Understanding the Impact of Underwater Noise to Preserve Marine Ecosystems and Manage Anthropogenic Activities
Abstract
1. Introduction
2. Underwater Noise as an Economic-Political Issue
3. The Impacts of Underwater Noise on Ecosystems
3.1. The Scientific Approach
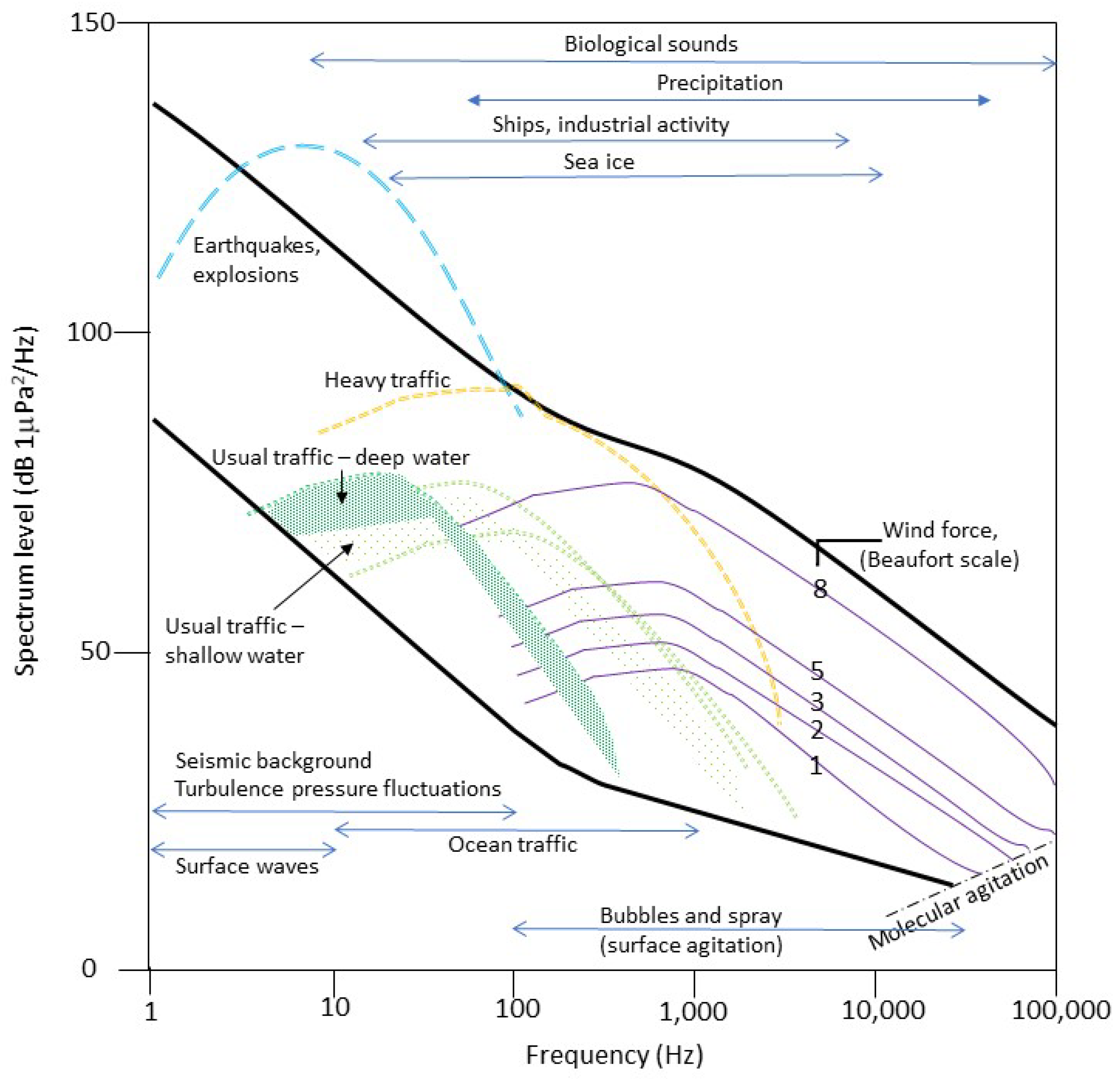
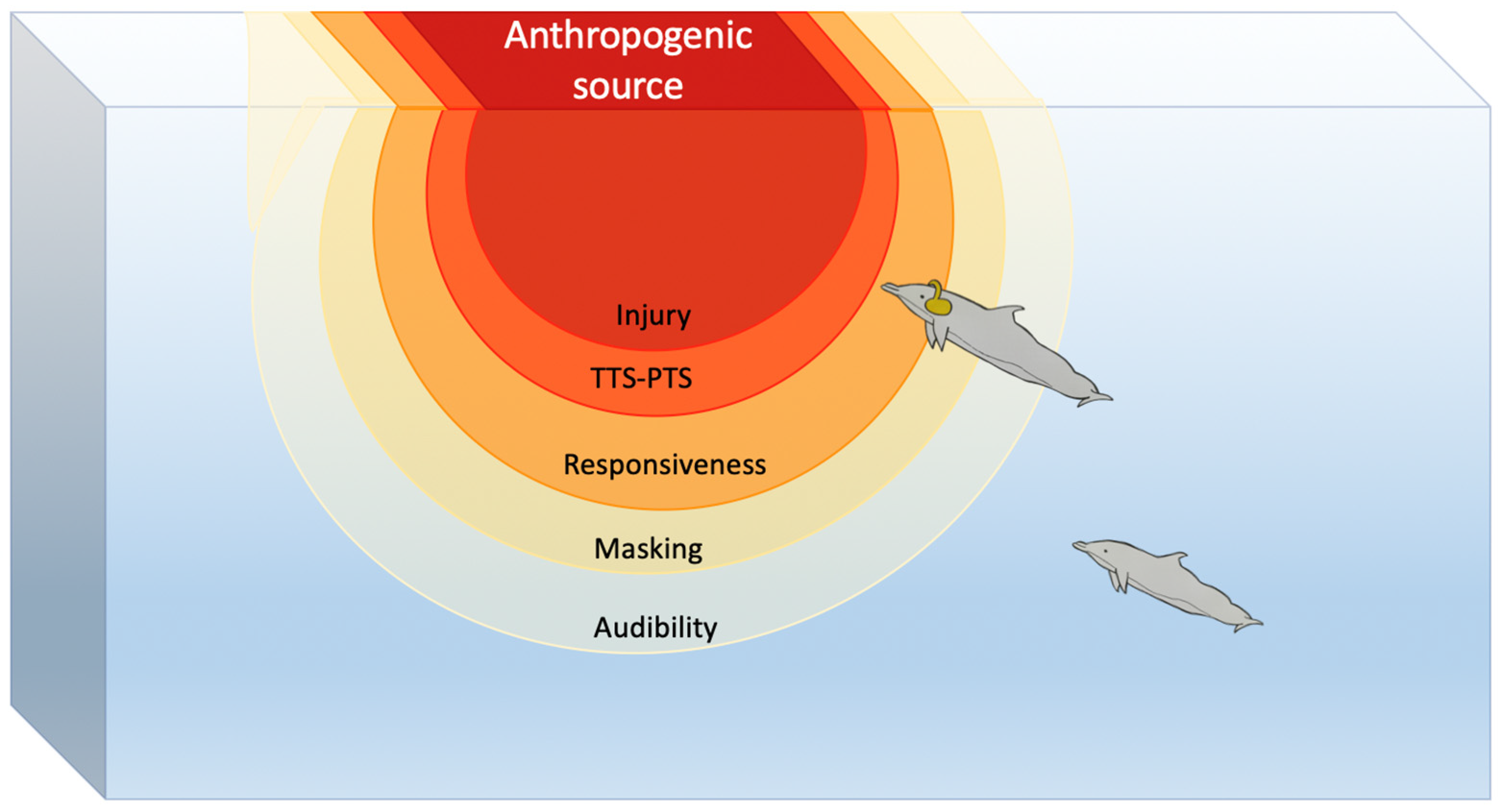
3.2. Impact on Marine Fauna and Ecosystems
3.3. The Other Side of the Coin: Stochastic Resonance
3.4. Communication and Cognition within the Ecosystem
4. SEAS-Mology: A Lesson Learnt from Helioseismology
5. Reflections
- Research to date has demonstrated that underwater noise has an impact on marine ecosystems, even though it is still difficult to quantify the problem.
- Different methodologies for the analysis of signals are used: spectrograms, correlations, counting of impulses, and waveform analysis.
- When the influence of noise on the behavior is investigated, we are not focusing on the disturbances introduced in the meaning of the message.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brundtland, G.H. Our Common Future Report of the World Commission on Environment and Development; UN-Document A/42/427. Available online: https://sustainabledevelopment.un.org/content/documents/5987our-common-future.pdf (accessed on 22 June 2023).
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development. 21 October 2015. A/RES/70/1. Available online: https://www.refworld.org/docid/57b6e3e44.html (accessed on 22 June 2023).
- Fogarty, M.J.; Gamble, R.; Perretti, C.T. Dynamic Complexity in Exploited Marine Ecosystems. Front. Ecol. Evol. 2016, 4. [Google Scholar] [CrossRef]
- de Salas, K.; Scott, J.L.; Schüz, B.; Norris, K. The super wicked problem of ocean health: A socio-ecological and behavioural perspective. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377. [Google Scholar] [CrossRef]
- Brown, C.L.; Hardy, A.R.; Barber, J.R.; Fristrup, K.M.; Crooks, K.R.; Angeloni, L.M. The Effect of Human Activities and Their Associated Noise on Ungulate Behavior. PLoS ONE 2012, 7, e40505. [Google Scholar] [CrossRef] [PubMed]
- Beaman, C.P. Auditory distraction from low-intensity noise: A review of the consequences for learning and workplace environments. Appl. Cogn. Psychol. 2005, 19, 1041–1064. [Google Scholar] [CrossRef]
- Directive 2002/49/EC of the European Parliament and of the Council of 25 June 2002 Relating to the Assessment and Management of Environmental Noise—Declaration by the Commission in the Conciliation Committee on the Directive Relating to the Assessment and Management of Environmental Noise. Available online: http://data.europa.eu/eli/dir/2002/49/oj (accessed on 22 June 2023).
- Jensen, F.H.; Bejder, L.; Wahlberg, M.; Soto, N.A.; Johnson, M.; Madsen, P.T. Vessel noise effects on delphinid communication. Mar. Ecol. Prog. Ser. 2009, 395, 161–175. [Google Scholar] [CrossRef]
- Chami, R.; Cosimano, T.; Fullenkamp, C.; Oztosun, S. Nature’s solution to climate change. Financ. Dev. Mag. 2019, 56, 34–38. [Google Scholar]
- Söderqvist, T. Economic Impact of Underwater Noise; Enveco Miljöekonomi AB Press: Bromma, Sweden, 2014. [Google Scholar]
- Precautionary Principle. Available online: https://eur-lex.europa.eu/EN/legal-content/glossary/precautionary-principle.html (accessed on 22 June 2023).
- Merchant, N.D. Underwater noise abatement: Economic factors and policy options. Environ. Sci. Policy 2018, 92, 116–123. [Google Scholar] [CrossRef]
- Luttenberger, L.R.; Slišković, M.; Ančić, I.; Boljat, H.U. Environmental Impact of Underwater Noise. J. Marit. Transp. Sci. 2022, 45–54. [Google Scholar] [CrossRef]
- Joy, R.; Tollit, D.; Wood, J.; MacGillivray, A.; Li, Z.; Trounce, K.; Robinson, O. Potential Benefits of Vessel Slowdowns on Endangered Southern Resident Killer Whales. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- MacGillivray, A.O.; Li, Z.; Hannay, D.E.; Trounce, K.B.; Robinson, O.M. Slowing deep-sea commercial vessels reduces underwater radiated noise. J. Acoust. Soc. Am. 2019, 146, 340–351. [Google Scholar] [CrossRef]
- Vancouver Fraser Port Authority, June 2018 –Summary Findings, ECHO Program, Voluntary Vessel Slowdown Trial Summary Findings. Available online: https://www.portvancouver.com/wp-content/uploads/2019/07/2019-06-Fact-sheet-ECHO-Program-2018-voluntary-vessel-slowdown-results.pdf (accessed on 22 June 2023).
- Underwater Noise in the Marine Environment. Available online: https://www.jpi-oceans.eu/en/underwater-noise-marine-environment (accessed on 22 June 2023).
- Addressing Underwater Noise from Ships-Draft Revised Guidelines Agreed. Available online: https://www.imo.org/en/MediaCentre/Pages/WhatsNew-1818.aspx (accessed on 22 June 2023).
- Merchant, N.D.; Putland, R.L.; André, M.; Baudin, E.; Felli, M.; Slabbekoorn, H.; Dekeling, R. A decade of underwater noise research in support of the European Marine Strategy Framework Directive. Ocean Coast. Manag. 2022, 228. [Google Scholar] [CrossRef]
- Hildebrand, J.A. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 2009, 395, 5–20. [Google Scholar] [CrossRef]
- Farcas, A.; Thompson, P.M.; Merchant, N.D. Underwater noise modelling for environmental impact assessment. Environ. Impact Assess. Rev. 2016, 57, 114–122. [Google Scholar] [CrossRef]
- Southall, B.L.; Finneran, J.J.; Reichmuth, C.; Nachtigall, P.E.; Ketten, D.R.; Bowles, A.E.; Ellison, W.T.; Nowacek, D.P.; Tyack, P. Marine Mammal Noise Exposure Criteria: Updated Scientific Recommendations for Residual Hearing Effects. Aquat. Mamm. 2019, 45, 125–232. [Google Scholar] [CrossRef]
- Readhead, M.L. Is underwater thermal noise useful? In Proceedings of the Inter-Noise and Noise-Con Congress and Conference Proceedings, Melbourne, Australia, 16–19 November 2014; Volume 2492, pp. 4978–4983. [Google Scholar]
- Slabbekoorn, H.; Bouton, N.; van Opzeeland, I.; Coers, A.; Cate, C.T.; Popper, A.N. A noisy spring: The impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 2010, 25, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.; Przeslawski, R.; Duncan, A.J.; Gunning, M.; Bruce, B. A critical review of the potential impacts of marine seismic surveys on fish & invertebrates. Mar. Pollut. Bull. 2017, 114, 9–24. [Google Scholar] [CrossRef]
- Andre, M.; van der Schaar, M.; Zaugg, S.; Houegnigan, L.; Sanchez, A.M. Sea Observatories and Acoustic Events: Towards a Global Monitoring of Ocean Noise. In Proceedings of the Institute of Acoustics, Tokyo, Japan, 5–8 April 2011; pp. 1–3. [Google Scholar] [CrossRef]
- Clark, C.W.; Ellison, W.T.; Southall, B.L.; Hatch, L.; Van Parijs, S.M.; Frankel, A.; Ponirakis, D. Acoustic masking in marine ecosystems: Intuitions, analysis, and implication. Mar. Ecol. Prog. Ser. 2009, 395, 201–222. [Google Scholar] [CrossRef]
- Weilgart, L. The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 2007, 85, 1091–1116. [Google Scholar] [CrossRef]
- Richardson, W.J.; Greene, C.R., Jr.; Malme, C.I.; Thomson, D.H. Marine Mammals and Noise; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, X.; Liu, G. Noise in the sea and its impacts on marine organisms. Int. J. Environ. Res. Public Health 2015, 12, 12304–12323. [Google Scholar] [CrossRef]
- Cunningham, K.A.; Mountain, D.C. Simulated masking of right whale sounds by shipping noise: Incorporating a model of the auditory periphery. J. Acoust. Soc. Am. 2014, 135, 1632–1640. [Google Scholar] [CrossRef]
- Pierzycki, R.H.; Seeber, B.U. Comodulation masking release in electric hearing. J. Assoc. Res. Otolaryngol. 2014, 15, 279–291. [Google Scholar] [CrossRef]
- Moore, B. Co-modulation masking release: Spectro-temporal pattern analysis in hearing. Br. J. Audiol. 1990, 24, 131–137. [Google Scholar] [CrossRef]
- Hall, J.W.; Tyler, R.; Fernandes, M.A. Factors Influencing the Masking Level Difference in Cochlear Hearing-Impaired and Normal-Hearing Listeners. J. Speech Lang. Hear. Res. 1984, 27, 145–154. [Google Scholar] [CrossRef]
- Bee, M.A. Sound source segregation in grey treefrogs: Spatial release from masking by the sound of a chorus. Anim. Behav. 2007, 74, 549–558. [Google Scholar] [CrossRef]
- Erbe, C.; Reichmuth, C.; Cunningham, K.; Lucke, K.; Dooling, R. Communication masking in marine mammals: A review and research strategy. Mar. Pollut. Bull. 2016, 103, 15–38. [Google Scholar] [CrossRef]
- Bee, M.A. Finding a mate at a cocktail party: Spatial release from masking improves acoustic mate recognition in grey treefrogs. Anim. Behav. 2008, 75, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Best, V.; Ozmeral, E.; Gallun, F.J.; Sen, K.; Shinn-Cunningham, B.G. Spatial unmasking of birdsong in human listeners: Energetic and informational factors. J. Acoust. Soc. Am. 2005, 118, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Hotchkin, C.; Parks, S. The Lombard effect and other noise-induced vocal modifications: Insight from mammalian communication systems. Biol. Rev. 2013, 88, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Lombard, E. Le signede le elevationde la voix. Ann. Malad. L’orielle Larynx. Nez. Pharynx 1911, 37, 101–119. [Google Scholar]
- Foote, A.D.; Osborne, R.W.; Hoelzel, A.R. Whale-Call response to masking boat noise. Nature 2004, 428, 910. [Google Scholar] [CrossRef]
- Holt, M.M.; Noren, D.P.; Emmons, C.K. Effects of noise levels and call types on the source levels of killer whale calls. J. Acoust. Soc. Am. 2011, 130, 3100–3106. [Google Scholar] [CrossRef] [PubMed]
- Gizon, L.; Barucq, H.; Duruflé, M.; Hanson, C.; Leguèbe, M.; Birch, A.; Chabassier, J.; Fournier, D.; Hohage, T.; Papini, E. Computational helioseismology in the frequency domain: Acoustic waves in axisymmetric solar models with flows. Astron. Astrophys. 2017, 600, A35. [Google Scholar] [CrossRef]
- Erbe, C.; Farmer, D.M. A software model to estimate zones of impact on marine mammals around anthropogenic noise. J. Acoust. Soc. Am. 2000, 108, 1327–1331. [Google Scholar] [CrossRef]
- Southall, B.; Bowles, A.; Ellison, W.; Finneran, J.J.; Gentry, R.; Greene CR, J.; Tyack, P. Marine mammal noise-exposure criteria: Initial scientific recommendations. Aquat. Mamm. 2007, 33, 411–521. [Google Scholar] [CrossRef]
- Popper, A.N.; Hastings, M. The effects of anthropogenic sources of sound on fishes. J. Fish Biol. 2009, 75, 455–489. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.J.; Deak, T.; Parsons, E.C.M. Concerns Related to Chronic Stress in Marine Mammals; Report of the Scientific Committee 61/E169; International Whalewatching Commission: Impington, UK; pp. 1–7.
- Erbe, C. Effects of Underwater Noise on Marine Mammals. Adv. Exp. Med. Biol. 2012, 730, 17–22. [Google Scholar] [CrossRef]
- Slabbekoorn, H. Habitat-Dependent ambient noise: Consistent spectral profiles in two African forest types. J. Acoust. Soc. Am. 2004, 116, 3727–3733. [Google Scholar] [CrossRef] [PubMed]
- van der Groen, O.; Wenderoth, N. Transcranial Random Noise Stimulation of Visual Cortex: Stochastic Resonance Enhances Central Mechanisms of Perception. J. Neurosci. 2016, 36, 5289–5298. [Google Scholar] [CrossRef]
- Weizheng, Z.; Xiaoqing, Z.; Rijie, Y.; Jianhui, H. Research on identification technology of ship radiated noise and marine biological noise. In Proceedings of the IEEE International Conference on Signal Processing, Communications and Computing (ICSPCC), Xiamen, China, 22–25 October 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Hänggi, P. Stochastic resonance in biology how noise can enhance detection of weak signals and help improve biological information processing. Chem. Phys. Chem. 2002, 3, 285–290. [Google Scholar] [CrossRef]
- Siddagangaiah, S.; Li, Y.; Guo, X.; Yang, K. On the dynamics of ocean ambient noise: Two decades later. Chaos: Interdiscip. J. Nonlinear Sci. 2015, 25, 103117. [Google Scholar] [CrossRef]
- Traer, J.; McDermott, J.H. Statistics of natural reverberation enable perceptual separation of sound and space. Proc. Natl. Acad. Sci. USA 2016, 113, E7856–E7865. [Google Scholar] [CrossRef] [PubMed]
- Klump, G.M. Bird communication in the noisy world. In Ecology and Evolution of Acoustic Communication in Birds; Kroodsma, D.E., Miller, E.H., Eds.; Cornell University Press: Ithaca, NY, USA, 2019; pp. 321–328. [Google Scholar]
- Brumm, H.; Slabbekoorn, H. Acoustic Communication in Noise. Adv. Study Behav. 2005, 35, 151–209. [Google Scholar] [CrossRef]
- Hulse, S.H. Auditory scene analysis in animal communication. Adv. Study Behav. 2002, 31, 163–200. [Google Scholar] [CrossRef]
- Spence, C.; Ranson, J.; Driver, J. Cross-modal selective attention: On the difficulty of ignoring sounds at the locus of visual attention. Percept. Psychophys. 2000, 62, 410–424. [Google Scholar] [CrossRef]
- Stocker, M. Fish, mollusks and other sea animals’ use of sound, and the impact of anthropogenic noise in the marine acoustic environment. J. Acoust. Soc. Am. 2002, 112, 2431. [Google Scholar] [CrossRef]
- Aubin, T.; Jouventin, P. Cocktail-party effect in king penguin colonies. Proc. R. Soc. B Boil. Sci. 1998, 265, 1665–1673. [Google Scholar] [CrossRef]
- Haykin, S.; Chen, Z. The Cocktail Party Problem. Neural Comput. 2005, 17, 1875–1902. [Google Scholar] [CrossRef]
- Keller, C.H.; Hartung, K.; Takahashi, T.T. Head-related transfer functions of the barn owl: Measurement and neural responses. Hear. Res. 1998, 118, 13–34. [Google Scholar] [CrossRef]
- Dall, S.; Giraldeau, L.; Olsson, O.; Mcnamara, J.; Stephens, D. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 2005, 20, 187–193. [Google Scholar] [CrossRef]
- Würsig, B. Intelligence and cognition. In Encyclopedia of Marine Mammals; Academic Press: Cambridge, MA, USA, 2009; pp. 616–623. [Google Scholar]
- Krause, J.; Lusseau, D.; James, R. Animal social networks: An introduction. Behav. Ecol. Sociobiol. 2009, 63, 967–973. [Google Scholar] [CrossRef]
- Biro, D.; Sasaki, T.; Portugal, S. Bringing a Time–Depth Perspective to Collective Animal Behaviour. Trends Ecol. Evol. 2016, 31, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.M.; Whalen, A.; Laland, K.N. Fish pool their experience to solve problems collectively. Nat. Ecol. Evol. 2017, 1, 135. [Google Scholar] [CrossRef] [PubMed]
- Couzin, I.D.; Krause, J. Self-Organization and Collective Behavior in Vertebrates. Adv. Study Behav. 2003, 32, 1–75. [Google Scholar] [CrossRef]
- Sumpter, D. The principles of collective animal behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2005, 361, 5–22. [Google Scholar] [CrossRef]
- Giraldeau, L.; Valone, T.J.; Templeton, J.J. Potential disadvantages of using socially acquired information. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 1559–1566. [Google Scholar] [CrossRef]
- Ioannou, C.C. Swarm intelligence in fish? The difficulty in demonstrating distributed and self-organised collective intelligence in (some) animal groups. Behav. Process 2017, 141, 141–151. [Google Scholar] [CrossRef]
- Ward, A.; Webster, M. Sociality: The Behaviour of Group-Living Animals; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Noad, M.J.; Cato, D.H.; Bryden, M.M.; Jenner, M.N.; Jenner, K.C.S. Cultural revolution in whale songs. Nature 2000, 408, 537. [Google Scholar] [CrossRef]
- Rendell, L.E.; Whitehead, H. Vocal clans in sperm whales (Physeter macrocephalus). Proc. R. Soc. B Boil. Sci. 2003, 270, 225–231. [Google Scholar] [CrossRef]
- Yurk, H.; Barrett-Lennard, L.; Ford, J.; Matkin, C. Cultural transmission within maternal lineages: Vocal clans in resident killer whales in southern Alaska. Anim. Behav. 2002, 63, 1103–1119. [Google Scholar] [CrossRef]
- Whitehead, H. Sperm Whales: Social Evolution in the Ocean; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Caldwell, M.C.; Caldwell, D.K. Individualized whistle contours in bottle-nosed dolphins (Tursiops truncatus). Nature 1965, 207, 434–435. [Google Scholar] [CrossRef]
- Janik, V.M.; Sayigh, L.S. Communication in bottlenose dolphins: 50 years of signature whistle research. J. Comp. Physiol. A 2013, 199, 479–489. [Google Scholar] [CrossRef]
- Leighton, R.B.; Noyes, R.W.; Simon, G.W. Velocity Fields in the Solar Atmosphere. I. Preliminary Report. Astrophys. J. 1962, 135, 474. [Google Scholar] [CrossRef]
- Ulrich, R.K. The Five-Minute Oscillations on the Solar Surface. Astrophys. J. 1970, 162, 993. [Google Scholar] [CrossRef]
- Kosovichev, A. Inversion methods in helioseismology and solar tomography. J. Comput. Appl. Math. 1999, 109, 1–39. [Google Scholar] [CrossRef]
- Kosovichev, A.G. HELIOSEISMIC response to the X2.2 solar flare of 2011 February 15. Astrophys. J. 2011, 734, L15. [Google Scholar] [CrossRef]
- Lindsey, C.; Braun, D. Seismic imaging of the Sun’s far hemisphere and its applications in space weather forecasting. Space Weather. 2017, 15, 761–781. [Google Scholar] [CrossRef] [PubMed]
- Wiegelmann, T.; Thalmann, J.K.; Solanki, S.K. The magnetic field in the solar atmosphere. Astron. Astrophys. Rev. 2014, 22, 78. [Google Scholar] [CrossRef]
- Moretti, P.F.; Jefferies, S.M.; Armstrong, J.D.; Mc Intosh, S.W. Observational signatures of the interaction between acoustic waves and the solar magnetic canopy. Astron. Astrophys. 2007, 471, 961–965. [Google Scholar] [CrossRef]
- Severino, G.; Straus, T.; Oliviero, M.; Steffen, M.; Fleck, B. The Intensity–Velocity Phase Spectra of Evanescent Oscillations and Acoustic Sources. Sol. Phys. 2012, 284, 297–314. [Google Scholar] [CrossRef]
- Broomhall, A.-M.; Chatterjee, P.; Howe, R.; Norton, A.A.; Thompson, M.J. The Sun’s Interior Structure and Dynamics, and the Solar Cycle. Space Sci. Rev. 2014, 186, 191–225. [Google Scholar] [CrossRef]
- Moretti, P.F.; Cacciani, A.; Hanslmeier, A.; Messerotti, M.; Otruba, W.; Warmuth, A. Full-disk magnetic oscillations in the solar photosphere. Astron. Astrophys. 2003, 403, 297–302. [Google Scholar] [CrossRef]
- Jefferies, S.M.; Fleck, B.; Murphy, N.; Berrilli, F. Observed Local Dispersion Relations for Magnetoacoustic-gravity Waves in the Sun’s Atmosphere: Mapping the Acoustic Cutoff Frequency. Astrophys. J. 2019, 884, L8. [Google Scholar] [CrossRef]
- Calchetti, D.; Jefferies, S.M.; Fleck, B.; Berrilli, F.; Shcherbik, D.V. A new method for detecting solar atmospheric gravity waves. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 379, 20200178. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, L. Patterns of Thought and of Etymology I. Nausea > of (>Eng.) Noise. Word 1945, 1, 260–276. [Google Scholar] [CrossRef]
- Gardner, W.A.; Robinson, E.A. Statistical Spectral Analysis—A Nonprobabilistic Theory. J. Dyn. Syst. Meas. Control. 1989, 111, 673. [Google Scholar] [CrossRef]
- Southall, B.L.; Nowacek, D.P.; Bowles, A.E.; Senigaglia, V.; Bejder, L.; Tyack, P.L. Marine Mammal Noise Exposure Criteria: Assessing the Severity of Marine Mammal Behavioral Responses to Human Noise. Aquat. Mamm. 2021, 47, 421–464. [Google Scholar] [CrossRef]
- Angluin, D.; Becerra-Bonache, L. Learning Meaning Before Syntax. In Grammatical Inference: Algorithms and Applications: Proceedings of the 9th International Colloquium, ICGI 2008, Saint-Malo, France, 22–24 September 2008; Clark, A., Coste, F., Miclet, L., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5278. [Google Scholar] [CrossRef]
- Balázsi, G.; van Oudenaarden, A.; Collins, J.J. Cellular Decision Making and Biological Noise: From Microbes to Mammals. Cell 2011, 144, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Parbery-Clark, A.; Strait, D.L.; Anderson, S.; Hittner, E.; Kraus, N. Musical Experience and the Aging Auditory System: Implications for Cognitive Abilities and Hearing Speech in Noise. PLoS ONE 2011, 6, e18082. [Google Scholar] [CrossRef]
- Berger, B.; Griesmayr, B.; Minarik, T.; Biel, A.L.; Pinal, D.; Sterr, A.; Sauseng, P. Dynamic regulation of interregional cortical communication by slow brain oscillations during working memory. Nat. Commun. 2019, 10, 4242. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Reljin, N.; Bolkhovsky, J.B.; Orjuela-Cañón, A.D.; Chon, K.H. Brain Activity Correlates with Cognitive Performance Deterioration During Sleep Deprivation. Front. Neurosci. 2019, 13, 1001. [Google Scholar] [CrossRef]
- Römer, H. Masking by Noise in Acoustic Insects: Problems and Solutions. In Animal Communication and Noise; Animal Signals and Communication, Brumm, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 2. [Google Scholar] [CrossRef]
- Buchanan, M. Ubiquity: Why Catastrophes Happen; Crown, Random House, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Mirollo, R.E.; Strogatz, S. Synchronization of Pulse-Coupled Biological Oscillators. SIAM J. Appl. Math. 1990, 50, 1645–1662. [Google Scholar] [CrossRef]
- Pikovsky, A.; Rosenblum, M.; Kurths, J.; Hilborn, R.C.; Pikovsky, A.; Rosenblum, M.; Kurths, J.; Hilborn, R.C. Synchronization: A Universal Concept in Nonlinear Science. Am. J. Phys. 2002, 70, 655. [Google Scholar] [CrossRef]
- Ashwin, P.; Wieczorek, S.; Vitolo, R.; Cox, P. Tipping points in open systems: Bifurcation, noise-induced and rate-dependent examples in the climate system. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1166–1184. [Google Scholar] [CrossRef] [PubMed]
- Strogatz, S.H. From Kuramoto to Crawford: Exploring the onset of synchronization in populations of coupled oscillators. Phys. D Nonlinear Phenom. 2000, 143, 1–20. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ’small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Selkoe, K.A.; Blenckner, T.; Caldwell, M.R.; Crowder, L.B.; Erickson, A.L.; Essington, T.E.; Estes, J.A.; Fujita, R.M.; Halpern, B.S.; Hunsicker, M.E.; et al. Principles for managing marine ecosystems prone to tipping points. Ecosyst. Health Sustain. 2015, 1, 1–18. [Google Scholar] [CrossRef]
- Dietz, S.; Rising, J.; Stoerk, T.; Wagner, G. Economic impacts of tipping points in the climate system. Proc. Natl. Acad. Sci. USA 2021, 118, e2103081118. [Google Scholar] [CrossRef] [PubMed]
- André, M.; van der Schaar, M.; Zaugg, S.; Houégnigan, L.; Sánchez, A.; Castell, J. Listening to the Deep: Live monitoring of ocean noise and cetacean acoustic signals. Mar. Pollut. Bull. 2011, 63, 18–26. [Google Scholar] [CrossRef]
- Mellinger, D.; Stafford, K.; Moore, S.; Dziak, R.; Matsumoto, H. An Overview of Fixed Passive Acoustic Observation Methods for Cetaceans. Oceanography 2007, 20, 36–45. [Google Scholar] [CrossRef]
- Levin, L.A.; Bett, B.J.; Gates, A.R.; Heimbach, P.; Howe, B.M.; Janssen, F.; McCurdy, A.; Ruhl, H.A.; Snelgrove, P.; Stocks, K.I.; et al. Global Observing Needs in the Deep Ocean. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Gagliardi, G.; Salza, M.; Ferraro, P.; De Natale, P.; Di Maio, A.; Carlino, S.; De Natale, G.; Boschi, E. Design and test of a laser-based optical-fiber Bragg-grating accelerometer for seismic applications. Meas. Sci. Technol. 2008, 19, 085306. [Google Scholar] [CrossRef]
- André, M.; Solé, M.; Lenoir, M.; Durfort, M.; Quero, C.; Mas, A.; Lombarte, A.; van der Schaar, M.; López-Bejar, M.; Morell, M.; et al. Low-frequency sounds induce acoustic trauma in cephalopods. Front. Ecol. Environ. 2011, 9, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Karaffa, P.T.; Draheim, M.M.; Parsons, E.C.M. What’s in a Name? Do Species’ Names Impact Student Support for Conservation. Hum. Dimens. Wildl. 2012, 17, 308–310. [Google Scholar] [CrossRef]
- Serpell, J. Factors influencing human attitudes to animals and their welfare. Anim. Welf. 2004, 13, S145–S151. [Google Scholar] [CrossRef]
- Levin, S.; Xepapadeas, T.; Crépin, A.-S.; Norberg, J.; de Zeeuw, A.; Folke, C.; Hughes, T.; Arrow, K.; Barrett, S.; Daily, G.; et al. Social-ecological systems as complex adaptive systems: Modeling and policy implications. Environ. Dev. Econ. 2012, 18, 111–132. [Google Scholar] [CrossRef]
- Gaucherel, C.; Théro, H.; Puiseux, A.; Bonhomme, V. Understand ecosystem regime shifts by modelling ecosystem development using Boolean networks. Ecol. Complex. 2017, 31, 104–114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, P.F.; Affatati, A. Understanding the Impact of Underwater Noise to Preserve Marine Ecosystems and Manage Anthropogenic Activities. Sustainability 2023, 15, 10178. https://doi.org/10.3390/su151310178
Moretti PF, Affatati A. Understanding the Impact of Underwater Noise to Preserve Marine Ecosystems and Manage Anthropogenic Activities. Sustainability. 2023; 15(13):10178. https://doi.org/10.3390/su151310178
Chicago/Turabian StyleMoretti, Pier Francesco, and Alice Affatati. 2023. "Understanding the Impact of Underwater Noise to Preserve Marine Ecosystems and Manage Anthropogenic Activities" Sustainability 15, no. 13: 10178. https://doi.org/10.3390/su151310178
APA StyleMoretti, P. F., & Affatati, A. (2023). Understanding the Impact of Underwater Noise to Preserve Marine Ecosystems and Manage Anthropogenic Activities. Sustainability, 15(13), 10178. https://doi.org/10.3390/su151310178





