Abstract
This article reviews the available research results of selected species of the genus Silphium L. (Asteraceae) as alternative plants for crops and industry. Silphium species have valuable qualities across a wide range of uses, which is very important in considering plant resources as a green alternative to a sustainable future. Species of the genus Silphium are tall perennials found in fields, prairies, open forests, and groves in the central and eastern parts of the United States and Canada. Various tribes of Native North American used Silphium for medicinal purposes. The cup plant Silphium perfoliatum L. is the most popular species of the genus Silphium due to its attractive ornamental, honey–giving, healing, and forage qualities. As the literature review shows, species of the genus Silphium are characterized by a high production potential in terms of yields and contain significant amounts of nutrients, i.e., carbohydrates, proteins, and L-ascorbic acid, as well as minerals and biologically active substances, e.g., terpenoids and essential oils, flavonoids, phenolic acids, and oleanosides. In addition, the research confirmed the possibility of using Silphium for fodder, as honeybee forage, phytoremediation plants, for reclamation of degraded land, as plants for energy purposes (biomass, biogas), and as plants that provide components with antimicrobial activity. This review largely takes into account many years of research experience conducted in Poland.
1. Introduction
In recent years, we can observe a number of processes taking place on our planet, which in the long run may have adverse effects on all forms of life. Certainly, the most severe of these are climate changes on Earth and the prospect of having to feed another 2 billion people by 2050 with limited access to water and arable land. These problems are also the most important challenges for modern agriculture. Even now, decisive and effective solutions are important, which will allow for combining the effects of improving the efficiency and quality of agricultural production with the rational use of natural resources. FAO has defined sustainable agricultural development as “the management and conservation of the natural resource base, and the orientation of technological change in such a manner as to ensure the attainment of continued satisfaction of human needs for present and future generations. Sustainable agriculture conserves land, water, and plant and animal genetic resources, and is environmentally non-degrading, technically appropriate, economically viable and socially acceptable” [1]. Sustainable agriculture fully responds to global challenges, providing a real perspective for conventional management that is no longer working [2]. Sustainable agricultural practices make it possible to use the means of production more efficiently and to better protect the environment and surroundings in which the farm operates. In the case of arable crops, yield, quality, and resistance to biological and physical stresses have been among the basic breeding requirements for many years. The importance of quality and stress resistance in modern cultivation of crops is constantly increasing [3]. This is due to the fact that the world’s food needs are met, with even an excess of food being produced. Limiting the cultivation area of many types of crops (cereals, sugar beets) is becoming a necessity. Increasingly larger areas of arable land appear here, released from the previous crops that are traditional for today’s agriculture. This creates opportunities for the cultivation of new plants or so-called “alternative plants” for various industries and energy [4].
The interest in alternative crops (new crops) will increase in the present century for many reasons that arose over the last years. Among the primary reasons that ought to be mentioned: increasing biological diversity of the food; production of novel food and dietary supplements; supply of medicines, antibodies, and vaccines; biological soil recultivation; production of biodegradable materials; production of renewable raw materials for industry and renewable energy sources; ecological pressure on agriculture and forming systems; etc. The research on new crops is carried out by a number of international and local organizations. Therefore, there is a tendency to search for plants that can be used in various areas of life and economy. There are dozens of alternative plant species that have already been introduced into practical cultivation in different countries. Moreover, it should be emphasized that there is an increase in interest in new plant species on the part of both farmers and users of plant raw materials.
When undertaking contemporary challenges in the field of introducing alternative plant species to cultivation, it is necessary to consider the widest possible use of raw materials from such agricultural production. There may be plants that can provide valuable raw materials for numerous industries at the same time, e.g., energy, pharmaceutical, food, fodder, paper, construction, etc. Such wide possibilities from using raw materials from the cultivation of valuable plant species will enable their full use in various economic conditions in a given period. When assessing the usability of such plant species, production efficiency should be taken into account in various environmental conditions, as well as botanical features, chemical composition and, of course, various utility concepts.
One interesting group of plants in this regard consists of plant species of the genus Sliphium L. (Asteraceae). The genus was brought to Europe from North America in the 18th century, due to the ornamental value of the plants [5]. The best-known species are Silphium perfoliatum L., S. integrifolium Michx., and S. laciniatum L., which have been known for a long time to the trappers of Mountain Men who would cross the prairies of North America as the compass plant, indicating with its leaves the north–south direction [6]. The cup plant Silphium perfoliatum L. is the most popular species, due to its attractive ornamental, melliferous, medicinal, and fodder values [7]. Introducing Silphium to Europe contributed to the interest in these species as plants with multidirectional utility potential, which is visible, among others, in a number of publications on S. perfoliatum published from 2000–2020: 96 publications came from Europe (31 publications from Germany and 30 from Poland), while in the USA (the area of natural occurrence of Silphium) there were only 12 publications [8].
Plants from the genus Silphium L. have raised, rarely branched stems which exude resin-like juice with a strong odor similar to that of turpentine [5]. Their opposite or alternate small leaves, sometimes all basal leaves, are acicular to oval or deltoid, with some of them serrate or serrulate. Sunflower-like yellow flower heads (Silphium albiflorum Gray produces white inflorescences) form in branched corymbs [9]. They are cold-hardy plants, resistant to diseases and pests [10]. Research shows that S. perfoliatum is an attractive plant that provides biomass for energy purposes (e.g., for the production of biogas and fodder plant, as well as raw material for the production of paper, office boards, as a source of biologically active substances for food and pharmaceutical purposes, or for the production of bio-protection preparations in organic farming. The review articles published so far focus mainly on the use of Silphium as an energy plant, but there is no comprehensive review article taking into account the multidirectional variants of the use of various Silphium species. In particular, there is no review of the state of knowledge about the chemical composition of these species, and yet the possibilities of using these plants result directly from the content of various groups of chemical compounds, broken down into primary metabolites and secondary metabolites.
Therefore, this review article presents the state of the art on this group of plants in terms of utility value with detailed botanical, cultivation, and chemical characteristics.
2. Methods
For the review, articles were obtained through three databases, namely Scopus, Science Direct, and Google Scholar. In addition, our own databases were used, mainly containing non-English language items (Russian, Polish). This review largely takes into account many years of research experience conducted in Poland. As a large portion of the articles written in Polish and Russian are very difficult to find, we believe that such consideration is important. As the focus of the review is on the broad aspect of research on species of the genus Silphium L., cup plant, the following key words were used: Silphium, anatomy, morphology, cultivation, chemical composition, nutritional values, biological activity, medicinal application, antioxidant properties, animal feeding, honeybee forage, ornamental plant, soil remediation, energy crop, biogas, forage, feed, material, composite, silage. The search was refined by reading the title and abstract of the included articles, and they were designed to meet the requirement of the current article. Articles published as research articles, reviews, and in book chapters were used. Articles published through 2022 were considered.
Information was collected on:
- anatomical and morphological features of selected Silphium species,
- biomass productivity in various geographic locations,
- chemical composition with division into primary metabolites, secondary metabolites, and minerals,
- multidirectional utility values of Silphium species biomass.
3. Results and Discussion
3.1. Genus Silphium L.
Species from the genus Silphium L. (family Asteraceae, subfamily Asteroideae, tribe Heliantheae) are tall perennial plants of the fields, prairies, open forests, and groves of the central and eastern parts of the United States and Canada [11,12,13,14]. The literature provides plentiful and often contradictory information on the number of species in the genus Silphium L. According to a study by Stuessy [15], the genus comprises 23 species, while other authors estimate that number as 33, 30, 25, 20, or 13–15 species [13,14,16,17]. These controversies are caused by the occurrence of polymorphism in the genus. Clevinger and Panero [13] distinguished two groups of species in the genus Silphium L.: Silphium and Composita, which differ from each other in the form of the plant and in the structure of the root. The most commonly known species of plants from the genus Silphium L. with their areas of natural occurrence are presented in Table 1.

Table 1.
Review of species found in the genus Silphium L. [11,12,13].
3.1.1. Description of Selected Silphium Species
Silphium perfoliatum L. (Figure 1) grows in natural conditions from Ontario to Oklahoma, in the states of North and South Dakota, Georgia, Mississippi, and Missouri (Table 1) [11,12]. The somatic number of chromosomes for the species is 2n = 14. Two botanical varieties are known within this species: Silphium perfoliatum L. var. connatum (L.) Cronq. and Silphium perfoliatum L. var. perfoliatum. In the literature, the plant is described under numerous popular names: Rosinweed, Cup Plant, Indian Cup (English) [11,12]; różnik przerośnięty, sylfia (Polish) [7]; sil’fia pronzennolistnaja (Russian) [18]; silfe (French) [19]; silfio (Italian) [20]; Durchwachsene Silphie (German) [21]; silfiu (Romanian) [14]; mácsonyás szilfium (Hungarian) [14].

Figure 1.
S. perfoliatum—different phases of development and major plant organs.
This species is encountered in Central Europe—in Germany (in the valley of the river Rhine, in the neighborhood of Dresden), in Austria (Graz), and in central Switzerland [14]—as well as in China [22]. S. perfoliatum is an erect herbaceous perennial plant growing up to 250 cm high. Stems of S. perfoliatum are naked and hollow inside, with a cross–section resembling a rhombus with concave walls, branching mainly in their upper part. Leaves are large (30 cm length × 20 cm width), dark green, with toothed edges, opposite, and joined together as if pierced by the stem. Lower leaves are set on long petioles, while upper leaves have winged petioles, fused at the base, forming a cup. On both the upper and lower side they are covered with rough, bristle-like hairs [7]. Although roots were found at maximum depths of 1.5–1.7 m, the greatest density of roots was found in the upper 0.3 m [23]. In Europe, this species is not classified as invasive plant species [24,25].
The species Silphium trifoliatum L. var. latifolium is commonly known as rosinweed or whorled rosinweed. It is native to eastern United States, east of the river Mississippi. Some authors claim that this species is a variety of Silphium asteriscus [13]. It is characterized by smooth stems and leaves which are often grouped in threes or fours around the stem, but can be single or paired [26]. At the end of the first year of cultivation, single S. trifoliatum one-year-old plants plants form a luxuriant leaf crown. S. trifoliatum plants differ considerably from S. perfoliatum and S. integrifolium plants with regard to morphology [26].
Silphium integrifolium is known as rosinweed, whole-leaf rosinweed, entire-leaf rosinweed, and prairie rosinweed. It is native to the eastern part of North America, and can be found in Ontario in Canada, in the eastern and central parts of the United States, and in New Mexico. The species produces stems up to 2 m tall, growing from characteristic clumps which can consist of up to 20 stems. The stems are hairless or slightly rough-haired, slightly waxy in texture. Leaves are stemless [27]. There are four varieties of the species: S. integifolium var. integrifolium—from 40 to 200 cm in height, S. integifolium var. leave—usually from 100 to 150 cm in height, with a larger number of flowers and with smooth leaves, and other varieties such as S. integifolium var. deamii Perry, S. integifolium var. gattingeri Perry, and S. integifolium var. neglectum Settle & T.R. Fisher.
After sowing in the first year, species of Silphium form a characteristic leaf rosette [28]. In subsequent years of vegetation, Silphium plants form multi-stem clumps. For example, two-year-old plants of S. trifoliatum can form clumps composed of 4 to 16 stems attaining the height of approx. 206 cm [26]. In autumn, two-year old plants of S. perfoliatum can have an average height of approx. 250 cm (average of 9 stems in a clump), while stems of S. integrifolium can be shorter compared to those of the two species mentioned earlier, and can attain an average height of approx. 184 cm (with an average of 19 stems in a clump) [7,26,27]. The underground part of the plant is a cylindrical rhizome, twisted and uneven, from which small roots grow [7,29]. The plants can easily multiply through the division of the underground part [7,26,27].
Blooming
Silphium species produce pseudo-dichotomous branching inflorescence shoots at the shoot top during the second year of vegetation. In Poland, the blooming of Silphium lasts from the beginning of July to the end of September, and can even extend into October [30]. Observations in Germany point to a flowering time from July to September [31]. In a study conducted in Lower Saxony (Germany) in the first year of flowering (which is the second year after planting), the plants of S. perfoliatum developed 6.3 stems on average (1–15 stems). In the first sampling period at the end of July, the flowering period had just started and a single stem held, on average, 1.3 flowering inflorescences. The stems then constantly developed new side branches and inflorescences, so that flower abundance was highest in August. In the last sampling in mid-September, the plants on some sites showed an ongoing branching, whereas, on other sites, the flowering was coming to an end [32]. The blooming of Silphium species is not uniform in time, i.e., when seeds are already ripening on the main stems, inflorescences are still blooming on lateral stems, which can be a hindrance for seed harvest [30]. Flowers are concentrated in yellow inflorescences of capitulum type, the diameter of which varies from 4.5 to 12 cm for S. perfoliatum, from 3.0 to 9.0 cm for S. trifoliatum, while in the case of S. integrifolium, from 4.5 cm to 10.5 cm, depending on the position on the inflorescence shoot [7,26,27]. In an entire clump, in the second year of cultivation, we observed the following number of inflorescences on the plants: for S. perfoliatum, approx. 468 inflorescences (51–54 on an individual shoot), for S. trifoliatum approx. 512 inflorescences (53 on an individual shoot), and in the case of S. integrifolium approx. 705 inflorescences (36 on an individual shoot) [7,27,33]. In research conducted in Germany, a single S. perfoliatum stem developed, on average, 1.5 inflorescences (range 0–5) until the end of July, 8 inflorescences (range 1–29) until mid-August, 26 inflorescences (range 1–121) until the end of August/beginning of September, and 33 inflorescences (range 4–108) until mid-September, with a mean number of approx. six stems per plant (range 1–15). Thus, total number of inflorescences per plant was estimated to be 10 (end of July), 55 (mid-August), 150 (end of August/beginning of September) and 188 (mid-September) [31].
Female ligulate flowers of the Silphium species situated on the edge of inflorescences had a shiny yellow corolla, and tubulate ones were placed in the middle part of the inflorescence and were functionally male, because they produced stamens and sterile pistils. Capitula S. trifoliatum contained an average of 34 ligulate and 168 tubulate flowers, while in the case of S. perfoliatum and S integrifolium, the corresponding numbers were 25 and 16 ligulate and 167 and 115 tubulate flowers, respectively. The morphological structure of inflorescences of S. perfoliatum, S. trifoliatum, and S. integrifolium, with special emphasis on the structure of pollen presenters and pollen grains, was the subject of a study by Weryszko-Chmielewska et al. [34]. The inflorescence is set on an abbreviated pedicel, covered from below with three rows of scale-like leaves arranged in a roof tile pattern. In the flower heads there are two kinds of flowers: ligulate, situated on the edges of the flower head, and tubulate, situated in the middle of the flower head, functionally male, which produce stamens and sterile pistils [34].
Inflorescences of Silphium are highly attractive to insect pollinators, especially bees. A study by Wróblewska [35] revealed a significant effect of pollinating insects on the setting of seeds of S. perfoliatum. Flower heads freely pollinated by those insects produced, on average, 69.7–80.3% of seeds (relative to the number of flowers), while isolated ones only 5.8–10.6%. S. perfoliatum is accepted to be a good melliferous plant in England [36], in Germany [37], in the regions of Leningrad and in Bashkiria [38,39], and in Bulgaria [35], where it provides late pollen supply for bees. In a Polish study, the estimated yield of honey per 1 ha was 152.8 kg, and the yield of pollen was 363.9 kg [35]. The cultivation of this species can provide considerable supply of both nectar and pollen from mid-summer until late autumn. An inflorescence of S. perfoliatum produced, on average, 122 disk florets (range 68–205) where a disk floret contained, on average, 14,200 pollen grains (range 6000–21,400), resulting in a mean number of 1.75 × 106 pollen grains per inflorescence [31]. Regarding pollen, it was shown that the estimated mean number of pollen grains per hectare of S. perfoliatum was higher than that of the maize [40].
Seeds
The maturation of the infructescences of Silphium seeds takes place irregularly and for a long time due to the continuous formation of new flowers, which results in the collection of mature, immature, and sterile instances [41]. The seed is a two-wing brown achene (Figure 1). Seeds of S. perfoliatum have a taste resembling that of sunflower seeds [42]. Seeds of three studied species are achenes, equipped with two alas that enable flight. No morphological differences, besides seed size and different color, were found among them. Moreover, seeds of the species studied have a soft seed coat. The seeds of S. perfoliatum are not uniform, which complicates the sowing process [43]. The dimensions of the seeds range from 9–10 mm in length, 4.5–6 mm in width, and 1–1.5 mm in thickness, with an average weight of 16–20 g per 1000 seeds [44,45,46]. S. perfoliatum was characterized with the highest seed yield in comparison to S. trifoliatum and S. integrifolium; about 185 g of seeds were achieved, thus it was considered as the most efficient of the three studied species in such a context [42]. Seed yields for other species (S. trifoliatum and S. integrifolium) accounted for 150 g and 94 g, respectively [42]. In this study, the 1000-seed weight was the highest in S. perfoliatum and amounted to about 21.5 g; in the case of S. trifoliatum and S. integrifolium, it was about 16.6 g and 12.5 g, respectively [42]. In other studies on S. perfoliatum, conducted in Poland and in Belarus, the authors obtained mean values of 1000-seed weight at the level of 9.2 g and 23.7–25.3 g, respectively [33,47].
3.1.2. Anatomy
Anatomical studies on the aboveground stems, leaves, and rhizomes of S. perfoliatum revealed the occurrence of inner secretory tissues in the form of annular-distributed conduits in stem cross-sections, most frequently surrounded with a single layer of epithelium [29]. The distribution of secretory vessels in the aboveground stems and in the rhizomes of S. perfoliatum is different. In aboveground stems they form two rings on the outer and inner side of the vascular bundles, while in rhizomes they are more compacted, forming rings around the phloem (Figure 2). The number of secretory vessels increases in the lower positioned parts of the stem, attaining a secondary increment. The secretory ducts are of schizogenous origin and they are composed of an exudate cavity of various sizes and a secretory epithelium comprising 1–3 layers of cells. The exudate of the secretory tissue of S. perfoliatum is essential oil or a resin solution in oil (balm). Essential oils are produced mostly as secondary metabolites and the places of their accumulation are the secretory structures found on the surface (secretory hairs) or inside the plant (secretory channels). The type of secretory structures is characteristic of a given botanical family [48,49]. The role of essential oils for the plant is, among others, protection against animals or insects, protection against pathogens, transmission of information in plants as well as for the environment (pollination), facilitating survival in unfavorable conditions, and inhibiting the growth of other plants growing nearby [49,50].
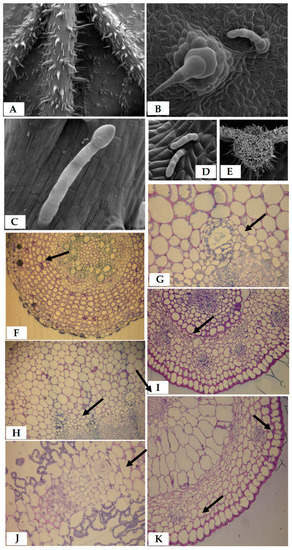
Figure 2.
Morphological and anatomical structure of Silphium species. (A) Covering trichomes in nerves of abaxial S. trifoliatum leaf blade surface [53]. (B) Non-glandular and glandular trichomes of S. trifoliatum [53]. (C,D) Structure of grandular trichomes of S. integrifolium and S. trifoliatum [53]. (E) Cross-section of S. trifoliatum leaf nerve [53]. (F) Cross-sections of S. perfoliatum rhizome [29]. (G,H) Cross-sections of upper part S. perfoliatum stem [29]. (I) Cross-sections of S. trifoliatum lingual flower. (J) Cross-sections of S. trifoliatum leaf blade [53]. (K) Cross-sections of S. trifoliatum tubular flower. Arrows, secretory ducts.
Rhizomes of the species of S. trifoliatum and S. integrifolium are similar to those of S. perfoliatum [29] (Figure 2) and S. laciniatum [51]. They display a primary structure and are covered with epidermis with visible protruding cells in the form of small hairs with thickened external walls. Parenchyma cells, forming a notable layer of bark, have thickened cellulose cell walls with straight cavities. Along with the growth of the rhizome, secretory vessels are formed in the inner bark layer. They are numerous, with irregular shapes, and are positioned close together, forming a characteristic layer. The surrounding epithelial cells are highly differentiated in size. The next tissues are the endodermis and the tissues of the axial cylinder with vascular bundles. The central part of the rhizome is filled with core parenchyma with thickened walls [52].
Microscope examinations of inflorescences of S. perfoliatum, S. trifoliatum, and S. integrifolium revealed the presence of hairs: glandular, non-glandular, and so-called mechanical [34]. Similar kinds of hairs was found on leaves of Silphium. Leaves of Silphium are characterized by a rough surface, which results from the considerable density of the cover hairs. In addition, on the adaxial and abaxial surfaces of leaves, single-row multi-cell club-shaped hairs were observed that perform secretory functions. Secretory ducts in the nerves of the leaf blades of S. perfoliatum, S. trifoliatum, and S. integrifolium are of schizogenous origin and occur in various numbers (1–5) around the vascular bundles [29,53]. Unfortunately, no data from anatomical studies conducted in other countries are available.
3.1.3. Cultivation
Species from the genus Silphium require moderately fertile, moist, and deep soil with neutral to slightly alkaline reaction. S. perfoliatum easily adapts to various conditions [28,54]. The described species of Silphium grow the best in full sunlight or in semi-shade with temperatures of around 20 °C and sandy soils close to water sources [55]. Studies conducted so far on the cultivation of S. perfoliatum [14,19,20,28,56,57,58,59] demonstrated easy adaptation of the species to various climatic conditions (Western Europe, Central Europe, Eastern Europe, Asia, New Zealand, South America). The plants can be multiplied generatively through seed sowing, or vegetatively through the division of root stocks of older plants [10].
Seeds can be sown into a hotbed or directly in the permanent growing site, but there is the problem of their low germination capacity of approx. 20%. To stimulate their germination strength, it is recommended to store the seeds prior to sowing, at a temperature of approx. 4 °C [14]. An increase of germination capacity, up to even as much as 90%, can be achieved by placing the sowings in a multiplicator, at a temperature of 30 °C, for 1–3 days, and then applying a temperature of 20 °C until the moment of germination [60]. Another method of improving the germination capacity of seeds of S. perfoliatum is through their stratification, e.g., through soaking, prior to the sowing, in a solution of gibberellic acid GA (1000 mg/dm3), which raises their germination capacity from approx. 28% to approx. 76% [61]. Von Gehren et al. [44] report that the assumption of the cultivation of S. perfoliatum in Central Europe by sowing is feasible when using pelleted seeds implemented with gibberellic acid and adhering to a sowing date at the end of April. Seeds of S. integrifolium soaked for 24 h in a ethephon/potassium nitrate solution followed by a 72 h drying step at 40 °C showed an increase of germination to up to 90 ± 2% compared to control seeds (3 ± 0% and 5 ± 1%) [62]. Cold stratification was nearly as good as the chemical treatment [62]. In the case of sowing seeds, Köhler and Biertümpfel [46] report that the optimal sowing time is not a specific date, but depends on optimal soil and weather conditions to ensure rapid germination of the seeds. There are reports of a special production of S. perfoliatum seeds for establishing plantations for energy purposes with patent protection [63].
S. perfoliatum plantations can also be established by replanting seedlings grown in the nursery [64]. The recommended distances between the rows ranged from 0.5 m to 0.6 m, 0.75 m, or 1 m. On the other hand, the distance between the plants in the rows ranged from 0.12 m to 0.50 m [65].
The simplest and the most effective method of multiplying Silphium plants is the division of root stocks of mother plants. Puia and Szabó [14] conducted vegetative multiplication of plants, dividing rhizomes of older plants into fragments containing from one to three buds, and from three to six buds, and concluded that those with the larger number of buds were better multiplication material.
The planting density is important. Pichard in Chile planted the field with a row spacing of 40 to 80 cm (from 104 to 208 plants/ha) [28]. The author reported that the DM yield was lower for the lowest plant densities, although not statistically significant (p ≥ 0.05), suggesting that the seeding rates need to be adjusted to produce at least 120,000 plants/ha [28]. The greater population densities did affect the plant morphology, but the yield components tended to compensate, and no significant effect (p ≥ 0.05) was observed on biomass yield [28]. In the experiments conducted so far, the planting of the area at the S. perfoliatum plantation ranged from 10,000 plants per ha to 140,000 plants per ha [65].
Fertilization of Silphium plants depends primarily on the content of mineral and organic substances in the soil. Taking into account the intensive growth of the plant and the high increase of green matter, S. perfoliatum is not a plant with particularly stringent requirements. Pavlov et al. [66] conducted a three-year experiment on the cultivation of S. perfoliatum in relation to the fertilization of plants until the moment of blooming, and demonstrated that the most beneficial was N, P, K fertilization at doses of 90 kg/ha of each component. Other authors cultivated plants by applying higher fertilization doses throughout the entire vegetation period: N, from 200 kg/ha to 300 kg/ha; P, 150 kg/ha; K, 150 kg/ha [20,59]. Pichard [28] in Chile applied the following fertilization: N, from 30 kg/ha to 200 kg/ha; P, 400 kg/ha; K, 100 kg/ha. Silphium are not demanding in terms of fertilization. Jemielin and Šjeluto [67] recommend liming acid soils, introducing organic (semi-liquid cattle manure) and mineral fertilization (nitrogen, phosphorus–potassium fertilizers) when establishing S. perfoliatum plantations. According to Šjeluto and Kostickaja [68], nitrogen fertilizers had a positive effect on the photosynthesis of S. perfoliatum. The photosynthetic activity was significantly higher when the increased N dose of 120 kg/ha was applied.
In the first year of cultivation, the plants do not bloom and only produce leaf rosettes at heights from approx. 16 cm to approx. 64 cm [7,28,57,69]. In subsequent years, Silphium species produce numerous stems and, depending on sowing density, form a very compact canopy. In the second year of cultivation, the height of the plants, in the phase of ripeness, can reach from approx. 188 cm to about 319 cm [7,28,57,69,70,71]. In the second and subsequent years of S. perfoliatum cultivation, 5 to 25 stems per plant have been observed [65]. The percentage share of leaves in a plant can vary from 20% (at the start of vegetation) to 93% (in the phase of blooming and fruition), and that of stems can vary from 7% to 80%, depending on the time of harvest [58,70,71,72,73,74,75]. Next-generation plants can produce, on average, from 6 to 112 flower heads with diameters from 4.5 to 12 cm [7,35,69]. Stem number of S. perfoliatum and thickness can vary due to plant density, but height and leaf to stem ratio may not be affected by density [28]. The leaf proportion of the plants can vary between 30% and 45% [28]. The available data concern mainly the species S. perfoliatum, which results from the great attractiveness of this species in terms of cultivation. S. perfoliatum requires very little crop management as there are few relevant pests and diseases. It requires much less pesticide after the first year of growth. Silphium is strong enough to outcompete weeds and does not require any herbicide measures from the second vegetation period onwards [63]. S. perfoliatum is a perennial plant that can be used for about 15 years without replanting, depending on the location [76]. Pre-sowing crops with weed-eliminating properties are recommended due to the low competition against weeds in the first year of cultivation. The effect of the preceding cultivation is not so significant in the following years, as S. perfoliatum can be used for many years [76]. As the cultivation age increases in the seventh year, with the formation of a large number of shoots and due to the drying of the leaves in the rosette of the base and lower leaves on the shoots, a decrease in the quality of green mass in terms of chemical composition has been shown [67]. Due to the increase in the share of shoots in the yield of green mass and due to the increase in fiber content, there is a decrease in indicators such as crude protein, metabolic energy, and feed units. The nitrogen dose of 120 kg/ha increases the concentration of crude protein (1–2%) and at the same time reduces the sugar content (2–3%) [67].
In crops of the species S. perfoliatum, sporadic diseases caused by fungi from the genera Sclerotinia, Botrytis, and Ascochyta silphii sp. nov., and the bacteria Pseudomonas syringae, have been observed [19,46,76,77,78]. There are also reports of insects attacking S. perfoliatum plants: Autographa gamma, Amphipyra tragopogonis, Hecatera bicolorata, Eucosma giganteana, Mordellistena cf. aethiops Smith, Uroleucon cf. ambrosiae, Acanthocaudus n.sp., Neotephritis finalis, and Eucosma giganteana Riley [21,76,79]. It should be emphasized that there are no detailed studies on the influence of diseases and pests on the yielding of Silphium species. Therefore, it is important to undertake research in this area.
3.1.4. Yields and Dry Matter (DM) Content
Yields of Silphium depend on plant age and on the time of harvest. Fresh matter weight obtained from one-year-old S. trifoliatum plants collected before mid-September was from 260 g to 762 g per plant. Single S. perfoliatum plants are distinguished with the highest green matter weight at 519 g, whereas S. integrifolium plants have given 345 g of fresh matter [7,26,27]. One-year-old S. perfoliatum plants have produced DM yields of up to 2.8 t/ha in a single cut [57]. Table 2 shows the DM yield of Silphhium species.

Table 2.
Yields of Silphium species from the second year of cultivation.
S. trifoliatum plants in the second year of cultivation in Poland, at the beginning of May (phase of intensive growth), produced 533 g of fresh matter (2.4 t/ha, DM) from a single clump, while at the beginning of July (start of blooming), green matter yield from a clump of plants was nearly 6.7-fold higher and amounted to 3591 g (28.8 t/ha, DM). In comparison, the corresponding yields of green matter of S. perfoliatum were about 676 g in May (2.9 t/ha, DM) and 5097 g (36.6 t/ha, DM)—a 7.5-fold increase—in July, and for S. integrifolium, 392 g (2.2 t/ha, DM) and 2141 g (19.8 t/ha, DM)—a 5.5-fold increase [7,26,27]. In other experiments, two-year-old and older S. perfoliatum plants produced the following yields of DM: in the phase of spring re-growth, from 5.4 t/ha to 24.7 t/ha; in the budding phase, from 8.0 t/ha to 32.9 t/ha; and in the blooming phase, from 27.7 t/ha to 36.6 t/ha [59,74,80]. In a cultivation in Chile, the authors obtained from 9.6 to 22.3 t/ha [28]. In a cultivation for energy generation purposes, dry matter yields of S. perfoliatum were obtained at the level of 14–19 t/ha [83] and 20.5–22.4 t/ha [84]. S. perfoliatum produces the highest yields compared to S. trifoliatum and S. integrifolium. Another alternative plant proposed for cultivation for energy purposes is Sida hermaphrodita, which gives a DM yield in the second and third years of cultivation with values ranging from 2.9 to 20 t/ha. Therefore, in terms of yield, S. perfoliatum may be competitive with S. hermaphrodita.
Silphium plants can be cut two or even three times during the period of their development/vegetation. After cutting, S. perfoliatum re-grows very fast, and even can bloom again [19,56,57]. Such a two-cut regime lowers the total yield but results in better nutritive value and higher voluntary intake by animals [28]. In the case of S. perfoliatum, in plant cultivation with 2–3 cuts during the vegetation season, the following yields were obtained: in the first cut, from 5.4 t/ha to 16.2 t/ha; in the second cut, from 0.2 t/ha to 11.2 t/ha; and in the third cut, up to 0.6 t/ha. The combined yields obtained from all three cuts were from 7.7 t/ha to 23.0 t/ha [19,56,57]. S. perfoliatum DM yield in Germany (Braunschweig) was 10.8 t/ha without additional irrigation and 16.1 t/ha with additional irrigation. The average DM yield of Silphium was 102% compared to the alfalfa yield and 66% of the maize yield [85]. S. perfoliatum has a high yielding potential [85].Thanks to deep and intensive rooting, it can also draw water from the deeper layers of the soil. Due to the high water demand, S. perfoliatum can yield DM comparable to maize only in places with good water supply (irrigation) [85]. Comparative studies show that yields of S. perfoliatum similar to that of maize can be obtained with sufficiently high rainfall in areas with a cool climate [78]. The DM yield of plants established with the sowing method was higher (approx. 13.9 t/ha) than the DM yield of plants established with the planting method (approx. 13.0 t/ha) due to higher number of shoots per unit area (1 m2) [86]. The results of research conducted in Poland showed that to obtain a well-developed plantation with higher Silphium biomass yields of good quality, a better and cheaper method of plantation establishment is the generative method of sowing high-quality seeds, compared to the more complicated and cost-intensive vegetative method of planting [86]. The literature reports that long-lasting drought had a negative effect on S. perfoliatum plants cultivated in southern France and in Romania, causing a significant reduction of yields [14,19,56].
DM content in green matter of S. perfoliatum, cultivated throughout the period of vegetation, increases from approx. 9.7% in the phase of spring re-growth to about 23.7% in the phase of blooming and fruition [19,20,57,59,80,98,99]. In the case of cultivation for two cuts, DM content in green matter constitutes 12.3–15.6% in the first cut, and 13.9–14.4% in the second cut [56]. As reported by [72], DM content in leaves is higher than that in stems; e.g., at the beginning of blooming of the plants, DM content in leaves is about 12.6%, while in stems it is about 7.2%. Leaves of one-year-old S. trifoliatum plants contained 16.28% DM, on average. Mean content of DM in two-year-old S. trifoliatum plant leaves increased from 13.95% at the beginning of May to 22.59% at the beginning of September. Leaves collected in spring (May) contained about twice as much DM as shoots; stems were characterized by a much higher DM content at the end of flowering and full fruition stage (September). Inflorescences collected in July and August had a similar DM content which amounted to 18.69%. Rhizomes and roots contained 33.64% DM, on average [26]. Leaves of one-year-old S. integrifolium plants contained 17.10% DM in 1998–2000, on average. Leaves collected in September 1998 contained the most DM (18.04%), while those harvested in 2000 contained the least (16.03%). Mean content of DM in 2-year-old S. integrifolium plant leaves increased from 16.26% at the beginning of May to 21.02% at the beginning of September. Leaves collected in spring (May) contained about twice as much DM as shoots; stems were characterized by a much higher DM content at the end of flowering and at full fruition stage (September). Inflorescences collected in July and August were characterized by similar DM content that amounted to 17.94%. Rhizomes and roots contained 32.46% DM, on average [27]. Study of DM in the aboveground part of six species of Silphium (S. lacinatum L., S. integrifolium Milchx., S. perfoliatum L., S. perfoliatum L. cv. Kanadchanka, S. scaberrinum Ell., S. trifoliatum L.) showed that this parameter was from 21.14% (S. lacinatum L. and S. perfoliatum L.) to 29.02% (S. scaberrinum Ell.) [100].
3.2. Chemical Composition
3.2.1. Carbohydrates
Analyses of the saccharide fraction of S. perfoliatum revealed the presence of water-soluble carbohydrates in the form of mono-, oligo- and polysaccharides. Total percentage content of reducing sugars in green matter varies from approx. 12% to about 21% DM, a major part of dry matter being concentrated in the stems—up to 24.5%, and only up to 9.9% in the leaves [59,66,101]. Monosaccharides constitute a more than double contribution in the saccharide fraction of S. perfoliatum compared to disaccharides (monosaccharides: in green matter—8.8–8.9%, in leaves—4.6–5.5%, in stems—12.5–15.4%; disaccharides: in green matter—3.7–4.2%, in leaves—2.2–3.5%, in stems—up to 5.6%) [72,73]. The leaves of one-year-old Silphium species contained up to 13.43% of total sugars in DM (S. trifoliatum) and up to 8.19% in DM (S. perfoliatum). Leaves of perennial plants contain the most total sugars in the spring re-growth phase, i.e., up to 14.67% DM and up to 5.55% DM reducing sugars (S. trifoliatum) [102]. Along with the growth of the plants, the content of sugars decreases. Seeds of S. perfoliatum, S. trifoliatum and S. integrifolium contain up to 9.58% (in conversion to DM) of water-soluble sugars [42]. The stems of Silphium species contain total and reducing sugars in amounts from 3.05% DM (S. integrifolium) to 19.13% (S. trifoliatum) and from 0.78% DM (S. integrifolium) to 17.74% DM (S. trifoliatum), respectively [102]. The total content of sugars in the aboveground part of six species of Silphium (S. lacinatum L., S. integrifolium Milchx., S. perfoliatum L., S. perfoliatum L. cv. Kanadchanka, S. scaberrinum Ell., S. trifoliatum L.) from Ukraine can be from 3.54% (S. integrifolium) to 12.17% (S. lacinatum), and crude fiber from 29.46 to 48.24% [100].
Dudkin et al. [103] demonstrated that simple sugars in leaves of S. perfoliatum are represented by glucose, galactose, arabinose, xylose, ribose, and rhamnose, and that large amounts of acids can also be found. High levels of the latter ones indicate the presence of an acidic component as a type of pectin substance or mucus [103]. The authors mentioned earlier concluded also that the polysaccharide fractions of leaves of S. perfoliatum after hydrolysis differ in terms of quality and quantity from the monosaccharide fraction, and that they are practically nearly pure polyuronides. In the aboveground part of S. perfoliatum dried by means of hot air (50 °C), the content of polysaccharides was 57.5%, and that of uronic acids was 2.5% [104].
Green matter of S. perfoliatum is characterized by a notable content of cellulose: from approx. 14.3% in the phase of spring re-growth to about 39.7% in the phase of fruition (in conversion to DM) [19,20,56,66,99]. Stems can contain even three-fold more cellulose relative to leaves, and thus e.g., at the beginning of the blooming phase, that content can be approx. 30–35% and about 11%, respectively, in conversion to DM [66,99]. The following cellulose levels were found in the leaves and stems of three species of Silphium (S. perfoliatum, S. trifoliatum and S. integrifolium) from perennial cultivation: up to approx. 28% DM and up to approx. 43% DM. Whereas, in the inflorescences and rhizomes, the cellulose level was about 25–26% [102]. Seeds of S. perfoliatum, S. trifoliatum, and S. integrifolium contained (in conversion to DM) up to 25.4% of cellulose [42]. The total content of crude fiber in the aboveground part of six species of Silphium (S. lacinatum L., S. integrifolium Milchx., S. perfoliatum L., S. perfoliatum L. cv. Kanadchanka, S. scaberrinum Ell., S. trifoliatum L.) from Ukraine was from 29.46% (S. integrifolium) to 48.24% (S. lacinatum). Witaszek et al. [105] showed that the content of lignocellulosic compounds in the dry matter of the aboveground parts of S. perfoliatum was 21.62% lignin, 30.96% cellulose, and 22.6% hemicellulose.
The composition of the saccharide fraction of underground organs of plants of the species S. perfoliatum, S. trifoliatum, and S. integrifolium includes an energy storage carbohydrate, inulin. Rhizomes of S. trifoliatum contained up to approx. 38% of inulin in conversion to DM, rhizomes of S. perfoliatum up to approx. 34%, and rhizomes of S. integrifolium up to approx. 33% [106]. Apart from inulin, the analyzed materials contained saccharose, from approx. 0.2% to approx. 2%, and fructose, from approx. 0.5% to about 4%. It was demonstrated that the content of fructose in inulin of Silphium oscillated in the range from approx. 71% (roots of S. perfoliatum) to approx. 94% (rhizomes of S. integrifolium and S. trifoliatum). Rhizomes of the studied Silphium species contain inulin, in which fructose has a higher percentage contribution compared to fructose content in the corresponding fructan isolated from the roots [106].
The average amount of sucrose in nectar of S. perfoliatum was 0.252 mol/L (0.029–0.468), that of glucose, 0.868 mol/L (range 0.054–1.515), and that of fructose, 1.043 mol/L (range 0.079–1.850) [40].
3.2.2. Protein and Amino Acids
Green matter of S. perfoliatum is characterized by a considerable content of protein, i.e., from 8.6% to 32.1% of total protein, including proper protein at from 7.3% to 21.9% in conversion to DM, depending on the stage of plant development [18,20,57,58,59,66,74,98,101,107]. The maximum content of total protein has been noted in the phase of spring re-growth and budding, and varied from 27.6% to 31.6% in the leaves, and from 16.1% to 21.1% in the stems [72,99,108,109,110]. Just before blooming and during the blooming phase of plants, total protein content decreases slightly and attains the lowest level in the phase of fruition [99]. The leaves of one-year-old S. perfoliatum contained, on average, up to 18.37% of total protein per DM, while the annual leaves of S. trifoliatum contained 12.70%. The leaves of S. perfoliatum from perennial cultivation in the spring re-growth phase contained up to 22.90% of total protein. The total protein content in S. trifoliatum and S. integrifolium leaves decreased during the growing season from 19.11% at the beginning of the vegetation to 12.95% at the end of the vegetation and from 18.40% to 12.03% respectively. The stems of these species were characterized by a lower protein content compared to the leaves [102]. Seeds of S. perfoliatum, S. trifoliatum, and S. integrifolium contain (in conversion to DM) up to 33.5% protein. It was determined that the dominant amino acids in the protein of Silphium seeds are glutamic acid (up to approx. 23%) and leucine (up to 7.76%) [42]. Study of protein content in the aboveground part of six species of Silphium (S. lacinatum L., S. integrifolium Milchx., S. perfoliatum L., S. perfoliatum L. cv. Kanadchanka, S. scaberrinum Ell., S. trifoliatum L.) showed that this parameter ranged from 14.18 to 26.08% [100]. The content of protein among forage plants was 36% (Agropyron cristatum), 34% (Lolium perenne), and 32% (Medicago sativa, Trifolium repens), etc. [111].
The proper protein fraction of S. perfoliatum is characterized by a content of poorly soluble proteins, the major amounts of which have been detected in the aboveground part of the plant (green matter) in the period of blooming and fruition (64.8% of protein nitrogen). It turned out that the sum of the water fractions and salt solutions of proteins exceeded the sum of the alcohol and alkaline fractions by a factor of two [112]. The proper protein fraction of S. perfoliatum displays a high content of the sum of albumins and globulins (36.4–53.1% of protein nitrogen), which is highly valuable as the fraction contains many exogenous amino acids [21,108,113]. The level of prolamins and glutelins in plant protein of S. perfoliatum is small. Glyaubertene and Marčyulyonis [114,115] demonstrated that a part of the protein complex of a plant is composed of a specific protein which, in the form of weakly acidic solutions, is characterized by considerable heterogeneity (large number of diverse protein fractions). Quantitative changes of the individual protein fractions were noted in the course of the process of plant growth and development, and also in various years of cultivation [114,115].
Eighteen protein amino acids have been identified in green matter of S. perfoliatum: glycine, alanine, valine, leucine, isoleucine, serine, threonine, cysteine, methionine, aspartic acid, glutamic acid, lysine, arginine, phenylalanine, tyrosine, tryptophan, histidine, and proline [20,80,116]. The amino acid composition of protein found in green matter of the plant is characterized by the presence of exogenous amino acids (including isoleucine), i.e., lysine (up to 5.2% of proper protein), phenylalanine (up to 7.2%), valine (up to 7.4%), leucine (up to 12.2%), isoleucine (from 5.0% to 5.2%), tryptophan (up to 1.9%), tyrosine (up to 4.1%), threonine (up to 6.9%), and methionine (up to 2.0%) [20,71]. Note should be taken of the high content of lysine, which is a deficit amino acid of plant proteins. In the leaves, lysine and phenylalanine account for approx. 6.9% and 4.4% of total protein, respectively, while in the stems the corresponding values are approx. 5.4% and 2.7% of total protein [116]. It has been determined that the qualitative composition of amino acids in the process of ontogenesis is constant [116]. The leaves and herbs of three species of S. perfoliatum, S. trifoliatum, and S. integrifolium are characterized by a similar quantitative composition of individual amino acids in the protein [102].
The above data on the content of protein in the aboveground part of S. perfoliatum, and its amino acid composition with a considerable level of lysine (the most deficit among plant protein amino acids), indicate a high biological value of the protein complex.
In pollen of S. perfoliatum, the mean total of free amino acids was 1.16 mg/mg (range 0.09–2.23) and the mean total of protein-bound amino acids was 79.5 mg/mg (range 70.6–88.4), corresponding to a protein content of approx. 8% (DM) [31]. Histidine and arginine were the most abundant amino acids in pollen. Essential amino acids accounted for 88% of free amino acids and 81% of all amino acids associated with proteins [31]. Regarding free amino acids in nectar of S. perfoliatum, the mean total was 1.11 mmol/mL (range 0.10–3.03). In contrast to amino acids in pollen, the amount and composition of free amino acids in nectar varied much more between samples. The histidine accounted for the largest amount. The essential amino acids was 61% of the mean total amino acids measured, due to the larger amounts of histidine and lysine [31].
3.2.3. Fat
The percentage content of crude fat in S. perfoliatum varies from 0.9% to 6.0% in green matter, from 3.6% to 7.7% in leaves, and from 3.2% to 5.8% in stems, in conversion to DM [18,57,66,72,99,110]. The leaves of one-year-old S. perfoliatum, S. trifoliatum, and S. integrifolium plants contained on average about 1.5% crude fat, while the leaves of perennial plants contained about 2% [102]. However, in the case of perennial organs, the inflorescences of S. perfoliatum contained the most raw fat: about 4% on average [102]. Study of crude fat content in the aboveground part of six species of Silphium (S. lacinatum L., S. integrifolium Milchx., S. perfoliatum L., S. perfoliatum L. cv. Kanadchanka, S. scaberrinum Ell., S. trifoliatum L.) showed that this parameter ranged from 2.34 to 4.73% [100]. The rhizomes of S. perfoliatum, S. trifoliatum, and S. integrifolium contained on average about 1%, while the stems contained about 0.6–0.9% fat [102]. Seeds of the three species (S. perfoliatum, S. trifoliatum and S. integrifolium) contained (in conversion to DM) up to 24.1% of fat [42]. The seeds of S. integrifolium from the USA contained up to 25.3% oil [117].
Duranti et al. [20], determined the content of fatty acids in the fat fraction of green matter of S. perfoliatum. The entire fat fraction accounted for 1.8% to 2.5% of DM. The main acids included in the composition of esters forming the fatty acids are the following: palmitic acid, from 27.9% to 30% of the fat fraction; linolenic acid, from 28% to 292%; linoleic acid, from 14.3% to 16.5%; vaccenic acid, from 5.1% to 6.6%; palmitic–oleic acid, from 3.3% to 4.0%; oleic acid, from 3.6% to 4.9%; arachidic acid, from 3.1% to 4.1%; myristoleic acid, from 2.1% to 3.4%; erucic acid, from 1.1% to 2.8%. Analysis of the lipid fraction of seeds of three species, S. perfoliatum, S. trifoliatum, and S. integrifolium, demonstrated that in the quantitative aspect, linoleic acid, with its content (in fat) of up to 44.4%, and oleic acid, with content of up to 13.2%, are the main fatty acids in soil acquired from the seeds [42]. In studies conducted in the USA, it was found that linoleic acid was dominant in S. integrifolium seed oil with levels up to 69.9% [117].
3.2.4. L-Ascorbic Acid
The highest content of L-ascorbic acid is characteristic of S. perfoliatum plants in the phase of spring re-growth: in green matter, from approx. 120 mg/100 g to approx. 500 mg/100 g in conversion to DM; in leaves, from approx. 140 mg/100 g to approx. 500 mg/100 g; and in stems, up to approx. 20 mg/100 g [101,110,118,119]. Studies on the species S. perfoliatum, S. trifoliatum, and S. integrifolium showed that the largest amount of L-ascorbic acid was contained in the leaves compared to other examined organs, i.e., about 300 mg/100 g for one-year plants and up to 779 mg/100 g leaves of perennial plants [102]. Along with the development of plants, the content of vitamin C decreased [102]. Inflorescences of S. perfoliatum, S. trifoliatum, and S. integrifolium species contained vitamin C in amounts from 156 mg/100 g to 339 mg/100 g [102]. In studies conducted in Ukraine among species in the flowering stage (S. lacinatum L., S. integrifolium Milchx., S. perfoliatum L., S. perfoliatum L. cv. Kanadchanka, S. scaberrinum Ell., S. trifoliatum L.), L-ascorbic acid was found in the range from 77.12 (S. perfoliatum L. cv. Kanadchanka) to 296.35 mg/100 g (S. lacinatum) in green mass [100].
3.2.5. Chlorophyll
The highest content of chlorophyll in the species S. perfoliatum, S. trifoliatum, and S. integrifolium was characteristic for leaves collected at the beginning of flowering, i.e., from 1.52% (S. integrifolium) to 2.29% (S. perfoliatum), while the stems contained lower chlorophyll levels [102].
3.2.6. Mineral Substances: Ash
In green matter of S. perfoliatum, ash accounts for from 6.8% to 17.9% of DM, and its mineral composition is as follows: Ca from 1.5% to 7.7%, P from 0.2% to 1.2%, Mg from 0.2% to 0.8%, K from 4.3% to 4.8%, and Na at approx. 0.01% [16,18,19,56,57,66,72,74,80,98,99,110]. According to Wever et al. [120], the content of ash for different samples was 8.86–9.40%. The total content of ash in the aboveground part of six species of Silphium (S. lacinatum L., S. integrifolium Milchx., S. perfoliatum L., S. perfoliatum L. cv. Kanadchanka, S. scaberrinum Ell., S. trifoliatum L.) from Ukraine was from 3.25% (S. scaberrinum) to 7.82% (S. perfoliatum cv. Kanadchanka) [100]. Analysis of the dynamics of accumulation of mineral components of ash showed that leaves and stems in the phase of spring, re-growth contained similar levels of ash (7–8%), in the phase of blooming, leaves contained approx. 14% of ash and stems approx. 7%, and in the phase of fruition, ash content in leaves was approx. 12% and in stems, 5% DM [99,110]. The percentage content of Ca in green matter of S. perfoliatum doubles in the period from the formation of flower buds to the phase of blooming. Whereas, the content of P in the ontogenesis of the plant decreases, but overall, throughout the vegetation period, the content of that element in the leaves is higher than in the stems [16]. Duranti et al. [20] determined the content of microelements in green matter of S. perfoliatum as follows: Fe from approx. 117 ppm to 161 ppm, Cu from approx. 12 ppm to 16 ppm, and Co in trace amounts, below 1 ppm.
Leaves and inflorescences of S. perfoliatum, S. trifoliatum, and S. integrifolium can be a supplementary source of mineral elements, e.g., K (3.0–3.8%), Ca (1.2–4.5%), or Mg (206–333 mg/100 g), as well as trace elements, e.g., Fe (8–27 mg/100 g) and Mn (3–6 mg) [102]. The content of minerals in seeds of three Silphium species, S. perfoliatum, S. trifoliatum, and S. integrifolium, and especially the level of K (from approx. 1.6 g/100 g to approx. 1.8 g/100 g DM), Ca (from approx. 1.0 g/100 g to approx. 2.1 g/100 g DM), or Mg (from 651 mg/100 g to 672 mg/100 g DM), and of trace elements, e.g., Fe (from approx. 28 mg/100 g to approx. 38 mg/100 g DM), is one of the more important characteristics affecting their potential nutritive value [42]. The content of mineral elements found in the aboveground part of six species of Silphium (S. lacinatum L., S. integrifolium Milchx., S. perfoliatum L., S. perfoliatum L. cv. Kanadchanka, S. scaberrinum Ell., S. trifoliatum L.) from Ukraine was in the range from 0.78% to 2.18% potassium, from 1.37% to 3.07% calcium, and from 0.13% to 0.35% phosphorus [100]. Plants from Asteraceae possessed a significantly high concentration of K in the vegetative stage than in the reproductive period [100]. In this case, the content of K in raw samples of some selected species was from 0.93% (Achillea wilhelmsii C. Koch) to 2.13% (Seriphidium quettense (Podleh) Ling, Bull) [100]. According to Achakzai et al. [121], the content of phosphorus was higher in the vegetative stage than in the reproductive growth stage.
3.2.7. Phenolic Acids
Phenolic compounds are composed of an aromatic ring containing a hydroxyl group as well as other substituents, such as a carboxyl or methoxy group [122]. Most phenolic compounds are combined with sugars, organic acids, and esters, although some of them are in the form of aglycones [123]. These substances are widely distributed in the plant world and therefore are an integral part of the daily diet. The general division of polyphenols includes two groups, phenolic acids and flavonoids, among which there are several more subclasses [122]. Phenolic compounds show the strongest antioxidant and anti-radical properties of all secondary metabolites. In plants, phenols fulfill multiple functions, including acting as substrates in biosynthetic reactions (e.g., caffeic acid is a precursor to lignin), protecting the plant from the harmful effects of ultraviolet radiation; compounds such as red or blue anthocyanins, yellow aurons, and chalcones attract pollinating insects. However, despite their positive effects, these compounds reduce transition metals, thus stimulating oxidative processes. Some flavonoids in the presence of nitric oxide show pro-oxidative activity [124].
Polyphenols can also enhance the effects of other antioxidants, including vitamins of fat-soluble and low-molecular-weight water-soluble substances [125]. Apart from antioxidant properties, these substances, due to their biological, chemical, and physical properties, also exhibit anti-inflammatory, anti-allergic, anti-hepatotoxic, anti-mutagenic, anti-tumor and anti-atherosclerotic activity [126,127].
The total content of polyphenols in the aerial parts of S. perfoliatum ranged from 11.33 mg GAE/g DM to 58.37 mg GAE/g DM [128]. Wojcińska and Drost-Karbowska studied (using TLC and HPLC methods) the presence of phenolic acids in the tubulate and ligulate flowers of S. perfoliatum [129]. They determined the occurrence of phenolic acids as derivatives of cinnamic acid (3.1) (Figure 3), i.e., caffeic acid (3.2), p-coumaric acid (3.3), and ferulic acid (3.4), and as derivatives of benzoic acid (3.6), i.e., protocatechuic acid (3.7), p-hydroxybenzoic acid (3.8), vanillic acid (3.9), and syringic acid (3.10). Phenolic acids occur both in the free form, and in glycoside bonds. The dominant phenolic acids are caffeic acid (4.379 mg/100 g DM) and p-coumaric acid (4.392 mg/100 g DM). The total content of free phenolic acids was 16.2 mg/100 g DM, while the content of phenolic acids liberated as a result of acidic and alkaline hydrolysis was 1.3 mg/100 g DM and 2.7 mg/100 g DM, respectively. Note should be taken of the fact that caffeic acid occurs primarily in the bound form, and is liberated mainly as a result of alkaline hydrolysis (2.4 mg/100 g DM) [129].
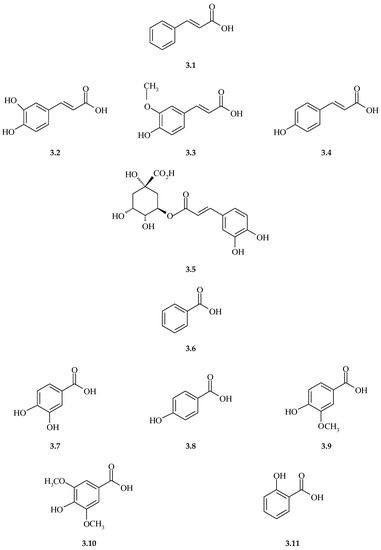
Figure 3.
Structures and names of phenolic acids as derivatives of cinnamic acid (3.1) (caffeic acid (3.2), p-coumaric acid (3.3), ferulic acid (3.4), and benzoic acid (3.6) (protocatechuic acid (3.7)), p-hydroxybenzoic acid (3.8), vanillic acid (3.9), syringic acid (3.10), salicillic acid (3.11), and depsides-chlorogenic acid (3.5).
In other studies, comprehensive analyses of the content of phenolic acids in the aboveground and underground organs of S. perfoliatum, S. trifoliatum, and S. integrifolium were performed [26,130,131,132,133]. In the fraction of free phenolic acids of S. perfoliatum, S. trifoliatum, and S. integrifolium (Table 3), the dominant one was caffeic acid, with levels up to 3.09 mg/100 g, up to 3.96 mg/100 g, and up to 1.95 mg/100 g (in leaves), up to 2.57 mg/100 g, up to 5.84 mg/100 g, and up to 2.49 mg/100 g (inflorescences), and up to 4.21 mg/100 g, up to 1.54 mg/100 g, and up to 1.96 mg/100 g (rhizomes), respectively [26,130,131,132,133]. In addition, in the three analyzed species and in all the plant organs, the following were identified in the fraction of free phenolic acids: p-coumaric acid, protocatechuic acid, p-hydroxybenzoic acid (Table 3). Ferulic acid was identified in the fraction of free phenolic acids of inflorescences and rhizomes of S. perfoliatum, leaves and inflorescences of S. trfoliatum, and in the leaves, inflorescences, and rhizomes of S. integrifolium (Table 3). Vanillic acid was present in the leaves and inflorescences of S. perfoliatum, inflorescences of S. trfoliatum, and in the leaves, inflorescences, and rhizomes of S. integrifolium (Table 3). Salicylic acid (3.11) was identified in the leaves and inflorescences of S. trfoliatum (Table 3) [26,130,131,132,133].

Table 3.
Phenolic acids in S. perfoliatum, S. trifoliatum, and S. integrifolium [26,130,131,132,133].
Phenolic acids are aromatic secondary metabolites of plants and are widely distributed throughout the plant kingdom. They are found mainly in fruits and vegetables, and particular interest in them is related to their potential protective role against ischemic heart disease, stroke or cancer [134]. Depending on their structure, we can distinguish two classes of phenolic acids, i.e., benzoic acid derivatives and cinnamic acid derivatives [135,136]. Phenolic acids are rarely found in free form and are generally esterified with quinic or tartaric acid derivatives. Esters of hydroxycinnamic acids and quinic acids are called chlorogenic acids (3.5). However, the most common 5-caffeoylquinic acid is also called chlorogenic acid. Hydroxycinnamic acids, found in many fruits and vegetables, contribute much more to total polyphenol intake than benzoic acid derivatives or flavonoids such as flavanols or flavones [135].
Several experimental and epidemiological studies suggest that phenolic acids contribute to protection against various degenerative diseases [137,138,139]. Their health impact has in particular been attributed to antioxidant properties.
The content of free phenolic acids was up to 7.93 mg/100 g in leaves of S. perfoliatum, up to 17.29 mg/100 g in inflorescences of S. trifoliatum, and up to 17.25 mg/100 g in inflorescences of S. integrifolium [26,130,131,132,133]. The phenolic acids mentioned above occur also in bound forms, and therefore the presence of those compounds was noted after acidic and alkaline hydrolysis. The fraction of phenolic acids after alkaline hydrolysis contributed the highest share in the group of phenolic acids, with levels of up to 14.61 mg/100 g in inflorescences of S. perfoliatum, up to 37.52 mg/100 g in inflorescences of S. trifoliatum, and up to 29.97 mg/100 g in inflorecneces of S. integrifolium [26,130,131,132,133].
As a result of acidic hydrolysis, a depside-chlorogenic acid was isolated from leaves of S. perfoliatum, with content of up to 0.18 mg/100 g. In addition, as a result of acidic hydrolysis, the lowest concentrations of phenolic acids could be obtained, compared to the remaining fractions: up to 1.43 mg/100 g in inflorescences of S. perfoliatum, up to 6.14 mg/100 g in inflorescences of S. trifoliatum, and up to 11.64 mg/100 g in leaves of S. integrifolium [26,130,131,132,133].
Phenolic acids were identified also in another study, in which 11 species from the genus Silphium were analyzed (Table 4, Figure 4): S. abliflorum, S. asteriscus, S. brachiatum, S. compositum, S. integrifolium, S. laciniatum, S. morhii, S. perfoliatum, S. radula, S. terebinthinaceum, and S. wasiotense [140,141]. The study demonstrated the presence of 16 phenolic acids, from two groups—that of benzoic acid and that of cinnamic acid—in the analyzed Silphium species. Williams [141] reports that the concentration of phenolic acids depended on the extraction solvent used (either ether or ethyl acetate), where the highest concentrations of phenolic acids were recorded in S. laciniatum, S. albiflorum and S. terebinthinaceum (Silphium section Composita) and S. radula (Silphium section Silphium).

Table 4.
Distribution of phenolic acids in leaf extracts of eleven species of Silphium [140].
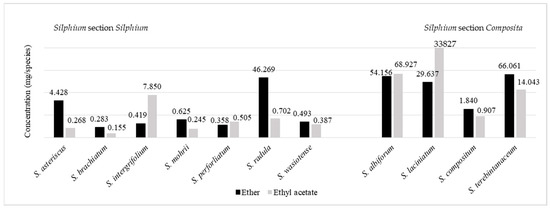
Figure 4.
The total concentration of phenolic acids (mg) extracted from leaves (1000 g) of eleven Silphium species using extraction with either ether or ethyl acetate [141].
3.2.8. Tannins
In the leaves of one-year of S. perfoliatum, S. trifoliatum, and S. integrifolium species, the tannin content ranged from 7.34% to 8.85%, and in perennial from 9.63% to 11.24% [102]. In the inflorescences, the content of tannins ranged from 8.44% tannins (S. perfoliatum) to 11.36% (S. integrifolium), while in rhizomes, tannins were found in the lowest concentrations, i.e., from an average of 5.73% (S. perfoliatum) to 7.46% (S. trifoliatum) [102].
3.2.9. Flavonoids
Flavonoids are, next to phenolic acids, the largest group of compounds representing polyphenols [142]. Particular groups of flavonoid compounds differ from each other in the number, type, and location of substituents in the molecule, which determine their chemical and physical properties, and also have an impact on individual metabolism and biological activity [143]. Flavonoids occur in the world of plants in two forms: free aglycones and glycosides (combination of aglycone and sugar part). Flavonoids occurring in the human diet most often occur as glycosides, and they are mainly flavanols and flavones [144]. Flavonoids in plants have many important functions. First of all, they give them the color, taste, and smell characteristic of a given species. They have a high ability to absorb UV radiation, which is why they are assigned a protective role against the harmful effects of this type of radiation. Their protective function also consists in capturing reactive oxygen species such as: superoxide anion, hydroxyl radical, hydrogen peroxide, and singlet oxygen, which are produced in plants in increased amounts [144]. Flavonoids regulate the activity of many enzymes, including by being involved in the formation of reactive oxygen species, e.g., peroxidases, lipoxygenases, and xanthine oxidases. These compounds are also known as attractants, i.e., substances attracting insects, which then transfer pollen and facilitate pollination of plants [145,146]. Flavonoids are also well-known for their varied therapeutic effects and the inhibition of mammalian carbonic anhydrase enzymes, which are implicated in a wide range of disorders, such as glaucoma, epilepsy, obesity, and cancers [147].
In the aboveground part of S. perfoliatum, the presence of nine known flavonoids was determined, including kaempferol, isoquercetin, and astragalin [16,148,149]. In addition, from the green matter of S. perfoliatum, three kaempferol triosides were isolated that contained the following apiosides: kaempferol–3–O–α–L–rhamnosyl–(1′′′→6′′)–O–β–D–galactopyranosyl–7–O–β–D–apiofuranoside, kaempferol 3–O–β–D–apiofuranoside 7–O–α–L–rhamnosyl–(1′′′′→6′″)–O–β–D–galactopyranoside, and kaempferol 3–O–β–D–apiofuranoside 7–O–α–L–rhamnosyl–(1⁗→6′″)–O–β–D (2′″–O–E–caffeoylgalactopyranoside [148,149]. The chemical formula of one of the kaempferol triosides, namely kaempferol 3–O–β–D–apiofuranoside 7–O–α–L–rhamnosyl–(1⁗→6′″)–O–β–D–galactopyranoside, is presented in Figure 5 [148].
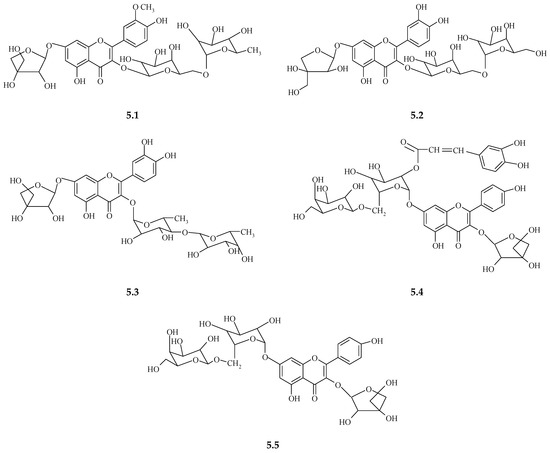
Figure 5.
Structures of the five flavonol triglycosides first isolated from Silphium asteriscus, S. albiflorum, and S. perfoliatum [141]: isorhamnetin 3–O–α–L–rhamnosyl (1′″→6″)–O–β–D–galactopyranoside 7–O–β–L–apiofuranoside (5.1), quercetin 3–O–α–L–rhamnosyl (1′″→6″)–O–β–D–galactopyranoside 7–O–β–L–apiofuranoside (5.2), quercetin 3–O–β–L–galactosyl (1′″→6″)–O–β–D–rhamnopyranoside 7–O–α–L–apiofuranoside (5.3), kaempferol 3–O–β–D–apiofuranoside 7–O–α–L–rhamnosyl (1″″→6″′)–O–β–D (2″′–O–E–caffeoylgalactopyranoside) (5.4), and kaempferol 3–O–β–D–apiofuranoside 7–O–α–L–rhamnosyl (1″″→6″′)–O–β–D–galactopyranoside (5.5).
It should be added that kaempferol triosides are characteristic for S. perfoliatum and have been isolated from that plant for the first time [148,149]. In a study on extracts from leaves of 11 species of Silphium, i.e., S. abliflorum, S. asteriscus, S. brachiatum, S. compositum, S. integrifolium, S. laciniatum, S. morhii, S. perfoliatum, S. radula, S. terebinthinaceum, and S. wasiotense, flavonoid glycosides have been identified, in the structures of which aglycones were represented by kaempferol, quercetin, and isorhamnetin. Table 5 presents 25 flavonoid glycosides which have been confirmed as occurring in the analyzed species of Silphium (Figure 5) [140]. The largest number of flavonoid glycosides (13) was identified in S. asteriskus, while the smallest number of flavonoid glycosides (4) was characteristic of leaves of S. brachatum [140]. The four species comprising section Composita showed the presence of various derivatives of the flavonols quercetin, isorhamnetin, and kaempferol [140]. Quercetin was common among all four species. In the section silphium, leaf extracts of S. asteriscus contained a total of 13 flavonol glycosides, the greatest number of flavonoids detected in all species examined [140].

Table 5.
Flavonoid glycosides identified in species from the genus Silphium. “+” indicates compounds detected in leaf extracts [140].
Of eleven species, detection of compound quercetin 3–O–β–L–galactosyl (1′″→6″)–O–β–D–rhamnopyranoside 7–O–α–L–apiofuranoside was specific to S. asteriscus. S. asteriscus shared the isorhamnetin triglycoside (Isorhamnetin 3–O–α–L–rhamnosyl (1′″→6″)–O–β–D–galactopyranoside 7–O–β–L–apiofuranoside) with the species S. brachiatum, S. integrifolium, S. mohrii and S. wasiotense [140]. Such complex flavonoid triglycosides have been little studied, not only because of their structural complexity, but also because research into natural products often focuses on other well-known classes of compounds. Williams reports that the triglycosides of isoramnetin, quercetin, and kaempferol have remarkably high abilities to inhibit the proliferation of breast cancer cells in laboratory studies [141].
Studies indicate that the analyzed species of Silphium contain significant levels of flavonoids [5,26,130,150]. The highest concentrations of flavonoids have been found in leaves of S. integrifolium (average of 1.05% DM) and the lowest in inflorescences of S. trifoliatum (mean of 0.13% DM), while the underground organs have contained only trace amounts of those compounds (Table 6) [5,26,130,150]. In another study, the total flavonoids content in the aerial parts of S. perfoliatum ranged from 1.39 mg QE/g DM (approx. 0.14%) to 7.28 mg QE/g DM (approx. 0.73%) [128].

Table 6.
Flavonoids content in S. perfoliatum, S. trifoliatum and S. integrifolium [5,26,130,150].
3.2.10. Carotenoids
The total content of carotenes in aboveground organs of S. perfoliatum is as follows: in green matter, from approx. 7 mg/100 g to 44 mg/100 g, converted to DM; in leaves, from approx. 14 mg/100 g to 100 mg/100 g; in stems, from approx. 1 mg/100 g to 5 mg/100 g. Generally, the variation of the content of carotenes in the ontogenesis of the plant displays decreasing tendency [16,18,57,66,101,110,118,119]. In addition, Davidyants and Abubakirov [16] report that lutein was identified in the group of carotenoids of S. perfoliatum. In studies conducted in Ukraine among the species S. lacinatum L., S. integrifolium Milchx., S. perfoliatum L., S. perfoliatum L. cv. Kanadchanka, S. scaberrinum Ell., and S. trifoliatum L., carotenes were found in the range from 0.23 mg/100 g (S. perfoliatum L. cv. Kanadchanka) to 1.54 mg/100 g (S. trifoliatum) in green mass [100].
3.2.11. Volatile Components (Essential Oil, Volatile Components of Extracts)
Essential oils are complex mixtures of volatile, liquid, and solid compounds found in plant structures (special secretory structures: outer secretory hairs or inner channels) that impart a characteristic smell/aroma [151,152]. Essential oils are extracted from plant tissues, mainly by steam distillation (in different variants) [153]. Other methods are also used that enable the isolation of volatile compounds as the dominant components in the complex of active substances, and research is carried out to increase efficiency by modifying conventional methods (extrusion, enfleurage, maceration, extraction with volatile and supercritical solvents, microextraction to the stationary phase), ultrasonic methods, and microwave-assisted extraction [154,155,156]. Essential oils contain from several dozen to several hundred chemical components, mainly terpenoids and phenylpropanoids from the group of hydrocarbons, alcohols, esters, aldehydes, ketones, phenols, ethers, and acids [156,157]. Essential oils show a wide spectrum of activity, including antiseptic, sedative, but also spasmolytic and irritating effects. Some of them have anaesthetic, expectorant, anti–inflammatory, cholesterol–lowering, anti–cancer, choleretic, and diuretic properties. Currently, they are used in medicine, aromatherapy, food production, and cosmetics [156,158,159]. Essential oils and their components are easily absorbed through the respiratory system when inhaled. Skin resorption and gastric absorption following oral administration are less effective but still possible [156,158,160].
There are numerous reports on the occurrence and composition of essential oil in three species: S. perfoliatum, S. trifoliatum, and S. integrifolium. Inflorescences of S. perfoliatum, S. trifoliatum, and S. integrifolium contain similar levels of essential oil, in the ranges of 0.15–0.28% v/w, 0.27–0.37% v/w, and 0.27–0.35% v/w, respectively [26,53,130,161,162,163]. In leaves of S. perfoliatum, S. trifoliatum, and S. integrifolium, essential oil is found in somewhat smaller amounts compared to inflorescences, with the corresponding ranges being 0.15–0.29% v/w, 0.12–0.32% v/w, and 0.22–0.30% v/w [26,130,161,162,163,164]. In contrast, the underground organs—rhizomes and roots—contain the largest amounts of essential oil (with characteristic blue color) compared to the aboveground parts, with the corresponding ranges for S. perfoliatum, S. trifoliatum, and S. integrifolium being as follows: 0.40–0.4% v/w, 0.36–0.46% v/w, and 0.46–0.69% v/w, respectively [26,52,130,161].
The composition of essential oil obtained from the aboveground organs, i.e., inflorescences and leaves of the individual species, displays qualitative similarity, with some differentiation in the shares of the particular components. In the chemical composition of the essential oil obtained from S. perfoliatum leaves, the dominant element is caryophyllene oxide (34.7%, 8.5%), germacrene D (6.4%, 24.3%), α-pinene (5.9%, 5.4%), spathulenol (4.6%, 3.9%), β-caryophyllene (1.5%, 2.8%, 4.0%), and trans-nerolidol (2.3%) [161,163]. In essential oil from leaves of S. trifoliatum, the dominant components were caryophyllene oxide (29.3%, 25.4%), germacrene D (16.1%, 8.3 g/100 cm3), silphiperfol–6–en–5–one (9.7%, 14.5%), (E)-nerolidol (8.2%, 6.3 g/100 cm3), β-caryophyllene (6.7%, 14.9%), spathulenol (4.9%, 2.7 g/100 cm3), α-humulene (3.2%, 4.7%), and α-pinene (1.2%, 6.0%) [53]. Whereas, the main components of essential oil from leaves of S. integrifolium include germacrene D (18.7%, 28.4%), α-pinene (8.8%), allo-aromadendr–9–ene (8.5%, 7.7%), caryophyllene oxide (6.1%, 12.4%), silphiperfol–6–en–5–one (3.7%, 5.1%), trans-α-bergamotene (3.1%), camphene (2.9%, 3.6%), β-caryophyllene (2.8%, 4.8%), limonene (2.4%), β-bourbonene (2.3%), spathulenol (2.1%, 3,2%), and trans-verbenol (2.0%) [53].
The main components of essential oil from inflorescences of S. perfoliatum were the following: α-pinene (20.9%), caryophyllene oxide (9.4%), trans-verbenol (6.4%), germacrene D (5.4%), camphene (3.6%), spathulenol (3.2%), myrtenal (2.4%), verbenone (2.4%) [161]. Dominant components in essential oil from inflorescences of S. trifoliatum were α-pinene (13.4%), bornyl acetate (6.5%), allo-aromadendr–9–ene (5.6%), camphene (5.5%), trans-verbenol (5.2%), caryophyllene oxide (5.0%), germacrene D (3.8%), limonene (3.4%), and silphiperfol–6–en–5–one (2.8%) [164]. Whereas, in essential oil from inflorescences of S. integrifolium, the main components were represented by caryophyllene oxide (19.0%), germacrene D (13.1%), α-pinene (8.0%), silphiperfol–6–en–5–one (7.3%), spathulenol (3.5%), camphene (2.7%), and trans-verbenol (2.0%) [164]. In general, in essential oil from inflorescences of Silphium, the dominant compounds are α-pinene (S. perfoliatum and S. trifoliatum) and caryophyllene oxide (S. integrifolium), while in the case of oils from leaves, the main components are caryophyllene oxide (S. perfoliatum and S. trifoliatum) and germacrene D (S. integrifolium).
In essential oil from the aboveground part of S. integrifolium from north Alabama, the authors identified the following components: α-pinene (58.6%), β-pinene (14.7%), myrcene (9.7%) [165].
The composition of essential oil obtained from rhizomes differed significantly from that of essential oil from leaves and inflorescences: among others, α-pinene is not present in essential oil distilled from Silphium rhizomes. In the case of essential oil obtained from S. perfoliatum rhizomes, the following compounds were identified as dominant ones: isocomene (14.4%, 2.8%), modhephene (9.9%, 2.3%), δ-selinene (4.7%, 4.0%), β-bisabolene (3.7%, 2.3%), β-caryophyllene (3.5%, 1.8%), silphiperfol–6–ene (3.0%), germacrene D (2.2%, 1.7%), δ-elemene (2.0%), α-humulene (1.5%, 2.9%), 7–α–H–silphiperfol–5–ene (1.2%, 9.7%), 7–β–H–silphiperfol–5–ene (1.2%, 5.7%, 14.9%), β-isocomene (3.8%, 0.5%), and a number of compounds which are derivatives of carterochaetol [161,163]. In essential oil isolated from S. trifoliatum rhizomes, the dominant components were the following: isocomene (12.3%, 3.2%), modhephene (10.8%, 2.9%), δ-selinene (7.2%, 0.2%), 7–β–H–silphiperfol–5–ene (7.0%, 5.2%), β-bisabolene (2.9%, 1.8%), β-caryophyllene (3.6%, 2.2%), silphiperfol–6–ene (3.4%, 0.4%), germacrene D (3.4%, 2.6%), δ-elemene (2.9%, 3.8%), 7–α–H–silphiperfol–5–ene (2.5%, 3.5%), β-isocomene (3.2%, 1.6%) [52]. Whereas, in essential oil from S. integrifolium rhizomes, the following volatile compounds represented the main components: β-himachalene (15.8%), isocomene (14.9%, 6.3%), modhephene (12.7%, 6.3%), allo-aromadendr–9–ene (11.0%, 12.5%), 7–β–H–silphiperfol–5–ene (4.4%, 1.4%), α-himachalene (4.2%), β-caryophyllene (4.0%, 3.0%), β-isocomene (3.0%, 2.4%) [52].
The content, composition, and properties of essential oils, as well as oil yield, depend on many factors, including plant phenotype, climatic conditions in which it grows, soil composition, and age of the plant, as well as the season and method of harvesting and the method of harvesting/obtaining essential oil. The composition of the oil is also influenced by the applied agrotechnical procedures, raw material processing, and the separation of the oil from the plant tissue [154,166,167].
The location of the essential oils in the various organs of the plant varies according to genus and species. It is noteworthy that the content and composition of essential oil for different organs of the same plant can vary significantly. During the entire life cycle of plants, these compounds change their chemical composition depending on the season, climatic conditions, or age itself. This variation sometimes also occurs in plants of the same species, but belonging to different chemotypes; therefore, plants intended for obtaining essential oil should only be harvested at a certain time. Taking into account the phenomenon of diffusion of these compounds in plants, it is very likely that oils play important functions in them, such as protection against other plants or insects [168].
Currently, many properties of essential oils are known, which are used not only in the food industry, but also in other industries, including in cosmetology (fragrances, preservatives), medicine (biologically active compounds), and agriculture (ingredients of plant protection products). Hence, due to their natural origin, low harmfulness, and the common tendency to replace chemical additives with substances of natural origin, essential oils are and will continue to be an object of interest for many industries [123,169,170,171].
In addition, the literature provides descriptions of volatile components occurring in extracts from S. perfoliatum, S. trifoliatum, and S. integrifolium that have been obtained with the use of the following solvents: petroleum ether, hexane, methanol, ethanol.
Extracts from inflorescences obtained with the use of petroleum ether, after the evaporation of petroleum ether, had brown coloring and were characterized by a greasy consistency, while the dry residue, for S. perfoliatum, S. trifoliatum, and S. integrifolium, was up to 4.67%, up to 4.56%, and up to 4.28% DM, respectively [164,172]. The dominant components of extracts from inflorescences of the analyzed species were α-pinene, germacrene D, and caryophyllene oxide. Apart from volatile compounds, the analyzed lipophilic extracts contain also significant amounts of sterols, i.e., γ-sitosterol and stigmasterol, as well as triterpene alcohols, i.e., α-amyrin and β-myrin. In addition, in ether extracts, higher alkanes, free fatty acids and their derivatives, and vitamin E were also identified. Extracts from flowers of S. perfoliatum, obtained with the use of solvents, i.e., hexane, ethanol, and methanol, contained analogous dominant volatile substances as in extracts obtained with the use of petroleum ether [5,173].
Extracts obtained from Silphium leaves with the use of petroleum ether, after the evaporation of the solvent, were characterized by a dark color and a greasy consistency, while the dry residue amounted to up to 1.93% for S. perfoliatum, up to 2.90% DM for S. trifoliatum, and up to 2.67% DM for S. integrifolium [53,172]. Extracts from leaves of the analyzed species display a high qualitative similarity to the corresponding essential oils, but differ from one another in terms of quantity. The main components of the analyzed extracts are: germacrene D, β-caryophyllene, and caryophyllene oxide, as well as silphiperfol–6–en–5–one. It should be mentioned that only extracts from leaves of S. integrifolium contained significant levels of allo-aromadendr–9–ene, which is found also in essential oils from the aboveground organs [53]. Apart from volatile compounds, the analyzed lipophilic extracts contain also significant amounts of sterols, i.e., γ-sitosterol and stigmasterol, as well as triterpene alcohols, i.e., α-amyrin and β-amyrin [53,172]. The compound α-Amyrine is characteristic for plants that produce resins [172]. In addition, in ether extracts from leaves, higher alkanes, free fatty acids and their derivatives, and vitamin E were also identified [53,172]. In other studies, in hexane, methanol, and ethanol extracts from leaves of S. perfoliatum, analogous volatile components were isolated as in extracts obtained with the use of petroleum ether [5,173].
After the evaporation of petroleum ether, extracts from rhizomes of S. perfoliatum, S. trifoliatum, and S. integrifolium were characterized by a resin-like consistency and had a flavor resembling the smell of turpentine, while the dry residue amounted to up to 2.62%, up to 1.84%, and up to 2.26% DM, respectively [52,172]. The main component of ether extract from rhizomes of S. perfoliatum, S. trifoliatum, and S. integrifolium is 16–acetoxycarterochaetol, the contribution of which amounts to approx. 45%, 41%, and 40%, respectively [52,172]. Derivatives of carterochaetol, including 16–acetoxycarterochaetol, have been identified in a mixture of sesquiterpene hydrocarbons obtained from the underground organs of three species of Silphium: S. asteriscus, S. perfoliatum, and S. terebinthinaceum [174]. Among the remaining dominant components of the extracts, the following sesquiterpene compounds should be enumerated: α-isocomene, modhephene, 7–β–H–silphiperfol–5–ene, β-caryophyllene, β-isocomene, germacrene D, 7–α–H–silphiperfol–5–ene, silphiperfol–6–ene, and β-bisabolene, as well as triterpene alcohols such as α- and β-amyrin [52,172]. It should be noted that only the extract from S. integrifolium contains a significant level of allo-aromadendr–9–ene, which can be found also in oil from the rhizomes, leaves, and inflorescences of the species [52]. In the extract from S. integrifolium, the presence of α- and β-himachalene was noted (also found in essential oil from S. integrifolium) [52]. In hexane and methanol extracts from rhizomes of S. perfoliatum, analogous volatile components were isolated as those in extracts obtained with the use of petroleum ether, but the methanol extracts had a significantly higher content of 16–acetoxycarterochaetol: 68.4%, compared to the hexane extracts at 45% [5]. A similar relationship was observed also between ethanol and hexane extracts from S. perfoliatum, S. trifoliatum, and S. integrifolium, which had higher concentrations of 16–acetoxycarterochaetol in the ethanol extracts compared to the hexane extracts, with the corresponding levels of the component being 310 and 62 µg/mL, 264 and 67 µg/mL, and 383 and 93 µg/mL, respectively [173]. In addition, the composition of fractions obtained during extraction with chloroform and ethanol from rhizomes of S. trifoliatum and S. integrifolium, in which the dominant compounds were 16–acetoxycarterochaetol and 7α–H–silphiperfol–5–ene, presilphiperfol–7–ene, 7–epi–silphiperfol–5–ene, modhephene, α- and β-isocomene, β-caryophyllene, α-humulene, germacrene D, presilphiperfolan–8–ol and α-amyrine, phytosterols, and compounds from the group of alkanes and esters of fatty acids, has also been studied [150]. Ethanol extracts from rhizomes of S. trifoliatum and S. integrifolium contained higher concentrations of 16–acetoxycarterochaetol compared to analogously obtained chloroform extracts, i.e., 7629 and 5776 µg/mL, 24,820 and 20,519 µg/mL, respectively [150].
Bohlmann and Jakupovic [174,175] performed detailed analysis of sesqui, di, and triterpenes in S. perfoliatum. From the rhizome of S. perfoliatum, they isolated the following sesquiterpene hydrocarbons: aplotaxene (6.1), bisabolene (6.2), germacrene C (6.3), germakrene D (6.4), humulene (6.5), caryophyllene (6.6), caryophyllene oxide (6.7), selina–4,6–diene (6.8), germacrene, eudesmane and guajane derivatives (6.9–6.14), precarabrone (6.15), isocomene (6.16), β-isocomene (6.17), modhephene (6.18), silphinene (6.19), silphiperfol–6–ene (6.20), 7–α–H–silphiperfol–5–ene (6.21), 7–β–H–silphiperfol–5–ene (6.22) (Figure 6). In addition, in rhizomes of S. perfoliatum, the presence was noted of the lactone sesquiterpene isoalantolactone (6.23) and compounds from the group of acyclic diterpenes dodeca-2t,4c,11–trien–1–al (7.1), 11,12–epoxy–heptadeca–1,9t,14c–trien–8–one (7.2) (Figure 6 and Figure 7) [175].
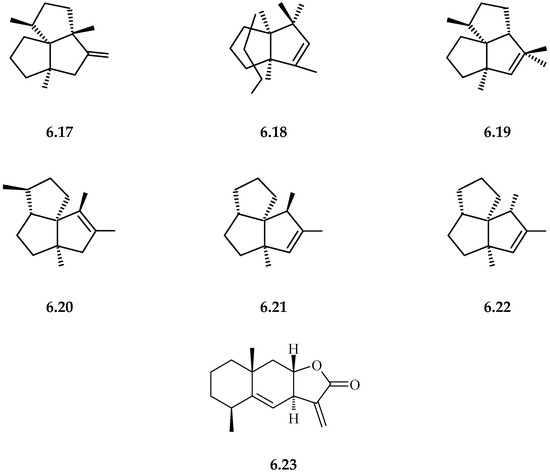
Figure 6.
Structure of major terpene compounds occurring in S. perfoliatum [16,174,175]: aplotaxene (6.1), bisabolene (6.2), germacrene C (6.3), germakrene D (6.4), humulene (6.5), caryophyllene (6.6), caryophyllene oxide (6.7), selina–4,6–diene (6.8), germacrene, eudesmane and guajane derivatives (6.9–6.14), precarabrone (6.15), isocomene (6.16), β-isocomene (6.17), modhephene (6.18), silphinene (6.19), silphiperfol–6–ene (6.20), 7–α–H–silphiperfol–5–ene (6.21), 7–β–H–silphiperfol–5–ene (6.22), isoalantolactone (6.23).
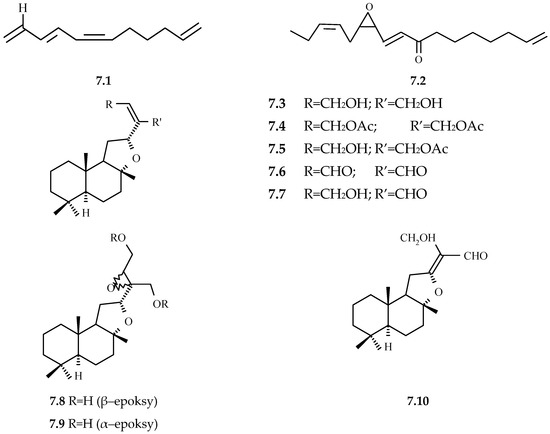
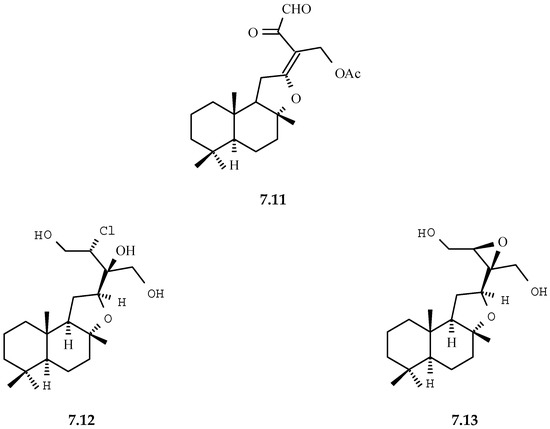
Figure 7.
Structure of major terpene compounds occurring in S. perfoliatum [16,174,175,176]: compounds from the group of acyclic diterpenes dodeca-2t,4c,11–trien–1–al (7.1), 11,12–epoxy–heptadeca–1,9t,14c–trien–8–one (7.2), 16–acetoxy–carterochaetol–acetate (7.3), 16–acetoxy–carterochaetol (7.4), 16–oxo–carterochaetal (7.5), 16–oxo–carterochaetol (7.6), 16–hydroxy–carterochaetol (7.7), 16–hydroxy–13,14H–13,14–epoxycarterochaetol (7.8,7.9), 16–oxo–13,14H–12,13–dehydrocarterochaetal (7.10), 16–acetoxy–14–okso–13,14H–12,13–dehydrocarterochaetal (7.11). Pcolinski et al. [176] isolated labdane-diterpenes of the type of carterochaetol as chlorosilphanol A (7.12) and silphanepoxol (7.13).
A large group of compounds of the rhizome of S. perfoliatum is that of diterpenes of the type of labdane, as derivatives of carterochaetol: 16–hydroxy–carterochaetol (7.7), 16–acetoxy–carterochaetol–acetate (7.3), 16–acetoxy–carterochaetol (7.4), 16–oxo–carterochaetal (7.5), 16–oxo–carterochaetol (7.6), 16–hydroxy–13,14H–13,14–epoxycarterochaetol (7.8,7.9), 16–oxo–13,14H–12,13–dehydrocarterochaetal (7.10), 16–acetoxy–14–okso–13,14H–12,13–dehydrocarterochaetal (7.11) (Figure 7). Pcolinski et al. [176] isolated the following labdane-diterpenes of the type of carterochaetol: chlorosilphanol A (7.12) and silphanepoxol (7.13) (Figure 7). In the aboveground part of the plant, together with sesquiterpenes 6.1 and 6.10 (Figure 6), lupenone triterpenes can be found: 3–oxy–lup–12, 13–en–28–al and 28–methyl–lup–12,13–en–28–ol.
3.2.12. Saponins
In the aboveground part of S. perfoliatum, the presence of the following triterpene glycosides was noted, where oleanolic acid is the aglycone (Figure 8): 3–O–β–D–glucuronopyranoside–oleanolic acid (glycoside F) (8.1); 3–O (6′–O–methyl)–β–D–glucuronopyranoside–28–O–β–D–glycopyranoside–oleanolic acid (silphioside A) (8.2); 3–O–β–D–glucuronopyranoside–28–O–β–D–glycopyranoside–oleanolic acid (glycoside G is identical with calenduloside F from Calendula officinalis L.) (8.3), 3,28–O–β–D– diglycopyranoside–oleanolic acid (silphioside B) (8.4); 3–O–β–D–glycopyranosyl–(1→2)–(6–O–acetyl)–β–D–glycopyranoside–28–O–β–D-glycopyranoside–oleanolic acid (silphioside C) (8.5); 3–O–β–D–glycopyranosyl (1→2)–O–β–D–glycopyranoside–28–O–β–D glycopyranoside –oleanolic acid (silphioside E) (8.6) [177,178,179,180,181]. In terms of quantitative composition, the primary compounds are silphioside B (0.21%), silphioside C (0.15%), silphioside E (0.16%), and glycoside G (0.11%) [16].
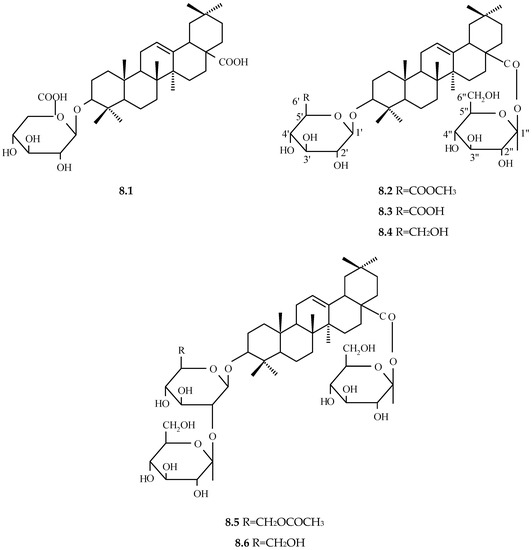
Figure 8.
Structure of triterpene saponins (derivatives of oleanolic acid) occurring in S. perfoliatum [16]: 3–O–β–D–glucuronopyranoside–oleanolic acid (glycoside F) (8.1); 3–O (6′–O–methyl)–β–D–glucuronopyranoside–28–O–β–D–glycopyranoside–oleanolic acid (silphioside A) (8.2); 3–O–β–D–glucuronopyranoside–28–O–β–D–glycopyranoside–oleanolic acid (glycoside G is identical with calenduloside F from Calendula officinalis L.) (8.3), 3,28–O–β–D–diglycopyranoside–oleanolic acid (silphioside B) (8.4); 3–O–β–D–glycopyranosyl–(1→2)–(6–O–acetyl)–β–D–glycopyranoside–28–O–β–D–glycopyranoside–oleanolic acid (silphioside C) (8.5); 3–O–β–D–glycopyranosyl (1→2)–O–β–D–glycopyranoside–28–O–β–D–glycopyranoside–oleanolic acid (silphioside E) (8.6).
Triterpenes are a diverse group of compounds, which include both chain forms, e.g., squalene, and polycyclic forms, e.g., steroids or saponins. Stigmasterol or β-sitosterol are structural elements of some plant membranes, performing a function analogous to cholesterol in the membranes of animal cells [182].
A comparative study conducted with the method of thin layer chromatography (TLC) on saponins isolated from green matter and rhizomes of S. perfoliatum demonstrated that the analyzed materials are characterized by an identical composition of the saponin fraction [16]. It was found that the qualitative composition of triterpene glycosides isolated from plants cultivated at various locations was identical. Therefore, the conditions of cultivation did not have any significant impact on the biosynthesis of triterpene glycosides in the genus Silphium L. [16]. It has been demonstrated that leaves of S. perfoliatum contained the largest amounts of oleanosides (mean of approx. 3.86% from harvests from June until September) relative to the inflorescences (3.70%) and rhizomes (1.71%). It was observed that with the development of plants, the level of oleanosides in leaves decreases, and therefore the optimum time for leaf harvest is the period prior to the blooming of plants (5.82%) [183]. Oleanosides occurred most abundantly in leaves of S. trifoliatum. Leaves of two-year-old plants, compared to one-year-old ones, contained more oleanosides. With the plant’s development, oleanoside content in leaves decreased. Kowalski [130] found that for S. integrifolium, contents of oleanosides were as follows: in leaves, up to 4.05% DM; in inflorescences, up to 4.84% DM; and in rhizomes, up to 2.48% DM (Table 7).

Table 7.
Oleanolic acid and oleanosides content in S. perfoliatum, S. trifoliatum and S. integrifolium [130,183].
In another study, it was demonstrated that as a result of hydrolysis of the saponin fraction of Silphium species, considerable levels of oleanolic and ursolic acids were obtained (oleanolic acid: from 1.55 mg/g DM in rhizomes of S. perfoliatum up to 22.08 mg/g DM in leaves of S. trifoliatum; ursolic acid: from 0.30 mg/g DM in rhizomes of S. trifoliatum up to 15.50 mg/g DM in leaves of S. trifoliatum) (Figure 9) [184]. The oleanolic acid content in ginseng (Panax quinquefolium) roots was 3.15 mg/g DM, while the content of oleanolic and ursolic acids in marigold (Calendula officinalis) flowers was respectively 20.52 mg/g DM and 0.58 mg/g [184]. Phytochemical studies indicate that tested materials may be an alternative source of triterpene saponins and their aglycones as compared to commonly known pot marigold flowers [184].
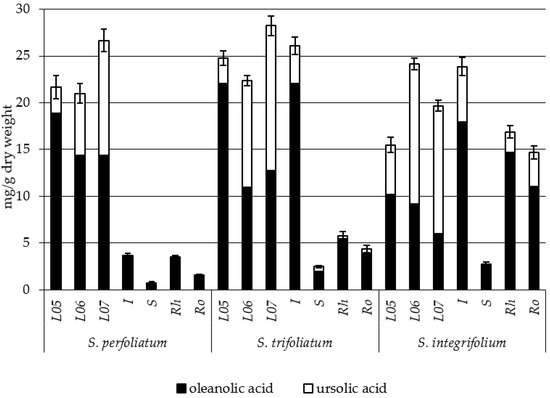
Figure 9.
Contents of glycoside-bonded oleanolic and ursolic acids (±SD) in S. perfoliatum, S. trifoliatum, and S. integrifolium. Key: L, leaves; I, inflorescences; S, seeds; Rh, rhizomes; Ro, roots; 5 May; 6 June; and 7 July [184].
Saponins, chemically speaking, are glycosides of triterpenoids or sterols. They are found in both edible plants (e.g., soybean, spinach) and inedible plants (e.g., horse chestnut, medical soapwort, ginseng). Steroid saponins have a high ability to hemolyze erythrocytes. Despite this negative influence, some of them are widely used in the pharmaceutical industry and medicine. Studies have shown that saponins have expectorant, anti-inflammatory, antiviral, antibacterial, and antifungal properties; they also show anti-mutagenic activity and are used in the treatment of atherosclerosis [185,186].
In terms of the amounts of saponin aglycones, the individual organs of Silphium differ significantly from one another [184]. The highest content of oleanolic acid was characteristic of Silphium leaves harvested in May, when they contained an average of 17.03 mg/g DM of that acid (from 10.15 mg/g in S. integrifolium to 22.08 mg/g in S. trifoliatum). Analyzing the dynamics of changes in the content of oleanolic acid in the leaves, one can conclude that the concentration of oleanolic acid in leaves generally decreases with the development of plants. Similar relationships concerning the changes in the content of oleanolic acid were observed in another study [130]. Inflorescences of S. trifoliatum and S. integrifolium contain oleanolic acid in amounts of 22.05 mg/g DM and 17.95 mg/g DM, respectively, while in inflorescences of S. perfoliatum, the concentration of the compound is notably lower at 3.68 mg/g DM [184]. Silphium species can be a competitive source of oleanolic acid relative to marigold Calendula officinalis L. For example, leaves of S. perfoliatum and S. trifoliatum harvested in the phase of intensive plant growth (May) and inflorescences of S. integrifolium and S. trifoliatum can be an alternative source of those compounds, relative to the commonly known marigold [184]. The content of oleanolic acid in Silphium roots and rhizomes varies from 1.55 mg/g DM in roots of S. perfoliatum to 14.7 mg/g DM in rhizomes of S. integrifolium. Seeds, on the other hand, contain oleanolic acid at levels from 0.75 mg/g DM in the case of S. perfoliatum to 2.78 mg/g DM in S. integrifolium. The presence of saponin glycosides in the seeds can be the main cause of reduced germination capacity of seeds of these species [61]. Another identified aglycone of saponins in the analyzed materials is ursolic acid, which is the most abundant in S. integrifolium and S. trifoliatum, with concentrations of up to 14.98 mg/g DM in leaves harvested prior to blooming (June) and up to 15.50 mg/g DM in leaves harvested in the stage of blooming of the plants [184].
In the aboveground and underground organs (leaves, inflorescences, seeds, rhizomes, and roots) of S. perfoliatum, S. trifoliatum, and S. integrifolium, Kowalski [184] noted the presence of 36 saponins, among which, glucuronide F was identified on the basis of an available standard; the occurrence of this saponin was confirmed in all organs of the analyzed species. Three compounds from the saponin fraction occurred simultaneously in the saponin complex isolated from inflorescences of marigold Calendula officinalis, and those were the aforementioned glucuronide F, glucuronide D2, and a substance with a characteristic mass spectrum (615 (100), 1171 (69), 585 (51)) [184]. In addition, a very detailed analysis was performed for the group of saponins isolated from 11 Silphium species, i.e., S. abliflorum, S. asteriscus, S. brachiatum, S. compositum, S. integrifolium, S. laciniatum, S. morhii, S. perfoliatum, S. radula, S. terebinthinaceum, and S. wasiotense; the result of the analysis was the isolation of approx. 90 new saponins which had not been identified before in the genus Silphium (Figure 10) [187]. The largest number of saponins was found in S. integrifolium (27 compounds), in S. radula (22 compounds), and in S. wasiotense (22 compounds), while the smallest number of saponins was found in S. terebinthinaceum (3 compounds) [187]. In the cited study, S. radula was characterized by the highest concentration of saponins (130 mg/g), with somewhat lower saponin concentrations being noted in S. integrifolium (116.6 mg/g), S asteriscus (105 mg/g), and S. compositum (102 mg/g) [187]. In the course of that study, nine new triterpene saponins were identified in S. radula and one in S. integrifolium.
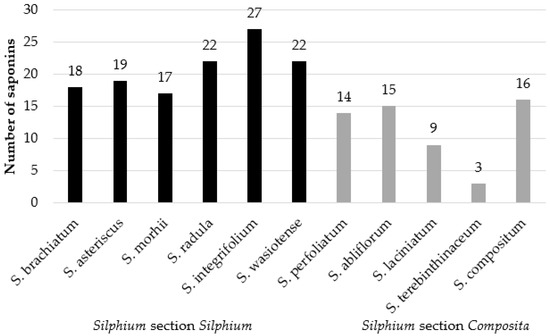
Figure 10.
The total number of saponins detected in crude extracts of Silphium [187].
In addition, several other known saponins were isolated and identified from S. integrifolium and S. morhii. The new saponins were identified as follows: 3β,6β,16β–trihydroxyolean–12–en–23–al–3–O–β–glucopyranosyl–16–O–β–glucopyranoside, urs–12–ene–3β,6β,16β–triol–3–O–β–galactopyranosyl–(1→2)–β–glucopyranoside, 3β,6β,16β––trihydroxyolean–12–en–23–oic acid–3–O–β–glucopyranosyl–16–O–β–glucopyranoside, urs–12–ene–3β,6β,16β,21β–tetraol–3–O–β–glucopyranoside, olean–12–ene–3β,6β,16β,21β–tetraol–3–O–β–glucopyranoside, olean–12–ene–3β,6β,16β,21β,23–pentaol–3–O–β–glucopyranosyl–16–O–β–glucopyranoside, olean–12ene–3β,6β,16β–triol–3–O–β–glucopyranosyl–16–O–α–arabinopyranosyl–(1→2)–β–glucopyranoside, olean–12–ene–3β,6β,16β,23–tetraol–3–O–β–glucopyranosyl–16–O–α–arabinopyranosyl–(1→2)–β–glucopyranoside, 3β,6β,16β,21β–tetrahydroxyolean–12–en–23–al–3–O–β–glucopyranoside, and ursolic acid 3–O–β–glucuronopyranosy–28–O–β–glucopyranoside [187]. The saponin 3–O–β–glucuronopyranosyloleanolic acid 28–O–β–glucopyranosyl 1–2–arabinopyranosyl, identified by Calabria [187] in S. perfoliatum, was previously isolated from Panax japonicus with the name chikusetsusaponin IV, which has shown to exhibit significant anti-obesity action in rats [188].
According to Calabria [187], the great variation in saponin profiles observed between species of Silphium represent a combination of environmental, developmental, and genetic factors.
3.3. Biological Activity
3.3.1. Historical Medicinal Applications
Various Native North American tribes have used S. perfoliatum for medicinal purposes. The Ojibwa (Chippewa) recommended infusion from its roots in the treatment of rheumatic diseases (lumbago and various pains with rheumatic background); decoction from the roots for lung diseases (lung hemorrhages), as a pain killer, and for abortion purposes; the plants were used also in the case of stomach disorders and in strong hemorrhaging [189,190]. Native Americans from the Fox tribe used infusions from the root of S. perfoliatum to reduce abundant menstrual hemorrhaging as a means of preventing vomiting during pregnancy and preventing premature childbirth [190]. The Iroquois recommended concoction from the roots as a vomitory preparation and as a bathing admixture in cases of paralysis; in addition, its roots have been used in rituals [191]. Native Americans from the tribes Winnebago, Ponca, and Omaha have used inhalations of smoke from burned rots and other parts of S. perfoliatum plants in the treatment of colds of the head and neuralgic and rheumatic pains; in addition, the Winnebago tribe has used root concoction as a vomitory agent [192,193].
In the American phytotherapy, the plant is used as a tonic, a diaphoretic, a diuretic, an expectorant in persistent cough and in asthma, in diseases of the liver and the spleen, against stomach ulcers, and in cases of internal injuries. The resinous secretion of S. perfoliatum and essential oil from flowers of the plant display stimulating and antispastic effects [16,194]. Fresh, blooming herbage has been used in homeopathy [129].
3.3.2. Research on Biological Activity In Vivo
Kuyanceva and Davidyants [195] conducted experiments on the regenerative activity of ethanol extracts from S. perfoliatum and demonstrated their properties for accelerating the healing of burn wounds in rats. In particular, it was demonstrated that in the experimental group of animals, obtaining compresses from liquid extracts from the plant, full recreation of the epithelium took place after 16 days of treatment, and in the control group after 20 days. In the case of application of the extract in the form of an ointment, the process of healing of the wounds in the experimental and control groups of animals took place after 9 and 14 days, respectively [195].
At the Institute of Chemistry of Plant Substances, Uzbekistan Academy of Sciences, a study was conducted on the pharmacological properties of a preparation obtained from leaves of S. perfoliatum on the base of purified sum of triterpene glycosides, i.e., silphiosides [196]. The toxicity of silphiosides was determined, for which the minimum lethal dose when administered to mice with food was, on average, 2200 mg/kg (from 1913 mg/kg to 2530 mg/kg). In addition, those authors demonstrated an anti-cholesterol activity of silphiosides [196]. After one-time “oral” administration of silphiosides to rats, at doses of 10 and 50 mg/kg, a reduction of the level of cholesterol in the blood was observed, by approx. 12% and 16%, respectively [196]. Administration of the preparation for a period of 10 days at the dose of 50 mg/kg produced even better effects: in this case, the level of cholesterol in the blood serum decreased by 19%. Also, a comparison was made between the activity of silphiosides and that of the preparation “Polisponin” obtained on the base of steroid saponins isolated from Dioscorea nipponica (Dioscoreaceae R. Br.), and was used in the treatment of atherosclerosis. After the administration of silphiosides (at the dose of 50 mg/kg), the levels of cholesterol and triglycerides dropped as low as to the level of 9.8% (from 37.4%) and 6.1% (from 26.5%), and after the administration of the preparation “Polisponin”, to the level of 18.3% and 12.2% [196]. The results of that study demonstrated that silphiosides, constituting the sum of triterpene glycosides obtained from leaves of S. perfoliatum, have a low level of toxicity and reduce the level of cholesterol and triglycerides in the blood serum, and are no less effective than the preparation “Polisponin” [196].
To analyze the estrogenic activity of saponins isolated from green matter of S. perfoliatum, experiments were conducted on sexually immature rat females. The results of the experiment were such that the saponin extract, at the dose of 50 mg/kg, displayed a strong estrogenic effect: a notable growth of the uterus was observed in the animals. Further studies demonstrated that preparations isolated from roots and seeds of S. perfoliatum also had a distinct estrogenic activity. Along with oral application of those preparations at the dose of 50 mg/kg, the increase of the mass of the uterus relative to the control was 199 ± 8.8% and 269.5 ± 19.3%, respectively, and that of the ovaries, 90.0 ± 1.5% and 7.5 ± 2.2%. It should be noted that the estrogenic activity of the preparation from the sum of silphiosides from Silphium seeds, at the dose of 10 m/kg, was higher than the activity of the sum of saponosides from alfalfa roots; when the preparations were administered to animals, the increase of uterus mass relative to the control was 40.5 ± 3.8% and 22.6 ± 7.7%, respectively [197].
3.3.3. Research on Biological Activity In Vitro
Experiments on Cancer Cells, against the HIV Virus, and Immunosuppressant Activity
A screening study was conducted on plants from the prairies in North America and it was found that organic extracts (prepared with the use of methanol and dichloromethane) from leaves, stems, and roots of S. perfoliatum and S. laciniatum displayed a moderate activity in anti-cancer screening tests (LC50 > or =20% of all tested cell lines). Water extracts from S. laciniatum had a strong anti-HIV activity (LC50 > 50% compared to the control); in addition, organic extracts from S. perfoliatum displayed a moderate activity (LC50 < 50% of the control) in tests against the HIV virus [198]. In another study, an attempt was undertaken at the estimation of the cytotoxic effect of polyhydroxylated pentacyclic oleanene and triterpene saponins of the type of ursane, isolated with the use of methanol from leaves of S. radula, relative to the human breast cancer cell line 25 MDA–MB-231. The study demonstrated that saponin I reduced the proliferation of the cells statistically significantly at 25 μg/mL [199]. Whereas, a sesquiterpene compound, (–)–alismoxide [200] and kaempferol trioside from S. perfoliatum, displayed cytotoxic activity towards cell cultures of animal and human cancers. In addition, kaempferol trioside was characterized by immunosuppressant activity towards mouse pancreas and thymus lymphocytes [149].
Research in Relation to Fungus- and Bacterium-Caused Diseases of Humans and Animals
Research was conducted on the biological activity of essential oil and extracts from S. perfoliatum, S.trifoliatum, and S. integrifolium in relation to bacteria and fungi [5,150]. Activity of essential oil and chloroform and ethanol extracts from rhizomes of S. trifoliatum and S. integrifolium was demonstrated in relation to Gram-positive microorganism strains of Staphylococcus aureus, as well as Gram-negative strains of Escherichia coli and the fungi Candida albicans and Malassezia pachydermatis [150]. In another study, activity of hexane and methanol extracts from leaves, inflorescences, and rhizomes of S. perfoliatum was demonstrated in relation to Gram-positive microorganism strains of Staphylococcus aureus and Enterococcus faecalis and Gram-negative strains of Escherichia coli and Pseudomonas aeruginosa [5].
Research in Relation to Fungus-Caused Plant Diseases
Davidyants et al. [201] conducted an in vitro study on the effect of silphiosides B, C, and E, and of the sum of saponins isolated from leaves of S. perfoliatum, on the growth of the phytopathogenic fungi Drechslera graminea (Rabh) Ito (barley leaf stripe), Rhizopus nodosus Namysl (dry rot of sunflower inflorescence), and Rhizopus nigricans Ehr (molding of food products). All preparations, at concentrations of 0.1% and 0.01%, inhibited the growth of mycelium of D. graminea, but the strongest activity was characteristic of the sum of saponosides. In the case of inhibition of overgrowth of spores of D. graminea, the strongest activity was displayed by silphiosides E and C. Whereas, for the fungi R. nodosus and R. nigricans, the sum of saponosides was the most inhibiting factor for mycelium growth and spore overgrowth. In addition, Davidyants et al. [201] demonstrated that anti-fungus activity is related with the number of sugar bonds with the aglycone (oleanolic acid). For this reason, silphiosides E (8.6) and C (8.5) (Figure 8), which have three sugar molecules in their structures, display a stronger activity relative to silphioside B (8.4), which has two sugar molecules.
Anti-fungal activity of raw extract from leaves of S. perfoliatum was confirmed also in relation to Fusarium oxysporum, Fusarium verticillioides, Penicillium expansum, Penicillium brevicompactum, Aspergillus flavus, and Aspergillus fumigatus [202]. Ethanol extract from leaves of S. perfoliatum was tested in relation to fungal isolates collected from cultivations of paprika [203]. In that experiment, isolates of Alternaria alternata, Botrytis cinerea, Colletotrichum coccodes, Fusarium oxysporum, Penicillium expansum, and Trichoderma harzianum extracted from paprika plants were used. Extract from Silphium was applied at concentrations of 5% and 10% [203]. The analyzed Silphium extracts significantly inhibited the growth of the tested fungal species relative to the control, with the exception of T. harzianum and B. cinerea at 5% extract concentration. The effect of 10% extract concentration lasted longer than that of 5%. A. alternata and C. coccodes were the fungi in relation to which the strongest inhibiting effect of the analyzed extracts from S. perfoliatum was noted [203].
3.3.4. The Effect of Triterpene Glycosides on Seed Germination and Catalase Activity
The use of physiologically active substances as plant growth regulators for pre-sowing treatment of agricultural plant seeds allows an increase in the absorption of water by seeds, stimulates enzymatic activity, growth, and metabolic processes in them, and increases the seed germination energy and ultimately plant productivity. The effect of purified amounts of triterpene glycosides containing, as major components, oleanolic acid glycosides, i.e, silphiosides B, C, E, G and extract enriched with them from S. perfoliatum leaves, on seed germination and catalase activity in them on two varieties of winter wheat (Tritium aestivum L.) has been studied [204]. It was shown that the treatment of seeds with triterpene glycosides solutions at concentrations of 0.0005 and 0.001% and extract at concentrations of 0.2 and 0.4% increases the intensity of their swelling within 48 h after soaking by 3.1–5.2% compared to the control, which leads to an earlier achievement of the threshold levels necessary for the activation of metabolic processes [204]. As a result of a study of changes in catalase activity in germinating seeds of winter wheat after 1, 3, and 7 days after soaking, it was revealed that the greatest effect of seed treatment with triterpene glycosides preparations manifested after 1 and 7 days of observation. The stimulating effect of TG preparations on catalase activity in germinating seeds of winter wheat was established. Under the influence of treatment with TG preparations, the germination energy and laboratory germination of seeds increased by 3–8% and 3–6%, respectively. The data obtained make it possible to consider the total preparation of triterpene glycosides isolated from leaves of S. perfoliatum as promising growth promoters for pre-sowing treatment of winter wheat seeds [204].
3.3.5. Extracts from Silphium as Additives Inhibiting Unfavorable Changes in Fats
A favorable effect of extracts from leaves, inflorescences, and rhizomes of S. perfoliatum, S. trifoliatum, and S. integrifolium added to sunflower oil on the preservation of stable quantitative composition in the fatty acid profile has been demonstrated. The analyzed extracts increased the values of inhibition of changes in relation to linoleic acid to a level comparable to that of butylated hydroxyanisole BHA, and sometimes, in suitable conditions, were even characterized by a more favorable value [173].
3.3.6. Antioxidant Properties of Extracts from Silphium
The polysaccharide fraction isolated from green matter of S. perfoliatum was characterized by a strong antioxidant activity. In the DPPH test, it scavenged free superoxide radicals in 76%, and in the ABTS test in 98%, of ascorbic acid compared to identical concentrations used as a control substance [104]. Ostolski et al. [128] showed that the antioxidant activity of the extracts from the aerial part of S. perfoliatum (test with the DPPH radical) ranged from 22.85 mg Trolox/g DM to 1110.64 mg Trolox/g DM. The antioxidant activity of the extracts was also significantly related to the content of polyphenolic compounds. In the period from April to September, the content of polyphenols and flavonoids decreased and then gradually increased [128].
3.3.7. Application in Animal Feeding
The plant’s high content of carbohydrates and proteins, favorable nutritive properties, high yields of green matter, resistance to diseases and pests, ability of acclimatization in various climate and soil conditions, and possibility of processing into silage and meal qualify it as suitable for fodder-feed purposes [57,64,66,73,80,98,99,101,108,109,110,114,115,116,205,206,207]. Conducted observations have revealed that, as opposed to silage, these plants in their green form are not overly happily consumed by ruminants [14], and hence the research has been oriented in the direction of using S. perfoliatum as a crop suitable for the production of silage [18,20,56,58,59,74,206,208,209]. In the period of vegetation from the vegetative phase to the beginning of seed setting, the value of the fermentation index of green forage from the three analyzed forms of S. perfoliatum was lower than 35, and only in the case of one form, harvested in the phase of the beginning of seed setting, the value of the fermentation index was 36.54, which guaranteed correct fermentation in the material [209]. Whereas, the high content of phenolic acids in S. perfoliatum puts a certain limitation on the use of the plant for fodder purposes [209]. Extracting proteins from S. perfoliatum biomass could also help replace soy with locally produced protein for feeding cattle [63].
In Moldova, a study was conducted on the composition of silage from S. perfoliatum, obtaining the following quality parameters: DM content, 13–6–16.4%; crude protein, 10.3–12.9% DM; crude fat, 2.1–3.7% DM; cellulose, 29.5–31.5% DM; nitrogen-free extractive substances, 36.9–46.3% DM; minerals, 11.46–15.66% DM; nutritive units of 1 kg silage, 01–0.2; metabolizable energy, 1.32–1.66 MJ/kg; digestible protein, 87–114 g/nutritive unit; pH of the silage 4.2–4.9; total organic acids, 1.9–3.2% DM; acetic acid, 0.8% DM; free acetic acid, 0.1–0.3% DM; fixed acetic acid, 0.7–0.5% DM; lactic acid, 1.1–2.4% DM; free lactic acid, 0.4–0.7% DM; fixed lactic acid, 0.7–1.6% DM [71]. Lehmkuhler et al. [210] state that cup plant silage has a nutritional value lower than corn silage, but it can be successfully incorporated as one component of the diet or be allocated to animals with lower energy requirements. According to Bernas et al. [211], S. perfoliatum can replace the yield and quality of silage maize, represents a lower environmental load per unit of production and unit of area, and generally carries many other benefits. Thus, cup plant is a recommendable option for dairy farming. S. perfoliatum can be considered an effective alternative to conventional silage [211]. Based on the results of Han and Albrecht [212], it has been concluded that S. perfoliatum silage can substitute mixture of alfalfa–corn silage at up to 30% of the forage portion in diets without substantial negative impacts on the performance of dairy cows, especially during late lactation. In one of the studies, the authors tested substitution of one-third and two-thirds of the silage for mid-lactation cows [212]. Increasing silage of S. perfoliatum to up to two-thirds of the forage portion in the diet reduced DM intakes and 4% fat-corrected milk production by 21.8% and 8.7%, respectively. The body weight of the cow decreased with increased addition of S. perfoliatum silage [212]. Moreover, a study with late-lactation cows indicated substitution of one-fourth of the silage-performed equivalent in DM intake, milk composition, and milk production to those of cows fed a low-forage diet (50% alfalfa–corn silage in diet), or a high-forage diet (66% alfalfa–corn silage in diet) [212].
The results of studies on the digestibility and nutritive value of silage from S. perfoliatum substantiate its attractiveness among fodders obtained from alternative crop plants. A study on dairy cows feeding with silage from green matter of S. perfoliatum demonstrated an increase of milking yield and content of vitamin A in the milk, and it was deemed likely that the effect observed was determined to a considerable extent by the occurrence of triterpene glycosides in the plant [16].
The DM yield and crude protein increased with increasing levels of N and P fertilization [28]. S. perfoliatum requires and absorbs low quantities of nitrogen, which renders it as a low-protein forage resource [28]. At seemingly low rates of nitrogen fertilization, it is an efficient biomass factory [28]. It was concluded that cup plant can be incorporated as a regular perennial summer fodder crop and also as a special feeding resource during summer drought as a supplement to existing pasture areas when its availability becomes limited [28]. Its adaptive and productive characteristics also make it suitable for low-input farming systems [28].
3.3.8. Ornamental Plant and Honeybee Forage
S. perfoliatum is also an attractive ornamental plant [213]. The species grows very intensively and thus can be a ornamentation of bowers, houses, and driveways, and also form lush hedges or fencing that flowers profusely through the summer. The plants are perfect for naturalization in natural or forest gardens [10].
In the context of improving agricultural landscapes for pollinating insects, energy crops with greater ecological value are required as an alternative to maize [214,215]. Energy plants proposed for biogas production can be a good supplement, and in this respect the species S. perfoliatum is very interesting [76]. After the first year from the establishment of the plantation, perennial crops can be used consecutively for at least ten years [31]. In contrast to maize, S. perfoliatum supplies both pollen and nectar. While the ray florets of S. perfoliatum hold the ovules, the disk florets produce the floral resources. In North America, its native range, S. perfoliatum is recommended as bee pasture [31].
S. perfoliatum has been accepted as a good melliferous plant in England [36], in Germany [37], in the regions of Leningrad and Bashkiria [38,39], and in Bulgaria [35], where it provides late forage for bees. Its attractiveness for bees is evidenced by the large numbers of those insects observed throughout the period of its blooming compared to other flowering plants [38,39]. S. perfoliatum can be used to produce about 560 kg, on average, of honey per hectare in Poland [216]. In the conditions at Lublin (Poland), the estimated honey yield per 1 ha has been recorded as 152.8 kg, with pollen yield of 363.9 kg [35]. Cultivation of the species can provide considerable amounts of both nectar and pollen forage from mid-summer until late autumn [30]. Nevertheless, S. perfoliatum has been proven to serve as a food plant for bees and butterflies [32]. S. perfoliatum can support certain hoverfly groups when it is harvested late to ensure a flower supply through to September and when semi-natural habitats are maintained in agricultural landscapes [32]. In studies conducted in Germany, it was shown that the production of pollen and nectar sugar by S. perfoliatum was the highest in the second half of August due to the high number of inflorescences per plant at this stage [31]. Pollen and nectar showed high amounts of some essential amino acids, in particular histidine, but the sum of the amino acids was low in concentration. Therefore, S. perfoliatum should be supplemented with various forage crops for bees to ensure a proper diet for these insects [31]. In addition, studies in Germany showed that irrigation of S. perfoliatum crops allowed for the increase of the production of nectar sugar, from 20 kg/ha to 58 kg/ha [217].
3.3.9. Application for Soil Remediation
The use of S. perfoliatum as a remediation plant in degraded and soil-less areas is an interesting possibility [30]. Although the scientific literature contains numerous studies on the use of species of the Asteraceae family as alternatives to the phytoremediation of heavy-metal contaminated soils, the species S. perfoliatum has so far been little-studied [218]. In the years 2010–2013, a study was conducted in Poland on the soil-forming effect and suitability of S. perfoliatum for the reclamation of areas after sulphur mining with the use of the Frasch method, by covering the area with flotation lime amended with municipal sewage sludge [219]. S. perfoliatum plants grew very well in the soil-less medium of flotation lime amended with municipal sewage sludge, forming a very stable and lush canopy and producing an average DM yield of 13.1 t/ha [219]. The use of S. perfoliatum for the reclamation of flotation lime amended with sewage sludge had a positive effect on the accumulation of organic matter and nutrients in the substrate and caused a lowering of its pH. The sludge fertilization of the calciferous substrate caused differentiation in the content of heavy metals, both in the substrate itself and in the S. perfoliatum plants growing on it. With increase of the concentration of heavy metals in the soil, a negative response of plants was observed, related with yield reduction to approx. 77% of the value obtained in the control cultivation. In addition, the authors concluded that the cultivation of S. perfoliatum on contaminated soil precludes the use of the plants for fodder purposes [220]. Biomass for non-food applications is considered as a substitute for petro-based materials such as expanded polystyrene (EPS) [221]. The collected biomass of plants previously used in phytoremediation can be used in the production of building materials. The results indicate that late-harvested S. perfoliatum biomass could be a biobased substitute for EPS in bonded leveling compounds [221]. Another application of S. perfoliatum after phytoremediation may be the use of this biomass in the production of paper and packaging materials. The paper strength of Silphium and Sida blends is comparable to the strength of the birch control [222]. Due to these promising results, these analyzed raw materials could find application, especially in the growing area of sustainable packaging materials.
In the cultivation of S. perfoliatum in soil with a high concentration of heavy metals, it was found that the roots are good Cu and Cr accumulators, while the leaf blade accumulates Zn and Pb well [218]. S. perfoliatum plants showed very high removal efficiency values, exceeding 85%, for the four analyzed heavy metals [218]. Du et al. [223] proposed pyrolyzing (at 350, 550 and 750 °C) biomass of S. perfoliatum previously used for phytoremediation. The long-term leaching risk of potentially toxic metals (PTMs) in the derived biochars from Silphium and the oxidation resistance of the biochars were investigated [223]. The results showed that PTMs in the biochar could transform into more stable and less toxic forms with the elevated pyrolysis temperature. The findings of this study demonstrated that high-temperature pyrolysis was able to reduce the potential risk of PTMs and ensure carbon stability [223]. Studies conducted in China have shown that the species S. perfoliatum can be used in phytoremediation of Cd [22].
3.3.10. Application as an Energy Crop
First studies considering S. perfoliatum as a biogas substrate were carried out in recent years by Conrad et al. [224], Aurbacher et al. [225,226], Stolzenburg and Monkos [227], Šiaudinis et al. [87], Slepetys et al. [228], and Mast et al. [229]. In the search for biogas alternatives to maize, S. perfoliatum is gaining more and more interest [85]. S. perfoliatum has so far been grown on around 400 ha in Germany [230], mainly by innovative farmers for local and regional bioenergy initiatives and energy supply companies. Silphium is a second-generation energy plant that is not used as food or forage, reduces environmental pollution by pesticides and fertilizers, increases soil humus, enriches the cultural landscape, and promotes biodiversity [231,232]. Silphium species are among the energy crops of the future [65,233]. In Moldova, experiments were conducted on the use of S. perfoliatum as an energy crop for the production of biogas. During the drying of plants on the field, the share of leaves in the plant and the moisture changed from approx. 31% and 71% (October) to 8% and 12% (March), respectively. The potential for gas generation from dry organic matter of S. perfoliatum was 471 L/kg, and the average content of biomethane in the biogas was about 52%. Methane production from silage from S. perfoliatum was found to be 3675 m3/ha. Biomass of S. perfoliatum was characterized by a high bulk density and moderate gross calorific values (18.3–18.7 MJ/kg) [234]. In another study, the potential for gas generation from dry organic matter of S. perfoliatum was from approx. 484 L/kg to 504 L/kg, and the average content of biomethane in the biogas was about 52% with production per hectare at levels from 5602 m3/ha to 12,146 m3/ha [81].
In Poland, analyses were performed for biomass of S. perfoliatum, harvested after the end of vegetation, at the heat-and-power generation plant, Electric Heating Plant Saturn Management, in Świecie. The analyses demonstrated that the biomass of the species is a valuable energy raw material, characterized by energy yields of 280–357 GJ/ha and heating value of approx. 15 MJ/kg, and that it can be competitive with willow cultivated for energy generation purposes [235]. In another experiment conducted in Poland, the following values of parameters characterizing biomass of S. perfoliatum as a plant cultivated for energy were obtained: specific density at moisture of 17%, 210 kg/m3; heating value at moisture of 13%, 15.23 MJ/kg; calorific value, 17.3 MJ/kg; ash content, 3.4% [83]. In an experiment conducted in Ukraine, the gross energy efficiency of S. perfoliatum cultivation was 356–385 GJ/ha [84]. Dry stems of S. perfoliatum can be easily collected mechanically in winter and they can be used for the production of a solid biofuel in the form of briquettes and pellets; biomass of this type has an energy value of approx. 18.3 MJ/kg DM and ash content of 2.5% [71]. Emissions of harmful gases, such as carbon monoxide, carbon dioxide, and nitrogen oxides, were determined in Lithuania, when pellets of S. perfoliatum were burned [236]. The determined emissions of harmful gases into the environment did not exceed the permissible values [236]. According to the European Environment Agency, biomass for combustion has the greatest potential among renewable energy sources in Poland [237]. Biomass is readily available and is economical as a fuel. The cheapest and simplest method of obtaining energy from biomass is usually to use it for the production of solid biofuels intended for combustion. Biomass is a fuel with a neutral influence on the emission of carbon dioxide, which results from the earlier use of carbon dioxide by plants in the photosynthesis process [238,239]. Sustainable biomass is an important fuel for mitigating climate change and decarbonizing the energy sector. Due to the high content of lignocellulosic fibers (cellulose, hemicellulose, and lignin) in the biomass of S. perfoliatum (about 90% dry weight), pre-treatment (extrusion or comminution) is necessary to increase biodegradability during anaerobic fermentation [105]. Research conducted in Poland has shown that S. perfoliatum can be used as a raw material for solid fuel and for anaerobic digestion. Witaszek et al. [105] showed that in the process of anaerobic fermentation, 1069 kWhe was obtained from 1 Mg of raw material crushed with an impact mill, 738.8 kWhe from 1 Mg of raw material extruded at 150 °C, and as much as 850.1 kWhe from 1 Mg of raw material extruded at 175 °C. On the other hand, the combustion of 1 Mg of S. perfoliatum pellets yielded 858.28 kWht [105].
S. perfoliatum has a high yielding potential [85]. Due to its deep and intensive rooting, it can also draw water from the deeper layers of the soil. Due to its high water demand, S. perfoliatum can yield DM comparable to maize only in places with good water supply (irrigation) [85]. Silphium essentially only has stems as an assimilation store. As the age of the plants increases, the number of shoots increases; at the same time, the illumination decreases, the thickness and stability of the stems decrease, and more and more leaves fall off before or during harvesting [85]. Perhaps these problems could be remedied by the occasional mechanical overtaking (e.g., of a rotary tiller) of overly dense plants. It should also be investigated whether the yield and yield stability of S. perfoliatum can be improved by breeding varieties with thicker and more stable stems and smaller leaves [85]. Although the reviewed studies show that the use of fertilizers can improve biomass yields, these operations can generate other costs which reduce energy efficiency [8]. The calorific value of S. perfoliatum depended on the plantation establishment method. The biomass from plants established by the generative method had a higher calorific value (16.95 MJ/kg) than biomass of plants established by the vegetative method (16.26 MJ/kg) [86].
In southwestern Germany, S. perfoliatum was investigated as a substrate for a large biogas plant with a biogas production of 100 m3/h [63]. It was found that the substitution of maize with Silphium can result in a methane yield reduction of 10% to 20% due to lower biomass yields. Delaying the harvest of S. perfoliatum is associated with a higher DM level, which is unfavorable for the fermentation carried out in the biogas plant [240]. Furthermore, S. perfoliatum provides food and shelter for open land animals, including birds and insects, and could hence be a suitable alternative to maize for large biogas plants, being more environmentally beneficial [63]. The results show that the methane yield per ha of S. perfoliatum lies in a range between grass and maize silage [24]. S. perfoliatum proved to have no negative effects on process stability for the ratio fed into the digester. Although S. perfoliatum cannot currently compete with maize silage, further research in plant breeding will help to increase DM yields, increase specific methane yield potential, and promote positive environmental effects such as a diverse energy crop rotation [24]. Experiments carried out in areas of the Czech Republic have confirmed that S. perfoliatum can be considered a promising novel crop for biogas production due to high yields of biomass (12–18 t/ha DM) and methane (3600–4250 Nm3/ha), competing with reference maize grown under the same soil and climatic conditions [96]. This corresponds with specific methane yields, which are about 5–10% higher in maize (269–319 Nm3/t VS) than in S. perfoliatum (254–298 Nm3/t VS) [96]. Moreover, the results of research conducted in Germany (Braunschweig) showed that S. perfoliatum proved suitable as a component in energy-cropping systems to reduce the risk of N leaching and soil erosion, which is particularly important for preventive flood protection in view of the more frequent occurrence of high-intensity rainfall under climate change conditions [241].
4. Conclusions
At present, one can observe a trend for the search for plants that can provide, e.g., food products with specific health-promoting properties, raw materials for the pharmaceutical industry, and renewable sources of energy. In addition, research on new crop plants is related to the protection of the natural environment, by way of organic agriculture, biological reclamation of soils, and counteracting the greenhouse effect, etc. These trends have caused an increase in the interest in new plant species both on the part of farmers and of the users of plant raw materials. Considering the above, it can be said that species of the genus Silphium can be so-called alternative plants that can be proposed for cultivation and for wide processing. The genus Silphium L. includes interesting plant species that are characterized by high yield of biomass, easy growth, and interesting chemical compositions in that they contain significant amounts of nutrients and biologically active substances. Therefore, taking into account the wide utility values of these plants, they can be proposed as alternatives for use in various industries. Certainly, the species of Silphium can be included in the category of so-called ‘new and promising plants’. Research on the use of these species for various purposes generally began in the 1960s, but it should be mentioned that they were previously employed by the indigenous peoples of North America. This is the first comprehensive review of a variety of studies on Silphium species (including non–English articles). As it results from the presented data, species of the genus Silphium are characterized by valuable utility features and can be used in a wide practical range. The studies conducted so far confirm that Silphium species can be used as fodder, as honey plants, as phytore-mediation plants, for reclamation of degraded land, as plants for energy purposes (biomass, biogas) and as plants that provide components with anti-microbial activity. In sum, species of the genus Silphium can be used as alternative plants for cultivation and industry.
Author Contributions
Conceptualization, G.K. and R.K.; methodology G.K. and R.K.; software, G.K. and R.K.; validation, G.K., T.B., R.K. and M.A.H.; formal analysis, G.K., T.B., R.K. and M.A.H.; investigation, G.K. and R.K.; resources, G.K., T.B., R.K. and M.A.H.; data curation, G.K. and R.K.; writing–original draft preparation, G.K. and R.K.; writing–review and editing, G.K., T.B., R.K. and M.A.H.; visualization, T.B.; supervision, R.K.; project administration, G.K. and R.K.; funding acquisition, G.K. and R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Agrobioengineering of University of Life Sciences in Lublin and for the Faculty of Food Science and Biotechnology of University of Life Sciences in Lublin.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Report of the FAO Council, 94th Session; FAO: Rome, Italy, 1988. [Google Scholar]
- Batmunkh, A.; Nugroho, A.D.; Fekete-Farkas, M.; Lakner, Z. Global challenges and responses: Agriculture, economic globalization, and environmental sustainability in Central Asia. Sustainability 2022, 14, 2455. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yıldız, M.; Tansı, S.; Sezen, M. New Plants with Commercial Potent. Available online: https://dergipark.org.tr/tr/download/article-file/142220 (accessed on 25 February 2022).
- Kowalski, R.; Kędzia, B. Antibacterial activity of Silphium perfoliatum extracts. Pharm. Biol. 2007, 45, 494–500. [Google Scholar] [CrossRef]
- Steyermark, J.A. Flora of Missouri; The Iova State University Press: Ames, IA, USA, 1962. [Google Scholar]
- Kowalski, R.; Wolski, T. Charakterystyka wzrostu i rozwoju rożnika przerośniętego Silphium perfoliatum L. w pierwszych latach uprawy. Ann. Univ. Mariae Curie-Sklodowska Sect. E 2001, 9, 311–317. [Google Scholar]
- Peni, D.; Stolarski, M.J.; Bordiean, A.; Krzyżaniak, M.; Dębowski, M. Silphium perfoliatum—A Herbaceous Crop with Increased Interest in Recent Years for Multi-Purpose Use. Agriculture 2020, 10, 640. [Google Scholar] [CrossRef]
- Worth, F.; Headwater, P.; Ecology, S.; Population, W.P.; Lizard, T.H. Viability of Silphium albiflorum in Tarrant County. Post Oak Prairie J. 2015, 1, 1–35. [Google Scholar]
- Brickell, C. Wielka Encyklopedia Roślin Ogrodowych od a do z; Muza SA: Warszaw, Poland, 1999. [Google Scholar]
- Rickett, H.W. Wild Flowers of the United States. Volume One. The Northeastern States. From the Atlantic to Minnesota and Missouri and From the Canadian Border to Virginia and Missouri, 1st ed.; McGraw-Hill for the The New York Botanical Garden: New York, NY, USA, 1966. [Google Scholar]
- Tutin, T.G.; Heywood, V.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europea; Cambrige University Press: Cambrige, UK, 1976; Volume 4. [Google Scholar]
- Clevinger, J.A.; Panero, J.L. Phylogenetic analysis of Silphium and subtribe Engelmanniinae (Asteraceae: Heliantheae) based on ITS and ETS sequence data. Am. J. Bot. 2000, 87, 565–572. [Google Scholar] [CrossRef]
- Puia, I.; Szabó, A.T. Culture experimentale d’ une nouvelle espece fourragére—Silphium perfoliatum L.—dans le Jardin Agrobotanique de Cluj-Napoca. Institutum Agronomicum „Dr. Petru Groza”, Cluj-Napoca (Romania). Not. Bot. Horti Agrobot. 1985, 15, 15–20. [Google Scholar]
- Stuessy, T.F. Heliantheae—Systematic review. In The Biology and Chemistry of the Compositae. II; Heywood, V.H., Harborne, J.B., Turner, B.L., Eds.; Academic Press: London, UK, 1977; pp. 621–672. [Google Scholar]
- Davidyants, E.S.; Abubakirov, N.K. Chimičeskij sostav i perspektivy ispol’zowaniya rasteniy r. Silphium L. Rastit. Resur. 1992, 28, 118–128. [Google Scholar]
- Huxley, A. Dictinary of Gardening, 4th ed.; Stockton Press: New York, NY, USA, 1992. [Google Scholar]
- Čubarova, G.V. Sil’fia pronzennolistnaja—odna iz perspektivnych silosnych kultur. Životnovodstvo 1971, 8, 17–18. [Google Scholar]
- Niqueux, M. Une nouvelle plante fourragére: Le silfe (Silphium perfoliatum L.). Fourrages 1981, 119–136. Available online: https://afpf-asso.fr/article/une-nouvelle-plante-fourragere-le-silfe-silphium-perfoliatum-l (accessed on 25 February 2022).
- Duranti, E.; Santilocchi, R.; Casoli, C. Composizione chimica e valore nutritivo di Silphium perfoliatum L. conservato mediante insilamento. Zootec. Nutr. Anim. 1988, 14, 349–356. [Google Scholar]
- Neumerkel, W.; Märtin, B.; Linke, G. Silphium perfoliatum L. eine Nutzpflanze? Wissenschaftliche Zeitschrift der Martin-Luther-Universität Halle 1978, 27, 31–38. [Google Scholar]
- Zhang, X.; Xia, H.; Li, Z.; Zhuang, P.; Gao, B. Potential of four forage grasses in remediation of Cd and Zn contaminated soils. Bioresour. Technol. 2010, 101, 2063–2066. [Google Scholar] [CrossRef]
- Schittenhelm, S.; Kottmann, L.; Schoo, B. Wasser als ertragsbegrenzender Faktor [Water as a limiting factor for crop yield]. J. Fur Kult. 2017, 69, 80–86. [Google Scholar]
- Haag, N.L.; Nägele, H.-J.; Reiss, K.; Biertümpfel, A.; Oechsner, H. Methane formation potential of cup plant (Silphium perfoliatum). Biomass Bioenergy 2015, 75, 126–133. [Google Scholar] [CrossRef]
- Matthews, J.; Beringen, R.; Huijbregts, M.A.J.; Van der Mheen, H.J.; Odé, B.; Trindade, L.; Van Valkenburg, J.L.C.H.; Van der Velde, G.; Leuven, R.S.E.W. Horizon Scanning and Environmental Risk Analyses of Non-Native Biomass Crops in The Netherlands. Available online: https://www.researchgate.net/publication/297136883_Horizon_scanning_and_environmental_risk_analyses_of_non-native_biomass_crops_in_the_Netherlands (accessed on 25 February 2022).
- Kowalski, R. Silphium trifoliatum L.—A new alternative cultivation herbal plant? Acta Agric. Scand. Sect. B Soil Plant Sci. 2007, 57, 155–166. [Google Scholar]
- Kowalski, R. Growth and development of Silphium integrifolium in the first 3 years of cultivation. N. Z. J. Crop Hortic. Sci. 2004, 32, 389–395. [Google Scholar] [CrossRef]
- Pichard, G. Management, production, and nutritional characteristics of cup-plant (Silphium perfoliatum) in temperate climates of southern Chile. Cienc. Investig. Agrar. 2012, 39, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Weryszko-Chmielewska, E.; Kowalski, R.; Wolski, T. Rożnik przerośnięty (Silphium perfoliatum L.) nowa roślina alternatywna. Część I. Badania morfologiczne i anatomiczne. Zesz. Probl. Postępów Nauk Rol. 1999, 468, 497–505. [Google Scholar]
- Woźniak, M.; Góral, S. Rożnik przerośnięty (Silphium perfoliatum) roślina na pastwiska pszczele. In Proceedings of the Materiały 35 Naukowej Konferencji Pszczelarskiej, Puławy, Poland, 11–12 March 1998; p. 90. [Google Scholar]
- Mueller, A.L.; Biertümpfel, A.; Friedritz, L.; Power, E.F.; Wright, G.A.; Dauber, J. Floral resources provided by the new energy crop, Silphium perfoliatum L. (Asteraceae). J. Apic. Res. 2020, 59, 232–245. [Google Scholar] [CrossRef]
- Mueller, A.L.; Dauber, J. Hoverflies (Diptera: Syrphidae) benefit from a cultivation of the bioenergy crop Silphium perfoliatum L. (Asteraceae) depending on larval feeding type, landscape composition and crop management. Agric. For. Entomol. 2016, 18, 419–431. [Google Scholar] [CrossRef]
- Figas, A.; Sawilska, A.K.; Rolbiecki, R.; Tomaszewska-Sowa, M. Morphological characteristics of achenes and fertility plants of cup plant (Silphium perfoliatum L.) Obtained from micropropagation growing under irrigation. Infrastruct. Ecol. Rural Areas 2016, 4, 1363–1372. [Google Scholar]
- Weryszko-Chmielewska, E.; Michońska, M.; Wolski, T.; Kowalski, R. Porównanie cech morfologicznych kwiatów trzech gatunków Silphium z uwzględnieniem prezenterów pyłkowych i ziarn pyłku. Bibl. Fragm. Agron. 1999, 6, 103–112. [Google Scholar]
- Wróblewska, A. Badania wartości pszczelarskiej Silphium perfoliatum L. In Proceedings of the Materiały I Ogólnopolskiej Konferencji Naukowej „Biologia kwitnienia, nektarowania i zapylania roślin”, Lublin, Poland, 13–14 November 1997; pp. 59–65. [Google Scholar]
- Hoves, F.N. Plants and Beekeeping. An Account of Those Plants, Wild and Cultivated, of Value to the Hive Bee, and for Honey Production in the British Isles. London: 197; Frazer Press: London, UK, 2017. [Google Scholar]
- Daniel, P. Silphium perfoliatum „Durchwachsene Silphie”—Eine neue Bienenweidepflanze. Biene 1984, 120, 196–199. [Google Scholar]
- Pelmenev, V.K. Nowyje kormowyje kul’tury. Nowyje Kormowyje kul’Tury; Medonosnyje Rastenija Rosselchozizdat: Moskva, Russia, 1985; pp. 121–122. [Google Scholar]
- Kučerov, E.V.; Siraeva, S.M. Silfia pronzennolistnaya. Pčelovodstvo 1987, 1, 15–16. [Google Scholar]
- Miller, P.D. Maize pollen: Collection and enzymology. In Maize for biological research; Sheridan, W.F., Ed.; University Press: Grand Forks, ND, USA, 1982; pp. 279–282. [Google Scholar]
- Schäfer, A.; Damerow, L.; Lammers Schulze, P. Durchwachsene Silphie: Bestandesetablierung mittels Aussaat Cup plant: Crop establishment by sowing. J. Kult. 2016, 68, 367–371. [Google Scholar]
- Kowalski, R.; Wierciński, J. Evaluation of chemical composition of some Silphium L. species seeds as alternative foodstuff raw materials. Polish J. Food Nutr. Sci. 2004, 13, 349–354. [Google Scholar]
- Schäfer, A.; Leder, A.; Graff, M.; Damerow, L.; Lammers, P.S. Determination and sorting of cup plant seeds to optimize crop establishment. Landtechnik 2018, 73, 97–105. [Google Scholar]
- von Gehren, P.; Gansberger, M.; Mayr, J.; Liebhard, P. The effect of sowing date and seed pretreatments on establishment of the energy plant Silphium perfoliatum by sowing. Seed Sci. Technol. 2016, 44, 310–319. [Google Scholar] [CrossRef]
- Schäfer, A.; Damerow, L.; Lammers, P.S. Determination of the seed geometry of cup plant as requirement for precision seeding. Landtechnik 2017, 72, 122–128. [Google Scholar]
- Köhler, J.; Biertümpfel, A. Wie die Saat, so die Ernte—Erfolgreiche Etablierung Durchwachsener Silphie durch Aussaat As the sowing, so the harvest—successful establishment of Cup Plant by sowing. J. Kult. 2016, 68, 356–362. [Google Scholar]
- Shalyuta, B.V.; Kostitskaya, E.V. The Yield of Silphium perfoliatum L. Depending on the Conditions of Cultivation. Agric. Eng. 2018, 22, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R.; Kowalska, G.; Jankowska, M.; Nawrocka, A.; Kałwa, K.; Pankiewicz, U.; Włodarczyk-Stasiak, M. Secretory structures and essential oil composition of selected industrial species of Lamiaceae. Acta Sci. Pol. Hortorum Cultus 2019, 18, 53–69. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.; Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.; Vazquez-Olivo, G.; Heredia, J. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Morris, L.J. Silphium laciniatum, Lin., Rosin Weed. Am. J. Pharm. 1881, 53, 487–491. [Google Scholar]
- Kowalski, R. The chemical composition of essential oils and lipophilic extracts of Silphium integrifolium Michx. and Silphium trifoliatum L. rhizomes. J. Essent. Oil Res. 2008, 20, 255–259. [Google Scholar] [CrossRef]
- Kowalski, R. The chemical composition of essential oils and lipophilic extracts of Silphium integrifolium Michx. and S. trifoliatum L. leaves. Flavour Fragr. J. 2008, 23, 164–171. [Google Scholar] [CrossRef]
- Van Tassel, D.L.; Albrecht, K.A.; Bever, J.D.; Boe, A.A.; Brandvain, Y.; Crews, T.E.; Gansberger, M.; Gerstberger, P.; González-Paleo, L.; Hulke, B.S.; et al. Accelerating Domestication: An Opportunity to Develop New Crop Ideotypes and Breeding Strategies Informed by Multiple Disciplines. Crop Sci. 2017, 57, 1274. [Google Scholar] [CrossRef] [Green Version]
- Stanford, G. Silphium perfoliatum (cup-plant) as a new forage. In Proceedings of the 12th North American Prairie Conference, Cedar Falls, IA, USA, 5–9 August 1990; Volume 1, pp. 33–37. [Google Scholar]
- Troxler, J.; Daccord, R. Le silfe (Silphium perfoliatum L.): Un fourrage intéressant? Rev. Suisse d’Agric. 1982, 14, 279–281. [Google Scholar]
- Filatov, V.I.; Baklanow, A.M.; Lavrov, B.V.A.; Komjagin, N. Produktivnost’ sil’fii pronzennolistnoy v zavisimosti ot prijemov agrotechniki na mieliorirovannych počvach. Izv. TSCHA 1986, 1, 58–63. [Google Scholar]
- Douglas, J.A.; Follett, J.M.; Halliday, J.R.; Hughes, J.W.; Parr, C.R. Silphium: Preliminary research on a possible new forage crop for New Zealand. Proc. Agron. Soc. N. Z. 1987, 17, 51–53. [Google Scholar]
- Daniel, P.; Rompf, R. Möglichkeiten und Grenzen der Nutzung der Durchwachsenen Silphie (Silphium perfoliatum L.) als Futter-, nachwachsenole Rohstoff- und Landschaftspflegepflanze. Agribiol. Res. 1994, 47, 345–353. [Google Scholar]
- Kolarova, M.; Grigorova, L. Laboratorna k”lnjaemost na silfiya (Silphium perfoliatum L.). Rastenev’dni Nauk. 1986, 23, 76–84. [Google Scholar]
- Procko, R.F.; Sidorenko, T.V. Doslidi po pidwišcennju schozosti nasinnja Silphium perfoliatum L. Ukr. Bot. Zhurnal 1991, 48, 64–67. [Google Scholar]
- Reinert, S.; Money, K.L.; Rockstad, G.B.G.; Kane, N.C.; Van Tassel, D.L.; Hulke, B.S. Two contrasting laboratory methods improve Silphium integrifolium Michx. germination rate to agronomically acceptable levels. Euphytica 2018, 214, 156. [Google Scholar] [CrossRef]
- von Cossel, M.; Amarysti, C.; Wilhelm, H.; Priya, N.; Winkler, B.; Hoerner, L. The replacement of maize (Zea mays L.) by cup plant (Silphium perfoliatum L.) as biogas substrate and its implications for the energy and material flows of a large biogas plant. Biofuels, Bioprod. Biorefin. 2020, 14, 152–179. [Google Scholar] [CrossRef] [Green Version]
- Han, K.J.; Albrecht, K.A.; Mertens, D.R.; Kim, D.A. Comparison of in vitro digestion kinetics of cup-plant and alfalfa. Asian-Australasian J. Anim. Sci. 2000, 13, 641–644. [Google Scholar] [CrossRef]
- Cumplido-Marin, L.; Graves, A.R.; Burgess, P.J.; Morhart, C.; Paris, P.; Jablonowski, N.D.; Facciotto, G.; Bury, M.; Martens, R.; Nahm, M. Two Novel Energy Crops: Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L.—State of Knowledge. Agronomy 2020, 10, 928. [Google Scholar] [CrossRef]
- Pavlov, V.S.; Pachomov, I.J.; Špakov, A.P.; Yakimčik, T.V. Produktivnost sil’fii pronzennolistnoj w zavisimosti ot azotnogo udobreniya. Chim. w Selskim Chozjajstwie 1984, 21, 24–26. [Google Scholar]
- Jemielin, V.A.; Šjeluto, B.V. Produktivnosť silfii pronziennolistnoj v zavisimosti ot posledjejstvija udobrjenij, pieriodičnosti podkormok i doz azota na sieďmoj hod žizni posievov. Viestn. Biełorusskoj Hosudarstviennoj Selskochoziajstviennoj Akadjemii 2021, 636.085.51, 107–112. [Google Scholar]
- Šjeluto, B.V.; Kostickaja, J.V. Fotosintjetičjeskaja djejatjelnosť rastjenij silfii pronziennolistnoj. Ispolz. Mieliorirovannych Zemiel 2021, 2, 55–61. [Google Scholar]
- Kuklina, A.G. Introdukcija silfii pronziennolistnoy (Silphium perfoliatum L.) v glavnom Botaničeskom Sadu RAN. Bjulletin Gl. Bot. Sada 1994, 170, 30–33. [Google Scholar]
- Klečkovskaya, M.S.; Muchin, N.G. Introdukcija sil’fii pronzennolistnoy v voronežskoy oblasti. Bjul. Glawnogo Bot. Sada 1990, 155, 12–16. [Google Scholar]
- Ţîţei, V. Biological Peculirities of cup plant (Silphium perfoliatum L.) and utilization possibilities in the republic of Moldova. Lucr. Ştiinţifice Ser. Agron. 2014, 57, 289–293. [Google Scholar]
- Edelšteyn, M.M.; Soloveva, I.W. Sravnitelnaja ocenka kormovych kačestv novych silosnych kultur v usloviyach moskovskoj oblasti. Dokl. TSCHA 1971, 168, 52–56. [Google Scholar]
- Edelšteyn, M.M.; Soloveva, I.W. Soderžanije sacharov v vegetatiwnych organach novych silosnych kultur. Dokl. TSCHA 1975, 209, 51–53. [Google Scholar]
- Maslinkov, M.; Donev, N. Sravnitelno izpitvane na novi mnogogodišni furažni kulturi pri uslovijata na plovdiv. Rastenev’dni Nauk. 1987, 24, 53–58. [Google Scholar]
- Andrejnski, Ž.; Angelow, L.; Andrejnska, I. Proučwane na nowi furažni kulturi. Rastenev’dni Nauk. 1989, 26, 41–45. [Google Scholar]
- Gansberger, M.; Montgomery, L.F.R.; Liebhard, P. Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind. Crop. Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- Bedlan, G. Ascochyta silphii sp. nov.—Eine neue Ascochyta-Art an Silphium perfoliatum [Ascochyta silphii sp. nov.—A new Ascochyta species on Silphium perfoliatum]. J. Kult. 2014, 66, 281–283. [Google Scholar]
- Schoo, B.; Kage, H.; Schittenhelm, S. Radiation use efficiency, chemical composition, and methane yield of biogas crops under rainfed and irrigated conditions. Eur. J. Agron. 2017, 87, 8–18. [Google Scholar] [CrossRef]
- Reinert, S.; Hulke, B.S.; Prasifka, J.R. Pest potential of Neotephritis finalis (Loew) on Silphium integrifolium Michx., Silphium perfoliatum L., and interspecific hybrids. Agron. J. 2020, 112, 1462–1465. [Google Scholar] [CrossRef]
- Stefanov, D.; Dimitrova, M.; Sanbev, S. Proučvane na vzmožnostite nyakoi rastenija da bdat otgleždani kato furažni kulturi. Životnov’dni Nauk. 1984, 21, 50–56. [Google Scholar]
- Siwek, H.; Włodarczyk, M.; Możdżer, E.; Bury, M.; Kitczak, T. Chemical Composition and Biogas Formation potential of Sida hermaphrodita and Silphium perfoliatum. Appl. Sci. 2019, 9, 4016. [Google Scholar] [CrossRef] [Green Version]
- Šiaudinis, G.; Karčauskienė, D.; Aleinikovienė, J. Assessment of a single application of sewage sludge on the biomass yield of Silphium perfoliatum and changes in naturally acid soil properties. Zemdirbyste-Agriculture 2019, 106, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Frączek, J.; Mudryk, K.; Wróbel, M. Rożnik przerośnięty Silphium perfoliatum l. Źródło biomasy do produkcji biopaliw stałych. Inżynieria Rolnicza 2011, 6, 21–28. [Google Scholar]
- Lopushniak, V.; Hrytsuliak, G.; Dzhus, G. Bioenergetic assessment of sewage sludge application under Silphium perfoliatum L. on sod-podzolic soils of Precarpathians. Agroecol. J. 2021, 126–134. [Google Scholar] [CrossRef]
- Schittenhelm, S.; Schoo, B.; Schroetter, S. Ertragsphysiologie von Biogaspflanzen: Vergleich von Durchwachsener Silphie, Mais und Luzernegras. J. Kult. 2016, 68, 378–384. [Google Scholar]
- Bury, M.; Możdżer, E.; Kitczak, T.; Siwek, H.; Włodarczyk, M. Yields, Calorific Value and Chemical Properties of Cup Plant Silphium perfoliatum L. Biomass, Depending on the Method of Establishing the Plantation. Agronomy 2020, 10, 851. [Google Scholar] [CrossRef]
- Šiaudinis, G.; Jasinskas, A.; Šlepetiene, A.; Karčauskiene, D. The evaluation of biomass and energy productivity of common mugwort (Artemisia vulgaris L.) and cup plant (Silphium perfoliatum L.) in albeluvisol. Žemdirbystė 2012, 99, 357–362. [Google Scholar]
- Boe, A.; Albrecht, K.A.; Johnson, P.J.; Wu, J. Biomass Production of Monocultures and Mixtures of Cup Plant and Native Grasses on Prime and Marginal Cropland. Am. J. Plant Sci. 2019, 10, 911–924. [Google Scholar] [CrossRef] [Green Version]
- Facciotto, G.; Bury, M.; Chiocchini, F.; Marín, L.C.; Czyż, H.; Graves, A.; Martens, R.; Morhart, C.; Paris, P.; Nahm, M.; et al. Preliminary results regarding yields of Virginia mallow (Sida hermaphrodita (L.) Rusby) and cup plant (Silphium perfoliatum L.) in different condition of Europe. In Proceedings of the Biomass Crops and Energy Grasses, Proceedings of the 27th European Biomass Conference and Exhibition, Lisbon, Portugal, 27–30 May 2019; pp. 350–352. Available online: https://www.researchgate.net/profile/Pierluigi-Paris/publication/334612043_Preliminary_results_regarding_yields_of_Virginia_mallow_Sida_hermaphrodity_L_Rusby_and_cup_plant_Silphium_perfomiatum_L_in_different_conditions_of_Europe/links/5d35c46fa6fdcc370a55e8f3/Preliminary-results-regarding-yields-of-Virginia-mallow-Sida-hermaphrodity-L-Rusby-and-cup-plant-Silphium-perfomiatum-L-in-different-conditions-of-Europe.pdf (accessed on 25 February 2022).
- Cossel, M.; Steberl, K.; Hartung, J.; Pereira, L.A.; Kiesel, A.; Lewandowski, I. Methane yield and species diversity dynamics of perennial wild plant mixtures established alone, under cover crop maize (Zea mays L.), and after spring barley (Hordeum vulgare L.). GCB Bioenergy 2019, 11, 1376–1391. [Google Scholar] [CrossRef] [Green Version]
- Ruidisch, M.; Nguyen, T.T.; Li, Y.L.; Geyer, R.; Tenhunen, J. Estimation of annual spatial variations in forest production and crop yields at landscape scale in temperate climate regions. Ecol. Res. 2015, 30, 279–292. [Google Scholar] [CrossRef]
- Schorpp, Q.; Schrader, S. Earthworm functional groups respond to the perennial energy cropping system of the cup plant (Silphium perfoliatum L.). Biomass Bioenergy 2016, 87, 61–68. [Google Scholar] [CrossRef]
- Boe, A.; Albrecht, K.A.; Johnson, P.J.; Wu, J. Biomass production of cup plant (Silphium perfoliatum L.) in response to variation in plant population density in the North Central USA. Am. J. Plant Sci. 2019, 10, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Franzaring, J.; Holz, I.; Kauf, Z.; Fangmeier, A. Responses of the novel bioenergy plant species Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L. to CO2 fertilization at different temperatures and water supply. Biomass Bioenergy 2015, 81, 574–583. [Google Scholar] [CrossRef]
- Šiaudinis, G. The investigation of three potential energy crops: Common mugwort, cup plant and virginia mallow on Western Lithuania’s Albeluvisol. Appl. Ecol. Environ. Res. 2017, 15, 611–620. [Google Scholar] [CrossRef]
- Ustak, S.; Munoz, J. Cup-plant potential for biogas production compared to reference maize in relation to the balance needs of nutrients and some microelements for their cultivation. J. Environ. Manag. 2018, 228, 260–266. [Google Scholar] [CrossRef]
- Heneman, P.; Červinka, J. Energy crops and bioenergetics in the Czech Republic. Ann. Warsaw Univ. Life Sci. 2007, 51, 73–78. [Google Scholar]
- Dimitrova, M.; Stefanov, D.; Sanbev, S.; Lazarov, W.; Christov, P. Technologija za schranenie na zelenata masa ot cherakleum i silfium. Životnov’dni Nauk. 1987, 24, 56–61. [Google Scholar]
- Glyaubertene, V.F.; Ivanovskaya, K.M. Biologičeskaja i biochimičeskaja charakteristika perspektivnych silosnych rastenij. (22. Soderžanie pitatel’nych veščestv v nadzemnoj masse sil’fii pronzennolistnoy i maral’ego kornja i ich izmenenie vo vremya vegetacii). Liet. TSR Moksl. Akad. Darbai 1987, 4, 110–118. [Google Scholar]
- Rakhmetov, D.B.; Vergun, O.M.; Stadnichuk, N.O.; Shymanska, O.V.; Rakhmetova, S.O.; Fishchenko, V.V. Biochemical study of plant raw material of Silphium L. spp. in M.M. Gryshko National Botanical Garden of the NAS of Ukraine. Plant Introd. 2019, 2019, 80–86. [Google Scholar]
- Bek, T.W.; Anikeenko, A.P. Chimičeskij sostaw zelenoj massy sil’fii pronzennolistnoj i sidy mnogoletnej. Naucz. Tech. Bjulleten Vsesojuznogo Instituta Rastenievod. 1983, 136, 50–52. [Google Scholar]
- Kowalska, G.; Pankiewicz, U.; Kowalski, R. Evaluation of Chemical Composition of Some Silphium L. Species as Alternative Raw Materials. Agriculture 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Dudkin, M.S.; Černo, N.K.; Škantova, N.G. Charakteristika vodorastvorimych polisacharidov listiev Silphium perfoliatum. Khimiya Prir. Soedin. 1979, 6, 771–774. [Google Scholar]
- Shang, H.-M.; Zhou, H.-Z.; Li, R.; Duan, M.-Y.; Wu, H.-X.; Lou, Y.-J. Extraction optimization and influences of drying methods on antioxidant activities of polysaccharide from cup plant (Silphium perfoliatum L.). PLoS ONE 2017, 12, e0183001. [Google Scholar]
- Witaszek, K.; Herkowiak, M.; Pilarska, A.A.; Czekała, W. Methods of Handling the Cup Plant (Silphium perfoliatum L.) for Energy Production. Energies 2022, 15, 1897. [Google Scholar] [CrossRef]
- Kowalski, R.; Wierciński, J. Ocena niektórych gatunków Silphium jako surowców inulinowych. Ann. Univ. Mariae Curie-Sklodowska Sect. E 2004, 59, 189–195. [Google Scholar]
- Utejš, J.A. Introdukcija i vprovadžennja v kul’turu sil’fii pronizanolistoy. Vestn. AN USSR 1975, 7, 61–65. [Google Scholar]
- Vavilov, P.P.; Edelšteyn, M.M.; Soloveva, I.V. Frakcjonnyj sostav bełka zelenoj massy novych silosnych kultur. Izv. TSCHA 1973, 5, 84–93. [Google Scholar]
- Glyaubertene, V.F. Biologičeskaja i biochimičeskaja charakteristika perspektivnych silosnych rasteniy. (18. Soderžanie i dinamika nakoplinija azotistych veščestv nadzemnoy časti gorca vejricha, okopnika šeršavogo, sil’fii pronzennolistnoy i maral’ego kornja v pieriod vegeta. Liet. TSR Moksl. Akad. Darbai 1986, 2, 101–108. [Google Scholar]
- Nedvaras, A.P.; Marčyuiyonis, V.I. Biologičeskaja i biochimičeskaja charakteristika perspektivnych silosnych rastenij. (20. Rost i razvitie, urožajnost, chimičeskij sostav nadzemnoj časti sil’fii pronzennolistnoy w usloviyach Litovskoy SSR). Liet. TSR Moksl. Akad. Darbai 1987, 3, 37–45. [Google Scholar]
- Lee, M.A. A global comparison of the nutritive values of forage plants grown in contrasting environments. J. Plant Res. 2018, 131, 641–654. [Google Scholar] [CrossRef]
- Glyaubertene, V.F.; Marčyulyonis, V.I. Biologičeskaja i biochimičeskaja charakteristika perspektiwnych silosnych rastenij. (17. Belkowye frakcii nadzemnoj časti gorca Wejricha, okopnika šeršawogo, sil’fii pronzennolistnoj i maral’ego kornja i ich izmenenie wo wremja wegetacii na VI godu wyrašč. Liet. TSR Moksl. Akad. Darbai 1985, 3, 103–109. [Google Scholar]
- Glyaubertene, V.F.; Marčyulyonis, V.I. Biologičeskaja i biochimičeskaja charakteristika perspektivnych silosnych rastenij. (11. Belkovye frakcii nadzemnoj časti gorca vejricha, okopnika šeršawogo, sil’fii pronzennolistnoy i maral’ego kornja i ich izmenenie vo vremja vegetacii). Liet. TSR Moksl. Akad. Darbai 1980, 3, 129–139. [Google Scholar]
- Glyaubertene, V.F.; Marčyulyonis, V.I. Biologičeskaja i biochimičeskaja charakteristika perspektivnych silosnych rastenij. (12. Elektroforez legkorastvorimych belkov nadzemnoj časti gorca vejricha, okopnika šeršavogo, sil’fii pronzennolistnoy i maral’ego kornya v poliakrilamidnom gele na IV go. Liet. TSR Moksl. Akad. Darbai 1981, 1, 115–121. [Google Scholar]
- Glyaubertene, V.F.; Marčyulyonis, V.I. Biologičeskaja i biochimičeskaja charakteristika perspektivnych silosnych rastenij. (16. Elektroforez v poliakrilamidnom gele legkorastvorimych belkov nadzemnoy časti sil’fii pronzennolistnoy i maral’ego kornja na VI godu vyraščivanija). Liet. TSR Moksl. Akad. Darbai 1984, 1, 68–75. [Google Scholar]
- Glyaubertene, V.F.; Ivanovskaya, K.M. Biologičeskaja i biochimičeskaja charakteristika perspektivnych silosnych rasteniy. (21. Soderžanie aminokislot w zelenoj masse gorca vejricha, okopnika šeršawogo, sil’fii pronzennolistnoy i maral’ego kornya w processe ontogeneza). Liet. TSR Moksl. Akad. Darbai 1987, 4, 102–109. [Google Scholar]
- Reinert, S.; Van Tassel, D.L.; Schlautman, B.; Kane, N.C.; Hulke, B.S. Assessment of the biogeographical variation of seed size and seed oil traits in wild Silphium integrifolium Michx. genotypes. Plant Genet. Resour. Charact. Util. 2019, 17, 427–436. [Google Scholar] [CrossRef]
- Edelšteyn, M.M.; Soloveva, I.V. Sodierżanije karotina i askorbinovoj kisloty v silosnych kulturach. Dokl. TSCHA 1974, 204, 51–54. [Google Scholar]
- Glyaubertene, V.F. Biologičeskaja i biochimičeskaja charakteristika perspektivnych silosnych rastenij. (13. Soderžanie karotina i askorbinovoy kisloty w nadzemnoj časti gorca vejricha, okopnika šeršawogo, sil’fii pronzennolistnoy i maral’ego kornja). Liet. TSR Moksl. Akad. Darbai 1982, 1, 81–87. [Google Scholar]
- Wever, C.; Höller, M.; Becker, L.; Biertümpfel, A.; Köhler, J.; van Inghelandt, D.; Westhoff, P.; Pude, R.; Pestsova, E. Towards high-biomass yielding bioenergy crop Silphium perfoliatum L.: Phenotypic and genotypic evaluation of five cultivated populations. Biomass and Bioenergy 2019, 124, 102–113. [Google Scholar] [CrossRef]
- Achakzai, A.K.K.; Masood, A.; Tareen, R.B. Mujeeb-Ur-Rahman Nutrients composition of common plant species of asteraceae in Quetta at two growth stages. J. Chem. Soc. Pakistan 2018, 40, 722–732. [Google Scholar]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Andrade, M.; das Graças Cardoso, M.; de Andrade, J.; Silva, L.; Teixeira, M.; Valério Resende, J.; da Silva Figueiredo, A.; Barroso, J. Chemical Composition and Antioxidant Activity of Essential Oils from Cinnamodendron dinisii Schwacke and Siparuna guianensis Aublet. Antioxidants 2013, 2, 384–397. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Olagaray, K.E.; Bradford, B.J. Plant flavonoids to improve productivity of ruminants—A review. Anim. Feed Sci. Technol. 2019, 251, 21–36. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Şentürk, M.; Gülçin, İ.; Beydemir, Ş.; Küfrevioğlu, Ö.İ.; Supuran, C.T. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem. Biol. Drug Des. 2011, 77, 494–499. [Google Scholar] [CrossRef]
- Ostolski, M.; Adamczak, M.; Brzozowski, B.; Stolarski, M.J. Screening of Functional Compounds in Supercritical Carbon Dioxide Extracts from Perennial Herbaceous Crops. Agriculture 2021, 11, 488. [Google Scholar] [CrossRef]
- Wojcińska, M.; Drost-Karbowska, K. Phenolic Acids in Silphium perfoliatum L. flowers (Asteraceae/Compositae). Acta Pol. Pharm. Drug Res. 1998, 55, 413–416. [Google Scholar]
- Kowalski, R. Secondary metabolites in Silphium integrifolium in the first 2 years of cultivation. N. Z. J. Crop Hortic. Sci. 2004, 32, 397–406. [Google Scholar] [CrossRef]
- Kowalski, R.; Wolski, T. Evaluation of phenolic acid content in Silphium perfoliatum L. leaves, inflorescences and rhizomes. Electron. J. Polish Agric. Univ. 2003, 6. Available online: http://www.ejpau.media.pl/volume6/issue1/horticulture/art-03.html (accessed on 25 February 2022).
- Kowalski, R.; Wierciński, J. Phenolic acids in leaves of three Silphium L. species. Polish J. Food Nutr. Sci. 2003, 53, 17–20. [Google Scholar]
- Kowalski, R.; Wolski, T. TLC and HPLC analysis of the phenolic acids in Silphium perfoliatum L. leaves, inflorescences and rhizomes. J. Planar Chromatogr. Mod. TLC 2003, 16, 230–236. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Clifford, M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Tomas-Barberan, F.A.; Clifford, M.N. Dietary hydroxybenzoic acid derivatives—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1024–1032. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B. Coffee consumption and risk of type 2 diabetes. JAMA 2005, 294, 97. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Wojcińska, M.; Calabria, L.M.; Linse, K.; Clevinger, J.A.; Mabry, T.J. The Flavonoids and Phenolic Acids of the Genus Silphium and Their Chemosystematic Value. Nat. Prod. Commun. 2009, 4, 1934578X0900400. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.D. The Flavonoids and Phenolic Acids of the Genus Silphium and Their Chemosystematic and Medicinal Value; University of Texas at Austin: Austin, TX, USA, 2006. [Google Scholar]
- Zaidi, M.A.; Crow, S.A. Biologically active traditional medicinal herbs from Balochistan, Pakistan. J. Ethnopharmacol. 2005, 96, 331–334. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Ding, H.; Zhang, G.; Hu, X.; Gong, D. Relationships of dietary flavonoid structure with its tyrosinase inhibitory activity and affinity. LWT 2019, 107, 25–34. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Res. Int. 2018, 111, 148–152. [Google Scholar] [CrossRef]
- Ekinci, D.; Karagoz, L.; Ekinci, D.; Senturk, M.; Supuran, C.T. Carbonic anhydrase inhibitors: In vitro inhibition of α isoforms (hCA I, hCA II, bCA III, hCA IV) by flavonoids. J. Enzyme Inhib. Med. Chem. 2013, 28, 283–288. [Google Scholar] [CrossRef]
- El-Sayed, N.H.; Wojcińska, M.; Drost-Karbowska, K.; Matławska, I.; Williams, J.; Mabry, T.J. Kaempferol triosides from Silphium perfoliatum. Phytochemistry 2002, 60, 835–838. [Google Scholar] [CrossRef]
- Feng, W.-S.; Pei, Y.-Y.; Zheng, X.-K.; Li, C.-G.; Ke, Y.-Y.; Lv, Y.-Y.; Zhang, Y.-L. A new kaempferol trioside from Silphium perfoliatum. J. Asian Nat. Prod. Res. 2014, 16, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R. Antimicrobial activity of essential oils and extracts of rosinweed (Silphium trifoliatum and Silphium integrifolium) plants used by the American Indians. Flavour Fragr. J. 2008, 23, 426–433. [Google Scholar] [CrossRef]
- Scur, M.C.; Pinto, F.G.S.; Pandini, J.A.; Costa, W.F.; Leite, C.W.; Temponi, L.G. Antimicrobial and antioxidant activity of essential oil and different plant extracts of Psidium cattleianum Sabine. Brazilian J. Biol. 2016, 76, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R.; Kowalska, G.; Jamroz, J.; Nawrocka, A.; Metyk, D. Effect of the ultrasound-assisted preliminary maceration on the efficiency of the essential oil distillation from selected herbal raw materials. Ultrason. Sonochem. 2015, 24, 214–220. [Google Scholar] [CrossRef]
- Palmieri, S.; Maggio, F.; Pellegrini, M.; Ricci, A.; Serio, A.; Paparella, A.; Lo Sterzo, C. Effect of the Distillation Time on the Chemical Composition, Antioxidant Potential and Antimicrobial Activity of Essential Oils from Different Cannabis sativa L. Cultivars. Molecules 2021, 26, 4770. [Google Scholar] [CrossRef]
- Kowalski, R.; Gagoś, M.; Kowalska, G.; Pankiewicz, U.; Sujka, M.; Mazurek, A.; Nawrocka, A. Effects of ultrasound technique on the composition of different essential oils. J. Anal. Methods Chem. 2019, 2019, 6782495. [Google Scholar] [CrossRef]
- Berka-Zougali, B.; Ferhat, M.-A.; Hassani, A.; Chemat, F.; Allaf, K.S. Comparative Study of Essential Oils Extracted from Algerian Myrtus communis L. Leaves Using Microwaves and Hydrodistillation. Int. J. Mol. Sci. 2012, 13, 4673–4695. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Riad, N.; Zahi, M.R.; Bouzidi, N.; Daghbouche, Y.; Touafek, O.; El Hattab, M. Occurrence of Marine Ingredients in Fragrance: Update on the State of Knowledge. Chemistry 2021, 3, 1437–1463. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.K.H.; Lau, B.W.M.; Ngai, S.P.C.; Tsang, H.W.H. Therapeutic Effect and Mechanisms of Essential Oils in Mood Disorders: Interaction between the Nervous and Respiratory Systems. Int. J. Mol. Sci. 2021, 22, 4844. [Google Scholar] [CrossRef] [PubMed]
- Wolski, T.; Kowalski, R.; Mardarowicz, M. Chromatographic analysis of essential oil occuring in inflorescences, leaves and rhizomes of Silphium perfoliatum L. Herba Pol. 2000, 46, 235–242. [Google Scholar]
- Kowalski, R.; Wierciński, J.; Mardarowicz, M. Essential oil in leaves and inflorescences of Silphium integrifolium michx. J. Essent. Oil Res. 2005, 17, 220–222. [Google Scholar] [CrossRef]
- Kowalski, R.; Wolski, T. The chemical composition of essential oils of Silphium perfoliatum L. Flavour Fragr. J. 2005, 20, 306–310. [Google Scholar] [CrossRef]
- Kowalski, R. Chemical composition of essential oils and lipophilic extracts of Silphium integrifolium and S. trifoliatum inflorescences. Chem. Nat. Compd. 2008, 44, 241–244. [Google Scholar] [CrossRef]
- Lawson, S.K.; Sharp, L.G.; Powers, C.N.; McFeeters, R.L.; Satyal, P.; Setzer, W.N. Volatile Compositions and Antifungal Activities of Native American Medicinal Plants: Focus on the Asteraceae. Plants 2020, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R.; Wawrzykowski, J. Essential oils analysis in dried materials and granulates obtained from Thymus vulgaris L., Salvia officinalis L., Mentha piperita L. and Chamomilla recutita L. Flavour Fragr. J. 2009, 24, 31–35. [Google Scholar] [CrossRef]
- Tomaino, A.; Cimino, F.; Zimbalatti, V.; Venuti, V.; Sulfaro, V.; De Pasquale, A.; Saija, A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005, 89, 549–554. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Filly, A.; Fabiano-Tixier, A.S.; Louis, C.; Fernandez, X.; Chemat, F. Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. C. R. Chim. 2016, 19, 707–717. [Google Scholar] [CrossRef]
- El Asbahani, A.; Jilale, A.; Voisin, S.N.; Aït Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.J.; Renaud, F.N.R. Chemical composition and antimicrobial activity of nine essential oils obtained by steam distillation of plants from the Souss-Massa Region (Morocco). J. Essent. Oil Res. 2015, 27, 34–44. [Google Scholar] [CrossRef]
- Hristova, Y.; Wanner, J.; Jirovetz, L.; Stappen, I.; Iliev, I.; Gochev, V. Chemical composition and antifungal activity of essential oil of Hyssopus officinalis L. from Bulgaria against clinical isolates of Candida species. Biotechnol. Biotechnol. Equip. 2015, 29, 592–601. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R. Analysis of lipophilic fraction from leaves, inflorescences and rhizomes of Silphium perfoliatum L. Acta Soc. Bot. Pol. 2005, 74, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R. Silphium L. extracts–composition and protective effect on fatty acids content in sunflower oil subjected to heating and storage. Food Chem. 2009, 112, 820–830. [Google Scholar] [CrossRef]
- Bohlmann, F.; Jakupovic, J. Neue labdan-derivate und sesquiterpene aus Silphium-arten. Phytochemistry 1979, 18, 1987–1992. [Google Scholar] [CrossRef]
- Bohlmann, F.; Jakupovic, J. Neue Sesquiterpen-Kohlenwasserstoffe mit anomalen Kohlenstoffgerüst aus Silphium-arten. Phytochemistry 1980, 19, 259–265. [Google Scholar] [CrossRef]
- Pcolinski, M.J.; Doskotch, R.W.; Lee, A.Y.; Clardy, J. Chlorosilphanol A and Silphanepoxol, Labdane Diterpenes from Silphium, perfoliatum. J. Nat. Prod. 1994, 57, 776–783. [Google Scholar] [CrossRef]
- Davidyants, E.S.; Putieva, Ż.M.; Bandyukova, W.A.; Abubakirov, N.K. Triterpenovye glikozidy Silphium perfoliatum. Khimiya Prir. Soedin. 1984, 1, 120–121. [Google Scholar]
- Davidyants, E.S.; Putieva, Ż.M.; Bandyukova, W.A.; Abubakirov, N.K. Triterpenovye glikozidy Silphium perfoliatum II. Khimiya Prir. Soedin. 1984, 5, 666–667. [Google Scholar]
- Davidyants, E.S.; Putieva, Ż.M.; Bandyukova, W.A.; Abubakirov, N.K. Triterpenovye glikozidy Silphium perfoliatum III. Strojenie sil’fiozida E. Khimiya Prir. Soedin. 1984, 6, 750–753. [Google Scholar]
- Davidyants, E.S.; Putieva, Ż.M.; Šaškov, A.S.; Bandyukova, W.A.; Abubakirov, N.K. Triterpenovye glikozidy Silphium perfoliatum IV. Chim. Prirod Soed 1985, 4, 519–522. [Google Scholar]
- Davidyants, E.S.; Putieva, Ż.M.; Bandyukova, W.A.; Abubakirov, N.K. Triterpenovye glikozidy Silphium perfoliatum V. Stroenie sil’fiozida A. Khimiya Prir. Soedin. 1986, 1, 63–66. [Google Scholar]
- Rogowska, A.; Szakiel, A. Enhancement of Phytosterol and Triterpenoid Production in Plant Hairy Root Cultures—Simultaneous Stimulation or Competition? Plants 2021, 10, 2028. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R. Ocena zawartości oleanozydów w organach nadziemnych i podziemnych rożnika przerośniętego Silphium perfoliatum L. Acta Sci. Pol. Hortorum Cultus 2002, 1, 5–15. [Google Scholar]
- Kowalski, R. Studies of selected plant raw materials as alternative sources of triterpenes of oleanolic and ursolic acid types. J. Agric. Food Chem. 2007, 55, 656–662. [Google Scholar] [CrossRef]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [Green Version]
- Khakimov, B.; Tseng, L.; Godejohann, M.; Bak, S.; Engelsen, S. Screening for Triterpenoid Saponins in Plants Using Hyphenated Analytical Platforms. Molecules 2016, 21, 1614. [Google Scholar] [CrossRef] [Green Version]
- Calabria, L.M. The Isolation and Characterization of Triterpene Saponins from Silphium and the Chemosystematic and Biological Significance of Saponins in the Asteraceae; University of Texas at Austin: Austin, TX, USA, 2008. [Google Scholar]
- Han, L.-K.; Zheng, Y.-N.; Yoshikawa, M.; Okuda, H.; Kimura, Y. Anti-obesity effects of chikusetsusaponins isolated from Panax japonicus rhizomes. BMC Complement. Altern. Med. 2005, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Densmore, F. Uses of Plants by the Chippewa Indians; U.S. G.P.O.: Washington, DC, USA, 1928; Volume 44. [Google Scholar]
- Smith, H.H. Etnobotany of the Ojibwe Indians. Bull. Public Museum Milwaukee 1932, 4, 365. [Google Scholar]
- Herrick, J.W. Iroquois Medical Botany; Syracuse University Press: Syracuse, NY, USA, 1977. [Google Scholar]
- Gilmore, M.R. A study in the ethnobotany of the Omaha Indians. Nebraska State Hist. Soc. Collect. 1913, 17, 314–357. [Google Scholar]
- Gilmore, M.R. Uses of Plants by the Indians of the Missouri River Region; Bison Books: Lincoln, NB, USA, 1919; Volume 47. [Google Scholar]
- Grieve, M. A Modern Herbal. Electric Newt.. 1995. Available online: http://www.botanical.com/botanical/mgmh/c/cuppl129.html (accessed on 25 February 2022).
- Kuyanceva, A.M.; Davidyants, E.S. Regenerirujuščaja aktivnost’ ekstrakta Silphium perfoliatum. Farm. Moskwa 1988, 6, 36–37. [Google Scholar]
- Syrov, W.N.; Khushabaktova, Z.A.; Davidyants, E.S. Triterpenowye glikozidy Silphium perfoliatum L. Gipolipidemičeskaja aktiwnost’ sil’fiozida. Khimiko-Farmatsevticheskii Zhurnal 1992, 26, 66–69. [Google Scholar]
- Davidyants, E.S. Triterpene Glycosides of Silphium perfoliatum L: Structure, Biological Activity, Possibility of Use; Stavropol State Agrarian University: Stavropol, Russia, 2017. [Google Scholar]
- Kindscher, K.; Manfredi, K.P.; Britton, M.; Demidova, M.; Phurlburt, D.P. Testing prairie plants with ethnobotanical importance for anti-cancer and anti-aids compounds. J. Ethnobiol. 1998, 18, 229–245. [Google Scholar]
- Calabria, L.M.; Piacente, S.; Kapusta, I.; Dharmawardhane, S.F.; Segarra, F.M.; Pessiki, P.J.; Mabry, T.J. Triterpene saponins from Silphium radula. Phytochemistry 2008, 69, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Blay, G.; García, B.; Molina, E.; Pedro, J.R. Syntheses of (+)-Alismoxide and (+)-4- e pi -Alismoxide. J. Org. Chem. 2006, 71, 7866–7869. [Google Scholar] [CrossRef]
- Davidyants, E.S.; Kartaševa, I.A.; Nešin, I.W. Vliyanie triterpenovych glikozidov Silphium perfoliatum L. na fitopatogennye griby. Rastit. Resur. 1997, 4, 93–98. [Google Scholar]
- Zabka, M.; Pavela, R.; Gabrielova-Slezakova, L. Promising antifungal effect of some Euro-Asiatic plants against dangerous pathogenic and toxinogenic fungi. J. Sci. Food Agric. 2011, 91, 492–497. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Kowalski, R. In vitro estimate of influence of Silphium perfoliatum L. leaves extract on some fungi colonizing the pepper plants. Acta Sci. Pol. Hortorum Cultus 2012, 11, 43–55. [Google Scholar]
- Davidyants, E.S. The effect of purified amount of triterpene glycosides and their enriched extract from leaves of Silphium perfoliatum L. on the germination of seeds of winter wheat and the activity of catalase in them. Chem. Plant Raw Mater. 2021, 2, 353–360. [Google Scholar] [CrossRef]
- Belyak, W.B. Mnogoletniye silosnye kultury dlya kormoproizvodstwa. Wiestnik Sielkochoziaisttwiennych Nauk 1981, 5, 66–70. [Google Scholar]
- Han, K.J.; Albrecht, K.A.; Muck, R.E.; Kim, D.A. Moisture effect on fermentation characteristics of cup-plant silage. Asian-Australasian J. Anim. Sci. 2000, 13, 636–640. [Google Scholar] [CrossRef]
- Varlamova K., A. Obogaščene kul’turnoj flory novymi vidami kormovych rastieniy. Vestn. Sielkochoziaisttviennych Nauk 1984, 4, 87–96. [Google Scholar]
- Mamedov, T.G.; Golubev, N.I. Vyraščivat’ na silos vygodno. Kormoproizvodstwo 1987, 7, 45–46. [Google Scholar]
- Piłat, P.; Majtkowski, W.; Majtkowska, G.; Mikołajczak, J.; Góralska, A. The usefulness for ensiling of chosen plant forms of species of Silphium genus. J. Cent. Eur. Agric. 2007, 8, 363–368. [Google Scholar]
- Lehmkuhler, J.W.; Ramos, M.H.; Albrecht, K.A. Cupplant Silage as a Replacement for Corn Silage in Growing Beef Cattle Diets. Forage Grazinglands 2007, 5, 1–6. [Google Scholar] [CrossRef]
- Bernas, J.; Bernasová, T.; Gerstberger, P.; Moudrý, J.; Konvalina, P.; Moudrý, J. Cup plant, an alternative to conventional silage from a LCA perspective. Int. J. Life Cycle Assess. 2021, 26, 311–326. [Google Scholar] [CrossRef]
- Han, K.-J.; Albrecht, K.A. Substitution Value of Cup Plant (Silphium perfoliatum L.) Silage in Dairy Cow Diet. J. Agric. Sci. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Weymar, H. Buch der Korbblüter; Neumann Verlag: Leipzig, Germany, 1969. [Google Scholar]
- Gardiner, M.A.; Tuell, J.K.; Isaacs, R.; Gibbs, J.; Ascher, J.S.; Landis, D.A. Implications of three biofuel crops for beneficial arthropods in agricultural landscapes. BioEnergy Res. 2010, 3, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Werling, B.P.; Dickson, T.L.; Isaacs, R.; Gaines, H.; Gratton, C.; Gross, K.L.; Liere, H.; Malmstrom, C.M.; Meehan, T.D.; Ruan, L.; et al. Perennial grasslands enhance biodiversity and multiple ecosystem services in bioenergy landscapes. Proc. Natl. Acad. Sci. USA 2014, 111, 1652–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabłoński, B.; Kołtowski, Z. Nectar secretion and honey potential of honey-plants growing under Poland’s conditions—Part XV. J. Apic. Sci. 2005, 49, 59–63. [Google Scholar]
- Mueller, A.L.; Berger, C.A.; Schittenhelm, S.; Stever-Schoo, B.; Dauber, J. Water availability affects nectar sugar production and insect visitation of the cup plant Silphium perfoliatum L. (Asteraceae). J. Agron. Crop Sci. 2020, 206, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Radu Sumalan, L.; Muntean, C.; Kostov, A.; Kržanović, D.; Noemi Jucsor, L.; Sorin Ciulca, I.; Renata Sumalan, M.; Gheju, M.; Cernicova-Buca, M. The cup plant (Silphium perfoliatum L.)—A viable solution for bioremediating soils polluted with heavy metals. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2095–2113. [Google Scholar] [CrossRef]
- Klimont, K.; Bulińska-Radomska, Z.; Górka, J.; Osińska, A. Badanie przydatności rożnika przerośniętego (Silphium perfoliatum L.) do rekultywacji terenów po otworowej eksploatacji złóż siarki. Probl. Inżynierii Rol. 2015, 23, 61–74. [Google Scholar]
- Antonkiewicz, J.; Jasiewicz, C. Effect of soil contamination with heavy metals on element contents in Silphium perfoliatum L. Chem. Inżynieria Ekol. 2003, 10, 205–210. [Google Scholar]
- Moll, L.; Höller, M.; Hubert, C.; Korte, C.A.C.; Völkering, G.; Wever, C.; Pude, R. Cup Plant (Silphium perfoliatum L.) Biomass as Substitute for Expanded Polystyrene in Bonded Leveling Compounds. Agronomy 2022, 12, 178. [Google Scholar] [CrossRef]
- Höller, M.; Lunze, A.; Wever, C.; Deutschle, A.L.; Stücker, A.; Frase, N.; Pestsova, E.; Spiess, A.C.; Westhoff, P.; Pude, R. Meadow hay, Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L. as potential non-wood raw materials for the pulp and paper industry. Ind. Crops Prod. 2021, 167, 113548. [Google Scholar] [CrossRef]
- Du, J.; Zhang, L.; Ali, A.; Li, R.; Xiao, R.; Guo, D.; Liu, X.; Zhang, Z.; Ren, C.; Zhang, Z. Research on thermal disposal of phytoremediation plant waste: Stability of potentially toxic metals (PTMs) and oxidation resistance of biochars. Process Saf. Environ. Prot. 2019, 125, 260–268. [Google Scholar] [CrossRef]
- Conrad, M.; Biertümpfel, A.; Vetter, A. Durchwachsene Silphie (Silphium perfoliatum L.)—Von der Futterpflanze zum Koferment F.N.R. Gülzower Fachgespräche. In Proceedings of the 2nd Symposium Energiepflanzen, Fachagentur Nachwachsende Rohstoffe; Fachagentur Nachwachsende Rohstoffe, Berlin, Germany, 17–18 November 2009; pp. 281–289. [Google Scholar]
- Aurbacher, J.; Bull, I.; Formowitz, B.; Gurgel, A.; Heiermann, M.; Heilmann, H.; Herrmann, C.; Idler, C.; Jänicke, H.; Kornatz, P.; et al. Energiepflanzen für Biogasanlagen. Available online: https://www.fnr.de/fileadmin/Projekte/2021/Mediathek/fnr_brosch.energiepflanzen-mv_web.pdf (accessed on 25 February 2022).
- Aurbacher, J.; Benke, M.; Formowitz, B.; Glauert, T.; Heiermann, M.; Herrmann, C.; Idler, C.; Kornatz, P.; Nehring, A.; Rieckmann, C.; et al. Energiepflanzen für Biogasanlagen. Available online: https://www.fnr.de/fileadmin/Projekte/2021/Mediathek/brosch.energiepflanzen-niedersachsen-webpdf_1.pdf (accessed on 25 February 2022).
- Stolzenburg, K.; Monkos, A. Erste Versuchsergebnisse mit der Durchwachsenen Silphie (Silphium perfoliatum L.) in Baden-Württemberg; Landwirtschaftliches Technologiezentrum Augustenberg: Karlsruhe, Germany, 2012; Available online: https://www.landwirtschaft-bw.info/pb/site/pbs-bw-mlr/get/documents_E545218469/MLR.LEL/PB5Documents/ltz_ka/Arbeitsfelder/Nachwachsende%20Rohstoffe/Kulturinformationen/Silphie_DL/Erste%20Versuchsergebnisse%20mit%20der%20Durchwachsenen%20Silphie%20in%20Baden-W%C3%BCrttemberg%20aktualisiert%202012-08.pdf?attachment=true (accessed on 25 February 2022).
- Slepetys, J.; Kadziuliene, Z.; Sarunaite, L.; Tilvikiene, V.; Kryzeviciene, A. Biomass potential of plants grown for bioenergy production. In Proceedings of the International Scientific Conference Renewable Energy and Energy Efficiency; Rivža, P., Rivža, S., Eds.; Latvia University of Agriculture: Jelgava, Latvia, 2012; pp. 66–72. [Google Scholar]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff-Hönninger, S. Methane yield potential of novel perennial biogas crops influenced by harvest date. Ind. Crops Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Biertümpfel, A.; Köhler, J.; Müller, R. Neues von der Silphie. Biogas J. 2015, 18, 42–43. [Google Scholar]
- Dauber, J.; Müller, A.L.; Schittenhelm, S.; Schoo, B.; Schorpp, Q.; Schrader, S.; Schroetter, S. Schlussbericht zum Vorhaben: Thema: Agrarökologische Bewertung der Durchwachsenen Silphie (Silphium perfoliatum L.) als eine Biomassepflanze der Zukunft; Teilvorhaben 1: Ober- und Unterirdische Biodiversität in Beständen der Durchwachsenen Silphie; Tei. Available online: https://literatur.thuenen.de/digbib_extern/dn056633.pdf (accessed on 25 February 2022).
- Sanderson, M.; Adler, P. Perennial Forages as Second Generation Bioenergy Crops. Int. J. Mol. Sci. 2008, 9, 768–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ende, L.M.; Knöllinger, K.; Keil, M.; Fiedler, A.J.; Lauerer, M. Possibly Invasive New Bioenergy Crop Silphium perfoliatum: Growth and Reproduction Are Promoted in Moist Soil. Agriculture 2021, 11, 24. [Google Scholar] [CrossRef]
- Ţîţei, V. The evaluation of biomass of the Sida hermaphrodita and Silphium perfoliatum for renewable energi in Moldova. Sci. Pap. Ser. A Agron. 2017, 60, 534–540. [Google Scholar]
- Majtkowski, W.; Piłat, J.; Szulc, P. Perspektywy uprawy i wykorzystania w Polsce rożnika przerosnietego Silphium perfoliatum L. Biul. Inst. Hod. i Aklim. Roślin 2009, 251, 283–291. [Google Scholar]
- Jasinskas, A.; Kleiza, V.; Streikus, D.; Domeika, R.; Vaiciukevičius, E.; Gramauskas, G.; Valentin, M.T. Assessment of Quality Indicators of Pressed Biofuel Produced from Coarse Herbaceous Plants and Determination of the Influence of Moisture on the Properties of Pellets. Sustainability 2022, 14, 1068. [Google Scholar] [CrossRef]
- Eurostat. Energy, Transport and Environment Indicators; Publications Office of the European Union: Luxembourg, 2014; ISBN 9789279412561. [Google Scholar]
- Arce, M.; Saavedra, Á.; Míguez, J.; Granada, E.; Cacabelos, A. Biomass fuel and combustion conditions selection in a fixed bed combustor. Energies 2013, 6, 5973–5989. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.A.A.; Salmiaton, A.; Wan Azlina, W.A.K.G.; Mohammad Amran, M.S.; Fakhru’l-Razi, A.; Taufiq-Yap, Y.H. Hydrogen rich gas from oil palm biomass as a potential source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 1258–1270. [Google Scholar] [CrossRef]
- Brendieck-Worm, C. Kurskorrektur in der Biogasbranche möglich? Zeitschrift für Ganzheitliche Tiermedizin 2017, 31, 50–53. [Google Scholar] [CrossRef]
- Grunwald, D.; Panten, K.; Schwarz, A.; Bischoff, W.; Schittenhelm, S. Comparison of maize, permanent cup plant and a perennial grass mixture with regard to soil and water protection. GCB Bioenergy 2020, 12, 694–705. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).