Co-Gasification Performance of Low-Quality Lignite with Woody Wastes Using Greenhouse Gas CO2—A TG–MS Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Fuels and Characterization
2.2. Char Production
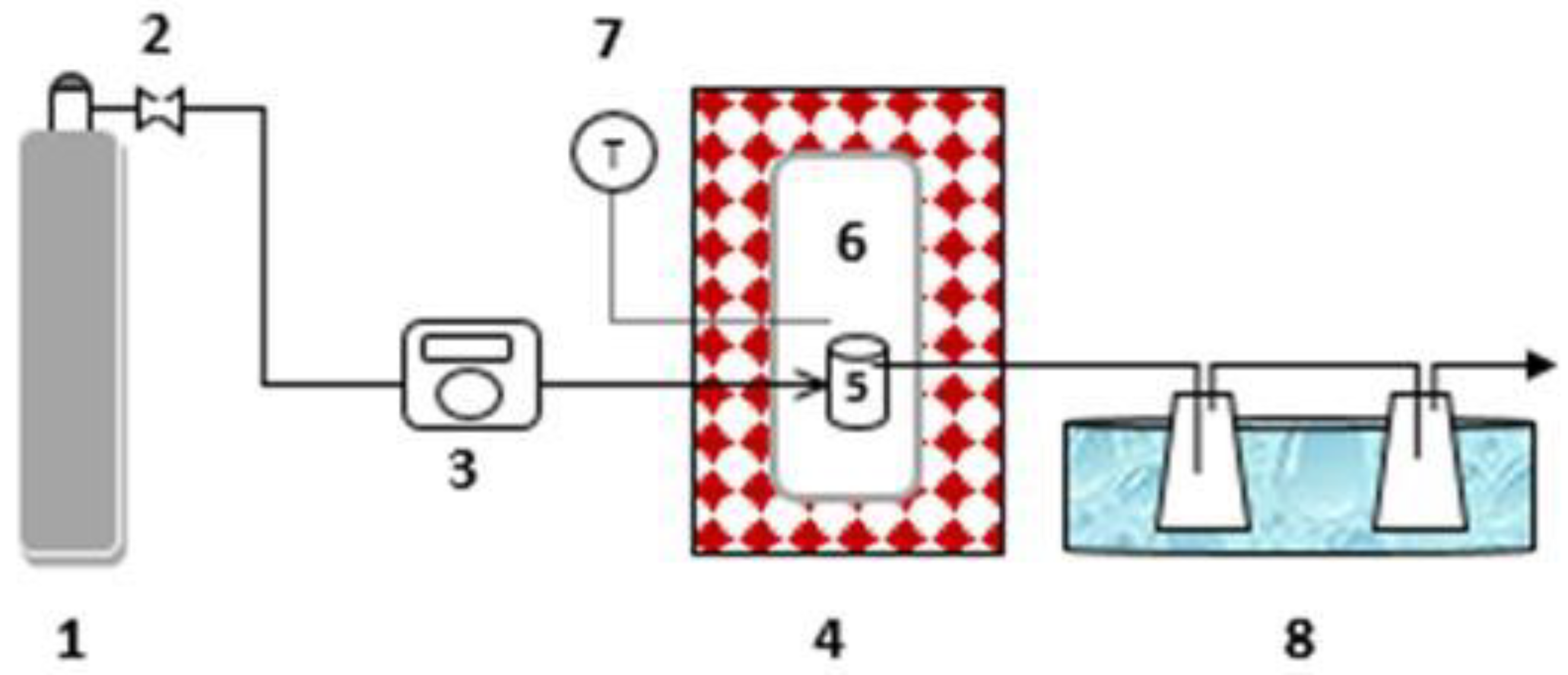
2.3. Carbon Dioxide Gasification Experiments
3. Results and Discussion
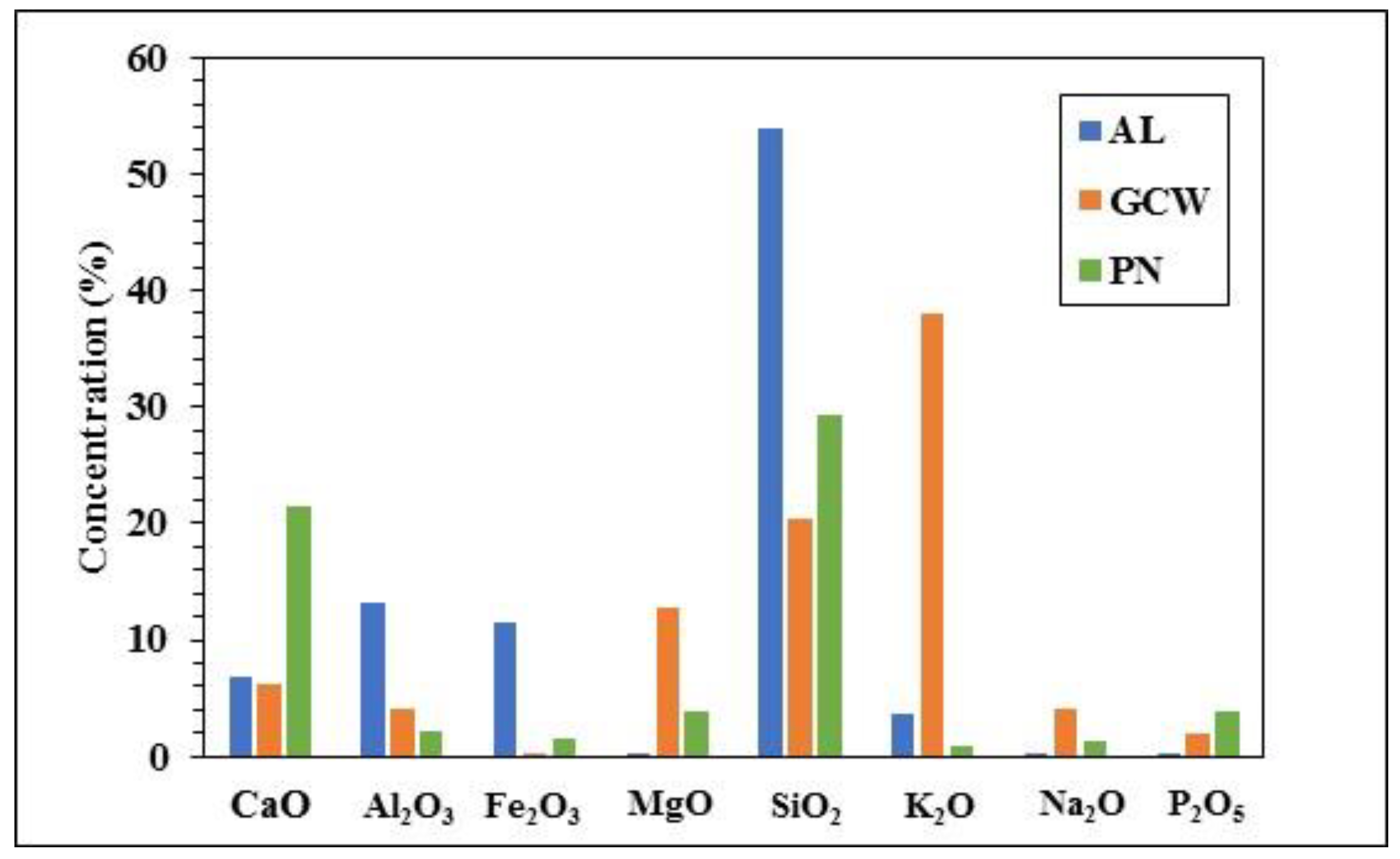
3.1. Raw Fuel Characterization
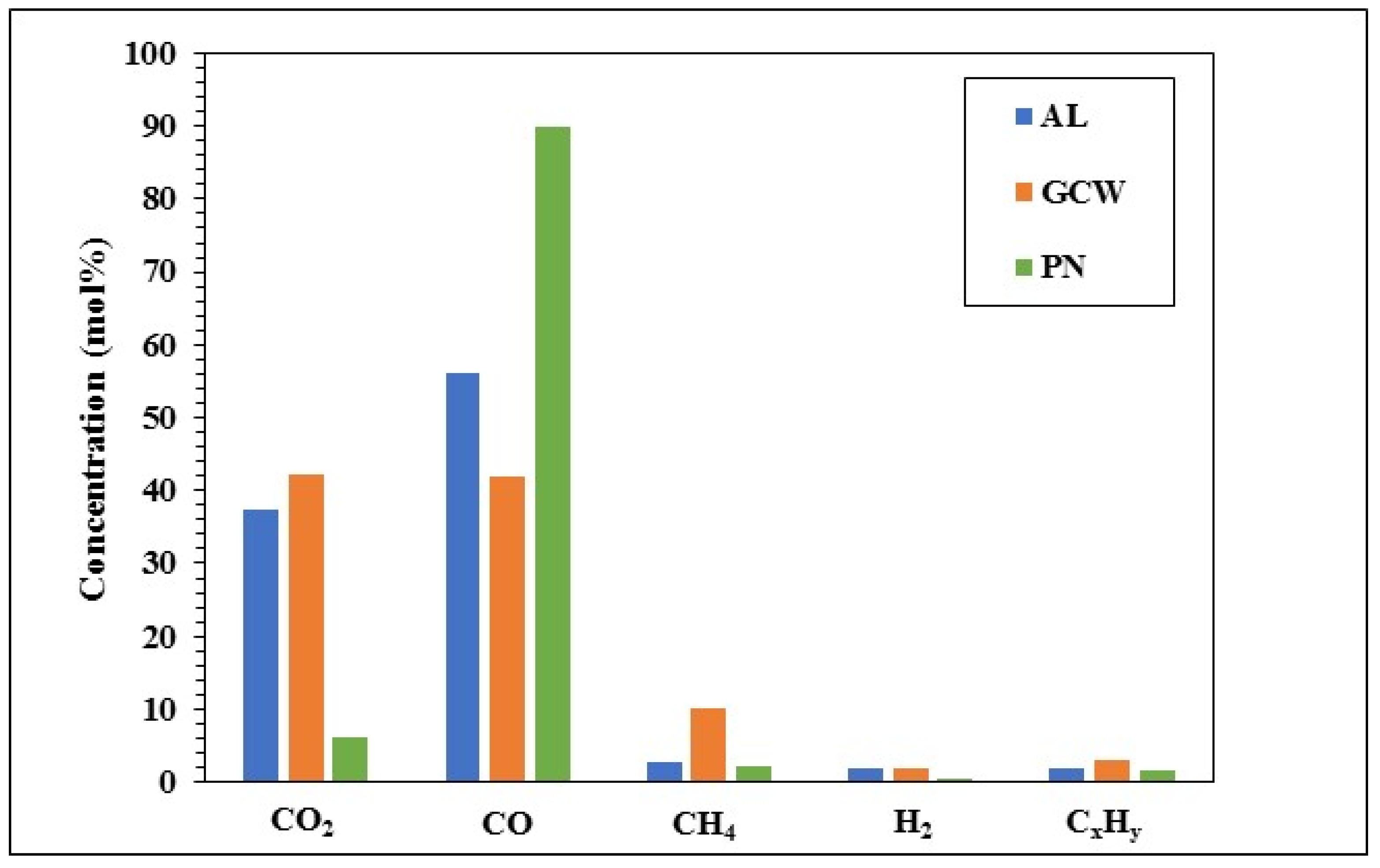
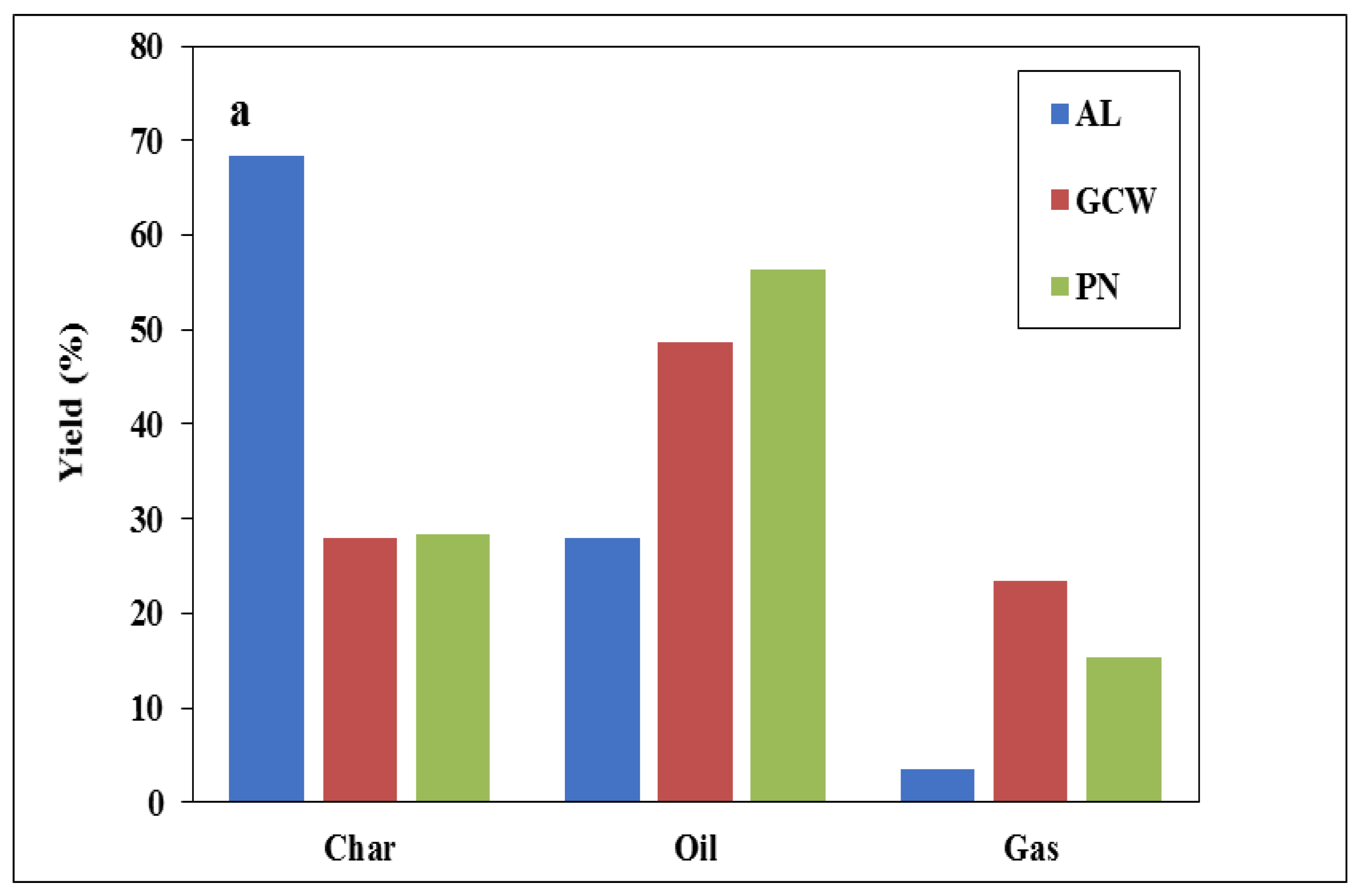
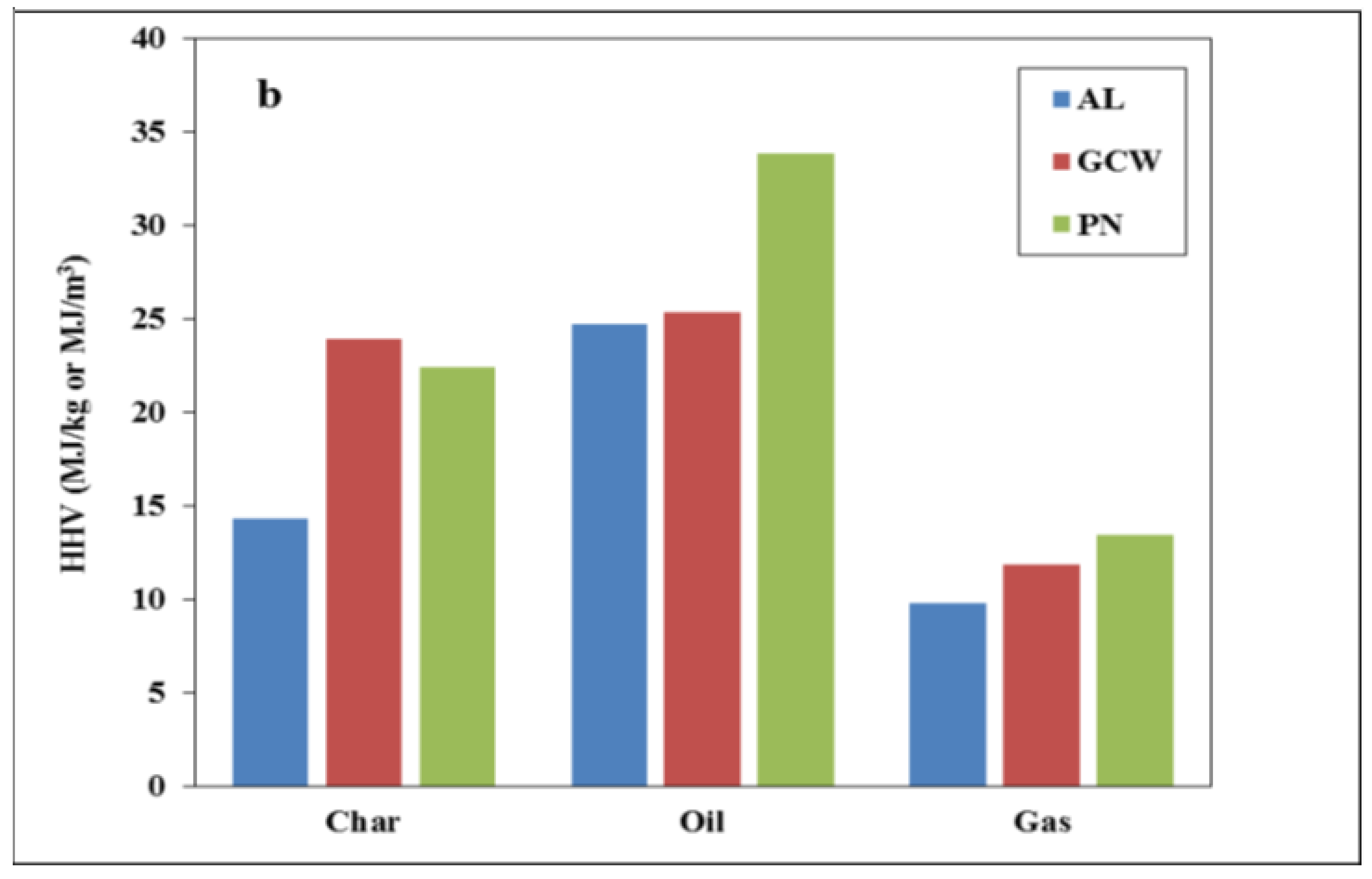
3.2. Pyrolysis Products Characterization
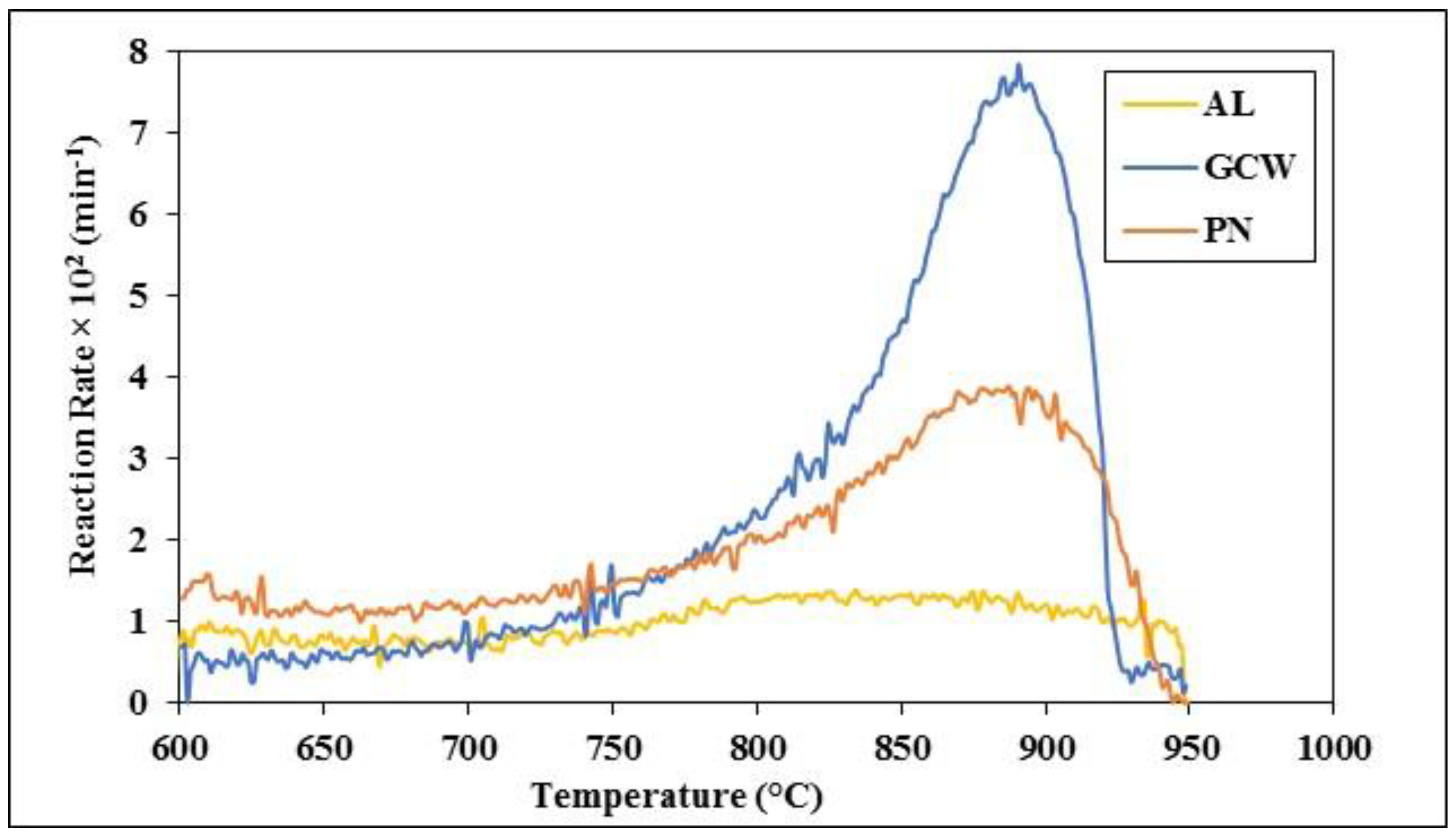
3.3. Gasification Characteristics of Lignite and Biomass Chars and Their Mixture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lahijani, P.; Zainal, Z.; Mohammadi, M.; Mohamed, A. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Beagle, E.; Wang, Y.; Bell, D.; Belmont, E. Co-gasification of pine and oak biochar with sub-bituminous coal in carbon dioxide. Bioresour. Technol. 2018, 251, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Vamvuka, D.; Teftiki, A.; Sfakiotakis, S. Increasing the reactivity of waste biochars during their co-gasification with carbon dioxide using catalysts and bio-oils. Thermochim. Acta 2021, 704, 179015. [Google Scholar] [CrossRef]
- Masnadi, M.; Grace, J.; Bi, X.; Lim, C.; Ellis, N. From fossil fuels towards renewable: Inhibitory and catalytic effects on carbon thermochemical conversion during co-gasification of biomass with fossil fuels. Appl. Energy 2015, 140, 196–209. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, J.; Zeng, X.; Li, X.; Yang, J.; Zou, Y.; Liu, S.; Dong, P.; Zhang, Y.; Liu, J. A high performance direct carbon solid oxide fuel cell-a green pathway for brown coal utilization. Appl. Energy 2019, 248, 679–687. [Google Scholar] [CrossRef]
- Santos, R.G.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Lampropoulos, A.; Kaklidis, N.; Athanasiou, C.; Montes-Moran, M.A.; Arenillas, A.; Menendez, J.A.; Binas, V.D.; Konsolakis, M.; Marnellos, G.E. Effect of olive kernel treatment (torrefaction vs. slow pyrolysis) on the physicochemical characteritics and the CO2 or H2O gasification performance of as-prepared biochars. Int. J. Hydrog. Prod. 2021, 46, 29126–29141. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S.; Pantelaki, O. Evaluation of gaseous and solid products from the pyrolysis of waste biomass blends for energetic and environmental applications. Fuel 2019, 236, 574–582. [Google Scholar] [CrossRef]
- Prasertcharoensuk, P.; Bull, S.J.; Phan, A.N. Gasification of waste biomass for hydrogen production: Effects of pyrolysis parameters. Renew. Energy 2019, 143, 112–120. [Google Scholar] [CrossRef]
- Zheng, X.; Ying, Z.; Wang, B.; Chen, C. Hydrogen and syngas production from municipal solid waste (MSW) gasification via reusing CO2. Appl. Therm. Eng. 2018, 144, 242–247. [Google Scholar] [CrossRef]
- Ziolkowski, P.; Madejski, P.; Amiri, M.; Kus, T.; Stasiak, K.; Subramanian, N.; Pawlak-Kruczek, H.; Badur, J.; Niedźwiecki, L.; Mikielewicz, D. Thermodynamic analysis of negative CO2 emission power plant using Aspen plus, Aspen hysys and Ebsilon software. Energies 2021, 14, 6304. [Google Scholar] [CrossRef]
- Chan, Y.H.; Rahman, S.N.F.S.A.; Lahuri, H.M.; Khalid, A. Recent progress on CO-rich syngas production via CO2 gasification of various wastes: A critical review on efficiency, challenges and outlook. Environ. Poll. 2021, 278, 116843. [Google Scholar] [CrossRef] [PubMed]
- Guizani, C.; Haddad, K.; Jeguirim, M.; Colin, B.; Limousy, L. Combustion characteristics and kinetics of torrefied olive pomace. Energy 2016, 107, 453–463. [Google Scholar] [CrossRef]
- Mafu, L.; Neomagus, H.; Everson, R.; Okolo, G.; Strydom, C.; Bunt, J. The carbon dioxide gasification characteristics of biomass char samples and their effect on coal gasification reactivity during co-gasification. Bioresour. Technol. 2018, 258, 70–78. [Google Scholar] [CrossRef]
- Eshun, J.; Wang, L.; Ansah, E.; Shahbazi, A.; Schimmel, K.; Kabadi, V.; Aravamudhan, S. Characterization of the physicochemical and structural evolution of biomass particles during combined pyrolysis and CO2 gasification. J. Energy Inst. 2019, 92, 82–93. [Google Scholar] [CrossRef]
- Maya, J.C.; Macias, R.; Gomez, C.A.; Chejne, F. On the evolution of pore microstructure during coal char activation with steam/CO2 mixtures. Carbon 2020, 158, 121–130. [Google Scholar] [CrossRef]
- Wang, M.; Wan, Y.; Guo, Q.; Bai, Y.; Yu, G.; Liu, Y.; Zhang, H.; Zhang, S.; Wei, J. Brief review on petroleum coke and biomass/coal co-gasification: Syngas production, reactivity characteristics and synergy behavior. Fuel 2021, 304, 121517. [Google Scholar] [CrossRef]
- Naidu, V.; Aghalayam, P.; Jayanti, S. Synergetic and inhibition effects in carbon dioxide gasification of blends of coals and biomass fuels of Indian origin. Bioresour. Technol. 2016, 209, 157–165. [Google Scholar] [CrossRef]
- Wei, J.; Gong, Y.; Guo, Q.; Chen, X.; Ding, L.; Yu, G. A mechanism investigation of synergy behavior variations during blended char co-gasification of biomass and different rank coals. Renew. Energy 2018, 131, 597–605. [Google Scholar] [CrossRef]
- Abdalazeez, A.; Li, T.; Wang, W.; Abuelgasim, S. A brief review of CO2 utilization for alkali carbonate gasification and biomass/coal co-gasification: Reactivity, products and process. J. CO2 Utiliz. 2021, 43, 101370. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, S.; Zhang, Q.; Hu, J.; Liu, R.; Huang, Z.; Gao, Y. Synergetic effect of the cotton stalk and high-ash coal on gas production during co-pyrolysis/gasification. Bioresour. Technol. 2021, 336, 125336. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J. Critical review of predictive coefficients for biomass ash deposition tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S.; Mpoumpouris, A. Slagging and fouling propensities of ashes from urban and industrial wastes. Rec. Innov. Chem. Eng. 2018, 11, 145–158. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, H.; Xu, K.; Xu, Q.; Li, L. Evaluation of the fusion and agglomeration properties of ashes from combustion of biomass, coal and their mixtures and the effects of K2CO3 additives. Fuel 2019, 255, 115829. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Xu, D.; Shi, L.; Li, B.; Bai, Y.; Yu, G.; Bao, W.; Fu, J.; Zhang, H.; et al. Migration and transformation of alkali/alkaline earth metal species during biomass and coal co-gasification: A review. Fuel Process. Technol. 2022, 235, 107376. [Google Scholar] [CrossRef]
- Mulvaney, D. Green new deal. In Sol Power; University of California Press: Berkley, CA, USA, 2019. [Google Scholar]
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008, on waste and repealing certain Directives. OJL 2008, 312, 3–30.
- Vamvuka, D. Biomass, Bioenergy and the Environment; Tziolas Publications: Salonica, Greece, 2009. [Google Scholar]
- European Association for Coal and Lignite—Eurocoal. Eurocoal Market. 2019. Available online: http://www.eurocoal.eu (accessed on 1 November 2022).
- REN21 Renewables 2020. Global Status Report. REN21 Secretariat: Paris, France. 2020. Available online: http://www.ren21.net/wp-content/uploads/2019/05/gsr_2020_full_report_en.pdf (accessed on 15 November 2021).
- International Energy Agency. Coal. 2018. Available online: http://www.iea.org/16topics/coal/ (accessed on 1 December 2020).
- Parvez, A.M.; Mujtaba, I.M.; Pang, C.; Lester, E.; Wu, T. Effect of the addition of different waste carbonaceous materials on coal gasification in CO2 atmosphere. Fuel Process. Technol. 2016, 149, 231–238. [Google Scholar] [CrossRef]
- Diao, R.; Li, S.; Deng, J.; Zhu, X. Interaction and kinetic analysis of co-gasification of bituminous coal with wasnut shell under CO2 atmosphere: Effect of inorganics and carbon structures. Renew. Energy 2021, 173, 177–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Yang, M.; Song, Y. Effect of fuel origin on synergy during co-gasification of biomass and coal in CO2. Bioresour. Technol. 2016, 200, 789–794. [Google Scholar] [CrossRef]
- Roni, M.S.; Chowdhury, S.; Mamun, S.; Marufuzzaman, M.; Lein, W.; Johnson, S. Biomass co-firing technology with policies and challenges and opportunities: A global review. Renew. Sustain. Energy Rev. 2017, 78, 1089–1101. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Wang, G.; Ning, X.; Wang, H.; Wang, P. Study on CO2 gasification reactivity of biomass char derived from high-temperature rapid pyrolysis. Appl. Therm. Eng. 2017, 121, 1022–1031. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gokalp, I.; Petrus, S.; Belandria, V.; Bostyn, S. Energy recovery analysis from sugar cane bagasse pyrolysis and gasification using thermogravimetry, mass spectrometry and kinetic models. J. Anal. Appl. Pyrolysis 2018, 132, 225–236. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, T.S.; Lee, S.M.; Choi, D.; Yeo, H.; Choi, I.G.; Choi, J.W. Comparison of physicochemical features of biooils and biochars produced from various woody biomasses by fast pyrolysis. Renew. Energy 2013, 50, 188–195. [Google Scholar] [CrossRef]
- Vamvuka, D.; Afthentopoulos, E.; Sfakiotakis, S. H2-rich gas production from steam gasification of a winery waste and its blends with industrial wastes. Effect of operating parameters on gas quality and efficiency. Renew. Energy 2022, 197, 1224–1232. [Google Scholar] [CrossRef]
- Roncancio, R.; Gore, J.P. CO2 char gasification: A systematic review from 2014 to 2020. Energy Convers. Manag. 2021, 10, 100060. [Google Scholar] [CrossRef]


| Sample | Volatiles | Fixed Carbon | Ash | C | H | N | O | S | GCV * (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|
| AL | 36.7 | 27.5 | 35.8 | 27.5 | 2.1 | 0.5 | 32.9 | 1.2 | 8.9 |
| GCW | 75.8 | 15.6 | 8.6 | 41.9 | 6.1 | 0.8 | 42.4 | 0.2 | 16.6 |
| PN | 76.8 | 17.5 | 5.7 | 47.8 | 6.9 | 0.2 | 39.3 | 0.05 | 20.1 |
| Sample | CaO | Al2O3 | Fe2O3 | MgO | SiO2 | K2O | Na2O | P2O5 |
|---|---|---|---|---|---|---|---|---|
| AL/GCW 70:30 | 6.6 | 10.4 | 8.1 | 3.9 | 43.8 | 14.0 | 1.3 | 0.8 |
| 50:50 | 6.5 | 8.6 | 5.8 | 6.4 | 37.1 | 20.9 | 2.0 | 1.1 |
| AL/PN 70:30 | 11.2 | 9.9 | 8.6 | 1.2 | 46.4 | 2.9 | 0.4 | 1.4 |
| 50:50 | 14.1 | 7.7 | 6.6 | 2.0 | 41.5 | 2.3 | 0.7 | 2.1 |
| Sample | AI | B/A | Rs | Sv | Fs | Deposition Tendency |
|---|---|---|---|---|---|---|
| AL | 0.32 | 0.38 | 74.4 | 1316 | low | |
| GCW | 0.19 | 2.51 | 0.50 | 51.6 | 1076 | high |
| PN | 0.006 | 0.93 | 0.05 | 51.9 | 1222 | medium to high |
| AL/GCW 70:30 | 0.70 | 0.63 | 69.6 | 1251 | medium | |
| 50:50 | 0.96 | 0.67 | 66.0 | 1200 | medium | |
| AL/PN 70:30 | 0.45 | 0.38 | 68.5 | 1296 | low to medium | |
| 50:50 | 0.55 | 0.34 | 64.1 | 1272 | medium |
| Sample | Organic Matter | Ash | C | H | N | O | S | GCV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|
| AL | 48.2 | 51.8 | 38.7 | 1.3 | 0.5 | 6.4 | 1.3 | 14.3 |
| GCW | 73.1 | 26.9 | 65.3 | 1.9 | 0.7 | 5.2 | - | 23.9 |
| PN | 79.9 | 20.1 | 63.5 | 1.9 | 0.8 | 13.7 | - | 22.4 |
| AL/GCW 70:30 | 55.0 | 45.0 | 46.5 | 1.6 | 0.5 | 5.4 | 1.0 | 17.4 |
| 50:50 | 60.9 | 39.1 | 52.4 | 1.6 | 0.6 | 5.5 | 0.8 | 19.3 |
| AL/PN 70:30 | 58.1 | 41.9 | 46.3 | 1.4 | 0.5 | 9.0 | 0.9 | 16.9 |
| 50:50 | 64.6 | 35.4 | 51.5 | 1.6 | 0.6 | 10.2 | 0.7 | 18.5 |
| Sample | AL | GCW | PN | AL/GCW 70:30 | AL/GCW 50:50 | AL/PN 70:30 | AL/PN 50:50 | |
|---|---|---|---|---|---|---|---|---|
| Tmax (°C) | 860 | 893 | 890 | 923 | 937 | 910 | 917 | |
| Rmax × 102 (min−1) | 1.4 | 8.0 | 3.9 | 1.7 | 2.0 | 2.0 | 2.4 | |
| RG × 104 (min−1/°C) | 0.16 | 0.89 | 0.44 | 0.19 | 0.21 | 0.22 | 0.26 | |
| Gas (mol%) | CO | 93.4 | 92.3 | 92.2 | 93.2 | 92.9 | 93.1 | 93.0 |
| H2O | 5.0 | 5.9 | 6.3 | 5.1 | 5.3 | 5.3 | 5.5 | |
| H2 | 1.3 | 1.5 | 1.1 | 1.4 | 1.5 | 1.3 | 1.2 | |
| CH4 | 0.1 | 0.08 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| CxHy | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | |
| HHV (MJ/m3) | 12.2 | 12.1 | 12.0 | 12.2 | 12.1 | 12.1 | 12.1 | |
| CE (%) | 90.0 | 100.0 | 100.0 | 92.4 | 94.1 | 92.0 | 94.5 | |
| CGE (%) | 31.8 | 41.2 | 40.1 | 30.9 | 31.5 | 33.6 | 35.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamvuka, D.; Tsagris, G.; Loulashi, C. Co-Gasification Performance of Low-Quality Lignite with Woody Wastes Using Greenhouse Gas CO2—A TG–MS Study. Sustainability 2023, 15, 9818. https://doi.org/10.3390/su15129818
Vamvuka D, Tsagris G, Loulashi C. Co-Gasification Performance of Low-Quality Lignite with Woody Wastes Using Greenhouse Gas CO2—A TG–MS Study. Sustainability. 2023; 15(12):9818. https://doi.org/10.3390/su15129818
Chicago/Turabian StyleVamvuka, Despina, George Tsagris, and Christia Loulashi. 2023. "Co-Gasification Performance of Low-Quality Lignite with Woody Wastes Using Greenhouse Gas CO2—A TG–MS Study" Sustainability 15, no. 12: 9818. https://doi.org/10.3390/su15129818
APA StyleVamvuka, D., Tsagris, G., & Loulashi, C. (2023). Co-Gasification Performance of Low-Quality Lignite with Woody Wastes Using Greenhouse Gas CO2—A TG–MS Study. Sustainability, 15(12), 9818. https://doi.org/10.3390/su15129818






