Corrosion Compatibility of Stainless Steels and Nickel in Pyrolysis Biomass-Derived Oil at Elevated Storage Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bio-Oil Exposure
2.3. ICP-MS and Microscopic Characterization
3. Results and Discussion
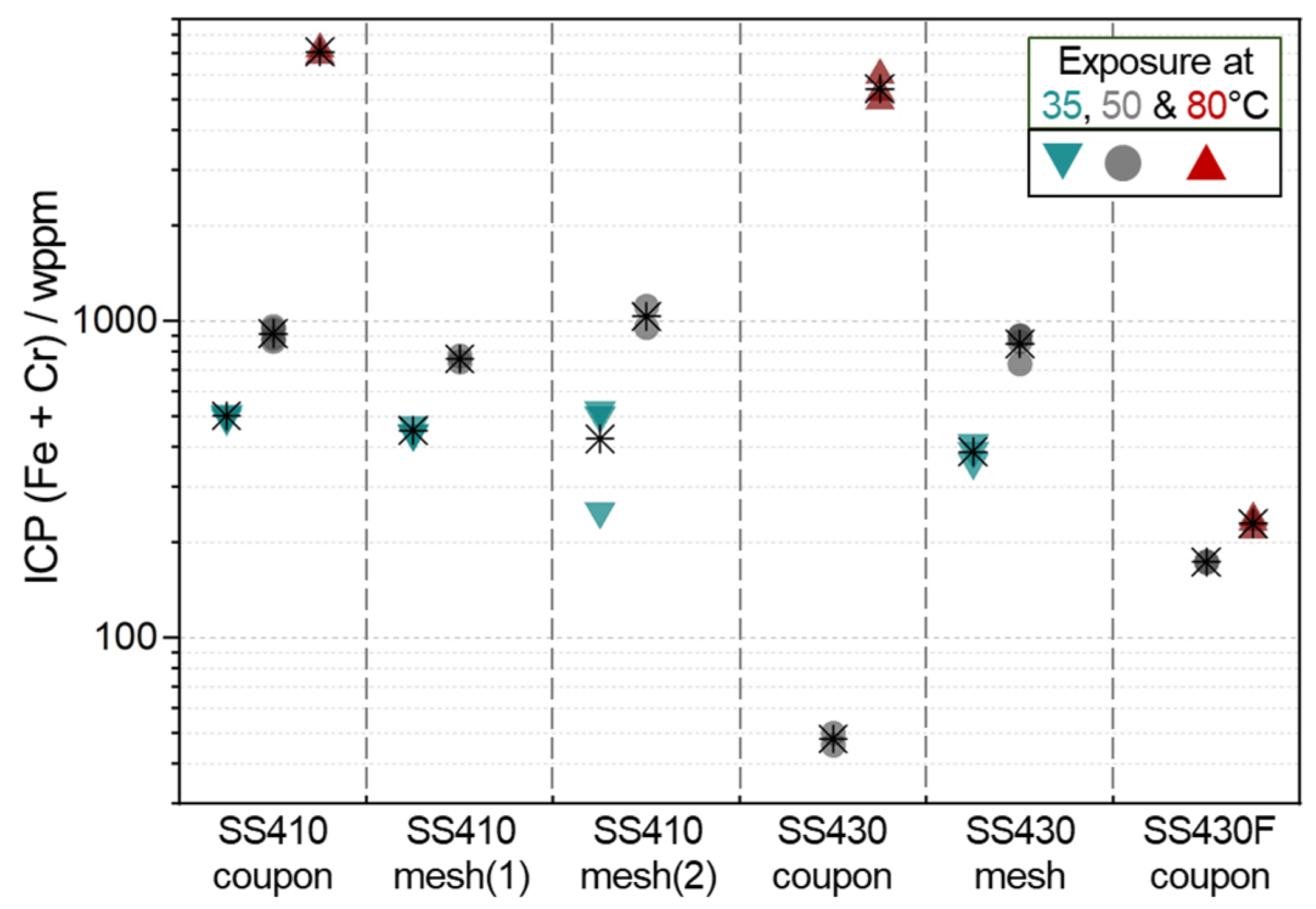
3.1. Mass Change and ICP Data
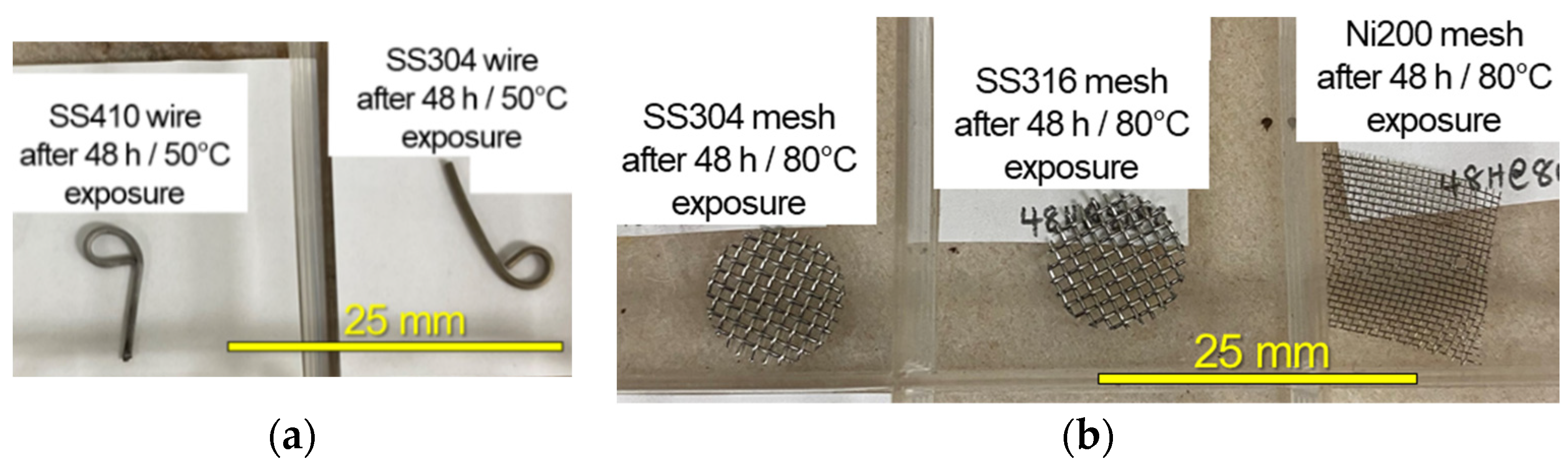
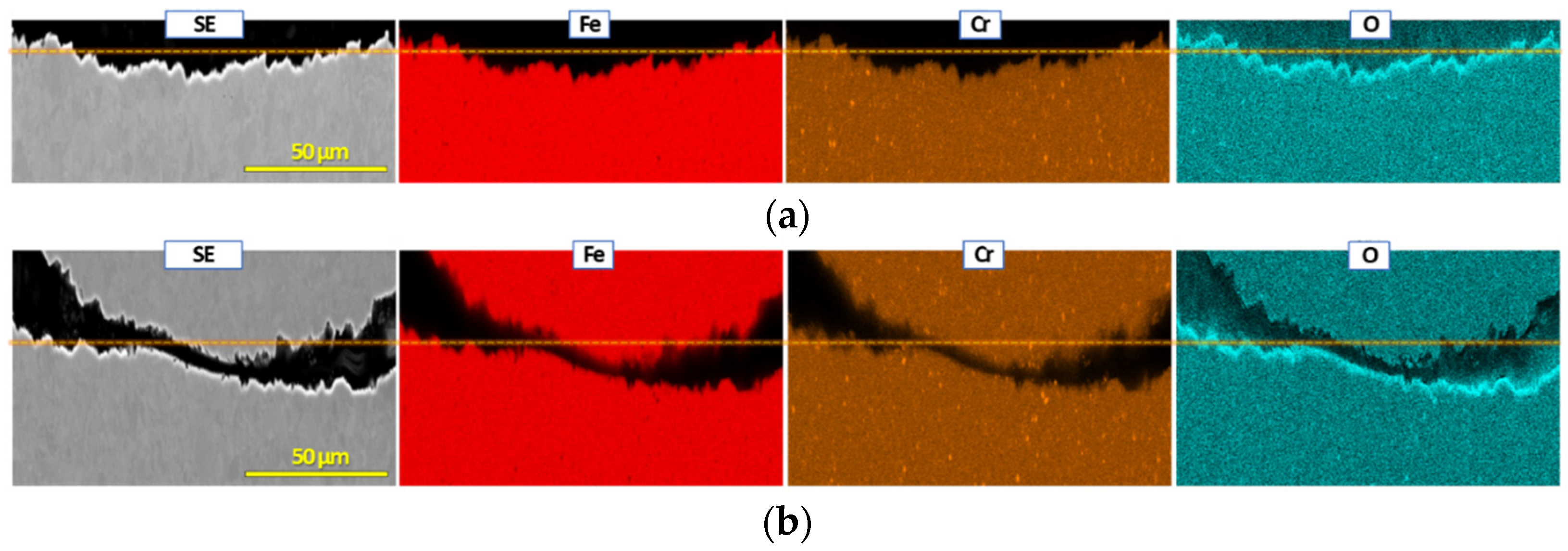
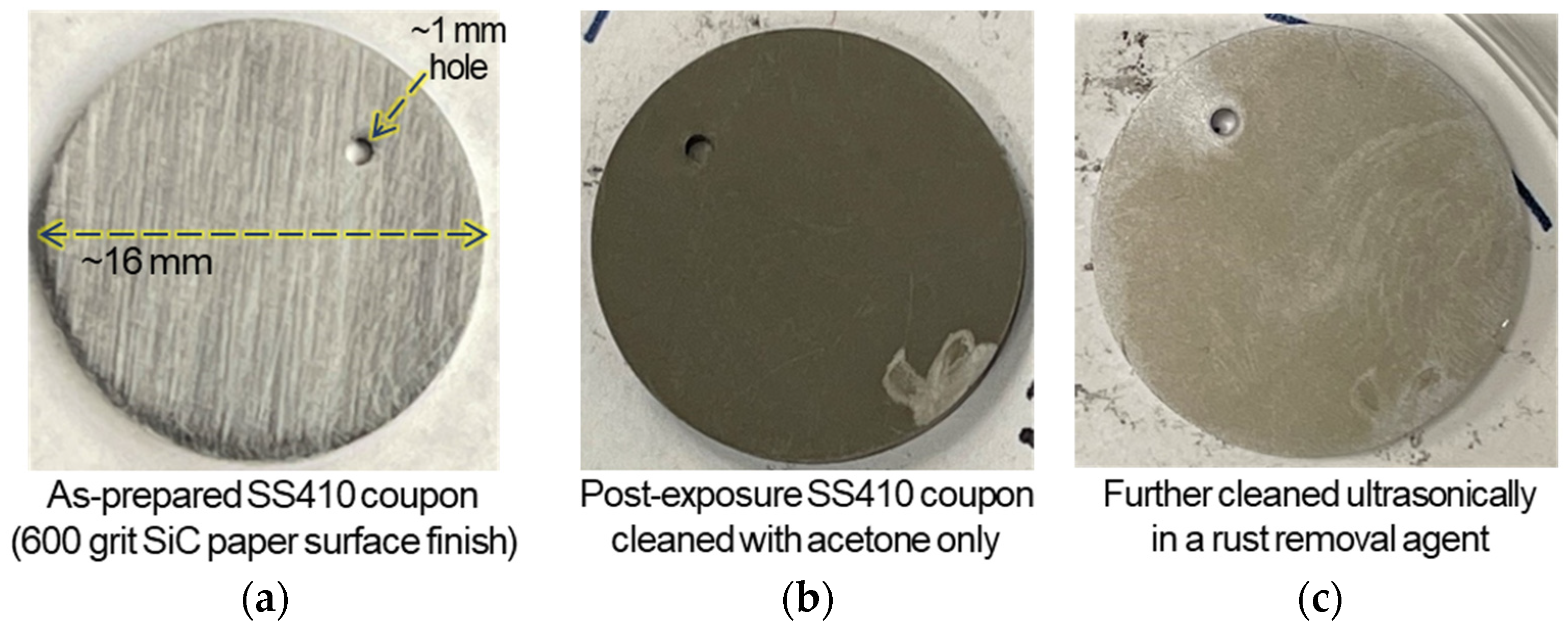
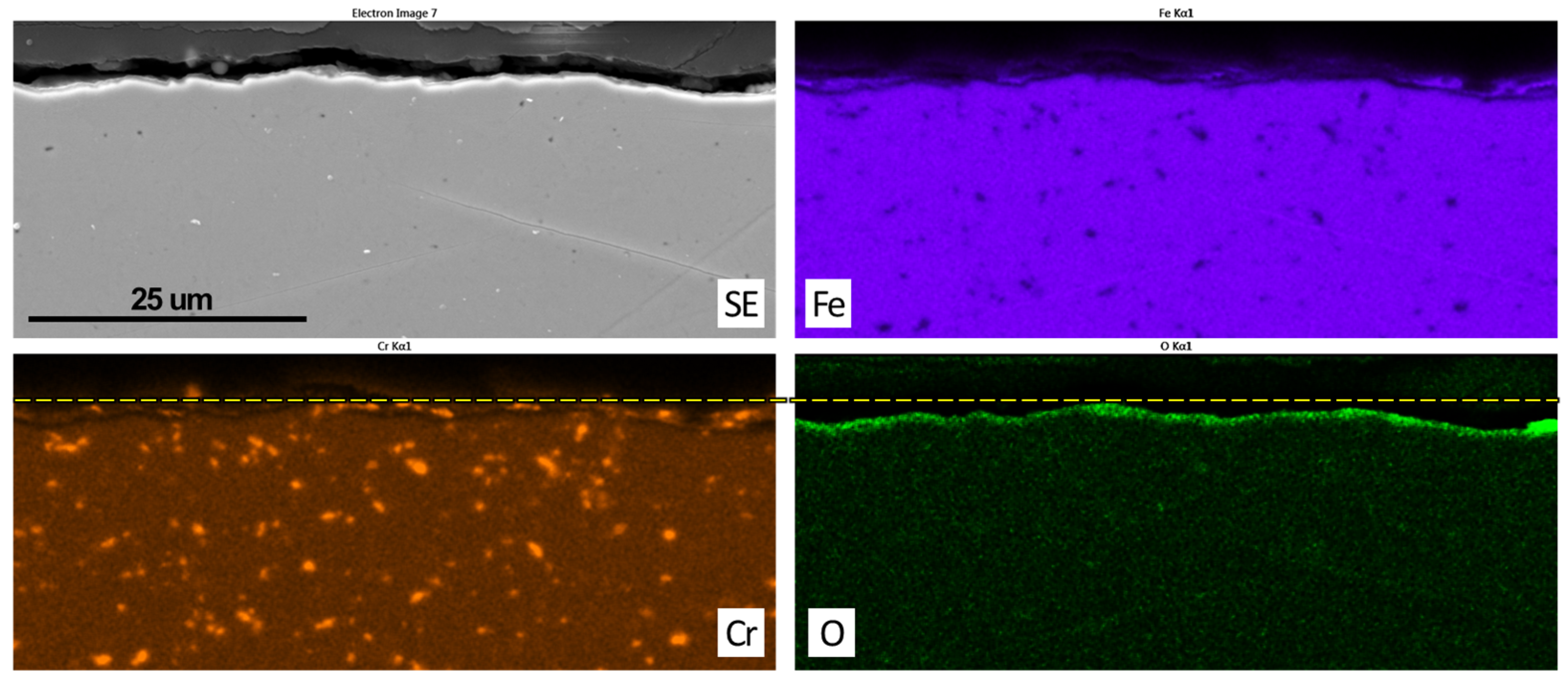
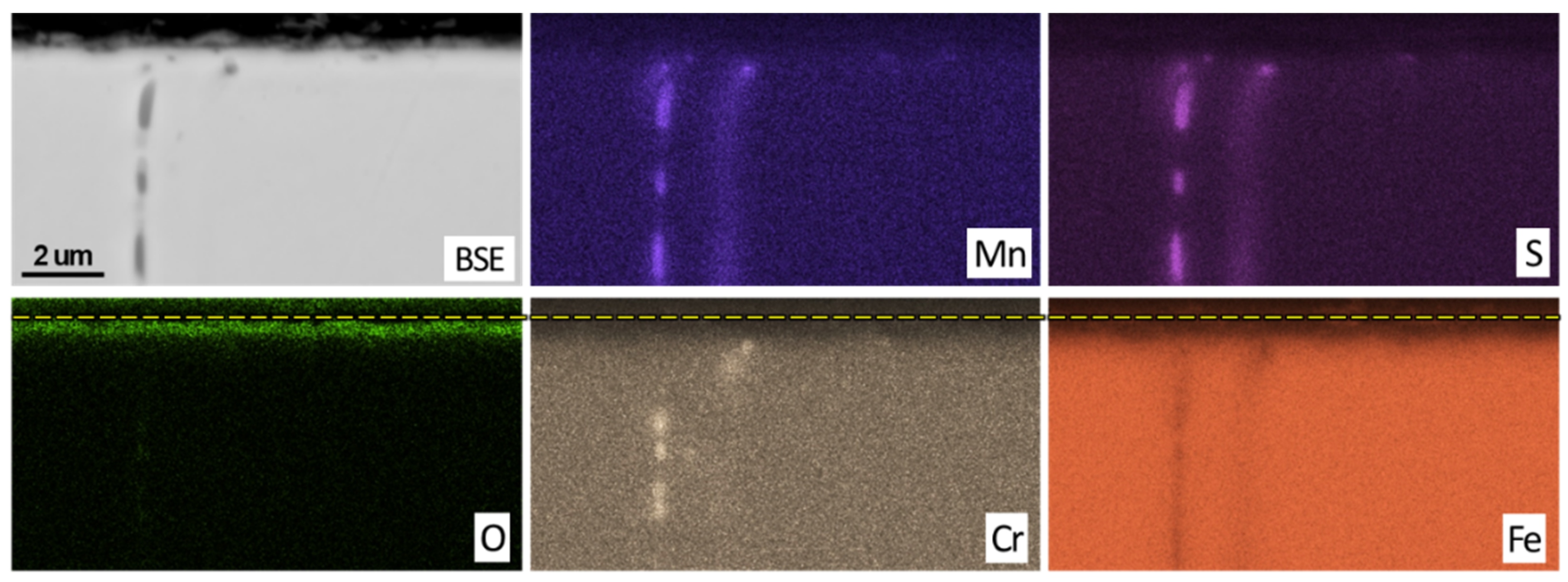
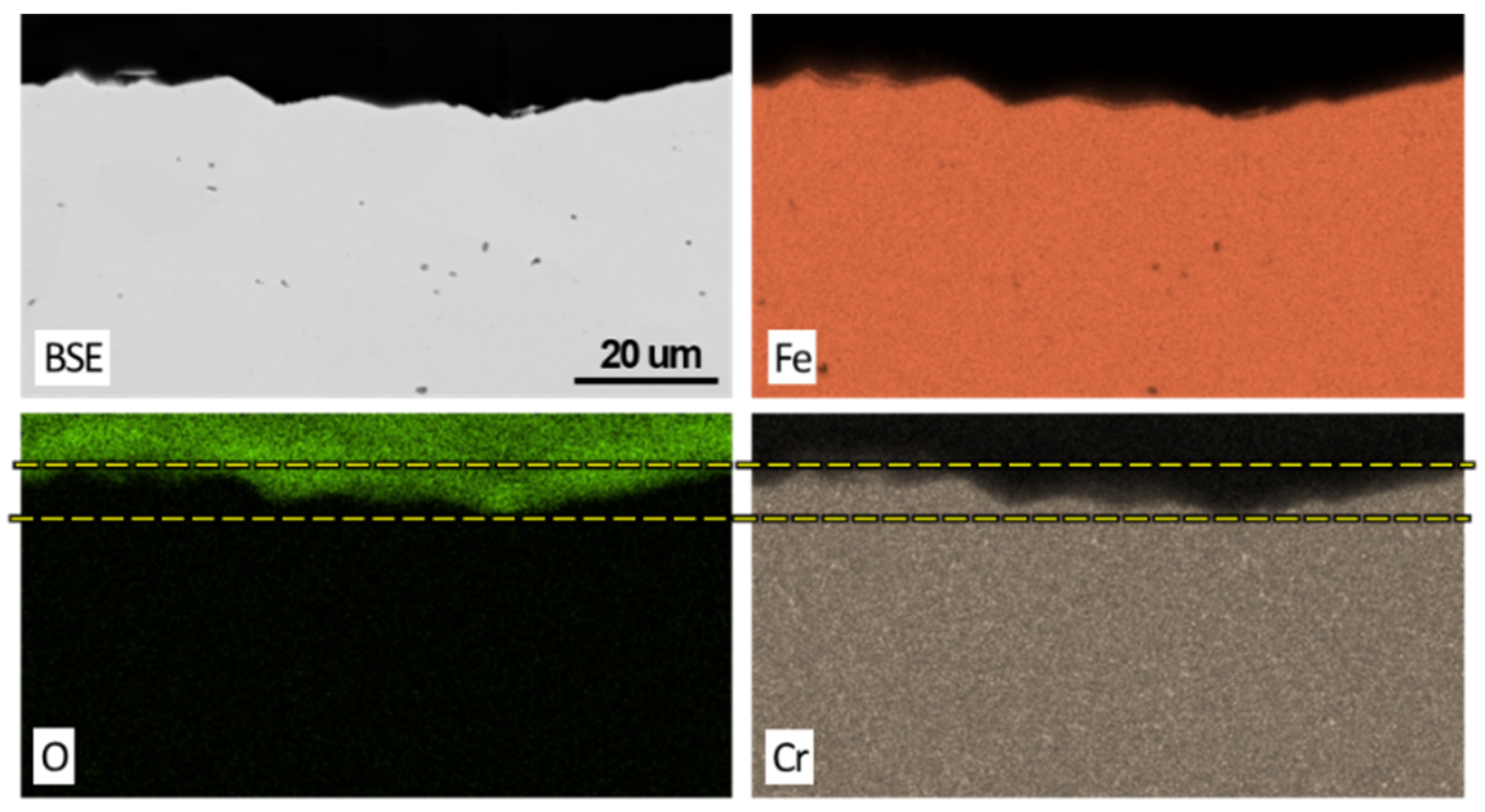
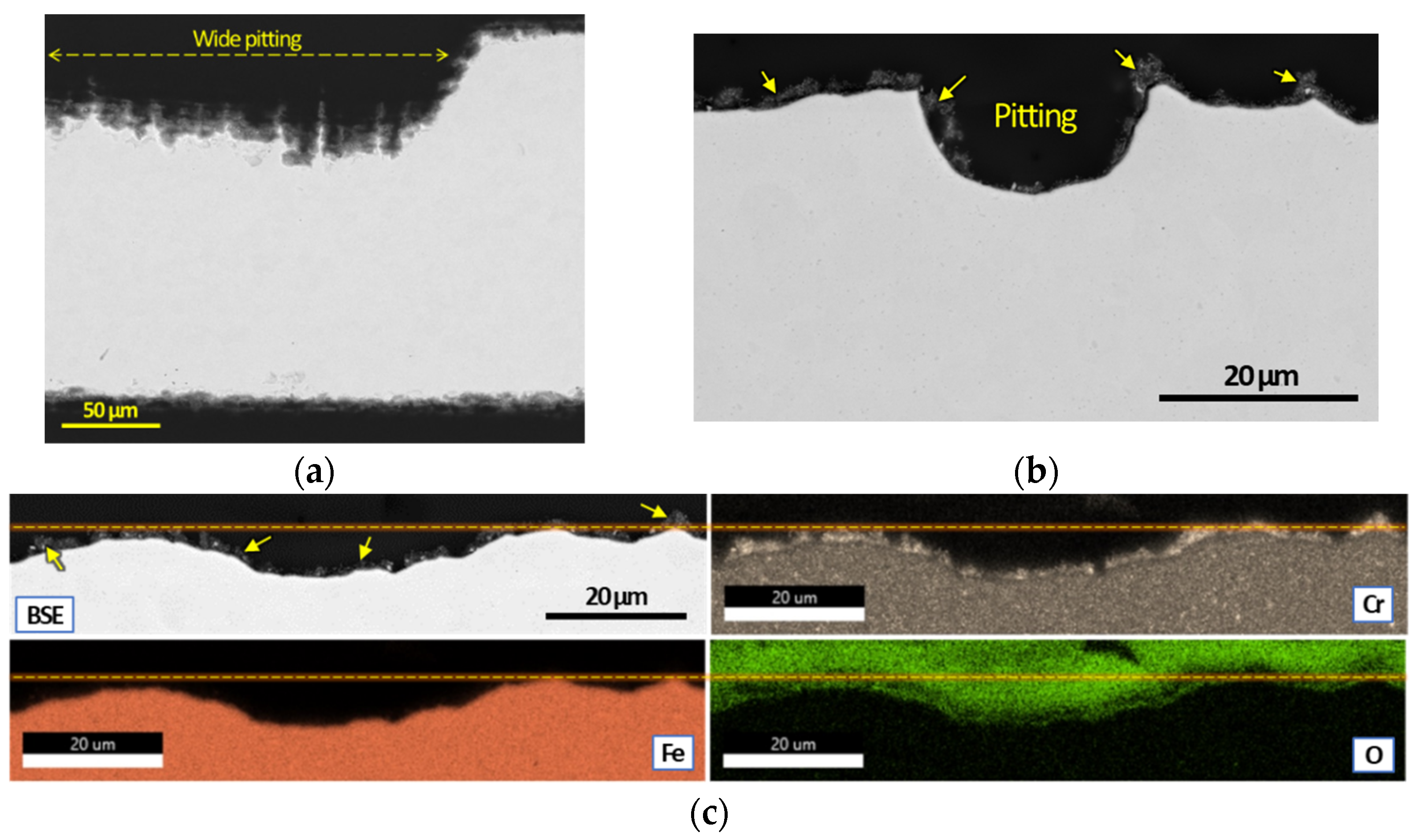
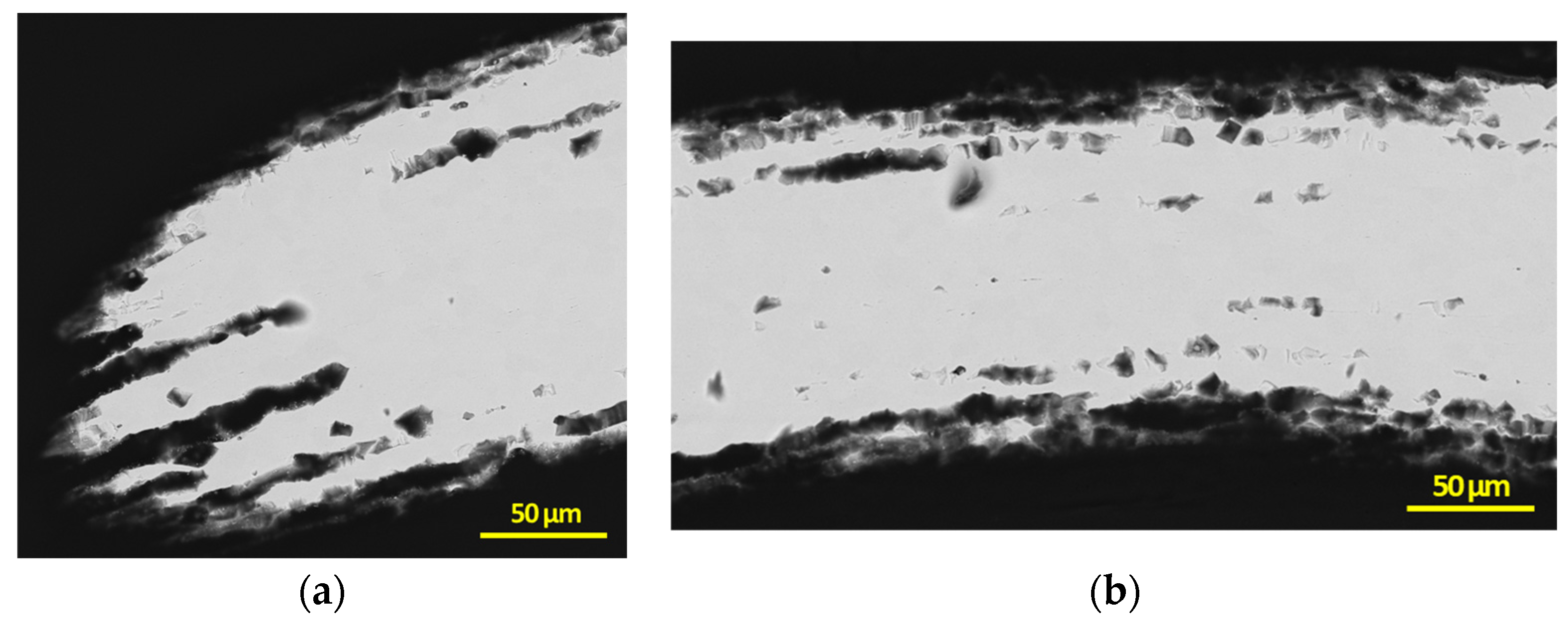
3.2. Post Exposure Sample Characterization
3.3. Comprehensive Data Analysis
4. Summary
- SS430F, SS316 and Ni200 exhibited the highest corrosion resistance in FR3 bio-oil, even for extended exposure (168 h) at 80 °C. SS304 was also corrosion-compatible but sensitive to exposure temperature in wire form. SS410 samples showed increasing mass loss in bio-oil exposure with increasing temperature and time.
- Fe/Cr values from ICP-MS data implied that the alloy samples with minimal mass change (associated with high corrosion resistance) formed protective surface oxide from Cr enrichment. In contrast, congruent metal dissolution, where Cr enrichment did not occur, is considered dominant in the alloy samples with large mass losses.
- SEM and EDS characterization showed the formation of a continuous oxide layer with uniform Cr distribution in a SS430F coupon that had minimal mass change after bio-oil exposure. For the alloy samples with notable mass losses, however, an oxide layer was absent or did not have uniform Cr distribution.
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Step | Description | Duration |
|---|---|---|
| 1 | Microwave-aided Teflon-bomb digestion of acid mixture | - |
| 2 | Stabilization of mixed acids at room temperature | 12~18 h |
| 3 | 0.25 mL bio-oil sample + 1.5 mL 16 M nitric acid (added in 0.1 mL increments), this initial digest was stored with a cover. | - |
| 4 | Stabilization of initial digests | 12~18 h |
| 5 | +0.5 mL 12 M hydrochloric acid + 0.2 mL concentrated hydrofluoric acid | - |
| 6 | Low-level digestion in high pressure microwave rotor (SK15) | - |
| 7 | Three-step heating of the digests to 205 °C | - |
| 8 | Digests diluted to 15 mL | - |
| Sample | Exposure Temp. in °C | Fe ppm | Cr ppm | (A) Fe + Cr ** | Mean Value of (A) ** | Fe/Cr *** | ||
|---|---|---|---|---|---|---|---|---|
| Average | Stdev | Average | Stdev | |||||
| SS410 coupon | 35 | 470 | ±87 | 35 | ±18 | 505 | 503 | 13.6 |
| 35 | 474 | ±62 | 36 | ±13 | 510 | 13.1 | ||
| 35 | 461 | ±60 | 34 | ±12 | 495 | 13.4 | ||
| 50 * | 795 | ±105 | 91 | ±33 | 886 | 914 | 8.7 | |
| 50 * | 771 | ±101 | 94 | ±34 | 865 | 8.2 | ||
| 50 * | 849 | ±113 | 93 | ±33 | 942 | 9.2 | ||
| 50 * | 867 | ±116 | 96 | ±35 | 963 | 9 | ||
| 80 | 6250 | ±815 | 689 | ±248 | 6939 | 7095 | 9.1 | |
| 80 | 6510 | ±1200 | 740 | ±376 | 7250 | 8.8 | ||
| SS410 mesh (1) | 35 | 439 | ±57 | 32 | ±11 | 471 | 452 | 13.9 |
| 35 | 416 | ±54 | 28 | ±10 | 444 | 15.1 | ||
| 35 | 411 | ±76 | 31 | ±16 | 442 | 13.4 | ||
| 50 | 656 | ±121 | 90 | ±45 | 745 | 761 | 7.4 | |
| 50 | 689 | ±91 | 90 | ±32 | 778 | 7.8 | ||
| SS410 mesh (2) | 35 | 484 | ±63 | 39 | ±14 | 523 | 426 | 12.4 |
| 35 | 468 | ±105 | 36 | ±22 | 504 | 13.1 | ||
| 35 | 240 | ±31 | 12 | ±4 | 252 | 20.5 | ||
| 50 | 845 | ±161 | 111 | ±57 | 956 | 1036 | 7.6 | |
| 50 | 994 | ±131 | 122 | ±44 | 1116 | 8.1 | ||
| SS430 coupon | 50 | 44 | ±8 | 2.3 | ±1.2 | 46 | 48 | 18.8 |
| 50 | 47 | ±9 | 3.1 | ±1.6 | 50 | 15 | ||
| 80 | 4070 | ±935 | 912 | ±570 | 4982 | 5420 | 4.5 | |
| 80 | 4330 | ±578 | 952 | ±345 | 5282 | 4.5 | ||
| 80 | 5050 | ±657 | 946 | ±340 | 5996 | 5.3 | ||
| SS430 mesh | 35 | 323 | ±42 | 36 | ±13 | 359 | 387 | 8.9 |
| 35 | 361 | ±67 | 29 | ±15 | 390 | 12.7 | ||
| 35 | 380 | ±70 | 33 | ±17 | 413 | 11.6 | ||
| 50 | 619 | ±141 | 115 | ±72 | 734 | 849 | 5.4 | |
| 50 | 770 | ±144 | 128 | ±65 | 898 | 6 | ||
| 50 | 738 | ±136 | 124 | ±64 | 862 | 6 | ||
| 50 | 771 | ±143 | 130 | ±66 | 901 | 5.9 | ||
| SS430F coupon | 50 | 163 | ±21 | 10 | ±4 | 173 | 174 | 15.8 |
| 50 | 165 | ±22 | 9 | ±3 | 174 | 17.9 | ||
| 80 | 222 | ±29 | 11 | ±4 | 233 | 230 | 21.1 | |
| 80 | 208 | ±38 | 11 | ±6 | 219 | 18.9 | ||
| 80 | 226 | ±42 | 13 | ±7 | 239 | 17.1 | ||
References
- Bridgwater, A.V. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Zacher, A.H.; Olarte, M.V.; Santosa, D.M.; Elliott, D.C.; Jones, S.B. A review and perspective of recent bio-oil hydrotreating research. Green Chem. 2014, 16, 491–515. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Zhang, H.; Chu, C.; Zheng, K.; Ju, M.; Liu, L. Liquefaction of Biomass and Upgrading of Bio-Oil: A Review. Molecules 2019, 24, 2250. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Pérez-Ramírez, J.; Yan, N. Biomass valorisation over polyoxometalate-based catalysts. Green Chem. 2021, 23, 18–36. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, V.; Kumar, P.; Mohanty, K. Hydrothermal liquefaction of biomass for bio-crude production: A review on feedstocks, chemical compositions, operating parameters, reaction kinetics, techno-economic study, and life cycle assessment. Fuel 2022, 316, 123377. [Google Scholar] [CrossRef]
- Kass, M.D.; Janke, C.J.; Lobodin, V.V.; Bras, W.; Keiser, J.R.; Sulejmanovic, D.; Jun, J. Compatibility of Fuel System Elastomers and Plastics with a Fast-Pyrolysis Oil (Bio-oil) at Room Temperature. Energy Fuels 2022, 36, 9158–9170. [Google Scholar] [CrossRef]
- Klinger, J.; Carpenter, D.L.; Thompson, V.S.; Yancey, N.; Emerson, R.M.; Gaston, K.R.; Smith, K.; Thorson, M.; Wang, H.; Santosa, D.M.; et al. Pilot Plant Reliability Metrics for Grinding and Fast Pyrolysis of Woody Residues. ACS Sustain. Chem. Eng. 2020, 8, 2793–2805. [Google Scholar] [CrossRef]
- Oasmaa, A.; Czernik, S. Fuel Oil Quality of Biomass Pyrolysis OilsState of the Art for the End Users. Energy Fuels 1999, 13, 914–921. [Google Scholar] [CrossRef]
- Oasmaa, A.; Kuoppala, E.; Solantausta, Y. Fast Pyrolysis of Forestry Residue. 2. Physicochemical Composition of Product Liquid. Energy Fuels 2003, 17, 433–443. [Google Scholar] [CrossRef]
- Zheng, J.-L. Pyrolysis oil from fast pyrolysis of maize stalk. J. Anal. Appl. Pyrolysis 2008, 83, 205–212. [Google Scholar] [CrossRef]
- Christensen, E.D.; Chupka, G.M.; Luecke, J.; Smurthwaite, T.; Alleman, T.L.; Iisa, K.; Franz, J.A.; Elliott, D.C.; McCormick, R.L. Analysis of Oxygenated Compounds in Hydrotreated Biomass Fast Pyrolysis Oil Distillate Fractions. Energy Fuels 2011, 25, 5462–5471. [Google Scholar] [CrossRef]
- Carpenter, D.; Westover, T.L.; Czernik, S.; Jablonski, W. Biomass feedstocks for renewable fuel production: A review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green Chem. 2014, 16, 384–406. [Google Scholar] [CrossRef]
- Ferrell, J.R.; Olarte, M.V.; Christensen, E.D.; Padmaperuma, A.B.; Connatser, R.M.; Stankovikj, F.; Meier, D.; Paasikallio, V. Standardization of chemical analytical techniques for pyrolysis bio-oil: History, challenges, and current status of methods. Biofuels Bioprod. Biorefin. 2016, 10, 496–507. [Google Scholar] [CrossRef]
- Connatser, R.M.; Frith, M.G.; Jun, J.; Lewis, S.A.; Brady, M.P.; Keiser, J.R. Approaches to Investigate the Role of Chelation in the Corrosivity of Biomass-Derived Oils. Biomass Bioenergy 2020, 133, 105446. [Google Scholar] [CrossRef]
- Black, S.; Ferrell, J.R. Accelerated aging of fast pyrolysis bio-oil: A new method based on carbonyl titration. RSC Adv. 2020, 10, 10046–10054. [Google Scholar] [CrossRef]
- Jun, J.; Frith, M.G.; Connatser, R.M.; Keiser, J.R.; Brady, M.P.; Lewis, S.A. Corrosion Susceptibility of Cr–Mo Steels and Ferritic Stainless Steels in Biomass-Derived Pyrolysis Oil Constituents. Energy Fuels 2020, 34, 6220–6228. [Google Scholar] [CrossRef]
- Jun, J.; Sulejmanovic, D.; Keiser, J.; Brady, M.; Kass, M. Corrosion Behavior of Alloy Structural Steels in Catechol and Biomass-Derived Pyrolysis Oils. In Proceedings of the NACE CORROSION Conference 2021, Virtual Meeting, 19 April 2021; p. 16361. [Google Scholar]
- Jun, J.; Sulejmanovic, D.; Keiser, J.R.; Brady, M.P.; Kass, M.D. Electrochemical Corrosion Analysis of Stainless Steels in LALM Pyrolysis Bio-Oil with Organic Corrodents. In Proceedings of the AMPP CORROSION Conference 2022, San Antonio, TX, USA, 6–10 March 2022; p. 17759. [Google Scholar]
- Scribner, L.A. Corrosion: Environments and Industries; ASM International: Almere, The Netherlands, 2006; Volume 13C. [Google Scholar]
- De Wolf, C.A.; Nasr-El-Din, H.A.; Bouwman, A.; Bang, E.R.A.; Naylor, E. Corrosion Rates of Cr- and Ni-Based Alloys With Organic Acids and Chelating Agents Used in Stimulation of Deep Wells. SPE Prod. Oper. 2017, 32, 208–217. [Google Scholar] [CrossRef]
- Scribner, L.A. Long-Term Corrosion by Organic Acids. In Proceedings of the NACE CORROSION Conference 2001, Houston, TX, USA, 11–16 March 2001; p. 01343. [Google Scholar]
- Badea, G.E.; Ionita, D.; Cret, P. Corrosion and passivation of the 304 stainless steel in formic acid solutions. Mater. Corros. 2014, 65, 1103–1110. [Google Scholar] [CrossRef]
- Singh, M.M.; Gupta, A. Corrosion behaviour of mild steel in formic acid solutions. Mater. Chem. Phys. 1996, 46, 15–22. [Google Scholar] [CrossRef]
- Talukdar, A.; Rajaraman, P.V. Investigation of Acetic Acid Effect on Carbon Steel Corrosion in CO2–H2S Medium: Mechanistic Reaction Pathway and Kinetics. ACS Omega 2020, 5, 11378–11388. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Mukherjee, A.K. Kinetics of Mild Steel Corrosion in Aqueous Acetic Acid Solutions. J. Mater. Sci. Technol. 2010, 26, 264–269. [Google Scholar] [CrossRef]
- Sekine, I.; Hatakeyama, S.; Nakazawa, Y. Corrosion behaviour of Type 430 stainless steel in formic and acetic acids. Corros. Sci. 1987, 27, 275–288. [Google Scholar] [CrossRef]
- Singh, S.K.; Mukherjee, A.K.; Singh, M.M. Kinetics of mild steel corrosion in aqueous formic acid solutions. Can. Metall. Q. 2011, 50, 186–194. [Google Scholar] [CrossRef]
- Demirbas, A. Competitive liquid biofuels from biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Diebold, J.P.; Czernik, S. Additives To Lower and Stabilize the Viscosity of Pyrolysis Oils during Storage. Energy Fuels 1997, 11, 1081–1091. [Google Scholar] [CrossRef]
- Garcia-Perez, M.; Adams, T.T.; Goodrum, J.W.; Geller, D.P.; Das, K.C. Production and Fuel Properties of Pine Chip Bio-oil/Biodiesel Blends. Energy Fuels 2007, 21, 2363–2372. [Google Scholar] [CrossRef]
- Gómez, N.; Banks, S.; Nowakowski, D.; Rosas, J.; Cara, J.; Sánchez, M.; Bridgwater, A. Effect of temperature on product performance of a high ash biomass during fast pyrolysis and its bio-oil storage evaluation. Fuel Process. Technol. 2018, 172, 97–105. [Google Scholar] [CrossRef]
- Khan, M.S.; Rafiq, M.; Sookrah, V.; Thomson, M.J. Effect of Dewatering Wood-Derived Fast Pyrolysis Oil on Its Fuel Properties for Power Generation. Energy Fuels 2019, 33, 12403–12420. [Google Scholar] [CrossRef]
- Talmadge, M.S.; Baldwin, R.M.; Biddy, M.J.; McCormick, R.L.; Beckham, G.T.; Ferguson, G.A.; Czernik, S.; Magrini-Bair, K.A.; Foust, T.D.; Metelski, P.D.; et al. A perspective on oxygenated species in the refinery integration of pyrolysis oil. Green Chem. 2014, 16, 407–453. [Google Scholar] [CrossRef]
- Keiser, J.; Warrington, G.; Jun, J.; Sulejmanovic, D.; Brady, M.; Kass, M.; Smith, K. Long-Term Corrosion Studies Of Pine Derived Bio-Oil And Blends With Heavy Fuel Oil. In Proceedings of the TAPPI PEERS 2020 Conference, Online, 2–4 November 2020. [Google Scholar]
- Keiser, J.R.; Warrington, G.L.; Lewis, S.A.; Connatser, R.M.; Jun, J.; Qu, J.; Lee, K.; Brady, M.P. Corrosion and Chemical Characterization of Bio-Oils from Biomass with Varying Ash and Moisture Contents. In Proceedings of the NACE CORROSION Conference 2021, Virtual Meeting, 19–30 April 2021; p. 16726. [Google Scholar]
- Jun, J.; Warrington, G.L.; Keiser, J.R.; Connatser, R.M.; Sulejmanovic, D.; Brady, M.P.; Kass, M.D. Corrosion of Ferrous Structural Alloys in Biomass Derived Fuels and Organic Acids. Energy Fuels 2021, 35, 12175–12186. [Google Scholar] [CrossRef]
- Jun, J.; Frith, M.G.; Connatser, R.M.; Keiser, J.R.; Brady, M.P.; Lewis, S. Corrosion of Ferrous Alloys by Organic Compounds in Simulated Bio-Oils. In Proceedings of the NACE CORROSION Conference 2019, Nashville, TN, USA, 24–28 March 2019; p. 12895. [Google Scholar]
- Jun, J.; Sulejmanovic, D.; Keiser, J.; Brady, M.; Kass, M. Evaluation of Corrosion Susceptibility of Structural Steels in Biomass Derived Pyrolysis Oil. In Proceedings of the NACE CORROSION Conference 2021, virtual meeting, 19 April 2021; p. 16502. [Google Scholar]
- Kass, M.D.; Armstrong, B.L.; Kaul, B.C.; Connatser, R.M.; Lewis, S.; Keiser, J.R.; Jun, J.; Warrington, G.; Sulejmanovic, D. Stability, Combustion, and Compatibility of High-Viscosity Heavy Fuel Oil Blends with a Fast Pyrolysis Bio-Oil. Energy Fuels 2020, 34, 8403–8413. [Google Scholar] [CrossRef]
- Darmstadt, H.; Garcia-Perez, M.; Adnot, A.; Chaala, A.; Kretschmer, A.D.; Roy, C. Corrosion of Metals by Bio-Oil Obtained by Vacuum Pyrolysis of Softwood Bark Residues. An X-ray Photoelectron Spectroscopy and Auger Electron Spectroscopy Study. Energy Fuels 2004, 18, 1291–1301. [Google Scholar] [CrossRef]
- Sulejmanovic, D.; Keiser, J.R.; Su, Y.-F.; Kass, M.D.; Ferrell, J.R.; Olarte, M.V.; Wade, J.E.; Jun, J. Effect of Carboxylic Acids on Corrosion of Type Stainless Steel in Pyrolysis Bio-Oil. Sustainability 2022, 14, 11743. [Google Scholar] [CrossRef]


| Fe | Cr | Ni | Mo | Mn | Cu | Si | C | N | O | S | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) SS410 Coupon * | 88.6 | 11.9 | 0.23 | 0.05 | 0.47 | 0.16 | 0.3 | 0.13 | 0.012 | 0.0026 | <0.001 | 0.06 V 0.02 Co |
| SS410 Mesh (1) | Bal. | 13.2 | 0.22 | 0.03 | 0.42 | 0.09 | 0.32 | 0.03 | 0.07 | 0.01 | 0.001 | 0.06 V 0.02 Co |
| SS410 Mesh (2) | Bal. | 13.1 | 0.2 | 0.02 | 0.46 | 0.05 | 0.31 | 0.04 | 0.063 | 0.0069 | 0.001 | 0.04 V |
| SS410 Wire ** | Bal. | 12.5 | - | - | 1 | - | 1 | <0.15 | - | - | <0.03 | |
| (A) SS430 Coupon * | 81.1 | 16.9 | 0.15 | 0.05 | 0.26 | 0.13 | 0.33 | 0.035 | - | - | 0.002 | 0.14 Ti 0.08 V 0.02 Co |
| SS430 Mesh | Bal. *** | 17.3 | 0.19 | <0.01 | 0.29 | 0.03 | 0.39 | 0.04 | 0.03 | 0.01 | 0.03 | 0.05 Co 0.02 V |
| SS430 Wire ** | Bal. | |||||||||||
| (B) SS430F Coupon * | 80.4 | 17.5 | 0.3 | 0.33 | 0.43 | 0.17 | 0.32 | 0.032 | 0.042 | 0.009 | 0.326 | 0.06 V |
| SS304 Mesh and wire ** | Bal. | 18 | 9 | - | 2 | - | 0.75 | <0.08 | 0.1 | - | <0.03 | |
| SS316 Mesh and wire ** | Bal. | 17 | 11 | 2.2 | 1 | - | 0.5 | <0.07 | - | - | <0.02 | |
| Ni200 Mesh and wire ** | <0.4 | - | Bal. | - | <0.35 | <0.25 | <0.35 | <0.15 | - | - | <0.01 | |
| (B) Ni200 Coupon ** |
| SS410 Mesh (1) | SS410 Mesh (2) | SS430 Mesh | SS304 Mesh | SS316 Mesh | Ni200 Mesh | SS410 Wire | SS430 Wire | SS304 Wire | SS316 Wire | Ni200 Wire | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wire radius (cm) | 0.03175 | 0.0179 | 0.03175 | 0.02032 | 0.02032 | 0.00635 | 0.0406 | 0.0406 | 0.0406 | 0.0406 | 0.0315 |
| Density (mg∙cm−3) | 7750 | 7750 | 7720 | 8000 | 8000 | 8900 | 7750 | 7720 | 8000 | 8000 | 8900 |
| Coupon Type | Mesh Type | Wire Type | |
|---|---|---|---|
| 5< and <16 | 65< and <270 | 60< and <320 | |
| Specimen surface area/cm2 | 4.45~5.44 | 2.19~10.8 | 0.57~2.37 |
| SS410 | SS430 | SS430F | Ni200 | |
|---|---|---|---|---|
| 96 h | - | −49.4, −48.7 [−49] | +0.002, −0.015 [−0.007] | - |
| 168 h | −113 | −47.6, −50.4, −45 [−47.7] | 0.037, 0.088, −0.004 [+0.04] | +0.04, −0.1 [−0.036] |
| Fe/Cr of Alloy (from Table 1) | Fe/Cr ICP (35 °C Exposure) | Fe/Cr ICP (50 °C Exposure) | Fe/Cr ICP (80 °C Exposure) | |
|---|---|---|---|---|
| Individual data | 7.4 | 13.1 13.4 13.6 | 8.2 8.7 9 9.2 | 8.8 9.1 |
| Note | - | More Fe dissolution | Slightly more Fe dissolution | Slightly more Fe dissolution |
| Avg. mass change in Figure 2 | - | −1.7 mg∙cm−2 | −5.7 mg∙cm−2 | −43 mg∙cm−2 |
| SS430F Coupon | SS430 Coupon | SS430 Mesh | |
|---|---|---|---|
| Fe/Cr of alloy (From Table 1) | 4.6 | 4.8 | 4.7 |
| Fe/Cr ICP | 15.8 17.9 | 15 18.8 | 5.4 5.9 6 |
| Note | More Fe dissolution | More Fe dissolution | Slightly more Fe dissolution |
| Avg. mass change in Figure 2 | 0 mg∙cm−2 | 0 mg∙cm−2 | −8.5 mg∙cm−2 |
| SS430F Coupon | SS430 Coupon | |
|---|---|---|
| Fe/Cr of alloy (From Table 1) | 4.6 | 4.8 |
| Fe/Cr ICP | 17.1 18.9 21.1 | 4.5 5.3 |
| Note | More Fe dissolution | Congruent dissolution of Fe and Cr |
| Avg. mass change in Figure 2 | 0 mg∙cm−2 | −37 mg∙cm−2 |
| Corrosion Risk | Low | FR3 Bio-Oil Exposure Temperature | ||
|---|---|---|---|---|
| Intermediate | 35 °C | 50 °C | 80 °C | |
| High | ||||
| SS410 | Mass loss (about −2 mg∙cm−2, see Figure 3) | High mass loss (<−5 mg∙cm−2, see Figure 3 and Table 4) | ||
| SS430 | Mass loss (about −1.8 mg∙cm−2, see Figure 3) | Could exhibit high mass loss (about −9 mg∙cm−2, see Figure 3) | High mass loss (about −50 mg∙cm−2, see Table 4) | |
| SS430F | No data, but high resistance expected | Low mass change (within ±0.1 mg∙cm−2, see Figure 3 and Table 4) | ||
| SS304 | Low mass change (within ±0.4 mg∙cm−2, see Figure 3) | Mass loss (about −1.6 mg∙cm−2, see Figure 3) | ||
| SS316 | No data, but high resistance expected | Low mass change (within ±0.1 mg∙cm−2, see Figure 3) | ||
| Ni200 | Low mass loss (<−1 mg∙cm−2, see Figure 3 and Table 4) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, J.; Su, Y.-F.; Keiser, J.R.; Wade, J.E., IV; Kass, M.D.; Ferrell, J.R., III; Christensen, E.; Olarte, M.V.; Sulejmanovic, D. Corrosion Compatibility of Stainless Steels and Nickel in Pyrolysis Biomass-Derived Oil at Elevated Storage Temperatures. Sustainability 2023, 15, 22. https://doi.org/10.3390/su15010022
Jun J, Su Y-F, Keiser JR, Wade JE IV, Kass MD, Ferrell JR III, Christensen E, Olarte MV, Sulejmanovic D. Corrosion Compatibility of Stainless Steels and Nickel in Pyrolysis Biomass-Derived Oil at Elevated Storage Temperatures. Sustainability. 2023; 15(1):22. https://doi.org/10.3390/su15010022
Chicago/Turabian StyleJun, Jiheon, Yi-Feng Su, James R. Keiser, John E. Wade, IV, Michael D. Kass, Jack R. Ferrell, III, Earl Christensen, Mariefel V. Olarte, and Dino Sulejmanovic. 2023. "Corrosion Compatibility of Stainless Steels and Nickel in Pyrolysis Biomass-Derived Oil at Elevated Storage Temperatures" Sustainability 15, no. 1: 22. https://doi.org/10.3390/su15010022
APA StyleJun, J., Su, Y.-F., Keiser, J. R., Wade, J. E., IV, Kass, M. D., Ferrell, J. R., III, Christensen, E., Olarte, M. V., & Sulejmanovic, D. (2023). Corrosion Compatibility of Stainless Steels and Nickel in Pyrolysis Biomass-Derived Oil at Elevated Storage Temperatures. Sustainability, 15(1), 22. https://doi.org/10.3390/su15010022









