Abstract
The cultivation of grain legumes (e.g., common bean) in sub-Saharan Africa contributes to the provision of food for a growing population and delivers environmental benefits such as inputs of nitrogen (N) to crops and soil via symbiotic nitrogen fixation (SNF). However, the success of SNF is constrained by several factors such as the poor efficiency of native rhizobial strains to fix N, the low availability of phosphorus (P) and the acidity of soils. Two trials have been conducted in low-fertility tropical soils at the smallholder farm scale in Madagascar to assess the effects of Rhizobium inoculation together with inputs of P and lime on the growth of the common bean. We showed that inoculation with native strains of Rhizobium had significant effects on bean root nodulation, which was increased by up to 15-fold on plant growth, which increased by 78% and on bean yield, which increased by 126%. Moreover, we observed positive and significant relationships between inoculation with Rhizobium and P fertilization on nodulation, plant growth and yield. However, the addition of dolomite lime did not show any effect in our study. The addition of P decreased the mycorrhization rate of roots. Additional research is still needed to improve our understanding of soil fertility conditions (mainly on nutrient availability, including micronutrients) allowing better efficiency of legume symbionts (rhizobium and mycorrhiza) in such low-fertility soils.
1. Introduction
The cultivation of grain legumes such as common bean, faba bean, cowpea, etc., in sub-Saharan Africa (SSA) contributes both to providing food for a growing population and to delivering environmental benefits to preserve natural resources. In most SSA countries, cropping systems and staple foods are based on cereals. Grain legumes are recognized as rich in proteins and in some essential amino acids and minerals lacking in cereals [1]. Therefore, legumes are an excellent complement and have high potential for the improvement of the nutritional quality of foods [2]. The common bean also offers ecosystem benefits such as inputs of nitrogen (N) to crops and soil via symbiotic nitrogen fixation (SNF) and assists with a decrease in climate change as N fertilization is responsible for half of all agricultural greenhouse gas emissions [3,4,5]. Despite these advantages, legume cultivation has declined worldwide in the last 50 years relative to cereals, with the notable exception of soybean production [6]. The low productivity of pulses is a general problem for most farming systems in SSA, leading to a decline in soil fertility and a reduction in N fixation related to biological and environmental factors [7].
The ability of grain legumes to use N2 through symbiotic nitrogen fixation (SNF) is constrained by several environmental factors. Low phosphorus (P) availability is considered to be the principal limiting factor for legume growth as it strongly reduces N fixation [8]. P plays a key role in the energy metabolism of N fixation [9], since this process uses high amounts of adenosine triphosphate. When P availability is limited, as it is often the case in tropical soils, symbioses between crop plants and arbuscular mycorrhizal fungi (AMF) can contribute to plant P nutrition [10,11]. SNF is also strongly limited by soil acidity and aluminum (Al) toxicity. Highly weathered tropical and subtropical soils are generally P deficient and subject to aluminum toxicity [12,13]. From an agronomic perspective, the former problem can be alleviated by P fertilization and the latter by liming [14].
Beside P deficiency and Al toxicity, the lack of efficient native strains of Rhizobium for nitrogen fixation could also limit SNF, since less efficient strains often out-compete the more efficient ones that only occupy a small proportion of nodules [15]. The beneficial effect of inoculation with efficient strains of Rhizobia on legume production has already been mentioned [16,17]. However, the success of efficient strain inoculation on SNF remains driven by the ability of scientists to select inoculants highly efficient for a specific soil within particular environmental conditions and adapted to bean genotypes. Thus, the inoculation of bean seeds with effective and competitive native Rhizobium strains and the supply of appropriate amounts of P and lime could markedly increase SNF and legume growth.
The aim of this study was to assess the effects of inoculation of bean seeds with suitable Rhizobium spp. together with inputs of P and dolomite lime on root nodulation and production of the common bean (Phaseolus vulgaris L.), growing on acid and P-depleted tropical soils. We hypothesized that (i) inoculation with efficient Rhizobium strains, well adapted to common bean, and (ii) improved soil conditions, more specifically a better soil P availability and a lower soil acidity, were needed to improve common bean growth and productivity. Experiments were conducted in two trials in field conditions of resource-poor, smallholder farms in the highlands of Madagascar.
2. Materials and Methods
2.1. Site Location and Description
The study was carried out in Ankazomiriotra, Vakinankaratra region, in the midwest of the Malagasy Highlands. The region has a humid subtropical climate, with a rainy season from October to April. Two sites belonging to different farmers were selected: Site 1 (S: 19°40′36.1″; E: 46°33′32.4″; 1171 m asl) and Site 2 (S: 19°39′40.6″; E: 046°33′24.2″; 1181 m asl). The trials were set up in 2015. The temperature and rainfall were measured daily with an automatic weather station (Cimel Electronique S.A.S, Paris, France) installed at Ivory, also in the midwest of the Malagasy Highlands (19°33′ S, 46°24′ E, 900 m asl) 20 km from the studied sites. The average annual rainfall and temperature (2007–2015) were, respectively, 1326 mm year−1 and 23 °C. The soils of the two sites were classified as Ferralic Cambisol [18].
2.2. Experimental Design
The field experiments were conducted during the second half of the rainy season, from February to May. The experiment design (Figure 1) included three treatments: (i) input of mineral P as triple super phosphate (TSP) with three application rates (0, 50 and 200 kg P2O5 ha−1, respectively, designated as P0, P1 and P2); (ii) input of dolomite lime with two application rates (0 or 500 kg ha−1, respectively, designated as L0 and L1) and (iii) Rhizobium inoculation (absent (I0) or present (I1)). Thus, for each site, we had a 3 × 2 × 2 factorial scheme replicated in 4 blocks, corresponding to 48 plots. Each plot measured 4 m × 2 m and was interspaced by 50 cm.
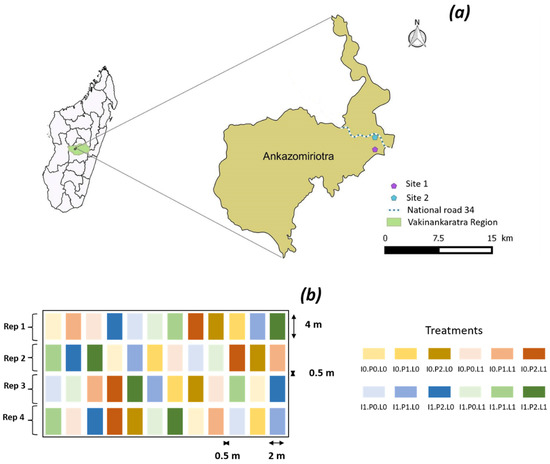
Figure 1.
Localization of the field trials in Ankazomiriotra, Vakinankaratra region, Madagascar (a) and schematic representation of the trial (b).
Common bean (Phaseolus vulgaris L.) was planted in the fields. We used a local cultivar, called “RI-5-2”, resulting from the crossing between two varieties, Ranjonomby and Ikinimba, which has a complete growth cycle of 80 days. Seeds were planted singly at a density of approximately 100,000 plants per ha. The space between rows was 50 cm and the interplant spacing was 20 cm.
Phosphorus and lime were applied after ploughing along the sowing line just before sowing. The seeds were inoculated with an inoculum provided by the Centre National de Recherches sur l’Environnement (CNRE), Laboratoire de Microbiologie de l’Environnement (LME), Antananarivo, Madagascar. It corresponded to a cocktail of ten Rhizobium spp. strains selected from bioassays carried out on a collection of infective isolates for their higher symbiotic effectiveness [19]. The strains are native strains from the highlands of Madagascar, collected in a rice/common bean intercropping trial conducted on a Ferralsol located in Lazaina (18°46′ S; 47°32′ E), near Antananarivo.
2.3. Plant and Soil Sampling
Before the start of the trials, bulk soil sampling was conducted on the 0–10 cm soil layer to characterize some physico-chemical properties and determine the mineralogy of the soils. The soil samples were air dried, sieved at 2 mm for the determination of soil P contents and pH and ground at 0.2 mm for the determination of total carbon and nitrogen contents and soil mineralogical content.
During field trials, samplings were conducted at two sampling dates for each experiment: at the flowering stage of the common bean (45 days after sowing), in which plants and soils were collected, and at the maturity of the plant (80 days after sowing), for the evaluation of the bean yield.
During the first sampling (at flowering), sixteen plants were randomly selected from a subplot in the middle of each plot. The plants were carefully dug out with their entire root system. The rhizospheric soil, defined as the soil attached to roots after gentle shaking by hand, was carefully separated from the plants and mixed together to form a composite sample. A subsample was frozen for the determination of mineral N content and another subsample was air dried and sieved to 2 mm for other soil analysis. From eight of the sixteen plants, the nodules were separated from the roots and counted to determine the nodule number. Shoots and roots were separated and washed to remove the remaining soil. Plant subsamples (shoot, root and nodule) were oven dried at 60 °C for 48 h and weighed to determine the shoot and root biomasses and the nodule weight. Another four plants with their root systems were washed and oven dried as previously and ground into a fine powder to assess N and P plant contents and SNF. Fresh roots from the remaining four plants were kept in the fridge for mycorrhization-rate determination.
Two other plant species (Urena lobata and Richardia scabra), common on the 2 sites, were also collected as reference plant for the assessment of the SNF. The samples’ treatment was the same as for the common bean samples.
At maturity, the bean pods were harvested by hand from ten plants and sun dried for three days before threshing and weighing.
2.4. Plant and Soil Analysis
Soil mineralogical contents (i.e., kaolinite, gibbsite and iron oxides (Fe2O3)) of the bulk soil samples were estimated by near infrared reflectance spectroscopy (NIRS), using the models developed by Ramaroson et al. [20]. The total carbon and nitrogen contents were determined by dry combustion in a Flash 2000 CHN Analyzer (Thermo Fisher Scientific GmbH., Dreieich, Germany). The remaining phosphorus (Prem), defined as the P concentration that remains in solution after shaking a soil sample for a certain period with a solution with known initial P concentration, was extracted according to the methodology of Alvarez et al. [21]. In brief, P content was determined at equilibrium after soil shaking in a solution of 0.01 mol L−1 CaCl2 containing 60 mg L−1 P, using a 1:10 soil-to-solution ratio for 1 h. Available P (Resin P) was extracted with anion exchange membrane (BDH no. 55164, 6 cm × 2 cm) in bicarbonate form [22]. Inorganic P was determined spectrophotometrically for Prem and Resin P, using the molybdenum blue-ascorbic acid method [23]. The soil pH was measured in water and in 1 M KCl suspensions using 1:5 (w:v) soil-to-solution ratio. The main mineralogical and physico-chemical soil properties of the two sites are shown in Table 1.

Table 1.
Physico-chemical properties and mineralogy of the soils of the two sites.
The mineral N content of the rhizospheric soil was determined by shaking 5 g of fresh soil in 50 mL 1 M KCl for one hour. After centrifugation and filtration, the solution was analyzed by colorimetry using a segmented flow analyzer (SAN++ SA3000/5000 Skalar Analytical, Breda, The Netherlands). The Resin P content and the soil pH water were determined as described previously. Total microbial activity in soil was estimated by measuring fluorescein diacetate (FDA) hydrolysis according to the method of Schnürer and Rosswall [24]. The acid phosphatase activity was measured by the method of Tabatabai [25] using hydrolysis of p-nitrophenylphosphate tetrahydrate (p-NPP) buffered at 6.0.
The total N content of a plant was determined by dry combustion in a Flash 2000 CHN Analyzer (Thermo Fisher Scientific GmbH, Dreieich, Germany). The P content was determined after calcination at 550 °C. After cooling, the ash was dissolved in warm 2% HCl. Total P was determined colorimetrically using the molybdenum blue-ascorbic acid method. The SNF was assessed by the natural abundance method. The 15N natural abundance (δ15N) of plant samples was measured with a mass spectrometer (Isoprime Precision, Elementar, Langenselbold, Germany) coupled with a CN elemental analyzer (Vario Pyro Cube, Elementar). The percentage of nitrogen derived by the atmosphere (%Ndfa) was calculated as follows [26]:
where δ15Nleg corresponds to the legume δ15N, δ15Nref is the nonfixing reference plant’s δ15N and the B value is the δ15N of the legume grown in a substrate without N. The δ15Nleg ref was the mean of two reference plants δ15N, Richardia scabra and Urena lobata. The δ15N for the reference plants were 6.62 and 6.16‰, respectively, for Site 1 and Site 2. The B-value was −0.482‰ [26]. The amount of N fixed by the crop was deduced by multiplying % Ndfa by the shoot N content and shoot biomass.
%Ndfa = ((δ15Nref − δ15Nleg)/(δ15Nref − B)) × 100
To determine the mycorrhization rates from the roots, fresh roots were stained with Trypan Blue [27] and the percentage of root length colonized by the mycorrhizal fungus was quantified using 15 × 1 cm root segments by the method of Giovannetti and Mosse [28].
2.5. Statistical Analysis
Statistical significance was set at p-value. All statistical tests were performed with the R software. Mean and standard deviation values were computed per treatment for all variables. A principal component analysis (PCA) was conducted on plant and soil variables using the package “FactoMineR”. Then, we performed a three-way ANOVA on the first two PCA axes as response variables (explaining 46% of total inertia) and with rhizobial inoculation, liming and P supply as factors. The normality and homogeneity of variance of the residuals were tested using the Shapiro’s test and Levene’s test (packages “stats” and “cars”). Because it was not significant, we did not include the effect of site in the ANOVA. We calculated the effect size for ANOVA using the eta-squared from the “lsr” package.
3. Results
3.1. Legume Symbioses
The inoculation with rhizobia and the supply of P had significant effects on the number of nodules. In the absence of inoculation, P and lime bean nodulation was nil (I0P0L0). After inoculation, the mean number of nodules increased from 6 nodules plant−1 for I0 to 91 nodules plant−1 for I1 (Figure 2a). However, even with inoculation, the number of nodules remained low without P (39 nodules plant−1), and it increased sharply with P to reach 116 to 118 nodules plant−1 for P1 and P2, respectively, with no significant difference between P1 and P2 (Figure 2b). A positive interaction was also observed between P and dolomite lime inputs, leading to a slight increase in the number of nodules per plant with dolomite lime (L1) (100 nodules plant−1 for I1L1) compared with L0 (82 nodules plant−1 for I1L0) but without significant differences between treatments with or without lime (see ANOVA and comparisons of means for all treatments on the data repository).
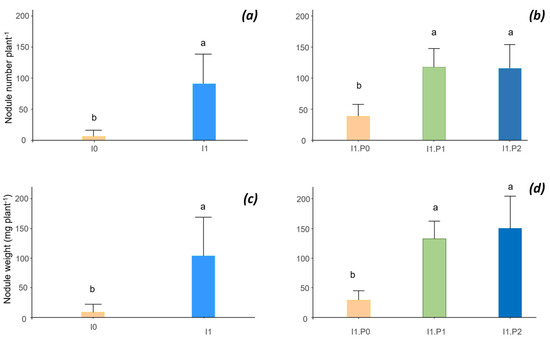
Figure 2.
Rhizobial inoculation (I) (a,c) and interaction effect between inoculation (I) and phosphorus (P) (b,d) on nodule number and nodule dry weight of common bean. Different letters indicate significant difference among the treatments according to post hoc Tukey HSD test at p-value < 0.05.
The nodule weight varied similarly to the number of nodules (Figure 2c,d). Nevertheless, the positive effect of the application of P was reinforced due to the increase in the average weight of the nodules with additional P, with an average weight of 0.90 mg per nodule for I0P0 and 1.2 mg per nodule for I0P1 and I0P2. Lime had no effect on nodule weight.
The rate of mycorrhization was not impacted by inoculation with rhizobia (see ANOVA and comparisons of means for all treatments on the data repository). However, an interaction was observed between P and dolomite inputs (Figure 3). In the absence of dolomite, the addition of P seemed to reduce mycorrhization, which decreased from 37% to 21%. With dolomite, the intermediate dose of P (P1) seemed to promote mycorrhization, with a mycorrhization rate of 43%.
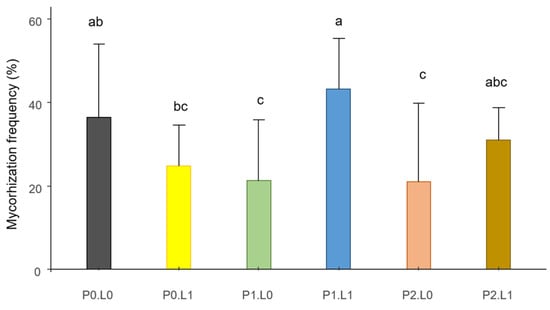
Figure 3.
Interaction between phosphorus (P) and lime (L) inputs on the rate of mycorrhization. Different letters indicate significant difference among the treatments according to post hoc Tukey HSD test at p-value < 0.05.
3.2. Legume Growth and Biological N2 Fixation
The sole significant effect was the interaction of inoculation and P supply, which had positive effects on biomass production at flowering and on bean yield (Figure 4a,b). The biomass was multiplied by a factor of 2.8 from I0P0 to I1P2, from 0.92 g plant−1 to 2.61 g plant−1. The bean yield increased by a factor of 3.5, from 82 to 287 kg ha−1.
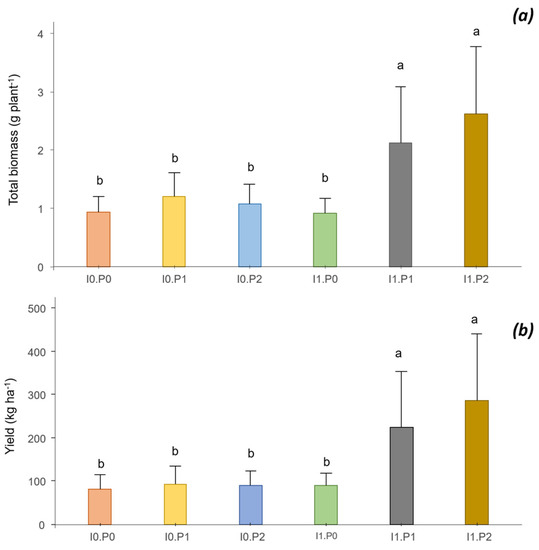
Figure 4.
Interaction between inoculation (I) and phosphorus (P) on the biomass (a) and the yield of common bean (b). Different letters indicate significant difference among the treatments according to post hoc Tukey HSD test at p-value < 0.05.
The inoculation significantly increased the N content of the biomass (by 17%), from 30 to 35 g kg−1 (Figure 5a). However, the lime decreased the N content of the plant. P input had a significant effect on plant P content, which almost doubled between P0 and P2, from 2.7 to 5 g kg−1 (Figure 5b).
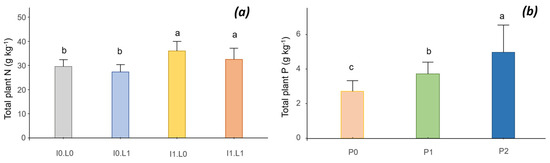
Figure 5.
Interaction between inoculation (I) and lime (L) on the total plant N (a) and phosphorus (P) effect on the total plant P of common bean (b). Different letters indicate significant difference among the treatments according to post hoc Tukey HSD test at p-value < 0.05.
Inoculation and interactions between inoculation, P and lime inputs had significant effects on the %Ndfa and the amount of N fixed by the plant (Figure 6). Without inoculation, the %Ndfa was low (%Ndfa < 30% for I0). Inoculation without P and lime inputs had no effect (%Ndfa = 35% for I1P0L0). After inoculation, the inputs of lime and P had positive effects, the %Ndfa reaching 68% for I1P0L1, 80% for I1P1L0 and I1P2L0 and up to 89% for I1P1L1 and I1P2L1. The amount of fixed N, which was negligible in the absence of inoculation (<1 kg ha−1), reached 9.5 kg ha−1 for I1P2L1.
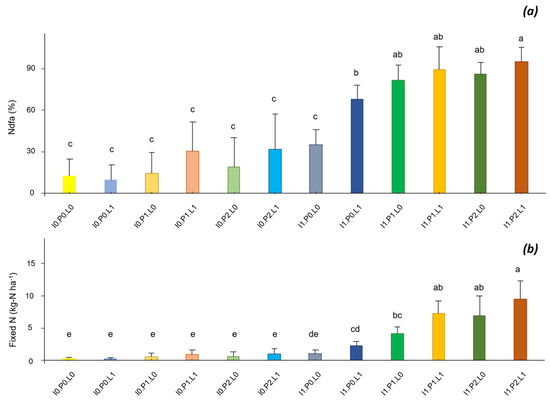
Figure 6.
Inoculation (I), phosphorus (P) and lime (L) interaction on the %Ndfa (a) and the amount of fixed N (b). Different letters indicate significant difference among the treatments according to post hoc Tukey HSD test at p-value < 0.05.
3.3. Rhizospheric Soil
Inoculation had no effect on soil properties. The main effects of the treatments were the effects of P and lime on Resin P and pH. The Resin P was very low without P addition (3.7 mg P kg−1, on average) and increased rapidly after the addition of P1 (96 mg P kg−1) and P2 (308 mg P kg−1). However, an interaction between P and lime was observed, resulting in a decrease in Resin P content at P0, after lime input (see ANOVA and comparison of means for all treatments on the data repository). Lime also had a noticeable effect on soil pH, which increased from 5 to 6 (Figure 7b). The treatments did not have marked effects either on the soil mineral nitrogen contents (N-NO3− and N-NH4+) or on the soil biological activities (total microbial activity and phosphatase acid activity). All treatments combined, the average nitrate and ammonium contents of the rhizospheric soil were, respectively, 15.6 mg N-NO3− kg−1 and 7.88 N-NH4+ kg−1 soil. The total microbial activity of the rhizospheric soil was estimated at 161 μg fluorescein g−1 h−1 and the acid phosphatase activity at 191 μg p-NP g−1 h−1.
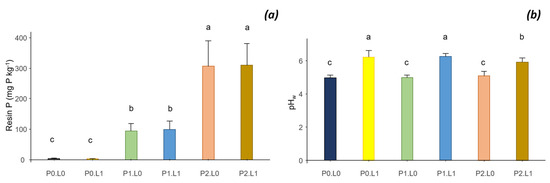
Figure 7.
Interaction between phosphorus (P) and lime (L) on the soil Resin P content (a) and soil pH (b). Different letters indicate significant difference among the treatments according to post hoc Tukey HSD test at p-value < 0.05.
3.4. Effects of Inoculation of Bean Seed and Inputs of P and Dolomite on Common Bean and Soil
The factorial map of the PCA (Figure 8a) showed a clear distinction between the noninoculated treatments, positioned on the positive scores of the first axis, and the inoculated treatments with the negative scores. However, only the inoculated treatments that received P had negative scores, whereas the treatments inoculated without supply of P had positive scores similar to the noninoculated treatments. The second axis of the PCA opposed the treatments following the level of P (the positive scores corresponding to the absence of P supply) and, to a lesser extent, according to the supply of lime (the negative scores corresponding to the absence of lime supply). The individual effects of the treatments were highlighted in Figure 8c–e and by the statistical results of the ANOVA carried out on the first two axes (Figure 8f).
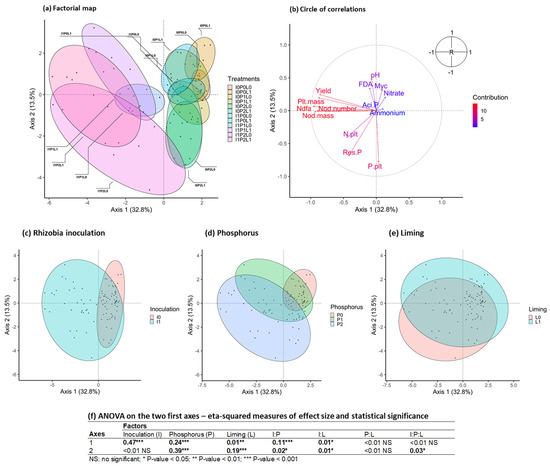
Figure 8.
Principal component analysis performed on plant and soil variables. (a) Individual factorial map with clustering based on rhizobial inoculation, liming and P treatments. (b) Circle of correlations. (c) Individual clustering based on rhizobial inoculation treatments. (d) Individual clustering based on P treatments. (e) Individual clustering based on liming treatments. (f) ANOVA performed on the first two PCA axes as response variables and with rhizobial inoculation, liming and P treatment as factors. Abbreviations in (b): plt.mass: plant biomass at flowering; yield: bean yield; N.plt: plant N content; P.plt: plant P content; Nod.number: nodule number; Nod.mass: nodule weight; Myc: rate of mycorrhization; pH: pH of the rhizospheric soil; nitrate: nitrate content of the rhizospheric soil; ammonium: ammonium content of the rhizospheric soil; Res.P: resin P content of the rhizospheric soil; FDA: total microbial activity of the rhizospheric soil; Aci.P: acid phosphatase activity of the rhizospheric soil. Principal component analysis performed on plant and soil variables. (a) Individual factorial map with clustering based on rhizobial inoculation, liming and P treatment. (b) Circle of correlations. (c) Individual clustering based on rhizobial inoculation treatments. (d) Individual clustering based on P treatments. (e) Individual clustering based on liming treatments. (f) ANOVA performed on the first two PCA axes as response variables and with rhizobial inoculation, liming and P treatment as factors.
Treatments inoculated and fertilized with P, associated with the negative scores of the first axis, corresponded to an increase in nodulation in %Ndfa as well as an increase in biomass at flowering and in bean yield (Figure 8b). On the second axis, negative values corresponded to an increase in soil P availability and an increase in plant P content. Positive values corresponded to an increase in soil pH.
4. Discussion
Our results indicate that inoculation with native strains of Rhizobium enhanced bean nodule production, biomass production and bean yield (Figure 2, Figure 3 and Figure 8). Many studies have highlighted the positive effects of rhizobial symbioses on legumes [29,30,31]. A meta-analysis showed an increase in bean yield of 59% in pot experiments and 16% in the field due to rhizobial inoculation [32]. Here, we showed an increase of 126% for the bean yield with inoculation in the field. The use of native rhizobia to increase SNF has been shown to be beneficial to the common bean [33,34], soybean [33], or cowpea [35]. According to Ouma et al. [33], it is preferable to use native rhizobia because they are better suited to local pedoclimatic conditions. The common bean is a relatively permissive host, nodulating with many rhizobial species [36]. The rhizobial strains we used were isolated in a companion study aiming to describe the diversity of the community of rhizobia associated with common beans cultivated in the Madagascar Highlands [37]. The selected strains were able to infect common bean root (infectivity) efficiently and had good effectiveness, i.e., a capacity to produce a larger amount of bean biomass compared with the noninoculated control [19]. At this time, the efficient rhizobia strains we used are not fully characterized, but they were able to multiply bean biomass by a factor of 2.6 to 3.1 [37]. Indeed, as most of Rhizobium native strains of soils are poorly infective or not efficient [31,38,39], there is a huge need to select efficient strains which are well adapted to each different soil condition.
The effectiveness of inoculation was very limited in the absence of P input. However, inoculation with rhizobia and fertilization with P had a highly significant effect on common bean nodulation and productivity (Figure 2 and Figure 3). While mean nodulation was only 8.7 ± 2.1 mg plant−1 in the absence of inoculation and 17.0 ± 3.1 mg plant−1 in the absence of P, it increased to 84.1 ± 13.8 mg plant−1 after inoculation and fertilization (Figure 2c,d). The increase in P availability (Resin P) (Figure 7a) and shoot P content (Figure 5b) suggests that SNF was strongly limited by the low P availability. It is widely accepted that the low availability of P in terrestrial ecosystems limits SNF [8]. The low content of available P in soils in Madagascar is one of the main constraints on crop production [40]. A survey of farm fields showed that 100% of rice flag leaf P content was below the critical value (2.4 g P kg−1 leaf biomass) in rainfed rice in highly weathered soils of Madagascar [41]. In these soils, without P input, the %Ndfa was very low (≅10%) and the amount of fixed N < 1 kg ha−1, whereas the %Ndfa was up to 95% and the amount of fixed N increased up to 10 kg ha−1 for I1P2L1. Indeed, legumes depending on symbiotic N2 fixation have a higher internal P requirement than plants dependent on combined N [42]. The conversion of N2 into ammonium by nitrogenase requires a large amount of energy in the form of ATP [43,44,45]. Then, P deficiency has a negative effect on the energy metabolism of legume nodules [46]. However, P deficiency is not the only nutrient deficiency that can impact nodulation effectiveness. Other nutrients, such as Ca, are very deficient in Malagasy soils [40]. Micronutrients are also often deficient in tropical soils. The supply of high-quality organic matter could therefore have a beneficial effect on nodulation through an increase in ecological processes involved in the supply of bioavailable P to plants and soil organisms [47,48].
Liming had no marked effect on bean nodulation or biomass. In fact, liming can have contrasting effects on the soil or living organisms (plants, microorganisms). On the one hand, the positive effects of liming on the fertility of tropical soils are well known [49]. Benefits are also provided to soil microorganisms; liming of acid soils provides nutrients (Ca and Mg) and improves soil conditions for bacterial growth by increasing soil pH and availability of P and molybdenum [50]. At pH 4.9 to 5.1 (Figure 7b), due to the close relationship between pH and exchangeable aluminum content, toxic effects of aluminum to biota are expected. Unfortunately, the exchangeable aluminum was not analyzed in our study. Nevertheless, at pH 4.7 in the bulk soil (Table 1) or pH 5.0 in the rhizospheric soil (Figure 7b), the conditions are highly favorable to the presence of exchangeable Al in tropical soils [51] and therefore to the solubilization of Al in toxic forms in the soil solution [52]. The rhizobial population can decline rapidly in soils with high aluminum saturation [50]. On the other hand, the increase of soil pH after liming influences soil Pi sorption, leading to the increase in soil P availability [53]. However, in our study, P availability (Resin P) was not increased by liming (Figure 7a). Indeed, the key role often attributed to pH on P availability can be questioned. In two recent studies [54,55] focusing on soil properties that control P availability, pH did not appear to be a major explanatory property. Moreover, phosphate uptake by roots increased when pH decreased [53], leading to a higher plant P content in the most acidic conditions (Figure 8b). Thus, the alleviation of the toxic effects of aluminum at pH 6 (Figure 7b) could be counteracted by a decrease in plant P uptake.
Interactions between rhizobia and arbuscular mycorrhizal fungi (AMF) can also be expected. AMF are symbionts that provide various compounds to plants, mainly P, in exchange for plant photosynthates [56]. Arbuscular mycorrhiza can enhance P uptake due to colonization by hyphae of soil volumes poorly accessible to roots, i.e., through a better mycorrhization rate and through mineralization of organic P by a higher phosphatase activity [57,58]. Our results did not show any clear effect of inoculation with Rhizobium on the mycorrhization rate (Figure 3). However, high soil available P was associated to a lower mycorrhization rate. Indeed, high P levels are known to affect AMF symbiosis negatively [59]. Anyway, to optimize the P plant uptake by the plant, it could be necessary to inoculate with both AMF and Rhizobium. The benefits of co-inoculation are reported by several authors [10,60]. Nevertheless, to optimize the beneficial effect of the co-inoculation, and increase the supply of P to rhizobia by mycorrhizae, it is necessary to manage the phosphate fertilization in an adequate way. Indeed, too-low or too-high availability of P both limit mycorrhization and thus promote plant nutrition by the direct route (roots) instead of the indirect route (mycorrhiza) [11].
5. Conclusions
The low crop productivity in grain legumes in sub-Saharan Africa limits the increase in their production in most farming systems. Low productivity is due to biological and environmental factors, i.e., the poor efficiency of native rhizobial strains, the low availability of P and the acidity of soils. Our results show that rhizobial inoculation with native strains enhanced bean root nodulation, and as a consequence, plant productivity through a better FSN. In addition, we showed a positive significant relationship between rhizobial inoculation and P input. However, we showed an antagonistic effect of P input on the rate of mycorrhization. We did not show a positive effect of the supply of dolomite lime in our study, the positive effects of Ca and Mg inputs being able to be offset by negative effects on the absorption of P by the plants. According to our results, we suggest that relatively low rates of P (50 kg P2O5 ha−1) and lime (200–300 kg dolomite ha−1, i.e., below the tested level L1 that led to a high soil pH increase), applied in the fertilizer banding line or in the sowing hole, are sufficient to increase P availability and reduce Al toxicity for the legume and rhizobium, with weak detrimental effects. Nevertheless, it remains for us to investigate further how the co-inoculation in the field, with both rhizobial and mychorrhiza, can contribute to the success of legumes, especially in harsh environments and low-fertility soils.
Author Contributions
Conceptualization, T.B., H.R. (Harimenja Razafintsalama) and B.R.; methodology, T.B., H.R. (Harimenja Razafintsalama) and B.R.; data acquisition, H.R. (Harimenja Razafintsalama) and A.T.E.R.; formal analysis, J.T. and H.R. (Harimenja Razafintsalama); writing—original draft preparation, T.B. and H.R. (Harimenja Razafintsalama); writing—review and editing, all authors; supervision, L.R. and T.B.; project administration, T.B.; funding acquisition, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Agropolis Fondation (France) under the reference ID 321 1001-009 through the “Investissements d’Avenir” program (Labex Agro: ANR-10-LABX-322 0001-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data were deposited on a figshare repository at the following DOI: 10.6084/m9.figshare.19433318 (Licence CC BY 4.0).
Acknowledgments
The authors are grateful to the two farmers from the Vakinankaratra region which allowed us to set up our trials in their plots. We thank Jean-Jacques Drevon for his leadership of the Fabatropimed project (Ecological services of legumes for nitrogen and phosphorus biogeochemical cycles and C sequestration in cereal cropping systems in Africa and the Mediterranean Basin), which funded this work. We are also thankful to M.P. Razafimanantsoa and A.H.D. Razafimahafaly (LRI, Antananarivo, Madagascar) for their technical assistance in the laboratory. We would also like to thank the DP SPAD (Dispositif en partenariat “Systèmes de Production d’Altitude et Durabilité à Madagascar), an interinstitutional scientific platform on highland agricultural systems and sustainability in Madagascar, for the meteorological data collected through the automatic weather station installed in the site of Ivory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Vaz Patto, M.C.; Amarowicz, R.; Aryee, A.N.; Boye, J.I.; Chung, H.J.; Martín-Cabrejas, M.A.; Domoney, C. Achievements and challenges in improving the nutritional quality of food legumes. Crit. Rev. Plant Sci. 2015, 34, 105–143. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.S.; Peoples, M.B.; Hauggaard-Nielsen, H. Faba bean in cropping systems. Field Crops Res. 2010, 115, 203–216. [Google Scholar] [CrossRef]
- Köpke, U.; Nemecek, T. Ecological services of faba bean. Field Crops Res. 2010, 115, 217–233. [Google Scholar] [CrossRef]
- Rubiales, D.; Mikic, A. Introduction: Legumes in sustainable agriculture. Crit. Rev. Plant Sci. 2015, 34, 2–3. [Google Scholar] [CrossRef]
- Chianu, J.N.; Chianu, J.N.; Mairura, F. Mineral fertilizers in the farming systems of sub-Saharan Africa. A review. Agron. Sustain. Dev. 2012, 32, 545–566. [Google Scholar] [CrossRef]
- Augusto, L.; Delerue, F.; Gallet-Budynek, A.; Achat, D.L. Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Glob. Biogeochem. Cycle 2013, 27, 804–815. [Google Scholar] [CrossRef]
- Olivera, M.; Tejera, N.; Iribarne, C.; Ocana, A.; Lluch, C. Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris): Effect of phosphorus. Physiol. Plant. 2004, 121, 498–505. [Google Scholar] [CrossRef]
- Larimer, A.L.; Bever, J.D.; Clay, K. The interactive effects of plant microbial symbionts: A review and meta-analysis. Symbiosis 2010, 51, 139–148. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, Q.; Zhou, J.; Rengel, Z.; Feng, G. Soil phosphorus availability determines the preference for direct or mycorrhizal phosphorus uptake pathway in maize. Geoderma 2021, 403, 115261. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Nitrogen fixation in perspective: An overview of research and extension needs. Field Crop. Res. 2000, 65, 93–106. [Google Scholar] [CrossRef]
- von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Ndakidemi, P.A.; Bambara, S.; Makoi, J.H. Micronutrient uptake in common bean (Phaseolus vulgaris L.) as affected by Rhizobium inoculation, and the supply of molybdenum and lime. Plant Omics 2011, 4, 40–52. [Google Scholar]
- Martinez-Romero, E. Diversity of Rhizobium-Phaseolus vulgaris symbiosis: Overview and perspectives. Plant Soil 2003, 252, 11–23. [Google Scholar] [CrossRef]
- Goedert, W.J. Management of the Cerrado soils of Brazil: A review. Eur. J. Soil Sci. 1983, 34, 405–428. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Hungria, M.; Kanampiu, F.; Giller, K.E. The role of legumes in the sustainable intensification of African smallholder agriculture: Lessons learnt and challenges for the future. Agric. Ecosyst. Environ. 2019, 284, 106583. [Google Scholar] [CrossRef]
- IUSS Working Group. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Razakatiana, A.T.E.; Trap, J.; Baohanta, R.H.; Raherimandimby, M.; Le Roux, C.; Duponnois, R.; Ramanankierana, H.; Becquer, T. Benefits of dual inoculation with arbuscular mycorrhizal fungi and rhizobia on Phaseolus vulgaris planted in a low-fertility tropical soil. Pedobiologia 2020, 83, 150685. [Google Scholar] [CrossRef]
- Ramaroson, V.H.; Becquer, T.; Sá, S.O.; Razafimahatratra, H.; Larvy Delarivière, J.; Blavet, D.; Vendrame, P.R.S.; Rabeharisoa, L.; Rakotondrazafy, A.F.M. Mineralogical analysis of ferralitic soils in Madagascar using NIR spectroscopy. Catena 2018, 168, 102–109. [Google Scholar] [CrossRef]
- Alvarez, V.V.H.; Novais, R.D.; Dias, L.E.; Oliveira, J.D. Determinação e uso do fósforo remanescente. Bol. Inf. Soc. Bras. Cienc. Solo 2000, 25, 27–34. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis, Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Schnürer, J.; Rosswall, T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analyses Part 2, Microbiological and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Samago, T.Y.; Anniye, E.W.; Dakora, F.D. Grain yield of common bean (Phaseolus vulgaris L.) varieties is markedly increased by rhizobial inoculation and phosphorus application in Ethiopia. Symbiosis 2018, 75, 245–255. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Díaz-Ambrona, C.H.; Mínguez, M.I. Cereal–legume rotations in a Mediterranean environment: Biomass and yield production. Field Crop. Res. 2001, 70, 139–151. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Kennedy, A.C. Grain legumes in northern Great Plains: Impacts on selected biological soil processes. Agron. J. 2007, 99, 1700–1709. [Google Scholar] [CrossRef]
- Zhong, Z.; Lemke, R.L.; Nelson, L.M. Nitrous oxide emissions associated with nitrogen fixation by grain legumes. Soil Biol. Biochem. 2009, 41, 2283–2291. [Google Scholar] [CrossRef]
- Kaschuk, G.; Alberton, O.; Hungria, M. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indications for improving sustainability. Soil Biol. Biochem. 2010, 42, 1–13. [Google Scholar] [CrossRef]
- Ouma, E.W.; Asango, A.M.; Maingi, J.; Njeru, E.M. Elucidating the potential of native rhizobial isolates to improve biological nitrogen fixation and growth of common bean and soybean in smallholder farming systems of Kenya. Int. J. Agron. 2016, 2016, 4569241. [Google Scholar] [CrossRef]
- Koskey, G.; Mburu, S.W.; Njeru, E.M.; Kimiti, J.M.; Ombori, O.; Maingi, J.M. Potential of native rhizobia in enhancing nitrogen fixation and yields of climbing beans (Phaseolus vulgaris L.) in contrasting environments of Eastern Kenya. Front. Plant Sci. 2017, 8, 443. [Google Scholar] [CrossRef]
- Mathu, S.; Herrmann, L.; Pypers, P.; Matiru, V.; Mwirichia, R.; Lesueur, D. Potential of indigenous bradyrhizobia versus commercial inoculants to improve cowpea (Vigna unguiculata L. walp.) and green gram (Vigna radiata L. wilczek.) yields in Kenya. Soil Sci. Plant Nutr. 2012, 58, 750–763. [Google Scholar] [CrossRef]
- Michiels, J.; Dombrecht, B.; Vermeiren, N.; Xi, C.W.; Luyten, E.; Vanderleyden, J. Phaseolus vulgaris is a non-selective host for nodulation. FEMS Microbiol. Ecol. 1998, 26, 193–205. [Google Scholar] [CrossRef][Green Version]
- Razakatiana, A.T.E. Rôle des Symbioses Mycorhizienne et Bactérienne: Biodisponibilité du Phosphore et Développement des Plantes de Haricot Sur le Sol Ferralitique de Madagascar. Doctoral Thesis, University of Antananarivo, Antananarivo, Madagascar, 26 July 2019. [Google Scholar]
- Catroux, G.; Hartmann, A.; Revellin, C. Trends in rhizobial inoculant production and use. Plant Soil 2001, 230, 21–30. [Google Scholar] [CrossRef]
- Hynes, R.K.; Jans, D.C.; Bremer, E.; Lupwayi, N.Z.; Rice, W.A.; Clayton, G.W.; Collins, M.M. Rhizobium population dynamics in the pea rhizosphere of rhizobial inoculant strain applied in different formulations. Can. J. Microbiol. 2001, 47, 595–600. [Google Scholar] [CrossRef]
- Raminoarison, M.; Razafimbelo, T.; Rakotoson, T.; Becquer, T.; Blanchart, E.; Trap, J. Multiple-nutrient limitation of upland rainfed rice in ferralsols: A greenhouse nutrient-omission trial. J. Plant Nutr. 2020, 43, 270–284. [Google Scholar] [CrossRef]
- Rabeharisoa, L.; Razanakoto, O.R.; Razafimanantsoa, M.P.; Rakotoson, T.; Amery, F.; Smolders, E. Larger bioavailability of soil phosphorus for irrigated rice compared with rainfed rice in Madagascar: Results from a soil and plant survey. Soil Use Manag. 2012, 28, 448–456. [Google Scholar] [CrossRef]
- Ribet, J.M.; Drevon, J.J. The phosphorus requirement of N2–fixing and urea-fed Acacia mangium. New Phytol. 1996, 132, 383–390. [Google Scholar] [CrossRef]
- Amâncio, S.; Stulen, I. Nitrogen Acquisition and Assimilation in Higher Plants; Plant ecophysiology; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA, 2004; Volume 3, 299p. [Google Scholar]
- Neila, A.; Adnane, B.; Mustapha, F.; Manel, B.; Imen, H.; Boulbaba, L.; Cherki, G.; Bouaziz, S. Phaseolus vulgaris—Rhizobia symbiosis increases the phosphorus uptake and symbiotic N2 fixation under insoluble phosphorus. J. Plant Nutr. 2014, 37, 643–657. [Google Scholar] [CrossRef]
- Zoundji, C.C.; Houngnandan, P.; Amidou, M.H.; Kouelo, F.A.; Toukourou, F. Inoculation and phosphorus application effects on soybean [Glycine max (L.) Merrill] productivity grown in farmers’ fields of Benin. J. Anim. Plant Sci. 2015, 25, 1384–1392. [Google Scholar]
- Sa, T.M.; Israel, D.W. Energy status and functioning of phosphorus-deficient soybean nodules. Plant Physiol. 1991, 97, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Trap, J.; Blanchart, E.; Ratsiatosika, O.; Razafindrakoto, M.; Becquer, T.; Andriamananjara, A.; Morel, C. Effects of the earthworm Pontoscolex corethrurus on rice P nutrition and plant-available soil P in a tropical Ferralsol. Appl. Soil Ecol. 2021, 160, 103867. [Google Scholar] [CrossRef]
- Trap, J.; Ranoarisoa, M.P.; Raharijaona, S.; Rabeharisoa, L.; Plassard, C.; Mayad, E.H.; Bernard, L.; Becquer, T.; Blanchart, E. Agricultural practices modulate the beneficial activity of bacterial-feeding nematodes on plant growth and nutrition: Evidence from an original intact soil core technique. Sustainability 2021, 13, 7181. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Ameliorating soil acidity of tropical Oxisols by liming for sustainable crop production. Adv. Agron. 2008, 99, 345–399. [Google Scholar]
- Andrade, D.S.; Murphy, P.J.; Giller, K.E. The diversity of Phaseolus-nodulating rhizobial populations is altered by liming of acid soils planted with Phaseolus vulgaris L. in Brazil. Appl. Environ. Microbiol. 2002, 68, 4025–4034. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, P.R.S.; Brito, O.R.; Martins, E.S.; Quantin, C.; Guimarães, M.F.; Becquer, T. Acidity control in Latosols under long-term pastures in the Cerrado region, Brazil. Soil Res. 2013, 51, 253–261. [Google Scholar] [CrossRef]
- Boudot, J.P.; Becquer, T.; Merlet, D.; Rouiller, J. Aluminium toxicity in declining forests: A general overview with a seasonal assessment in the Vosges mountains (France). Ann. Sci. For. 1994, 51, 27–51. [Google Scholar] [CrossRef]
- Barrow, N.J. The effects of pH on phosphate uptake from the soil. Plant Soil 2017, 410, 401–410. [Google Scholar] [CrossRef]
- Achat, D.L.; Pousse, N.; Nicolas, M.; Brédoire, F.; Augusto, L. Soil properties controlling inorganic phosphorus availability: General results from a national forest network and a global compilation of the literature. Biogeochemistry 2016, 127, 255–272. [Google Scholar] [CrossRef]
- Khaledian, Y.; Quinton, J.N.; Brevik, E.C.; Pereira, P.; Zeraatpisheh, M. Developing global pedotransfer functions to estimate available soil phosphorus. Sci. Total Environ. 2018, 644, 1110–1116. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; 800p. [Google Scholar]
- Joner, E.J.; van Aarle, I.M.; Vosatka, M. Phosphatase activity of extra-radical arbuscular mycorrhizal hyphae: A review. Plant Soil 2000, 226, 199–210. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected byroot-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Balzergue, C.; Puech-Pagès, V.; Bécard, G.; Rochange, S.F. The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J. Exp. Bot. 2011, 62, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Aryal, U.K.; Xu, H.L.; Fujita, M. Rhizobia and AM fungal inoculation improve growth and nutrient uptake of bean plants under organic fertilization. J. Sustain. Agric. 2003, 21, 27–39. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).