Polyphenol Characterization and Antioxidant Capacity of Multi-Species Swards Grown in Ireland—Environmental Sustainability and Nutraceutical Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Polyphenol Extraction
2.3. Total Polyphenol Content and Total Flavonoid Content
2.4. Characterisation of Polyphenols Using LC-ESI-QqQ-MS
2.5. Antioxidant Studies
2.5.1. Ferric Reducing Antioxidant Power (FRAP)
2.5.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH••)
2.5.3. Oxygen Radical Antioxidant Capacity (ORAC)
2.6. Statistical Analysis
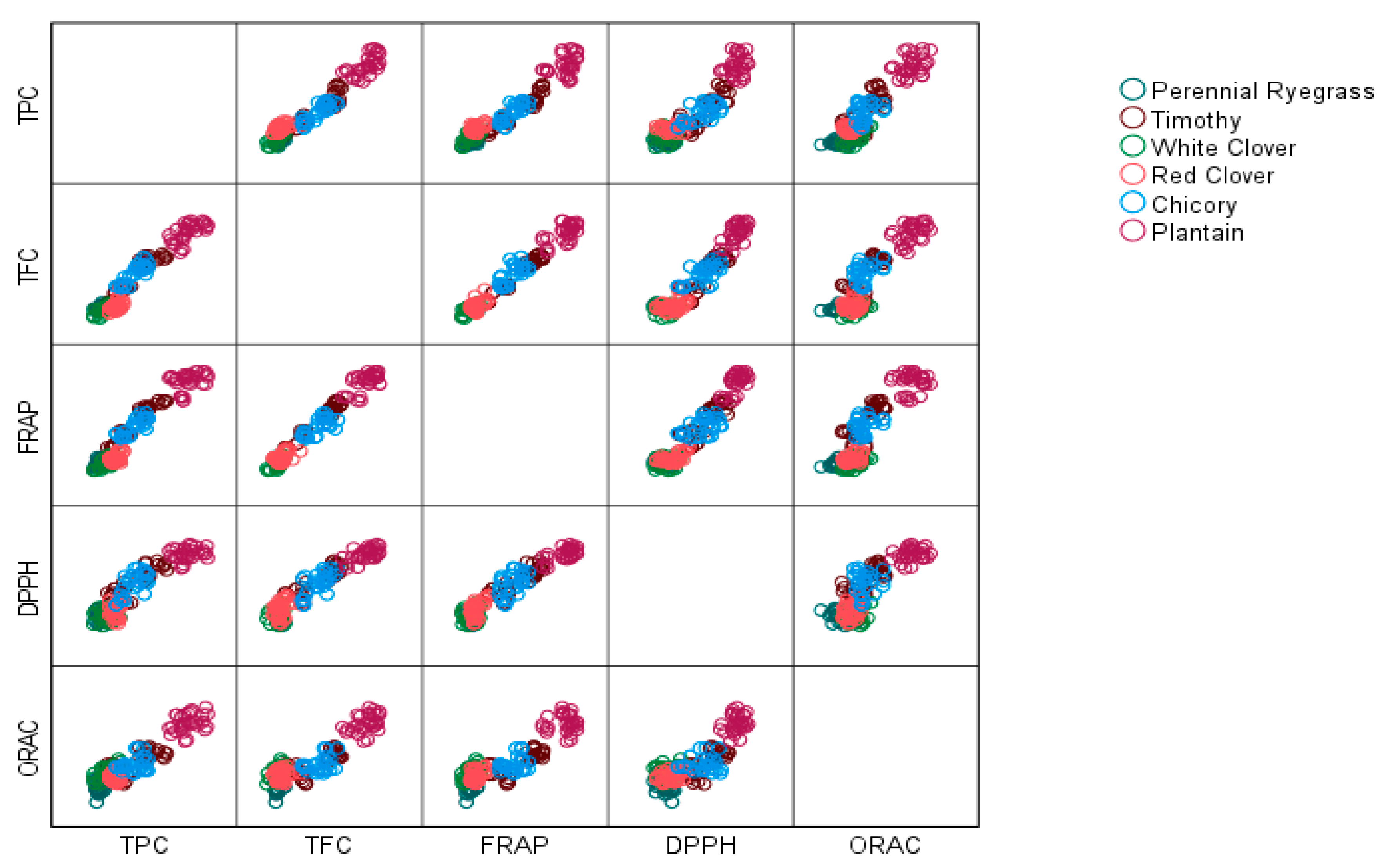
3. Results
3.1. Total Polyphenol and Flavonoid Content
3.2. Characterisation of Polyphenols Using LC-ESI-QqQ-MS
3.3. Antioxidant Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DCCAE (Department of Communications, Climate Action and the Environment). National Energy and Climate Plan 2021–2030. 2019. Available online: https://ec.europa.eu/info/energy-climate-change-environment/implementation-eu-countries/energy-and-climate-governance-and-reporting/national-energy-and-climate-plans_en (accessed on 15 November 2022).
- EPA (Environmental Protection Agency). Latest Emissions Data. 2021. Available online: https://www.epa.ie/our-services/monitoring—Assessment/climate-change/ghg/latest-emissions-data/ (accessed on 15 November 2022).
- EPA (Environmental Protection Agency). Understanding Global Warming Potentials. 2021. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials#:~:text=Nitrous%20Oxide%20(N2O,than%20100%20years%2C%20on%20average (accessed on 15 November 2022).
- Jaramillo, D.M.; Sheridan, H.; Soder, K.; Dubeux, J.C.B., Jr. Enhancing the Sustainability of Temperate Pasture Systems through More Diverse Swards. Agronomy 2021, 11, 1912. [Google Scholar] [CrossRef]
- Fustec, J.; Lesuffleur, F.; Mahieu, S.; Cliquet, J.B. Nitrogen rhizodeposition of legumes. A review. Agron. Sustain. Dev. 2010, 30, 57–66. [Google Scholar] [CrossRef]
- Cranston, L.M.; Kenyon, P.R.; Morris, S.T.; Kemp, P.D. A review of the use of chicory, plantain, red clover and white clover in a sward mix for increased sheep and beef production. J. N. Z. Grassl. 2015, 77, 89–94. [Google Scholar] [CrossRef]
- Distel, R.A.; Arroquy, J.I.; Lagrange, S.; Villalba, J.J. Designing diverse agricultural pastures for improving ruminant production systems. Front. Sustain. Food Syst. 2020, 4, 596869. [Google Scholar] [CrossRef]
- Harlow, B.E.; Flythe, M.D.; Kagan, I.A.; Goodman, J.P.; Klotz, J.L.; Aiken, G.E. Isoflavone supplementation, via red clover hay, alters the rumen microbial community and promotes weight gain of steers grazing mixed grass pastures. PLoS ONE 2020, 15, e0229200. [Google Scholar] [CrossRef]
- Stoldt, A.-K.; Derno, M.; Nürnberg, G.; Weitzel, J.M.; Otten, W.; Starke, A.; Wolffram, S.; Metges, C.C. Effects of a 6-wk intraduodenal supplementation with quercetin on energy metabolism and indicators of liver damage in periparturient dairy cows. J. Dairy Sci. 2015, 98, 4509–4520. [Google Scholar] [CrossRef]
- Burmańczuk, A.; Hola, P.; Milczak, A.; Piech, T.; Kowalski, C.; Wojciechowska, B.; Grabowski, T. Quercetin decrease somatic cells count in mastitis of dairy cows. Res. Vet. Sci. 2018, 117, 255–259. [Google Scholar] [CrossRef]
- Clarkson, T.B. Soy, Soy Phytoestrogens and Cardiovascular Disease. Nutr. Bull. 2006, 31, 150–159. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Fraisse, D.; Carnat, A.; Viala, D.; Pradel, P.; Besle, J.M.; Coulon, J.B.; Felgines, C.; Lamaison, J.L. Polyphenolic composition of a permanent pasture: Variations related to the period of harvesting. Sci. Food Agric. 2007, 87, 2427–2435. [Google Scholar] [CrossRef]
- Gupta, S.; Rajauria, G.; Abu-Ghannam, N. Study of the microbial diversity and antimicrobial properties of Irish edible brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 482–489. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. A comparative study on the polyphenolic content, antibacterial activity and antioxidant capacity of different solvent extracts of Brassica oleracea vegetables. Int. J. Food Sci. Technol. 2011, 47, 223–231. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Kaurinović, B.; Popović, M.; Vasiljević, S. Profile of phenolic compounds in Trifolium pratense L. extracts at different growth stages and their biological activities. Int. J. Food Prop. 2016, 20, 3090–3101. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Shannon, E.; Jaiswal, A.K.; Abu-Ghannam, N. Polyphenolic content and antioxidant capacity of white, green, black, and herbal teas: A kinetic study. Food Res. 2017, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Dalar, A.; Uzun, Y.; Turker, M.; Mukemre, M.; Konczak, I. Health attributes of ethnic vegetables consumed in the Eastern Anatolia region of Turkey: Antioxidant and enzyme-inhibitory properties. J. Ethn. Foods 2016, 3, 142–149. [Google Scholar] [CrossRef][Green Version]
- Dalar, A.; Konczak, I. Cichorium intybus from Eastern Anatolia: Phenolic composition, antioxidant and enzyme inhibitory activities. Ind. Crops Prod. 2014, 60, 79–85. [Google Scholar] [CrossRef]
- Milala, J.; Grzelak, K.; Król, B.; Juśkiewicz, J.; Zduńczyk, Z. Composition and properties of chicory extracts rich in fructans and polyphenols. Pol. J. Food Nutr. Sci. 2009, 59, 35–43. [Google Scholar]
- Saviranta, N.; Julkunen-Tiitto, R.; Oksanen, E.; Karjalainen, R.O. Red clover (Trifolium pratense L.) isoflavones: Root phenolic compounds affected by biotic and abiotic stress factors. J. Sci. Food Agric. 2010, 90, 418–423. [Google Scholar] [CrossRef]
- Qawasmeh, A.; Obied, H.K.; Raman, A.; Wheatley, W. Influence of Fungal Endophyte Infection on Phenolic Content and Antioxidant Activity in Grasses: Interaction between Lolium perenne and Different Strains of Neotyphodium lolii. J. Agric. Food Chem. 2012, 60, 3381–3388. [Google Scholar] [CrossRef] [PubMed]
- Besle, J.M.; Viala, D.; Martin, B.; Pradel, P.; Meunier, B.; Berdagu’e, J.L.; Fraisse, D.; Lamaison, J.L.; Coulon, J.B. Ultraviolet-absorbing compounds in milk are related to forage polyphenols. J. Dairy Sci. 2010, 93, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Papadopoulos, Y.; Yang, R.; Young, J.C.; McRae, K. Isoflavone Profiles of Red Clovers and Their Distribution in Different Parts Harvested at Different Growing Stages. J. Agric. Food Chem. 2006, 54, 5797–5805. [Google Scholar] [CrossRef]
- Iqbal, Y.; Ponnampalam, E.N.; Suleria, H.A.R.; Cottrell, J.J.; Dunshea, F.R. LC-ESI/QTOF-MS Profiling of Chicory and Lucerne Polyphenols and Their Antioxidant Activities. Antioxidants 2021, 10, 932. [Google Scholar] [CrossRef]
- Beara, I.N.; Lesjak, M.M.; Orčić, D.Z.; Simin, N.D.; Četojević-Simin, D.D.; Božin, B.N.; Mimica-Dukić, N.M. Comparative analysis of phenolic profile, antioxidant, anti-inflammatory and cytotoxic activity of two closely related Plantain species: Plantago altissima L. and Plantago lanceolata. Food Sci. Technol. 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Valente, A.H.; Thamsborg, S.M.; Simonsen, H.T.; Boas, U.; Enemark, H.L. Antiparasitic activity of chicory (Cichorium intybus) and its natural bioactive compounds in livestock: A review. Parasites Vectors 2018, 11, 475. [Google Scholar] [CrossRef]
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef]
- Kagan, I.A. Soluble phenolic compounds of perennial ryegrass (Lolium perenne L.): Potential effects on animal performance, and challenges in determining profiles and concentrations. Anim. Feed. Sci. Technol. 2020, 277, 114960. [Google Scholar] [CrossRef]
- Diago, M.P.; Ayestarán, B.; Guadalupe, Z.; Poni, S.; Tardáguila, J. Impact of prebloom and fruit set basal leaf removal on the flavonol and anthocyanin composition of Tempranillo grapes. Am. J. Enol. Vitic. 2012, 63, 367–376. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Olmos Colmenero, J.J.O.; Winters, A.L.; Scollan, N.D.; Minchin, F.R. Polyphenol oxidase activity in grass and its effect on plant-mediated lipolysis and proteolysis of Dactylis glomerata (cocksfoot) in a simulated rumen environment. J. Sci. Food Agric. 2006, 86, 1503–1511. [Google Scholar] [CrossRef]
- Gong, X.X.; Su, X.S.; Zhan, K.; Zhao, G.Q. The protective effect of chlorogenic acid on bovine mammary epithelial cells and neutrophil function. J. Dairy Sci. 2018, 101, 10089–10097. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, S. Teagasc Dairy Manual. 2016; Volume 6, p. 34. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.teagasc.ie/media/website/animals/dairy/FeedingDiaryCow.pdf (accessed on 15 November 2022).
- Křížová, L.; Křešťáková, V.; Dadáková, K.; Kašparovský, T. Production of Bovine Equol-Enriched Milk: A Review. Animals 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Hoikkala, A.; Mustonen, E.; Saastamoinen, I. High levels of equol in organic skimmed Finnish cow milk. Mol. Nutr. Food Res. 2007, 51, 782–786. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Oskoueian, A. Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. BioMed Res. Int. 2013, 2013, 349129. [Google Scholar] [CrossRef]
- Sinz, S.; Kunz, C.; Liesegang, A.; Braun, U.; Marquardt, S.; Soliva, C.R.; Kreuzer, M. In vitro bioactivity of various pure flavonoids in ruminal fermentation, with special reference to methane formation. Czech J. Anim. Sci. 2018, 63, 293–304. [Google Scholar]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Wang, D.; Huang, J.; Zhang, Z.; Tian, X.; Huang, H.; Yu, Y.; Zhang, G.; Ding, J.; Huang, R. Influences of Portulaca oleracea extracts on in vitro methane emissions and rumen fermentation of forage. J. Food Agric. Environ. 2013, 11, 483–488. [Google Scholar]
- Minneé, E.M.K.; Waghorn, G.C.; Lee, J.M.; Clark, C.E.F. Including chicory or plantain in a perennial ryegrass/white clover-based diet of dairy cattle in late lactation: Feed intake, milk production and rumen digestion. Anim. Feed. Sci. Technol. 2017, 227, 52–61. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Beaulieu, D.; Barbano, A.D.M. Feed and Animal Factors Influencing Milk Fat Composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef] [PubMed]
- Teagasc. Grassland Re-Seeding: How to Establosh Multispecies Swards. 2020. Available online: https://www.teagasc.ie/publications/2020/grassland-re-seeding-how-to-establish-multi-species-swards.php (accessed on 15 November 2022).
| Targets | Molecular Formula | Retention Time (min) | Fragmentor Voltage (V) | Collision Energy (V) | Precursor Ion (m/z) | Product Ion (m/z) |
|---|---|---|---|---|---|---|
| Chlorogenic acid | C16H18O9 | 1.090 | 165 | 10 | 353.2 | 191.0 |
| Naringin | C27H32O14 | 2.137 | 225 | 33 | 579.4 | 271.2 |
| Daidzein | C15H10O4 | 3.700 | 145 | 31 | 253.0 | 208.0 |
| Quercetin | C15H10O7 | 4.094 | 130 | 15 | 301.1 | 151.1 |
| Kaempferol | C15H10O6 | 4.640 | 130 | 0 | 285.0 | 285.0 |
| Luteolin | C15H10O6 | 4.640 | 135 | 25 | 285.2 | 133.0 |
| Formononetin | C16H12O4 | 6.947 | 112 | 10 | 267.0 | 252.0 |
| Biochanin A | C16H12O5 | 8.636 | 135 | 17 | 283.0 | 268.0 |
| Perennial Ryegrass | Timothy | ||||||
|---|---|---|---|---|---|---|---|
| April | TPC | 26.93 a | ±3.16 | April | TPC | 52.37 c | ±4.32 |
| TFC | 14.57 a | ±1.25 | TFC | 35.72 b | ±2.01 | ||
| May | TPC | 29.43 a | ±1.72 | May | TPC | 37.71 c | ±3.18 |
| TFC | 13.8 a | ±2.20 | TFC | 23.11 b | ±4.86 | ||
| June | TPC | 25.15 a | ±3.43 | June | TPC | 95.45 c | ±4.26 |
| TFC | 12.97 a | ±3.82 | TFC | 67.12 b | ±2.57 | ||
| July | TPC | 23.01 a | ±2.93 | July | TPC | 75.41 c | ±3.08 |
| TFC | 10.70 a | ±0.78 | TFC | 60.29 b | ±2.98 | ||
| August | TPC | 19.95 a | ±1.74 | August | TPC | 76.95 c | ±4.33 |
| TFC | 11.73 a | ±0.93 | TFC | 64.62 b | ±3.68 | ||
| White Clover | Red Clover | ||||||
| April | TPC | 38.04 a | ±5.26 | April | TPC | 40.57 b | ±4.92 |
| TFC | 17.06 a | ±1.07 | TFC | 12.62 a | ±4.15 | ||
| May | TPC | 20.16 a | ±3.85 | May | TPC | 41.70 b | ±2.81 |
| TFC | 6.27 a | ±4.06 | TFC | 13.06 a | ±2.88 | ||
| June | TPC | 33.17 a | ±5.58 | June | TPC | 47.49 b | ±2.97 |
| TFC | 12.33 a | ±3.30 | TFC | 21.84 a | ±5.79 | ||
| July | TPC | 39.63 a | ±4.13 | July | TPC | 38.60 b | ±3.38 |
| TFC | 16.01 a | ±0.83 | TFC | 15.52 a | ±5.27 | ||
| August | TPC | 31.70 a | ±3.99 | August | TPC | 43.49 b | ±2.54 |
| TFC | 12.64 a | ±1.63 | TFC | 13.68 a | ±2.60 | ||
| Chicory | Plantain | ||||||
| April | TPC | 71.01 c | ±5.89 | April | TPC | 118.58 d | ±1.43 |
| TFC | 58.95 b | ±4.64 | TFC | 81.01 c | ±7.37 | ||
| May | TPC | 74.94 c | ±3.14 | May | TPC | 137.11 d | ±9.40 |
| TFC | 53.67 b | ±2.52 | TFC | 102.42 c | ±3.72 | ||
| June | TPC | 67.77 c | ±6.47 | June | TPC | 138.69 d | ±8.11 |
| TFC | 55.94 b | ±6.82 | TFC | 101.12 c | ±3.36 | ||
| July | TPC | 52.57 c | ±4.24 | July | TPC | 112.27 d | ±8.50 |
| TFC | 40.55 b | ±5.27 | TFC | 87.77 c | ±5.60 | ||
| August | TPC | 47.50 c | ±4.40 | August | TPC | 120.29 d | ±7.20 |
| TFC | 35.56 b | ±0.93 | TFC | 96.24 c | ±5.83 | ||
| Perennial Ryegrass | Timothy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| April | BcA | 0.01 | ±0.01 | Kae | 0.05 | ±0.02 | April | BcA | 0.01 | ±0.01 | Kae | 0.07 | ±0.04 |
| CgA | 6.62 | ±0.41 | Lu | 0.03 | ±0.03 | CgA | 14.61 | ±1.21 | Lu | 0.04 | ±0.03 | ||
| Dz | 0.05 | ±0.06 | Nar | 0.05 | ±0.07 | Dz | 0.04 | ±0.04 | Nar | 0.04 | ±0.05 | ||
| Fmnt | 0.05 | ±0.02 | Que | 0.03 | ±0.03 | Fmnt | 0.04 | ±0.02 | Que | 0.01 | ±0.01 | ||
| May | BcA | 0.05 | ±0.04 | Kae | 0.02 | ±0.01 | May | BcA | 0 | ±0.01 | Kae | 0.02 | ±0.01 |
| CgA | 5.64 | ±0.87 | Lu | 0.01 | ±0.01 | CgA | 13.83 | ±0.57 | Lu | 0.04 | ±0.01 | ||
| Dz | 0.03 | ±0.03 | Nar | 0 | ±0.01 | Dz | 0.02 | ±0.01 | Nar | 0.02 | ±0.01 | ||
| Fmnt | 0.04 | ±0.01 | Que | 0.01 | ±0.01 | Fmnt | 0.06 | ±0.01 | Que | 0.01 | ±0.01 | ||
| June | BcA | 0.02 | ±0.02 | Kae | 0.02 | ±0.01 | June | BcA | 0.03 | ±0.03 | Kae | 0.02 | ±0.02 |
| CgA | 4.51 | ±0.48 | Lu | 0.01 | ±0.00 | CgA | 16.61 | ±0.29 | Lu | 0.01 | ±0.02 | ||
| Dz | 0.02 | ±0.02 | Nar | 0.01 | ±0.01 | Dz | 0.01 | ±0.02 | Nar | 0.01 | ±0.00 | ||
| Fmnt | 0.06 | ±0.03 | Que | 0.01 | ±0.00 | Fmnt | 0.04 | ±0.04 | Que | 0.01 | ±0.02 | ||
| July | BcA | 0.01 | ±0.01 | Kae | 0.01 | ±0.01 | July | BcA | 0.06 | ±0.05 | Kae | 0.02 | ±0.01 |
| CgA | 4.63 | ±0.57 | Lu | 0.03 | ±0.03 | CgA | 17.07 | ±1.35 | Lu | 0.03 | ±0.01 | ||
| Dz | 0.01 | ±0.02 | Nar | 0.01 | ±0.01 | Dz | 0.01 | ±0.01 | Nar | 0.01 | ±0.00 | ||
| Fmnt | 0.01 | ±0.01 | Que | 0.01 | ±0.00 | Fmnt | 0.15 | ±0.03 | Que | 0 | ±0.00 | ||
| August | BcA | 0.01 | ±0.01 | Kae | 0.01 | ±0.01 | August | BcA | 0.09 | ±0.07 | Kae | 0.02 | ±0.01 |
| CgA | 1.94 | ±0.33 | Lu | 0.01 | ±0.01 | CgA | 20.69 | ±0.56 | Lu | 0.05 | ±0.04 | ||
| Dz | 0.01 | ±0.01 | Nar | 0.01 | ±0.01 | Dz | 0.01 | ±0.00 | Nar | 0.03 | ±0.03 | ||
| Fmnt | 0.03 | ±0.01 | Que | 0.01 | ±0.02 | Fmnt | 0.25 | ±0.03 | Que | 0.01 | ±0.00 | ||
| White Clover | Red Clover | ||||||||||||
| April | BcA | 0.02 | ±0 | Kae | 0.03 | ±0.01 | April | BcA | 1.6 | ±0.11 | Kae | 0.05 | ±0.04 |
| CgA | 1.05 | ±0.01 | Lu | 0.06 | ±0.02 | CgA | 0.11 | ±0.05 | Lu | 0.03 | ±0.02 | ||
| Dz | 0.02 | ±0.01 | Nar | 0.02 | ±0.03 | Dz | 1.07 | ±0.15 | Nar | 0.01 | ±0.02 | ||
| Fmnt | 0.57 | ±0.07 | Que | 0.01 | ±0.01 | Fmnt | 4.29 | ±0.69 | Que | 0.01 | ±0.00 | ||
| May | BcA | 0.02 | ±0.01 | Kae | 0.02 | ±0.01 | May | BcA | 2.22 | ±0.55 | Kae | 0.03 | ±0.02 |
| CgA | 0.21 | ±0.05 | Lu | 0.02 | ±0.01 | CgA | 0.11 | ±0.03 | Lu | 0.05 | ±0.00 | ||
| Dz | 0.01 | ±0 | Nar | 0.01 | ±0.01 | Dz | 0.43 | ±0.02 | Nar | 0.02 | ±0.02 | ||
| Fmnt | 0.1 | ±0.02 | Que | 0.01 | ±0.01 | Fmnt | 5.64 | ±0.68 | Que | 0 | ±0.01 | ||
| June | BcA | 0.02 | ±0.02 | Kae | 0.03 | ±0.01 | June | BcA | 2.44 | ±0.55 | Kae | 0.03 | ±0.03 |
| CgA | 0.19 | ±0.07 | Lu | 0.03 | ±0.01 | CgA | 0.06 | ±0.01 | Lu | 0.07 | ±0.03 | ||
| Dz | 0.01 | ±0.02 | Nar | 0.01 | ±0.01 | Dz | 0.9 | ±0.03 | Nar | 0.01 | ±0.01 | ||
| Fmnt | 0.19 | ±0.05 | Que | 0.01 | ±0.01 | Fmnt | 5.65 | ±0.76 | Que | 0.01 | ±0.01 | ||
| July | BcA | 4.04 | ±0.84 | Kae | 0.06 | ±0.03 | July | BcA | 2.57 | ±0.53 | Kae | 0.05 | ±0.06 |
| CgA | 0.17 | ±0.03 | Lu | 0.05 | ±0.05 | CgA | 0.04 | ±0.00 | Lu | 0.03 | ±0.00 | ||
| Dz | 0.04 | ±0.03 | Nar | 0.01 | ±0.01 | Dz | 0.44 | ±0.05 | Nar | 0.01 | ±0.00 | ||
| Fmnt | 7.25 | ±1.11 | Que | 0.01 | ±0.00 | Fmnt | 5.86 | ±0.76 | Que | 0.01 | ±0.00 | ||
| August | BcA | 0.04 | ±0.02 | Kae | 0.02 | ±0.02 | August | BcA | 3.03 | ±0.46 | Kae | 0.02 | ±0.01 |
| CgA | 0.08 | ±0.04 | Lu | 0.04 | ±0.01 | CgA | 0.07 | ±0.06 | Lu | 0.04 | ±0.01 | ||
| Dz | 0.01 | ±0 | Nar | 0.01 | ±0.01 | Dz | 0.61 | ±0.03 | Nar | 0.01 | ±0.01 | ||
| Fmnt | 1.71 | ±0.29 | Que | 0.04 | ±0.06 | Fmnt | 8.23 | ±0.81 | Que | 0.01 | ±0.01 | ||
| Chicory | Plantain | ||||||||||||
| April | BcA | 0.07 | ±0.01 | Kae | 0.84 | ±0.13 | April | BcA | 0.06 | ±0.01 | Kae | 0.04 | ±0.02 |
| CgA | 0.77 | ±0.05 | Lu | 0.9 | ±0.23 | CgA | 7.36 | ±0.69 | Lu | 0.02 | ±0.02 | ||
| Dz | 0.35 | ±0.59 | Nar | 0.02 | ±0.02 | Dz | 0.02 | ±0.01 | Nar | 0.01 | ±0.01 | ||
| Fmnt | 0.3 | ±0.01 | Que | 0.01 | ±0.01 | Fmnt | 0.35 | ±0.09 | Que | 0.01 | ±0.01 | ||
| May | BcA | 0.06 | ±0.01 | Kae | 1.23 | ±0.25 | May | BcA | 0.05 | ±0.02 | Kae | 0.03 | ±0.02 |
| CgA | 1.73 | ±0.30 | Lu | 1.29 | ±0.17 | CgA | 9.58 | ±0.30 | Lu | 0.03 | ±0.02 | ||
| Dz | 0.01 | ±0.01 | Nar | 0.01 | ±0.01 | Dz | 0.01 | ±0.01 | Nar | 0 | ±0.01 | ||
| Fmnt | 4.36 | ±0.40 | Que | 0.04 | ±0.05 | Fmnt | 0.11 | ±0.02 | Que | 0.01 | ±0.01 | ||
| June | BcA | 0.57 | ±0.10 | Kae | 0.89 | ±0.00 | June | BcA | 0.06 | ±0.04 | Kae | 0.02 | ±0.02 |
| CgA | 1.55 | ±0.20 | Lu | 0.87 | ±0.00 | CgA | 8.08 | ±0.58 | Lu | 0.05 | ±0.03 | ||
| Dz | 0.01 | ±0.01 | Nar | 0.02 | ±0.01 | Dz | 0.01 | ±0.01 | Nar | 0.02 | ±0.01 | ||
| Fmnt | 1.38 | ±0.35 | Que | 0.01 | ±0.00 | Fmnt | 0.14 | ±0.02 | Que | 0.01 | ±0.01 | ||
| July | BcA | 0.38 | ±0.14 | Kae | 1.27 | ±0.26 | July | BcA | 0.21 | ±0.03 | Kae | 0.15 | ±0.12 |
| CgA | 1.16 | ±0.27 | Lu | 1.25 | ±0.27 | CgA | 7.43 | ±0.26 | Lu | 0.39 | ±0.27 | ||
| Dz | 0.02 | ±0.01 | Nar | 0.01 | ±0.01 | Dz | 0.01 | ±0.01 | Nar | 0.01 | ±0.01 | ||
| Fmnt | 1.51 | ±0.44 | Que | 0.01 | ±0.00 | Fmnt | 0.74 | ±0.24 | Que | 0 | ±0.00 | ||
| August | BcA | 3.25 | ±0.00 | Kae | 0.03 | ±0.00 | August | BcA | 0.45 | ±0.18 | Kae | 0.13 | ±0.05 |
| CgA | 0.83 | ±0.06 | Lu | 0.06 | ±0.03 | CgA | 5.49 | ±0.89 | Lu | 0.1 | ±0.06 | ||
| Dz | 0.28 | ±0.00 | Nar | 0.01 | ±0.00 | Dz | 0.01 | ±0.02 | Nar | 0 | ±0.01 | ||
| Fmnt | 5.68 | ±0.00 | Que | 0.01 | ±0.00 | Fmnt | 0.86 | ±0.30 | Que | 0.01 | ±0.01 | ||
| Perennial Ryegrass | Timothy | ||||||
|---|---|---|---|---|---|---|---|
| April | FRAP | 90.04 a | ±3.01 | April | FRAP | 216.75 b | ±7.92 |
| DPPH•• | 20.93 a | ±3.19 | DPPH•• | 42.96 c | ±7.51 | ||
| ORAC | 1053.55 a | ±111.18 | ORAC | 1027.61 c | ±106.38 | ||
| May | FRAP | 80.26 a | ±5.38 | May | FRAP | 154.15 b | ±8.03 |
| DPPH•• | 19.27 a | ±3.54 | DPPH•• | 43.90 c | ±2.75 | ||
| ORAC | 1038.14 a | ±109.19 | ORAC | 1243.69 c | ±132.59 | ||
| June | FRAP | 102.77 a | ±3.92 | June | FRAP | 352.00 b | ±6.97 |
| DPPH•• | 23.01 a | ±5.98 | DPPH•• | 68.07 c | ±2.81 | ||
| ORAC | 960.15 a | ±100.64 | ORAC | 1586.34 c | ±68.96 | ||
| July | FRAP | 79.04 a | ±6.76 | July | FRAP | 307.18 b | ±8.98 |
| DPPH•• | 25.87 a | ±3.31 | DPPH•• | 62.48 c | ±6.50 | ||
| ORAC | 769.36 a | ±46.27 | ORAC | 1510.63 c | ±73.57 | ||
| August | FRAP | 67.36 a | ±7.96 | August | FRAP | 326.84 b | ±4.62 |
| DPPH•• | 23.50 a | ±4.97 | DPPH•• | 60.05 c | ±1.93 | ||
| ORAC | 755.08 a | ±156.06 | ORAC | 1665.46 c | ±94.46 | ||
| White Clover | Red Clover | ||||||
| April | FRAP | 108.57 a | ±6.43 | April | FRAP | 97.25 a | ±8.43 |
| DPPH•• | 23.30 ab | ±5.54 | DPPH•• | 23.74 b | ±4.57 | ||
| ORAC | 1251.90 b | ±108.17 | ORAC | 1082.85 b | ±81.19 | ||
| May | FRAP | 50.58 a | ±4.33 | May | FRAP | 89.54 a | ±7.22 |
| DPPH•• | 24.40 ab | ±5.52 | DPPH•• | 29.22 b | ±3.76 | ||
| ORAC | 1009.17 b | ±85.25 | ORAC | 1176.31 b | ±102.53 | ||
| June | FRAP | 97.95 a | ±6.20 | June | FRAP | 136.05 a | ±5.35 |
| DPPH•• | 27.21 ab | ±3.85 | DPPH•• | 37.58 b | ±2.87 | ||
| ORAC | 1185.22 b | ±65.08 | ORAC | 1220.49 b | ±71.11 | ||
| July | FRAP | 94.53 a | ±6.20 | July | FRAP | 90.91 a | ±7.62 |
| DPPH•• | 30.19 ab | ±4.34 | DPPH•• | 30.39 b | ±4.51 | ||
| ORAC | 1319.97 b | ±82.71 | ORAC | 1051.56 b | ±93.29 | ||
| August | FRAP | 69.07 a | ±3.26 | August | FRAP | 92.02 a | ±7.28 |
| DPPH•• | 18.44 ab | ±2.71 | DPPH•• | 23.36 b | ±6.86 | ||
| ORAC | 1219.08 b | ±107.26 | ORAC | 988.17 b | ±53.15 | ||
| Chicory | Plantain | ||||||
| April | FRAP | 270.24 b | ±10.53 | April | FRAP | 368.70 c | ±9.40 |
| DPPH•• | 54.96 c | ±8.00 | DPPH•• | 71.59 d | ±5.22 | ||
| ORAC | 1594.22 c | ±132.29 | ORAC | 2200.39 d | ±123.70 | ||
| May | FRAP | 286.71 b | ±12.19 | May | FRAP | 448.14 c | 17.78 |
| DPPH•• | 56.22 c | ±4.92 | DPPH•• | 79.94 d | ±5.22 | ||
| ORAC | 1289.67 c | ±112.20 | ORAC | 2478.93 d | ±125.47 | ||
| June | FRAP | 244.75 b | ±8.35 | June | FRAP | 482.49 c | ±6.75 |
| DPPH•• | 58.98 c | ±6.78 | DPPH•• | 80.94 d | ±3.50 | ||
| ORAC | 1224.31 c | ±71.76 | ORAC | 2230.17 d | ±73.90 | ||
| July | FRAP | 194.44 b | ±4.14 | July | FRAP | 457.90 c | ±7.12 |
| DPPH•• | 50.83 c | ±6.47 | DPPH•• | 78.21 d | ±3.80 | ||
| ORAC | 1306.99 c | ±69.78 | ORAC | 2032.51 d | ±132.40 | ||
| August | FRAP | 211.46 b | ±8.90 | August | FRAP | 450.94 c | ±14.06 |
| DPPH•• | 35.74 c | ±2.16 | DPPH•• | 79.82 d | ±4.00 | ||
| ORAC | 1301.14 c | ±13.97 | ORAC | 2394.90 d | ±109.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapisarda, S.; Abu-Ghannam, N. Polyphenol Characterization and Antioxidant Capacity of Multi-Species Swards Grown in Ireland—Environmental Sustainability and Nutraceutical Potential. Sustainability 2023, 15, 634. https://doi.org/10.3390/su15010634
Rapisarda S, Abu-Ghannam N. Polyphenol Characterization and Antioxidant Capacity of Multi-Species Swards Grown in Ireland—Environmental Sustainability and Nutraceutical Potential. Sustainability. 2023; 15(1):634. https://doi.org/10.3390/su15010634
Chicago/Turabian StyleRapisarda, Samuel, and Nissreen Abu-Ghannam. 2023. "Polyphenol Characterization and Antioxidant Capacity of Multi-Species Swards Grown in Ireland—Environmental Sustainability and Nutraceutical Potential" Sustainability 15, no. 1: 634. https://doi.org/10.3390/su15010634
APA StyleRapisarda, S., & Abu-Ghannam, N. (2023). Polyphenol Characterization and Antioxidant Capacity of Multi-Species Swards Grown in Ireland—Environmental Sustainability and Nutraceutical Potential. Sustainability, 15(1), 634. https://doi.org/10.3390/su15010634





