Abstract
A large part of municipal solid waste (MSW) still goes to landfills, representing an environmental concern. A circular economy approach can enable safe management of MSW while mitigating the increasing energy needs when waste is used as a feedstock in energy production processes (waste to energy). Currently, MSW can be converted into refuse-derived fuel (RDF) through mechanical and biological treatment processes. This study analyzes the status of MSW and RDF production, as well as its main destinations in Portugal and Europe. The legislation applied, possible energy-recovery routes, and challenges associated with energy recovery are discussed throughout this paper. This research finds that the production of RDF in Portugal has been neglected, mostly because of RDF composition being quite heterogeneous and its poor fuel properties. Therefore, the need to improve and upgrade the characteristics and properties of RDF for waste-to-energy processes was detected. RDF can be pretreated to be further applied to waste-to-energy and waste-to-gas processes, such as incineration and gasification. The technology readiness level data, costs, and SWOT analysis allowedto assess that although incineration is the most mature and widely used technology, gasification becomes more attractive, having lower costs and gaseous emissions, proving to be more efficient and sustainable for MSW and RDF conversion.
1. Introduction
With population growth, expanding urban density, economic developments, and rising community living standards, waste is constantly being produced in increasing records of generation and volume. Waste is produced all over the world, and it is an environmental issue whose mismanagement carries adverse socioeconomic, health, and environmental costs [1]. Currently, municipal solid waste (MSW) management is a real problem. These wastes represent approximately between 7–10% of the total waste produced in the European Union (EU) [2]. The challenges posed by MSW management result from its extremely complex and undifferentiated composition, the direct proximity of the waste produced to citizens, the high public visibility of this issue, and its impact on the environment and human health [2]. Even with recent waste-recycling technologies, a considerable part is still disposed in sanitary landfills because of the relatively low cost of this disposal solution (41.3 EUR/t MSW) [3]. Landfills represent a long-term environmental concern because of the potential contamination of soil and water with toxins that are leached out and emissions of greenhouse gases (GHG) such as methane (CH4) and carbon dioxide (CO2). In addition, the use of landfills represents a great loss of material and energy resources, as well as land, with potential for other applications [4,5].
Overall, specific economic and regulatory instruments have been created to promote waste management efficiency and to reduce landfilling and associated environmental impacts. Waste management is one of the key priorities set by the European Commission in the context of the circular economy [6]. Directive 2008/98/EC establishes the waste hierarchy applicable as a general principle of waste prevention and management legislation and policy: prevention, reduction, preparation for reuse, recycling, recovery (material and energy), and disposal. According to this hierarchy, waste is regarded as a resource. The top priority is the prevention of waste generation. When production cannot be minimized, the priority is reuse and, subsequently, recycling and other types of recovery, namely energy recovery. According to Directive 2018/850/EC, which transposes Directive 1999/31/EC, landfilling should be minimized and considered the last option for waste treatment. High landfill taxes and other charges on, for example, the amount of waste produced and not reused (“pay as you throw”) can contribute not only to achieve the targets set by Council Directives 2018/850/EC and 2018/851/EC, but it can also provide an economic opportunity to create value by enabling waste recovery [7]. These two European directives provide important steps toward more sustainable waste management: (a) preparation for reuse and recycling of MSW should increase to a minimum of 60% in 2030 and 65% by weight in 2035 (Directive 2018/851/EC); (b) by 2035, the amount of MSW landfilled must be reduced to 10% or less of the total amount of MSW produced (by weight) (Directive 2018/850/EC); (c) restrictions on landfilling of waste streams that are subject to separate collection (Directive 2018/850/EC); (d) specific criteria for by-products and end-of-waste status should be considered (Directive 2018/851/EC).
MSW has no clear definition. These wastes are broadly classified into organic and inorganic compounds and generally consist of everyday items that are used and disposed of, such as product packaging, food waste, paper, plastics, wood, textiles, metals, and glass [8]. The quantity and composition of MSW reflect on the economic evolution of the population [8,9]. In addition to the economic power of populations, other factors such as geographical location, number of inhabitants, and climate also influence the amount and composition of MSW [10]. Other factors can also modify MSW production and composition. During the COVID-19 pandemic, some cities in China reduced their production by about 30%. On the other hand, the United States showed MSW production 20% above normal [11]. Thus, considering that this work aims to make a comprehensive and realistic approach to the production of MSW and RDF in Portugal, policies, and potential energy routes, only data on the production and composition of MSW until 2020 will be highlighted. Figure 1 demonstrates the physical composition of MSW in mainland Portugal in 2020 [12].
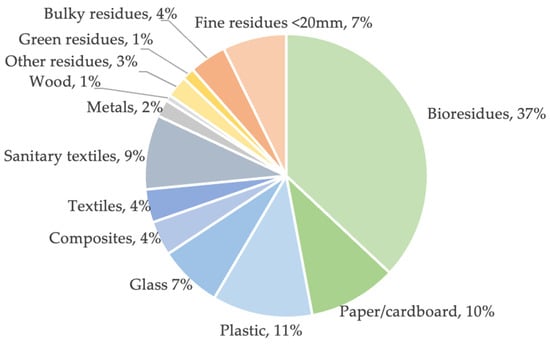
Figure 1.
Physical composition of MSW produced in mainland Portugal in 2020 [12].
MSW in Portugal’s mainland is mainly composed of biological waste (about 37%) and waste from selective collection (paper/cardboard, plastic, and glass: around 28%). In 2020, 5.014 million tons of MSW were produced in mainland Portugal, corresponding to an annual uptake of 512 kg/inhabitant/year, e.g., a daily production of 1.4 kg per habitant (Table 1). MSW production in Portugal has been increasing when compared to previous years, reaching higher values than the EU average in 2018, 2019, and 2020 [12,13].

Table 1.
MSW production in Europe and mainland Portugal between 2016 and 2020.
Of the total MSW treated in 2020, 37.7% was disposed of in landfills in the EU and 64.2% in Portugal. Table 2 presents the main final destinations of waste in the EU and Portugal [12,14].

Table 2.
Destination of MSW in Europe and Portugal in 2020. Source: authors’ calculations using Eurostat data for Europe.
In Portugal, waste recovery (including energy, organic, and multi-material recovery) represents about 35.7%, which is a significant percentage but still below the EU average. However, analyzing only the energy and organic recovery, Portugal represents more interesting values than the EU average, which means that it had committed itself to finding more sustainable destinations for MSW in 2020. Regarding landfilling, more than half of MSW produced is directly sent to landfills and the rest comes from the rejects of mechanical–biological treatment (MBT) and mechanical treatment (MT), which means that a significant percentage of the waste and rejects from the treatments cannot be recovered [12].
2. RDF Production in Portugal
Not all waste can be recycled efficiently (e.g., without excessive resource consumption), and only some waste can be recycled multiple times. In fact, recycling is not a process that applies to all waste streams. This means that for waste that cannot be properly recycled, another approach is needed that avoids immediate waste disposal [15]. Waste recovery before disposal, a key concept in the framework of waste management policy in Europe and in Portugal, is one of the approaches for nonrecyclable waste.
RDF is defined as a solid fuel produced by processing nonhazardous mono- or mixed waste streams (such as MSW, construction and demolition waste, or industrial waste) to make them a suitable feedstock for energy recovery, in strict compliance with the legislation [16,17,18].
In general, MSW can be processed by physical technologies and divided into three main fractions: combustible, incombustible, and moisture, with the combustible fraction corresponding to the RDF. The processing of MSW to produce RDF starts with the separation of waste at the MBT plant [4,5,19,20]. The aim of the MBT plant is separating and stabilizing the quickly biodegradable fraction (including food waste) of the waste, as well as recovering recyclables from mixed-waste streams. This is one of the main technologies used in MSW management and consists of two processes: mechanical–biological pretreatment (MBP) and mechanical–biological stabilization (MBS). In MBP, the organic fraction is separated and biologically stabilized before forwarding to the landfill, and recyclables and RDF are recovered from the residual coarse fraction. In the MBS or bio-drying process, the waste is dried prior to extraction of a larger fraction of RDF. MBP aims to stabilize the organic fraction to minimize landfill gas and leachate emissions, while MBS maximizes the RDF and material recovery [21]. In general, the RDF is produced by applying a combination of processes that may include screening, shredding, sanitizing, drying, and densification [22].
As mentioned, the materials to produce RDF are mainly produced in the process of MBT; however, remaining quantities are produced along the waste-processing line. Table 3 shows the annual evolution of the amounts of material for RDF in sorting units, MT and MBT, declared by the Portuguese Municipal Solid Waste Management Systems (MSWMS) between the years 2015 and 2019 [12]. There are no data after 2019.

Table 3.
Quantity of RDF material produced in Portugal (t).
It should be noted that not all the material produced is converted into RDF. Table 4 represents the quantities of RDF produced and sent to waste management operators (WMO) between the years 2015 and 2019 in Portugal [23]. In 2020, there was no production in Portugal, so figures are presented up to 2019.

Table 4.
Quantities of RDF destined to WMO (t).
A general analysis shows a decrease in the amount of RDF produced over the years compared to 2015. According to the “Relatório Anual Resíduos Urbanos” of the Portuguese Environment Agency (APA), the low production of RDF can be explained by the unavailability of the WMO for the reception of this material from the MSWMS because of the high moisture content that RDF presents [22]. For this reason, many existing RDF production units did not operate, and those that did not use their full processing capacity.
Currently, there are five RDF production units in Portugal, while there are 34 landfills. These five existing units had not recorded any activity after 2019, as previously mentioned. The last recorded RDF production occurred in 2019, considering that 57.6% of MSW went to landfills in the same year, the produced RDF corresponds to only 0.024% of the material that was unavailable [23]. Despite not taking advantage of all the material available to produce RDF, Portugal is known to import this resource from several EU countries. In 2016 alone, the United Kingdom accounted for about 75% of the RDF imported into Portugal, followed by Italy and the Netherlands. The imported RDF was mainly consumed by the cement industry, about 137,000 t in that same year [16,18]. In Portugal, in addition to the cement industry, other facilities such as the pulp and paper industry and ceramics are the main stakeholders in RDF use [24].
3. RDF Quality Requirements
RDF exhibits high heterogeneity, moisture, ash, and chlorine contents and low friability. This means that energy conversion can be problematic because its properties can lead to harmful emissions (hydrochloric acid and dioxins), slagging, and fouling that cause equipment corrosion, hindering RDF application in waste-to-energy (WtE) and waste-to-gas (WtG) [17] processes.
Current regulations are setting high quality standards for RDF so that it can be readily accepted as a substitute or auxiliary fuel in most thermochemical energy production systems, with small modifications. Therefore, regulations specifying strict quality standards for RDF are being issued in most countries [25]. In Europe, RDF is classified as waste according to the code “19 12 10—Combustible waste (waste-derived fuels)” of the European Waste List (EWL). However, Directive 2018/851/EC, which repeals Directive 2008/98/EC, provides that certain specific wastes may leave the status of waste if they have undergone a recovery operation and meet a set of requirements set out in Article 6 (1) of Directive 2008/98/EC and amended by this directive [2]. RDF is the material that does not comply with the established current standard, EN ISO 21640:2021, and the declaration of conformity [16]. The European Standardization Commission (CEN) established CEN/TC 343 for solid recovered fuels (SRF) that correspond to an RDF alternative with recognized quality to standardize fuels produced from nonhazardous waste at the European level. The CEN focus with this standard is to “provide a common classification and specification system for SRF to enable efficient trade, to promote their safe use in energy conversion activities, and to increase user confidence. The document facilitates a good understanding between seller and buyer, supports purchasing, cross-border movements, use and supervision, and effective communication with equipment manufacturers. It also aims for the classification and specification system to support authorities’ authorization procedures and facilitate reporting on environmental issues” [26].
CEN/TC 343 standards and technical specifications are separated into five different groups (GW 1 to 5), as shown Table 5 [26,27,28]. These standards impose mandatory and optional specifications for SRF. The specifications include class code, origin, particle shape, ash content, moisture content, calorific value, chemical properties, biomass content, physical composition, and fuel preparation, as well as physical properties such as bulk density, volatile content, and chemical properties regarding major and minor elements [26,27,28].

Table 5.
CEN/TC 343 standards for SRF.
The European standard EN ISO 21640:2021 (CEN/TC 343/WG2) presents the main quality specifications, based on properties such as calorific value, chlorine, and mercury content, with class 1 being the most demanding and highest quality and class 5 the minimum quality required, as can be seen in Table 6 [29,30].

Table 6.
SRF classification system according to EN ISO 21640:2021. ar: as received, db: dry basis.
Fuel that does not meet the specifications and classification conditions showed in Table 6 and does not have the declaration of compliance is referred to as RDF. This does not necessarily imply that SRF is always of better quality than RDF, but that quality is known and defined according to standards, while RDF is a broader term not necessarily covered by any standards [16]. The classification system, classes, and specifications that are proposed by CEN/TC 343 should help authorities in licensing, be an aid for the end user to easily understand what must be considered when it comes to SRF and RDF and should increase the public’s positive perception of using these fuels. Figure 2 illustrates a simplified flow chain for SRF, from nonhazardous waste input to SRF end-use [30].
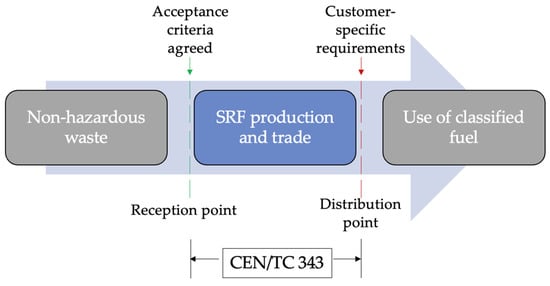
Figure 2.
SRF schematics from production to delivery point.
Figure 3 shows a demonstration of what could be considered RDF or SRF [16].
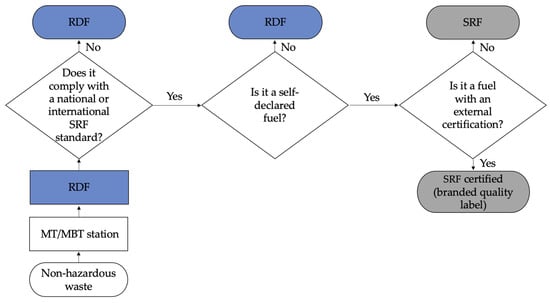
Figure 3.
A schematic approach to distinguish RDF from SRF.
Figure 3 shows that RDF can still become a certified SRF (quality mark) if produced according to a defined quality assurance procedure to meet a market demand (fuel with a well-defined quality). In Portugal, quality assurance with recommended certification is defined by the NP 4486:2008, which transposed the already established European standard CEN/TS 15359:2006, revoked to EN ISO 21640:2021. Producing certified SRF, developed according to the standard users’ specifications and declaration of conformity, is more rigorous and costly than simply producing uncertified secondary fuels. For consumers of secondary fuels (SRF and RDF), the most critical parameters that determine their viability in replacing fossil fuels are calorific value chlorine content (which generally comes from the plastic fraction, responsible for the formation of dioxins associated with corrosion problems), sulfur (responsible for the emission of SOX and associated with corrosion problems), heavy metals (especially mercury), ash, moisture, and biomass contents (that determine the emissions that can be considered null for industries that are within the emissions trading) [31].
4. RDF Pretreatment Technologies
There are basically two pathways for RDF management, which include disposal and energy recovery (Figure 4).
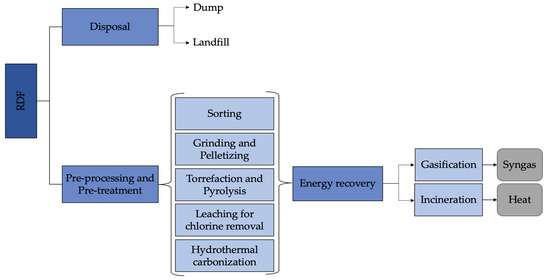
Figure 4.
RDF energy recovery scheme.
Although RDF is a value-added product when compared to the original waste, it is characterized by high variability in its morphological and quantitative composition. This means that its processing can be problematic, even leading to operational problems (slagging, fouling, and corrosion) or contribute to toxic emissions. RDF mainly consists of carbon derivatives such as organics, plastic, paper, wood, and textiles. Plastic and paper are the main fractions of RDF and consist of about 50–80%, the remaining fractions are mostly organics, wood, and textiles [32]. Typically, RDF has the following proximate composition: moisture content between 4–23 wt.%, volatile matter between 19–81 wt.%, fixed carbon around 4–24 wt.%, and ash content in the range of 5–60 wt.%. As for the ultimate composition, RDF characterization studies have found the following values: carbon (41–58 wt.%), hydrogen (5–10 wt.%), nitrogen (0.8–2.5 wt.%), oxygen (1–50 wt.%), and sulfur (0.1–0.4 wt.%) [20,33,34,35,36,37].
A typical RDF has around 5 to 20% organics, which contain about 75 wt.% moisture, representing the main source of moisture in RDF. For example, RDF with a higher amount of food waste demands more energy to remove moisture because of its composition. The application of measures such as the selective collection of food waste could improve the process of RDF production in the MBT plant and the quality of the RDF, since it would have a lower presence of food wastes in the composition of the RDF and decrease the fuel’s moisture content [5,38].
In this context, RDF preprocessing and pretreatment can be a suitable solution for most of these problems, making the use of RDF in energy recovery (WtE or WtG) more attractive [33,39].
4.1. Physical, Chemical, and Thermochemical Pretreatment of RDF
4.1.1. Sorting
Sorting is a physical pretreatment and one of the primary steps in the production of RDF. In this step, the RDF is obtained after several MSW processing techniques (drying, mechanical separation, and screening) removing most of the biodegradable fractions, metals, glass, fine articles, those with high concentrations of chlorine, and other inorganic materials, resulting in a mixture of organic materials with small amounts of inorganics [32,38].
The RDF obtained from sorting facilities typically has four main mass fractions of 15–35% plastic, 20–50% paper and cardboard, 2–10% wood, 5–20% organics, and about 5–10% noncombustibles [5]. This first approach to mixed waste results, in extreme cases, in an increase in calorific value from 8.4 (MSW) to 27.0 MJ/kg (RDF) [40].
4.1.2. Particle Size Reduction and Pelletizing
Grinding and particle size distribution are important factors in the valorization of RDF. Due to the high proportion of bulky, elastic, and fibrous materials in the RDF, dedicated crushing devices are required. RDF is also screened to determine the particle size distribution, an important parameter affecting densification and the selection of the energy conversion process. Moisture content is another important parameter in densification, which should vary from 10 to 20 wt.% [41]. According to Rezaei et al. (2020), the 4 mm mill produced RDF with optimal particle size distribution for pelletizing, and the RDF sample with 20% moisture content consumed the lowest pelletizing energy [5].
The morphology of MSW-based RDF is different from biomass, where lignin acts as a natural binder during pelletizing. This does not occur in the pelletization of RDF, so the introduction of artificial binders that do not alter the quality and increase the cost of the fuel should be considered. Temperature also plays a key role in RDF pelletizing, so a special matrix should be used. The temperature condition of 120 °C results in pellets with high durability and apparent density, like biomass pellets. The production of RDF pellets leads to densification, uniformity, and homogenization of the produced fuel quality [41].
4.1.3. Thermochemical Pretreatments
Torrefaction and Pyrolysis
Torrefaction and pyrolysis have been explored as a pretreatments to increase the calorific value, hydrophobicity, and friability of the feedstock [42]. These are eminent thermochemical recycling techniques that convert waste into energy or value-added products such as char, gases, and liquid fuel (pyrolytic oil) [43]. Pyrolysis is the process by which a long-chain polymer is converted into smaller molecules through thermal degradation (300 to 900 °C) under an inert or oxygen-deficient atmosphere, with or without the presence of catalysts [44,45]. When a lower temperature, slower heating rate, and longer reaction time are applied, the process has been called slow pyrolysis or torrefaction, where mostly solid product (char) is obtained [42].
The yields of liquid (pyrolytic oil) or gaseous (fuel gas) products can be increased using fast pyrolysis or flash pyrolysis, which occur at high temperatures (over 900 °C) under very high heating rates and for short residence times. Table 7 highlights the main differences between these three types of pyrolysis [46].

Table 7.
Operating conditions for slow, fast, and flash pyrolysis of MSW.
Therefore, parameters such as temperature, heating rate, residence time in the reaction zone, and size of materials affect the torrefaction and pyrolysis of biomass, MSW, and RDF [44]. The effects of these parameters have been studied by several authors. Yuan et al. (2015) investigated the torrefaction of MSW and stated that the pretreatment increased the product’s energy density and calorific value from 23.5 to 31.1 MJ/kg at 400 °C, 30 min. Another observation was of better grinding, lower chlorine content for temperatures below 450 °C, and better overall incineration properties [47]. Białowiec et al. (2017) showed that RDF torrefaction (200–300 °C, for 1 h) allowed a reduction in the moisture content from 22.9 to 1.4% and an increase in the lower heating value (LHV) from 19.6 to 25.3 MJ/kg at 300 °C. However, the increase in torrefaction temperature enabled a slight increase in ash and sulfur contents. These results suggest that torrefaction can increase the attractiveness of RDF as an energy source; however, the methods capable of removing inorganics from the chars of this fuel need to be studied [35]. In another work, Nobre et al. (2019) studied RDF torrefaction in a temperature range of 200–400 °C and residence times of 15 to 60 min. The process allowed the obtaining of char with better fuel characteristics. The higher heating value (HHV) of the RDF chars reached 26.2 MJ/kg. The produced chars presented high ash content, and the leaching with water allowed significant reductions in the inorganic fraction [39]. Chen et al. (2008) investigated the slow pyrolysis of two RDF samples by thermogravimetric analysis and Fourier transform infrared spectrometer and concluded that biomass degradation occurs in the temperature zones of 220–430 °C, the plastic fraction is degraded between 430–520 °C, and at a temperature above 650 °C, the carbonates are degraded [48]. Slow pyrolysis of blends of RDF and high-density polyethylene (HDPE) with biomass was investigated by Chavando et al. (2022), and the authors concluded that the effect of adding RDF and HDPE was an improvement in the LHV of the pyrolysis gas. However, pyrolysis of 100 wt.% of RDF and HDPE produces a heavy molecular tar fraction that condenses and solidifies, causing blockages and operational problems. Blending RDF and HDPE with biomass significantly mitigates this negative impact, so blends of RDF/HDPE with biomass are recommended as a feedstock for pyrolysis [38]. Manyà et al. (2015) investigated the effect of adding 10% RDF to olive pomace during slow pyrolysis (400 and 600 °C, for 30 min) with heating rates of 5 °C/min and 40 °C/min. The results showed that the addition of RDF to olive pomace led to an increase in the specific surface area of the resulting chars, this being more pronounced in pyrolysis at 40 °C/min and 600 °C. The increase in the oxygen reactivity of the chars was particularly high at this condition, as well, because of the catalytic effect of the inorganic components contained in the RDF. In any case, all the chars produced at a peak temperature of 600 °C exhibited low H:C ratios (less than 0.4), so these high-temperature chars would have a high carbon sequestration potential [36].
The use of catalysts in the pyrolysis of MSW and RDF has also been studied, aiming to minimize the production of tars and increase the conversion of feedstock into chars or gas. A pyrolysis process of MSW (550 °C, for 30 min), coupled with a thermal or catalytic cracking stage with dolomite (350 °C and 900 °C), was carried out by Veses et al. (2020). The results showed that the noncondensable gas produced consisted of more than 80 vol.% of CO and H2, with a heating value of 16 MJ/Nm3 when the temperature of 900 °C was reached in the catalytic cracking reactor. The gas obtained after the catalytic process can be considered a derived syngas that can be used for energy or chemical production. The obtained char had a heating value of 7.2 MJ/kg and high ash content, so its application as fuel in cement plants may be an interesting option [49]. Whyte et al. (2015) studied catalytic pyrolysis options (500 °C, 10 °C/min, for 30 min) of RDF char using spent and regenerated catalyst. The catalyst had a nominal 400 mol ratio of SiO2/Al2O3 (ZSM-5). They found that the catalysts were effective in reducing tar; however, they did not have much effect on yield and quality of char. The catalytic oil had density, heating value, and viscosity comparable to conventional diesel fuel. The three most significant compounds present in the oil were 2,4-dimethyl-1-heptene, ethylbenzene, and styrene, making the liquid suitable as a source of valuable chemicals. Catalyst use allowed an increase in CO2 production; still, the gases from catalytic pyrolysis had significant heating values ranging from 17.1 to 21.9 MJ/m3 [50]. Gandidi et al. (2018) used MSW composed of 52% plastics to produce bio-crude oil (BCO) under thermal and catalytic pyrolysis using activated natural zeolite as catalyst at 400 °C and reaction time of 60 min. The results showed that catalytic pyrolysis produced 21.4% BCO, while thermal pyrolysis produced 15.2% BCO, thus showing better production performance [51].
In general, the heating value of pyrolysis gas is typically between 5 MJ/m3 and 15 MJ/m3 based on MSW and between 15 MJ/m3 and 30 MJ/m3 based on RDF [52]. The chars produced by torrefaction or pyrolysis of these feedstocks can have heating values of 28–30 MJ/kg [37].
Given the energy content of the products, the large-scale pyrolysis process incorporates the gas into a boiler to generate power, while the liquids produced are processed and upgraded for use as combustible fuel. Torrefied and pyrolytic char can be used as a solid fuel [38].
Hydrothermal Carbonization (HTC)
For feedstocks with high moisture and ash content, hydrothermal carbonization (HTC) emerges as a more suitable thermochemical pretreatment process, allowing the conversion of these feedstocks into high-calorific solid fuel (hydrochar) without resorting to pre-drying. HTC occurs in the presence of water, with temperatures between 180 °C and 350 °C, under high pressure (0.8–4.0 mPa), with residence times from one to several hours (typically up to 72 h), and under an inert atmosphere to avoid excessive oxidative reactions [53]. HTC has hydrochar as its main product, but it also produces gas and an aqueous phase (process water), where polar inorganic and organic products resulting from feedstock decomposition are dissolved. One of the main concerns of the HTC technology is process-water treatment and/or recycling. Recent studies report several potential solutions for this issue using different feedstocks and HTC conditions, namely, process-water recirculation [54], nutrient recovery [55], using HTC process water as liquid fertilizer [56], or process-water treatment using electrochemical oxidation, distillation, or wet oxidation [57,58]. HTC of solid wastes produces hydrochars with higher calorific values from 14 to 20 MJ/kg and low chlorine, sulfur, and nitrogen contents, as well as high carbon concentrations [37].
Some HTC studies have been developed using SRF and RDF as feedstock. For example, Alves et al. (2021) investigated the HTC of pure SRF and SRF mixed with used cooking oil (UCO). The operating conditions were 275 and 300 °C, residence time of 30 min and SRF:water ratios of 1:10, 1:5, and 1:2.5. A significant improvement in the HHV of pure SRF hydrochar was observed compared to the original SRF, increasing by 48% to a final average value of 29.4 MJ/kg (t 300 °C, 30 min, SRF:water ratio 1:2.5). The optimum hydrochar characteristics were obtained at 300 °C, SRF:water ratio of 1:5, and 20 wt.% of UCO. The incorporation of UCO in the SRF samples further increased the heating value of the hydrochars because of the predominance of hydrocarbon chains in these materials. Some of the organic and inorganic compounds migrated to the liquid phase or transformed into other products, decreasing the ash content present in the hydrochar [37]. In addition, Nobre et al. (2021) investigated HTC t of RDF at temperatures between 250 and 300 °C, residence time of 30 min and 120 min, and RDF:water ratios of 1:15 and 1:5. The hydrochar produced at 300 °C for 120 min showed the lowest ash content (3.3%), while the highest HHV was found for the hydrochar obtained at 275 °C for 120 min (28.1 MJ/kg). The authors found that the HTC process was responsible for a significant reduction in chlorine concentration, showing dichlorination efficiencies between 69.2 and 77.9% from the RDF to the produced chars. The process water showed an acidic pH and significant chemical oxygen demand (COD), needing further treatment and valorization to increase the sustainability of the HTC process. The HTC process paired with leaching for inorganic and chlorine removal appears to be an effective RDF pretreatment solution for energy recovery [59].
Leaching for Chlorine Removal
RDF has a high inorganic content (around 14–17 wt.%), which tends to increase with torrefaction or pyrolysis (up to 22–29 wt.%) [33,35], with chlorine being one of the most problematic elements present in the inorganic profile of RDF given its harmful characteristics [33,60]. Water leaching or chemical leaching to remove inorganic compounds such as chlorine is a pretreatment method that has been applied mainly to biomass, waste, and chars. Ash and chlorine removal efficiencies by water leaching of coastal wood-waste samples were investigated by Lee et al. (2021). The treatments achieved 80–90% chlorine removal and 35–80% ash removal from the samples, and the treated waste met the chlorine limit of ISO 17225-2 I3 for wood pellets [61]. A study of the leaching of Cl in coal gangue at different pH values reported that, with increasing acidity of the leaching solution, the reaction rates of most matrix compositions in coal gangue increased, and more Cl was leached in less time. Under the condition of pH 8.1, the rate of Cl leaching was 18.98%; this rate increased to 57.21% (pH of 2.1) with decreasing pH. However, acid leaching results in an aqueous effluent that needs remediation and treatment since it represents a risk to the environment [62]. Nobre et al. (2019) evaluated the Cl removal potential with water at ambient temperature and with water near its boiling point on RDF chars. Both leaching tests caused a reduction in Cl content in the RDF chars, but leaching with water near its boiling point proved to be more effective in removing chlorine (~55–70%), indicating that Cl is present in the RDF chars in the form of water-soluble compounds [33]. These results show the effectiveness of Cl removal by leaching, thus representing a solution to consider in the pretreatment of waste rich in chlorinated species for WtE or WtG applications.
5. Energy Recovery from RDF
Energy recovery from RDF can be grouped into electricity, heat, or combined heat and power (CHP), and fuels’ production. Heat and electricity can be generated from the direct incineration of RDF or from the incineration of syngas obtained from the gasification of RDF. In addition, RDF can be gasified and syngas upgraded and converted into fuels. Thus, performing WtE and WtG through thermal treatment is the most practiced route for RDF valorization. They are considered better options for segregation, separation, and processing of waste materials because of numerous advantages such as a large reduction in waste volume (70–80%), potential as an alternative energy source, ease of landfill rehabilitation, reclamation of valuable land, and reduction in carbon footprints. The main recipients of RDF are cement plants. The use in the cement industry stands out among the potential applications since the main environmental impacts in cement production are associated with high energy demand [24,63]. RDF is typically used as a substitute for coal in the cement industry to reduce 40% of CO2 [32]. In India, it has been suggested to use RDF to fill 5% of the total energy requirements in cement industries that are located within 100 km of the waste-processing facility [64]. The second largest consumers of RDF are coal-fired and electric power plants and other industrial boilers [34].
WtE and WtG technologies can only be implemented if there is a consistent chain of collection, pretreatment, and, finally, energy recovery of the waste. The main challenges of these technologies are related to economics, as well as to their environmental and social aspects. Thus, by-products such as syngas and biochar must have market value to make MSW and RDF handling economically attractive. Technologies such as gasification represent fewer polluting alternatives when compared to incineration, but it has considerable operation and maintenance costs and, in addition, results in unwanted by-products such as unconverted material (solid fraction), tar (liquid fraction), and complex mixtures of hydrochars, which can be minimized or removed by the action of catalysts. The performance of these technologies is very sensitive to the characteristics of the feedstock requiring pretreatment before feeding [65].
5.1. Incineration
Incineration is the simplest and most widely used thermochemical conversion technology. From all the electricity and heat production capacity that has been installed worldwide, more than 90% has been based on incineration [66,67]. Incineration treats about 15–20% of MSW in the Organization for Economic Cooperation and Development (OECD) countries [68]. Waste incineration aims to treat waste to reduce its volume and hazardous characteristics by capturing (and thus concentrating) or destroying potentially harmful substances. Incineration processes can also provide a means to allow recovery of the energy, mineral, and chemical content of waste [69]. Almost 500–600 kWh of electricity can be generated from 1 t of waste per incineration [63].
Incineration is defined as a complete process of waste oxidation in a furnace at high temperatures (above 1000 °C) that produces flow gases (mainly CO2 and H2O, which contain most of the thermal energy) and ash as by-products, resulting in a waste volume reduction of up to 80% [46,63]. In complete incineration, the main constituents of the flue gases are H2O, N2, CO, and O2. Depending on the composition of the combusted material and the operating conditions, smaller amounts of CO2, SOX, NOX, dioxins and furans, and heavy metals are formed [52]. Therefore, greater attention is paid to meeting limits for gaseous emissions and pollution control [46].
Currently, several technical and operational advances in incineration have been achieved, including good incineration efficiency and sophisticated emission control to avoid the emission of hazardous gases. The use of incineration technology is fully established and is commonly used in energy production from waste [70]. Giraud et al. (2021) conducted a study estimating a typical incineration operating condition by characterizing MSW WtE facilities operating in the United States of America (USA). One hundred and eighty-eight boilers were identified in the studied WtE facilities (more than 70). These boilers were stratified into nine categories by incineration technology. The results of the survey show that typical operating conditions for WtE incineration in the US are furnace temperatures above 1160 °C, gas residence time above 2.4 s, outlet gas concentrations of nearly 10%, db for oxygen, and more than 16% for moisture [71].
Dong et al. (2018) indicated that an MSW incineration plant in France, of the mechanical-grate type, could produce 361 kWh/tMSW of electricity, 238 kg of bottom ash, and 34.3 kg of fly ash and air pollution control residues. The same incinerator emitted 927.0 g NOX/tMSW and 51.2 g SO2/tMSW. Bottom ash recycling for metals and materials is highly applicable regarding this incineration process [72].
Brożek et al. (2022) investigated the RDF incineration process and showed that, for each kilogram of RDF, about 3.85 kWh (13,860 kJ) of heat can be obtained. As for emissions, each kilogram of incinerated RDF generated 12.95 mmolNOX/gRDF, 0.0328 mmolSOX/gRDF, and 49.31 mmolCO2/gRDF [73].
A modernized incineration plant located in Milan (Italy), equipped with three mobile incineration grid lines and MSW incineration capacity of 450,000 t/year at a temperature of 850 °C, allows the recovery of 24% of electricity. About 88% of the ash produced in the process is used for road construction [74]. Despite being considered as a proven low-cost but highly reliable technology, incineration has low energy conversion efficiency and high GHG emissions, as mentioned above, such as NOX, SOX, CO2, and particulate matter, as well as ash [75,76]. Although the evolution of modern incineration technology has notably reduced its environmental impacts, it still faces strong opposition from society because of its potential health risks from emissions of pollutants such as dioxins and furans [74].
5.2. Gasification
Gasification is the most promising and efficient technique for converting waste into gas. It provides great flexibility, employing various types of waste, and is used in the production of a variety of products. It is a widely accepted technology that harnesses the energy stored in waste [77,78]. It is a thermochemical conversion process involving various chemical reactions, heat and mass transfers, and pressure dependencies [76]. It occurs by reacting a sub-stoichiometric amount of oxygen (oxygen deficit) with the carbonaceous feedstock at temperatures in the range of 800–1200 °C, producing mostly syngas [79]. It is important that the properties of the feedstock are kept within certain predefined limits. This often requires special pretreatment, especially for MSW and RDF [52]. Selecting a type of gasifier with suitable feedstock characteristics is very important because the gasification performance is different with various feedstock components [80]. Gasification technologies have been described and are available in several research articles [79,81].
Syngas is composed of CO, H2, CH4, and other hydrocarbons, along with a significant amount of inert CO2 gas. If the gasification agent is air and not pure O2, a significant amount of N2 will also be present [76,79]. Syngas from RDF gasification composition is shown Table 8.

Table 8.
Chemical composition of syngas from RDF gasification. Adapted from [82].
Syngas can be employed as a feedstock in the chemical industry or as a fuel to produce heat and electricity [43]. Several papers in the literature report the production of syngas from waste.
Lin et al. (2020) studied the application HTC to MSW prior to gasification. MSW hydrochar was gasified in a downdraft reactor between 600–1000 °C under air, CO/O2, and steam/O2 atmospheres. The results showed that hydrothermal treatment at 220 °C for 30 min can remove 90.5 wt.% of chlorine from MSW. In gasification with air, the best heating value of syngas (8.5 MJ/kg) was achieved at 600 °C and at 900 °C in gasification with CO/O2 (9.6 MJ/kg) and with steam/O2 (16.6 MJ/kg). The quality of the syngas was improved, while the tar yield was generally reduced with reaction temperature, regardless of the atmosphere. Overall, the results indicated that HTC coupled with gasification was an effective approach to produce hydrogen-rich gas from MSW [83]. Gasification using pure O2 as the gasifying agent of waste mixed with plastic and cellulosic materials was carried out by Na et al. (2003), in the temperature range of 1100–1450 °C in a fixed-bed gasifier to investigate the gasification behavior with the operating conditions. The waste mixture was previously pelletized. The results showed the syngas had about 30–40 vol.% H2 and 15–30 vol.% CO, depending on the oxygen/waste ratio. By increasing the bed height, H2 and CO formation was increased, while CO2 formation was decreased by the char–CO2 reaction and cracking of the plastics. The cold gas efficiency was around 61%, and the heating values of the gases were in the range of 2800–3200 kcal/Nm3 [80].
Gasification of RDF at 900 °C yielded 0.67 kg/kg RDF syngas with 20 vol.% H2, 16.5 vol.% CO, 9 vol.% CH4, and 14.5 vol.% CO2 and a relatively high yield of heavy tars and waxes. The tar and wax fraction can amount to up to 10 wt.% of the feed, which can cause fouling in downstream processes. A secondary catalytic stage can significantly reduce the tar content in gas [84].
Pio et al. (2020) investigated the direct co-gasification of RDF with pine biomass in an 80 kWth pilot scale autothermal bubbling fluidized bed reactor using air as the gasifying agent. The operating conditions were as follows: average bed temperature between 785 and 829 °C, equivalence ratio between 0.21 and 0.36, and percentage of RDF incorporation in the feedstock of 0, 10, 20, 50, and 100 wt.%. Increasing the percentage of RDF in the feedstock led to an increase in the concentration of CH4 and C2H4 in the syngas, reaching a percentage of 29.9 vol.% CH4 and 78.2 vol.% C2H4, respectively, and, consequently, to an increase in the LHV of the gas to a maximum value of 6.4 MJ/Nm3 using 100% RDF. The cold gas efficiency was between 32.6 and 53.5% and the carbon conversion efficiency between 56.0 and 84.1%, seeing a slight increase in cold gas efficiency with the increasing weight percentage of RDF in the fuel mixture. The results were justified by the synergistic effect of co-gasification of RDF and pine biomass. The authors concluded that RDF is a promising feedstock for co-gasification with biomass [70]. According to Yang et al. (2021), the co-gasification process is considered more beneficial over the pure RDF gasification process in terms of exploiting the synergistic effects between RDF and biomass, minimal tar formation, improved process efficiency, and reduced pollutant emissions [32].
Among the limitations of MSW and derived-fuels (RDF and SRF) gasification are the difficulty in converting the feedstock that is quite heterogeneous, the difficulty to perform complete gasification of chars (from torrefaction, pyrolysis, or HTC) at low temperature (<700 °C), and the formation of tar, an undesirable by-product that adheres to the surfaces of equipment and pipes and can cause problems in downstream processes [85]. Therefore, studies regarding catalytic gasification of MSW, RDF, and SRF have been conducted to minimize tar formation, reduce activation energy, improve carbon conversion, and maximize the production of a given gas (e.g., H2). Alkali metal and alkaline-earth metal such as, K, Na, Ca, and Mg and transition metal (Fe) compounds are the most widely used catalysts in the gasification process because of their catalytic activity, availability, and low cost [86]. Lazzarotto et al. (2020) performed steam gasification of mixed plastic waste (28.77 wt.% polypropylene, 23.53 wt.% polystyrene, and 16.67 wt.% polyethylene) in a fluidized bed reactor at 800–900 °C in the presence of CaO. The presence of the CaO catalyst increased the dry gas and H2 yields, reaching maximum values of 3.12 Nm3/kgwaste and 104 mol/kgwaste at 900 °C, respectively [87]. The catalytic effect of adding natural minerals (lime, calcined dolomite, and olivine) in SRF gasification was evaluated by Pinto et al. (2014). The best results were obtained in the presence of dolomite, where higher gas yield and H2 and CO content, lower H2S content, and 46% reduction in tar content were obtained. This can be justified by the fact that dolomite generally exhibits a catalytic effect in promoting hydrocarbon destruction by cracking reactions, steam-reforming reactions, and CO2-reforming reactions [88].
The use of waste materials for catalyst development has also been investigated, as it promotes an environment-friendly and sustainable approach toward an economical catalyst development. Irfan et al. (2021) used waste marble powder (WMP) that has high proportion of Ca species to prepare catalysts. Catalysts of WMP, Ni-doped WMP, and Ni-doped WMP promoted by different transition metals (Fe, Cu, Co, and Zn) were developed, and their performances were evaluated for gasification of wet MSW (50 wt.% moisture). The results revealed that the addition of Ni-WMP catalyst greatly enhanced the dry gas yield (0.73 to 1.16 Nm3/kg), H2 concentration (212 to 509 mL/g), and carbon conversion efficiency (61.7% to 76.4%) and reduced the tar content (9.11 to 3.9 wt.%). In contrast to the Ni-WMP catalyst, the transition-metal-promoted catalysts showed higher catalytic activity toward H2 concentration (549–629 mL/g), dry gas yield (1.19–1.30 Nm3/kg), and lower tar content (3.45–2.93 wt%). The results revealed that the Co-promoted bimetallic catalyst performed better than Fe-, Cu-, and Zn-promoted catalysts in MSW gasification [89]. Overall, catalytic gasification of MSW and RDF can potentially become a widely used process on an industrial scale if the selected catalysts are cheap and efficient.
5.3. Waste Incineration vs. Waste Gasification
Albeit incineration being the most mature technology for energy recovery from waste, gasification has been described as the more attractive technology [78]. The potential advantages of gasification over incineration are associated with the production of a clean gas with a significant heating value, the higher electrical conversion efficiencies that can be achieved, and the lower contaminant emissions associated with gaseous emissions and solid wastes allowing compliance with current pollutant-emission standards [90,91]. A comprehensive strengths, weaknesses, opportunities, and threats (SWOT) analysis of waste incineration and waste gasification technologies is presented in Table 9 and Table 10.

Table 9.
SWOT analysis of waste incineration.

Table 10.
SWOT analysis of waste gasification.
Statistical data reported an increasing number of incineration plants. Europe alone had about 499 plants operating in 2019, which were responsible for the thermal treatment of 99,000,000 t of waste [97]. According to the European Technology and Innovation Platform Bioenergy, currently there are about 21 gasification plants in operation worldwide, 6 plants under construction, and 19 plants planned for construction [98]. Table 11 compares some technical aspects of these two thermochemical technologies, such as technology readiness level (TRL), associated costs, emissions, and some operational facilities.

Table 11.
Technology readiness level, costs, and available installations of gasification and incineration.
6. Conclusions
This paper assesses the current MSW generation, as well as the main destinations and the production of RDF focusing on the Portuguese case, and discusses the possible routes and the main challenges for the energetic valorization of RDF. A significant potential to produce RDF in Portugal can be identified in the share of MSW that is sent to landfills (57.6% in 2019 and higher values in the following years). Of the materials available in 2019, only 0.024% was processed into RDF. Among the reasons for the low RDF production, high moisture content that decreases RDF fuel properties is the most evident. The production of RDF contributes to a reduction in waste sent to landfills; as such, there is great potential to optimize environmental performance of waste management, changing the perception of waste from “waste for disposal” to “carrier of resources and energy”. The energy application of RDF is difficult, depending on various factors such as MSW composition, the production and quality of RDF, heating value, conversion technology, and associated gaseous emissions. The composition of RDF is quite heterogeneous, as it contains numerous materials such as plastic, paper/cardboard, wood, rubber, textiles, and some metals and fine contaminating particles that escape the sorting process, making its valorization a very complex process. Therefore, it is necessary to improve the quality of RDF as solid fuel through pretreatments such as sorting, griding, pelletizing, torrefaction, pyrolysis, leaching of inorganic compounds, and hydrothermal carbonization. These pretreatments allow greater RDF homogenization, reduced moisture, increased apparent and energy density, high hydrophobicity and friability, and reduced inorganic and chlorine contents. Of the available energy recovery technologies, incineration is the most mature and widely used; however, its environmental impact is higher when compared to the other technologies such as gasification. The incineration process is available on an industrial scale (TRL 9), while gasification availability is comparatively limited (TRL 6–8). Moreover, gasification of RDF is an expanding field with several research and development issues to be addressed. It is legitimate to prioritize improving the quality of RDF as a solid fuel and developing cheap and efficient catalysts to reduce tar formation, so that it can be fed into available gasifiers or other existing thermochemical energy converters without much modification.
Author Contributions
Conceptualization, S.M.S.; methodology, S.M.S. and C.N.; validation, S.M.S. and C.N.; formal analysis, C.N.; investigation, S.M.S.; writing—original draft preparation, S.M.S.; writing—review and editing, S.M.S., C.N., P.B. and M.G.; visualization, C.N.; supervision, C.N., P.B. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the FUNDAÇÃO PARA A CIÊNCIA E A TECNOLOGIA, I.P. (FCT), grant 2022.09990.BD, and also by the EUROPEAN REGIONAL DEVELOPMENT FUND, Project number 39838, the SI I&DT Projects in a co-promotion and research grant IPP/ProjetoAmbWTE/0001/2021, and by national funds through the FUNDAÇÃO PARA A CIÊNCIA E TECNOLOGIA, I.P. (FCT) from FCT/MCTES UIDB/04077/2020–2023 and UIDP/04077/2020–2023 (MEtRICs—Mechanical Engineering and Resource Sustainability Center), UIDB/05064/2020 (VALORIZA—Research Centre for Endogenous Resource Valorization).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oyedotun, T.D.T.; Moonsammy, S.; Oyedotun, T.D.; Nedd, G.A.; Lawrence, R.N. Evaluation of waste dynamics at the local level: The search for a new paradigm in national waste management. Environ. Chall. 2021, 4, 100130. [Google Scholar] [CrossRef]
- Directive 2018/851/UE, Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018. Off. J. Eur. Union 2018, 61, 109–140. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/?uri=CELEX%3A32018L0851 (accessed on 8 October 2021).
- Valorsul. Quanto Custa Depositar? 2021. Available online: https://www.valorsul.pt/pt/cliente/quanto-custa-depositar/ (accessed on 8 October 2021).
- Rezaei, H.; Panah, F.Y.; Lim, C.J.; Sokhansanj, S. Pelletization of Refuse-Derived Fuel with Varying Compositions of Plastic, Paper, Organic and Wood. Sustainability 2020, 12, 4645. [Google Scholar] [CrossRef]
- Rezaei, H.; Yazdanpanah, F.; Lim, C.J.; Sokhansanj, S. Pelletization properties of refuse-derived fuel—Effects of particle size and moisture content. Fuel Process. Technol. 2020, 205, 106437. [Google Scholar] [CrossRef]
- Alves, L.; Silva, S.; Soares, I. Waste management in insular areas: A Pay-As-You-Throw system in Funchal. Energy Rep. 2020, 6, 31–36. [Google Scholar] [CrossRef]
- Guerrero, L.A.; Maas, G.; Hogland, W. Solid waste management challenges for cities in developing countries. Waste Manag. 2013, 33, 220–232. [Google Scholar] [CrossRef]
- Sipra, A.T.; Gao, N.; Sarwar, H. Municipal solid waste (MSW) pyrolysis for bio-fuel production: A review of effects of MSW components and catalysts. Fuel Process. Technol. 2018, 175, 131–147. [Google Scholar] [CrossRef]
- Doña-Grimaldi, V.; Palma, A.; Montoya, M.R.; Morales, E.; Díaz, M. Energetic valorization of MSW compost valorization by selecting the maturity conditions. J. Environ. Manag. 2019, 238, 153–158. [Google Scholar] [CrossRef]
- Worldbank. Waste Composition. 16–21. Available online: worldbank.org (accessed on 8 October 2022).
- Kulkarni, B.N.; Anantharama, V. Repercussions of COVID-19 pandemic on municipal solid waste management: Challenges and opportunities. Sci. Total. Environ. 2020, 743, 140693. [Google Scholar] [CrossRef]
- APA. Relatório Anual Resíduos Urbanos 2020; Agência Portuguesa do Ambiente: Amadora, Portugal, 2021; Available online: https://apambiente.pt/sites/default/files/_Residuos/Producao_Gest%C3%A3o_Residuos/Dados%20RU/RARU%202018.pdf (accessed on 23 May 2022).
- Eurostat. Municipal Waste by Waste Management Operations. 2021. Available online: https://ec.europa.eu/eurostat/databrowser/view/ENV_WASMUN/default/table?lang=en (accessed on 8 September 2021).
- Eurostat. Management of Waste by Waste Management Operations and Type of Material. 2021. Available online: https://ec.europa.eu/eurostat/databrowser/view/ENV_WASSD__custom_1181470/default/table?lang=en (accessed on 8 October 2021).
- Arena, U.; Di Gregorio, F.; De Troia, G.; Saponaro, A. A techno-economic evaluation of a small-scale fluidized bed gasifier for solid recovered fuel. Fuel Process. Technol. 2015, 131, 69–77. [Google Scholar] [CrossRef]
- IEA Bioenergy. Trends in the Use of Solid Recovered Fuels. 2020. Available online: https://www.ieabioenergy.com/wp-content/uploads/2020/05/Trends-in-use-of-solid-recovered-fuels-Main-Report-Task36.pdf (accessed on 22 July 2022).
- Nobre, C. Thermochemical Upgrading of Refuse Derived Fuel; Faculdade de Ciências e Tecnologias da Universidade Nova de Lisboa: Caparica, Portugal, 2019; Available online: https://run.unl.pt/bitstream/10362/77043/1/Nobre_2019.pdf (accessed on 22 July 2022).
- Vaz, A.S.; Silva, F.; Bacalhau, R.; Mesquita, A.; Teixeira, C.A.; Borges, C.; Mil-Homens, F.; Pássaro, M. PERSU2020+ Reflexão Estratégica e Ajustamentos às Medidas do PERSU 2020. 2020, pp. 1–165. Available online: https://participa.pt/contents/consultationdocument/PERSU2020+.pdf (accessed on 22 July 2022).
- Kaur, A.; Bharti, R.; Sharma, R. Municipal solid waste as a source of energy. Mater. Today Proc. 2021, 81, 904–915. [Google Scholar] [CrossRef]
- Khosasaeng, T.; Suntivarakorn, R. Effect of Equivalence Ratio on an Efficiency of Single Throat Downdraft Gasifier Using RDF from Municipal solid waste. Energy Procedia 2017, 138, 784–788. [Google Scholar] [CrossRef]
- Montejo, C.; Tonini, D.; Márquez, M.C.; Astrup, T.F. Mechanical–biological treatment: Performance and potentials. An LCA of 8 MBT plants including waste characterization. J. Environ. Manag. 2013, 128, 661–673. [Google Scholar] [CrossRef]
- Robinson, T.; Bronson, B.; Gogolek, P.; Mehrani, P. Sample preparation for thermo-gravimetric determination and thermo-gravimetric characterization of refuse derived fuel. Waste Manag. 2016, 48, 265–274. [Google Scholar] [CrossRef]
- APA. Relatório Anual Resíduos Urbanos 2019. 2020. Available online: https://apambiente.pt/sites/default/files/_Residuos/Producao_Gest%C3%A3o_Residuos/Dados%20RU/RARU%202019.pdf (accessed on 2 August 2022).
- Veolia. Combustível Derivado de Resíduos. 2022. Available online: https://www.veolia.pt/solucoes/combustivel-derivado-de-residuos#no-back (accessed on 22 July 2022).
- Caputo, A.C.; Pelagagge, P.M. RDF production plants: I Design and costs. Appl. Therm. Eng. 2002, 22, 423–437. [Google Scholar] [CrossRef]
- BS EN ISO 21640:2021; Standards Publication Solid Recovered Fuels—Specifications and Classes. CEN: Geneva, Switzerland, 2021.
- CEN/TC 343; Solid Recovered Fuels. CEN—European Committee for Standardization: Brussels, Belgium, 2021. Available online: https://standards.iteh.ai/catalog/tc/cen/ea946eb8-b158-4ab3-87b3-83415b1f48a9/cen-tc-343 (accessed on 25 August 2022).
- ISO. When Waste Becomes Worthwhile: New Standards for Solid Recovered Fuels Just Published. Junho de 2021. 2021. Available online: https://www.iso.org/news/ref2690.html (accessed on 26 August 2022).
- Flamme, S.; Geiping, J. Quality standards and requirements for solid recovered fuels: A review. Waste Manag. Res. J. A Sustain. Circ. Econ. 2012, 30, 335–353. [Google Scholar] [CrossRef]
- Frankenhaeuser, M. European Standardisation of Solid Recovered Fuels; VTT Symposium (Valtion Teknillinen Tutkimuskeskus): Dublin, Ireland, 2011; pp. 283–285. [Google Scholar]
- ERFO. International Workshop on Solid Recovered Fuel: SRF Market Views in Europe. 2010. Available online: https://www.yumpu.com/en/document/read/5066076/international-workshop-on-solid-recovered-fuel-helsinki-31-erfo (accessed on 29 July 2022).
- Yang, Y.; Liew, R.K.; Tamothran, A.M.; Foong, S.Y.; Yek, P.N.Y.; Chia, P.W.; Van Tran, T.; Peng, W.; Lam, S.S. Gasification of refuse-derived fuel from municipal solid waste for energy production: A review. Environ. Chem. Lett. 2021, 19, 2127–2140. [Google Scholar] [CrossRef]
- Nobre, C.; Alves, O.; Longo, A.; Vilarinho, C.; Gonçalves, M. Torrefaction and carbonization of refuse derived fuel: Char characterization and evaluation of gaseous and liquid emissions. Bioresour. Technol. 2019, 285, 121325. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Dziok, T.; Magdziarz, A.; Nowak, W. Composition and properties of fly ash collected from a multifuel fluidized bed boiler co-firing refuse derived fuel (RDF) and hard coal. Energy 2021, 234, 121229. [Google Scholar] [CrossRef]
- Białowiec, A.; Pulka, J.; Stępień, P.; Manczarski, P.; Gołaszewski, J. The RDF/SRF torrefaction: An effect of temperature on characterization of the product—Carbonized Refuse Derived Fuel. Waste Manag. 2017, 70, 91–100. [Google Scholar] [CrossRef]
- Manyà, J.J.; García-Ceballos, F.; Azuara, M.; Latorre, N.; Royo, C. Pyrolysis and char reactivity of a poor-quality refuse-derived fuel (RDF) from municipal solid waste. Fuel Process. Technol. 2015, 140, 276–284. [Google Scholar] [CrossRef]
- Alves, O.; Nobre, C.; Durão, L.; Monteiro, E.; Brito, P.; Gonçalves, M. Effects of dry and hydrothermal carbonisation on the properties of solid recovered fuels from construction and municipal solid wastes. Energy Convers. Manag. 2021, 237, 114101. [Google Scholar] [CrossRef]
- Chavando, J.A.M.; Silva, V.B.; Tarelho, L.A.; Cardoso, J.S.; Eusébio, D. Snapshot review of refuse-derived fuels. Util. Policy 2022, 74, 101316. [Google Scholar] [CrossRef]
- Nobre, C.; Vilarinho, C.; Alves, O.; Mendes, B.; Gonçalves, M. Upgrading of refuse derived fuel through torrefaction and carbonization: Evaluation of RDF char fuel properties. Energy 2019, 181, 66–76. [Google Scholar] [CrossRef]
- Azam, M.; Jahromy, S.S.; Raza, W.; Raza, N.; Lee, S.S.; Kim, K.-H.; Winter, F. Status, characterization, and potential utilization of municipal solid waste as renewable energy source: Lahore case study in Pakistan. Environ. Int. 2020, 134, 105291. [Google Scholar] [CrossRef]
- Jewiarz, M.; Mudryk, K.; Wróbel, M.; Frączek, J.; Dziedzic, K. Parameters Affecting RDF-Based Pellet Quality. Energies 2020, 13, 910. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Sharma, B.; Goswami, Y.; Sharma, S.; Shekhar, S. Inherent roadmap of conversion of plastic waste into energy and its life cycle assessment: A frontrunner compendium. Renew. Sustain. Energy Rev. 2021, 146, 111070. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Kurniawan, W.; Aziz, M. Technological review on thermochemical conversion of COVID-19-related medical wastes. Resour. Conserv. Recycl. 2021, 167, 105429. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Aboughaly, M.; Ayoub, N. Comparative study of MSW heat treatment processes and electricity generation. J. Energy Inst. 2018, 91, 481–488. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Y.; Kobayashi, N.; Zhao, D.; Xing, S. Study of Fuel Properties of Torrefied Municipal Solid Waste. Energy Fuels 2015, 29, 4976–4980. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.-W.; Zhang, X. Pyrolysis Analysis of RDF by TG-FTIR Techniques. Environ. Sci. Technol. 2008, 31, 29–32. [Google Scholar]
- Veses, A.; Sanahuja-Parejo, O.; Callén, M.S.; Murillo, R.; García, T. A combined two-stage process of pyrolysis and catalytic cracking of municipal solid waste for the production of syngas and solid refuse-derived fuels. Waste Manag. 2020, 101, 171–179. [Google Scholar] [CrossRef]
- Whyte, H.E.; Loubar, K.; Awad, S.; Tazerout, M. Pyrolytic oil production by catalytic pyrolysis of refuse-derived fuels: Investigation of low cost catalysts. Fuel Process. Technol. 2015, 140, 32–38. [Google Scholar] [CrossRef]
- Gandidi, I.M.; Susila, M.D.; Mustofa, A.; Pambudi, N.A. Thermal—Catalytic cracking of real MSW into Bio-Crude Oil. J. Energy Inst. 2018, 91, 304–310. [Google Scholar] [CrossRef]
- Neuwahl, F.; Cusano, G.; Benavides, J.G.; Holbrook, S.; Serge, R. Best Available Techniques (BAT) Reference Document for Waste Treatment Industries; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Yan, W.; Perez, S.; Sheng, K. Upgrading fuel quality of moso bamboo via low temperature thermochemical treatments: Dry torrefaction and hydrothermal carbonization. Fuel 2017, 196, 473–480. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, C.; Qin, S.; Wang, B.; Zhao, P.; Cui, X. Effects of process water recirculation on yields and quality of hydrochar from hydrothermal carbonization process of rice husk. J. Anal. Appl. Pyrolysis 2022, 166, 105618. [Google Scholar] [CrossRef]
- Motavaf, B.; Dean, R.A.; Nicolas, J.; Savage, P.E. Hydrothermal carbonization of simulated food waste for recovery of fatty acids and nutrients. Bioresour. Technol. 2021, 341, 125872. [Google Scholar] [CrossRef]
- Shan, G.; Li, W.; Bao, S.; Liu, J.; Zhu, L.; Tan, W. Evaluating the aqueous phase obtained from hydrothermal carbonization of municipal sludge as possible liquid fertilizer for plant growth: An analysis of heavy metals and their molecular composition. J. Clean. Prod. 2023, 404, 136989. [Google Scholar] [CrossRef]
- Wilk, M.; Czerwińska, K.; Śliz, M.; Imbierowicz, M. Hydrothermal carbonization of sewage sludge: Hydrochar properties and processing water treatment by distillation and wet oxidation. Energy Rep. 2023, 9, 39–58. [Google Scholar] [CrossRef]
- González-Arias, J.; de la Rubia, M.; Sánchez, M.; Gómez, X.; Cara-Jiménez, J.; Martínez, E. Treatment of hydrothermal carbonization process water by electrochemical oxidation: Assessment of process performance. Environ. Res. 2023, 216, 114773. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.; Alves, O.; Durão, L.; Şen, A.; Vilarinho, C.; Gonçalves, M. Characterization of hydrochar and process water from the hydrothermal carbonization of Refuse Derived Fuel. Waste Manag. 2021, 120, 303–313. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Reddy, V.M.; Nagarajan, R. Ultrasonic coal washing to leach alkali elements from coals. Ultrason. Sonochem. 2015, 27, 235–240. [Google Scholar] [CrossRef]
- Lee, J.; Ghiasi, B.; Lau, A.; Sokhansanj, S. Chlorine and ash removal from salt-laden woody biomass by washing and pressing. Biomass- Bioenergy 2021, 155, 106272. [Google Scholar] [CrossRef]
- Peng, B.; Li, X.; Zhao, W.; Yang, L. Study on the release characteristics of chlorine in coal gangue under leaching conditions of different pH values. Fuel 2018, 217, 427–433. [Google Scholar] [CrossRef]
- Rajendran, N.; Gurunathan, B.; Han, J.; Krishna, S.; Ananth, A.; Venugopal, K.; Priyanka, R.S. Recent advances in valorization of organic municipal waste into energy using biorefinery approach, environment and economic analysis. Bioresour. Technol. 2021, 337, 125498. [Google Scholar] [CrossRef]
- Goli, V.S.N.S.; Singh, D.N.; Baser, T. A critical review on thermal treatment technologies of combustible fractions from mechanical biological treatment plants. J. Environ. Chem. Eng. 2021, 9, 105643. [Google Scholar] [CrossRef]
- Istrate, I.-R.; Medina-Martos, E.; Galvez-Martos, J.-L.; Dufour, J. Assessment of the energy recovery potential of municipal solid waste under future scenarios. Appl. Energy 2021, 293, 116915. [Google Scholar] [CrossRef]
- Cullen, J.M.; Allwood, J.M. The efficient use of energy: Tracing the global flow of energy from fuel to service. Energy Policy 2010, 38, 75–81. [Google Scholar] [CrossRef]
- Kirkels, A.F.; Verbong, G.P. Biomass gasification: Still promising? A 30-year global overview. Renew. Sustain. Energy Rev. 2011, 15, 471–481. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Du, M.A.; Huang, I.-T.; Liu, I.-H.; Chang, E.-E.; Chiang, P.-C. Strategies on implementation of waste-to-energy (WTE) supply chain for circular economy system: A review. J. Clean. Prod. 2015, 108, 409–421. [Google Scholar] [CrossRef]
- Bosmans, A.; Helsen, L. Energy From Waste: Review of Thermochemical Technologies for Refuse Derived Fuel (RDF) Treatment. In Proceedings of the Third International Symposium on Energy from Biomass and Waste, Venice, Italy, 8–11 November 2010; pp. 8–11. [Google Scholar]
- Pio, D.; Tarelho, L.; Tavares, A.; Matos, M.; Silva, V. Co-gasification of refused derived fuel and biomass in a pilot-scale bubbling fluidized bed reactor. Energy Convers. Manag. 2020, 206, 112476. [Google Scholar] [CrossRef]
- Giraud, R.J.; Taylor, P.H.; Huang, C.-P. Combustion operating conditions for municipal Waste-to-Energy facilities in the U.S. Waste Manag. 2021, 132, 124–132. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Chi, Y.; Weiss-Hortala, E.; Ni, M.; Zhou, Z. Comparison of waste-to-energy technologies of gasification and incineration using life cycle assessment: Case studies in Finland, France and China. J. Clean. Prod. 2018, 203, 287–300. [Google Scholar] [CrossRef]
- Brożek, P.; Złoczowska, E.; Staude, M.; Baszak, K.; Sosnowski, M.; Bryll, K. Study of the Combustion Process for Two Refuse-Derived Fuel (RDF) Streams Using Statistical Methods and Heat Recovery Simulation. Energies 2022, 15, 9560. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Chi, Y.; Weiss-Hortala, E.; Ni, M. Life cycle assessment of pyrolysis, gasification and incineration waste-to-energy technologies: Theoretical analysis and case study of commercial plants. Sci. Total. Environ. 2018, 626, 744–753. [Google Scholar] [CrossRef]
- Safar, M.; Lin, B.-J.; Chen, W.-H.; Langauer, D.; Chang, J.-S.; Raclavska, H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Energy 2018, 235, 346–355. [Google Scholar] [CrossRef]
- Widjaya, E.R.; Chen, G.; Bowtell, L.; Hills, C. Gasification of non-woody biomass: A literature review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef]
- Hameed, Z.; Aslam, M.; Khan, Z.; Maqsood, K.; Atabani, A.; Ghauri, M.; Khurram, M.S.; Rehan, M.; Nizami, A.-S. Gasification of municipal solid waste blends with biomass for energy production and resources recovery: Current status, hybrid technologies and innovative prospects. Renew. Sustain. Energy Rev. 2020, 136, 110375. [Google Scholar] [CrossRef]
- Safar, K.M.; Bux, M.R.; Faria, U.; Pervez, S. Integrated model of municipal solid waste management for energy recovery in Pakistan. Energy 2021, 219, 119632. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Alam, T.; Krishna, B.B.; Bhaskar, T.; Perkins, G. A review on the production of renewable aviation fuels from the gasification of biomass and residual wastes. Bioresour. Technol. 2020, 312, 123596. [Google Scholar] [CrossRef]
- Na, J.I.; Park, S.J.; Kim, Y.K.; Lee, J.G.; Kim, J.H. Characteristics of oxygen-blown gasification for combustible waste in a fixed-bed gasifier. Appl. Energy 2003, 75, 275–285. [Google Scholar] [CrossRef]
- Chanthakett, A.; Arif, M.; Khan, M.; Oo, A.M. Performance assessment of gasification reactors for sustainable management of municipal solid waste. J. Environ. Manag. 2021, 291, 112661. [Google Scholar] [CrossRef] [PubMed]
- Rajca, P. Innovative Technologies in the Development of Alternative RDF Fuel. Eng. Prot. Environ. 2018, 21, 251–260. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, J.; Zhao, P.; Wang, Z.; Yang, M.; Cui, X.; Tian, H.; Guo, Q. Gasification of real MSW-derived hydrochar under various atmosphere and temperature. Thermochim. Acta 2020, 683, 178470. [Google Scholar] [CrossRef]
- Haydary, J.; Šuhaj, P.; Šoral, M. Semi-Batch Gasification of Refuse-Derived Fuel (RDF). Processes 2021, 9, 343. [Google Scholar] [CrossRef]
- Ge, Z.; Guo, L.; Jin, H. Catalytic supercritical water gasification mechanism of coal. Int. J. Hydrogen Energy 2020, 45, 9504–9511. [Google Scholar] [CrossRef]
- Xu, B.; Cao, Q.; Kuang, D.; Gasem, K.A.; Adidharma, H.; Ding, D.; Fan, M. Kinetics and mechanism of CO2 gasification of coal catalyzed by Na2CO3, FeCO3 and Na2CO3–FeCO3. J. Energy Inst. 2020, 93, 922–933. [Google Scholar] [CrossRef]
- Lazzarotto, I.; Ferreira, S.; Junges, J.; Bassanesi, G.; Manera, C.; Perondi, D.; Godinho, M. The role of CaO in the steam gasification of plastic wastes recovered from the municipal solid waste in a fluidized bed reactor. Process. Saf. Environ. Prot. 2020, 140, 60–67. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Carolino, C.; Miranda, M.; Abelha, P.; Direito, D.; Perdikaris, N.; Boukis, I. Gasification improvement of a poor quality solid recovered fuel (SRF). Effect of using natural minerals and biomass wastes blends. Fuel 2014, 117, 1034–1044. [Google Scholar] [CrossRef]
- Irfan, M.; Li, A.; Zhang, L.; Ji, G.; Gao, Y.; Khushk, S. Hydrogen-rich syngas from wet municipal solid waste gasification using Ni/Waste marble powder catalyst promoted by transition metals. Waste Manag. 2021, 132, 96–104. [Google Scholar] [CrossRef]
- Cardoso, J.; Silva, V.; Eusébio, D. Techno-economic analysis of a biomass gasification power plant dealing with forestry residues blends for electricity production in Portugal. J. Clean. Prod. 2019, 212, 741–753. [Google Scholar] [CrossRef]
- Couto, N.D.; Silva, V.B.; Rouboa, A. Thermodynamic Evaluation of Portuguese municipal solid waste gasification. J. Clean. Prod. 2016, 139, 622–635. [Google Scholar] [CrossRef]
- Foster, W.; Azimov, U.; Gauthier-Maradei, P.; Molano, L.C.; Combrinck, M.; Munoz, J.; Esteves, J.J.; Patino, L. Waste-to-energy conversion technologies in the UK: Processes and barriers—A review. Renew. Sustain. Energy Rev. 2020, 135, 110226. [Google Scholar] [CrossRef]
- Arafat, H.A.; Jijakli, K. Modeling and comparative assessment of municipal solid waste gasification for energy production. Waste Manag. 2013, 33, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Liu, Y.; Xia, B.; Jiang, X.; Skitmore, M. Overview of public-private partnerships in the waste-to-energy incineration industry in China: Status, opportunities, and challenges. Energy Strat. Rev. 2020, 32, 100584. [Google Scholar] [CrossRef]
- Directive (UE) 2018/850, Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018 Amending Directive 1999/31/EC on the Landfill of Waste. 2018. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/?uri=CELEX%3A32018L0850. (accessed on 28 February 2022).
- Solis, M.; Silveira, S. Technologies for chemical recycling of household plastics—A technical review and TRL assessment. Waste Manag. 2020, 105, 128–138. [Google Scholar] [CrossRef]
- CEWEP. Waste-to-Energy Plants in Europe in 2019; Confederation of European Waste-to-Energy Plants: Düsseldorf, Germany, 2019; Available online: https://www.cewep.eu/waste-to-energy-plants-in-europe-in-2019/ (accessed on 22 July 2022).
- ETIP Bioenergy. Production Facilities. European Technology and Innovation Platform Bioenergy. 2022. Available online: https://www.etipbioenergy.eu/databases/production-facilities (accessed on 22 July 2022).
- GOV.UK. Task 2 Report: Review of Current Status of Advanced Gasification Technologies. 2021. Available online: https://www.gov.uk/government/publications/advanced-gasification-technologies-review-and-benchmarking (accessed on 22 July 2022).
- Aracil, C.; Haro, P.; Fuentes-Cano, D.; Gómez-Barea, A. Implementation of waste-to-energy options in landfill-dominated countries: Economic evaluation and GHG impact. Waste Manag. 2018, 76, 443–456. [Google Scholar] [CrossRef]
- WtERT. Silla 2 Waste-to-Energy Plant, Milano, Italy. WtERT Germany GmbH. 2022. Available online: https://www.wtert.net/bestpractice/8/Silla-2-Waste-to-Energy-Plant-Milano-Italy.html (accessed on 22 July 2022).
- SIERRA ENERGY. FastOx® Gasification is the Future. Available online: https://sierraenergy.com/ (accessed on 14 July 2022).
- Kauno Kogeneracinė Jėgainė. About the Power Plant Kauno CHP. 2018. Available online: https://kkj.lt/apie-mus/apie-jegaine/16 (accessed on 22 July 2022).
- Power Technology. Amager Bakke Waste-to-Energy Plant. 2022. Available online: https://www.power-technology.com/projects/amager-bakke-waste-energy-plant/ (accessed on 2 May 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).