Response of Leaf Photosynthesis–Transpiration Coupling to Biotic and Abiotic Factors in the Typical Desert Shrub Artemisia ordosica
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
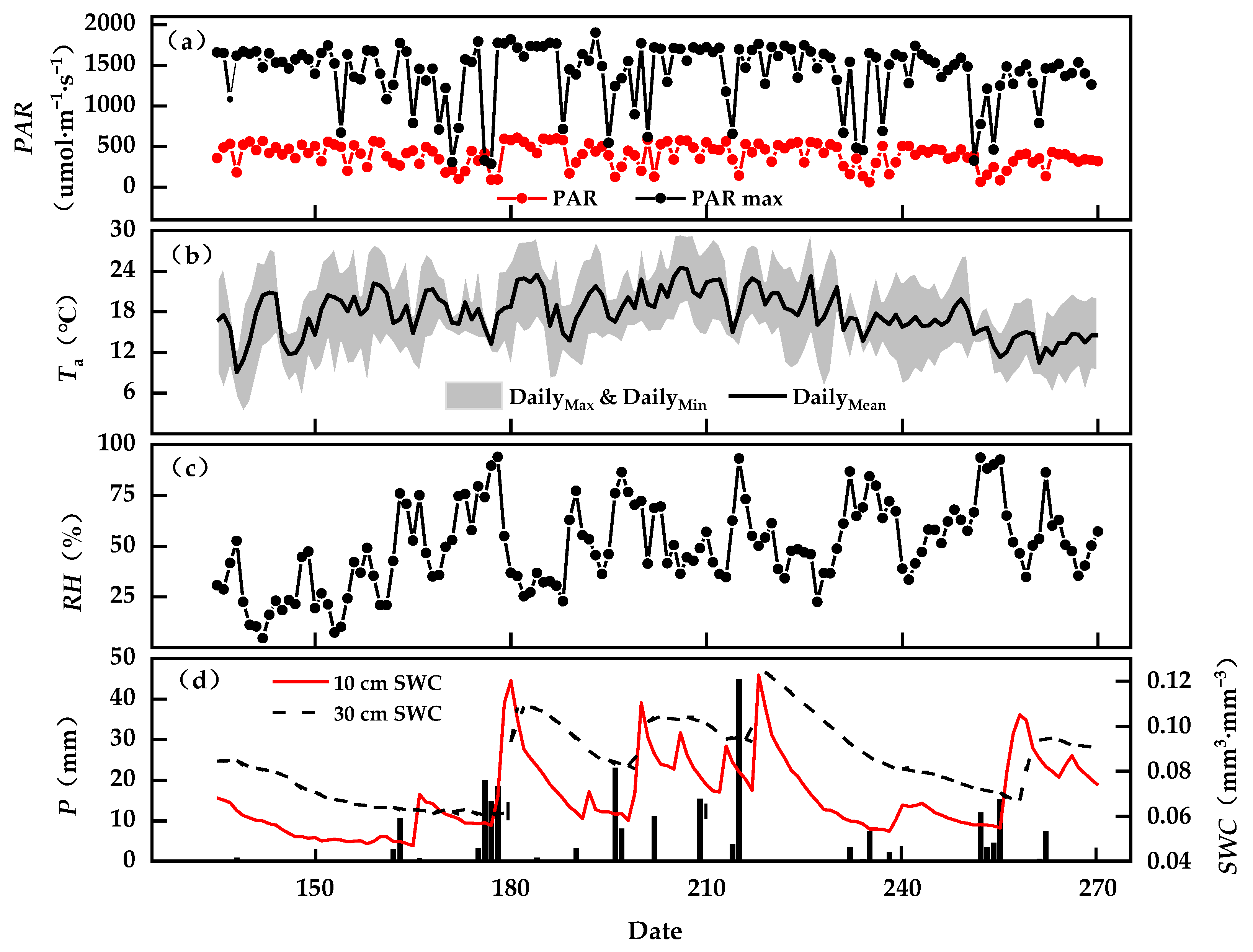
2.1.1. Study Site
2.1.2. Determination of Gas Exchange Parameters
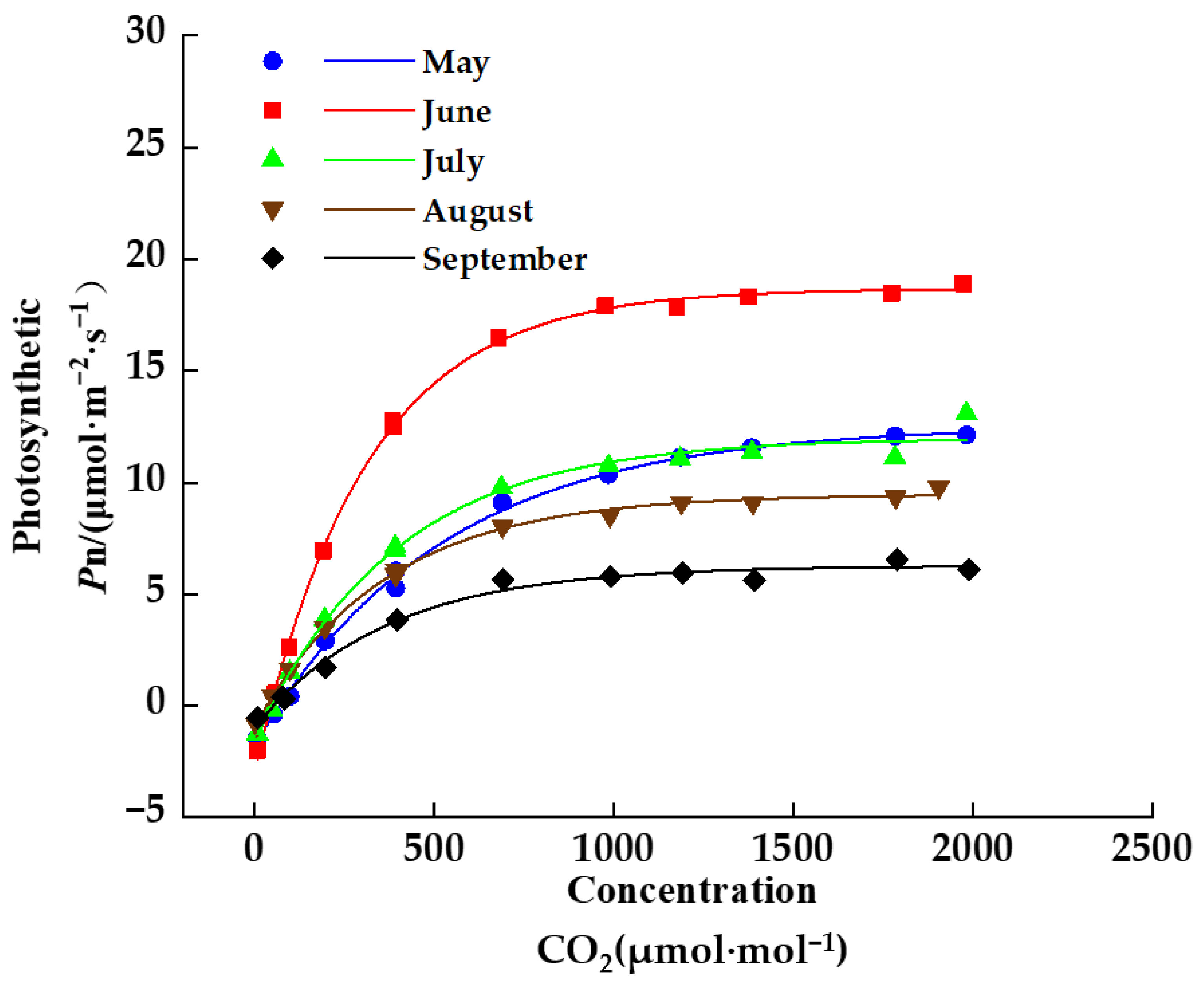
2.1.3. Measurement of CO2 Response Curve
2.1.4. Determination of Environmental Factors
2.2. Statistical Analyses
3. Results
3.1. Seasonal Dynamics of Environmental Factors
3.2. Seasonal Dynamics of Gas Exchange Parameters
3.3. Seasonal Dynamics of Photosynthetic Parameters
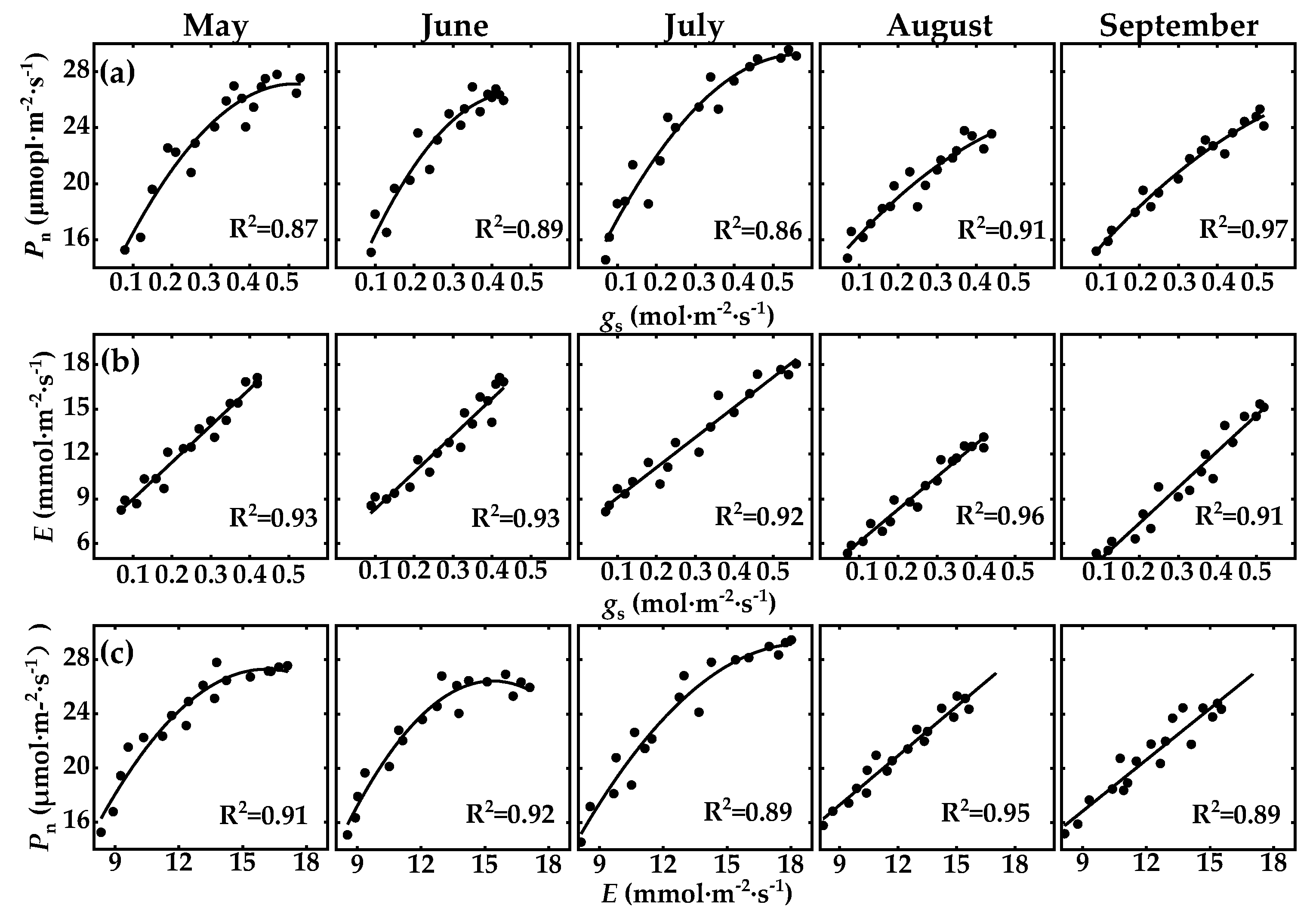
3.4. Response of Photosynthetic Parameters to Environmental Factors
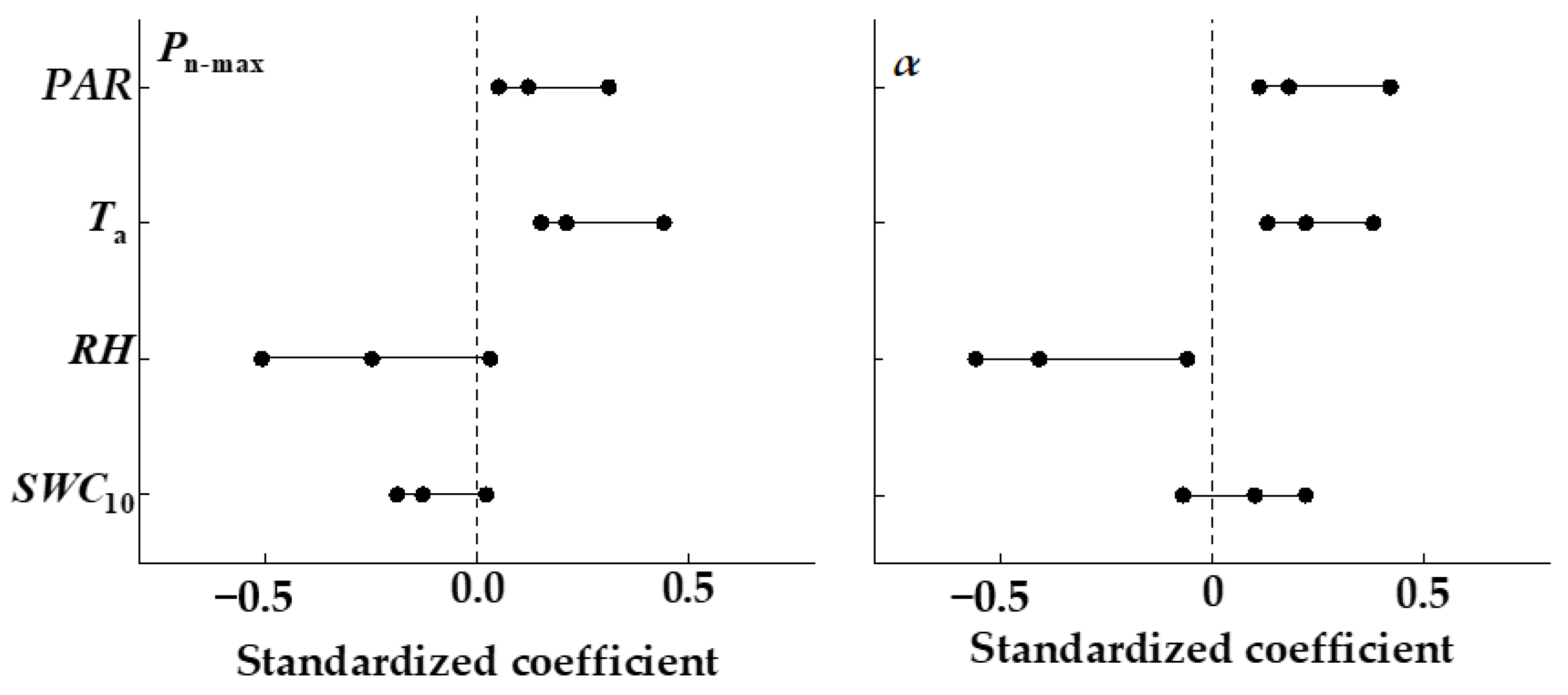
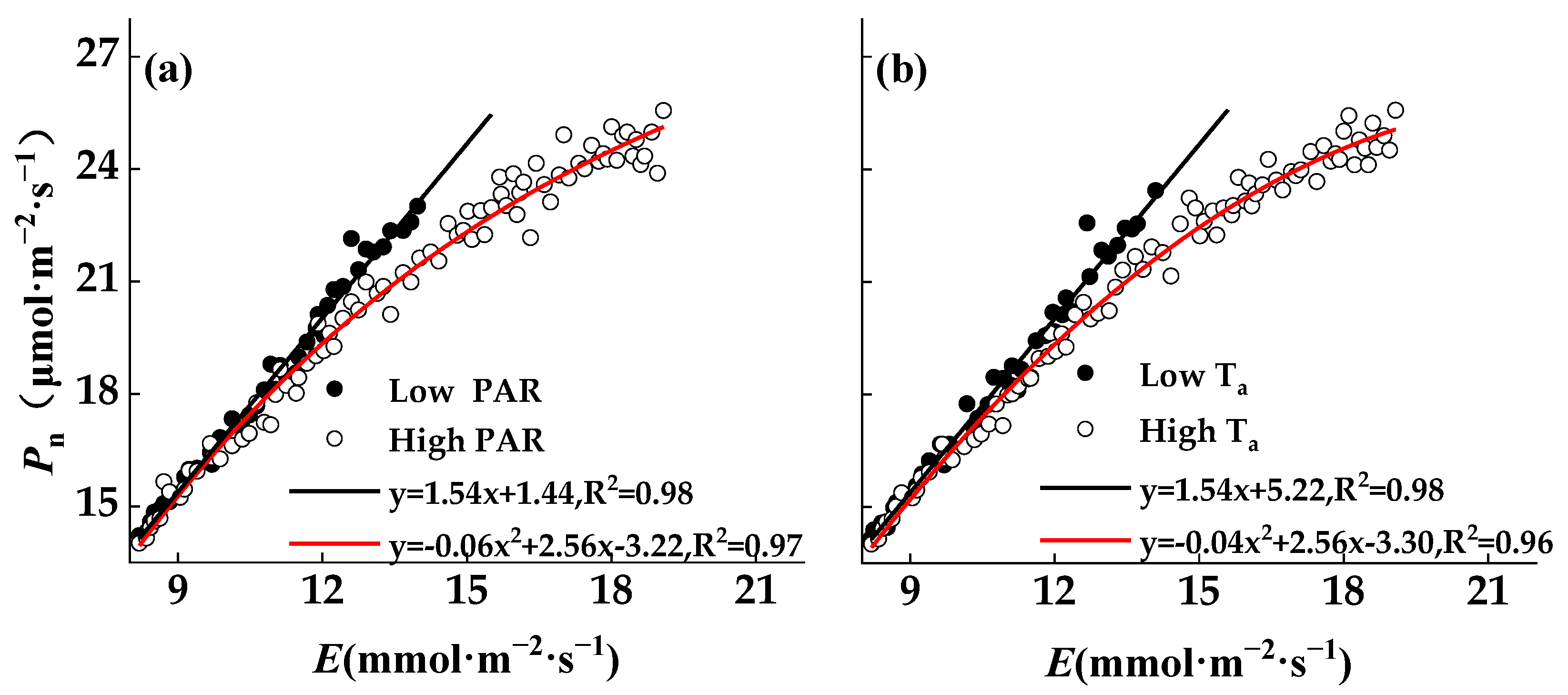
3.5. Effects of Environmental Factors on Photosynthesis and Transpiration Coupling
4. Discussion
4.1. Correlation between Pn, gs, and E
4.2. Effects of Environmental Factors on Plant Photosynthesis
4.3. Effects of Environmental Factors on Pn−E Coupling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zha, T.S.; Wu, Y.J.; Jia, X.; Zhang, M.Y.; Bai, Y.J.; Liu, P.; Ma, J.Y.; Bourque, C.P.; Peltola, H. Diurnal response of effective quantum yield of PSII photochemistry to irradiance as an indicator of photosynthetic acclimation to stressed environments revealed in a xerophytic species. Ecol. Indic. 2017, 74, 191–197. [Google Scholar] [CrossRef]
- Li, Y.Z.; Xu, Y.J.; Zhang, W.Q.; Zhuang, Q.W.; Zhang, Y.; Cai, P. Carbon and water fluxes are more sensitive to drought than heat in terrestrial ecosystems in China. J. Hydrol. 2021, 603, 127177. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Richards, R.A.; Jin, Y.; Siddique, K.H.M.; Li, F.M.; He, J. Yield and water-use related traits in landrace and new soybean cultivars in arid and semi-arid areas of China. Field Crop. Res. 2022, 283, 108559. [Google Scholar] [CrossRef]
- Huang, J.P.; Yu, H.P.; Dai, A.G.; Wei, Y.; Kang, L. Drylands face potential threat under 2 degrees C global warming target. Nat. Clim. Chang. 2017, 7, 417–422. [Google Scholar] [CrossRef]
- Liu, P.; Zha, T.S.; Jia, X.; Black, T.A.; Jassal, R.S.; Ma, J.Y.; Bai, Y.J.; Wu, Y.J. Different effects of spring and summer droughts on ecosystem carbon and water exchanges in a semi-arid shrubland ecosystem in northwest China. Ecosystems 2019, 22, 1869–1885. [Google Scholar] [CrossRef]
- Barros, V.; Stocker, T.F. Managing the risks of extreme events and disasters to advance climate change adaptation: Special report of the Intergovernmental Panel on climate change. J. Clin. Endocr. Metab. 2012, 18, 586–599. [Google Scholar]
- Du, W.T.; Kang, S.C.; Qin, X.; Ji, Z.M.; Sun, W.J.; Chen, J.Z.; Yang, J.H.; Chen, D.L. Can summer monsoon moisture invade the Jade Pass in Northwestern China. Clim. Dyn. 2020, 55, 3101–3115. [Google Scholar] [CrossRef]
- Chambers, J.C.; Bradley, B.A.; Brown, C.S.; Antonio, C.D.; Germino, M.J.; Grace, J.B.; Hardegree, S.P.; Miller, R.F.; Pyke, D.A. Resilience to stress and disturbance, and resistance to Bromus tectorum L. invasion in cold desert shrublands of western North America. Ecosystems 2013, 17, 360–375. [Google Scholar] [CrossRef]
- Grant, O.M.; Tronina, L.; García-Plazaola, J.L.; Esteban, R.; Prtrira, J.S.; Chaves, M.M. Resilience of a semi-deciduous shrub, Cistus salvifolius, to severe summer drought and heat stress. Funct. Plant Biol. 2014, 42, 219–228. [Google Scholar] [CrossRef]
- Ye, Z.P.; Duan, D.H.; An, T.; Kang, H.J. Construction of CO2-response model of electron transport rate in C4 crop and its application. Chin. J. Plant Ecol. 2018, 42, 1000–1008. [Google Scholar] [CrossRef]
- Makino, A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef]
- Zhang, Y.E.; Yu, X.X.; Chen, L.H.; Jia, G.D. Comparison of the partitioning of evapotranspiration-numerical modeling with different isotopic models using various kinetic fractionation coefficients. Plant Soil 2018, 430, 307–328. [Google Scholar] [CrossRef]
- Zhang, Y.E.; Yu, X.X.; Chen, L.H.; Jia, G.D. Whole-plant instantaneous and short-term water-use efficiency in response to soil water content and CO2 concentration. Plant Soil 2019, 444, 281–298. [Google Scholar] [CrossRef]
- Little, C.; Lara, A.; McPhee, J.; Urrutia, R. Revealing the impact of forest exotic plantations on water yield in large scale watersheds in South-Central Chile. J. Hydrol. 2009, 374, 162–170. [Google Scholar] [CrossRef]
- Yu, G.R.; Zhuang, J.I.E.; Yu, Z.L. An attempt to establish a synthetic model of photosynthesis-transpiration based on stomatal behavior for maize and soybean plants grown in field. J. Plant Physiol. 2001, 158, 861–874. [Google Scholar] [CrossRef]
- Niu, S.L.; Song, L.; Wang, J.S.; Luo, Y.Q.; Yu, G.R. Dynamic carbon-nitrogen coupling under global change. Sci. China Life Sci. 2023, 66, 771–782. [Google Scholar] [CrossRef]
- Jin, C.; Zha, T.S.; Bourque, C.P.A.; Liu, P.; Jia, X.; Zhang, F.; Yu, H.Q.; Tian, Y.; Li, X.H.; Kang, X.Y.; et al. Multi-year trends and interannual variation in ecosystem resource use efficiencies in a young mixedwood plantation in northern China. Agric. For. Meteorol. 2023, 330, 109318. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.Q.; Oren, R.; Wu, X.Y. Hyposensitive canopy conductance renders ecosystems vulnerable to meteorological droughts. Global Chang. Biol. 2023, 29, 1890–1904. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Ma, J.Y.; Feng, Q.; Sun, J.H.; Sun, W. Water sources and water-use efficiency of desert plants in different habitats in Dunhuang, NW China. Ecol. Res. 2017, 32, 243–258. [Google Scholar] [CrossRef]
- Farquhar, G.; Richards, R. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Jia, X.; Zha, T.S.; Gong, J.N.; Wang, B.; Zhang, Y.Q.; Wu, B.; Qin, S.G.; Peltola, H. Carbon and water exchange over a temperate semi-arid shrubland during three years of contrasting precipitation and soil moisture patterns. Agric. For. Meteorol. 2016, 228, 120–129. [Google Scholar] [CrossRef]
- Boese, S.; Jung, M.; Carvalhais, N.; Teuling, A.J.; Reichstein, M. Carbon–water flux coupling under progressive drought. Biogeosciences 2019, 16, 2557–2572. [Google Scholar] [CrossRef]
- Drake, G.E.; Joelker, M.G.; Varhammar, A.V.; Medlyn, B.E.; Reich, P.B.; Leign, A.; Pfautsch-Blackman, C.J.; López, R.; Aspinwall, M.J. Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Global Chang. Biol. 2018, 24, 2390–2402. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zha, T.S.; Jia, X.; Wang, B.; Guo, X.N.; Zhang, Y.Q.; Wu, B.; Yang, Q.; Peltola, H. Diurnal freeze-thaw cycles modify winter soil respiration in a desertshrub-land ecosystem. Forests 2016, 7, 161. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.Z.; Wang, Z.H.; Wang, X.; Jia, Z.; Liu, L.L. High aerosol loading decreases the transpiration of poplars both in the day- and night-time. Agric. For. Meteorol. 2022, 327, 109225. [Google Scholar] [CrossRef]
- Hayat, M.; Zha, T.S.; Jia, X.; Iqbal, S.; Qian, D.; Bourque, C.P.A.; Khan, A.; Tian, Y.; Bai, Y.J.; Liu, P.; et al. A Multiple-Temporal Scale Analysis of Biophysical Control of Sap Flow in Salix Psammophila Growing in a Semi-arid Shrubland Ecosystem of Northwest China. Agric. For. Meteorol. 2020, 288, 107985. [Google Scholar] [CrossRef]
- Law, B.E.; Falge, E.; Gu, L.; Baldocchi, D.D. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric. For. Meteorol. 2002, 113, 97–120. [Google Scholar] [CrossRef]
- Li, X.Y. Soil-vegetation-hydrological coupling, response and adaptation mechanisms in arid regions. Earth Sci. 2011, 41, 1721–1730. [Google Scholar] [CrossRef]
- Yu, G.R.; Gao, Y.; Wang, Q.F.; Shen, W.J. Discussion on the key processes of carbon-nitrogen-water coupling cycles and biological regulation mechanisms in terrestrial ecosystem. Chin. J. Eco-Agric. 2013, 21, 1–13. [Google Scholar]
- Rogers, A.; Serbin, S.P.; Ely, K.S.; Sloan, V.L.; Wullschleger, S.D. Terrestrial biosphere models underestimate photosynthetic capacity and CO2 assimilation in the Arctic. New Phytol. 2017, 216, 1090–1103. [Google Scholar] [CrossRef]
- Prentice, I.C.; Dong, N.; Gleason, S.M.; Maire, V.; Wright, I.J.; Penuelas, J. Balancing the costs of carbon gain and water transport: Testing a new theoretical framework for plant functional ecology. Ecol. Lett. 2013, 17, 82–91. [Google Scholar] [CrossRef]
- Bahar, N.A.; Ishida, F.Y.; Weerasinghe, L.K. Leaf-level photosynthetic capacity in lowland Amazonian and high-elevation Andean tropical moist forests of Peru. New Phytol. 2017, 214, 1002–1018. [Google Scholar] [CrossRef]
- Smith, N.G.; Dukes, J.S. Drivers of leaf carbon exchange capacity across biomes at the continental scale. Ecology 2018, 99, 1610–1620. [Google Scholar] [CrossRef]
- Chen, W.; Li, G.C.; Wang, D.L.; Yang, Z.; Wang, Z.; Zhang, X.P.; Peng, B.; Bi, P.S.; Zhang, F.J. Influence of the ecosystem conversion process on the carbon and water cycles in different regions of China. Ecol. Indic. 2023, 148, 110040. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, F.; Gou, X.H.; Fonti, P.; Xia, J.Q.; Cao, Z.Y.; Liu, J.G.; Wang, Y.F.; Zhang, J.Z. Seasonal variations in leaf-level photosynthesis and water use efficiency of three isohydric to anisohydric conifers on the Tibetan Plateau. Agric. For. Meteorol. 2021, 308, 108581. [Google Scholar] [CrossRef]
- Lindqvist, J.; Bankestad, D.; Carstensen, A.M.; Lundin, B.; Wik, T. Complexity of chlorophyll fluorescence dynamic response as an indicator of excessive light intensity. IFAC-PapersOnLine 2016, 16, 392–397. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Köcher, P.; Horna, V.; Leuschner, C. Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. Tree Physiol. 2012, 32, 1021–1032. [Google Scholar] [CrossRef]
- Ripullone, R.; Guerrieri, M.R.; Nole, A.; Magnani, F.; Borghetti, M. Stomatal conductance and leaf water potential responses to hydraulic conductance variation in Pinus pinaster seedlings. Trees 2007, 21, 371–378. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 120–128. [Google Scholar] [CrossRef]
- Yu, G.Y.; Wang, Q.F.; Yu, Z.L. Study on water-carbon coupling cycle and process management ecosystem. Adv. Earth Sci. 2004, 5, 831–839. [Google Scholar]
- Lu, S.; Lu, Y.L.; Peng, W.; Ju, A.Q.; Ren, T.S. A generalized relationship between thermal conductivity and matric suction of soils. Geoderma 2019, 337, 491–497. [Google Scholar] [CrossRef]
- Prins, A.; Orr, D.J.; Andralojc, P.J.; Reynolds, M.P.; Carmo-Silva, E.; Parry, M.A. Rubisco catalytic properties of wild and domesticated relatives provide scope for improving wheat photosynthesis. Environ. Exp. Bot. 2016, 67, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.B.; Chen, Y.; Lu, J.; Yan, W.D. Water uptake pattern by coniferous forests in two habitats linked to precipitation changes in subtropical monsoon climate region, China. Forests 2022, 13, 708. [Google Scholar] [CrossRef]
- Xu, M.Z.; Zha, T.S.; Tian, Y.; Liu, P.; Jia, X.; Bourque, C.P.A.; Jin, C.; Wei, X.S.; Zhao, H.X.; Guo, Z.F. Elevated physiological plasticity in xerophytic-deciduous shrubs as demonstrated in their variable maximum carboxylation rate. Ecol. Indic. 2022, 144, 109475. [Google Scholar] [CrossRef]


| Pn | E | gs | |
|---|---|---|---|
| Month | (μmol·mol−1) | (mmol·m−2·s−1) | (mol·m2·s−1) |
| May | 24.04 (2.01) | 12.35 (0.07) | 0.51 (0.14) |
| June | 23.04 (3.97) | 13.62 (2.27) | 0.45 (0.12) |
| July | 28.09 (1.13) | 13.58 (2.30) | 0.57 (0.15) |
| August | 20.25 (3.23) | 8.98 (2.43) | 0.44 (0.11) |
| September | 23.54 (4.23) | 9.03 (2.37) | 0.51 (0.19) |
| Month | α | Pn−max | Csat | C0 | Rp | R2 |
|---|---|---|---|---|---|---|
| (mol·mol−1) | (μmol·m−2·s−1) | (μmol·m−2·s−1) | (μmol·m−2·s−1) | (μmol·m−2·s−1) | ||
| May | 0.027 (0.009) | 12.076 (2.132) | 1877.883 (78.321) | 67.658 (12.212) | 1.749 (0.811) | 0.991 |
| June | 0.080 (0.032) | 18.723 (4.321) | 1536.522 (65.322) | 43.334 (9.443) | 3.133 (0.951) | 0.997 |
| July | 0.044 (0.021) | 12.388 (3.572) | 2758.173 (100.275) | 45.567 (5.251) | 1.816 (0.342) | 0.992 |
| August | 0.035 (0.045) | 9.521 (1.946) | 2031.811 (89.312) | 33.051 (8.347) | 1.081 (0.310) | 0.998 |
| September | 0.020 (0.010) | 6.214 (1.523) | 1568.160 (72.143) | 50.921 (7.215) | 0.955 (0.241) | 0.987 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Luo, Y.; Jin, C.; Xu, M.; Li, X.; Tian, Y. Response of Leaf Photosynthesis–Transpiration Coupling to Biotic and Abiotic Factors in the Typical Desert Shrub Artemisia ordosica. Sustainability 2023, 15, 10216. https://doi.org/10.3390/su151310216
Mao J, Luo Y, Jin C, Xu M, Li X, Tian Y. Response of Leaf Photosynthesis–Transpiration Coupling to Biotic and Abiotic Factors in the Typical Desert Shrub Artemisia ordosica. Sustainability. 2023; 15(13):10216. https://doi.org/10.3390/su151310216
Chicago/Turabian StyleMao, Jun, Yu Luo, Chuan Jin, Minze Xu, Xinhao Li, and Yun Tian. 2023. "Response of Leaf Photosynthesis–Transpiration Coupling to Biotic and Abiotic Factors in the Typical Desert Shrub Artemisia ordosica" Sustainability 15, no. 13: 10216. https://doi.org/10.3390/su151310216
APA StyleMao, J., Luo, Y., Jin, C., Xu, M., Li, X., & Tian, Y. (2023). Response of Leaf Photosynthesis–Transpiration Coupling to Biotic and Abiotic Factors in the Typical Desert Shrub Artemisia ordosica. Sustainability, 15(13), 10216. https://doi.org/10.3390/su151310216






