; Hussain, C.M. Thin-Film Nanocomposite Devices for Renewable Energy Current Status and Challenges. Sustain. Mater. Technol. 2020, 26, e00233. [Google Scholar] [CrossRef]
- Serrano-Garcia, W.; Bonadies, I.; Thomas, S.W.; Guarino, V. New Insights to Design Electrospun Fibers with Tunable Electrical Conductive–Semiconductive Properties. Sensors 2023, 23, 1606. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Wei, D. Two-Dimensional Metal-Organic Frameworks and Covalent Organic Frameworks. Chin. J. Chem. 2022, 40, 1359–1385. [Google Scholar] [CrossRef]
- Abdelhamid, A.A.; Badr, M.H.; Mohamed, R.A.; Saleh, H.M. Using Agricultural Mixed Waste as a Sustainable Technique for Removing Stable Isotopes and Radioisotopes from the Aquatic Environment. Sustainability 2023, 15, 1600. [Google Scholar]
- Dawoud, M.M.A.; Hegazi, M.M.; Saleh, H.M.; El Helew, W.K. Removal of Stable and Radio Isotopes from Wastewater by Using Modified Microcrystalline Cellulose Based on Taguchi L16. Int. J. Environ. Sci. Technol. 2022, 20, 1289–1300. [Google Scholar] [CrossRef]
- Saleh, H.M.; Moussa, H.R.; El-Saied, F.A.; Dawod, M.; Bayoumi, T.A.; Abdel Wahed, R.S. Mechanical and Physicochemical Evaluation of Solidifed Dried Submerged Plants Subjected to Extreme Climatic Conditions to Achieve an Optimum Waste Containment. Prog. Nucl. Energy 2020, 122, 103285. [Google Scholar] [CrossRef]
- Saleh, H.M.; Aglan, R.F.; Mahmoud, H.H. Qualification of Corroborated Real Phytoremediated Radioactive Wastes under Leaching and Other Weathering Parameters. Prog. Nucl. Energy 2020, 219, 103178. [Google Scholar] [CrossRef]
- Saleh, H.M. Some Applications of Clays in Radioactive Waste Management. In Clays and Clay Minerals: Geological Origin, Mechanical Properties and Industrial Applications; Wesley, L.R., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2014; pp. 403–415. ISBN 978-1-63117-779-8. [Google Scholar]
- Bayoumi, T.A.; Saleh, H.M.; Eskander, S.B. Solidification of hot real radioactive liquid scintillator waste using cement–clay composite. Monatshefte Für Chem.-Chem. Mon. 2013, 144, 1751–1758. [Google Scholar] [CrossRef]
- Saleh, H.M.; Bondouk, I.I.; Salama, E.; Esawii, H.A. Consistency and Shielding Efficiency of Cement-Bitumen Composite for Use as Gamma-Radiation Shielding Material. Prog. Nucl. Energy 2021, 137, 103764. [Google Scholar] [CrossRef]
- Reda, S.M.; Saleh, H.M. Calculation of the Gamma Radiation Shielding Efficiency of Cement-Bitumen Portable Container Using MCNPX Code. Prog. Nucl. Energy 2021, 142, 104012. [Google Scholar] [CrossRef]
- Saleh, H.M.; Bondouk, I.I.; Salama, E.; Mahmoud, H.H.; Omar, K.; Esawii, H.A. Asphaltene or Polyvinylchloride Waste Blended with Cement to Produce a Sustainable Material Used in Nuclear Safety. Sustainabilitiy 2022, 14, 3525. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Faheim, A.A.; Salman, A.A.; Saleh, H.M. Sustainable Lightweight Concrete Made of Cement Kiln Dust and Liquefied Polystyrene Foam Improved with Other Waste Additives. Sustainability 2022, 14, 15313. [Google Scholar] [CrossRef]
- Eid, M.S.; Bondouk, I.I.; Saleh, H.M.; Omar, K.M.; Diab, H.M. Investigating the Effect of Gamma and Neutron Irradiation on Portland Cement Provided with Waste Silicate Glass. Sustainability 2022, 15, 763. [Google Scholar] [CrossRef]
- Eid, M.S.; Bondouk, I.I.; Saleh, H.M.; Omar, K.M.; Sayyed, M.I.; El-Khatib, A.M.; Elsafi, M. Implementation of Waste Silicate Glass into Composition of Ordinary Cement for Radiation Shielding Applications. Nucl. Eng. Technol. 2021, 54, 1456–1463. [Google Scholar] [CrossRef]
- Ehab, M.; Salama, E.; Ashour, A.; Attallah, M.; Saleh, H.M. Optical Properties and Gamma Radiation Shielding Capability of Transparent Barium Borosilicate Glass Composite. Sustainability 2022, 14, 13298. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Li, Y.; Jin, Z.-Y.; Yang, Y.; Yang, M.-B.; Yin, B. Light-and Magnetic-Responsive Synergy Controlled Reconfiguration of Polymer Nanocomposites with Shape Memory Assisted Self-Healing Performance for Soft Robotics. J. Mater. Chem. C 2021, 9, 5515–5527. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Lyu, Q.; Zhu, J. Bioinspired Structural Color Nanocomposites with Healable Capability. Polym. Chem. 2020, 11, 6413–6422. [Google Scholar] [CrossRef]
- Lu, C.; Fang, R.; Chen, X. Single-atom Catalytic Materials for Advanced Battery Systems. Adv. Mater. 2020, 32, 1906548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Burke, A.F. Electrochemical Capacitors: Materials, Technologies and Performance. Energy Storage Mater. 2021, 36, 31–55. [Google Scholar] [CrossRef]
- Verma, K.D.; Sinha, P.; Banerjee, S.; Kar, K.K. Characteristics of Electrode Materials for Supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials I: Characteristics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 269–285. [Google Scholar]
- Forouzandeh, P.; Pillai, S.C. Two-Dimensional (2D) Electrode Materials for Supercapacitors. Mater. Today Proc. 2021, 41, 498–505. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.M. Two-Dimensional Nanosheets and Membranes for Their Emerging Technologies. Curr. Opin. Chem. Eng. 2023, 39, 100893. [Google Scholar] [CrossRef]
- Tyagi, D.; Wang, H.; Huang, W.; Hu, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent Advances in Two-Dimensional-Material-Based Sensing Technology toward Health and Environmental Monitoring Applications. Nanoscale 2020, 12, 3535–3559. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Beack, S.; Han, S.; Shin, M.; Lee, T.; Park, Y.; Kim, K.S.; Yetisen, A.K.; Yun, S.H.; Kwon, W. Multifunctional Photonic Nanomaterials for Diagnostic, Therapeutic, and Theranostic Applications. Adv. Mater. 2018, 30, 1701460. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, S.; Bian, X.; Feng, J.; An, Y.; Yuan, C. Morphology-and Porosity-Tunable Synthesis of 3D Nanoporous SiGe Alloy as a High-Performance Lithium-Ion Battery Anode. ACS Nano 2018, 12, 2900–2908. [Google Scholar] [CrossRef]
- Salahdin, O.D.; Sayadi, H.; Solanki, R.; Parra, R.M.R.; Al-Thamir, M.; Jalil, A.T.; Izzat, S.E.; Hammid, A.T.; Arenas, L.A.B.; Kianfar, E. Graphene and Carbon Structures and Nanomaterials for Energy Storage. Appl. Phys. A 2022, 128, 703. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, J.; Sim, D.; Shi, W.; Tay, Y.Y.; Ma, J.; Hng, H.H.; Yan, Q. In Situ Growth of Si Nanowires on Graphene Sheets for Li-Ion Storage. Electrochim. Acta 2012, 74, 176–181. [Google Scholar] [CrossRef]
- Dong, Q.; Ryu, H.; Lei, Y. Metal Oxide Based Non-Enzymatic Electrochemical Sensors for Glucose Detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Hong, S.; Wang, Y.; Song, N.; Han, Z.; Dong, H. 0D/2D Heterojunction Constructed by High-Dispersity Mo-Doped Ni2P Nanodots Supported on g-C3N4 Nanosheets towards Enhanced Photocatalytic H2 Evolution Activity. Int. J. Hydrogen Energy 2020, 45, 22556–22566. [Google Scholar] [CrossRef]
- Jin, X.; Gu, T.-H.; Lee, K.-G.; Kim, M.J.; Islam, M.S.; Hwang, S.-J. Unique Advantages of 2D Inorganic Nanosheets in Exploring High-Performance Electrocatalysts: Synthesis, Application, and Perspective. Coord. Chem. Rev. 2020, 415, 213280. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, M.; Pan, Y.; Li, Y.; Wang, K.; Yuan, J.; Sun, Y.; Zhang, Q. Carbon Nanodots Memristor: An Emerging Candidate toward Artificial Biosynapse and Human Sensory Perception System. Adv. Sci. 2023, 10, 2207229. [Google Scholar] [CrossRef] [PubMed]
- Rasal, A.S.; Yadav, S.; Yadav, A.; Kashale, A.A.; Manjunatha, S.T.; Altaee, A.; Chang, J.-Y. Carbon Quantum Dots for Energy Applications: A Review. ACS Appl. Nano Mater. 2021, 4, 6515–6541. [Google Scholar] [CrossRef]
- Baruah, B.; Kumar, A. Platinum-Free Anode Electrocatalysts for Methanol Oxidation in Direct Methanol Fuel Cells. In Ceramic and Specialty Electrolytes for Energy Storage Devices; CRC Press: Boca Raton, FL, USA, 2021; pp. 261–283. ISBN 1003144810. [Google Scholar]
- Niu, H.; Xia, C.; Huang, L.; Zaman, S.; Maiyalagan, T.; Guo, W.; You, B.; Xia, B.Y. Rational Design and Synthesis of One-Dimensional Platinum-Based Nanostructures for Oxygen-Reduction Electrocatalysis. Chin. J. Catal. 2022, 43, 1459–1472. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Ayoko, G.A.; Bell, J.; Sun, Z. Cobalt Oxide-Based Nanoarchitectures for Electrochemical Energy Applications. Prog. Mater. Sci. 2019, 103, 596–677. [Google Scholar] [CrossRef]
- Inkson, B.J. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) for Materials Characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Elsevier: Amsterdam, The Netherlands, 2016; pp. 17–43. [Google Scholar]
- Cao, D.; Gong, S.; Shu, X.; Zhu, D.; Liang, S. Preparation of ZnO Nanoparticles with High Dispersibility Based on Oriented Attachment (OA) Process. Nanoscale Res. Lett. 2019, 14, 210. [Google Scholar] [CrossRef]
- Wartel, F.; Kosmidis, L.; Gogonel, A.; Baldovino, A.; Stephenson, Z.; Triquet, B.; Quinones, E.; Lo, C.; Mezzetta, E.; Broster, I. Timing Analysis of an Avionics Case Study on Complex Hardware/Software Platforms. In Proceedings of the 2015 Design, Automation & Test in Europe Conference & Exhibition (DATE), Grenoble, France, 9–13 March 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 397–402. [Google Scholar]
- Shrivastava, S.; Trung, T.Q.; Lee, N.-E. Recent Progress, Challenges, and Prospects of Fully Integrated Mobile and Wearable Point-of-Care Testing Systems for Self-Testing. Chem. Soc. Rev. 2020, 49, 1812–1866. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Rodrigo, M.A. Renewable Energies Driven Electrochemical Wastewater/Soil Decontamination Technologies: A Critical Review of Fundamental Concepts and Applications. Appl. Catal. B Environ. 2020, 270, 118857. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and Its Derived Composite Electrodes toward Supercapacitors: Fabrication, Properties, and Challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, M.O.; Ansari, S.A.; Kumar, P. Nanostructured Titanium Nitride and Its Composites as High-Performance Supercapacitor Electrode Material. Nanomaterials 2022, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin Derived Nitrogen-Doped Porous Carbons with Ultrahigh Specific Surface Area and Tailored Hierarchical Porosity for High Performance Supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Liu, Z.-G.; Huang, X.-J. Nanostructured Metal Oxides/Hydroxides-Based Electrochemical Sensor for Monitoring Environmental Micropollutants. Trends Environ. Anal. Chem. 2014, 3, 28–35. [Google Scholar] [CrossRef]
- Obodo, R.M.; Shinde, N.M.; Chime, U.K.; Ezugwu, S.; Nwanya, A.C.; Ahmad, I.; Maaza, M.; Ejikeme, P.M.; Ezema, F.I. Recent Advances in Metal Oxide/Hydroxide on Three-Dimensional Nickel Foam Substrate for High Performance Pseudocapacitive Electrodes. Curr. Opin. Electrochem. 2020, 21, 242–249. [Google Scholar] [CrossRef]
- Yang, T.; Xu, C.; Liu, C.; Ye, Y.; Sun, Z.; Wang, B.; Luo, Z. Conductive Polymer Hydrogels Crosslinked by Electrostatic Interaction with PEDOT: PSS Dopant for Bioelectronics Application. Chem. Eng. J. 2022, 429, 132430. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, Synthesis, and Characterization of Graphene–Nanoparticle Hybrid Materials for Bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef]
- Li, B.L.; Setyawati, M.I.; Zou, H.L.; Dong, J.X.; Luo, H.Q.; Li, N.B.; Leong, D.T. Emerging 0D Transition-metal Dichalcogenides for Sensors, Biomedicine, and Clean Energy. Small 2017, 13, 1700527. [Google Scholar] [CrossRef]
- Rajesh, D.; Francis, M.K.; Bhargav, P.B.; Nafis, A.; Balaji, C. 2D Layered Nickel-Cobalt Double Hydroxide Nano Sheets@ 1D Silver Nanowire-Graphitic Carbon Nitrides for High Performance Super Capacitors. J. Alloys Compd. 2022, 898, 162803. [Google Scholar] [CrossRef]
- Qian, C.; Guo, X.; Zhang, W.; Yang, H.; Qian, Y.; Xu, F.; Qian, S.; Lin, S.; Fan, T. Co3O4 Nanoparticles on Porous Bio-Carbon Substrate as Catalyst for Oxygen Reduction Reaction. Microporous Mesoporous Mater. 2019, 277, 45–51. [Google Scholar] [CrossRef]
- Mary, B.; Vijaya, J.J.; Nair, R.R.; Mustafa, A.; Selvamani, P.S.; Saravanakumar, B.; Bououdina, M.; Kennedy, L.J. Reduced Graphene Oxide-Tailored CuFe2O4 Nanoparticles as an Electrode Material for High-Performance Supercapacitors. J. Nanomater. 2022, 2022, 9861440. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid Interface Sci. 2021, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, M.; Li, X.; Liu, X.; Zhu, L.; Annie Bligh, S.W. Multifluid Electrospinning for the Generation of Complex Nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2020, 12, e1601. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Botlhoko, O.J.; Setshedi, K.; Scriba, M.; Masukume, M.; Ray, S.S. Top-down Synthesis of Graphene: A Comprehensive Review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Marcì, G.; Palmisano, L. Heterogeneous Photocatalysis: Relationships with Heterogeneous Catalysis and Perspectives; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 0444640169. [Google Scholar]
- Gavrilović, T.V.; Jovanović, D.J.; Dramićanin, M.D. Synthesis of Multifunctional Inorganic Materials: From Micrometer to Nanometer Dimensions. In Nanomaterials for Green Energy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 55–81. [Google Scholar]
- Zou, Y.; Wang, S. An Investigation of Active Sites for Electrochemical CO2 Reduction Reactions: From in Situ Characterization to Rational Design. Adv. Sci. 2021, 8, 2003579. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A. Importance and Challenges of Hydrothermal Technique for Synthesis of Transition Metal Oxides and Composites as Supercapacitor Electrode Materials. J. Energy Storage 2021, 44, 103295. [Google Scholar] [CrossRef]
- Mao, X.; Wang, Y.; Xiang, C.; Zhan, D.; Zhang, H.; Yan, E.; Xu, F.; Hu, X.; Zhang, J.; Sun, L.; et al. Core-Shell Structured CuCo2S4@CoMoO4 Nanorods for Advanced Electrode Materials. J. Alloys Compd. 2020, 844, 156133. [Google Scholar] [CrossRef]
- Yuan, Y.; Jia, H.; Liu, Z.; Wang, L.; Sheng, J.; Fei, W. A highly conductive Ni(OH)2 nano-sheet wrapped CuCo2S4 nano-tube electrode with a core–shell structure for high performance supercapacitors. Dalton Trans. 2021, 50, 8476–8486. [Google Scholar] [CrossRef]
- Yadav, K.; Kumar, P.; Teja, D.R.; Chakraborty, S.; Chakraborty, M.; Mohapatra, S.S.; Sahoo, A.; Chou, M.M.C.; Liang, C.-T.; Hang, D.-R. A Review on Low-Dimensional Nanomaterials: Nanofabrication, Characterization and Applications. Nanomaterials 2022, 13, 160. [Google Scholar]
- Tran, D.T.; Kshetri, T.; Chuong, N.D.; Gautam, J.; Van Hien, H.; Kim, N.H.; Lee, J.H. Emerging Core-Shell Nanostructured Catalysts of Transition Metal Encapsulated by Two-Dimensional Carbon Materials for Electrochemical Applications. Nano Today 2018, 22, 100–131. [Google Scholar] [CrossRef]
- Low, S.; Shon, Y.-S. Molecular Interactions between Pre-Formed Metal Nanoparticles and Graphene Families. Adv. Nano Res. 2018, 6, 357. [Google Scholar]
- Sinha, P.; Banerjee, S.; Kar, K.K. Transition Metal Oxide/Activated Carbon-Based Composites as Electrode Materials for Supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials II; Springer: Berlin/Heidelberg, Germany, 2020; pp. 145–178. [Google Scholar]
- Deng, X.; Zou, K.; Momen, R.; Cai, P.; Chen, J.; Hou, H.; Zou, G.; Ji, X. High Content Anion (S/Se/P) Doping Assisted by Defect Engineering with Fast Charge Transfer Kinetics for High-Performance Sodium Ion Capacitors. Sci. Bull. 2021, 66, 1858–1868. [Google Scholar] [CrossRef]
- Banerjee, A.N.; Anitha, V.C.; Joo, S.W. Improved Electrochemical Properties of Morphology-Controlled Titania/Titanate Nanostructures Prepared by in-Situ Hydrothermal Surface Modification of Self-Source Ti Substrate for High-Performance Supercapacitors. Sci. Rep. 2017, 7, 13227. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, N.; Wang, S.; Zhou, T.; Tong, Y.; Ao, C.; Yan, W.; Zhang, L.; Chu, W.; Wu, C. Interfacial Engineering of Cobalt Sulfide/Graphene Hybrids for Highly Efficient Ammonia Electrosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 6635–6640. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Huang, J.-K.; Jin, L.; Hsu, Y.-T.; Yu, S.F.; Li, L.-J.; Yang, H.Y. Selective Decoration of Au Nanoparticles on Monolayer MoS2 Single Crystals. Sci. Rep. 2013, 3, 1839. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ma, Y.; Yuan, Q.; Hu, H.; Hu, X.; Qian, Z.; Rolle, J.K.; Gu, Y.; Li, S. Enhanced Ferroptosis by Oxygen-Boosted Phototherapy Based on a 2-in-1 Nanoplatform of Ferrous Hemoglobin for Tumor Synergistic Therapy. ACS Nano 2020, 14, 3414–3425. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, H.; Xiao, J.; Tu, Y.; Deng, D.; Yang, H.; Tian, H.; Li, J.; Ren, P.; Bao, X. Triggering the Electrocatalytic Hydrogen Evolution Activity of the Inert Two-Dimensional MoS 2 Surface via Single-Atom Metal Doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Yu, C.; Wang, H.; Li, Q. Recent Progress of Hollow Carbon Nanocages: General Design Fundamentals and Diversified Electrochemical Applications. Adv. Sci. 2023, 10, 2206605. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, P.; Fan, J.; Yu, H.; Yu, J. Hetero-Phase MoC-Mo2C Nanoparticles for Enhanced Photocatalytic H2-Production Activity of TiO2. Nano Res. 2021, 14, 1095–1102. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Chen, S.; Surendran, A.; Wu, X.; Lee, S.Y.; Stephen, M.; Leong, W.L. Recent Technological Advances in Fabrication and Application of Organic Electrochemical Transistors. Adv. Mater. Technol. 2020, 5, 2000523. [Google Scholar] [CrossRef]
- Hansora, D.P.; Shimpi, N.G.; Mishra, S. Performance of Hybrid Nanostructured Conductive Cotton Materials as Wearable Devices: An Overview of Materials, Fabrication, Properties and Applications. Rsc. Adv. 2015, 5, 107716–107770. [Google Scholar] [CrossRef]
- Perdoménico, J.; Ruiz, M.M.; Codesido, N.O.; De Candia, A.G.; Marcolongo, J.P.; Slep, L.D. Helpful Correlations to Estimate the p K a of Coordinated HNO: A Potential–PH Exploration in a Pendant-Arm Cyclam-Based Ruthenium Nitroxyl. Dalt. Trans. 2021, 50, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly Imprinted Polymer Based Sensors for Medical Applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef] [PubMed]
- Md Jani, A.M.; Habiballah, A.S.; Budiman Abdul Halim, M.Z.; Ahmad Zulkifli, F.W.; Mahmud, A.H.; Yazid, H. Nanoporous Anodic Aluminum Oxide (NAAO) for Catalytic, Biosensing and Template Synthesis Applications (A Review). Curr. Nanosci. 2019, 15, 49–63. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical Sensing Based on Carbon Nanoparticles: A Review. Sensors Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Li, W.; Nie, Z.; Yao, S. Surface Nanocrystallization of Glassy Carbon Electrode: Application in Direct Electrochemistry of Glucose Oxidase. Electrochem. Commun. 2008, 10, 1459–1462. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical Glucose Sensors in Diabetes Management: An Updated Review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable Electrochemical Biosensors in North America. Biosens. Bioelectron. 2021, 172, 112750. [Google Scholar] [CrossRef]
- Saei, A.A.; Najafi-marandi, P.; Abhari, A.; De Guardia, M.; Ezzati, J.; Dolatabadi, N. Electrochemical Biosensors for Glucose Based on Metal Nanoparticles. Trends Anal. Chem. 2012, 9, 66. [Google Scholar] [CrossRef]
- Sedaghat, S.; Piepenburg, C.R.; Zareei, A.; Qi, Z.; Peana, S.; Wang, H.; Rahimi, R. Laser-Induced Mesoporous Nickel Oxide as a Highly Sensitive Nonenzymatic Glucose Sensor. ACS Appl. Nano Mater. 2020, 3, 5260–5270. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Wu, F.; Cao, X.; Li, Z.; Alharbi, M.; Abbas, A.N.; Amer, M.R.; Zhou, C. Highly Sensitive and Wearable In2O3 Nanoribbon Transistor Biosensors with Integrated On-Chip Gate for Glucose Monitoring in Body Fluids. ACS Nano 2018, 12, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Li, H.; Yan, X.; Lin, Y.; Lu, G. Biosensors Based on Fluorescence Carbon Nanomaterials for Detection of Pesticides. TrAC Trends Anal. Chem. 2020, 134, 116126. [Google Scholar] [CrossRef]
- Yola, M.L.; Göde, C.; Atar, N. Determination of Rutin by CoFe2O4 Nanoparticles Ionic Liquid Nanocomposite as a Voltammetric Sensor. J. Mol. Liq. 2017, 246, 350–353. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Brunton, N.P.; Brennan, C. Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 1118464680. [Google Scholar]
- Cao, X.; Halder, A.; Tang, Y.; Hou, C.; Wang, H.; Duus, J.Ø.; Chi, Q. Engineering Two-Dimensional Layered Nanomaterials for Wearable Biomedical Sensors and Power Devices. Mater. Chem. Front. 2018, 2, 1944–1986. [Google Scholar] [CrossRef]
- Janssen, J.; Lambeta, M.; White, P.; Byagowi, A. Carbon Nanotube-Based Electrochemical Biosensor for Label-Free Protein Detection. Biosensors 2019, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Hong, J.T.; Choi, S.J.; Kim, H.S.; Park, W.K.; Han, S.T.; Park, J.Y.; Lee, S.; Kim, D.S.; Ahn, Y.H. Detection of Microorganisms Using Terahertz Metamaterials. Sci. Rep. 2014, 4, 4988. [Google Scholar] [CrossRef]
- Güell, A.G.; Meadows, K.E.; Dudin, P.V.; Ebejer, N.; Macpherson, J.V.; Unwin, P.R. Mapping Nanoscale Electrochemistry of Individual Single-Walled Carbon Nanotubes. Nano Lett. 2014, 14, 220–224. [Google Scholar] [CrossRef]
- Wanekaya, A.K.; Chen, W.; Myung, N.V.; Mulchandani, A. Nanowire-based Electrochemical Biosensors. Electroanalysis 2006, 18, 533–550. [Google Scholar] [CrossRef]
- Shashaani, H.; Faramarzpour, M.; Hassanpour, M.; Namdar, N.; Alikhani, A.; Abdolahad, M. Silicon Nanowire Based Biosensing Platform for Electrochemical Sensing of Mebendazole Drug Activity on Breast Cancer Cells. Biosens. Bioelectron. 2016, 85, 363–370. [Google Scholar] [CrossRef]
- Falina, S.; Syamsul, M.; Rhaffor, N.A.; Sal Hamid, S.; Mohamed Zain, K.A.; Abd Manaf, A.; Kawarada, H. Ten Years Progress of Electrical Detection of Heavy Metal Ions (HMIs) Using Various Field-Effect Transistor (FET) Nanosensors: A Review. Biosensors 2021, 11, 478. [Google Scholar] [CrossRef]
- Adhikari, J.; Rizwan, M.; Keasberry, N.A.; Ahmed, M.U. Current Progresses and Trends in Carbon Nanomaterials-based Electrochemical and Electrochemiluminescence Biosensors. J. Chin. Chem. Soc. 2020, 67, 937–960. [Google Scholar] [CrossRef]
- Lawal, A.T. Progress in Utilisation of Graphene for Electrochemical Biosensors. Biosens. Bioelectron. 2018, 106, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sui, C.; Wang, C.; Wang, Y.; He, D.; Sun, Y.; Zhang, Y.; Meng, Q.; Ma, T.; Song, X.-M. Gold Nanoparticles/Single-Stranded DNA-Reduced Graphene Oxide Nanocomposites Based Electrochemical Biosensor for Highly Sensitive Detection of Cholesterol. Front. Chem. Sci. Eng. 2021, 15, 1572–1582. [Google Scholar] [CrossRef]
- Soldado, A.; Barrio, L.C.; Díaz-Gonzalez, M.; de la Escosura-Muñiz, A.; Costa-Fernandez, J.M. Advances in Quantum Dots as Diagnostic Tools. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 107, pp. 1–40. ISBN 0065-2423. [Google Scholar]
- Kumar, S.; Bukkitgar, S.D.; Singh, S.; Pratibha; Singh, V.; Reddy, K.R.; Shetti, N.P.; Venkata Reddy, C.; Sadhu, V.; Naveen, S. Electrochemical Sensors and Biosensors Based on Graphene Functionalized with Metal Oxide Nanostructures for Healthcare Applications. ChemistrySelect 2019, 4, 5322–5337. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-Performance Monoclinic WO3 Nanospheres with the Novel NH4+ Diffusion Behaviors for Aqueous Ammonium-Ion Batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like Chitosan Porous Carbon Spheres/MXene Composite with High Specific Capacitance and Rate Performance for Supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Askari, S.; Bozcheloei, Z.A. Piezoelectric Composites in Neural Tissue Engineering: Material and Fabrication Techniques. J. Compos. Compd. 2022, 4, 37–46. [Google Scholar] [CrossRef]
- Hou, J.; Qu, S.; Yang, M.; Zhang, J. Materials and Electrode Engineering of High Capacity Anodes in Lithium Ion Batteries. J. Power Sources 2020, 450, 227697. [Google Scholar] [CrossRef]
- Browne, M.P.; Sofer, Z.; Pumera, M. Layered and Two Dimensional Metal Oxides for Electrochemical Energy Conversion. Energy Environ. Sci. 2019, 12, 41–58. [Google Scholar] [CrossRef]
- Xu, R.; Du, L.; Adekoya, D.; Zhang, G.; Zhang, S.; Sun, S.; Lei, Y. Well-defined Nanostructures for Electrochemical Energy Conversion and Storage. Adv. Energy Mater. 2021, 11, 2001537. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium Ion, Lithium Metal, and Alternative Rechargeable Battery Technologies: The Odyssey for High Energy Density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard Carbons for Sodium-Ion Batteries: Structure, Analysis, Sustainability, and Electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. The Chemistry of Redox-flow Batteries. Angew. Chemie Int. Ed. 2015, 54, 9776–9809. [Google Scholar] [CrossRef]
- Liu, C.-F.; Liu, Y.-C.; Yi, T.-Y.; Hu, C.-C. Carbon Materials for High-Voltage Supercapacitors. Carbon 2019, 145, 529–548. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Zhang, H.; Baakes, F.; Desmaizieres, G.; Hayun, H.; Yang, L.; Kolek, M.; Küpers, V.; Janek, J.; Mandler, D. Side by Side Battery Technologies with Lithium-Ion Based Batteries. Adv. Energy Mater. 2020, 10, 2000089. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the Future Transportation Fuel: From Production to Applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Notton, G.; Nivet, M.-L.; Voyant, C.; Paoli, C.; Darras, C.; Motte, F.; Fouilloy, A. Intermittent and Stochastic Character of Renewable Energy Sources: Consequences, Cost of Intermittence and Benefit of Forecasting. Renew. Sustain. Energy Rev. 2018, 87, 96–105. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of Current Development in Electrical Energy Storage Technologies and the Application Potential in Power System Operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent Progress in Redox Flow Battery Research and Development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Sagadevan, S.; Johan, M.R.; Marlinda, A.R.; Akbarzadeh, O.; Pandian, K.; Shahid, M.M.; Mohammad, F.; Podder, J. Background of energy storage. In Advances in Supercapacitor and Supercapattery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–26. [Google Scholar]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-Dimensional, One-Dimensional, Two-Dimensional and Three-Dimensional Nanostructured Materials for Advanced Electrochemical Energy Devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for Electrochemical Capacitors and Related Devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Xu, Q. Metal–Organic Frameworks for Energy. Adv. Energy Mater. 2019, 9, 1801307. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Sivanesan, I. A Comprehensive Compilation of Graphene/Fullerene Polymer Nanocomposites for Electrochemical Energy Storage. Polymers 2023, 15, 701. [Google Scholar] [CrossRef]
- Hemmati, S.; Li, G.; Wang, X.; Ding, Y.; Pei, Y.; Yu, A.; Chen, Z. 3D N-Doped Hybrid Architectures Assembled from 0D T-Nb2O5 Embedded in Carbon Microtubes toward High-Rate Li-Ion Capacitors. Nano Energy 2019, 56, 118–126. [Google Scholar] [CrossRef]
- Zhang, X.; Ju, Z.; Zhu, Y.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C.; Yu, G. Multiscale Understanding and Architecture Design of High Energy/Power Lithium-ion Battery Electrodes. Adv. Energy Mater. 2021, 11, 2000808. [Google Scholar] [CrossRef]
- Hamdan, A.; Glad, X.; Cha, M.S. Synthesis of Copper and Copper Oxide Nanomaterials by Pulsed Electric Field in Water with Various Electrical Conductivities. Nanomaterials 2020, 10, 1347. [Google Scholar] [CrossRef]
- Shuai, C.; Wang, B.; Yang, Y.; Peng, S.; Gao, C. 3D Honeycomb Nanostructure-Encapsulated Magnesium Alloys with Superior Corrosion Resistance and Mechanical Properties. Compos. Part B Eng. 2019, 162, 611–620. [Google Scholar] [CrossRef]
- Abed, S.M.; Reda, S.E.M.; Muhsin, N.M.B. Review on Nanotechnology Applications in Removing Chemical Pollutants and Their Application in Health. Trends Pharm. Nanotechnol. 2022, 11, 31–38. [Google Scholar]
- Zhang, J.; You, C.; Lin, H.; Wang, J. Electrochemical Kinetic Modulators in Lithium–Sulfur Batteries: From Defect-Rich Catalysts to Single Atomic Catalysts. Energy Environ. Mater. 2022, 5, 731–750. [Google Scholar] [CrossRef]
- Yang, X.; Luo, J.; Sun, X. Towards High-Performance Solid-State Li–S Batteries: From Fundamental Understanding to Engineering Design. Chem. Soc. Rev. 2020, 49, 2140–2195. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Zerrin, T.; Dong, B.; Ozkan, C.S.; Ozkan, M. Sustainable and Low-Cost Lithium-Ion Batteries: Nonconventional Electrode Chemistries and State of Health Characterization. Technol. Innov. 2020, 21, 1–23. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Z.; Siegenthaler, J.; Zheng, B.; Tong, Y.; Li, J.; Schuelke, T.; Fan, Q.H.; Wang, K.; Xu, H. Review on Recent Advances in Two-dimensional Nanomaterials-based Cathodes for Lithium-sulfur Batteries. EcoMat 2023, 5, e12286. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-Based Anodes for Lithium-Ion Batteries: Effectiveness of Materials Synthesis and Electrode Preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Luo, L.; He, Y.; Li, B.; Cai, Y.; Hu, S.; Xiao, X. Hierarchical Porous Silicon Structures with Extraordinary Mechanical Strength as High-Performance Lithium-Ion Battery Anodes. Nat. Commun. 2020, 11, 1474. [Google Scholar] [CrossRef]
- Pattadar, D.K.; Sharma, J.N.; Mainali, B.P.; Zamborini, F.P. Impact of the Assembly Method on the Surface Area-to-Volume Ratio and Electrochemical Oxidation Potential of Metal Nanospheres. J. Phys. Chem. C 2019, 123, 24304–24312. [Google Scholar] [CrossRef]
- Imran, M.; Prakash, O.; Pushkar, P.; Mungray, A.; Kailasa, S.K.; Chongdar, S.; Mungray, A.K. Performance Enhancement of Benthic Microbial Fuel Cell by Cerium Coated Electrodes. Electrochim. Acta 2019, 295, 58–66. [Google Scholar] [CrossRef]
- Dwivedi, S. Solid Oxide Fuel Cell: Materials for Anode, Cathode and Electrolyte. Int. J. Hydrogen Energy 2020, 45, 23988–24013. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Guo, Z.; Zhang, W.; Li, H.; Huang, W. Recent Developments and Advances in Boron-Doped Diamond Electrodes for Electrochemical Oxidation of Organic Pollutants. Sep. Purif. Technol. 2019, 212, 802–821. [Google Scholar] [CrossRef]
- Ko, J.; Zhao, Z.-J.; Hwang, S.H.; Kang, H.-J.; Ahn, J.; Jeon, S.; Bok, M.; Jeong, Y.; Kang, K.; Cho, I. Nanotransfer Printing on Textile Substrate with Water-Soluble Polymer Nanotemplate. ACS Nano 2020, 14, 2191–2201. [Google Scholar] [CrossRef]
- Malik, M.; Chan, K.H.; Azimi, G. Review on the Synthesis of LiNixMnyCo1-x-YO2 (NMC) Cathodes for Lithium-Ion Batteries. Mater. Today Energy 2022, 28, 101066. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, K.; Hu, Z.; Tao, Z.; Mai, L.; Kang, Y.; Chou, S.; Chen, J. Recent Developments on and Prospects for Electrode Materials with Hierarchical Structures for Lithium-ion Batteries. Adv. Energy Mater. 2018, 8, 1701415. [Google Scholar] [CrossRef]
- Ajdari, F.B.; Kowsari, E.; Shahrak, M.N.; Ehsani, A.; Kiaei, Z.; Torkzaban, H.; Ershadi, M.; Eshkalak, S.K.; Haddadi-Asl, V.; Chinnappan, A. A Review on the Field Patents and Recent Developments over the Application of Metal Organic Frameworks (MOFs) in Supercapacitors. Coord. Chem. Rev. 2020, 422, 213441. [Google Scholar] [CrossRef]
- Boisset, A.; Athouël, L.; Jacquemin, J.; Porion, P.; Brousse, T.; Anouti, M. Comparative Performances of Birnessite and Cryptomelane MnO2 as Electrode Material in Neutral Aqueous Lithium Salt for Supercapacitor Application. J. Phys. Chem. C 2013, 117, 7408–7422. [Google Scholar] [CrossRef]
- Shimizu, W.; Makino, S.; Takahashi, K.; Imanishi, N.; Sugimoto, W. Development of a 4.2 V Aqueous Hybrid Electrochemical Capacitor Based on MnO2 Positive and Protected Li Negative Electrodes. J. Power Sources 2013, 241, 572–577. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Chen, L.; Huang, X. Structural and Electrochemical Characterizations of Surface-Modified LiCoO2 Cathode Materials for Li-Ion Batteries. Solid State Ion. 2002, 148, 335–342. [Google Scholar] [CrossRef]
- Dong, W.; Zhao, Y.; Cai, M.; Dong, C.; Ma, W.; Pan, J.; Lv, Z.; Dong, H.; Dong, Y.; Tang, Y. Nanoscale Borate Coating Network Stabilized Iron Oxide Anode for High-Energy-Density Bipolar Lithium-Ion Batteries. Small 2023, 19, 2207074. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Lu, Y.; Zhu, J.; Zhang, X. Silica/Polyacrylonitrile Hybrid Nanofiber Membrane Separators via Sol-Gel and Electrospinning Techniques for Lithium-Ion Batteries. J. Power Sources 2016, 313, 205–212. [Google Scholar] [CrossRef]
- Lee, S.J.; Joe, Y.S.; Yeon, J.S.; Min, D.H.; Shin, K.H.; Baek, S.H.; Xiong, P.; Nakhanivej, P.; Park, H.S. Hierarchically Structured Silicon/Graphene Composites Wrapped by Interconnected Carbon Nanotube Branches for Lithium-ion Battery Anodes. Int. J. Energy Res. 2022, 46, 15627–15638. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium-and Sodium-ion Batteries. Transit. Met. Oxides Electrochem. Energy Storage 2022, 10, 55–99. [Google Scholar]
- Huang, J.; Wang, J.; Wang, C.; Zhang, H.; Lu, C.; Wang, J. Hierarchical Porous Graphene Carbon-Based Supercapacitors. Chem. Mater. 2015, 27, 2107–2113. [Google Scholar] [CrossRef]
- Rajasekaran, S.J.; Grace, A.N.; Jacob, G.; Alodhayb, A.; Pandiaraj, S.; Raghavan, V. Investigation of Different Aqueous Electrolytes for Biomass-Derived Activated Carbon-Based Supercapacitors. Catalysts 2023, 13, 286. [Google Scholar] [CrossRef]
- Wang, R.; Han, M.; Zhao, Q.; Ren, Z.; Guo, X.; Xu, C.; Hu, N.; Lu, L. Hydrothermal Synthesis of Nanostructured Graphene/Polyaniline Composites as High-Capacitance Electrode Materials for Supercapacitors. Sci. Rep. 2017, 7, 44562. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D Carbon-Based Networks Combined with Pseudocapacitive Electrode Material for High Energy Density Supercapacitor: A Review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Patnaik, P.; Mondal, R.; Sarkar, S.; Choudhury, A.; Chatterjee, U. Proton Exchange Membrane from the Blend of Poly (Vinylidene Fluoride) and Functional Copolymer: Preparation, Proton Conductivity, Methanol Permeability, and Stability. Int. J. Hydrogen Energy 2022, 47, 41920–41931. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.; Li, C.; Lei, Y. Mechanisms for Enhanced Performance of Platinum-based Electrocatalysts in Proton Exchange Membrane Fuel Cells. ChemSusChem 2014, 7, 361–378. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Liang, Y.-Y. Assessment of Thermochemically Stable Apatite La10(SiO4)6O3 as Electrolyte for Solid Oxide Fuel Cells. Ceram. Int. 2012, 38, 3955–3961. [Google Scholar] [CrossRef]
- Jeon, S.; Seo, J.; Shin, J.W.; Lee, S.; Seo, H.G.; Lee, S.; Tsvetkov, N.; Kim, J.; An, J.; Jung, W. Metal-Oxide Nanocomposite Catalyst Simultaneously Boosts the Oxygen Reduction Reactivity and Chemical Stability of Solid Oxide Fuel Cell Cathode. Chem. Eng. J. 2023, 455, 140611. [Google Scholar] [CrossRef]
- Vallejos, S.; Di Maggio, F.; Shujah, T.; Blackman, C. Chemical Vapour Deposition of Gas Sensitive Metal Oxides. Chemosensors 2016, 4, 4. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Wen, K.; Jiang, W.; Li, Q. Immobilized Enzymes in Inorganic Hybrid Nanoflowers for Biocatalytic and Biosensing Applications. J. Mater. Chem. B 2021, 9, 7597–7607. [Google Scholar] [CrossRef]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon Nanomaterials as Versatile Platforms for Biosensing Applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xie, H.; Shen, L.; Xu, Y.; Zhang, M.; Zhou, M.; Li, B.; Li, R.; Lin, H. Covalent Organic Frameworks: The Rising-Star Platforms for the Design of CO2 Separation Membranes. Small 2023, 19, 2207313. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, X.; Li, Z.; Meng, J.; Chu, X.; Zhang, P.; Sun, B.; Zhang, J.; Gao, Y.; Xu, W. Multifunctional Integrated Superhydrophobic Coatings with Unique Fluorescence and Micro/Micro/Nano-Hierarchical Structures Enabled by In Situ Self-Assembly. ACS Appl. Mater. Interfaces 2023, 15, 7442–7453. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, X.; Zhan, F.; He, Q.; Wang, H.; Chen, J.; Wang, H.; Ren, X.; Chen, L. Toward Emerging Two-Dimensional Nickel-Based Materials for Electrochemical Energy Storage: Progress and Perspectives. Energy Storage Mater. 2022, 53, 79–135. [Google Scholar] [CrossRef]
- Wei, Y.; Zou, L.; Wang, H.; Wang, Y.; Xu, Q. Micro/Nano-scaled Metal-organic Frameworks and Their Derivatives for Energy Applications. Adv. Energy Mater. 2022, 12, 2003970. [Google Scholar] [CrossRef]
- Sharanyakanth, P.S.; Radhakrishnan, M. Synthesis of Metal-Organic Frameworks (MOFs) and Its Application in Food Packaging: A Critical Review. Trends Food Sci. Technol. 2020, 104, 102–116. [Google Scholar] [CrossRef]
- Zhou, D.; Guo, X.; Zhang, Q.; Shi, Y.; Zhang, H.; Yu, C.; Pang, H. Nickel-based Materials for Advanced Rechargeable Batteries. Adv. Funct. Mater. 2022, 32, 2107928. [Google Scholar] [CrossRef]
- Sakar, M.; Do, T. Metal–Organic Frameworks for Photocatalytic Environmental Remediation. Photocatalytic Funct. Mater. Environ. Remediat. 2019, 309–341. [Google Scholar] [CrossRef]
- Wang, M.; Dong, X.; Meng, Z.; Hu, Z.; Lin, Y.; Peng, C.; Wang, H.; Pao, C.; Ding, S.; Li, Y. An Efficient Interfacial Synthesis of Two-Dimensional Metal–Organic Framework Nanosheets for Electrochemical Hydrogen Peroxide Production. Angew. Chemie Int. Ed. 2021, 60, 11190–11195. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xu, Y.; Xue, H.; Liu, C.; Pang, H. Ultrathin Two-Dimensional Cobalt-Organic Frameworks Nanosheets for Electrochemical Energy Storage. Chem. Eng. J. 2019, 373, 1319–1328. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L. Research Progress on Applications of Polyaniline (PANI) for Electrochemical Energy Storage and Conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Ahmaruzzaman, M. Recent Progress on Synthesis and Modifications of ZnIn2S4 Based Novel Hybrid Materials for Potential Applications. Mater. Sci. Eng. B 2023, 292, 116418. [Google Scholar] [CrossRef]
- Patial, S.; Raizada, P.; Hasija, V.; Singh, P.; Thakur, V.K.; Nguyen, V.-H. Recent Advances in Photocatalytic Multivariate Metal Organic Frameworks-Based Nanostructures toward Renewable Energy and the Removal of Environmental Pollutants. Mater. Today Energy 2021, 19, 100589. [Google Scholar] [CrossRef]
- Gupta, R.K. Metal Phosphates and Phosphonates: Fundamental to Advanced Emerging Applications; Springer Nature: Berlin/Heidelberg, Germany, 2023; ISBN 3031270622. [Google Scholar]
- Yan, C.; Jin, J.; Wang, J.; Zhang, F.; Tian, Y.; Liu, C.; Zhang, F.; Cao, L.; Zhou, Y.; Han, Q. Metal–Organic Frameworks (MOFs) for the Efficient Removal of Contaminants from Water: Underlying Mechanisms, Recent Advances, Challenges, and Future Prospects. Coord. Chem. Rev. 2022, 468, 214595. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, M.; Wang, H.; Han, C.; Pan, F.; Sun, J. Covalent Organic Framework for Rechargeable Batteries: Mechanisms and Properties of Ionic Conduction. Adv. Energy Mater. 2022, 12, 2200057. [Google Scholar] [CrossRef]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and Applications of Three Dimensional Covalent Organic Frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Ahmed, N.; Amjad, M.W.; Hussain, M.A.; Elsherif, M.A.; Ejaz, H.; Alotaibi, N.H. Covalent Organic Frameworks (COFs) as Multi-Target Multifunctional Frameworks. Polymers 2023, 15, 267. [Google Scholar] [CrossRef]
- Afshari, M.; Dinari, M. Materials, Chemistry, and Synthesis of Covalent Organic Frameworks. In Covalent Organic Frameworks; CRC Press: Boca Raton, FL, USA, 2023; pp. 19–38. ISBN 1003206506. [Google Scholar]
- Wang, K.; Zhang, Z.; Lin, L.; Hao, K.; Chen, J.; Tian, H.; Chen, X. Cyanine-Assisted Exfoliation of Covalent Organic Frameworks in Nanocomposites for Highly Efficient Chemo-Photothermal Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 39503–39512. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Niu, S.; Li, C.; Chen, C.; Liu, Q.; Huo, J. Microwave-Assisted Rapid Synthesis and Activation of Ultrathin Trimetal–Organic Framework Nanosheets for Efficient Electrocatalytic Oxygen Evolution. J. Colloid Interface Sci. 2021, 603, 148–156. [Google Scholar] [CrossRef]
- Dogru, M.; Sonnauer, A.; Zimdars, S.; Döblinger, M.; Knochel, P.; Bein, T. Facile Synthesis of a Mesoporous Benzothiadiazole-COF Based on a Transesterification Process. CrystEngComm 2013, 15, 1500–1502. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical Synthesis of Nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Kim, J.; Cho, H.-Y.; Kim, S.; Ahn, W.-S. Facile Synthesis of Covalent Organic Frameworks COF-1 and COF-5 by Sonochemical Method. RSC Adv. 2012, 2, 10179–10181. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Thomas, A. Covalent Organic Frameworks (COFs) for Electrochemical Applications. Chem. Soc. Rev. 2021, 50, 6871–6913. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; He, L.; Zhuang, Q.; Li, B.; Wang, C.; Lv, Y.; Chu, J.; Song, Y. Recent Advances in Confining Polyoxometalates and the Applications. Small 2023, 19, 2207315. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, W.; Yang, S.; Wang, L.; Yu, G. 2D Covalent Organic Frameworks Based on Heteroacene Units. Small 2023, 19, 2207876. [Google Scholar] [CrossRef]
- Wen, J.; Huang, L.; Huang, Y.; Luo, W.; Huo, H.; Wang, Z.; Zheng, X.; Wen, Z.; Huang, Y. A Lithium-MXene Composite Anode with High Specific Capacity and Low Interfacial Resistance for Solid-State Batteries. Energy Storage Mater. 2022, 45, 934–940. [Google Scholar] [CrossRef]
- Sang, D.K.; Wang, H.; Guo, Z.; Xie, N.; Zhang, H. Recent Developments in Stability and Passivation Techniques of Phosphorene toward Next-generation Device Applications. Adv. Funct. Mater. 2019, 29, 1903419. [Google Scholar] [CrossRef]
- Neupane, G.P.; Yildirim, T.; Zhang, L.; Lu, Y. Retracted: Emerging 2D MXene/Organic Heterostructures for Future Nanodevices. Adv. Funct. Mater. 2020, 30, 2005238. [Google Scholar] [CrossRef]
- Long, Y.; Tao, Y.; Shang, T.; Yang, H.; Sun, Z.; Chen, W.; Yang, Q. Roles of Metal Ions in MXene Synthesis, Processing and Applications: A Perspective. Adv. Sci. 2022, 9, 2200296. [Google Scholar] [CrossRef]
- Xu, X.; Yang, L.; Zheng, W.; Zhang, H.; Wu, F.; Tian, Z.; Zhang, P.; Sun, Z. MXenes with Applications in Supercapacitors and Secondary Batteries: A Comprehensive Review. Mater. Reports Energy 2022, 2, 100080. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.; Li, S.; Urbankowski, P.; Li, J.; Xu, Y.; Gogotsi, Y. An Ultrafast Conducting Polymer@ MXene Positive Electrode with High Volumetric Capacitance for Advanced Asymmetric Supercapacitors. Small 2020, 16, 1906851. [Google Scholar] [CrossRef] [PubMed]
- Nahirniak, S.; Ray, A.; Saruhan, B. Challenges and Future Prospects of the MXene-Based Materials for Energy Storage Applications. Batteries 2023, 9, 126. [Google Scholar] [CrossRef]
- Yang, Q.; Eder, S.J.; Martini, A.; Grützmacher, P.G. Effect of Surface Termination on the Balance between Friction and Failure of Ti3C2T x MXenes. Npj. Mater. Degrad. 2023, 7, 6. [Google Scholar] [CrossRef]
- Wyatt, B.C.; Rosenkranz, A.; Anasori, B. 2D MXenes: Tunable Mechanical and Tribological Properties. Adv. Mater. 2021, 33, 2007973. [Google Scholar] [CrossRef]
- Li, H.; Fan, R.; Zou, B.; Yan, J.; Shi, Q.; Guo, G. Roles of MXenes in Biomedical Applications: Recent Developments and Prospects. J. Nanobiotech. 2023, 21, 73. [Google Scholar] [CrossRef]
- Carey, M.; Hinton, Z.; Natu, V.; Pai, R.; Sokol, M.; Alvarez, N.J.; Kalra, V.; Barsoum, M.W. Dispersion and Stabilization of Alkylated 2D MXene in Nonpolar Solvents and Their Pseudocapacitive Behavior. Cell Reports Phys. Sci. 2020, 1, 100042. [Google Scholar] [CrossRef]
- Pal, M.; Ayele, Y.; Hadush, M.; Panigrahi, S.; Jadhav, V.J. Public Health Hazards Due to Unsafe Drinking Water. Air Water Borne Dis 2018, 7, 2. [Google Scholar]
- Nair, K.V.K.; Satpathy, K.K.; Venugopalan, V.P. Biofouling Control; Current Methods and New Approaches with Emphasis on Power Plant Cooling Water Systems. In Fouling Organisms of the Indian Ocean; CRC Press: Boca Raton, FL, USA, 2020; pp. 159–188. ISBN 1003077994. [Google Scholar]
- Li, Z.; Ding, J.; Guo, C.; Lefebvre, J.; Malenfant, P.R.L. Decomposable S-Tetrazine Copolymer Enables Single-Walled Carbon Nanotube Thin Film Transistors and Sensors with Improved Sensitivity. Adv. Funct. Mater. 2018, 28, 1705568. [Google Scholar]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Zhang, C.; Ding, Y.; Han, J.; Jiang, S.; He, S. In Situ Growth of N/O-Codoped Carbon Nanotubes in Wood-Derived Thick Carbon Scaffold to Boost the Capacitive Performance. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 662, 131018. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Hybrid Technologies: The Future of Energy Efficient Desalination–A Review. Desalination 2020, 495, 114659. [Google Scholar] [CrossRef]
- Kavitha, J.; Rajalakshmi, M.; Phani, A.R.; Padaki, M. Pretreatment Processes for Seawater Reverse Osmosis Desalination Systems—A Review. J. Water Process Eng. 2019, 32, 100926. [Google Scholar] [CrossRef]
- Amadi, C.N.; Igweze, Z.N.; Orisakwe, O.E. Heavy Metals in Miscarriages and Stillbirths in Developing Nations. Middle East Fertil. Soc. J. 2017, 22, 91–100. [Google Scholar] [CrossRef]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The Sequence of the Human Genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef] [PubMed]
- Blandin, G.; Verliefde, A.R.D.; Comas, J.; Rodriguez-Roda, I.; Le-Clech, P. Efficiently Combining Water Reuse and Desalination through Forward Osmosis—Reverse Osmosis (FO-RO) Hybrids: A Critical Review. Membranes 2016, 6, 37. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R. Nanocomposite Membranes for Water Separation and Purification: Fabrication, Modification, and Applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Khan, N.A.; Ahmed, S.; Farooqi, I.H.; Ali, I.; Vambol, V.; Changani, F.; Yousefi, M.; Vambol, S.; Khan, S.U.; Khan, A.H. Occurrence, Sources and Conventional Treatment Techniques for Various Antibiotics Present in Hospital Wastewaters: A Critical Review. TrAC Trends Anal. Chem. 2020, 129, 115921. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Nycz, M.; Arkusz, K.; Pijanowska, D.G. Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response. Nanomaterials 2019, 9, 1072. [Google Scholar] [CrossRef]
- Shashikala, V.; Kumar, V.S.; Padmasri, A.H.; Raju, B.D.; Mohan, S.V.; Sarma, P.N.; Rao, K.S.R. Advantages of Nano-Silver-Carbon Covered Alumina Catalyst Prepared by Electro-Chemical Method for Drinking Water Purification. J. Mol. Catal. A Chem. 2007, 268, 95–100. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Zaidi, S.; Khalil, M.J.; Khan, M.A.; Alam, M.A.; Masood, F.; Bazli, L.; Chelliapan, S. Current Trends in Hydrogen Production, Storage and Applications in India: A Review. Sustain. Energy Technol. Assessments 2022, 53, 102677. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of Microplastics in Wastewater Treatment Plants and Their Environmental Dispersion with Effluent and Sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef] [PubMed]
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials 2019, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Camarca, A.; Varriale, A.; Capo, A.; Pennacchio, A.; Calabrese, A.; Giannattasio, C.; Murillo Almuzara, C.; D’Auria, S.; Staiano, M. Emergent Biosensing Technologies Based on Fluorescence Spectroscopy and Surface Plasmon Resonance. Sensors 2021, 21, 906. [Google Scholar] [CrossRef] [PubMed]
- Besha, A.T.; Liu, Y.; Fang, C.; Bekele, D.N.; Naidu, R. Assessing the Interactions between Micropollutants and Nanoparticles in Engineered and Natural Aquatic Environments. Crit. Rev. Environ. Sci. Technol. 2020, 50, 135–215. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, M.; Malik, N.; Cho, M.H.; Khan, M.M. Recent Progress of Algae and Blue–Green Algae-Assisted Synthesis of Gold Nanoparticles for Various Applications. Bioprocess Biosyst. Eng. 2019, 42, 1–15. [Google Scholar] [CrossRef]
- Singh, T.; Atieh, M.A.; Al-Ansari, T.; Mohammad, A.W.; Mckay, G. The Role of Nanofluids and Renewable Energy in the Development of Sustainable Desalination Systems: A Review. Water 2019, 12, 2002. [Google Scholar] [CrossRef]
- Mostafavi, E.; Soltantabar, P.; Webster, T.J. Nanotechnology and Picotechnology. Biomater. Transl. Med. 2019, 191–212. [Google Scholar] [CrossRef]
- Pandey, G. Nanotechnology for Achieving Green-Economy through Sustainable Energy. Rasayan J. Chem. 2018, 11, 942–950. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-Enabled Strategies to Enhance Crop Nutrition and Protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Nanostructured Metal Oxides and Its Hybrids for Biomedical Applications. Adv. Colloid Interface Sci. 2020, 281, 102178. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomedicine 2020, 15, 3447. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.H.; Ding, R.; Yuan, Q.; Wei, Y.; Liang, H. Facile Synthesis of Alcalase-Inorganic Hybrid Nanoflowers Used for Soy Protein Isolate Hydrolysis to Improve Its Functional Properties. Food Chem. 2019, 289, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Duan, W.; Liu, J.; Zhuo, K.; Chen, Y.; Wang, J. Electrochemical Synthesis of AuPt Nanoflowers in Deep Eutectic Solvent at Low Temperature and Their Application in Organic Electro-Oxidation. Sci. Rep. 2018, 8, 13141. [Google Scholar] [CrossRef] [PubMed]
- Sanzò, G.; Taurino, I.; Puppo, F.; Antiochia, R.; Gorton, L.; Favero, G.; Mazzei, F.; Carrara, S.; De Micheli, G. A Bimetallic Nanocoral Au Decorated with Pt Nanoflowers (Bio) Sensor for H2O2 Detection at Low Potential. Methods 2017, 129, 89–95. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Li, G.; Row, K.H. Utilization of Deep Eutectic Solvents in Dispersive Liquid-Liquid Micro-Extraction. TrAC Trends Anal. Chem. 2019, 120, 115651. [Google Scholar] [CrossRef]
- Phillips, A.M.F.; Pombeiro, A.J.L. Electrochemical Asymmetric Synthesis of Biologically Active Substances. Org. Biomol. Chem. 2020, 18, 7026–7055. [Google Scholar] [CrossRef]
- Juska, V.B.; Pemble, M.E. A Dual-Enzyme, Micro-Band Array Biosensor Based on the Electrodeposition of Carbon Nanotubes Embedded in Chitosan and Nanostructured Au-Foams on Microfabricated Gold Band Electrodes. Analyst 2020, 145, 402–414. [Google Scholar] [CrossRef]
- Mahmudunnabi, R.G.; Farhana, F.Z.; Kashaninejad, N.; Firoz, S.H.; Shim, Y.-B.; Shiddiky, M.J.A. Nanozyme-Based Electrochemical Biosensors for Disease Biomarker Detection. Analyst 2020, 145, 4398–4420. [Google Scholar] [CrossRef]
- Ngo, Y.L.T.; Jana, J.; Chung, J.S.; Hur, S.H. Electrochemical Biosensors Based on Nanocomposites of Carbon-Based Dots. Korean Chem. Eng. Res. 2020, 58, 499–513. [Google Scholar] [CrossRef]
- Bertel, L.; Miranda, D.A.; García-martín, J.M. Nanostructured Titanium Dioxide Surfaces for Electrochemical Biosensing. Sensors 2021, 21, 6167. [Google Scholar] [CrossRef] [PubMed]
- Son, G.; Son, Y.; Jeon, H.; Kim, J.-Y.; Lee, S. A Three-Dimensional Monte Carlo Model for Coarsening Kinetics of the Bi-Continuous System via Surface Diffusion and Its Application to Nanoporous Gold. Scr. Mater. 2020, 174, 33–38. [Google Scholar] [CrossRef]
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Mikhaylov, V.; Demchenko, A.V.; Stine, K.J. Preparation, Modification, Characterization, and Biosensing Application of Nanoporous Gold Using Electrochemical Techniques. Nanomaterials 2018, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, J. Fabrication of Nanoporous Au Films with Ultra-High Surface Area for Sensitive Electrochemical Detection of Glucose in the Presence of Cl−. Appl. Surf. Sci. 2014, 297, 84–88. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver Nanoparticles: Various Methods of Synthesis, Size Affecting Factors and Their Potential Applications–a Review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A Review on Various Electrochemical Techniques for Heavy Metal Ions Detection with Different Sensing Platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Starowicz, M.; Stypuła, B.; Banaś, J. Electrochemical Synthesis of Silver Nanoparticles. Electrochem. Commun. 2006, 8, 227–230. [Google Scholar] [CrossRef]
- Rajkumar, T.; Sapi, A.; Das, G.; Debnath, T.; Ansari, A.; Patra, J.K. Biosynthesis of Silver Nanoparticle Using Extract of Zea Mays (Corn Flour) and Investigation of Its Cytotoxicity Effect and Radical Scavenging Potential. J. Photochem. Photobiol. B Biol. 2019, 193, 1–7. [Google Scholar] [CrossRef]
- Mavaei, M.; Chahardoli, A.; Shokoohinia, Y.; Khoshroo, A.; Fattahi, A. One-Step Synthesized Silver Nanoparticles Using Isoimperatorin: Evaluation of Photocatalytic, and Electrochemical Activities. Sci. Rep. 2020, 10, 1762. [Google Scholar] [CrossRef]
- Subhan, M.A.; Neogi, N.; Choudhury, K.P. Industrial Manufacturing Applications of Zinc Oxide Nanomaterials: A Comprehensive Study. Nanomanufacturing 2022, 2, 265–291. [Google Scholar] [CrossRef]
- Akhter, P.; Arshad, A.; Saleem, A.; Hussain, M. Recent Development in Non-Metal-Doped Titanium Dioxide Photocatalysts for Different Dyes Degradation and the Study of Their Strategic Factors: A Review. Catalysts 2022, 12, 1331. [Google Scholar] [CrossRef]
- Lim, H.; Kim, H.S.; Qazi, R.; Kwon, Y.; Jeong, J.; Yeo, W. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2020, 32, 1901924. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.R.; Wagner, A.M.; Shin, S.R.; Hassan, S.; Khademhosseini, A.; Peppas, N.A. Modular Fabrication of Intelligent Material-Tissue Interfaces for Bioinspired and Biomimetic Devices. Prog. Mater. Sci. 2019, 106, 100589. [Google Scholar] [CrossRef]
- Kacimi, R.; Dhaou, R.; Beylot, A.-L. Load Balancing Techniques for Lifetime Maximizing in Wireless Sensor Networks. Ad Hoc. Networks 2013, 11, 2172–2186. [Google Scholar] [CrossRef]
- Fennell, J.F., Jr.; Liu, S.F.; Azzarelli, J.M.; Weis, J.G.; Rochat, S.; Mirica, K.A.; Ravnsbæk, J.B.; Swager, T.M. Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chem. Int. Ed. 2016, 55, 1266–1281. [Google Scholar] [CrossRef] [PubMed]
- Prosa, M.; Bolognesi, M.; Fornasari, L.; Grasso, G.; Lopez-Sanchez, L.; Marabelli, F.; Toffanin, S. Nanostructured Organic/Hybrid Materials and Components in Miniaturized Optical and Chemical Sensors. Nanomaterials 2020, 10, 480. [Google Scholar] [CrossRef]
- Kumar, S.; Pavelyev, V.; Mishra, P.; Tripathi, N. A Review on Chemiresistive Gas Sensors Based on Carbon Nanotubes: Device and Technology Transformation. Sens. Actuators A Phys. 2018, 283, 174–186. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon Nanomaterials and Their Application to Electrochemical Sensors: A Review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Tewari, B.B. Critical Reviews on Engineered Nanoparticles in Environmental Remediation. Curr. J. Appl. Sci. Technol. 2019, 36, 1–21. [Google Scholar] [CrossRef]
- Bie, C.; Yu, H.; Cheng, B.; Ho, W.; Fan, J.; Yu, J. Design, Fabrication, and Mechanism of Nitrogen-Doped Graphene-Based Photocatalyst. Adv. Mater. 2021, 33, 2003521. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, V.; Jeon, H.C.; Kang, T.W.; Kumar, S. Structural, optical and electrical properties of Ni doped ZnO nanostructures synthesized by solution combustion method. J. Mater. Sci. Mater. Electron. 2018, 29, 1327–1332. [Google Scholar] [CrossRef]
- Kaur, H.; Dahake, R.; Maddigapu, P.R.; Hippargi, G.; Pophali, G.R.; Bansiwal, A. Enhanced photocatalytic degradation of antimicrobial triclosan using rGO–TiO2 composite under natural solar illumination. J. Mater. Sci. Mater. Electron. 2020, 31, 6045–6058. [Google Scholar] [CrossRef]
- Durmus, Z.; Kurt, B.Z.; Durmus, A. Synthesis and Characterization of Graphene Oxide/Zinc Oxide (GO/ZnO) Nanocomposite and Its Utilization for Photocatalytic Degradation of Basic Fuchsin Dye. ChemistrySelect 2019, 4, 271–278. [Google Scholar] [CrossRef]
- Krishnamurthy, V.; Gopinath, K.P.; Gnanaprakash, D.; Vanathi, G.; Shivanirudh, S.G.; Ahamed, M.I. Importance and ILL-Effects of Nanoparticles: Sensors for Their Identification. Curr. Anal. Chem. 2020, 16, 244–259. [Google Scholar] [CrossRef]
- Khraisheh, M.; Elhenawy, S.; Almomani, F.; Al-Ghouti, M.; Hassan, M.K.; Hameed, B.H. Recent Progress on Nanomaterial-Based Membranes for Water Treatment. Membranes 2021, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Mao, Y. A Review on Molten Salt Synthesis of Metal Oxide Nanomaterials: Status, Opportunity, and Challenge. Prog. Mater. Sci. 2021, 117, 100734. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Ma, R.; Zhang, X.; Rao, J.; Yin, Y.; Wang, X.; Yi, F. Flexible Temperature Sensors Based on Carbon Nanomaterials. J. Mater. Chem. B 2021, 9, 1941–1964. [Google Scholar] [CrossRef]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2020, 14, 166. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; He, B.; Pan, R.; Wang, Z.; Chen, M.; Wang, Z.; Yin, K.; Yao, Y.; Wei, L. Advanced Multifunctional Aqueous Rechargeable Batteries Design: From Materials and Devices to Systems. Adv. Mater. 2022, 34, 2104327. [Google Scholar] [CrossRef]
- Chadha, U.; Selvaraj, S.K.; Ashokan, H.; Hariharan, S.P.; Mathew Paul, V.; Venkatarangan, V.; Paramasivam, V. Complex Nanomaterials in Catalysis for Chemically Significant Applications: From Synthesis and Hydrocarbon Processing to Renewable Energy Applications. Adv. Mater. Sci. Eng. 2022, 2022, 1552334. [Google Scholar] [CrossRef]
- Hughes, S.; Asmatulu, E. Nanotoxicity and Nanoecotoxicity: Introduction, Principles, and Concepts. Nanotoxicol. Nanoecotoxicol. 2021, 1, 1–19. [Google Scholar] [CrossRef]
- Ghosh, D.; Sarkar, K.; Devi, P.; Kim, K.-H.; Kumar, P. Current and Future Perspectives of Carbon and Graphene Quantum Dots: From Synthesis to Strategy for Building Optoelectronic and Energy Devices. Renew. Sustain. Energy Rev. 2021, 135, 110391. [Google Scholar] [CrossRef]
- Xu, L.; Xu, L.; Luo, J.; Yan, Y.; Jia, B.; Yang, X.; Gao, Y.; Wang, Z.L. Hybrid All-in-One Power Source Based on High-Performance Spherical Triboelectric Nanogenerators for Harvesting Environmental Energy. Adv. Energy Mater. 2020, 10, 2001669. [Google Scholar] [CrossRef]
- Zheng, B.; Lin, X.; Zhang, X.; Wu, D.; Matyjaszewski, K. Emerging Functional Porous Polymeric and Carbonaceous Materials for Environmental Treatment and Energy Storage. Adv. Funct. Mater. 2020, 30, 1907006. [Google Scholar] [CrossRef]
- Zheng, L.; Teng, F.; Ye, X.; Zheng, H.; Fang, X. Photo/Electrochemical Applications of Metal Sulfide/TiO2 Heterostructures. Adv. Energy Mater. 2020, 10, 1902355. [Google Scholar] [CrossRef]
- Mdletshe, L.S.; Makgwane, P.R.; Ray, S.S. Fabrication of Bimetal CuFe2O4 Oxide Redox-Active Nanocatalyst for Oxidation of Pinene to Renewable Aroma Oxygenates. Nanomaterials 2019, 9, 1140. [Google Scholar] [CrossRef]
- Periyasamy, M.; Kar, A. Modulating the Properties of SnO2 Nanocrystals: Morphological Effects on Structural, Photoluminescence, Photocatalytic, Electrochemical and Gas Sensing Properties. J. Mater. Chem. C 2020, 8, 4604–4635. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Nanoparticles in Reverse Osmosis Membranes for Desalination: A State of the Art Review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Nagar, A.; Pradeep, T. Clean Water through Nanotechnology: Needs, Gaps, and Fulfillment. ACS Nano 2020, 14, 6420–6435. [Google Scholar] [CrossRef]
- Sharma, V.K.; Ma, X.; Guo, B.; Zhang, K. Environmental Factors-Mediated Behavior of Microplastics and Nanoplastics in Water: A Review. Chemosphere 2021, 271, 129597. [Google Scholar] [CrossRef]
- Tallec, K.; Blard, O.; González-Fernández, C.; Brotons, G.; Berchel, M.; Soudant, P.; Huvet, A.; Paul-Pont, I. Surface Functionalization Determines Behavior of Nanoplastic Solutions in Model Aquatic Environments. Chemosphere 2019, 225, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Un-Noor, F.; Padmanaban, S.; Mihet-Popa, L.; Mollah, M.N.; Hossain, E. A Comprehensive Study of Key Electric Vehicle (EV) Components, Technologies, Challenges, Impacts, and Future Direction of Development. Energies 2017, 10, 1217. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Kandy, M.M.; Sankaralingam, M. Chapter 1. A Fundamental Approach towards Carbon Dioxide Conversion to Chemicals and Fuels: Current Trends for CO2 Utilization Technologies; The Royal Society of Chemistry: London, UK, 2022; ISBN 9781839165542. [Google Scholar]



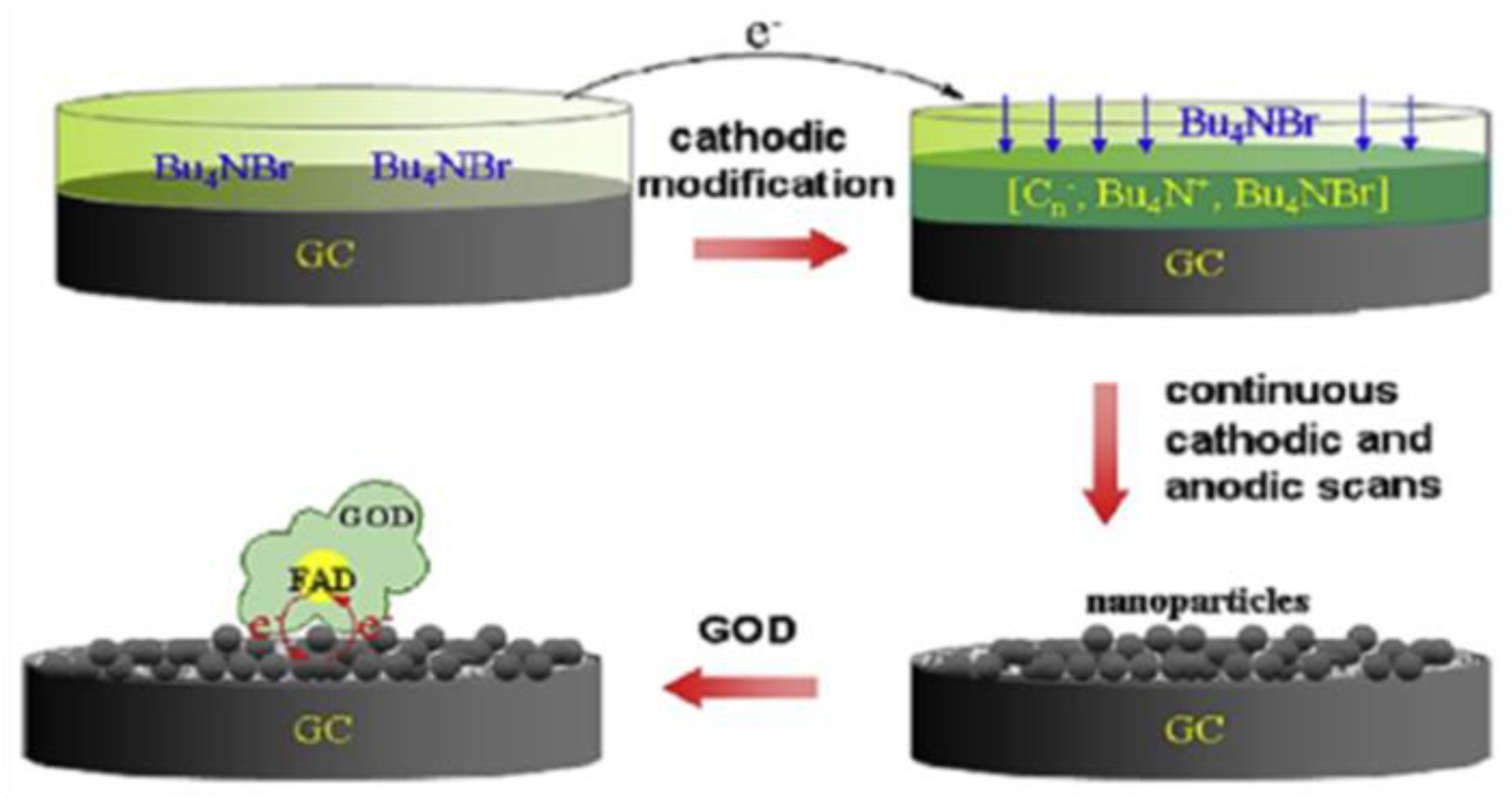
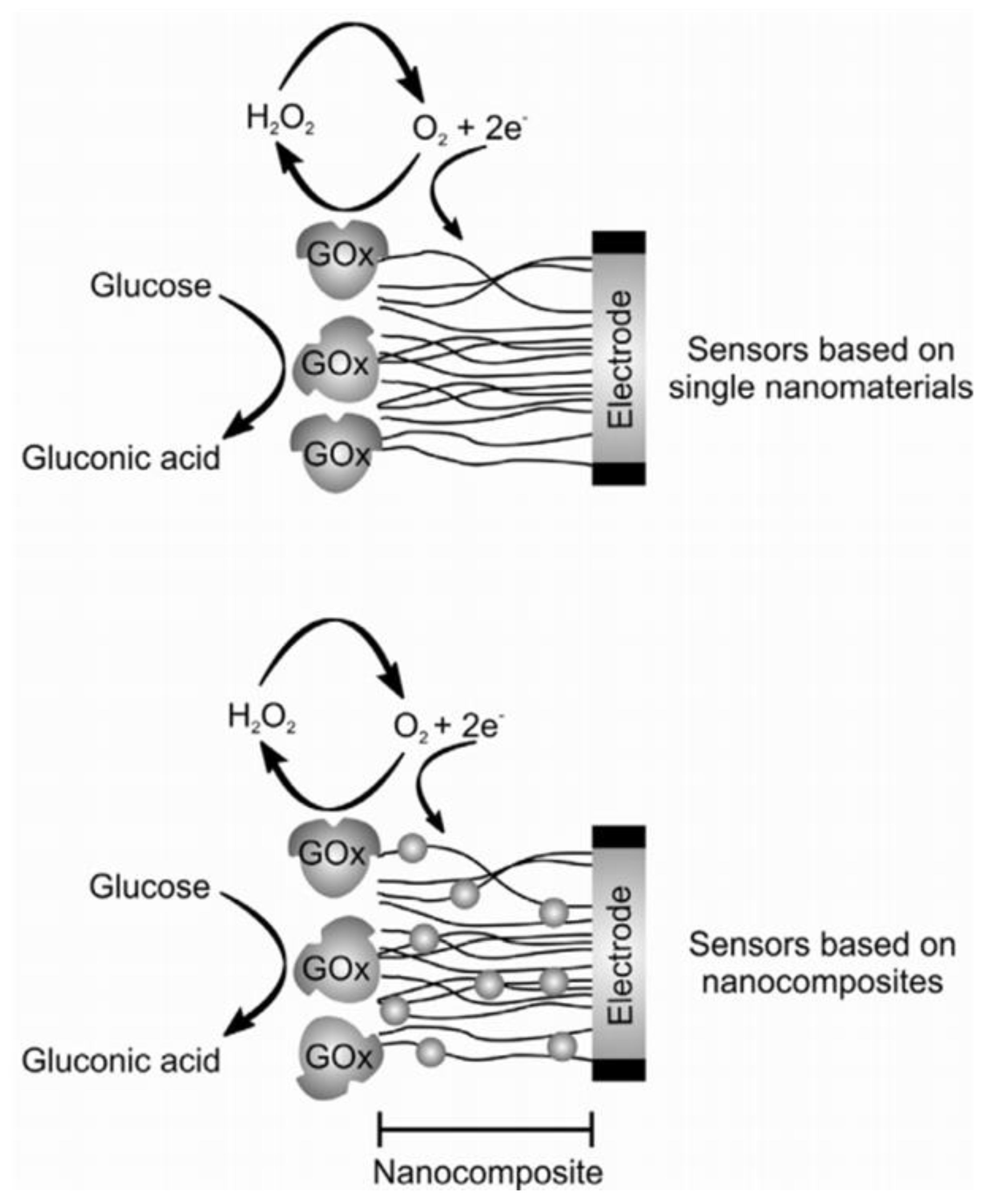
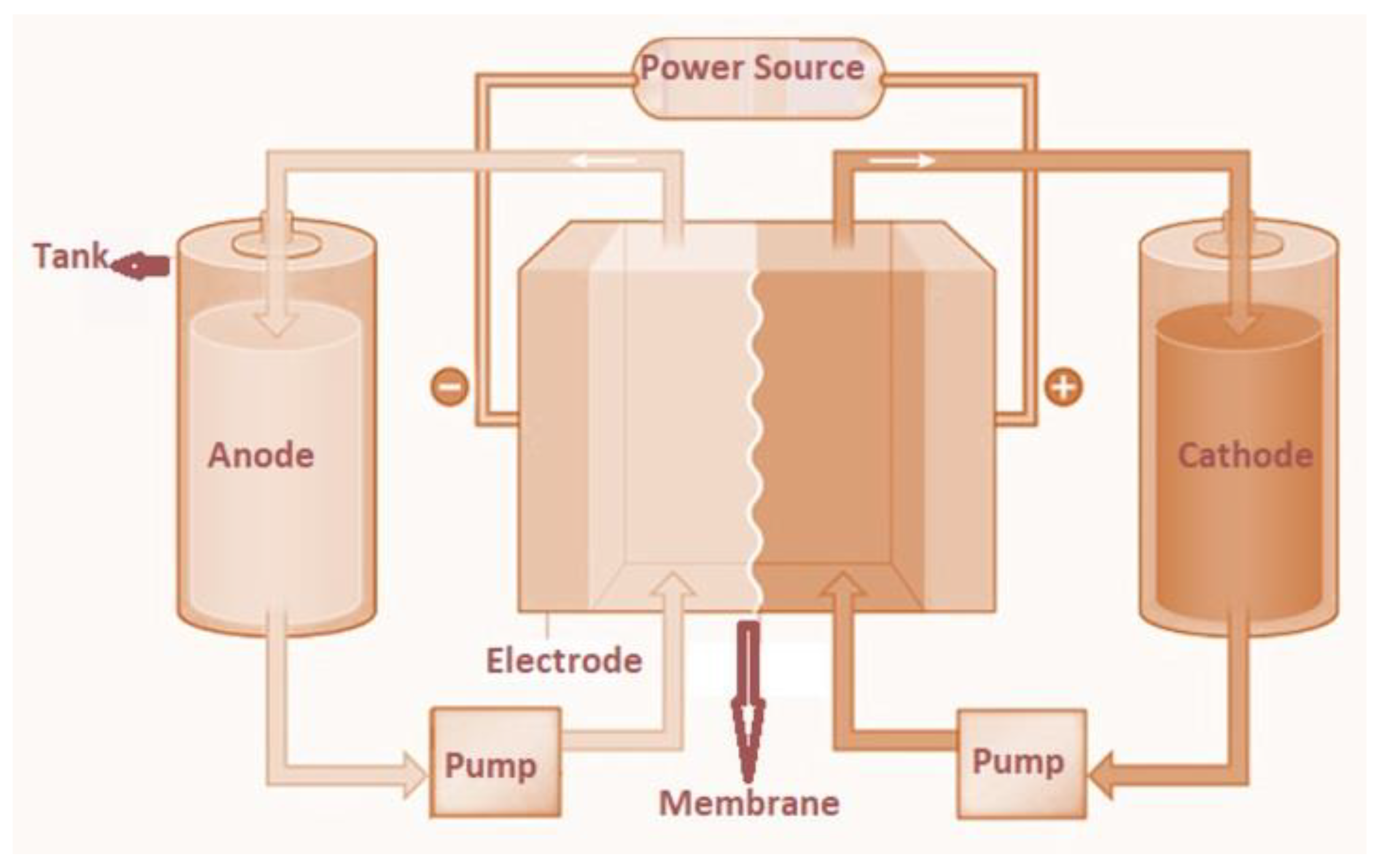

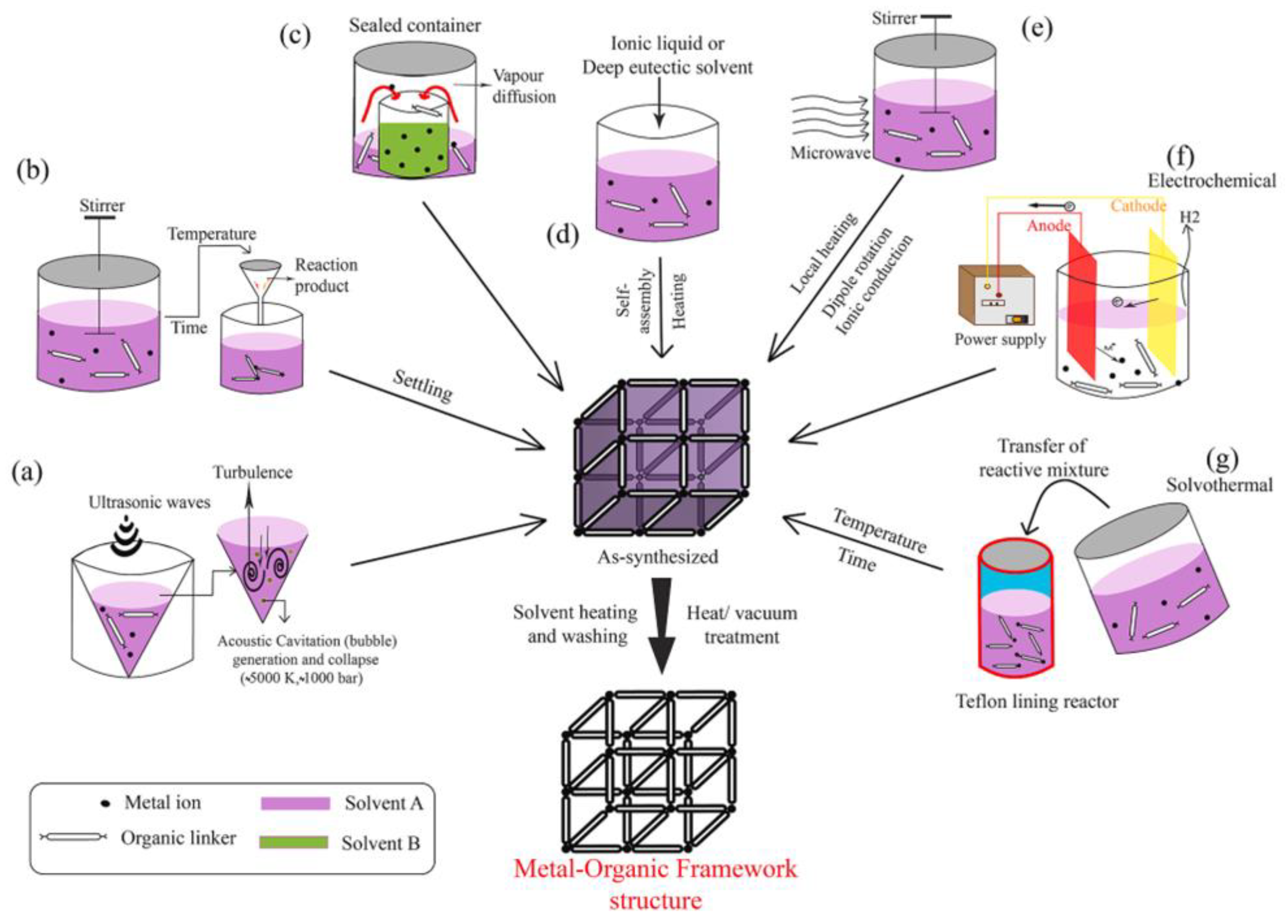
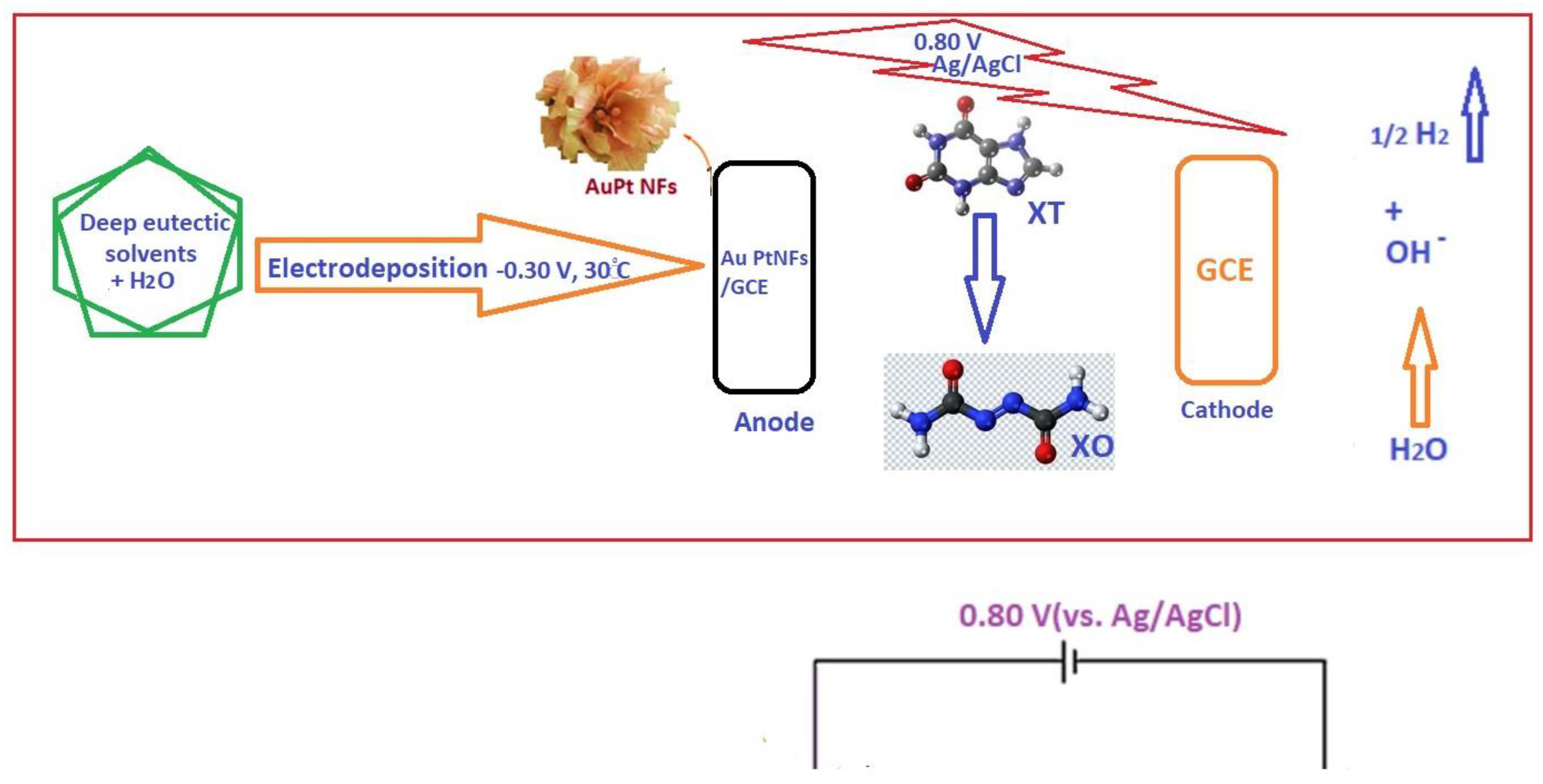
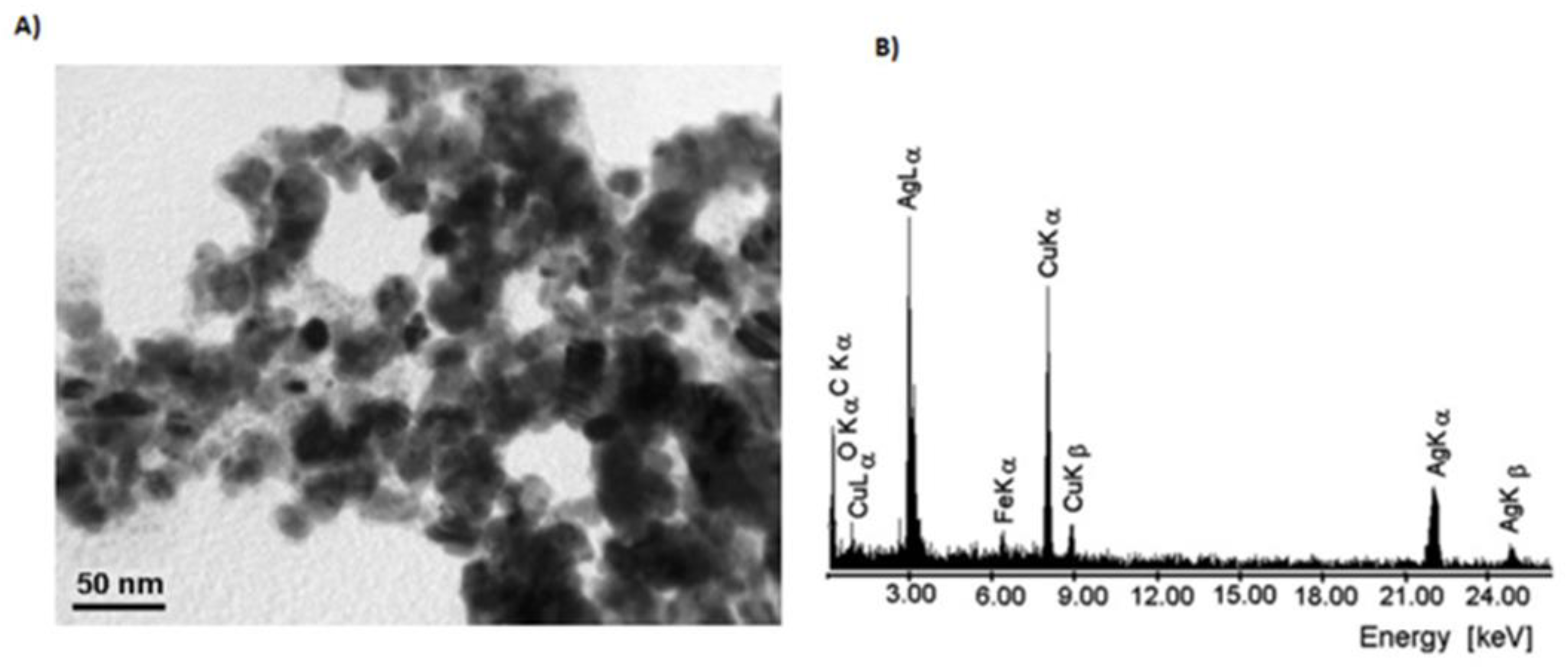
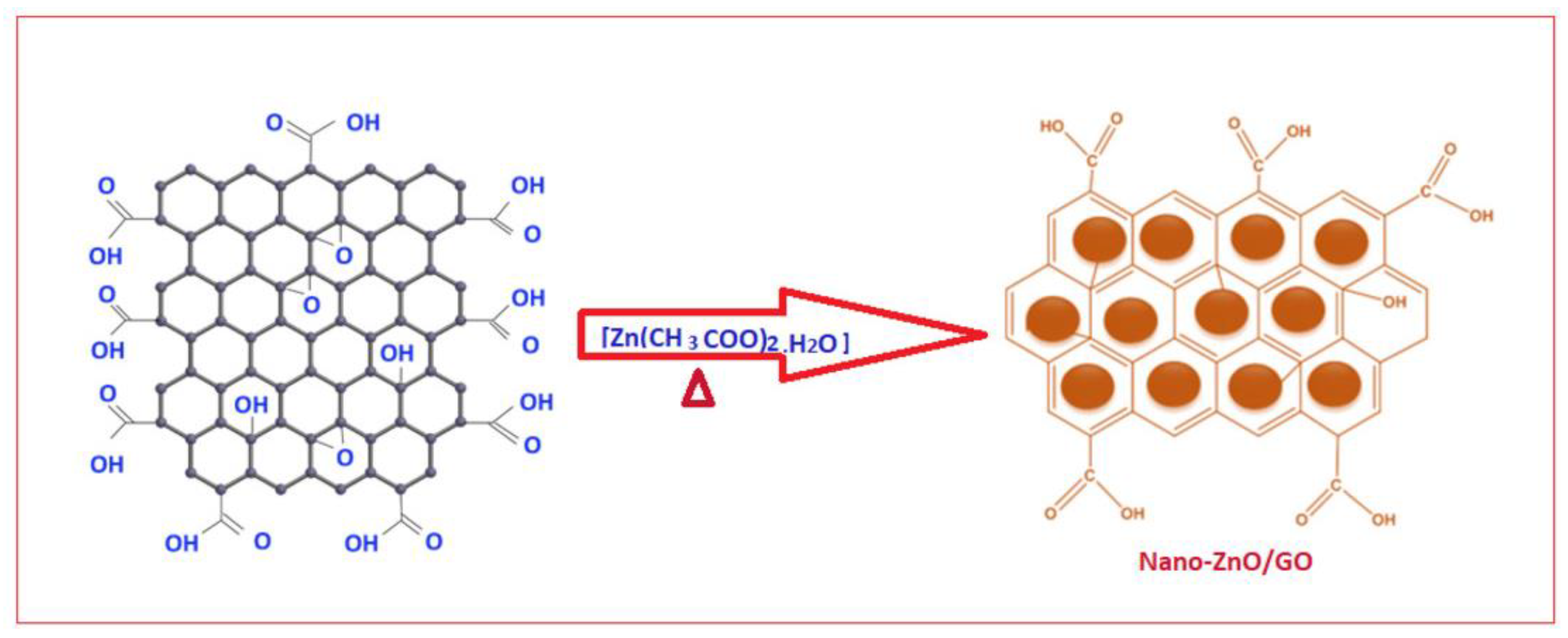
| Electrochemical Device | Nanomaterials Used | Applications | References |
|---|---|---|---|
| Batteries | Nanostructured metals, metal oxides, and carbon-based materials, MOFs, COFs, MXenes. | Electrode materials to improve performance, increase surface area, improve conductivity, and provide higher energy and power densities | [20,21] |
| Supercapacitors | Carbon nanotubes, graphene, and other carbon-based materials, MOFs, COFs, and MXenes. | Electrode materials to increase surface area, improve conductivity, and provide high power and energy densities | [22,23,24,25,26] |
| Fuel Cells | Platinum, gold, and other metal nanoparticles, MOFs, COFs, MXenes. | Catalysts to improve the efficiency of electrochemical reactions that generate electricity | [27] |
| Sensors | Metal nanoparticles, metal oxides, and carbon-based materials, MOFs, COFs, MXenes | Sensing elements to improve sensitivity and selectivity due to their high surface area, high catalytic activity, and unique optical and electrical properties | [28] |
| Nanomaterials | Synthesis Method | Morphological Features | Properties | References |
|---|---|---|---|---|
| Metal oxides and hydroxides | Various | High surface area, redox properties | Suitable for electrochemical devices | [46,47,49,50] |
| Conducting polymers (e.g., polyaniline, polypyrrole) | Various | High conductivity, tunable redox potential | Versatile for electrochemical applications | [51] |
| Hybrid materials | Combination of nanomaterial classes | Improved performance and functionality | Enhanced properties through synergy | [52] |
| 0D nanoparticles and nanodots | Various synthesis methods | Small size, high surface area | Promising for batteries, sensors, catalysts, supercapacitors | [53] |
| 1D nanowires and 2D nanosheets | Various synthesis methods | Large surface area, excellent conductivity | Suitable for supercapacitors, fuel cells, and other electrochemical devices | [54] |
| 3D nanostructures (nanocubes, nanorods, mesoporous silica) | Various synthesis methods | High surface area, unique porosity | Potential for batteries, supercapacitors, fuel cells | [55] |
| Nanomaterials for electrode materials | Various synthesis methods | Tailored properties for high-performance devices | Potential for cost-effective electrochemical devices | [56] |
| Properties/Performance Characteristics | Carbon-Based Nanomaterials | Metal-Based Nanomaterials | Metal Oxide Nanomaterials | Semiconductor Nanomaterials | Composite Nanomaterials |
|---|---|---|---|---|---|
| High electrical conductivity | ✓ | ||||
| Large surface area | ✓ | ✓ | |||
| Excellent mechanical strength | ✓ | ||||
| Unique catalytic properties | ✓ | ||||
| High surface-to-volume ratios | ✓ | ||||
| Semiconducting behavior | ✓ | ✓ | |||
| Diverse functionalities | ✓ | ||||
| Size-dependent optical and electronic properties | ✓ | ||||
| Enhanced conductivity | ✓ | ||||
| Tunable bandgap | ✓ | ||||
| Efficient charge separation | ✓ | ||||
| Improved photocatalytic activity | ✓ | ||||
| High electrocatalytic activity | ✓ | ||||
| Fast charge transport | ✓ | ||||
| High stability | ✓ | ||||
| Efficient light absorption | ✓ | ||||
| Mechanical strength | ✓ |
| Nanostructure | Method of Manufacture | References |
|---|---|---|
| Carbon nanotubes | Chemical vapor deposition | [95] |
| Arc discharge | ||
| Laser ablation | ||
| Gas-phase catalytic growth | ||
| Polymer nanowires | Electrochemical deposition | [96] |
| Template filling | ||
| Reactive ion etching | ||
| Graphene nanostructures | Exfoliation | [97] |
| Chemical vapor deposition | ||
| Epitaxial growth | ||
| Metallic nanowires | Template-assisted electrodeposition | [98] |
| Electrochemical deposition | ||
| Electroless deposition | ||
| 4- Template filling | ||
| Si nanowires | Reactive ion etching | [99] |
| Photolithography |
| Nanomaterials | Properties | Application | References |
|---|---|---|---|
| Carbon nanotubes (CNTs) | High surface area, good electrical conductivity, and excellent mechanical properties The development of (bio)sensors capable of tackling future biosensing challenges in clinical diagnostics, environmental monitoring, and security control represents a very good alternative when the special properties of CNTs are combined with the potent biomolecule recognition properties and the known benefits of the electrochemical techniques. | Detection of glucose, cholesterol, and DNA | [101] |
| Graphene (GR) | With high sensitivities, broad linear detection ranges, low detection limits, and long-term stabilities, GR-based biosensors displayed exceptional performance. | Detection of glucose, dopamine, and DNA | [102] |
| Metal nanoparticles | High surface area and excellent catalytic activity | Detection of glucose, cholesterol, and DNA | [103] |
| Quantum dots | Unique optical and electronic properties | Detection of biomolecules such as proteins and DNA | [104] |
| Metal oxide nanoparticles | High surface area and excellent catalytic activity | Detection of glucose, cholesterol, and DNA | [105] |
| Material Name | Material Class | Synthesis Method | Performance Parameters | Application | References |
|---|---|---|---|---|---|
| Lithium Cobalt Oxide (LiCoO2) | Metal Oxide | Solid-state reaction | High specific capacity, good cycling stability | Lithium-ion batteries | [148,149] |
| Lithium Iron Phosphate (LiFePO4) | Metal Phosphate | Sol–gel method | High energy density, long cycle life | Lithium-ion batteries | [150] |
| Silicon/Graphene Composites | Composite | Chemical vapor deposition (CVD) | High specific capacity, enhanced stability | Lithium-ion batteries | [151] |
| Sodium-ion Intercalation Materials | Metal Oxide | Hydrothermal synthesis | Good rate capability, low cost | Sodium-ion batteries | [152] |
| Graphene | Carbon-based Material | Mechanical exfoliation | High specific capacitance, fast charge–discharge rate | Supercapacitors | [153] |
| Activated Carbon | Carbon-based Material | Chemical activation | High energy density, long cycle life | Supercapacitors | [154] |
| Polyaniline | Conductive Polymer | Chemical oxidation | High capacitance, good stability | Supercapacitors | [155] |
| Carbon Nanotubes | Carbon-based Material | Chemical vapor deposition (CVD) | High power density, excellent cycling stability | Supercapacitors | [156] |
| Proton Exchange Membrane (PEM) | Polymer Electrolyte | Solution casting | High proton conductivity, Low permeability | Polymer electrolyte fuel cells | [157] |
| Platinum-based Catalysts | Noble Metal | Wet chemical synthesis | High catalytic activity, good durability | Polymer electrolyte fuel cells | [158] |
| Solid Oxide Electrolyte | Ceramic | Solid-state sintering | High ionic conductivity, stable at high temperatures | Solid oxide fuel cells | [159] |
| Perovskite Oxides | Metal Oxide | Sol–gel method | Good oxygen reduction reaction, thermal stability | Solid oxide fuel cells | [160] |
| Metal Oxide Semiconductors | Metal Oxide | Chemical vapor deposition (CVD) | High sensitivity, selective detection | Gas sensors | [161] |
| Enzymes | Biocatalyst | Enzyme immobilization | High specificity, rapid response | Biosensors | [162] |
| Nanomaterials (e.g., Carbon nanotubes, Graphene) | Various | Various synthesis methods | High sensitivity, versatile applications | Biosensors | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, H.M.; Hassan, A.I. Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices. Sustainability 2023, 15, 10891. https://doi.org/10.3390/su151410891
Saleh HM, Hassan AI. Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices. Sustainability. 2023; 15(14):10891. https://doi.org/10.3390/su151410891
Chicago/Turabian StyleSaleh, Hosam M., and Amal I. Hassan. 2023. "Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices" Sustainability 15, no. 14: 10891. https://doi.org/10.3390/su151410891
APA StyleSaleh, H. M., & Hassan, A. I. (2023). Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices. Sustainability, 15(14), 10891. https://doi.org/10.3390/su151410891







