Abstract
Nutrient recovery technologies have been constantly developed and optimised to address challenges in water and wastewater management, sanitation, and agri-food systems, while promoting sustainable management of resources and circular phosphorous economy. However, these technologies have been rarely explored beyond the laboratory-scale in developing countries where it is mostly needed. In this study, a nutrient recovery batch reactor system was installed at a local farm in the Philippines to process raw septage from an onsite sanitation system, a septic tank, to recover a high-value fertiliser for local crop production. The batch reactor was used for two processes, namely acid hydrolysis for pre-treatment of septage and chemical precipitation for recovered phosphorous fertiliser (RPF). The recovered fertiliser was then applied to produce eggplants and tomatoes, which are the common crops grown in the farm. Results show that an average of 290 g of RPF was produced for every 100 L of raw septage processed. With hydrolysis, 77% of the phosphate concentration were released as phosphates from the solid component of the raw septage. About 98.5% of phosphates were recovered from the hydrolysed septage. The RPF when applied to the farm’s eggplants and tomatoes has yields comparable to that of the commercial fertilisers. This study was able to demonstrate the potential of a resource-oriented sanitation system that promotes nutrient recycling towards sustainable agriculture that further leads to meeting the United Nations sustainable development goals, particularly zero hunger (goal 2), clean water and sanitation (goal 6), sustainable cities and communities (goal 11), and responsible consumption and production (goal 12).
1. Introduction
Rapid urbanisation, population growth, and socio-economic development have caused a 3–4% increase in phosphorus (P) demand annually, leading to an accelerated P supply depletion [1,2]. Hence, it is expected that the future global security of agriculture and food (agri-food) systems will be compromised by year 2050 if P extraction continues to increase [3]. Moreover, the P in the agri-food supply chains following a linear pathway from phosphate rock mining for fertiliser production would eventually end up in various waste streams that cause adverse environmental impacts, such as eutrophication in water resources [4]. Developing countries with emerging economies are greatly affected with eutrophication and problems with sanitation due to high population density, increase in anthropogenic activities, and lack of appropriate sewage treatment systems or sanitation systems [5]. Particularly in the Philippines, 84% of the households use a septic tank as an onsite sanitation system to dispose of and treat human wastes since it is cheaper, but the design commonly installed does not incorporate a leach field; hence without regular desludging, the raw septage would overflow and leak directly into the environment posing health risks [6].
A paradigm-shift to a resource-oriented sanitation system could play an important role in addressing the challenges and risks with the linear P pathway through the concept of circular economy. Circular P economy is the sustainable management of P from resources, materials, and products within the economy while minimising waste generation through recycling and recovery from the waste stream endpoints from the linear P flows [7]. This implies that P can be recovered from waste streams to be used back as a high-value product for various economic sectors. Agri-food systems connected to wastewater streams and water body sinks have the most potential for recovery [8]. This will supplement the decreasing supply of P while minimising the end-point wastes [9].
An established nutrient recovery process that is already implemented in some wastewater treatment plants to recover P is chemical precipitation to form calcium phosphates (Ca5(PO4)3OH) and struvite MgNH4PO4·6H2O [10]. Although calcium phosphate could be an alternative source of P for industries, it needs further processing to be used efficiently for agriculture [11]. For agri-food systems, phosphorus recovered as struvite is preferred because of production efficiency while promoting economic sustainability [12,13]. Struvite is a high-value slow-release fertiliser that can be typically recovered from wastewater through the addition of magnesium salts at alkaline condition [14,15]. Several studies have reported on the use of struvite as fertiliser for different kinds of crops where it produced higher or similar crop yields compared to other commercial fertilisers [16,17,18]. A lot of studies were conducted solely in the production of struvite from wastewater feedstock but these studies do not usually proceed to its application to crops [19].
The typical wastewater input feedstock used for P recovery via precipitation are dewatering liquor, digestate (from anaerobic digestion), and waste activated sludge liquor from domestic wastewater and animal manure [20]. Although recovery from urine has been widely studied [21], the use of human wastes from septic tank sludge or septage has been rarely explored because of potential microbial contaminants and low social acceptance [22]. Septage or fecal sludge is generated from raw or partially digested blackwater in an onsite sanitation system (e.g., septic tanks after collection, storage, or treatment) [23]. It contains an average of 215 mg/L of phosphate, 96.3 mg/L of total phosphorus, 1300 mg/L of total nitrogen, and 1805 mg/L ammonia nitrogen [24]. Septage has minimal heavy metal concentrations and hence, it can be used as a non-hazardous phosphorous source for agriculture application and a clean raw material for industry use [24,25,26]. It was also found that organic contamination from organic toxins in fecal sludge, such as hexachlorobenzene and naphthalene, is around 95% lower than in regulations on fertiliser for some European countries [27,28]. Therefore, the potential of P and N recovery from septage for agri-food applications can be considered advantageous compared to other wastewater streams.
Struvite has been successfully recovered as a macronutrient fertiliser from blackwater effluent or supernatant with around a 90% recovery rate [27,29]. The struvite produced contained minimal quantities of heavy metals, and a reduction in pathogen levels was observed during precipitation. These few studies on struvite recovery from septage are focused on the liquid component, either the supernatant, effluent, or liquor, but the potential of the solid component or sludge of the septage has been hardly explored. Particularly for septic tanks, the wastewater undergoes partial aerobic-anaerobic digestion that captures significant P and N content in the sludge [26]. Hence, the maximum potential of nutrient recovery from septage is not yet thoroughly investigated. Consequently, hydrolysis could be used as a pre-treatment method for septage to release the nutrients from the sludge into liquid soluble components, as what has been performed in sewage sludge to maximise nutrient recovery.
Hydrolysis is typically used as a pre-treatment of sludge to release soluble organics that can be an additional carbon source for a subsequent nutrient removal processes [30]. To optimise nutrient recovery from wastewater, this process has also been applied in some studies to release phosphorus into a soluble form for nutrient recovery and removal processes. Hydrolysis could release around 75% of P (800–900 mg TP/L) from initial sludge (700–800 mg P/L was dissolved from 1100–1200 mg P/L sludge) [31]. Though there were few studies on hydrolysis of sewage sludge, there are no known studies conducted yet on the hydrolysis of septage, considering that it has lower, if at all, heavy metal contamination compared to sewage sludge [25]. Moreover, most of the studies on hydrolysis focus more on the release of soluble organic components, such as a soluble chemical oxygen demand and volatile fatty acids, yet only a few focus more on the release phosphates and ammonium for nutrient recovery [32].
In this study, the sustainability and challenges of integrating a nutrient recovery system for the localised production of fertilisers were assessed through the installation of a nutrient recovery batch reactor at a local operating farm, where the produced fertiliser would be applied. The study involves four major processes: septage collection, hydrolysis, chemical precipitation, and farm application. The batch reactor installed was used to treat the raw septage and to recover a high-value fertiliser through hydrolysis and chemical precipitation, respectively. The efficiency of the recovered phosphorous fertiliser (RPF) as a P-source fertiliser was also evaluated through its application for eggplant and tomato production. There is a lack of demonstration projects beyond laboratory-scale to evaluate the sustainability of existing technologies for nutrient recovery from wastewater feedstock. Through this study, the concept of a circular phosphorous (P) economy would be applied in a real scenario to promote a paradigm shift to a resource-oriented sanitation system for sustainable communities in developing countries, such as the Philippines.
2. Materials and Methods
2.1. Nutrient Recovery Batch Reactor
A fabricated batch reactor for the recovery of nutrients (i.e., RPF) from septage was installed at Salikneta Farm (Salikneta), Upper Ciudad Real, City of San Jose Del Monte, Bulacan, Philippines (14°48′09.3″ N 121°07′38.5″ E), a 63 hectare-farm managed by a university. Various crops are being grown inside the farm, such as tomatoes (Solanum lycopersicum) and eggplants (Solanum melongena), and livestock are being bred for research purposes and for local food distribution and consumption. The nutrient recovery batch reactor was designed with a maximum capacity of 100 L (0.10 m3) per batch with an overall dimension of 0.762 m (L) × 0.762 m (W) × 1.859 m (H), shown in Figure 1. Its conical tank with a diameter of 0.610 m and depth of 0.644 m is supported by a stand and frame. The reactor mixer has a motor drive (0.37 kW, 220/440 V, 60 Hz, 3-phase) and a variable frequency drive to adjust the mixer speed between 20.94 and 41.89 rad/s (0–60 Hz).
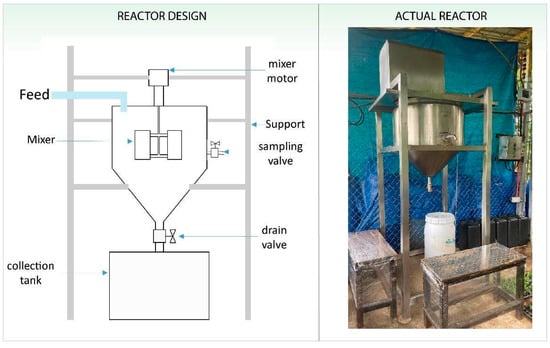
Figure 1.
Nutrient recovery batch reactor design and the actual fabricated reactor.
2.2. Septage Sources
The septage was collected from two different sources, namely the septic tanks at Salikneta farm and from a septage treatment plant (SpTP) in Metro Manila, to recover the required amount of fertiliser for farm application. The septic tanks at Salikneta and from a septage treatment plant (SpTP) were collected using a slurry pump. Salikneta has about 10 functional septic tanks with a cumulative capacity of 200 L. For the business-as-usual scenario, the septage is being hauled twice a year by a third party for desludging and septage treatment, thus accumulating an average of 400 L of septage every year. The SpTP, located 35 km from Salikneta, has an operational treatment capacity of 240 m3/day (240,000 L/day) at 16 hrs/day operation, where the raw septage is typically hauled from residential buildings and houses within Metro Manila. Prior to the operation of the batch reactor, the liquid and solid components of each septage source were characterised, and the analyses are shown in Table 1.

Table 1.
Characterisation of raw septage (liquid and solid components).
The raw septage was characterised showing that it contains the basic components of a fertiliser, such as phosphorus (P), nitrogen (N), and potassium (K). The solid component of septage from Salikneta has an average of 4830 mg/kg of total phosphorus (TP), 10,800 mg/kg of total nitrogen (TN), and 1010 mg/kg of potassium (K), while the septage from the SpTP contains an average of 12,700 mg/kg TP, 15,700 mg/kg TN, and 780 mg/kg of K. The raw septage was also analysed to have 54,410 mg/L total solids. It is evident that both septage sources contain high concentrations of nutrients that could be potentially recovered. Consequently, the liquid component of septage from Salikneta only contains an average of 7.7 mg/L PO4-P and 77 mg/L NH4-N while the SpTP has 7.9 mg/L PO4-P and 129 mg/L NH4-N. This is lower than the required average soluble PO4-P needed to economically precipitate struvite, that is 50 mg/L PO4-P [33]. Moreover, both septage sources have high amounts of micronutrients, such as calcium (Ca), iron (Fe), magnesium (Mg), and zinc (Zn) which are usually bonded with phosphate ions [34]. Hence, it would be difficult to apply the current nutrient recovery technologies directly without the pre-treatment of septage through hydrolysis, releasing P from the solid components into a soluble form as phosphates.
Heavy metals were also analysed and showed to have very low concentrations to no detection of arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb), and mercury (Hg). In addition, the raw septage also contains a high amount of microbiological components, such as Escherichia coli (E. coli), which is expected based on the conventional parameters of septage characteristics reported by the United States Environmental Protection Agency (US EPA) [35], but these parameters could be reduced throughout the nutrient recovery process [27].
2.3. Batch Reactor Operations
2.3.1. Hydrolysis of Septage
The first process in the batch reactor was hydrolysis for the pre-treatment of raw septage that releases the P and N from the solid component of the septage into soluble forms in the liquid component. The operating conditions for the hydrolysis stage are summarised in Table 2. The lower the pH the more phosphorus could be dissolved efficiently, and the product is assumed to be generally pathogen-free [31]. Hence, the septage was treated with 37% hydrochloric acid (HCl) gradually, to achieve the target pH of 2.0 under continuous mixing at 200 to 250 rpm (20 to 25 Hz). After every addition of HCl, a 10-min homogeneous mixing was performed, and samples were collected to measure the pH. When the target pH was achieved, hydrolysis continued for around 2 h. Then, the hydrolysed septage was drained into a settling drum. Settling was performed for two to three days to separate the hydrolysed septage (i.e., supernatant) from the solid waste sludge. The solid waste sludge was transferred into the drying pan for air drying. The dried waste sludge was then characterised for its future use as a soil conditioner. The supernatant was collected and transferred back to the batch reactor for the subsequent struvite precipitation process.

Table 2.
Operating conditions for each production process.
2.3.2. Recovery of Phosphorous Fertiliser
The recovery of phosphorous fertiliser was conducted in the batch reactor through chemical precipitation to promote struvite formation. The precipitation occurs at a basic condition, thus the initial pH of the supernatant was first analysed to determine the amount of sodium hydroxide (NaOH) needed. The calculated amount of NaOH solution was poured into the batch reactor while being subjected to continuous mixing until pH 9.0 was achieved [36]. The supernatant was analysed for phosphate and ammonium concentrations to determine the amount of magnesium chloride hexahydrate (MgCl2·6H2O) and ammonium chloride (NH4Cl) needed to form struvite according to Equation (1). However, the MgCl2·6H2O added is calculated based on the Mg:P molar ratio of 2:1, since it is recommended to apply a ratio higher than to that of the stoichiometric value of 1:1 [37], while the NH4Cl added is calculated based on the N:P molar ratio of 4:1 [36]. The mixture was subjected to continuous mixing for one to two hours at 100 to 150 RPM (10–15 Hz) for the struvite crystallisation process. Then, the precipitates were left to settle at the conical bottom of the batch reactor for 18–24 h. The settled precipitates at the bottom were flushed and transferred to drying pans subjected to air and sun drying. The dried precipitates were collected for characterisation. The recovered precipitates were used as recovered phosphorous fertiliser for farm application research. The remaining effluent from the batch reactor was also drained and stored in storage drums, and samples were analysed to identify the proper treatment process prior to application, such as an added water source for crop irrigation or hydroponics.
Mg2+ + PO43− + NH4+ + 6H2O → MgNH4PO4·6H2O
2.3.3. Batch Reactor Performance
The performance of the batch reactor was evaluated based on the material balance calculated throughout the process flows for every 100 L or raw septage processed. Thirty-eight batches were run, processing around of 3682 L of raw septage from Salikneta and SpTP to produce 10,252 g of RPF for farm application studies [38]. Thirty-one batches (batch numbers: 1–31) were evaluated using the septage from SpTP and Salikneta with a ratio of 90:10, two batches were evaluated using the septage from Salikneta farm (batch numbers: 32–33), and five batches was evaluated using the septage from SpTP (batch numbers: 34–38). The batches were run to accommodate the required phosphorous fertiliser needed for the crop yield studies. To evaluate holistically the performance of the batch reactor in the context of a circular economy, an overall material balance was calculated considering 1 batch or 100 L of raw septage processed from collection to recovery. The calculations are based on the analyses of raw septage, including septage density (1.006 kg/L) and total solids (54,410 mg/L), and characterisation of RPF, effluent, and waste sludge. Losses throughout the processes were also considered in the calculations, estimating 20% losses-to-septage ratio due to the attached water/moisture and manual collection of intermediate products (i.e., wet waste sludge and wet precipitates) during the drying process. The efficiency of hydrolysis is evaluated as the amount of PO4-P released, that is the difference in PO4-P concentration before and after hydrolysis, as shown in Equation (2):
where [PO4-P]h is the PO4-P concentration after hydrolysis and [PO4-P]sl is the initial PO4-P concentration in the liquid component of the raw septage. The nutrient recovery efficiency in the form of RPF is evaluated by the percent of PO4-P recovered based on changes in PO4-P concentration from the hydrolysed septage and the effluent shown in Equation (3):
where [PO4-P]e is the concentration of PO4-P in the effluent.
2.4. Application of the Recovered Phosphorous Fertiliser to Farm
Experiments were conducted at Salikneta under field andgreenhouse condition, to compare the growth and performance of eggplant (Solanum melongena, Calixto variety) and tomato (Solanum lycopersicum, Diamante Max F1 variety) in response to the application of RPF and a commercial fertiliser (ammonium phosphate). Tomatoes and eggplants were tested as these crops responded with the phosphorous fertiliser application through increased yield, quality, and resistance to diseases [39]. P is an essential plant macro-element and a component of nucleic acids and phospholipids that plays an important role in photosynthesis, root development, and nutrient and water absorption. The application of fertiliser for every treatment in the experiments is based on the initial soil analysis of 80 kg N, 120 kg P2O5, 30 kg K2O and recommended rates for eggplant and tomato.
2.4.1. Field Experiments for Eggplant
Eggplants were planted in 12 m2 plots laid out in a randomized complete block design (RCBD) with three replicates of four fertiliser treatments: control or no fertiliser application (E-T1); application of 16 g ammonium phosphate per plant (E-T2); application of 42 g RPF per plant (E-T3); and application of 21 g RPF and 8 g ammonium phosphate (E-T4).
The field plots were ploughed twice at a depth of 15–20 cm deep at a one-week interval, followed by harrowing to further break the soil peds into the desired soil tilth and level the field. Plastic mulch was placed over each plot and was fastened to the soil using 6–8 cm long bamboo slats placed 30 cm apart. Planting holes were distanced at 75 cm between columns and 50 cm between rows.
For seedling production, one to two seeds were placed in each hole of the holed seedling tray filled with soil growing media. The seedlings were watered regularly in the morning for soil moisture. Thinning was conducted 3–5 days after emergence, only the robust seedling was left in each hole. The seedlings were drenched weekly with urea solution (10 g of urea diluted in 1 gal of water) for one month. The seedlings were kept in the greenhouse for three-weeks and cared against weeds, pests, and diseases. Seedling hardening was performed one week before transplanting. Twenty-eight eggplant seedlings were transplanted in each plot. Each seedling was gently pulled out from the seedling tray and carefully transplanted into the prepared holes of each plot to avoid root–shoot injury. Re-planting of the missing hills was performed three to five days after transplanting. During the dry season, the plants were watered thrice a week, and during the wet season the eggplant was watered when soil was dry. The plants were strictly monitored against wilting through pruning and weeding.
The recommended rate of fertiliser that was applied to eggplants has an NPK ratio of 80-80-0. The first application of fertilisers was performed 14 days after transplanting (DAT). The second application with urea fertiliser was performed 30 DAT. Urea was used for sufficing the nitrogen requirements and the calculated rate of 2.5 g, 6.5 g, and 5.0 g for E-T2, E-T3, and E-T4, respectively. Harvesting started two weeks after flowering and was performed twice a week. The harvested eggplants are usually 8–10 cm long and should be soft.
2.4.2. Field Experiments for Tomato
Tomato grown in polyethylene bags was laid out in RCBD with four replicates of six fertiliser treatments: control or no fertiliser application (T-T1); application of 13.50 g of N, 16.20 of P, 1.35 of K, NPK fertiliser per plant (T-T2); application of 42.95 g RPF per plant (T-T3); application of 42.95 g RPF and 1.35 g K per plant (T-T4); application of 16.20 g ammonium phosphate per plant (T-T5); and application of 16.20 g ammonium phosphate and 1.35 g K per plant (T-T6).
Seeds were sown in a seedling tray with a planting medium having a ratio of 2:2:1 of compost, soil, and perlite. Seedlings were watered twice a day. Seedlings were hardened through full sunlight exposure for 10 days. Tomato seedlings was transplanted 30 days after sowing. Seedlings were then transferred in a 9 in × 16 in polyethylene bag with 20 kg of soil. Watering of the plants was performed every other day to avoid waterlogging that may lead to development of diseases and even death of plants. Weeding was also performed to avoid competition for water, space, and especially nutrients; and to reduce the possibility of the occurrence of pests and diseases. Trellising was also performed 15 days after transplanting to provide support for the growing tomatoes.
Application of ammonium phosphate was performed during transplanting. By contrast, RPF was applied during planting and 14 days after transplanting using the ring method. To supply plants their needed requirement for N and K, urea and muriate of potash were applied using split application at 15 and 30 days after transplanting, one-inch deep and 3 inches away from the base of the plant. Harvesting of tomato fruits was performed at the light red stage. The frequency of harvesting is twice a week early in the morning for five harvestings.
2.5. Analyses
The ammonia–nitrogen (NH3-N), orthophosphate–phosphorus (PO4-P), and nitrate–nitrogen (NO3-N) of raw septage, supernatant, and effluent were analysed using a portable spectrophotometer (DR 1900, HACH, Loveland, CO, USA). The raw septage, effluent, and recovered phosphates were outsourced to accredited third-party laboratories for analyses of various parameters, such as wet chemistry (biological oxygen demand, chemical oxygen demand, NH3-N, PO4-P, NO3-N, total nitrogen, total phosphorous, potassium, total organic carbon, total suspended solids, calcium and magnesium), heavy metals through inductively coupled plasma optical emission spectroscopy (ICP-OES, Agilent Technologies, Santa Clara, CA, USA) (arsenic, cadmium, iron, mercury, lead, and zinc), and microbiology (E. coli and fecal coliform). Scanning electron microscopy (SEM) with energy dispersive X-ray (EDX) analysis, and X-ray diffraction (XRD) analysis were conducted to identify the elemental composition and mineralogical phases of composite samples from the recovered P-fertiliser processed from the raw septage of Salikneta and SpTP.
For the application of fertilisers to tomato, titratable acidity (TA) was measured after every harvest following the standard procedure by the Association of Official Analytical Chemists [40]; the total soluble solids (TSS) of tomato fruits were measured using refractometer; the number of seeds per treatment was recorded; and the yield per plant was measured every harvest. The data collected were subjected to analysis of variance (ANOVA) using SPSS 22 and Microsoft Excel data packages. Comparison among means was performed using Tukey’s honestly significant difference (HSD) at a 5% level of significance.
3. Results
3.1. Process Flow and Material Balance
The performance of the nutrient recovery batch reactor was evaluated based on the movement of all the materials entering and leaving the identified system boundary as shown in Figure 2. The whole production is divided into four major processes: septage collection, hydrolysis, nutrient recovery via chemical precipitation, and product application to farm for local crops (i.e., eggplant and tomato). The material balance of raw materials, by-products, and the RPF was calculated with the basis of processing 100 L of raw septage, having the characteristics presented in Table 1. The material balance for every component of the raw materials, by-products, and RPF is presented in Table A1. The detailed data and results of the material balance are presented in the Supplemental Information (Tables S1–S3). An average of 290 g of RPF for every 100 L of raw septage processed, or for every batch, was produced. Moreover, the whole system would produce 38.37 L of effluent and 1.87 kg of dried waste sludge as by-products.
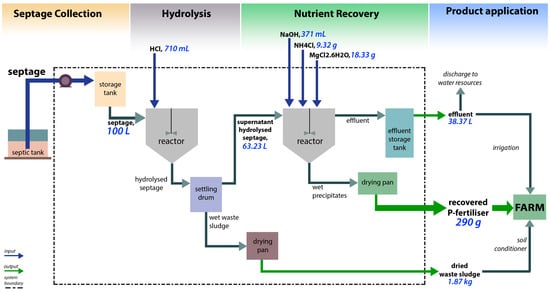
Figure 2.
Process flow diagram of the nutrient recovery system with overall material balance.
Effect of Hydrolysis
To maximise the recovery from the raw septage, hydrolysis was performed as a pre-treatment to increase the concentration of dissolved phosphates needed for struvite precipitation. Figure 3 shows the trend of phosphates initially present in the raw septage liquor and the total concentration of phosphates after hydrolysis (i.e., hydrolysed septage), for every batch. The initial raw septage liquor has an average of 17.84 ± 4.62 mg/L PO4-P. After hydrolysis, the phosphate concentration of the supernatant or the hydrolysed septage increased to an average of 76.74 ± 13.63 mg/L PO4-P. Hence, the pre-treatment managed to release about 58.90 ± 9.33 mg/L PO4-P from the septage sludge, that is 77% of the total phosphate concentration in the hydrolysed septage (details in Table A2). The fluctuation of results observed in Figure 3 reflects the varying characteristics of each batch of raw septage processed, which mainly depends on the source and type of septic tank, especially for the septage collected from the SpTP. Generally, lower pH could dissolve more phosphorus but in actual scenarios, the phosphorous dissolution rate varies due to the presence of different phosphorous compounds in various types of wastewater sludge [31].
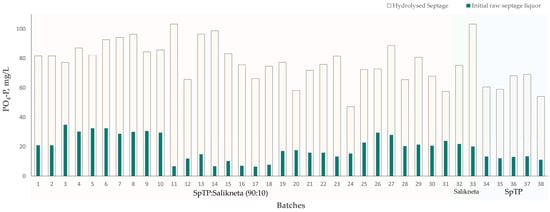
Figure 3.
Phosphates released through hydrolysis for all batches.
3.2. Recovered Phosphate Fertiliser (RPF)
For struvite precipitation using the batch reactor, an average of 98.50% was recovered from the hydrolysed septage as phosphate fertiliser leaving an effluent that passed the regulatory standards as shown in Table A2. The phosphates were successfully recovered as phosphate fertilisers with characteristics shown in Table 3. Based on the definition given by the Philippine government through the Fertilizer and Pesticides Authority (FPA), the RPF can be classified either as: non-traditional inorganic fertiliser, wherein the major nutrients (i.e., NPK) are supplied by synthetic or chemical compounds; or a fortified organic fertiliser defined as any decomposed organic product of plant or animal origin that is enriched with chemical ingredients to increase its nutrient content to a minimum total NPK of 8% [41]. Thus, the characterisation results of the RPF are compared to both types of fertilisers. The recovered precipitates contain 2010 mg/kg TN, 32,900 mg/kg TP, and 2400 mg/kg K, resulting in a total NPK of 8.03% by weight. The value of total NPK is above the minimum limits by the Philippine National Standards (PNS) for organic fertilizer; hence, the recovered precipitates can be qualified as a fertiliser [42]. The precipitates also have high contents of micronutrients (Ca, Fe, Mg, Zn) that are supplemental for plant growth [43]. Moreover, the E. coli, fecal coliform, and heavy metals are of lower values than the standards set by the FPA and the Association of American Plant Food Control Officials (AAPFCO) for inorganic fertilisers [41,44]. The subsequent analyses would discuss the quality and purity of struvite produced.

Table 3.
Recovered phosphorous fertiliser (RPF) analysis.
The scanning electron microscopy (SEM) images for the recovered precipitates and waste sludge showed various crystal shapes as shown in Figure 4. The image for the commercial struvite fertiliser was observed to have more orthorhombic crystal shapes with lesser contamination of other compounds, as shown in Figure 4a, while the image of waste sludge from the hydrolysis process (Figure 2b) expectedly showed more heterogeneous shapes. It could also be observed that the images of RPF processed from the septage of Salikneta do not differ from the septage processed from the SpTP. There are some orthorhombic shapes that represent struvite, but there were other irregular course shapes that could be attributed to other phosphate precipitates, such as amorphous calcium phosphates [37], given that the initial concentration of calcium is relatively high in the raw septage (see Table 1). Since the precipitates produced were not pure struvite, the term recovered phosphorous fertiliser (RPF) would better describe the main product as it can still be classified as fertiliser based on the characterisation results in Table 3. Some studies utilising animal manure [45] and municipal wastewater [46] produced similar SEM images.

Figure 4.
SEM images of (a) commercial struvite, (b) waste sludge, (c) RPF from Salikneta farm septage, and (d) RPF from SpTP septage.
X-ray diffraction (XRD) analysis for the RPF from Salikneta (blue line) and SpTP sludge (black line) are illustrated in Figure 5. The distinct peaks in both samples are observed at around 2Ө = 31, 45, and 56°. The peaks observed at 2Ө = 31.9372, 45.6552, and 56.6247° have almost similar intensities for the RPF from Salikneta sludge. By contrast, the peak at 2Ө = 31.7463° had the highest intensity for the RPF from SpTP sludge. Comparing the results with the XRD analysis of struvite presented in the recent study of de Souza Meira et al. [47] and Sun et al. [29], it could be concluded that the RPF mostly contain impurities. The distinct peaks in Figure 5 refer to the presence of halite and small peaks are attributed to the presence of struvite and other precipitates. By contrast, the distinct peaks illustrated in the XRD plots in the study of Sun et al. [29] and de Souza Meira et al. [45] refer to the presence of struvite. The presence of other micronutrients may have affected the morphology and composition of the recovered fertilisers. The impact of the micronutrients, such as Ca2+ and Al3+, is explained in the study of Acelas et al. [48], by investigating the struvite purity and morphology at varying Mg2+:Ca2+ and Mg2+:Al3+ molar ratios. The XRD analysis showed that the peaks for struvite crystals became less distinct at increasing concentrations of Ca2+ and Al3+.
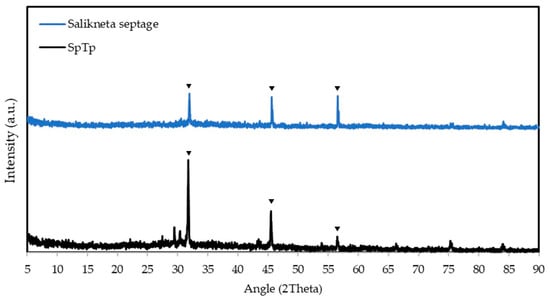
Figure 5.
XRD plots of RPF processed from the raw septage of Salikneta farm and SpTP, where distinct peaks are indicating the presence of struvite and other precipitates.
Based on the SEM and XRD analyses, there is a need to further improve the operating conditions to achieve high quality and purity of struvite in the RPF. One possible solution is to reduce the presence of the other micronutrients, such as calcium and iron, in the hydrolysed samples but at the expense of higher production costs.
By-Products
As shown in the process flow and material balance in Figure 2, two by-products were produced, the wastewater effluent and the dried waste sludge. The effluent is typically discharged in water bodies while the waste sludge is transported to landfills for disposal. However, in this study, both the effluent and the waste sludge were characterised, shown in Table 4, to evaluate its potential as by-products of the proposed circular phosphorous economy application. The effluent characteristics passed the Philippine Department of Environment and Natural Resources (DENR) Administrative Order (DAO) 2016-08 and DAO 2021-19 General Effluent Standard (GES) for Class C, shown in Table 4, providing evidence that the batch reactor could efficiently treat the septage [49,50]. Moreover, this shows the effluent’s potential reuse as irrigation water in the farm, avoiding a significant amount of water use and thus saving water costs. The dried waste sludge was also analysed and compared to the US EPA standard for biosolids, as shown in Table 4, to evaluate its potential as a by-product rather than as waste [51]. Considering the characteristics of the raw septage processed presented in Table 1, the dried waste sludge was expected to contain minimal to no detection of heavy metals (i.e., As, Cd, Hg, and Pb), fecal coliform, and E. coli while having significant amounts of nitrogen, phosphorus, and potassium (NPK), and other nutrients (i.e., Ca, Fe, Mg, Zn), thus showing its potential as a soil conditioner or supplemental fertiliser for local farm crops.

Table 4.
Characterisation of by-products: effluent and waste sludge from the nutrient recovery system.
3.3. Crop Yield
Field experiments were conducted to compare the growth and performance of eggplant and tomato in response to an application of RPF and commercial fertiliser (i.e., ammonium phosphate). The results presented in Table 5 show that differences in eggplant yields ranged from 9 to 11 tons compared with the yield from the control. It was found that the yields of treatments E-T3 and E-T4 containing RPF as a phosphorous source does not have a significant difference with E-T2 containing commercial fertilisers. Hence, this research was able to demonstrate that eggplant fertilised with RPF and urea could have a comparable yield with eggplants fertilised with commercial fertilisers.

Table 5.
Eggplant plot yield and yield per hectare.
For the quality of tomato produced, Table 6 presents that the highest titratable acidity (TA) of 5.82 measured from T-T2 plants applied with NPK followed by T-T5, T-T6, T-T4, and T-T3. This study also shows that T-T2 had the highest recorded total soluble solids (TSS) but was comparable to T-T3, T-T4, T-T5, and T6 but statistically different to T-T1. It was found that potassium (K) has a profound influence on fruit quality, particularly size, appearance, colour, soluble solids, acidity, and vitamin contents; thus, TSS and TA significantly increased with increasing rates of K [52].

Table 6.
Tomato titratable acidity, total soluble solids, number of seeds, and yield per plant.
On the number of seeds, the study showed that amongst treatments T-T2 and T-T4 had the highest number of seeds followed by T-T3, T-T6, and T-T5. Statistical analysis shows that T-T2, T-T3, T-T4, T-T5, and T-T6 are comparable but are statistically different from T-T1. According to Xie et al. [53], the addition of P fertiliser showed an increased seed yield of oilseed flax. Oloyede et al. [54] reported in their study that an application of NPK on pumpkin had significantly improved its seed yield.
The yield per plant was measured every harvest. The highest yield was obtained from T-T2 (1870 g) followed by T-T6, T-T5, T-T4, and T-T3. This result can be attributed to the nutrients supplied to the plants. T-T2 received complete and high nutrient content of N, P, and K. However, statistically, T-T3, T-T5 and T-T6 yields were comparable to T-T2. The lowest yield was still obtained from T-T1 with no fertiliser application. In general, the improved vegetative growth, yield and chemical content of tomatoes was attributed to receiving complete NPK nutrients.
The RPF produced from the nutrient recovery reactor contained significant amounts of P and N required for crop growth, but the P in the RPF is not just in struvite form, but other phosphates were also formed based on the SEM results (see Figure 4). However, the crops fertilised with RPF managed to produce comparable yields with crops fertilised with commercial and conventional fertilisers.
4. Discussion
The potential of integrating a local nutrient recovery system for onsite sanitation systems to create a paradigm shift from the business-as-usual scenario of a linear P pathway to a circular economy was exhibited and evaluated, hence resulting in a sustainable management of waste and resources within a community. A circular P economy considers sustainability wherein socio-economic and material aspects are integrated into the circular pathway of P systems management [55]. Ultimately, a circular P economy utilises nutrient recovery systems to divert P flows before reaching the water resources back to agri-food systems while by-passing further extraction of fossilised P [9]. Through the results of this research, the integration of the nutrient recovery systems generally played a critical role in transforming the value and supply chains from the linear P pathway to circular P economy flows as summarised in Figure 6.
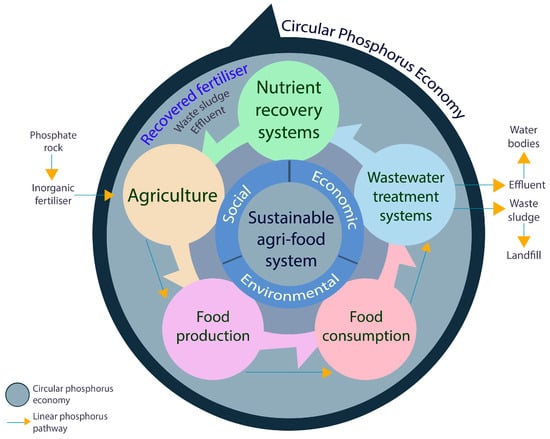
Figure 6.
Circular phosphorous economy.
For nutrient recovery systems, the processes and technologies are being constantly developed and optimised. However, there is a lack of demonstration projects beyond the laboratory-scale and application to real scenarios while considering most, if not all, of the value and supply chains in the circular economy [56]. In this real-case study, waste feedstock (i.e., septage) from a farm–school community and a treatment facility were processed and converted to a high-value product (i.e., RPF) while the by-products (i.e., effluent and waste sludge), typically considered as waste, could potentially be recycled for other purposes within the circular flow. Results show a favourable recovery of P from waste for the local effluent standard; however, economic production of RPF should be further investigated and improved. Additionally, the application and use of the RPF were evaluated for the local production of crops for food. The yields for both eggplants and tomatoes are comparable to that of crops grown using commercial fertilisers. These results are congruent with previous studies stating that utilising pure struvite is not agronomically viable, but when blended with other phosphate fertilisers, it could maintain crop yields that are comparable to conventional P fertilisers [15,57]. Though RPF application to crops producing promising yields and fruit quality, other factors still needed to be explored in future works, such as the variable release patterns of P through the soil and nutrient uptake of the crops. Consequently, the periodic effects of heavy metals on the soil and crops should also be taken into consideration, even if the produced RPF passed the government standards for fertiliser [44] since weathering could also occur among other external factors. Conversely, since one of the components of RPF is struvite, there is also lesser chance that heavy metals could attach due to its crystalline structure [58,59]. In general, this study shows that septage management could be turned into a circular economy industry that the local government and community could support to reduce environmental pollution and improve food production without heavy reliance on fertiliser importation.
Stakeholder engagements through workshops and focus group discussions were conducted to understand challenges in the implementation of a nutrient recovery system in the Philippines [38]. The farmers, in particular, gave positive perspective of the system as it produces a high-value product that could be used locally in their crops; however, their main issue is their technical capacity for the whole production process. This shows the potential of recovered fertiliser as an alternative for local farmers being affected by the exponential increase in fertiliser prices. Furthermore, acceptability of fertiliser obtained from human waste will have more social acceptability as compared to the direct application of human waste for food production. Indeed, social impacts are important for sustainable development through involvement of potential users to improve the marketability of the product while minimising the complexity of the technology [20]. Hence, there is a high potential to apply the circular phosphorous economy concept in a localised setting for sustainable sanitation and sustainable management of resources and wastes in vulnerable areas.
Further research needs to be performed to assess and quantify the avoided environmental impacts due to the application of a circular P economy (nutrient recovery system in a localised setting), addressing current challenges in sanitation, waste management, and phosphorus resource depletion. Sustainability assessment tools could be applied, such as a life-cycle sustainability assessment or multi-criteria decision-making analyses, to assess and quantify the socio-economic impacts holistically for all value and supply chains within the circular economy scope. This will further address the challenges on the agri-food sector, such as the increase in prices for fertiliser and thus food, eventually increasing poverty; and it will provide support for policies to improve the local socio-economic endeavours for sustainable development programmes.
5. Conclusions
This study involves local production of recovered fertiliser and its application to the local farm; thus, it provides a clear understanding of the potential and challenges brought about by the system in promoting a resource-oriented sanitation system for sustainability, especially in developing countries, such as the Philippines. The overall nutrient recovery process using the batch reactor could produce an average of 290 g of recovered phosphorous fertiliser (RPF) for every 100 L of raw septage processed. The acid hydrolysis of raw septage could increase the soluble phosphate concentration to 76.74 ± 13.63 mg/L PO4-P (supernatant of the hydrolysed septage) from 17.84 ± 4.62mg/L PO4-P (the initial concentration of the raw septage liquor). As the result of hydrolysis, about 77% of the phosphate concentration of hydrolysed septage comes from the released phosphates from the solid component of the raw septage. The chemical precipitation resulted to about 98.5% of phosphate being recovered as fertiliser from the hydrolysed septage. Moreover, the effluent could be used as irrigation water for the crops in the farm, and the waste sludge could be used as soil conditioner or supplemental fertiliser based on the characterisation results. Consequently, the RPF was applied to eggplant and tomatoes having comparable yields with commercial fertilisers. Having an onsite nutrient recovery batch reactor could incur savings for both septage desludging and fertiliser costs, helping farmers and the local community. Further assessments are needed as social and economic factors are equally important for the sustainable development. In general, this research actualised the proof-of-concept of the circular phosphorous economy towards achieving the sustainable development goals identified by the United Nations, particularly zero hunger (goal 2), clean water and sanitation (goal 6), sustainable cities and communities (goal 11), and responsible consumption and production (goal 12), to improve the planetary health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15139904/s1, Table S1: Material balance for raw materials and intermediate products for every 100 L of raw septage processed (1 batch process); Table S2: Material balance for recovered phosphorous fertiliser and by-products for every 100 L of raw septage processed (1 batch process); Table S3: Ratio of raw materials, by-products, and recovered phosphorous fertiliser per L of septage; Figure S1: Fabricated nutrient recovery batch reactor installed at Salikneta Farm; Figure S2: Tomato and eggplant cultivated using the recovered phosphorous fertiliser from septage.
Author Contributions
Conceptualization, D.S.; methodology, C.M.J.P., D.S., M.A.B.P. and M.E.A.A.S.; validation, D.S. and M.A.B.P.; formal analysis, M.A.B.P., C.M.J.P., R.G.D., A.H.O., A.B.B. and M.E.A.A.S.; investigation, A.L.L.J., C.M.J.P., R.G.D., A.H.O., A.B.B., M.E.A.A.S., M.A.B.P. and D.S.; resources, D.S., M.A.B.P., C.M.J.P., R.G.D., A.L.L.J., A.H.O., A.B.B. and M.E.A.A.S.; data curation, A.L.L.J., C.M.J.P. and M.A.B.P.; writing—original draft preparation, C.M.J.P.; writing—review and editing, C.M.J.P., A.B.B., D.S., M.A.B.P., R.G.D., A.L.L.J., A.H.O. and M.E.A.A.S.; visualization, C.M.J.P.; supervision, D.S. and M.A.B.P.; project administration, D.S., M.A.B.P. and C.M.J.P.; funding acquisition, D.S. and M.A.B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following funding agencies: The UK BEIS funded Newton Prize (UK Newton Fund Grant Reference NP2PB\100028) for UK-Philippines research excellence; EPSRC supported Newton fund project (EP/P018513/1) “Water-Energy-Nutrient Nexus in the Cities of the Future”; Newton-Agham Fund PhD (grant number 537006268) was funded by the British Council and Department of Science and Technology-Philippines.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in within the article and Supplementary Materials.
Acknowledgments
The authors would like to acknowledge the NexCities Team of Salikneta Farm of Agri-vet Sciences Institute, De La Salle Araneta University for the support in operations and monitoring of the pilot-scale reactor throughout the duration of the research. The authors would also like to thank the Society for the Conservation of Philippine Wetlands, Inc. (SCPW) for their support and assistance in engaging the surrounding communities and other relevant stakeholders for this research.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Material balance for every component of the raw materials, by-product, and product.
Table A1.
Material balance for every component of the raw materials, by-product, and product.
| Material Balance | Raw Materials | By-Product | Product | |||||
|---|---|---|---|---|---|---|---|---|
| Raw Septage | 37% HCl | 8.35 M NaOH | MgCl2·6H2O | NH4Cl | Waste Sludge | Effluent | Recovered Phosphorous Fertiliser | |
| Volume, L | 100.00 | 0.71 | 0.37 | - | - | - | 38.37 | - |
| Mass, kg | 100.60 | 0.85 | 0.48 | 0.018 | 0.009 | 1.87 | 38.37 | 0.29 |
| NPK | ||||||||
| Total Nitrogen, g | 101.72 | - | - | - | 2.39 | 28.56 | 4.76 | 0.59 |
| Total Phosphorous, g | 69.89 | - | - | - | - | 9.61 | 0.00 | 9.61 |
| Potassium, g | 49.31 | - | - | - | - | 1.01 | 1.15 | 0.71 |
| Calcium, g | 172.41 | - | - | - | - | 11.67 | 60.24 | 22.03 |
| Magnesium, g | 19.97 | - | - | 2.15 | - | 2.91 | 2.95 | 4.53 |
| Heavy Metals | ||||||||
| Arsenic, g | 0.03 | - | - | - | - | 0.01 | 0.00 | ND |
| Cadmium, g | 0.04 | - | - | - | - | 0.01 | <0.001 | 0.00 |
| Iron, g | 135.71 | - | - | - | - | 23.52 | 0.00 | 12.80 |
| Mercury, g | <0.0002 | - | - | - | - | <0.0002 | <0.0002 | <0.0002 |
| Lead, g | 0.63 | - | - | - | - | 0.19 | <0.005 | 0.01 |
| Zinc, g | 16.27 | - | - | - | - | 4.31 | 0.00 | 0.15 |
| Fecal Coliform, MPN/100mL | 9.20 × 105 | - | - | - | - | 0.21 | 2.00 | 0.25 |
| E. coli, MPN/100mL | 9.20 × 105 | - | - | - | - | 0.21 | 2.00 | 0.25 |

Table A2.
Phosphates released via hydrolysis of raw septage and percent phosphate recovered.
Table A2.
Phosphates released via hydrolysis of raw septage and percent phosphate recovered.
| Batch | Raw Septage, PO4-P, mg/L | Hydrolysed Septage, PO4-P, mg/L | PO4-P Released, PO4-P, mg/L | % of Phosphate Released | Effluent, PO4-P, mg/L | % PO4-P Recovered |
|---|---|---|---|---|---|---|
| SpTP:Salikneta (90:10) | ||||||
| 1 | 20.90 | 81.60 | 60.70 | 74.39 | 1.03 | 98.74 |
| 2 | 20.90 | 81.60 | 60.70 | 74.39 | 1.03 | 98.74 |
| 3 | 34.85 | 77.17 | 42.32 | 54.84 | 4.36 | 94.35 |
| 4 | 30.19 | 87.12 | 56.93 | 65.35 | 4.36 | 95.00 |
| 5 | 32.52 | 82.14 | 49.62 | 60.41 | 6.14 | 92.53 |
| 6 | 32.52 | 92.63 | 60.11 | 64.89 | 6.14 | 93.37 |
| 7 | 28.77 | 94.15 | 65.38 | 69.44 | 0.61 | 99.35 |
| 8 | 30.03 | 96.28 | 66.26 | 68.81 | 0.61 | 99.37 |
| 9 | 30.54 | 84.45 | 53.92 | 63.84 | 0.41 | 99.51 |
| 10 | 29.49 | 85.64 | 56.15 | 65.56 | 0.41 | 99.52 |
| 11 | 6.62 | 103.22 | 96.60 | 93.59 | 0.38 | 99.63 |
| 12 | 11.84 | 65.65 | 53.81 | 81.96 | 0.38 | 99.42 |
| 13 | 14.79 | 96.45 | 81.66 | 84.66 | 0.49 | 99.49 |
| 14 | 6.71 | 98.71 | 91.99 | 93.20 | 0.49 | 99.50 |
| 15 | 10.16 | 83.12 | 72.95 | 87.77 | 0.43 | 99.49 |
| 16 | 6.96 | 75.56 | 68.60 | 90.79 | 0.43 | 99.44 |
| 17 | 6.39 | 66.24 | 59.85 | 90.35 | 0.32 | 99.51 |
| 18 | 7.63 | 74.59 | 66.95 | 89.77 | 0.32 | 99.57 |
| 19 | 17.02 | 77.28 | 60.25 | 77.97 | 0.46 | 99.41 |
| 20 | 17.53 | 58.11 | 40.58 | 69.83 | 0.46 | 99.21 |
| 21 | 15.90 | 71.77 | 55.87 | 77.84 | 0.37 | 99.48 |
| 22 | 15.90 | 75.83 | 59.93 | 79.03 | 0.37 | 99.51 |
| 23 | 13.30 | 81.47 | 68.17 | 83.67 | 0.33 | 99.59 |
| 24 | 15.33 | 47.08 | 31.75 | 67.43 | 0.33 | 99.29 |
| 25 | 22.78 | 72.43 | 49.65 | 68.55 | 1.39 | 98.08 |
| 26 | 29.42 | 72.80 | 43.38 | 59.59 | 1.39 | 98.09 |
| 27 | 27.85 | 88.69 | 60.84 | 68.60 | 1.31 | 98.53 |
| 28 | 20.41 | 65.49 | 45.08 | 68.83 | 1.31 | 98.00 |
| 29 | 21.36 | 80.60 | 59.24 | 73.50 | 0.74 | 99.08 |
| 30 | 20.70 | 67.98 | 47.28 | 69.55 | 0.74 | 98.91 |
| 31 | 23.87 | 57.38 | 33.51 | 58.40 | 1.01 | 98.23 |
| Average | 20.10 ± 8.87 | 78.81 ± 12.94 | 58.71 ± 14.59 | 74.09 ± 10.98 | 1.24 ± 1.64 | 98.45 ± 1.91 |
| Salikneta farm | ||||||
| 32 | 21.70 | 75.23 | 53.53 | 71.15 | 0.53 | 99.29 |
| 33 | 20.09 | 103.22 | 83.13 | 80.53 | 0.53 | 99.48 |
| Average | 20.90 ± 1.14 | 89.23 ± 19.79 | 68.33 ± 20.93 | 75.84 ± 6.53 | 0.53 ± 0.00 | 99.39 ± 0.14 |
| SpTP | ||||||
| 34 | 13.24 | 60.57 | 47.33 | 78.14 | 1.73 | 97.15 |
| 35 | 12.02 | 58.95 | 46.94 | 79.62 | 1.73 | 97.07 |
| 36 | 12.91 | 68.20 | 55.30 | 81.08 | 1.57 | 97.69 |
| 37 | 13.44 | 69.19 | 55.75 | 80.58 | 1.57 | 97.73 |
| 38 | 11.00 | 54.06 | 43.06 | 79.65 | 0.90 | 98.34 |
| Average | 12.52 ± 1.01 | 62.20 ± 6.41 | 49.67 ± 5.60 | 79.81 ± 1.12 | 1.50 ± 0.35 | 97.60 ± 0.51 |
| Total Average | 17.84 ± 4.62 | 76.74 ± 13.63 | 58.90 ± 9.33 | 76.58 ± 2.93 | 1.09 ± 0.50 | 98.48 ± 0.90 |
References
- Cordell, D.; Drangert, J.O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Mo, W.; Zhang, Q. Energy-Nutrients-Water Nexus: Integrated Resource Recovery in Municipal Wastewater Treatment Plants. J. Environ. Manag. 2013, 127, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Gerten, D.; Heck, V.; Jägermeyr, J.; Bodirsky, B.L.; Fetzer, I.; Jalava, M.; Kummu, M.; Lucht, W.; Rockström, J.; Schaphoff, S.; et al. Feeding Ten Billion People Is Possible within Four Terrestrial Planetary Boundaries. Nat. Sustain. 2020, 3, 200–208. [Google Scholar] [CrossRef]
- Smol, M.; Preisner, M.; Bianchini, A.; Rossi, J.; Hermann, L.; Schaaf, T.; Kruopiene, J.; Pamakštys, K.; Klavins, M.; Ozola-Davidane, R.; et al. Strategies for Sustainable and Circular Management of Phosphorus in the Baltic Sea Region: The Holistic Approach of the InPhos Project. Sustainability 2020, 12, 2567. [Google Scholar] [CrossRef]
- Sotto, L.P.A.; Beusen, A.H.W.; Villanoy, C.L.; Bouwman, L.F.; Jacinto, G.S. Nutrient Load Estimates for Manila Bay, Philippines Using Population Data. Ocean Sci. J. 2015, 50, 467–474. [Google Scholar] [CrossRef]
- Baltazar, D.E.; Harada, H.; Fujii, S.; Tan, M.F.; Akib, S. A Comparative Analysis of Septage Management in Five Cities in the Philippines. Eng 2021, 2, 12–26. [Google Scholar] [CrossRef]
- Barquet, K.; Järnberg, L.; Rosemarin, A.; Macura, B. Identifying Barriers and Opportunities for a Circular Phosphorus Economy in the Baltic Sea Region. Water Res. 2020, 171, 115433. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Elser, J.J.; Hilton, J.; Ohtake, H.; Schipper, W.J.; Van Dijk, K.C. Greening the Global Phosphorus Cycle: How Green Chemistry Can Help Achieve Planetary P Sustainability. Green Chem. 2015, 17, 2087–2099. [Google Scholar] [CrossRef]
- Withers, P.J.A. Closing the Phosphorus Cycle. Nat. Sustain. 2019, 2, 1001–1002. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Zhang, J.; Liang, S. Nutrient Recovery from Wastewater: From Technology to Economy. Bioresour. Technol. Rep. 2020, 11, 100425. [Google Scholar] [CrossRef]
- Cichy, B.; Kużdżał, E.; Krztoń, H. Phosphorus Recovery from Acidic Wastewater by Hydroxyapatite Precipitation. J. Environ. Manag. 2019, 232, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Ashley, K.; Cordell, D.; Mavinic, D. A Brief History of Phosphorus: From the Philosopher’s Stone to Nutrient Recovery and Reuse. Chemosphere 2011, 84, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Batstone, D.J.; Hülsen, T.; Mehta, C.M.; Keller, J. Platforms for Energy and Nutrient Recovery from Domestic Wastewater: A Review. Chemosphere 2015, 140, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.A.; Doyle, J.D. Struvite Formation, Control and Recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A Slow-Release Fertiliser for Sustainable Phosphorus Management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef]
- Arcas-Pilz, V.; Parada, F.; Villalba, G.; Rufí-Salis, M.; Rosell-Melé, A.; Gabarrell Durany, X. Improving the Fertigation of Soilless Urban Vertical Agriculture Through the Combination of Struvite and Rhizobia Inoculation in Phaseolus Vulgaris. Front. Plant Sci. 2021, 12, 649304. [Google Scholar] [CrossRef]
- González, C.; Fernández, B.; Molina, F.; Camargo-Valero, M.A.; Peláez, C. The Determination of Fertiliser Quality of the Formed Struvite from a WWTP. Water Sci. Technol. 2021, 83, 3041–3053. [Google Scholar] [CrossRef]
- Valle, S.F.; Giroto, A.S.; Dombinov, V.; Robles-Aguilar, A.A.; Jablonowski, N.D.; Ribeiro, C. Struvite-Based Composites for Slow-Release Fertilization: A Case Study in Sand. Sci. Rep. 2022, 12, 14176. [Google Scholar] [CrossRef]
- González-Ponce, R.; López-de-Sá, E.G.; Plaza, C. Lettuce Response to Phosphorus Fertilization with Struvite Recovered from Municipal Wastewater. HortScience 2009, 44, 426–430. [Google Scholar] [CrossRef]
- Shaddel, S.; Bakhtiary-Davijany, H.; Kabbe, C.; Dadgar, F.; Østerhus, S.W. Sustainable Sewage Sludge Management: From Current Practices to Emerging Nutrient Recovery Technologies. Sustainability 2019, 11, 3435. [Google Scholar] [CrossRef]
- Tilley, E.; Atwater, J.; Mavinic, D. Recovery of Struvite from Stored Human Urine. Environ. Technol. 2008, 29, 797–806. [Google Scholar] [CrossRef]
- Ignacio, J.J.; Malenab, R.A.; Pausta, C.M.; Beltran, A.; Belo, L.; Tanhueco, R.M.; Promentilla, M.A.; Orbecido, A. A Perception Study of an Integrated Water System Project in a Water Scarce Community in the Philippines. Water 2019, 11, 1593. [Google Scholar] [CrossRef]
- Penn, R.; Ward, B.J.; Strande, L.; Maurer, M. Review of Synthetic Human Faeces and Faecal Sludge for Sanitation and Wastewater Research. Water Res. 2018, 132, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Ofori-Amanfo, D.; Awuah, E.; Cobbold, F. A Comprehensive Study on the Physicochemical Characteristics of Faecal Sludge in Greater Accra Region and Analysis of Its Potential Use as Feedstock for Green Energy. J. Renew. Energy 2019, 2019, 8696058. [Google Scholar] [CrossRef]
- De Graaff, M.S.; Temmink, H.; Zeeman, G.; Buisman, C.J.N. Energy and Phosphorus Recovery from Black Water. Water Sci. Technol. 2011, 63, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- McGaughy, K.; Reza, M.T. Recovery of Macro and Micro-Nutrients by Hydrothermal Carbonization of Septage. J. Agric. Food Chem. 2018, 66, 1854–1862. [Google Scholar] [CrossRef]
- Gell, K.; Ruijter, F.J.d.; Kuntke, P.; de Graaff, M.; Smit, A.L. Safety and Effectiveness of Struvite from Black Water and Urine as a Phosphorus Fertilizer. J. Agric. Sci. 2011, 3, 67–80. [Google Scholar] [CrossRef]
- Moges, M.E.; Todt, D.; Heistad, A. Treatment of Source-Separated Blackwater: A Decentralized Strategy for Nutrient Recovery towards a Circular Economy. Water 2018, 10, 463. [Google Scholar] [CrossRef]
- Sun, H.; Mohammed, A.N.; Liu, Y. Phosphorus Recovery from Source-Diverted Blackwater through Struvite Precipitation. Sci. Total Environ. 2020, 743, 140747. [Google Scholar] [CrossRef]
- Hatziconstantinou, G.J.; Yannakopoulos, P.; Andreadakis, A. Primary Sludge Hydrolysis for Biological Nutrient Removal. Water Sci. Technol. 1996, 34, 417–423. [Google Scholar] [CrossRef]
- Antakyali, D.; Meyer, C.; Preyl, V.; Maier, W.; Steinmetz, H. Large-Scale Application of Nutrient Recovery from Digested Sludge as Struvite. Water Pract. Technol. 2013, 8, 256–262. [Google Scholar] [CrossRef]
- Bi, W.; Li, Y.; Hu, Y. Recovery of Phosphorus and Nitrogen from Alkaline Hydrolysis Supernatant of Excess Sludge by Magnesium Ammonium Phosphate. Bioresour. Technol. 2014, 166, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cornel, P.; Schaum, C. Phosphorus Recovery from Wastewater: Needs, Technologies and Costs. Water Sci. Technol. 2009, 59, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Law, K.P.; Pagilla, K.R. Reclaimed Phosphorus Commodity Reserve from Water Resource Recovery Facilities—A Strategic Regional Concept towards Phosphorus Recovery. Resour. Conserv. Recycl. 2019, 150, 104429. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Guide to Septage Treatment and Disposal Guide to Septage Treatment Disposal; U.S. Environmental Protection Agency: Washington, DC, USA, 1994. Available online: https://www.epa.gov/sites/default/files/2018-11/documents/guide-septage-treatment-disposal.pdf (accessed on 25 April 2023).
- Goel, S.; Kansal, A. Phosphorous Recovery from Septic Tank Liquor: Optimal Conditions and Effect of Tapered Velocity Gradient. J. Clean. Prod. 2020, 275, 124056. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Molinari, R. Advances in Struvite Precipitation Technologies for Nutrients Removal and Recovery from Aqueous Waste and Wastewater. Sustainability 2020, 12, 7538. [Google Scholar] [CrossRef]
- Promentilla, M.A.B.; Longos, A.L.; Orbecido, A.H.; Suplido, M.E.A.A.; Rosales, E.M.E.; Pausta, C.M.J.; Damalerio, R.G.; Lecciones, A.J.M.; Devanadera, M.C.E.; Saroj, D.P. Nutrient Recycling from Septage Toward a Green Circular Bioeconomy: A Case Study in Salikneta Farm, Philippines. Chem. Eng. Trans. 2022, 94, 1075–1080. [Google Scholar] [CrossRef]
- Filho, A.B.C.; Trevizaneli, B.; Rugeles-Reyes, S.M. Phosphorus (P) Improves Industrial Tomato Quality and Yield in Soil with High Phosphorus Content. Aust. J. Crop Sci. 2020, 14, 1335–1341. [Google Scholar] [CrossRef]
- Saad, A.G.; Jaiswal, P.; Jha, S.N. Non-Destructive Quality Evaluation of Intact Tomato Using VIS-NIR Spectroscopy Related Papers Non-Destructive Quality Evaluation of Intact Tomato Using VIS-NIR Spectroscopy. Int. J. Adv. Res. 2014, 2, 632–639. [Google Scholar]
- Fertilizer and Regulations Division. Fertilizer Regulatory Policies and Implementing Guidelines; Fertilizer and Pesticide Authority: Quezon City, Philippines, 2019.
- PNS BAFS 40:2014; Philippine National Standard for Organic Fertilizer. Bureau of Product Standards: Makati City, Philippines, 2014. Available online: http://spsissuances.da.gov.ph/attachments/article/1106/PNS-BAFS40-2014-OrganicFertilizer.pdf (accessed on 25 April 2023).
- De Mello Gabriel, G.V.; Pitombo, L.M.; Rosa, L.M.T.; Navarrete, A.A.; Botero, W.G.; do Carmo, J.B.; de Oliveira, L.C. The Environmental Importance of Iron Speciation in Soils: Evaluation of Classic Methodologies. Environ. Monit. Assess. 2021, 193, 63. [Google Scholar] [CrossRef]
- Association of American Plant Food Control Officials. Statement of Uniform Interpretation and Policy (SUIP) #25—The ‘Heavy Metal Rule’. 2019. Available online: https://www.aapfco.org/rules.html (accessed on 25 April 2023).
- Rahman, M.M.; Liu, Y.; Kwag, J.-H.; Ra, C. Recovery of Struvite from Animal Wastewater and Its Nutrient Leaching Loss in Soil. J. Hazard. Mater. 2011, 186, 2026–2030. [Google Scholar] [CrossRef] [PubMed]
- Mavhungu, A.; Masindi, V.; Foteinis, S.; Mbaya, R.; Tekere, M.; Kortidis, I.; Chatzisymeon, E. Advocating Circular Economy in Wastewater Treatment: Struvite Formation and Drinking Water Reclamation from Real Municipal Effluents. J. Environ. Chem. Eng. 2020, 8, 103957. [Google Scholar] [CrossRef]
- de Souza Meira, R.C.; da Paz, S.P.A.; Corrêa, J.A.M. XRD-Rietveld Analysis as a Tool for Monitoring Struvite Analog Precipitation from Wastewater: P, Mg, N and K Recovery for Fertilizer Production. J. Mater. Res. Technol. 2020, 9, 15202–15213. [Google Scholar] [CrossRef]
- Acelas, N.Y.; Flórez, E.; López, D. Phosphorus Recovery through Struvite Precipitation from Wastewater: Effect of the Competitive Ions. Desalin. Water Treat. 2014, 54, 2468–2479. [Google Scholar] [CrossRef]
- Department of Environment and Natural Resources. Water Quality Guidelines and General Effluent Standards; Department of Environment and Natural Resources: Quezon City, Philippines, 2016. Available online: https://emb.gov.ph/wp-content/uploads/2019/04/DAO-2016-08_WATER-QUALITY-GUIDELINES-AND-GENERAL-EFFLUENT-STANDARDS.pdf (accessed on 25 April 2023).
- Department of Environment and Natural Resources. Updated Water Quality Guidelines (WQG) and General Effluent Standars (GES) for Selected Parameters; Department of Environment and Natural Resources: Quezon City, Philippines, 2021. Available online: https://emb.gov.ph/wp-content/uploads/2021/07/DAO-2021-19-UPDATED-WQG-AND-GES-FOR-SELECTED-PARAM.pdf (accessed on 25 April 2023).
- United States Environmental Protection Agency. A Plain English Guide to the EPA Part 503 Biosolids Rule; United States Environmental Protection Agency: Washington, DC, USA, 1994. Available online: https://www.epa.gov/sites/default/files/2018-12/documents/plain-english-guide-part503-biosolids-rule.pdf (accessed on 25 April 2023).
- Javaria, S.; Khan, M.Q.; Bakhsh, I. Effect of Potassium on Chemical and Sensory Attributes of Tomato Fruit. J. Anim. Plant Sci. 2012, 22, 1081–1085. [Google Scholar]
- Xie, Y.; Niu, X.; Niu, J. Effect of Phosphorus Fertilizer on Growth, Phosphorus Uptake, Seed Yield, Yield Components, and Phosphorus Use Efficiency of Oilseed Flax. Agron. J. 2016, 108, 1257–1266. [Google Scholar] [CrossRef]
- Oloyede, F.M.; Agbaje, G.O.; Obisesan, I.O. Effect of NPK Fertilizer on Fruit Development of Pumpkin (Cucurbita Pepo Linn.). Am. J. Exp. Agric. 2013, 3, 403–411. [Google Scholar] [CrossRef]
- Jedelhauser, M.; Mehr, J.; Binder, C.R. Transition of the Swiss Phosphorus System towards a Circular Economy-Part 2: Socio-Technical Scenarios. Sustainability 2018, 10, 1980. [Google Scholar] [CrossRef]
- Suárez-Eiroa, B.; Fernández, E.; Méndez-Martínez, G.; Soto-Oñate, D. Operational Principles of Circular Economy for Sustainable Development: Linking Theory and Practice. J. Clean. Prod. 2019, 214, 952–961. [Google Scholar] [CrossRef]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A Review and Meta-Analysis of the Agricultural Potential of Struvite as a Phosphorus Fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]
- Ahmed, N.; Shim, S.; Won, S.; Ra, C. Struvite Recovered from Various Types of Wastewaters: Characteristics, Soil Leaching Behaviour, and Plant Growth. Land Degrad. Dev. 2018, 29, 2864–2879. [Google Scholar] [CrossRef]
- Kern, J.; Heinzmann, B.; Markus, B.; Kaufmann, A.C.; Soethe, N.; Engels, C. Recycling and Assessment of Struvite Phosphorus from Sewage Sludge. Agric. Eng. Int. CIGR J. 2008, 10, 1–13. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).