Abstract
Agricultural waste negatively impacts the environment and generates economic difficulties for agro-industrial companies and farmers. As a result, it is necessary for an eco-friendly and sustainable alternative to managing this type of waste. Therefore, the research aimed to investigate lettuce waste as an alternative substrate to generate bioelectricity in single-chamber microbial fuel cells (scMFCs). It was possible to report voltage and electric current peaks of 0.959 ± 0.026 V and 5.697 ± 0.065 mA on the fourteenth day, values that were attained with an optimum pH of 7.867 ± 0.147 and with an electrical conductivity of 118.964 ± 8.888 mS/cm. Moreover, as time passed the values began to decline slowly. The calculated value of maximum power density was 378.145 ± 5.417 mW/cm2 whose current density was 5.965 A/cm2, while the internal resistance reported using Ohm’s Law was 87.594 ± 6.226 Ω. Finally, it was possible to identify the Stenotrophomonas maltophilia bacterium (99.59%) on a molecular scale, as one of the microorganisms present in the anodic biofilm. The three microbial fuel cells were connected in series and demonstrated that they were capable of lighting an LED bulb, with a voltage of 2.18 V.
1. Introduction
The consumption of food is of vital importance for human beings and ecosystems and can occur in processed products (with added value) or raw (vegetables) [1]. However, the world population is experiencing exponential growth, and it is estimated that by 2050 it will reach 9.7 billion inhabitants [2]. This increase in population will lead to a great demand for food and, in turn, will generate a large amount of food or organic waste, which requires good management and disposal [1,3,4]; however, this large amount of waste can pollute the environment and is susceptible to anaerobic degradation, releasing greenhouse gases (methane and CO2), which contribute to global warming [5,6].
In 2018, Rethink Food Waste Through Economics (ReFED) reported approximately 1.3 billion tons of waste from improperly managed food at each stage of its process [7]. These stages range from the post-harvest stage by farmers or companies dedicated to food harvests to the waste generated in homes, commercial establishments, and restaurants [8,9]. One of the products that must have optimal conditions for their durability are vegetables and fruits. According to the report of the Food and Agriculture Organization (FAO), the production in 2019 was 314.5 million tons [10], approximately 13% more than in the year 2018 (FAOSTAT-FAO statistical database, 2019); China, India, the United States, Turkey, Russia, Vietnam, and Mexico produced more than 67% of the world’s horticultural products [11,12].
Among the great variety of vegetables, lettuce is widely consumed in people’s salads. In addition, it contains a large number of vitamins (A, C, E, and K), antioxidant compounds, and polyphenols. Recent studies have demonstrated the beneficial effects of lettuce in the prevention of cardiovascular diseases [13,14,15]. Similarly, being a fast-producing vegetable (compared to other vegetables), it is economically attractive for entrepreneurs, with the United States, Europe, and China being the countries with the highest production [16]. Owing to the high production of this vegetable, large amounts of lettuce waste are also generated worldwide, becoming a major problem for society and businessmen [17].
This problem related to the generation of organic waste has led to the search for new technologies that allow the reuse of organic waste. There is a promising alternative to microbial combustion cells (MFCs). The MFCs are low-cost bioelectrochemical devices that directly convert organic substrates (anolyte) into electrical energy because electrons are generated in oxidation reactions by the metabolic activities of the electrogenic microorganisms present in the anolyte. Additionally, MFCs have good versatility as they use a wide range of substrates as organic waste [18,19,20,21,22,23].
Vegetable waste has been investigated as a substrate for bioelectricity production by MFCs since it is a lignocellulolytic biomass, which represents a source of carbon and energy for electrochemically active bacteria (electrogenic). These bacteria can transmit electrons to the anode surfaces through different mechanisms, such as direct electron transfer through conductive pili (nanowires) or electron transfer mediated by naturally released redox mediators (flavins and pyocyanin) [19].
There are a wide variety of designs, however, single-chamber MFCs are the most economical and versatile for manufacturing, and they have also been shown to generate large values of electric current with some substrates [23,24]. Javed et al. [22] fabricated MFCs with graphite electrodes, using vegetable and kitchen waste as a substrate, and managed to generate voltage peaks and power densities of approximately 500 mV and 104,400 mW/m2, respectively, with a resistance of 500 Ω. Likewise, Vera Natalia Ginting et al. (2019) used vegetable mustard waste in their microbial fuel cells, using graphite as electrodes to generate electrical current and voltage peaks of 20.1 mA and 72.13 mV [25]. Kalagbor et al. (2020) managed to generate peak voltage and electric current of 3.7 V and 3.2 A, respectively, using a mixture of tomato and banana waste (20 Kg) as a substrate in their single-chamber microbial fuel cells [26]. In the literature review, it was observed that metallic electrodes are currently being used to improve electrical conductivity and obtain higher values of electrical current, and the identification of electrogenic microorganisms is of vital importance in explaining the possible sources of electrons in the process of electric power generation [27,28,29].
The main objective of this study was to determine the potential of yeast waste as an alternative substrate for bioelectricity generation in single-chamber fuel cells using zinc- and copper-based electrodes. The physicochemical parameters of voltage, electric current, electrical conductivity, and pH were monitored for this. The power density (DP), current density (DC), and internal resistance of the cells are calculated. On the other hand, the FTIR (fundamental Fourier transform infrared spectroscopy) spectra of the substrate were obtained. Finally, the initial and final micrographs were obtained by Scanning Electron Microscopy (SEM) of the anodic electrode, as well as the molecularly identified microorganisms adhered to the electrode. This research will provide great opportunities to farmers and companies dedicated to the planting, harvesting, and sale of this vegetable because they will be able to use their waste for the generation of electrical energy, which will reduce energy costs and can be used in places far from cities. However, transferring this technology to farmers in places where illiteracy exists can be challenging. On the other hand, it provides knowledge in the field of bioelectrogenesis.
2. Materials and Methods
2.1. Design and Construction of scMFCs
Three single-chamber microbial fuel cells (scMFCs) were built. The chambers were made of polyvinyl chloride, to which a 10 × 20 cm rectangular cut was made on one side where the zinc (Zn) cathodic electrode was placed, and the copper (Cu) anodic electrode was placed on the left side, inside of the chamber. The electrodes had 145 and 200 cm2 areas for Cu and Zn, respectively. For the external circuit, a copper wire with a thickness of 6 mm and a resistance of 100 Ω was used, and Nafion 117 (Merck, Tampa, FL, USA) was used as the proton exchange membrane (Figure 1).
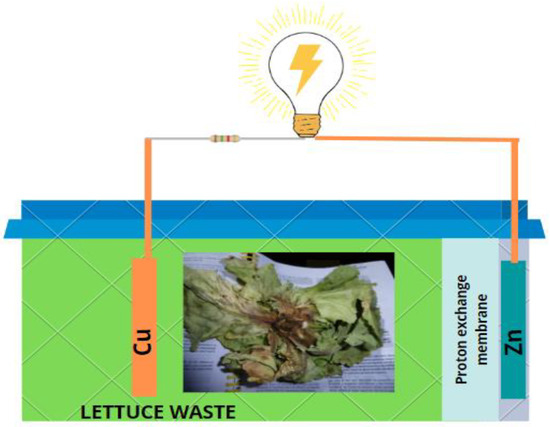
Figure 1.
Schematic layout of a single-chamber microbial fuel cell (scMFC).
2.2. Samples of Lettuce Waste
Lettuce waste was collected from the Palermo Ex Mayorista market, Trujillo, Peru. A total of 3.5 kg was collected and placed in Ziploc hermetic bags for transfer to the laboratory, where it was washed several times to eliminate any contaminants that adhered from the environment. The waste was liquefied for 20 min (Labtron, LDO-B10, Royal Oak, MI, USA) to obtain approximately 720 mL.
2.3. Characterization of Microbial Fuel Cells
The current and voltage values were monitored for 30 days using a multimeter (Prasek Premium PR-85, MI, USA). The voltage and current values are presented with the mean ± standard deviation. The power and current densities (mean ± standard) were measured using the method described by Rojas-Flores et al. (2023), where the external resistances were 10 ± 0.15, 35 ± 3.5, 60 ± 4.8, 100 ± 6.2, 300 ± 6.2, 390 ± 7.2, 560 ± 10, 680 ± 14.5, 840 ± 16.5, 1000 ± 24.6 Ω [30]. The internal resistance was determined using an energy sensor (Vernier- ± 30 V, ± 1000 mA, USA) and Ohm’s law (V = R × I). Likewise, the pH and electrical conductivity values were monitored with a pH meter (110 Series Oakton, MI, USA) and a conductivity meter (CD-4301, MI, USA) during 30 days of operation.
2.4. Isolation of Anodic Microbes
Isolation of the microorganisms involved in the bioelectrogenesis (or electrogenic) of MFC was possible using conventional microbiology techniques. Subsequently, the copper plates were removed from the MFC after physicochemical parameters were measured. A swab was used to collect samples from the anode plate with evidence of microbial growth (biofilm formation). The swab with the sample was streaked on brain-heart infusion agar (BHI agar) (Merck) contained in a Petri dish and incubated at 35 ± 2 °C for 24 h. After incubation, colony growth was observed. Possible colonies were sown in a selective medium, McConkey Agar (Merck). Microscopic characteristics were observed using Gram staining. Finally, axenic cultures were prepared on slanted nutrient agar for subsequent identification.
2.5. Molecular Identification
For molecular identification, the axenic culture of the isolated bacteria was sent to the EcoBiotech LAB SAC laboratory (Trujillo, Peru). The cetyltrimethylammonium bromide (CTAB) buffer was used for the DNA extraction of the isolated bacterium. Once the DNA was extracted, multiple copies of the specific segment of DNA were obtained using the Polymerase Chain Reaction (PCR) technique. Universal bacterial primers were employed (27f/1492r; 5′-AGAGTTTGATCCTGGCTCAG-3′/5′-CTACGGCTACCTTGTTACGA-3′). Identification was performed using the 16S rRNA gene and the Sanger sequencing method. The sequences obtained were analyzed and aligned in Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 15 April 2023) to obtain the isolated bacterium’s identity percentage.
2.6. Statistics Analysis
For the analysis of the data for the continuous variables (voltage, current), Pearson’s linear regression was used, employing the equation of the line where the values of electrical resistance were cleared. The data fit of the regression model was evaluated using the coefficient of determination (R2). In addition, to compare the results of this study with those obtained by other authors, the 95% confidence intervals were calculated using an alpha value of 0.05, for the three variables studied (voltage, current, DP), and then comparisons made in pairs. Likewise, from the data of the size of the group (n), standard deviation (SD), and average provided by some authors, it was possible to calculate the size of the effect using Cohen’s D, thus determining whether the results obtained when using lettuce waste were superior (greater efficiency) compared to those obtained experimentally when using other types of residues.
3. Results and Analysis
3.1. Electrical Parameters Measurement
Figure 2a shows the voltage values shown during monitoring. The values from the first day (0.348 ± 0.005 V) increased until day 14 (0.959 ± 0.026 V) and then fell to day 30 (0.589 ± 0.023 V). The increase in voltage values is due to the reduction and oxidation processes that occur in the anodic and cathodic chambers, generating a potential differential and an increase in these types of reactions. As the reactions decrease, the potential differential also decreases. This decrease in these types of reactions can be due to various causes, temperature variation, pH, organic matter, etc. [31,32,33]. Dziegielowski et al. (2023) reported peak voltage values of 550 mV in single-chamber microbial fuel cells using graphite electrodes and agricultural soil waste as fuel, mentioning that the oxygenation of the substrate allows the increase in potential up to certain limits [34]. Similarly, Abazarian et al. (2023) generated voltage peaks of 283.7 ± 2.8 mV in their fuel cells using fishpond sediment as fuel, mentioning that substrates with high carbon and glucose contents enhance the performance of MFCs [35]. Bhattacharya et al. (2023) used a mixture of cucumber, banana, and carrot waste as fuel, managing to generate 480 ± 20 mV voltage peaks in single-chamber fuel cells, mentioning that the potential drop is due to the depletion of the substrate used, leading to a decrease in the metabolism of bacteria [36]. The values of the electric current are shown in Figure 2b, which increased from the first day (0.495 ± 0.004 mA), with a maximum value (5.697 ± 0.065 mA) on day 14, and then decreased until day 30 (1.556 ± 0.083 mA). The increases in the values of the electric current have been reported to be due to the generation of electrons in the fermentation process of the substrate and the good adhesion of the microorganisms for the formation of the biofilm. A key point in the process is the metallic character of the electrodes used because it allows a better transition of electrons from the anodic to the cathodic chamber. The decrease is due to the decrease of the substrate volume in the last days; as well as to the corrosion observed in the last days of monitoring. Din et al. (2023) in their research mentioned that the bacteria that are generated within fuel cells produce a greater amount of electrical current when they are under natural environmental conditions because they carry out their metabolism better, which generates better growth and good formation of biofilms [37]. Likewise, Abubakar et al. (2023) mentioned in their research that the absence of exoelectrogenic microorganisms and other types of bacteria with high organic loads could lead to a decrease in the efficiency of MFCs, and the absence of these types of microorganisms is due to a poor choice of pH, which will operate the MFCs [38].
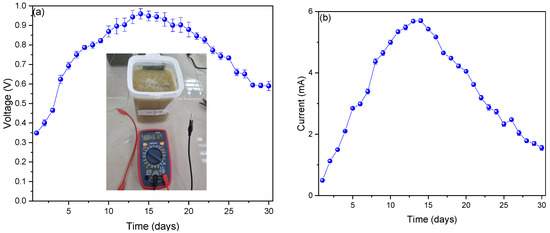
Figure 2.
Monitoring values of (a) voltage and (b) electric current of microbial fuel cells.
The monitored pH values are shown in Figure 3a, where it can be seen that the values vary from slightly neutral to slightly acidic, showing an optimal operating pH on day 14 of 7.867 ± 0.147. It has been reported that the pH values of substrates such as vegetables or fruits vary due to fermentation, that each type of substrate will have a different pH, and that environmental conditions are a determining factor for this [39,40]. For this reason, the optimal operating pH of each substrate must be found, so that when this value is found, it can be adjusted in future work and the efficiency of each microbial fuel cell can be optimized [41]. Zonfa et al. (2023) used cheese whey as a substrate in their microbial fuel cells, whose operating pH was 3.6 ± 0.1, managing to generate current peaks of 1.8 mA [42]. Likewise, the use of onion waste has been reported in single-chamber microbial fuel cells, whose optimum operating pH was 6.968 ± 0.286, managing to generate voltage peaks and electric current of 0.991 ± 0.02 V and 4.459 ± 0.0608 mA, respectively [43]. The values of the electrical conductivity of the substrate are shown in Figure 3b), whose behavior increased from day 1 (57,894 ± 1732 mS/cm) to day 15 (118,964 ± 8888 mS/cm), and then decreased slowly until day 30 (55,908 ± 4509 mS/cm). Previous investigations have shown that the increase in electrical conductivity is due to the decrease in the internal resistance of fuel cells, and this decrease could be due in the first days to the oxidation and reduction processes that occurred in the anodic and cathodic chambers, which generates bioelectricity. On the other hand, the decrease in electrical conductivity values may be due to the increase in resistance in recent days due to precipitation and sedimentation of the organic matter of the substrate during the fermentation process [44,45].
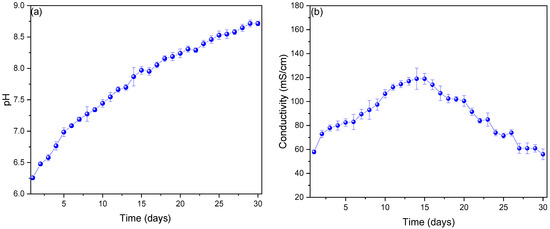
Figure 3.
Monitoring of (a) pH and (b) conductivity values of microbial fuel cells.
Figure 4a shows the graphs that obey Ohm’s Law. Based on the formula for Ohm’s Law, it can be established that the voltage is directly proportional to the current so a linear function can be made (y = mx + b), and from the slope (m) the resistance (Ω) of the MFC can be calculated [46,47,48]. In the same way, the coefficient of determination (R2) was 0.99, which indicates the good fit of the regression model. Calculated Internal Resistance (Rint) was measured on the fourteenth day because this was the day in which the highest values of current and voltage were found, giving a value of Rint of 87,594 ± 6226 Ω, which is relatively low compared with other investigations. Al Lawati et al. (2019) mentioned in their research that the values of the internal resistance can vary depending on the area of the electrodes and the separation between them, as well as the microorganisms present in the substrates used [49]. Similarly, Tremouli et al. (2019) reported that a low internal resistance of MFCs increased anodic reaction rates, owing to the possibility of direct electron transfer and microbial consortia operating in the same MFC configuration, directly affecting cell performance for microbial fuel [50]. However, the increase in the internal resistance of MFCs may originate from poor biofilm formation on the bioanode surface, resulting in a low electron transfer rate at the bioanode electrolyte interface, which may allow a slower biocatalytic activity of the bioanode and a lower number of electrons [51]. In this sense, Din et al. (2020) reported that their fuel cells showed a resistance of 90 Ω, managing to generate peaks of 1.12 V and 8.8 mA using potato waste as a substrate and working at an optimal pH of 7.28 (during the entire investigation), mentioning that the low resistance optimized the efficiency of MFCs [52]. Likewise, an internal resistance value of 75,581 ± 5892 Ω has also been reported in single-chamber MFCs using coriander waste, where they mentioned that this low internal resistance and high values of electric current were due to the good formation of the electrode biofilm anodic [53]. Figure 4b shows the power density values as a function of the current density, where a maximum power density of 378.145 ± 5.417 mW/cm2 was observed at a current density of 5.965 A/cm2 with a peak voltage at 917,444 ± 18,194 mV, which are higher than those reported in the literature. For example, Wang et al. (2023) generated power density peaks of 24.75 mW/m2 at a current density of 17.48 mA/cm2 using river sediment and carbon felt electrodes as a substrate, where they mentioned that the power density values can be improved by embedding metallic nanoparticles to improve conductivity in electron transfer [54]. Likewise, Hirose et al. (2023) made electrodes from the vegetable sponge, which were used in microbial fuel cells with a single chamber, managing to generate power density peaks of 0.3 µW/cm2, mentioning that the porosity in the electrodes increases the possibility of adhesion of microorganisms, leading to good biofilm formation [55]. Fadhil et al. (2023) generated a maximum power density of 543.28 mW/m2 using photosynthetic algae as a substrate, where they observed a dense layer of biofilm after 120 d of operation [56].
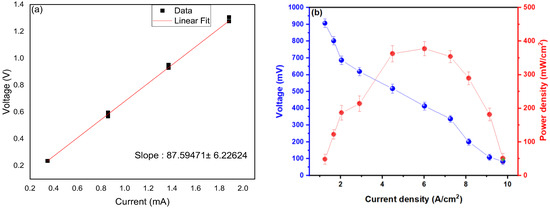
Figure 4.
Values of (a) internal resistance and (b) power density as a function of current density.
The maximum electrical values obtained in scMFC using lettuce waste (0.959 ± 0.026 V; 5.697 ± 0.065 mA; 378,145 ± 5417 mW/cm2) can be contrasted with similar studies with vegetable waste and fruit waste, as summarized in Table 1. Possibly the different values may vary according to the type of electrode used and the MFC (dual-chamber or single-chamber MFC) used. The copper material is known to be a good conductor of electrons despite its antimicrobial activity.

Table 1.
Comparison of electrical parameters obtained in MFC using other organic waste with the values obtained with lettuce waste.
Table 2 shows that concerning the voltage parameter obtained from lettuce waste (0.959 ± 0.026), it was statistically different from that obtained with spinach residues; organic substrate, market vegetables, Kiwi fruit, pitahaya (0.804, 0.110, 0.208, 0.993, 0.46 V, respectively). This value was superior to the others because all these experiments obtained voltages below the lower limit of the 95% CI calculated. However, they were not higher than those obtained by potato and mandarin (1120, 1191, respectively, as these values were above the calculated upper range. Regarding the current parameter (mA), there was a difference with the residues of spinach, organic substrate, Kiwi fruit, pitahaya, and mandarin (2.37, 0.8, 3.807, 2.86, 1.43 mA, respectively), in turn, these values were below the lower confidence interval, being only surpassed by what is obtained with potato waste (12.45 mA).

Table 2.
Confidence intervals of lettuce waste parameters.
Fruit waste generally has higher electrical values due to its greater fermentable sugar content. However, vegetable waste shows lower electrical values in MFC due to the complex chemical structure they present. This can be improved if cellulolytic microorganisms are used. Finally, the values of the power density parameter obtained in the study were compared with other values obtained from published studies, finding differences with Kiwi fruit and pitahaya waste (212.68 and 304.33 mW/m2, respectively), all of which are below the lower limit of the interval. However, only in the tangerine waste was superiority found for this parameter.
In the comparison with pitahaya waste, Cohen’s D = 17.77; 42; 6 was obtained, which could indicate superiority with the electricity values produced by MFC with lettuce waste in all three parameters (Cohen’s D ≥ 0.80). In addition, it was compared with Kiwi fruit waste, finding a Cohen’s D of 0.73; 22; 8.55, where there is a slight superiority of moderate magnitude (Cohen’s D: 0.5–0.7) in the voltage obtained with this residue; while for the other two electrical parameters (current and power density), lettuce waste was higher. Finally, in the comparison with mandarin fruit residues, the obtained values of Cohen’s D of 7.53; 70; 5.46 would reflect a superiority of this waste concerning lettuce for obtaining voltage and power density, but not in obtaining current.
3.2. Isolation and Identification of the Electrogenic Microorganisms
Concerning the isolation of electrogenic microorganisms in BHI agar, smooth colonies were obtained, and in McConkey agar, the colonies were colorless and lactose-negative (lactose does not ferment), whereas, with Gram staining, negative Bacillus Gram was observed.
Table 3 shows molecular identification where Blast characterization revealed that the bacterium isolates correspond to Stenotrophomonas maltophilia with an identity percentage of 99.59%. This bacterium belongs to the class Gammaproteobacteria. Stenotrophomonas maltophilia is a cosmopolitan bacterium, which can be found in a wide variety of habitats, and is usually isolated from the environment, particularly bodies of water, sewage, soils, and salads, and in nature is associated with the roots of many plants; this may explain its isolation from lettuce residues [62,63,64]. It is a non-lactose fermenting Gram-negative bacillus, so its colonies on McConkey agar are colorless because it uses the peptone of the medium producing ammonia increasing the pH and resulting in a yellow coloration [62]. On the other hand, it is a strict aerobe, and is oxidase negative, however, it has been shown that it can show positive oxidase activity, which can allow it to grow under anaerobic conditions such as in the anodic chamber of the MFC [62]. Likewise, it is known that it can develop and grow in environments with a low concentration of oxygen; on the other hand, in another study, it is mentioned that it retains its ability to form biofilms under anaerobic conditions [65].

Table 3.
Blast characterization of the bacterium identified from an anode plate of an MFC with lettuce waste.
3.3. Bioelectricity Generation from Lettuce Waste by scMFC
On the other hand, the great ability of Stenotrophomonas maltophilia to adhere and form biofilms on abiotic surfaces allows it to develop on the anode plate of the MFC [62,63]. This step is important in electricity generation as it allows the transfer of electrons (product of metabolism) to the anode to generate electricity [66]. The biofilm formation starts with adherence to the surface of the anodic plate (electrode), followed by irreversible attachment, then final maturation. During this process of adherence, its motile flagella, interfere with the fimbriae/pili, adhesins, and the outer membrane lipopolysaccharide positively charged surface. Their mobile structures can be used as nanowires, which electrons transport from substrates (lettuce) degradation to anodic plate such as demonstrated in other studies with Geobacter and its role in electricity generation [67,68]. This bacterium is Gram-negative and belongs to the phylum Proteobacteria and class Gammaproteobacteria, which supports other studies where it is mentioned that this group of bacteria is associated with the generation of electricity in MFC [69,70]. Finally, this species has been isolated and investigated in MFC with different organic and inorganic substrates, where its role as a remediator and at the same time as an electrogenic bacterium has been demonstrated [71,72,73,74]. An important aspect is that only this bacterium could be isolated, possibly due to the antimicrobial activity of the anode plate, which is copper [75]. However, it is a controversial issue as copper is a good electron conductor, and its conductivity is 900-times greater than that of polycrystalline graphite, which represents an advantage over carbon-based electrodes. On the other hand, the bacterium Geobacter sp. is electrochemically active and can colonize copper; this could support the fact that the three types of bacteria isolated are electrogenic [76]. Finally, the three single-chamber microbial fuel cells were connected in series, managing to generate 2.81 V on day fourteen, enough to light an LED (red) as shown in Figure 5.
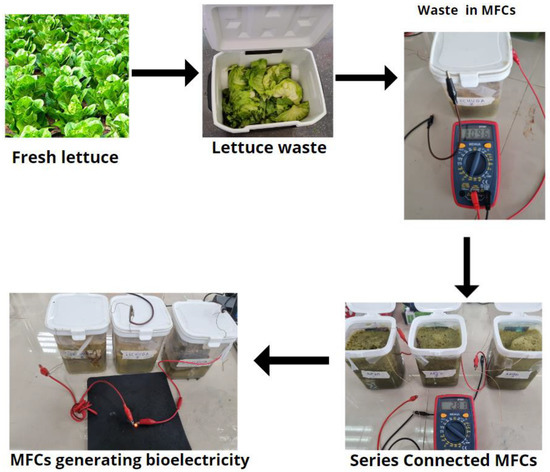
Figure 5.
Bioelectricity generation process using lettuce waste by scMFCs.
The results are promising as clean and eco-friendly energy (bioelectricity) can be generated. On the other hand, the results support the idea that bioenergy will come from organic biomass in the future, which was mentioned by Destouni and Frank [77]. Likewise, other researchers maintain that this type of bioenergy leads to sustainable development [78,79]. Finally, the generation of bioelectricity from lettuce waste along with other organic waste contributes to achieving the seventh objective of sustainable development, affordable and non-polluting energy, as mentioned in an article by Fagunwa and Olanbiwoninu (2020), that MFCs and the science of microbiology contribute to achieving this goal [80]. It should be noted that there are still certain technical challenges of MFCs, such as the low amount of electricity that is produced and the limitations of their scaling, which requires future interdisciplinary research covering areas such as physics, electronics, microbiology, and biochemistry.
4. Conclusions
This study successfully generated bioelectricity, showing the potential of leaching waste in single-chamber microbial fuel cells on a laboratory scale using zinc and copper electrodes. On day 14, electrical current (5.697 ± 0.065 mA) and voltage peaks (0.959 ± 0.026 V) were generated by scMFC. These values were obtained at pH and substrate electrical conductivity values of 7.867 ± 0.147 and 118.964 ± 8.888 mS/cm, respectively. Similarly, the internal resistance of MFCs had a low value of 87.594 ± 6226 Ω, and it was possible to calculate a maximum power and current density of 378,145 ± 5417 mW/cm2 and 5.965 A/cm2, respectively. The electrogenic bacterium Stenotrophomonas maltophilia was isolated and identified from the biofilm developed on the surface of the anode. Finally, the microbial combustion cells were connected in series, enabling a 2.81 V LED lamp, which represents an encouraging result in the advances to generate clean and sustainable energy.
The research also suggests some recommendations to optimize results concerning bioelectricity generation; for example, using metal electrodes coated with a compound that is not harmful to microorganisms to prevent corrosion observed in zinc (cathode) electrodes. Another recommendation could be that the optimal pH value has to be maintained for a longer time to optimize the electricity values produced by the MFC.
Author Contributions
Conceptualization, S.M.B.; Methodology, W.R.-V.; Software, M.G.-C.; Validation, W.R.-V., M.G.-C., D.D.-N. and F.D.; Formal analysis, W.R.-V., S.R.-F. and F.D.; Investigation, W.R.-V., S.R.-F., R.N.-N., M.G.-C. and E.M.-T.; Resources, D.D.-N.; Data curation, D.D.-N. and E.M.-T.; Writing—original draft, S.R.-F. and C.V.R.; Writing—review & editing, S.M.B. and C.V.R.; Supervision, R.N.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of Fruit and Vegetable Waste to Produce Value-Added Products: Conventional Utilization and Emerging Opportunities-A Review. Chemosphere 2022, 287, 132221. [Google Scholar] [CrossRef] [PubMed]
- United Nations World Population Prospects 2019: Highlights|Naciones Unidas. Available online: https://www.un.org/es/desa/world-population-prospects-2019-highlights (accessed on 25 May 2023).
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent Advances in Extraction Technologies for Recovery of Bioactive Compounds Derived from Fruit and Vegetable Waste Peels: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 719–752. [Google Scholar] [CrossRef] [PubMed]
- Mortula, M.M.; Ahmed, A.; Fattah, K.P.; Zannerni, G.; Shah, S.A.; Sharaby, A.M. Sustainable Management of Organic Wastes in Sharjah, UAE through Co-Composting. Methods Protoc. 2020, 3, 76. [Google Scholar] [CrossRef] [PubMed]
- Introduction—Food Loss. Available online: http://www.fao.org/platform-food-loss-waste/food-loss/introduction/en/ (accessed on 25 May 2023).
- Salihoglu, G.; Salihoglu, N.K.; Ucaroglu, S.; Banar, M. Food Loss and Waste Management in Turkey. Bioresour. Technol. 2018, 248, 88–99. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and Vegetable Waste Management: Conventional and Emerging Approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Gonçalves Neto, J.; Vidal Ozorio, L.; Campos de Abreu, T.C.; Ferreira dos Santos, B.; Pradelle, F. Modeling of Biogas Production from Food, Fruits and Vegetables Wastes Using Artificial Neural Network (ANN). Fuel 2021, 285, 119081. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Rosello, C.; Bélanger, R.; Ratti, C. Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing. Foods 2020, 9, 1468. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive Potential of Fruit and Vegetable Wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar] [CrossRef]
- Mao, G.; Wu, D.; Wei, C.; Tao, W.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Reconsidering Conventional and Innovative Methods for Pectin Extraction from Fruit and Vegetable Waste: Targeting Rhamnogalacturonan I. Trends Food Sci. Technol. 2019, 94, 65–78. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef]
- Han, R.; Truco, M.J.; Lavelle, D.O.; Michelmore, R.W. A Composite Analysis of Flowering Time Regulation in Lettuce. Front. Plant Sci. 2021, 12, 632708. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Tang, Y.-X.; Yang, Q.-C. Optimal Control of Environmental Conditions Affecting Lettuce Plant Growth in a Controlled Environment with Artificial Lighting: A Review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Chao, L.; Zeb, A.; Sun, Y. Foliar-Applied Polystyrene Nanoplastics (PSNPs) Reduce the Growth and Nutritional Quality of Lettuce (Lactuca sativa L.). Environ. Pollut. 2021, 280, 116978. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Tian, Y.; Lu, B.; Hang, Y.; Chen, Q. Hyperspectral Technique Combined with Deep Learning Algorithm for Detection of Compound Heavy Metals in Lettuce. Food Chem. 2020, 321, 126503. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Dong, Y.; Song, Z. Effect of Polyethylene Particles on Dibutyl Phthalate Toxicity in Lettuce (Lactuca sativa L.). J. Hazard. Mater. 2021, 401, 123422. [Google Scholar] [CrossRef]
- Boas, J.V.; Oliveira, V.B.; Simões, M.; Pinto, A.M.F.R. Review on Microbial Fuel Cells Applications, Developments and Costs. J. Environ. Manag. 2022, 307, 114525. [Google Scholar] [CrossRef]
- Ramya, M.; Senthil Kumar, P. A Review on Recent Advancements in Bioenergy Production Using Microbial Fuel Cells. Chemosphere 2022, 288, 132512. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. Microbial Fuel Cell: A Green Approach for the Utilization of Waste for the Generation of Bioelectricity. Bioresour. Bioprocess. 2016, 3, 38. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial Fuel Cells: From Fundamentals to Applications. A Review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Javed, M.M.; Nisar, M.A.; Ahmad, M.U.; Yasmeen, N.; Zahoor, S. Microbial Fuel Cells as an Alternative Energy Source: Current Status. Biotechnol. Genet. Eng. Rev. 2018, 34, 216–242. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial Fuel Cells, a Renewable Energy Technology for Bio-Electricity Generation: A Mini-Review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Guerrero-Barajas, C. Modern Trend of Anodes in Microbial Fuel Cells (MFCs): An Overview. Environ. Technol. Innov. 2021, 23, 101579. [Google Scholar] [CrossRef]
- Vera Natalia Ginting, C.; Sari Nasution, J.; Alfatah Sembiring, M.; Simorangkir, M. The Effect of Composition and Substrate Fermentation Duration on Microbial Fuel Cell Electrical Energy. J. Pendidik. Kim. 2019, 11, 116–121. [Google Scholar] [CrossRef]
- Kalagbor, I.A.; Azunda, B.I.; Igwe, B.C.; Akpan, B.J. Electricity Generation from Waste Tomatoes, Banana, Pineapple Fruits and Peels Using Single Chamber Microbial Fuel Cells (SMFC). Open Access J. Waste Manag. Xenobiotics 2020, 3, 141. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and Modification of Materials to Build Cost-Effective Anodes for Microbial Fuel Cells (MFCs): An Overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K. Biomass-Derived Composite Anode Electrode: Synthesis, Characterizations, and Application in Microbial Fuel Cells (MFCs). J. Environ. Chem. Eng. 2021, 9, 106111. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.-Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.-H. A Comprehensive Review on Microbial Fuel Cell Technologies: Processes, Utilization, and Advanced Developments in Electrodes and Membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Benites, S.M.; Nazario-Naveda, R.; Delfín-Narciso, D.; Gallozzo-Cardemas, M.; Díaz, F.; Murga-Torres, E.; Rojas-Villacorta, W. Use of Kiwi Waste as Fuel in MFC and Its Potential for Use as Renewable Energy. Fermentation 2023, 9, 446. [Google Scholar] [CrossRef]
- Yang, W.; Du, M.; Liu, H.; Bao, J.; Tang, J.; Li, J. Full Cell Mathematical Models of Air Cathode Microbial Fuel Cells. Exp. Comput. Multiph. Flow 2023, 5, 111–121. [Google Scholar] [CrossRef]
- Safwat, S.M.; Khaled, A.; Elawwad, A.; Matta, M.E. Dual-Chamber Microbial Fuel Cells as Biosensors for the Toxicity Detection of Benzene, Phenol, Chromium, and Copper in Wastewater: Applicability Investigation, Effect of Various Catholyte Solutions, and Life Cycle Assessment. Process Saf. Environ. Prot. 2023, 170, 1121–1136. [Google Scholar] [CrossRef]
- Ray, S.; Pandey, S.; Mohanty, M.; Padhee, S. Comparative Analysis of Power Management System for Microbial Fuel Cell. In Communications in Computer and Information Science; Springer Nature: Cham, Switzerland, 2022; pp. 127–133. ISBN 9783031217494. [Google Scholar]
- Dziegielowski, J.; Mascia, M.; Metcalfe, B.; Di Lorenzo, M. Voltage Evolution and Electrochemical Behaviour of Soil Microbial Fuel Cells Operated in Different Quality Soils. Sustain. Energy Technol. Assess. 2023, 56, 103071. [Google Scholar] [CrossRef]
- Abazarian, E.; Gheshlaghi, R.; Mahdavi, M.A. Interactions between Sediment Microbial Fuel Cells and Voltage Loss in Series Connection in Open Channels. Fuel 2023, 332, 126028. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Bose, D.; Yadav, J.; Sharma, B.; Sangli, E.; Patel, A.; Mukherjee, A.; Ashutosh Singh, A. Bioremediation and Bioelectricity from Himalayan Rock Soil in Sediment-Microbial Fuel Cell Using Carbon Rich Substrates. Fuel 2023, 341, 127019. [Google Scholar] [CrossRef]
- Din, M.I.; Ahmed, M.; Ahmad, M.; Iqbal, M.; Ahmad, Z.; Hussain, Z.; Khalid, R.; Samad, A. Investigating the Activity of Carbon Fiber Electrode for Electricity Generation from Waste Potatoes in a Single-Chambered Microbial Fuel Cell. J. Chem. 2023, 2023, 8520657. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Biryol, İ.; Ayol, A. Yeast Industry Wastewater Treatment with Microbial Fuel Cells: Effect of Electrode Materials and Reactor Configurations. Int. J. Hydrogen Energy 2023, 48, 12424–12432. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Lin, H. Effects of PH on Simultaneous Cr(VI) and p-Chlorophenol Removal and Electrochemical Performance in Leersia Hexandra Constructed Wetland-Microbial Fuel Cell. Environ. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Vemuri, B.; Chilkoor, G.; Dhungana, P.; Islam, J.; Baride, A.; Koratkar, N.; Ajayan, P.M.; Rahman, M.M.; Hoefelmeyer, J.D.; Gadhamshetty, V. Oxygen Reduction Reaction with Manganese Oxide Nanospheres in Microbial Fuel Cells. ACS Omega 2022, 7, 11777–11787. [Google Scholar] [CrossRef]
- Sharma, R.; Kumari, R.; Pant, D.; Malaviya, P. Bioelectricity Generation from Human Urine and Simultaneous Nutrient Recovery: Role of Microbial Fuel Cells. Chemosphere 2022, 292, 133437. [Google Scholar] [CrossRef]
- Zonfa, T.; Kamperidis, T.; Falzarano, M.; Lyberatos, G.; Polettini, A.; Pomi, R.; Rossi, A.; Tremouli, A. Two-Stage Process for Energy Valorization of Cheese Whey through Bio-Electrochemical Hydrogen Production Coupled with Microbial Fuel Cell. Fermentation 2023, 9, 306. [Google Scholar] [CrossRef]
- Segundo, R.-F.; De La Cruz-Noriega, M.; Milly Otiniano, N.; Benites, S.M.; Esparza, M.; Nazario-Naveda, R. Use of Onion Waste as Fuel for the Generation of Bioelectricity. Molecules 2022, 27, 625. [Google Scholar] [CrossRef]
- Agrahari, R.; Bayar, B.; Abubackar, H.N.; Giri, B.S.; Rene, E.R.; Rani, R. Advances in the Development of Electrode Materials for Improving the Reactor Kinetics in Microbial Fuel Cells. Chemosphere 2022, 290, 133184. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Calay, R.K.; Eregno, F.E. Role and Important Properties of a Membrane with Its Recent Advancement in a Microbial Fuel Cell. Energies 2022, 15, 444. [Google Scholar] [CrossRef]
- Potrykus, S.; León-Fernández, L.F.; Nieznański, J.; Karkosiński, D.; Fernandez-Morales, F.J. The Influence of External Load on the Performance of Microbial Fuel Cells. Energies 2021, 14, 612. [Google Scholar] [CrossRef]
- Rossi, R.; Logan, B.E. Unraveling the Contributions of Internal Resistance Components in Two-Chamber Microbial Fuel Cells Using the Electrode Potential Slope Analysis. Electrochim. Acta 2020, 348, 136291. [Google Scholar] [CrossRef]
- Koók, L.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. The Influential Role of External Electrical Load in Microbial Fuel Cells and Related Improvement Strategies: A Review. Bioelectrochemistry 2021, 140, 107749. [Google Scholar] [CrossRef]
- Al Lawati, M.J.; Jafary, T.; Baawain, M.S.; Al-Mamun, A. A Mini Review on Biofouling on Air Cathode of Single Chamber Microbial Fuel Cell; Prevention and Mitigation Strategies. Biocatal. Agric. Biotechnol. 2019, 22, 101370. [Google Scholar] [CrossRef]
- Tremouli, A.; Karydogiannis, I.; Pandis, P.K.; Papadopoulou, K.; Argirusis, C.; Stathopoulos, V.N.; Lyberatos, G. Bioelectricity Production from Fermentable Household Waste Extract Using a Single Chamber Microbial Fuel Cell. Energy Procedia 2019, 161, 2–9. [Google Scholar] [CrossRef]
- Daud, N.N.M.; Ahmad, A.; Yaqoob, A.A.; Ibrahim, M.N.M. Application of Rotten Rice as a Substrate for Bacterial Species to Generate Energy and the Removal of Toxic Metals from Wastewater through Microbial Fuel Cells. Environ. Sci. Pollut. Res. Int. 2021, 28, 62816–62827. [Google Scholar] [CrossRef]
- Din, M.I.; Iqbal, M.; Hussain, Z.; Khalid, R. Bioelectricity Generation from Waste Potatoes Using Single Chambered Microbial Fuel Cell. Energy Sources Recovery Util. Environ. Eff. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Nazario-Naveda, R.; Gallozzo-Cardenas, M.; Diaz, F.; Murga-Torres, E. Potential Use of Coriander Waste as Fuel for the Generation of Electric Power. Sustainability 2023, 15, 896. [Google Scholar] [CrossRef]
- Wang, H.; Chai, G.; Zhang, Y.; Wang, D.; Wang, Z.; Meng, H.; Jiang, C.; Dong, W.; Li, J.; Lin, Y.; et al. Copper Removal from Wastewater and Electricity Generation Using Dual-Chamber Microbial Fuel Cells with Shrimp Shell as the Substrate. Electrochim. Acta 2023, 441, 141849. [Google Scholar] [CrossRef]
- Hirose, S.; Inukai, K.; Nguyen, D.T.; Taguchi, K. Use of Loofah Electrodes Coated with Rice Husk Smoked Charcoal and Japanese Ink in a Microbial Fuel Cell for Muddy Water Treatment. Energy Rep. 2023, 9, 160–167. [Google Scholar] [CrossRef]
- Fadhil, S.H.; Ismail, Z.Z. Bioremediation of Real-Field Slaughterhouse Wastewater Associated with Power Generation in Algae-Photosynthetic Microbial Fuel Cell. Bioremediat. J. 2021, 27, 75–83. [Google Scholar] [CrossRef]
- Mulyono, T.; Misto; Cahyono, B.E.; Fahmidia, N.H. The Impact of Adding Vegetable Waste on the Functioning of Microbial Fuel Cell. In Proceedings of the 3rd International Conference on Physical Instrumentation and Advanced Materials (ICPIAM) 2021, Jember, Indonesia, 27 October 2021. [Google Scholar]
- Yaakop, A.S.; Ahmad, A.; Hussain, F.; Oh, S.-E.; Alshammari, M.B.; Chauhan, R. Domestic Organic Waste: A Potential Source to Produce the Energy via a Single-Chamber Microbial Fuel Cell. Int. J. Chem. Eng. 2023, 2023, 2425735. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Mohanakrishna, G.; Sarma, P.N. Composite Vegetable Waste as Renewable Resource for Bioelectricity Generation through Non-Catalyzed Open-Air Cathode Microbial Fuel Cell. Bioresour. Technol. 2010, 101, 970–976. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; Cabanillas-Chirinos, L.; Nazario-Naveda, R.; Gallozzo-Cardenas, M.; Diaz, F.; Delfin-Narciso, D.; Rojas-Villacorta, W. Use of Tangerine Waste as Fuel for the Generation of Electric Current. Sustainability 2023, 15, 3559. [Google Scholar] [CrossRef]
- Segundo, R.-F.; Benites, S.M.; De La Cruz-Noriega, M.; Vives-Garnique, J.; Otiniano, N.M.; Rojas-Villacorta, W.; Gallozzo-Cardenas, M.; Delfín-Narciso, D.; Díaz, F. Impact of Dragon Fruit Waste in Microbial Fuel Cells to Generate Friendly Electric Energy. Sustainability 2023, 15, 7316. [Google Scholar] [CrossRef]
- Said, M.S.; Tirthani, E.; Lesho, E. Stenotrophomonas Maltophilia. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572123/ (accessed on 22 May 2023).
- An, S.-Q.; Berg, G. Stenotrophomonas maltophilia. Trends Microbiol. 2018, 26, 637–638. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, P. Genomic Potential of Stenotrophomonas maltophilia in Bioremediation with an Assessment of Its Multifaceted Role in Our Environment. Front. Microbiol. 2016, 7, 967. [Google Scholar] [CrossRef]
- Bonaventura, G.D.; Stepanović, S.; Picciani, C.; Pompilio, A.; Piccolomini, R. Effect of Environmental Factors on Biofilm Formation by Clinical Stenotrophomonas maltophilia isolates. Folia Microbiol. 2007, 52, 86–90. [Google Scholar] [CrossRef]
- Greenman, J.; Gajda, I.; You, J.; Mendis, B.A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial Fuel Cells and Their Electrified Biofilms. Biofilm 2021, 3, 100057. [Google Scholar] [CrossRef] [PubMed]
- Malvankar, N.S.; Lovley, D.R. Microbial Nanowires for Bioenergy Applications. Curr. Opin. Biotechnol. 2014, 27, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G. Microbial Nanowires and Electroactive Biofilms. FEMS Microbiol. Ecol. 2018, 94, fiy086. [Google Scholar] [CrossRef] [PubMed]
- Juang, D.F.; Yang, P.C.; Lee, C.H.; Hsueh, S.C.; Kuo, T.H. Electrogenic Capabilities of Gram Negative and Gram Positive Bacteria in Microbial Fuel Cell Combined with Biological Wastewater Treatment. Int. J. Environ. Sci. Technol. 2011, 8, 781–792. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El-Taweel, G.E.; Naha, S.; Goswami, P. Bacterial Community Structure of Electrogenic Biofilm Developed on Modified Graphite Anode in Microbial Fuel Cell. Sci. Rep. 2023, 13, 1255. [Google Scholar] [CrossRef]
- Segundo, R.-F.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Angelats-Silva, L.; Díaz, F. Golden Berry Waste for Electricity Generation. Fermentation 2022, 8, 256. [Google Scholar] [CrossRef]
- Venkidusamy, K.; Megharaj, M. Identification of Electrode Respiring, Hydrocarbonoclastic Bacterial Strain Stenotrophomonas maltophilia MK2 Highlights the Untapped Potential for Environmental Bioremediation. Front. Microbiol. 2016, 7, 1965. [Google Scholar] [CrossRef]
- Galai, S.; Pérez de los Ríos, A.; Hernández-Fernández, F.J.; Kacem, S.H.; Ramírez, F.M.; Quesada-Medina, J. Microbial Fuel Cell Application for Azoic Dye Decolorization with Simultaneous Bioenergy Production Using Stenotrophomonas sp. Chem. Eng. Technol. 2015, 38, 1511–1518. [Google Scholar] [CrossRef]
- Silva-Palacios, F.; Salvador-Salinas, A.; Quezada-Alvarez, M.A.; Rodriguez-Yupanqui, M.; Segundo, R.F.; Renny, N.N.; Cabanillas-Chirinos, L. Bioelectricity generation through Microbial Fuel Cells using Serratia fonticola bacteria and Rhodotorula glutinis yeast. Energy Rep. 2023, 9, 295–301. [Google Scholar] [CrossRef]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial Properties of a Novel Copper-Based Composite Coating with Potential for Use in Healthcare Facilities. Antimicrob. Resist. Infect. Control 2019, 8, 3. [Google Scholar] [CrossRef]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schröder, U. Does It Have to Be Carbon? Metal Anodes in Microbial Fuel Cells and Related Bioelectrochemical Systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef]
- Destouni, G.; Frank, H. Renewable Energy. Ambio 2010, 39 (Suppl. S1), 18–21. [Google Scholar] [CrossRef]
- Seetharaman; Moorthy, K.; Patwa, N.; Saravanan; Gupta, Y. Breaking Barriers in Deployment of Renewable Energy. Heliyon 2019, 5, e01166. [Google Scholar] [CrossRef]
- Dhanya, B.S.; Mishra, A.; Chandel, A.K.; Verma, M.L. Development of Sustainable Approaches for Converting the Organic Waste to Bioenergy. Sci. Total Environ. 2020, 723, 138109. [Google Scholar] [CrossRef]
- Fagunwa, O.E.; Olanbiwoninu, A.A. Accelerating the Sustainable Development Goals through Microbiology: Some Efforts and Opportunities. Access Microbiol. 2020, 2, acmi000112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).