Ammonium and Phosphate Recovery from Biogas Slurry: Multivariate Statistical Analysis Approach
Abstract
1. Introduction
2. Methodology
2.1. Molar Ratio and pH Adjustment
2.2. Details of the Experiment
2.2.1. Preparation of Biochars
- Sample number: Biochar type
- CK: ——
- DG: Bean stem biochar
- SH-1: Water hyacinth root biochar
- SH-2: Water hyacinth stem and leaf biochar
- HSK: Peanut shell biochar
- MG-1: Mushroom soil biochar (first batch)
- MG-2: Mushroom soil biochar (second batch)
- SD: Rice biochar
- JM: Jimei block sludge biochar
- TA-1: Tongan city sludge biochar
- TA-2: Tongan hydrothermal sludge biochar
- TA-3: Tongan hydrogel sludge biochar
- XJ: Rubber wood biochar
2.2.2. Preparation of Simulated Biogas Slurry
2.2.3. Preparation of Stock Solution
2.2.4. Analytical Methods
2.2.5. Chemical Analysis
2.3. Statistical Analysis
3. Results
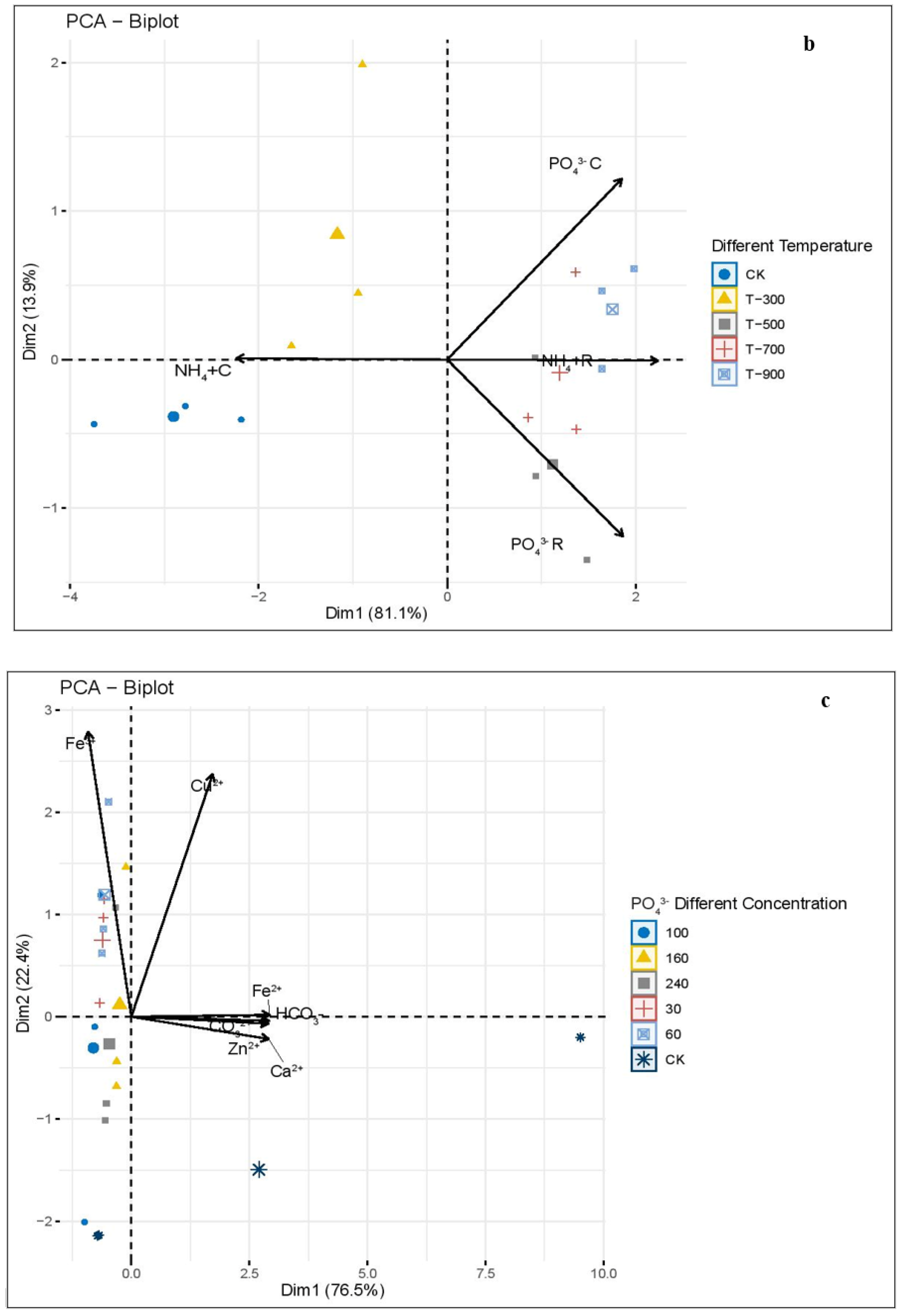
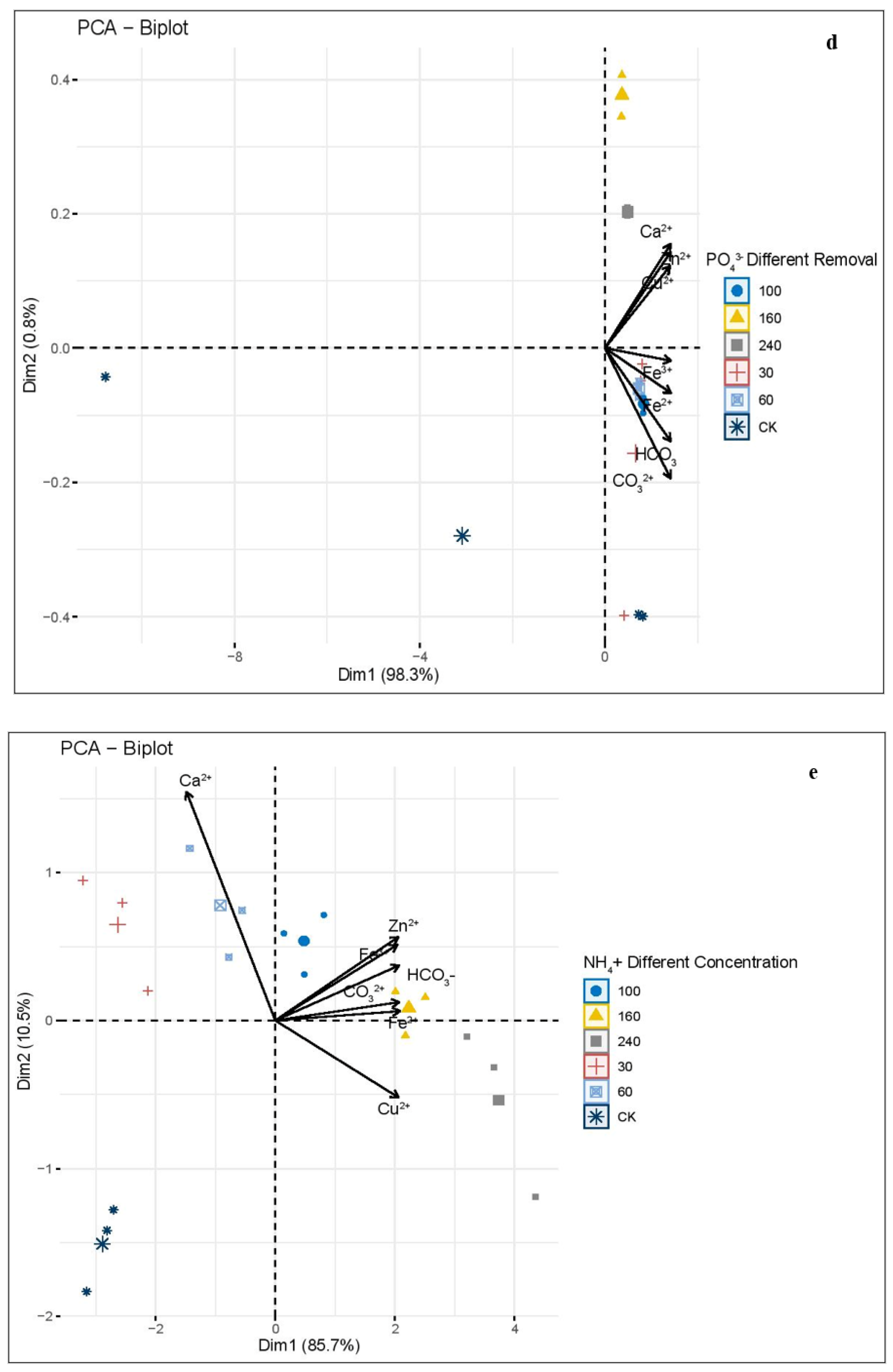
3.1. Principal Component Analysis (PCA) of Phosphate and Ammonium Concentration, and the Removal Effect of Different Biochars, Pyrolysis Temperatures, and Phosphate and Ammonium Different Element and Removal Concentration
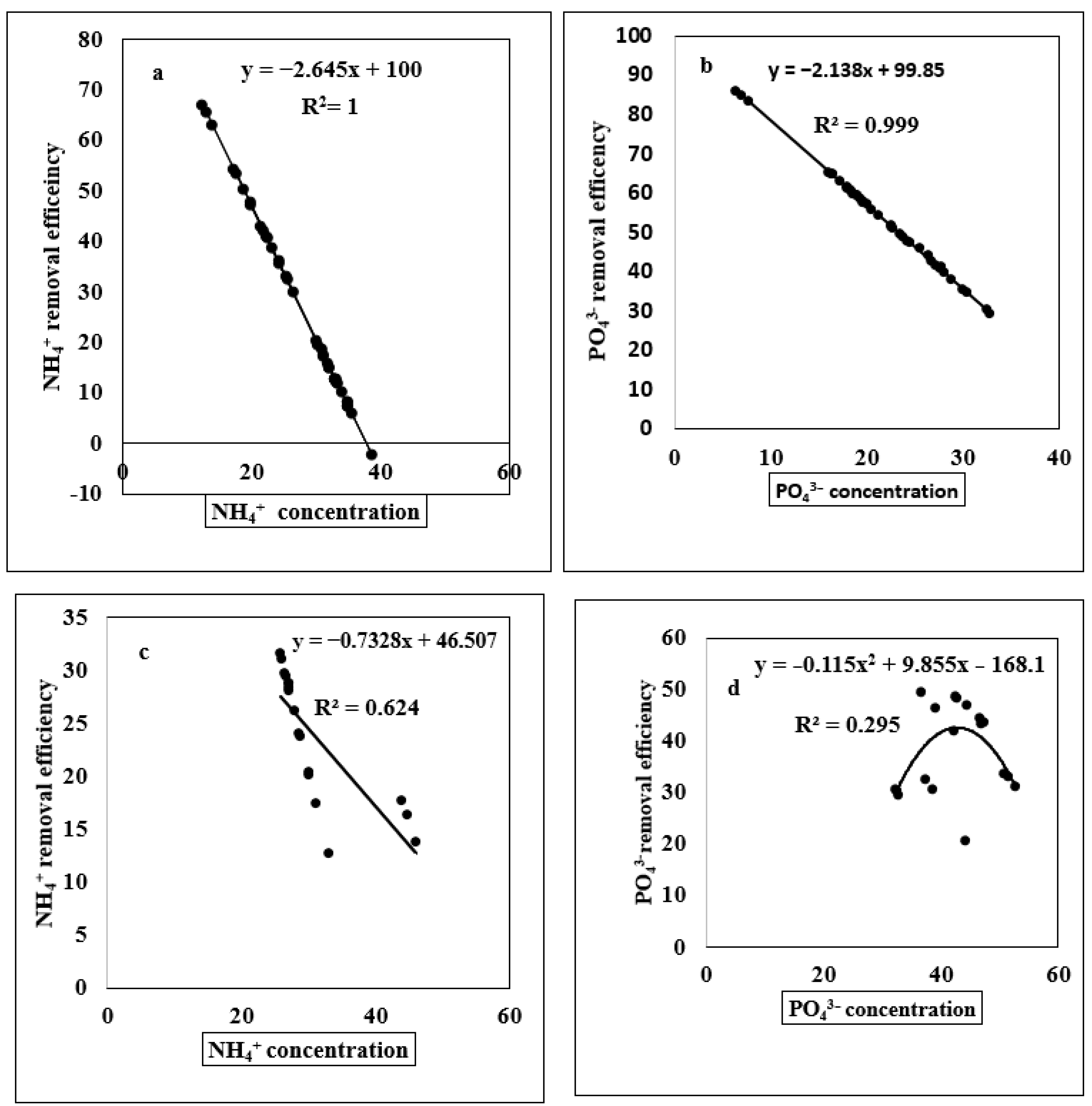
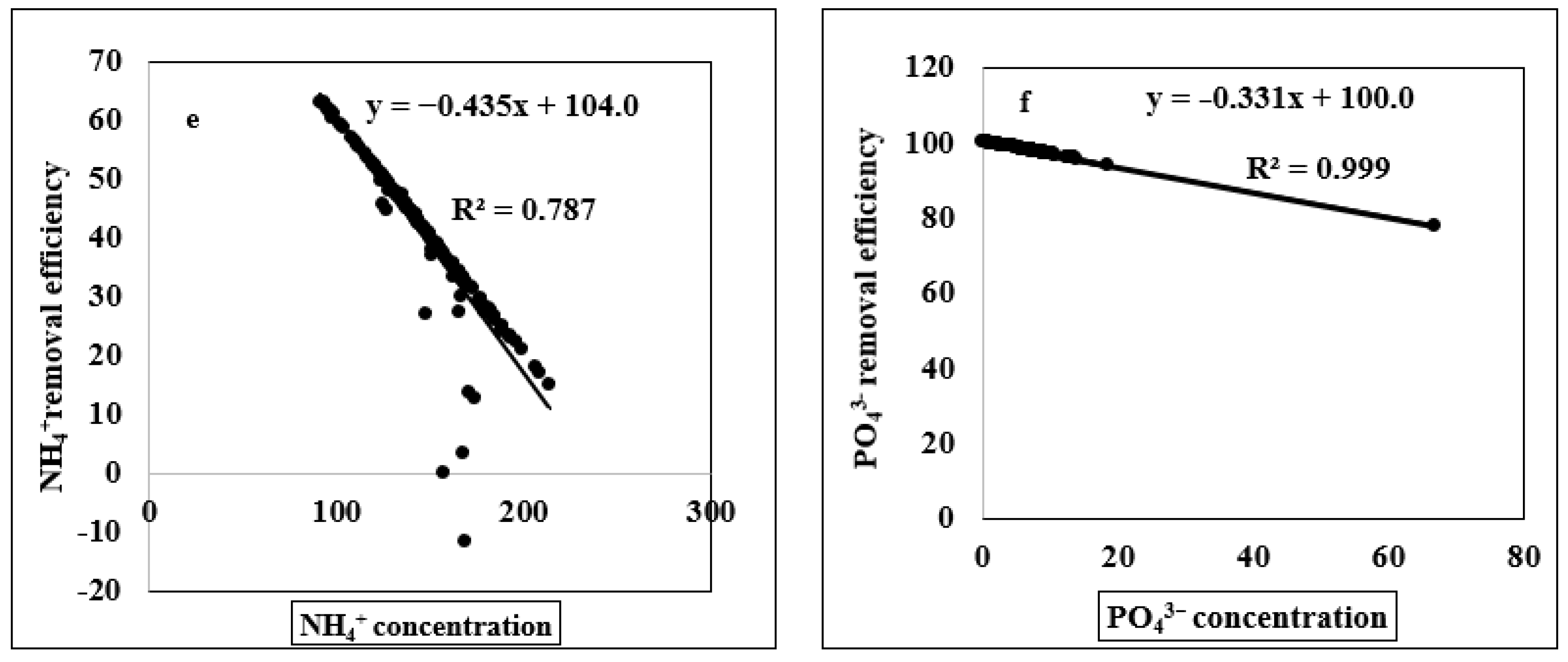
3.2. Relationship of Ammonium and Phosphate Concentration with Ammonium and Phosphate Removal Efficiency, Pyrolysis Temperature, Removal Efficiency and Different Elemental Concentrations
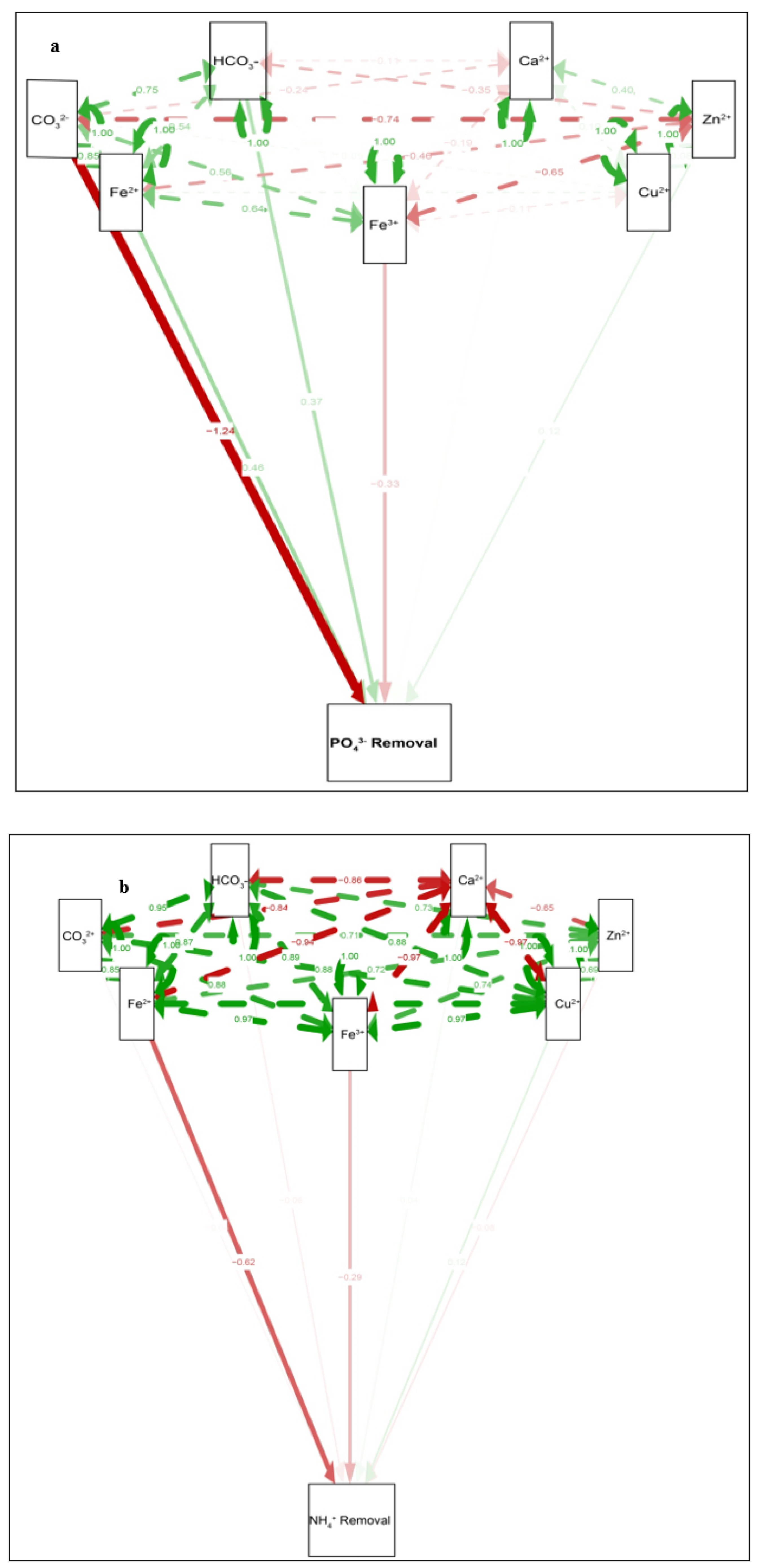
3.3. The Key Route Main Path of Removal: (A) Phosphate Removal, (B) Ammonium Removal
3.4. Pearson Correlation under Ammonium Concentration and Removal Efficiency, and Phosphate Removal Concentration and Removal Efficiency
4. Discussion
4.1. Ammonium and Phosphate Recovery from Biogas Slurry by Struvite and Biochar Precipitation
4.2. Principal Component Analysis (PCA) Analysis of Different Indicators
4.3. Regression Analysis of Different Indicators
4.4. Route Main Path Analysis of Different Indicators
4.5. Pearson Correlation of Different Indicators
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, L.; Liu, J.; Wang, Z. Pig farm wastewater treatment, and comprehensive utilization technology. Chin. J. Anim. Sci. 2015, 10, 51–57. [Google Scholar]
- Wu, L.; Zhang, S.; Wang, J.; Ding, X. Phosphorus retention using iron (II/III) modified Biochar in saline-alkaline soils: Adsorption, column, and field tests. J. Environ. Pollut. 2020, 261, 114223. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Zhang, D.; Lin, C.; Zhang, Y.; Zhao, L.; Liu, H.; Liu, Z. Effect of organic loading rate on anaerobic digestion of pig manure: Methane production, mass flow, reactor scale and heating scenarios. J. Environ. Manag. 2019, 231, 646–652. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, T.; Ren, H.; Li, G.; Ding, L.; Pawlowski, L. Phosphorus recovery from biogas slurry by ultrasound/H2O2 digestion coupled with HFO/biochar adsorption process. Waste Manag. 2017, 1, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Scarlat, N.; Fahl, F.; Dallemand, J.F.; Monforti, F.; Motola, V. A spatial analysis of biogas potential from manure in Europe. J. Ren. Sustain. Energy Rev. 2018, 1, 915–930. [Google Scholar] [CrossRef]
- Bergland, W.H.; Dinamarca, C.; Toradzadegan, M.; Nordgård, A.S.; Bakke, I.; Bakke, R. High rate manure supernatant digestion. J. Water Res. 2015, 76, 1–9. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, D.; Shen, X.; Liu, W.; Yi, W.; Li, Z.; Liu, S. Synchronously electricity generation and degradation of biogas slurry using a microbial fuel cell. J. Renew. Energy 2019, 142, 158–166. [Google Scholar] [CrossRef]
- Pivato, A.; Vanin, S.; Raga, R.; Lavagnolo, M.C.; Barausse, A.; Rieple, A.; Laurent, A.; Cossu, R. Use of digestate from a decentralized on-farm biogas plant as fertilizer in soils: An ecotoxicological study for future indicators in risk and life cycle assessment. J. Waste Manag. 2016, 1, 378–389. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Mandale, S.J.; Williams, P.M.; Lovitt, R.W. Nanofiltration of treated digested agricultural wastewater for recovery of carboxylic acids. J. Clean Prod. 2016, 20, 4749–4761. [Google Scholar] [CrossRef]
- Shang, D.; Geissler, B.; Mew, M.; Satalkina, L.; Zenk, L.; Tulsidas, H.; Barker, L.; El-Yahyaoui, A.; Hussein, A.; Taha, M. Unconventional uranium in China’s phosphate rock: Review and outlook. J. Renew. Sustain. Energy Rev. 2021, 140, 110740. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Xiang, S.; Huang, Z.; Ruan, R.; Liu, Y. Nutrient removal from digested swine wastewater by combining ammonia stripping with struvite precipitation. J. Environ. Sci. Pollut. Res. 2019, 26, 6725–6734. [Google Scholar] [CrossRef] [PubMed]
- Lupton, S. Markets for waste and waste-derived fertilizers. An empirical survey. J. Rural. Stud. 2017, 55, 83–99. [Google Scholar] [CrossRef]
- Aziz, M.; Yaseen, M.; Naveed, M.; Wang, X.; Fatima, K.; Saeed, Q.; Mustafa, A. Polymer-Paraburkholderia phytofirmans PsJN coated diammonium phosphate enhanced microbial survival, phosphorous use efficiency, and production of wheat. Agronomy 2020, 10, 1344. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Zessner, M. Overview and description of technologies for recovering phosphorus from municipal wastewater. J. Resour. Conserv. Recycl. 2015, 105, 325–346. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Huu, H.N.; Wenshan, G.; Yiwen, L.; Soon, W.C.; Dinh, D.N.; Heng, L.; Jie, W. A critical review on ammonium recovery from wastewater for sustainable wastewater management. J. Bioresour. Technol. 2018, 268, 749–758. [Google Scholar]
- Liu, B.; Giannis, A.; Zhang, J.; Chang, V.W.; Wang, J.Y. Characterization of induced struvite formation from source-separated urine using seawater and brine as magnesium sources. J. Chemosphere 2013, 11, 2738–2747. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, C.; Wang, Y. Process conditions for removal of nitrogen and phosphorus from pig farm wastewater by MAP crystallization method. J. Ecol. Rural. Environ. 2010, 3, 268–272. [Google Scholar]
- Luján-Facundo, M.; Iborra-Clar, M.; Mendoza-Roca, J.; Also-Jesús, M. Alternatives for the management of pig slurry: Phosphorous recovery and biogas generation. J. Water Proc. Eng. 2019, 30, 100473. [Google Scholar] [CrossRef]
- Zhang, K.E.; Bowers, J.; Harrison, H. Releasing phosphorus from calcium for struvite fertilizer production from anaerobically digested dairy effluent. J. Water Environ. Res. 2010, 1, 32–42. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, C.; Shi, W.; Huang, X.; Ye, Y.; Jiang, W.; Kang, J.; Liu, D.; Ren, Y.; Li, D. Preferable phosphate removal by nano-La (III) hydroxides modified mesoporous rice husk biochars: Role of the host pore structure and point of zero charges. J. Sci. Total Environ. 2019, 662, 511–520. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Q.; Zhang, J.; Wu, Y.; Zhang, L.; Wang, L. Study on the technology of chemical precipitation treatment of high concentration ammonia nitrogen wastewater. J. Indust. Water Treat. 2010, 6, 31–34. [Google Scholar]
- Dongmei, Z.; Lihua, C.; Xiaohui, G. Research on the influencing factors of struvite precipitation method used in pig farm wastewater pretreatment. J. South China Norm. Univ. 2012, 2, 99–102. [Google Scholar]
- Ackerman, J.N.; Zvomuya, F.; Cicek, N.; Flaten, D. Evaluation of manure-derived struvite as a phosphorus source for canola. J. Plant Sci. 2013, 3, 419–424. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Huang, H.M.; Munir, T.; Wang, G.Q.; Young, B.R. Phosphorus recovery through struvite crystallization: Challenges for future design. Sci. Total Environ. 2019, 648, 1244–1256. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Ding, L. Recovery of phosphate and ammonia nitrogen from the anaerobic digestion supernatant of activated sludge by chemical precipitation. J. Clear Prod. 2015, 102, 437–446. [Google Scholar] [CrossRef]
- Nelson, N.O.; Mikkelsen, R.L.; Hesterberg, D.L. Struvite precipitation in anaerobic swine lagoon liquid: Effect of pH and Mg:P ratio and determination of the rate constant. J. Biore Technol. 2003, 89, 229–236. [Google Scholar] [CrossRef]
- Adnan, A.; Dastur, M.; Donald, S.M.; Frederic, A.K. Preliminary investigation into factors affecting controlled struvite crystallization at the bench scale. J. Environ. Eng. Sci. 2004, 3, 195–202. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Yuan, P.; Peng, J.; Fan, M. Modeling the crystallization of magnesium ammonium phosphate for phosphorus recovery. J. Chemosphere 2006, 65, 1182–1187. [Google Scholar] [CrossRef]
- Safaa, M.R. Phosphate removal from aqueous solution using slag and fly ash. HBRC J. 2013, 3, 270–275. [Google Scholar]
- Chinos, J.M.; Fernández, A.I.; Villalba, G.; Segarra, M.; Urruticoechea, A.; Artaza, B.; Espiell, F. Removal of ammonium and phosphates from wastewater resulting from the process of cochineal extraction using MgO-containing by-product. J. Water Res. 2003, 37, 1601–1607. [Google Scholar] [CrossRef]
- Stratful, I.; Scrimshaw, M.; Lester, J. Conditions influencing the precipitation of magnesium ammonium phosphate. J. Water Res. 2001, 35, 4191–4199. [Google Scholar] [CrossRef]
- Wang, J.; Burken, J.G.; Zhang, X.J.; Surampalli, R. Engineered struvite precipitation: Impacts of component-ion molar ratios and pH. J. Environ. Eng. 2005, 131, 1433–1440. [Google Scholar] [CrossRef]
- Perera, P.W.A.; Han, Z.Y.; Chen, Y.X.; Wu, W.X. Recovery of nitrogen and phosphorous as struvite from swine waste biogas digester effluent. Biomed. Environ. Sci. 2007, 20, 343–350. [Google Scholar] [PubMed]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-liquid separation of animal slurry in theory and practice: A review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef]
- Prabhu, M.; Mutnuri, S. Cow urine as a potential source for struvite production. J. Recycl. Org. Waste Agric. 2014, 3, 49. [Google Scholar] [CrossRef]
- Jiang, D.; Yoshimasa, A.; Motoi, M. Removal and recovery of phosphate from water by calcium-silicate composites-novel adsorbents made from waste glass and shells. J. Environ. Sci. Pollut. Res. 2017, 24, 8210–8218. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Hassan, M.A.; Irshad, S.; Talib, M.A.; Zhang, H. Efficient capture of phosphate and cadmium using Biochar with multifunctional amino and carboxylic moieties: Kinetics and mechanism. J. Water Air Soil. Pollut. 2020, 231, 25. [Google Scholar]
- Rafeah, W.; Zainab, N.; Veronica, U. Removal of mercury, lead, and copper from aqueous solution by activated carbon of palm oil empty fruit bunch. J. World Appl. Sci. 2009, 5, 84–91. [Google Scholar]
- Zheng, J. Influencing factors and application of nitrogen and phosphorus removal by struvite method. J. Urban Roads Bridge Flood Cont. 2009, 5, 254–257. [Google Scholar]
- Gadekar, S.; Pullammanappallil, P. Validation and applications of a chemical equilibrium model for struvite precipitation. Environ. Model. Assess 2010, 15, 201–209. [Google Scholar] [CrossRef]
- Booker, N.A.; Priestley, A.J.; Fraser, I.H. Struvite formation in wastewater treatment plants: Opportunities for nutrient recovery. J. Environ. Technol. 1999, 20, 777–782. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control, and recovery. J. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry; Wiley-Inter Science: New York, NY, USA, 1970; p. 583. [Google Scholar]
- Arge, E.; Bruaset, A.; Langtangen, H.P. Modern Software Tools for Scientific Computing; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Kolaczyk, E.D.; Csárdi, G. Statistical Analysis of Network Data with R; Springer: New York, NY, USA, 2014; p. 65. [Google Scholar]
- Kubar, A.A.; Qing, H.; Sajjad, M.; Chen, Y.; Lian, F.; Wang, J.; Kubar, A.A. Recovery of phosphate and ammonium from the biogas slurry as value-added fertilizer by biochar and struvite co-precipitation. J. Sustain. 2021, 7, 3827. [Google Scholar] [CrossRef]
- Asada, T.; Ishihara, S.; Yamane, T.; Toba, A.; Yamada, A.; Oikawa, K. Science of bamboo charcoal: Study on carbonizing temperature of bamboo charcoal and removal capability of harmful gases. J. Health Sci. 2002, 48, 473–479. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. A wood based low-temperature biochar captures NH3 –N generated from ruminant urine-N, retaining its bioavailability. Plant Soil. 2012, 353, 73–84. [Google Scholar] [CrossRef]
- Sajjad, M.; Sardar, K.; Shams, A.B.; Saduf, M.; Alia, N.; Sheikh, S.A.; Anwarzeb, K. Removal of potentially toxic elements from aqueous solutions and industrial wastewater using activated carbon. J. Water Sci. Technol. 2017, 75, 2571–2579. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, T.; Li, J.; Xu, K.; Wang, C. Biochar as a Carrier of Struvite Precipitation for Nitrogen and Phosphorus Recovery from Urine. J. Environ. Eng. 2018, 144, 2018. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Venterea, R.T. Biochar’s role as an alternative N-fertilizer: Ammonia capture. Plant Soil. 2012, 350, 35–42. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Yang, L.; Pan, B. Removal of ammonium from rare-earth wastewater using natural brucite as a magnesium source of struvite precipitation. Water Sci. Technol. 2011, 63, 468–474. [Google Scholar] [CrossRef]
- Türker, M.; Çelen, I. Removal of ammonia as struvite from anaerobic digester effluents and recycling of magnesium and phosphate. Bioresour. Technol. 2007, 8, 1529–1534. [Google Scholar] [CrossRef]
- Bhargava, D.; Sheldarkar, S. Use of TNSAC in phosphate adsorption studies and relationships. Effects of adsorption operating variables and related relationships. Water Res. 1993, 27, 313–324. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Bayramoglu, M. Modeling the effects of adsorbent dose and particle size on the adsorption of Cr (VI) ions from aqueous solutions. J. Adsorp. Sci. Technol. 2004, 22, 583–594. [Google Scholar] [CrossRef]
- Kara, S.; Aydiner, C.; Demirbas, E.; Kobya, M.; Dizge, N. Modeling the effects of adsorbent dose and particle size on the adsorption of reactive textile dyes by fly ash. J. Desalination 2007, 212, 282–293. [Google Scholar] [CrossRef]
- Taubner, S.; Klasenand, J.; Munder, T. Why do psychotherapists participate in psychotherapy research and why not? results of the attitudes to psychotherapy research questionnaire with a sample of experienced German psychotherapists. J. Psychol. Res. 2016, 26, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Geng, Y.; Sarkis, J.; Yang, M.; Xi, F.; Zhang, Y.; Xue, B.; Luo, Q.; Renand, W.; Bao, T. Measurement of polycyclic aromatic hydrocarbons (PAHs) in a Chinese brown field re development site: The case of Shenyang. J. Ecol. Eng. 2013, 53, 115–119. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. J. Rev. Comput. Statain. 2002, 2, 433–459. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Wangand, D.; Wang, Y. Assessment of temporal and spatial variations in water quality using multivariate statistical methods: A case study of the Xin’anjiang river, China. J. Front Environ. Sci. Eng. 2014, 8, 895–904. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Wellenand, C.; Wang, Y. A Bayesian approach of high impaired river reaches identification and total nitrogen load estimation in a sparsely monitored basin. J. Environ. Sci. Pollut. Res. Int. 2017, 24, 987–996. [Google Scholar] [CrossRef]
- Petr, P. Principal component weighted index for wastewater quality monitoring. J. Water. 2019, 11, 2376. [Google Scholar]
- Sial, M.H.; Awan, M.S.; Waqas, M. Role of institutional credit on agricultural production: A time series analysis of Pakistan. J. Econ. Fin. 2011, 3, 126. [Google Scholar] [CrossRef][Green Version]
- Hussain, A. Impact of credit disbursement, area under cultivation, fertilizer consumption and water availability on rice production in Pakistan (1988–2010). Sarhad J. Agric. 2012, 28, 95–101. [Google Scholar]
- Faridi, M.Z.; Chaudhry, M.O.; Tahir, N. Institutional credit and agricultural productivity an evidence from Pakistan. J. Life 2015, 13, 183–188. [Google Scholar]
- Chandio, A.A.; Jiang, Y.; Joyo, M.A.; Rehman, A. Impact of area under cultivation, water availability, credit disbursement, and fertilizer off-take on wheat production in Pakistan. J. Appl. Environ. Biol. Sci. 2016, 6, 10–18. [Google Scholar]
- Rehman, A.; Chandio, A.A.; Hussain, I.; Jingdong, L. Fertilizer consumption, water availability and credit distribution: Major factors affecting agricultural productivity in Pakistan. J. Saudi Soc. Agric. Sci. 2019, 18, 269–274. [Google Scholar] [CrossRef]
- Zhang, H.; Schroder, J.L.; Fuhrman, J.K.; Basta, N.T.; Storm, D.E.; Payton, M.E. Path and multiple regression analyses of phosphorus sorption capacity. J. Soil. Sci. Soci. Am. 2005, 69, 96. [Google Scholar] [CrossRef]
- Dodor, D.E.; Oya, K. Phosphate sorption characteristics of major soils in Okinawa, Japan. J. Commun. Soil Sci. Plant Anal. 2000, 31, 277–288. [Google Scholar] [CrossRef]
- Singh, B.B.; Tabatabai, M.A. Effects of soil properties on phosphate sorption. J. Commun. Soil. Sci. Plant Anal. 1977, 8, 97–107. [Google Scholar] [CrossRef]
- Sanyal, S.K.; Datta, D.S.K.; Chan, P.Y. Phosphate sorption desorption behavior of some acidic soils of south and southeast Asia. J. Soil Sci. Soc. Am. 1993, 57, 937–945. [Google Scholar] [CrossRef]
- Borling, K.; Ottabong, E.; Barberis, E. Phosphorus sorption in relation to soil properties in some cultivated Swedish soils. J. Nutr. Cycl. Agro Ecosyst. 2001, 59, 39–46. [Google Scholar] [CrossRef]
- Loeppert, R.L.; Inskeep, W.P. Iron methods of soil analysis. In Part 3. SSSA Book Ser. 5.; Sparks, D.L., Ed.; ASA and SSSA: Madison, WI, USA, 1996; pp. 639–664. [Google Scholar]
- Schoumans, O.F. Determination of the degree of phosphateorus in non-calcareous soil. In Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters. Bull. No. 369. Southern Extension Research Activity-Information Exchange Group (SERA-IEG-17); Pierzynski, G.M., Ed.; Kansas State University: Manhattan, KS, USA, 2000; pp. 31–34. [Google Scholar]
- Kleinman, P.J.A.; Sharpley, A.N. Estimating phosphorus sorption saturation from Mehlich-3 data. J. Commun. Soil. Sci. Plant Anal. 2002, 33, 1825–1839. [Google Scholar] [CrossRef]
- Wang, B.; Lehmann, J.; Hanley, K.; Hestrin, R.; Enders, A. Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. J. Chemosphere 2015, 138, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, Y.; Zhao, Y.; Li, W.; Song, W.; Miao, Z. Avian influenza H9N2 sero prevalence among pig population and pig farm staff in Shandong, China. J. Virol. 2015, 121, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Petersen, D.; Blazewicz, S.; Firestone, M.; Herman, D.; Turetsky, M.; Waldrop, M. Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. J. Environ. Microb. 2012, 14, 993–1008. [Google Scholar] [CrossRef]
- Zhi, W.; Ji, G. Quantitative response relationships between nitrogen transformation rates and nitrogen functional genes in a tidal flow constructed wetland under C/N ratio constraints. J. Water Res. 2014, 64, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Haloi, N.; Sarma, H.P. Heavy metal contaminations in the groundwater of Brahmaputra flood plain: An assessment of water quality in Barpeta district, Assam (India). J. Environ. Moni Assess 2012, 184, 6229–6237. [Google Scholar] [CrossRef]
- Mansouri, B.; Salehi, J.; Etebari, B.; Moghaddam, H.K. Metal concentrations in the groundwater in Birjand flood plain, Iran. J. Bull. Environ. Toxi 2012, 89, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, A.; David, P.; Arrighi, J.F.; Chiarenza, S.; Thibaud, M.C.; Nussaume, L.; Marin, E. Reducing the genetic redundancy of Arabidopsis phosphate transporter1 transporters to study phosphate uptake and signaling. J. Plant Phys. 2015, 167, 1511–1526. [Google Scholar] [CrossRef]
- Jaimie, B.P.; Miller, A.C.V.; Alison, B. Uncoupling growth from phosphorus uptake in Lemna: Implications for use of duckweed in wastewater remediation and P recovery in temperate climates. J. Food Eng. Secur. 2020, 9, 244. [Google Scholar]
- Adoonsook, D.; Chia, Y.C.; Wongrueng, A.; Pumas, C. A simple way to improve a conventional A/O-MBR for high simultaneous carbon and nutrient removal from synthetic municipal wastewater. PLoS ONE 2019, 14, e0214976. [Google Scholar] [CrossRef]


| Ca2+ | Zn2+ | Cu2+ | Fe3+ | Fe2+ | CO32− | HCO3− | K+ | SO42− | |
|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | 1 | −0.476 * | −0.822 ** | −0.484 * | −0.643 ** | −0.618 ** | −0.546 * | −0.377 | −0.593 ** |
| Zn2+ | −0.476 * | 1 | 0.849 ** | 0.966 ** | 0.915 ** | 0.936 ** | 0.968 ** | 0.982 ** | 0.362 |
| Cu2+ | −0.822 ** | 0.849 ** | 1 | 0.855 ** | 0.924 ** | 0.905 ** | 0.864 ** | 0.798 ** | 0.13 |
| Fe3+ | −0.484 * | 0.966 ** | 0.855 ** | 1 | 0.959 ** | 0.881 ** | 0.920 ** | 0.983 ** | 0.347 |
| Fe2+ | −0.643 ** | 0.915 ** | 0.924 ** | 0.959 ** | 1 | 0.892 ** | 0.912 ** | 0.913 ** | −0.116 |
| CO32− | 0.618 ** | 0.936 ** | 0.905 ** | 0.881 ** | 0.892 ** | 1 | 0.964 ** | 0.879 ** | −0.113 |
| HCO3− | −0.546 * | 0.968 ** | 0.864 * | 0.920 * | 0.912 ** | 0.964 ** | 1 | 0.926 ** | −0.217 |
| K+ | −0.377 | 0.982 ** | 0.798 ** | 0.983 ** | 0.913 ** | 0.879 ** | 0.926 * | 1 | −0.466 |
| SO42− | −0.593 ** | −0.362 | 0.13 | −0.347 | −0.116 | −0.113 | −0.217 | −0.466 | 1 |
| Ca2+ | Zn2+ | Cu2+ | Fe3+ | Fe2+ | CO32− | HCO3− | K+ | SO42− | |
|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | 1 | 0.900 ** | 0.980 ** | 0.973 ** | 0.947 ** | 0.989 ** | 0.987 ** | 0.980 ** | 0.987 ** |
| Zn2+ | 0.900 ** | 1 | 0.948 ** | 0.955 ** | 0.962 ** | 0.939 ** | 0.943 ** | 0.950 ** | 0.938 ** |
| Cu2+ | 0.980 ** | 0.948 ** | 1 | 0.999 ** | 0.990 ** | 0.998 ** | 0.998 ** | 1.000 ** | 0.998 ** |
| Fe3+ | 0.973 ** | 0.955 ** | 0.999 ** | 1 | 0.994 ** | 0.995 ** | 0.996 ** | 0.999 ** | 0.997 ** |
| Fe2+ | 0.947 ** | 0.962 ** | 0.990 ** | 0.994 ** | 1 | 0.981 ** | 0.984 ** | 0.990 ** | 0.985 ** |
| CO32− | 0.989 ** | 0.939 ** | 0.998 ** | 0.995 ** | 0.981 ** | 1 | 1.000 ** | 0.998 ** | 0.998 ** |
| HCO3− | 0.987 ** | 0.943 ** | 0.998 ** | 0.996 ** | 0.984 ** | 1.000 ** | 1 | 0.999 ** | 0.998 ** |
| K+ | 0.980 ** | 0.950 ** | 1.000 ** | 0.999 ** | 0.990 ** | 0.998 ** | 0.999 ** | 1 | 0.998 ** |
| SO42− | 0.987 ** | 0.938 ** | 0.998 ** | 0.997 ** | 0.985 ** | 0.998 ** | 0.998 ** | 0.998 ** | 1 |
| Ca2+ | Zn2+ | Cu2+ | Fe3+ | Fe2+ | CO32− | HCO3− | K+ | SO42− | |
|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | 1 | 1.000 ** | 0.528 * | −0.385 | 0.981 ** | 0.964 ** | 0.972 ** | 0.998 ** | 0.994 ** |
| Zn2+ | 1.000 ** | 1 | 0.528 * | −0.385 | 0.981 ** | 0.964 ** | 0.972 ** | 0.998 ** | 0.994 ** |
| Cu2+ | 0.528 * | 0.528 * | 1 | 0.581 * | 0.586 * | 0.551 * | 0.564 * | 0.523 * | 0.543 * |
| Fe3+ | −0.385 | −0.385 | 0.581 * | 1 | −0.303 | −0.325 | 0.319 | −0.387 | 0.362 |
| Fe2+ | 0.981 ** | 0.981 ** | 0.586 * | −0.303 | 1 | 0.993 ** | 0.990 ** | 0.989 ** | 0.994 ** |
| CO32− | 0.964 ** | 0.964 ** | 0.551 * | −0.325 | 0.993 ** | 1 | 0.992 ** | 0.977 ** | 0.983 ** |
| HCO3− | 0.972 ** | 0.972 ** | 0.564 * | −0.319 | 0.990 ** | 0.992 ** | 1 | 0.980 ** | 0.986 ** |
| K+ | 0.998 * | 0.998 ** | 0.523 * | −0.387 | 0.989 ** | 0.977 ** | 0.980 ** | 1 | 0.999 ** |
| SO42− | 0.994 ** | 0.994 ** | 0.543 * | −0.362 | 0.994 ** | 0.983 ** | 0.986 ** | 0.999 ** | 1 |
| Ca2+ | Zn2+ | Cu2+ | Fe3+ | Fe2+ | CO32− | HCO3− | K+ | SO42− | |
|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | 1 | 0.99 * | 0.97 * | 0.98 * | 0.98 * | 0.96 * | 0.97 * | 0.99 * | 0.99 * |
| Zn2+ | 0.99 * | 1 | 0.97 * | 0.98 | 0.98 * | 0.96 * | 0.97 * | 0.99 * | 0.99 * |
| Cu2+ | 0.97 * | 0.97 * | 1 | 0.97 * | 0.97 * | 0.96 * | 0.96 * | 0.97 * | 0.97 * |
| Fe3+ | 0.98 * | 0.98 * | 0.97 * | 1 | 0.99 * | 0.98 * | 0.98 * | 0.99 * | 0.99 * |
| Fe2+ | 0.98 * | 0.98 * | 0.97 * | 0.99 * | 1 | 0.99 * | 0.99 * | 0.98 * | 0.99 * |
| CO32− | 0.96 * | 0.96 * | 0.96 * | 0.98 * | 0.99 | 1 | 0.99 * | 0.97 * | 0.98 * |
| HCO3− | 0.97 * | 0.97 * | 0.96 * | 0.98 * | 0.99 * | 0.99 * | 1 | 0.98 * | 0.98 * |
| K+ | 0.99 * | 0.99 * | 0.97 * | 0.99 * | 0.98 * | 0.97 * | 0.98 * | 1 | 0.99 * |
| SO42− | 0.99 * | 0.99 * | 0.97 * | 0.99 * | 0.99 * | 0.98 * | 0.98 * | 0.99 * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubar, A.A.; Huang, Q.; Kubar, K.A.; Khan, M.A.; Sajjad, M.; Gul, S.; Yang, C.; Wang, Q.; Guo, G.; Kubar, G.M.; et al. Ammonium and Phosphate Recovery from Biogas Slurry: Multivariate Statistical Analysis Approach. Sustainability 2022, 14, 5617. https://doi.org/10.3390/su14095617
Kubar AA, Huang Q, Kubar KA, Khan MA, Sajjad M, Gul S, Yang C, Wang Q, Guo G, Kubar GM, et al. Ammonium and Phosphate Recovery from Biogas Slurry: Multivariate Statistical Analysis Approach. Sustainability. 2022; 14(9):5617. https://doi.org/10.3390/su14095617
Chicago/Turabian StyleKubar, Aftab Ali, Qing Huang, Kashif Ali Kubar, Muhammad Amjad Khan, Muhammad Sajjad, Sumaira Gul, Chen Yang, Qingqing Wang, Genmao Guo, Ghulam Mustafa Kubar, and et al. 2022. "Ammonium and Phosphate Recovery from Biogas Slurry: Multivariate Statistical Analysis Approach" Sustainability 14, no. 9: 5617. https://doi.org/10.3390/su14095617
APA StyleKubar, A. A., Huang, Q., Kubar, K. A., Khan, M. A., Sajjad, M., Gul, S., Yang, C., Wang, Q., Guo, G., Kubar, G. M., Kubar, M. I., & Wahocho, N. A. (2022). Ammonium and Phosphate Recovery from Biogas Slurry: Multivariate Statistical Analysis Approach. Sustainability, 14(9), 5617. https://doi.org/10.3390/su14095617







