Heterogeneous Catalyzed Biodiesel Production Using Cosolvent: A Mini Review
Abstract
1. Introduction
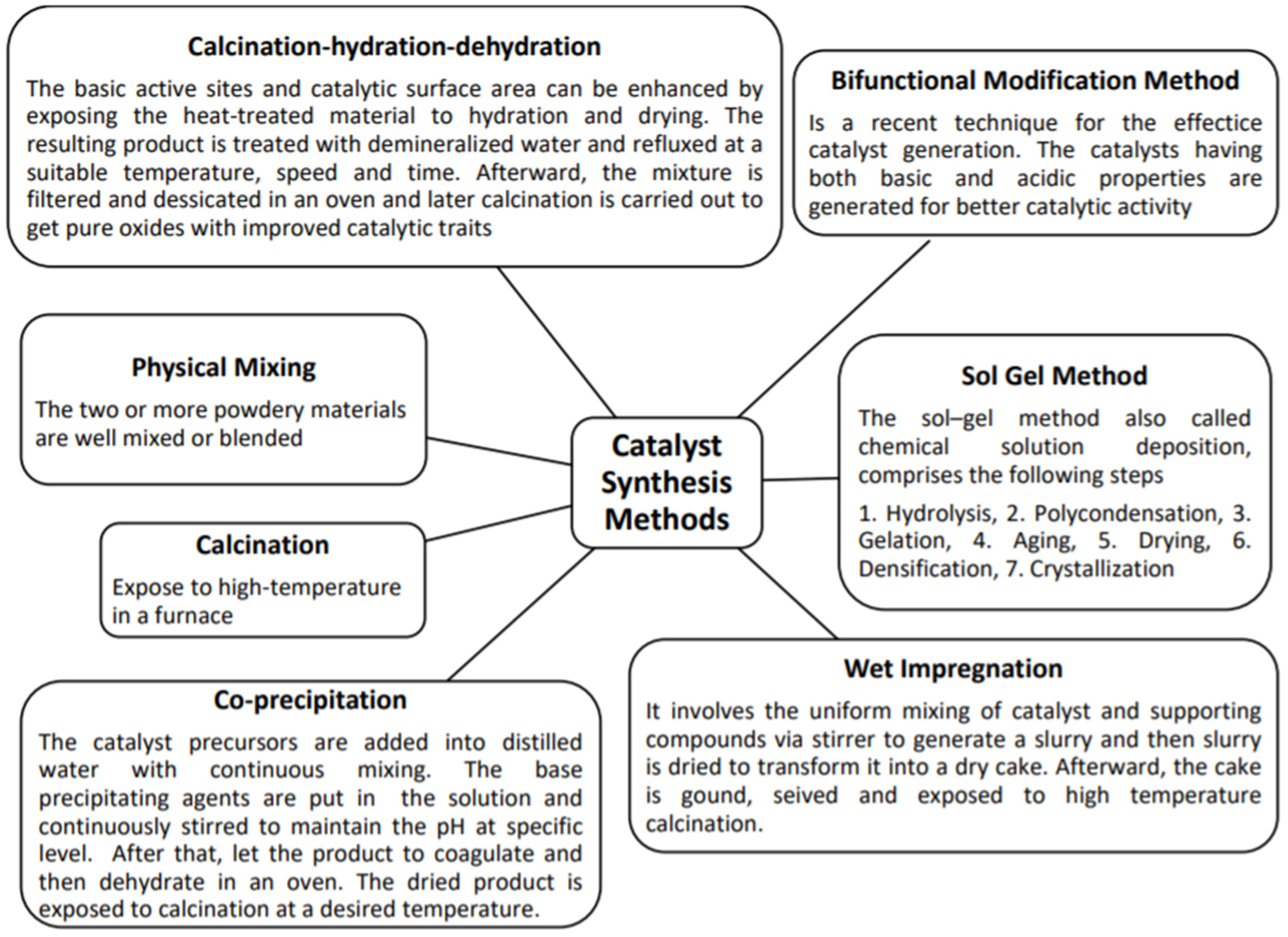
2. Techniques for Heterogeneous Catalyst Synthesis
3. Heterogeneous Catalyzed Biodiesel Generation Using Cosolvents
| Catalyst | Catalyst Preparation | Feedstock | Cosolvent | Reaction Parameters (MeOH/O, Catalyst wt. %, Time, Temperature, Cosolvent) | Yield | References |
|---|---|---|---|---|---|---|
| CaO as heterogeneous catalyst | The catalyst was synthesized from waste snail shells via calcination for the specific time and temperature. | Soybean oil | Acetone | 6, 3, 120, 28, 20 wt. % of oil | Y = 98 | [31] |
| River snail shells-derived CaO | The biomaterial was scrubbed with water and dried followed by grinding, sieving and calcination at various temperatures (600–1000 °C) for 3 h. | Palm oil | Tetrahydrofuran (THF) | 12, 5, 90, 65, 10% v/v (THF in methanol) | Y = 98.5 ± 1.5 | [67] |
| Scalable imidazolium salts-based solid acid | 1,3-disulfonic acid imidazolium chloride and anhydrous ferric chloride were used to prepare the 1,3-disulfonic acid imidazolium tetrachloroferrate. | Firmiana platanifolia oil | Biomass-derived tetrahydrofuran (THF) | 15, 5, 480, 120, 48.8 mmol THF | Y = 97 | [68] |
| Calcium aluminium oxide catalyst | Solid state technique was applied for the synthesis of calcium aluminium oxide using CaCO3 and Al2O3 calcined at 900 °C. | Waste vegetable oil | Acetone | 6, 1.2, 25, 55, 20 wt. % of acetone | C = 97.98 | [55] |
| OH-impregnated CaO as heterogeneous catalyst | OH-impregnated CaO was synthesized by wet impregnation method | Refined coconut oil | Tetrahydrofuran (THF) | 6, 5, 10, 60, 0.58 mol% of oil | C = 81.70 | [59] |
| Strontium lanthanum mixed metal oxide | Co-precipitation method was used to prepare strontium lanthanum mixed metal oxide | Schleichera oleosa oil | Di-isopropyl ether (DPE) | 14, 1.5, 40, 60, cosolvent to alcohol molar ratio, 1/1 | C = 96.37 | [56] |
| SO4−2/SnO2–SiO2 (solid acid catalyst) | Sulfated tin oxide carried with silica was synthesized as per method reported by Lam et al. [30] | Waste cooking oil | Biodiesel | 15, 6, 90, 150, biodiesel as cosolvent | Y = 88.2 | [69] |
| Na/NaOH/γ-Al2O3 heterogeneous base catalyst | The successive processing of γ-Al2O3 with sodium hydroxide and sodium at 320 °C under nitrogen was carried out to prepare the Na/NaOH/γ-Al2O3 catalyst accompanied by the technique suggested by Suzukamo et al. [70]. | Soybean oil | n-hexane | 9, 1 g, 120, 60, 5:1 (oil to n-hexane molar ratio) | Y = 94 | [71] |
| Mg–Fe mixed oxides | Hydrotalcite was used to prepare Mg–Fe mixed oxides through calcination for 3 h | Rapeseed oil | Butanol | 24, 1, 240, 120, methanol and butanol at a molar ratio of 1/1 | C = 97.5 | [29] |
| Limescale (kettle limescale deposit) | The catalyst was synthesized from kettle limescale deposit after crushing, grinding and sieving followed by oven drying. Subsequently, the catalyst was calcined at 900 °C. | Waste cooking oil | Acetone | 2.15/5 (v/v), 7.875, 12.5, 60, acetone concentration (pure oil-based) of 13.95 wt. % | C = 97.16 | [72] |
| Calcium oxide (CaO) derived from eggshell | The eggshell material was heat-treated at designated temperature of 800 °C in air for 3 h to generate CaO materials | Palm oil | Acetone | 12, 13, 600, 75 ± 2, 10% v/v of acetone | Y = 97.5 | [73] |
| Mg–Al hydrotalcites | Hydrotalcites were synthesized by the co-precipitation technique | Canola oil. | Isopropyl alcohol | 6, 3, 540, 60, cosolvent content: 10 wt. % | C > 60 | [74] |
| SrO doped SiO2 (SrO/SiO2) | The sol-gel approach was applied to prepare SrO-doped CaO and SrO-doped SiO2 catalysts | Refined olive oil | Hexane | 6, 5, 30, 45, hexane to olive oil (volume basis)/1/2 | Y > 90 | [75] |
| Novel catalyst (β-tricalcium phosphate) | The catalyst was produced by the calcination of ground fish bones at various temperatures, ranging from 600 °C to 900 °C for 4 h | Karanja oil | Tetrahydrofuran | 10, 2.5, 90, 65, (THF): methanol 1:1 | Y = 97 | [58] |
| Calcium-based catalysts | The scallop shells were calcined at 800 °C for 3 h to derive Ca-based catalysts | Mixture of 50 wt. % (soybean oil and non-edible beef tallow) | Acetone | 12, 5, 150, 65, (Vsolvent/V methanol = 0.36 | Y = 85.3 | [27] |
| Modified ZrO2 | WO3–ZrO2 was produced through incipient wetness impregnation of sulfuric acid or ammonium meta tungstate over zirconium oxide (ZrO2), prepared via the precipitation technique | Palm fatty acid distillate (PFAD) | Toluene | 9, 0.5, 120, 80, 10% v/v | Y > 90 | [57] |
| CaO | Calcined at 700 °C for 5 h and stored in oven. | Rapeseed oil | Tetrahydrofuran (THF) | 12, 2.5, 360, 60, THF (30.0 wt. %) | Y > 90 | [76] |
| Calcium methoxide catalyst | The calcined quick lime powder was utilized to prepare calcium methoxide by reacting it with methanol at a specific temperature for 2 h | Waste cooking oil | Tetrahydrofuran (THF) | 11.6, 2.83, 100.14, 65, 8.65% v/v of THF in methanol concentration | C = 99.13 | [77] |
| Sr–Al double oxides | The sol-gel citrate method was applied to prepare Sr–Al mixed oxides | Lard oil | Tetrahydrofuran (THF) | 5.5, 0.9, 45, 50, 5 wt. % THF | Y = 99.7 | [26] |
| Quaternized polysulfone alkali-catalyzed membrane | The solvent evaporation phase inversion method was employed to synthesize a series of alkalized polysulfones APSF membrane. | Soybean oil | n-hexane | 10 g soybean oil/10 g methanol, 25 wt. % of soybean oil mass, 240, 60, 50 wt. % hexane as cosolvent | C = 95.3 | [78] |
| Calcium methoxide | The quick lime was employed to make calcium methoxide catalyst | Palm stearin | Tetrahydrofuran (THF) | 9.39, 2.33, 102, 65, 9.07% v/v based on methanol of THF cosolvent | C = 98.23 | [79] |
| Eggshell-derived catalyst | Calcined at 850 °C for 3 h | Jatropha oil | Acetone | 9, 7, 120, 65, 1:1 (acetone/oil) weight ratio | Y = 93 | [80] |
| TiO2-supported ZnO catalyst | The catalyst was manufactured by the impregnation of titanium support with zinc nitrate accompanied by drying and calcination | WCO | Hexane | 18, 10, 60, 200, hexane to oil mole ratio of 1/1 | C > 90 | [28] |
| Zeolite Y interchanged with CsCl | The zeolites Y411 and Y756 were treated with CsCl using 1 M and 0.5 M solutions. | Waste vegetable oil | Tetrahydrofuran (THF) | 80, 2.5, 270, 65, 10 wt. % concentration of cosolvent | Conversion increase = 9 to 18% | [81] |
| CaO/scoria (a kind of ignition rock) | The wet impregnation technique and calcination were applied to prepare the catalyst | Waste cooking oil | n-hexane | 14.76, 12, 262, 59.7, n-hexane to oil volume ratio = 0.905/1 | C = 97.7 | [32] |
| CaO | Anhydrous calcium oxide (99.99% purity) was procured | Soybean oil | Glymes | 430 μL/700μ L, 0.03g, 240, 60, 300μ L glyme | C = 99 | [82] |
| CaO | The CaO catalyst was procured and activated using methanol | Soybean oil | Iso-propanol | 20, 30 mg, 6.5, 65, 14.5 wt. % | Y = 99 | [83] |
| CaO as heterogeneous catalyst | CaO catalyst was procured and used | Linseed oil | Diethyl ether (DEE) | 9.48, 160 g CaO, 180, 30, DEE/methanol ratio of 1.19:1 (continuous mode) | Y = 98.08 | [84] |
| Carbon based heterogeneous catalyst | The catalyst was synthesized using hydrothermal carbonization, sulfonation and combination of two. | Palm fatty acid distillate (PFAD) | Tetrahydrofuran (THF) | 6, 3, 180, 333K, THF: 0.2% of PFAD feedstock, ultrasonic assisted 120 W | C = 86 | [61] |
| Calcined sodium silicate as heterogeneous catalyst | The sodium silicate was purchased, dehydrated and calcined in furnace | Refined soybean oil | Petrodiesel | 9, 3, 120, -, 10 wt. % of cosolvent, ultrasonic assisted 20 KHz | Y = 97 | [62] |
4. Optimization of Reaction Parameters for Heterogeneously Catalyzed Biodiesel Generation Using Cosolvents
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Sharma, B.; Larroche, C.; Dussap, C.-G. Comprehensive assessment of 2G bioethanol production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Masjuki, H.H.; Kalam, M.A.; Ong, H.C.; Gul, M.; Farooq, M.; Soudagar, M.E.M.; Ahmed, W.; Harith, M.H.; Yusoff, M.N.A.M. Ultrasound-assisted process optimization and tribological characteristics of biodiesel from palm-sesame oil via response surface methodology and extreme learning machine-Cuckoo search. Renew. Energy 2020, 158, 202–214. [Google Scholar] [CrossRef]
- Krawczyk, T. Biodiesel-alternative fuel makes inroads but hurdles remain. Inform 1996, 7, 801–815. [Google Scholar]
- Yusuf, A.A.; Dandakouta, H.; Yahuza, I.; Yusuf, D.A.; Mujtaba, M.A.; El-Shafay, A.S.; Soudagar, M.E.M. Effect of low CeO2 nanoparticles dosage in biodiesel-blends on combustion parameters and toxic pollutants from common-rail diesel engine. Atmos. Pollut. Res. 2022, 13, 101305. [Google Scholar] [CrossRef]
- Chuah, L.F.; Bokhari, A.; Yusup, S.; Klemeš, J.J.; Akbar, M.M.; Saminathan, S. Optimisation on pretreatment of kapok seed (Ceiba pentandra) oil via esterification reaction in an ultrasonic cavitation reactor. Biomass Convers. Biorefinery 2017, 7, 91–99. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Yasin, S.; Ali, C.H.; Abbas, M.M.; Jamil, M.A.; Hussain, A.; Soudagar, M.E.M.; Rahman, M.M. Application of Agricultural Waste as Heterogeneous Catalysts for Biodiesel Production. Catalysts 2021, 11, 1215. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.M.A.; Soudagar, M.E.M.; Fattah, I.M.R. Current State and Perspectives on Transesterification of Triglycerides for Biodiesel Production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Gad, M.S.; He, Z.; L-Shafay, A.S.E.; L-Seesy, A.I.E. Combustion characteristics of a diesel engine running with Mandarin essential oil-diesel mixtures and propanol additive under different exhaust gas recirculation: Experimental investigation and numerical simulation. Case Stud. Therm. Eng. 2021, 26, 101100. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Mujtaba, M.A.; Soudagar, M.E.M.; Veza, I.; Fattah, I.M.R. Microwave Assisted Biodiesel Production Using Heterogeneous Catalysts. Energies 2021, 14, 8135. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Mandari, V.; Devarai, S.K. Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: A critical review. Bioenergy Res. 2021, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic Applications in the Production of Biodiesel from Vegetable Oils. ChemSusChem 2009, 2, 278–300. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in Enzymatic Biodiesel Production and Commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- Luque, R.; Lovett, J.C.; Datta, B.; Clancy, J.; Campelo, J.M.; Romero, A.A. Biodiesel as feasible petrol fuel replacement: A multidisciplinary overview. Energy Environ. Sci. 2010, 3, 1706–1721. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, M.P. Selection of potential oils for biodiesel production. Renew. Sustain. Energy Rev. 2016, 56, 1129–1138. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous catalysts for biodiesel production. Energy Fuels 2008, 22, 207–217. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Hussain, F.; Alshahrani, S.; Abbas, M.; Khan, H.; Jamil, A.; Yaqoob, H.; Soudagar, M.; Imran, M.; Ahmad, M.; Munir, M. Waste Animal Bones as Catalysts for Biodiesel Production; A Mini Review. Catalysts 2021, 11, 630. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Ali, C.H.; Javaid, A.; Cheema, I.I. Sustainable biodiesel production from waste cooking oil utilizing waste ostrich (Struthio camelus) bones derived heterogeneous catalyst. Fuel 2020, 277, 118091. [Google Scholar] [CrossRef]
- Zanjani, N.G.; Kamran-Pirzaman, A.; Khalajzadeh, M. Synthesis of modified layered double hydroxide of MgAl catalyst with Ba and Li for the biodiesel production. Clean Technol. Environ. Policy 2020, 22, 1173–1185. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Jiang, F.; Xiao, G. In situ synthesis and characterization of Ca–Mg–Al hydrotalcite on ceramic membrane for biodiesel production. Chin. J. Chem. Eng. 2015, 23, 1035–1040. [Google Scholar] [CrossRef]
- Kostić, M.D.; Djalović, I.G.; Stamenković, O.S.; Mitrović, P.M.; Adamović, D.S.; Kulina, M.K.; Veljković, V.B. Kinetic modeling and optimization of biodiesel production from white mustard (Sinapis alba L.) seed oil by quicklime-catalyzed transesterification. Fuel 2018, 223, 125–139. [Google Scholar] [CrossRef]
- Zanjani, N.G.; Pirzaman, A.K.; Yazdanian, E. Biodiesel production in the presence of heterogeneous catalyst of alumina: Study of kinetics and thermodynamics. Int. J. Chem. Kinet. 2020, 52, 472–484. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Iftekhar, S.; Haapaniemi, E.; Sillanpää, M. Effect of different co-solvents on biodiesel production from various low-cost feedstocks using Sr–Al double oxides. Renew. Energy 2020, 146, 2158–2169. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Ramos, M.; Catarino, M.; Puna, J.; Gomes, J. Solvent Assisted Biodiesel Production by Co-processing Beef Tallow and Soybean Oil over Calcium Catalysts. Waste Biomass Valoriz. 2020, 11, 6249–6259. [Google Scholar] [CrossRef]
- Emeji, I.C.; Afolabi, A.S.; Abdulkareem, A.S.; Kalala, J. Characterization and kinetics of biofuel produced from waste cooking oil. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 21–23 October 2015. [Google Scholar]
- Hájek, M.; Vávra, A.; Mück, J. Butanol as a co-solvent for transesterification of rapeseed oil by methanol under homogeneous and heterogeneous catalyst. Fuel 2020, 277, 118239. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Sulfated tin oxide as solid superacid catalyst for transesterification of waste cooking oil: An optimization study. Appl. Catal. B Environ. 2009, 93, 134–139. [Google Scholar] [CrossRef]
- Laskar, I.B.; Deshmukhya, T.; Bhanja, P.; Paul, B.; Gupta, R.; Chatterjee, S. Transesterification of soybean oil at room temperature using biowaste as catalyst; an experimental investigation on the effect of co-solvent on biodiesel yield. Renew. Energy 2020, 162, 98–111. [Google Scholar] [CrossRef]
- Mohadesi, M.; Moradi, G.; Ghanbari, M.; Moradi, M.J. Investigating the effect of n-hexane as solvent on waste cooking oil conversion to biodiesel using CaO on a new support as catalyst. Measurement 2019, 135, 606–612. [Google Scholar] [CrossRef]
- Alhassan, Y.; Kumar, N.; Bugaje, I.; Pali, H.S.; Kathkar, P. Co-solvents transesterification of cotton seed oil into biodiesel: Effects of reaction conditions on quality of fatty acids methyl esters. Energy Convers. Manag. 2014, 84, 640–648. [Google Scholar] [CrossRef]
- Luu, P.D.; Takenaka, N.; Van Luu, B.; Pham, L.N.; Imamura, K.; Maeda, Y. Co-solvent method produce biodiesel form waste cooking oil with small pilot plant. Energy Procedia 2014, 61, 2822–2832. [Google Scholar] [CrossRef][Green Version]
- Luu, P.D.; Truong, H.T.; Van Luu, B.; Pham, L.N.; Imamura, K.; Takenaka, N.; Maeda, Y. Production of biodiesel from Vietnamese Jatropha curcas oil by a co-solvent method. Bioresour. Technol. 2014, 173, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Awogbemi, O.; Von Kallon, D.V.; Aigbodion, V.S. Trends in the development and utilization of agricultural wastes as heterogeneous catalyst for biodiesel production. J. Energy Inst. 2021, 98, 244–258. [Google Scholar] [CrossRef]
- EEtim, A.O.; Musonge, P.; Eloka-Eboka, A.C. Effectiveness of biogenic waste-derived heterogeneous catalysts and feedstock hybridization techniques in biodiesel production. Biofuels Bioprod. Biorefin. 2020, 14, 620–649. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.; Rashid, U.; Saiman, M.I.; Tan, Y.P.; Alsultan, A.; Taufiq-Yap, Y.H. Modified waste egg shell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil. A review. Renew. Sustain. Energy Rev. 2018, 82, 3645–3655. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Wongweang, C.; Khamsrisuk, S. Application of eggshell wastes as a heterogeneous catalyst for biodiesel production. Sustain. Energy 2013, 1, 7–13. [Google Scholar]
- Qu, T.; Niu, S.; Zhang, X.; Han, K.; Lu, C. Preparation of calcium modified Zn-Ce/Al2O3 heterogeneous catalyst for biodiesel production through transesterification of palm oil with methanol optimized by response surface methodology. Fuel 2021, 284, 118986. [Google Scholar] [CrossRef]
- Rezania, S.; Mahdinia, S.; Oryani, B.; Cho, J.; Kwon, E.E.; Bozorgian, A.; Nodeh, H.R.; Darajeh, N.; Mehranzamir, K. Biodiesel production from wild mustard (Sinapis Arvensis) seed oil using a novel heterogeneous catalyst of LaTiO3 nanoparticles. Fuel 2022, 307, 121759. [Google Scholar] [CrossRef]
- Yoosuk, B.; Udomsap, P.; Puttasawat, B.; Krasae, P. Modification of calcite by hydration–dehydration method for heterogeneous biodiesel production process: The effects of water on properties and activity. Chem. Eng. J. 2010, 162, 135–141. [Google Scholar] [CrossRef]
- Laca, A.; Laca, A.; Díaz, M. Eggshell waste as catalyst: A review. J. Environ. Manag. 2017, 197, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Ahmad, Z.; Hameed, B. Highly active alumina-supported Cs–Zr mixed oxide catalysts for low-temperature transesterification of waste cooking oil. Appl. Catal. A Gen. 2014, 487, 16–25. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. ChemInform Abstract: Heterogeneous Catalysis for Sustainable Biodiesel Production via Esterification and Transesterification. ChemInform 2014, 46, 7887–7916. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Xu, Y.; Liu, Q.; Qian, G. CaFeAl mixed oxide derived heterogeneous catalysts for transesterification of soybean oil to biodiesel. Bioresour. Technol. 2015, 190, 438–441. [Google Scholar] [CrossRef]
- Paterson, G.; Issariyakul, T.; Baroi, C.; Bassi, A.; Dalai, A. Ion-exchange resins as catalysts in transesterification of triolein. Catal. Today 2013, 212, 157–163. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Marwaha, A.; Dhir, A.; Mahla, S.K.; Mohapatra, S.K. An overview of solid base heterogeneous catalysts for biodiesel production. Catal. Rev. 2018, 60, 594–628. [Google Scholar] [CrossRef]
- Marwaha, A.; Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Waste materials as potential catalysts for biodiesel production: Current state and future scope. Fuel Process. Technol. 2018, 181, 175–186. [Google Scholar] [CrossRef]
- Tobío-Pérez, I.; Domínguez, Y.D.; Machín, L.R.; Pohl, S.; Lapuerta, M.; Piloto-Rodríguez, R. Biomass-based heterogeneous catalysts for biodiesel production: A comprehensive review. Int. J. Energy Res. 2021, 46, 3782–3809. [Google Scholar] [CrossRef]
- Encinar, J.M.; Pardal, A.; Sánchez, N. An improvement to the transesterification process by the use of co-solvents to produce biodiesel. Fuel 2016, 166, 51–58. [Google Scholar] [CrossRef]
- Maeda, K.; Kuramochi, H.; Asakuma, Y.; Fukui, K.; Tsuji, T.; Osako, M.; Sakai, S.-I. De-emulsification of mixtures containing glycerin and fatty acid methyl ester promoted by dimethyl ether co-solvent. Chem. Eng. J. 2011, 169, 226–230. [Google Scholar] [CrossRef]
- Maeda, Y.; Thanh, L.T.; Imamura, K.; Izutani, K.; Okitsu, K.; Van Boi, L.; Lan, P.N.; Tuan, N.C.; Yoo, Y.E.; Takenaka, N. New technology for the production of biodiesel fuel. Green Chem. 2011, 13, 1124–1128. [Google Scholar] [CrossRef]
- Singh, V.; Yadav, M.; Sharma, Y.C. Effect of co-solvent on biodiesel production using calcium aluminium oxide as a reusable catalyst and waste vegetable oil. Fuel 2017, 203, 360–369. [Google Scholar] [CrossRef]
- Sahani, S.; Banerjee, S.; Sharma, Y.C. Study of ‘co-solvent effect’ on production of biodiesel from Schleichera Oleosa oil using a mixed metal oxide as a potential catalyst. J. Taiwan Inst. Chem. Eng. 2018, 86, 42–56. [Google Scholar] [CrossRef]
- Mongkolbovornkij, P.; Champreda, V.; Sutthisripok, W.; Laosiripojana, N. Esterification of industrial-grade palm fatty acid distillate over modified ZrO2 (with WO3–, SO4–and TiO2–): Effects of co-solvent adding and water removal. Fuel Process. Technol. 2010, 91, 1510–1516. [Google Scholar] [CrossRef]
- Madhu, D.; Sharma, Y.C. Synthesis of a reusable novel catalyst (β-tricalcium phosphate) for biodiesel production from a common Indian tribal feedstock. Resour. Technol. 2017, 3, 144–157. [Google Scholar] [CrossRef]
- Bambase, M.E., Jr.; Almazan, R.A.R.; Demafelis, R.B.; Sobremisana, M.J.; Dizon, L.S.H. Biodiesel production from refined coconut oil using hydroxide-impregnated calcium oxide by cosolvent method. Renew. Energy 2021, 163, 571–578. [Google Scholar] [CrossRef]
- Macawile, M.C.; Auresenia, J. Utilization of Supercritical Carbon Dioxide and Co-solvent n-hexane to Optimize Oil Extraction from Gliricidia sepium Seeds for Biodiesel Production. Appl. Sci. Eng. Prog. 2022, 15. [Google Scholar] [CrossRef]
- Gaikwad, N.D.; Gogate, P.R. Synthesis and application of carbon based heterogeneous catalysts for ultrasound assisted biodiesel production. Green Process. Synth. 2015, 4, 17–30. [Google Scholar] [CrossRef]
- Parida, S. Improving heterogeneously catalyzed transesterification reaction for biodiesel production using ultrasound energy and petro-diesel as cosolvent. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 1–13. [Google Scholar] [CrossRef]
- Mamtani, K.; Shahbaz, K.; Farid, M.M. Deep eutectic solvents—Versatile chemicals in biodiesel production. Fuel 2021, 295, 120604. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Mjalli, F.S.; Hayyan, M.; AlNashef, I.M. A novel phosphonium-based deep eutectic catalyst for biodiesel production from industrial low grade crude palm oil. Chem. Eng. Sci. 2013, 92, 81–88. [Google Scholar] [CrossRef]
- Hayyan, A.; Rashid, S.N.; Hayyan, M.; Zulkifliy, M.Y.; Hashim, M.A.; Osman, N.A. Synthesis of novel eutectic catalyst for the esterification of crude palm oil mixed with sludge palm oil. J. Oil Palm Res. 2017, 29, 373–379. [Google Scholar] [CrossRef]
- Taysun, M.B.; Sert, E.; Atalay, F.S. Physical properties of benzyl tri-methyl ammonium chloride based deep eutectic solvents and employment as catalyst. J. Mol. Liq. 2016, 223, 845–852. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Kaewpuang, T.; Yoosuk, B.; Promarak, V. Economical and green biodiesel production process using river snail shells-derived heterogeneous catalyst and co-solvent method. Bioresour. Technol. 2016, 209, 343–350. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Zhang, H.; Wang, A.; Jin, D.; Yang, S. Effective production of biodiesel from non-edible oil using facile synthesis of imidazolium salts-based Brønsted-Lewis solid acid and co-solvent. Energy Convers. Manag. 2018, 166, 534–544. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Accelerating transesterification reaction with biodiesel as co-solvent: A case study for solid acid sulfated tin oxide catalyst. Fuel 2010, 89, 3866–3870. [Google Scholar] [CrossRef]
- Suzukamo, G.; Fukao, M.; Minobe, M. Preparation of New Solid Superbase and Its Catalytic Activity. Chem. Lett. 1987, 16, 585–588. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kang, B.-S.; Kim, M.-J.; Park, Y.M.; Kim, D.-K.; Lee, J.-S.; Lee, K.-Y. Transesterification of vegetable oil to biodiesel using heterogeneous base catalyst. Catal. Today 2004, 93, 315–320. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Maleki, M.; Ansari, A. Study of the transesterification of waste cooking oil for the production of biodiesel in a microreactor pilot: The effect of acetone as the co-solvent. Fuel 2020, 273, 117736. [Google Scholar] [CrossRef]
- Roschat, W.; Najai, K.; Kaewpuang, T.; Moonsin, P. Biodiesel production via ethanolysis catalyzed by CaO derived from eggshell as low-cost basic heterogeneous catalyst. Reson. (1H NMR) 2017, 6, 10. [Google Scholar]
- İlgen, O.; Dincer, I.; Yildiz, M.; Alptekin, E.; Boz, N.; Canakci, M.; Akin, A.N. Investigation of biodiesel production from canola oil using Mg-Al hydrotalcite catalysts. Turk. J. Chem. 2007, 31, 509–514. [Google Scholar]
- Chen, C.-L.; Huang, C.-C.; Tran, D.-T.; Chang, J.-S. Biodiesel synthesis via heterogeneous catalysis using modified strontium oxides as the catalysts. Bioresour. Technol. 2012, 113, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Bonfanti, L.; Capelli, S.; Manenti, F.; Bianchi, C.L.; Patience, G.S.; Pirola, C. Heterogeneous Oil Transesterification in a Single-Phase Liquid Mixture using a Co-Solvent for Improved Biofuels Production. Energy Technol. 2015, 3, 1170–1173. [Google Scholar] [CrossRef]
- Chumuang, N.; Punsuvon, V. Response Surface Methodology for Biodiesel Production Using Calcium Methoxide Catalyst Assisted with Tetrahydrofuran as Cosolvent. J. Chem. 2017, 2017, 4190818. [Google Scholar] [CrossRef]
- Shi, W.; Li, H.; Zhou, R.; Zhang, H.; Du, Q. Biodiesel production from soybean oil by quaternized polysulfone alkali-catalyzed membrane. Bioresour. Technol. 2016, 210, 43–48. [Google Scholar] [CrossRef]
- Songoen, W.; Punsuvon, V.; Arirop, W.; Timyamprasert, A. Production of Biodiesel from Palm Stearin Using Solid Catalyst Assisted with Co-Solvent. Appl. Mech. Mater. 2018, 876, 9–14. [Google Scholar] [CrossRef]
- Shi, Z.; Jiang, Y.; Zhou, L.; Gao, J. Eggshell-derived catalyst for biodiesel production in the presence of acetone as co-solvent. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 320–325. [Google Scholar] [CrossRef]
- Brito, A.; Borges, M.E.; Arvelo, R.; Garcia, F.; Diaz, M.C.; Otero, N. Reuse of Fried Oil to Obtain Biodiesel: Zeolites Y as a Catalyst. Int. J. Chem. React. Eng. 2007, 5. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, H.; Song, Z.; Olubajo, O. Glymes as benign co-solvents for CaO-catalyzed transesterification of soybean oil to biodiesel. Bioresour. Technol. 2013, 139, 107–112. [Google Scholar] [CrossRef]
- Chueluecha, N.; Kaewchada, A.; Jaree, A. Enhancement of biodiesel synthesis using co-solvent in a packed-microchannel. J. Ind. Eng. Chem. 2017, 51, 162–171. [Google Scholar] [CrossRef]
- Gargari, M.H.; Sadrameli, S. Investigating continuous biodiesel production from linseed oil in the presence of a Co-solvent and a heterogeneous based catalyst in a packed bed reactor. Energy 2018, 148, 888–895. [Google Scholar] [CrossRef]
- Jiang, J.-J.; Tan, C.-S. Biodiesel production from coconut oil in supercritical methanol in the presence of cosolvent. J. Taiwan Inst. Chem. Eng. 2012, 43, 102–107. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, H.M.; Iqbal, T.; Yasin, S.; Irfan, M.; Abbas, M.M.; Veza, I.; Soudagar, M.E.M.; Abdelrahman, A.; Kalam, M.A. Heterogeneous Catalyzed Biodiesel Production Using Cosolvent: A Mini Review. Sustainability 2022, 14, 5062. https://doi.org/10.3390/su14095062
Khan HM, Iqbal T, Yasin S, Irfan M, Abbas MM, Veza I, Soudagar MEM, Abdelrahman A, Kalam MA. Heterogeneous Catalyzed Biodiesel Production Using Cosolvent: A Mini Review. Sustainability. 2022; 14(9):5062. https://doi.org/10.3390/su14095062
Chicago/Turabian StyleKhan, Haris Mahmood, Tanveer Iqbal, Saima Yasin, Muhammad Irfan, Muhammad Mujtaba Abbas, Ibham Veza, Manzoore Elahi M. Soudagar, Anas Abdelrahman, and Md. Abul Kalam. 2022. "Heterogeneous Catalyzed Biodiesel Production Using Cosolvent: A Mini Review" Sustainability 14, no. 9: 5062. https://doi.org/10.3390/su14095062
APA StyleKhan, H. M., Iqbal, T., Yasin, S., Irfan, M., Abbas, M. M., Veza, I., Soudagar, M. E. M., Abdelrahman, A., & Kalam, M. A. (2022). Heterogeneous Catalyzed Biodiesel Production Using Cosolvent: A Mini Review. Sustainability, 14(9), 5062. https://doi.org/10.3390/su14095062










