Abstract
To compare the relative importance of the biomass ratio hypothesis and the niche complementarity hypothesis in explaining changes in soil respiration (Rs), and to explore whether the relationship between biodiversity and Rs was affected by both biotic and abiotic factors, dynamic plant community monitoring was conducted in the Ebinur Lake Wetland Nature Reserve. By calculating the functional diversity (FD), community-weighted mean functional traits (CWM), and soil factors, the correlation between FD and Rs was compared using a linear regression model and a structural equation model. The results showed that (1) the CWM traits could better explain the changes of Rs than the FD, indicating that the biomass ratio hypothesis was more suitable for explaining changes in Rs in arid desert areas; and (2) the correlation between biodiversity and Rs was affected by the interaction between biological factors and environmental factors. Soil water content and species richness also affected Rs. Research on the relationship between biodiversity and Rs should examine both biotic and abiotic factors and clarify and explore various factors affecting Rs, which is of great significance to evaluate the community dynamics and variation characteristics of Rs. The study of various factors affecting Rs in this region is helpful to elucidate the process of the soil carbon cycle in arid desert areas.
1. Introduction
Soil respiration (Rs) is an important part of terrestrial ecosystem carbon cycling and the main route of soil carbon output to the atmosphere [1]. Rs refers mainly to the ecochemical process of releasing CO2 from the soil through root respiration, as well as the decomposition of litter and soil organic matter by soil microorganisms combined with the respiration of soil animals [2]. As an important component of the carbon cycle, Rs can reflect the influence of environmental changes on vegetation in ecosystems [3,4]. In addition, Rs is one of the main pathways for changes in the soil carbon pool and an important source of atmospheric carbon in the form of CO2. The global soil carbon bank has reached 2.2–3 × 103 Pg (1 Pg = 1 × 1015 g), which is twice the size of the global atmospheric carbon pool [5]. More than 75 Pg of carbon is released into the atmosphere each year through Rs [6]. Changes in atmospheric CO2 concentrations caused by subtle changes in the soil carbon pool and respiration can affect the balance of the global carbon cycle and thus influence global climate change. The relationship between Rs and the greenhouse effect will influence future terrestrial ecosystem functions and global change trends [7]. As a result, Rs is also a key process that affects important ecosystem functions such as climate regulation, nutrient cycling, and vegetation productivity [8].
Functional diversity (FD) refers to the value, range, distribution, or dispersion of functional traits in plant communities [9], mainly including the FD index and community-weighted means (CWM). The FD index is usually used to compensate for the deficiencies of plant functional group division and explain the relationship between biodiversity and ecosystem functions [10]. CWM is defined as the weighted average value of plant characteristics in the community, which is calculated based on the characteristic value of each species and the relative richness of species [11]. FD, as an important component of biodiversity, can more accurately predict the changes and processes of ecosystem functions than species diversity and is the main determinant of ecosystem functions and processes [12,13,14,15]. Reports have shown that FD is positively correlated with ecosystem function based on studies of the relationship between natural secondary forest diversity and ecosystem function in Yunnan, and that FD is a better explanation for changes in ecosystem function than species diversity [16]. As an important process in ecosystem function, Rs is a major indicator of soil biological activity, fertility, and permeability [17]. FD not only affects ecosystem functions but also affects changes in Rs.
A growing body of evidence has shown that trait characteristics are the main factors affecting ecosystem functions. Therefore, indices based on functional characteristics, such as the FD index and CWM, can be used to test two hypotheses together with the species richness. One of these hypotheses is the “niche complementarity” hypothesis [18], which proposes that the increase in species richness improves the efficiency of resource utilization, thus enhancing the ecosystem function [18]. This hypothesis is usually characterized by diversity indices (such as species diversity and FD index). The second hypothesis is the “biomass ratio hypothesis” [19], which holds that the ecosystem function at a certain time point is mainly determined by the value of the functional traits of the dominant plant species. When this hypothesis is dominant, it can be characterized by CWM [20,21]. Although it is generally believed that the “niche complementarity hypothesis” and “biomass ratio hypothesis” are not mutually exclusive, their relative importance is still controversial. Both hypotheses aim to discuss the mechanism of diversity affecting ecosystem function. Rs is an important component of ecosystem function, as well as an important ecosystem process [17]. This study’s two hypotheses may explain Rs in addition to ecosystem function.
Globally, arid and semi-arid regions occupy more than two-fifths of terrestrial regions and are characterized by fragile environments and extreme abiotic ecological factors. The daily and annual temperature ranges are large, the atmospheric and soil water contents are low, the nutrients and soil organic matter are lacking, the vegetation distribution is relatively sparse, the community structure and function are simple, and the types of vegetation are mainly drought-tolerant shrubs and herbs. Owing to the low content of soil organic carbon in arid and semi-arid regions, Rs is extremely sensitive to temperature and moisture changes and thus becomes one of the important indicators that can be used to measure regional climate change. As Rs is the main pathway of soil carbon loss in arid and semi-arid areas, this study sought to analyze the response process of Rs to various environmental factors, reveal the changes in Rs under different factors, and deeply explore the response intensity and mechanism of Rs in response to global change in these areas. It is undoubtedly of great significance to clarify the source and sink functions of terrestrial ecosystems in the carbon cycle and accurately estimate the carbon budget under the condition of future climate change.
Numerous studies have focused on Rs in arid regions. Most of these studies discussed the main factors influencing spatiotemporal changes in Rs and their relationships with Rs [11], whereas studies addressing the relationships between functional traits and diversity and Rs are relatively lacking. In this study, the plant communities in the Ebinur Lake Basin were taken as the research object. This study focused on the effects of the FD index of plant communities, the mean weights of community traits, plant traits, and soil factors on Rs, using linear and structural equation models (SEMs) as methods to address the following questions:
(1) Which of these could better explain the changes in Rs: the mean weights of community traits (biomass ratio hypothesis) or the FD index (niche complementarity hypothesis)?
(2) Do other biotic or abiotic factors affect the relationship between diversity and Rs? What is the relative importance of the two?
2. Methods and Materials
2.1. Overview of Study Area
This study focused on typical desert ecosystems in the Ebinur Lake Basin of China. The study area was located in the Ebinur Lake Wetland National Nature Reserve (44°30′–45°09′ N, 82°36–83°50′ E). The climate in this area is dry, belonging to a typical temperate continental climate. The annual sunshine hours are about 2800 h, and the annual precipitation (100–200 mm) is much less than the annual evaporation (1500–2000 mm). The annual average temperature is 7.8 °C, the extreme highest temperature is 44 °C, and the extreme lowest temperature is −33 °C [22,23].
The nature reserve is located in a low-lying basin, with aeolian sand, gray-brown desert soil, and gray desert soil as the soil parent material, all of which are seriously affected by desertification. The vegetation in the study area is mainly composed of sandy vegetation, including mesophytes and halophytes. The dominant species include Populus euphratica, Haloxylon ammodendron, Halimodendron halodendron, Alhagi sparsifolia, Reaumuria soongarica, Nitraria roborowskii, Apocynum venetum, Phragmites australis, Halodendron strobilaceum, Salsola collina, and Suaeda glauca [24].
2.2. Sampling
The test began in July 2018. A 1 ha (100 m × 100 m) large sample plot was set up 150 m perpendicular to the bank of the Acheson River. The selected large sample plots were divided into 100 10 m × 10 m and 400 5 m × 5 m continuous quadrat units (Figure 1).
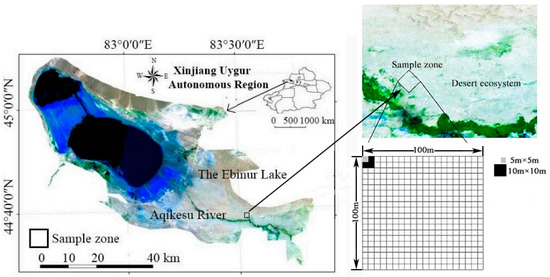
Figure 1.
Study area and location of the plots.
2.3. Rs Rate Monitoring
The observation time of the Rs rate was from 10:00 to 12:00 Beijing time. The LI-8100 open-circuit soil carbon flux system (LI-8100, LI-COR Inc., Lincoln, NE, USA) was used to measure the Rs rate. The total time taken to measure the Rs rate was 120 s, including a 1 s sampling period, a 30 s recording interval, and an effective measurement time of 90 s.
For observation, two points in each 5 m × 5 m plot were randomly selected and Rs rings were installed. To reduce the disturbance of Rs by the Rs rings, a polyvinyl chloride cylinder with an outer diameter of 22 cm and an inner diameter of 19.5 cm was embedded 24 h in advance, with an embedding depth of 5–8 cm and the upper part extending 2–3 cm above the ground. The aboveground plants were cut off uniformly near the sample sites.
2.4. Plant Community Survey
In July 2018, the species names of all species in the sample plots were recorded, and the indicators of plant samples were measured and collected. The plant height of each plant species in each plot was measured with a ruler. Five intact leaves (assimilating branches) were selected for each plant according to the inconsistent leaf sizes of different plants, and the length, width, and thickness of the leaves were measured with a Vernier caliper. After fixation in an oven for 1 h at 105 °C, the leaves were dried to a constant weight at 70 °C, and the dry weight of the leaves was determined using a balance with an accuracy of 0.0001 g to calculate the dry matter content (LDMC) of the leaves. The specific leaf area was calculated based on the ratio of the leaf area to the weight of the same leaf. Finally, the leaf was pulverized using a grinder (Laichi, MM400 hybrid model). The leaf carbon content (LCC) was determined by the potassium dichromate volumetric method, the leaf nitrogen content (LNC) was determined by the Kjeldahl nitrogen method, and the leaf phosphorus content (LPC) was determined by the molybdenum antimony colorimetric method [25].
2.5. Determination of Soil Physical and Chemical Properties
In each 5 m × 5 m sample square, the diagonal sampling method was used to collect soil samples from the upper 0–10 cm layer [26,27]. The soil water content (SWC) was measured by the drying method. About 10 g of fresh soil was dried at 105 °C for 48 h to constant weight [28]. During SWC sampling, additional 0–10 cm soil samples were collected to determine the physical and chemical properties of the soil after removing plant roots and debris. Soil salt content (SSC) was determined by the gravimetric method, the soil pH value was determined by the potentiometric method, the soil organic carbon (SOC) content was determined by the potassium dichromate dilution heat method, the soil ammonium nitrogen (AN) [29] content was determined by diphenol blue colorimetry, the soil nitrate nitrogen (NN) content was determined by dual-wavelength ultraviolet spectrophotometry [30], and the soil total nitrogen (TN) content was determined by the Kjeldahl method. The concentrations of total phosphorus (TP) and available phosphorus (AP) in the soil were determined by molybdenum antimony anti-colorimetry [31]. While monitoring the Rs rate, an MS-10 soil temperature sensor (Rain Root Technology Co., Ltd., Beijing, China) was used to measure the soil temperature.
2.6. Diversity Index and Average Calculation of Community Character Weight
Based on the nine measured plant functional traits, the functional evenness index (FEve), functional volume index (FRic), and functional dispersion index (FDis) of each small quadrat were calculated. The weighted mean values of community traits included the CWM of maximum tree height (CWMMH), the CWM of leaf length (CWMLL), the CWM of leaf width (CWMLW), the CWM of leaf thickness (CWMLT), the CWM for leaf dry matter content (CWMLDMC), the CWM for specific leaf area (CWMSLA), the CWM for leaf carbon content (CWMLCC), the CWM for leaf nitrogen content (CWMLNC), the CWM for leaf phosphorus content (CWMLPC), and the weighted mean value of all nine traits (CWM). The calculation formula of each index is provided below.
The functional volume index (FRic) is calculated as follows:
where SFic is the space occupied by a species in community i for functional traits, and RC is the absolute value range of characteristic C.
The functional evenness index (FEve) is calculated as follows:
where EW represents uniformity, dist(i,j) represents the Euclidean distance between species i and j, and wi represents the number of species i.
The functional dispersion index (FDis) is calculated as follows:
where zj represents the weighted Euclidean distance from species j to the center of gravity C in the community.
The CWM was defined as the mean weight of the functional traits of species within a community. The CWM indicated that the ecosystem function or process was mainly driven by the functional traits of dominant species in the community, and each trait was calculated separately. The specific calculation formula is as follows:
where Pi is the relative abundance of species i in the community, and traiti is the value of the functional traits of species i.
2.7. Multiple Collinearity Test
While the linear regression model was constructed with Rs as the response variable and FD and environmental variables as the explanatory variables, the dataset needed to be subjected to a multicollinearity test, which was reflected by the variance expansion factor (VIF). Multicollinearity affects the interpretation and prediction ability of the explanatory variables to the response variables. The multicollinearity between variables was tested based on the VIF. Based on previous reports, there was considered to be no multicollinearity between explanatory variables when the VIF was less than 10 [32,33].
2.8. Construction of Linear Regression Model
The Rs was taken as the response variable; the species diversity index, the FD index, the weighted mean of community traits, and the environmental factors were taken as the explanatory variables; the linear regression model was constructed with productivity; and the correlation between the explanatory variables and Rs in each model was compared. For the six models with different indices, the determination coefficient (R2) and Akachi information criterion (AIC) were used to judge the goodness of fit of the model; compare the correlation between the CMW, FD, and Rs; and judge the difference between the impact of the CMW and FD on Rs.
2.9. Structural Equation Modeling
The direct and indirect relationships between FD indices, environmental factors, and Rs were analyzed by the SEM, and the relative importance of the biomass ratio hypothesis and the niche complementarity hypothesis to Rs in this arid area was compared.
According to the results of variable screening, the FD index and potential environmental variables with the highest variable importance and the variables most related to productivity were selected and incorporated into the SEM. The SEM was fitted by the maximum likelihood method, and the goodness of fit of the model was evaluated by comparing the fitting index (CFI), the mean square sum square root of progressive residuals (RMSEA), and the significant probability value (p) [34]. The critical values of goodness of fit were CFI > 0.9, RMSEA < 0.08, and p > 0.05. The relative effects of different factors on Rs were quantified using the standardization coefficient of each path in the model, and the direct, indirect, and total standardization effects of each variable on Rs were quantified [35] so as to compare the applicability of the niche complementarity hypothesis and the biomass ratio hypothesis to Rs in arid areas.
2.10. Statistical Analysis
The relationships between Rs and soil factors, FD index, and CMW were assessed through regression analysis. Regression analyses and multicollinearity tests were performed in SPSS 24.0, the CMW values were calculated in R4.0.2, and the SEM was established in AMOS 24.0.0. Principal component analysis (PCA) was performed with Origin 2021 for analytical mapping.
3. Results and Analysis
3.1. Correlations between Rs, FD, and Environmental Factors
The correlation regression analysis of Rs, the FD index, and soil factors showed that the FRic, FEve, and FDis were significantly correlated with Rs (p < 0.05) (Figure 2). There was no significant correlation between specific leaf area, leaf N, leaf p, and Rs among the mean weights of nine community traits (p > 0.05) (Figure 3), and the mean weights of other community traits were significantly correlated with Rs. Among the 10 soil environmental factors (SWC, SSC, pH, SOC, AN, NN, TN, TP, AP, and soil temperature), soil pH and volumetric water content had a significant correlation with Rs (p < 0.05), while the other soil environmental factors had no significant correlation with Rs (p > 0.05) (Figure 4).
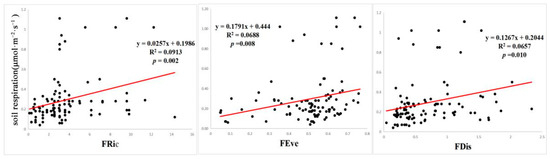
Figure 2.
Relationship between functional diversity and soil respiration.
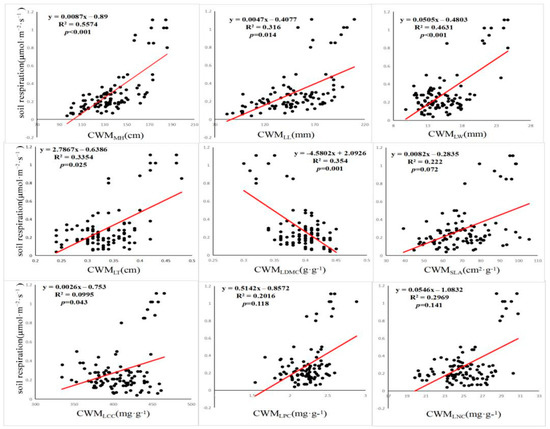
Figure 3.
Relationship between community-weighted means and soil respiration.
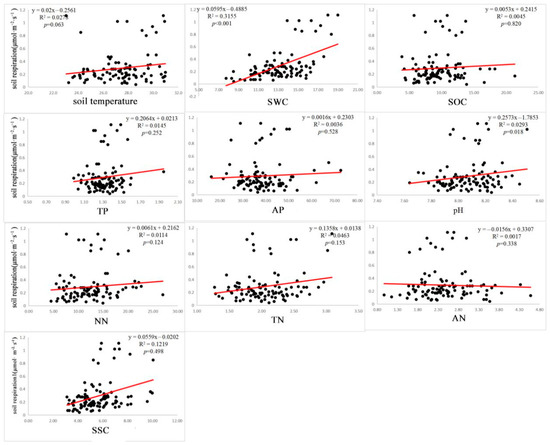
Figure 4.
Relationship between soil factors and soil respiration.
In the linear regression model for evaluating diversity and Rs based on the diversity-Rs models, the decision coefficient of the multiple regression model for the FD index and Rs (model I ) was R2 = 0.300, the decision coefficient of the multiple regression model for the CMW and Rs (model II ) was R2 = 0.841, and the decision coefficient of the multiple regression model for the mean value of the FD index and the CMW and Rs (model III) was R2 = 0.841. The multiple regression model between the FD index and Rs was lower than that between the FD index, the CMW, and Rs. The decision coefficient and FD index of the multiple regression model of the CMW and Rs were the same as those of Rs. The AIC value of model I (AIC = 27.916) and the AIC value of model II (AIC = 26.789) were lower than the AIC value of model III (AIC = 38.789) (Table 1).

Table 1.
Evaluation of linear regression models for soil respiration (Rs) and diversity. Functional diversity (FD) and community-weighted mean (CWM) models include three independent models. Model I represents multiple regression models of FD index and Rs. Model II represents multiple regression models of community-weighted mean and Rs. Model III represents multiple regression models of FD index, CWM, and Rs. R2 represents the coefficient of determination; AIC represents the Akaike information criterion.
3.2. Screening Optimal Variables for Rs Interpretation through a Linear Model
When there were more variables, the SEMs did not easily converge. Therefore, the diversity index and soil factors were added one by one to construct an optimal SEM. After variable screening and comparison, the FRic was finally used to represent FD, the mean weights of 9 community traits were reduced to one community trait mean weight, and the variables of 10 soil factors were reduced to one soil factor. Finally, the species richness index was added to construct the optimal SEM, which had a CFI of 0.997, an RMSEA of 0.063, and a p of 0.248 (Figure 5).
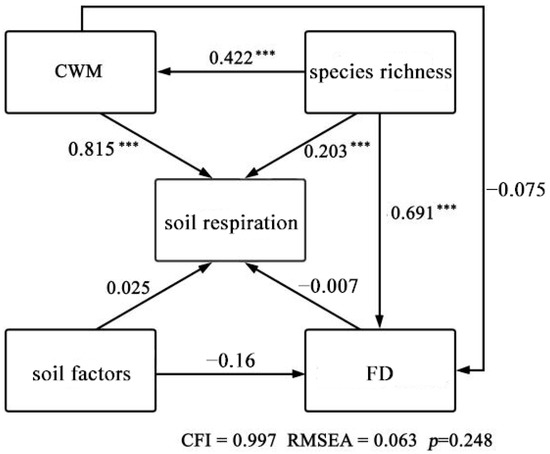
Figure 5.
Relationships between functional diversity index, the mean weight of community traits, soil factors, and species richness in the structural equation model. The arrow indicates the causal direction of the hypothesis. *** indicates significance at p < 0.001.
3.3. Effects of Each Factor on Rs
Each variable explained 84.1% of community Rs (SEM, R2 = 0.841). The FRic had a significant direct effect on Rs (p > 0.05), and the path coefficient reflecting the effect was −0.007. The CMW had a very significant direct effect on Rs (p < 0.001), and the path coefficient reflecting the effect was 0.815. Rs was also directly affected by soil environmental factors and species richness index, and the path coefficients were 0.025 and 0.203, respectively (Table 2). As can be seen from the PCA plots, the first and second PCA were able to characterize 51.8% and 21.4% of the multifactorial variations, respectively (Figure 6), with RS and CWM showing better positive correlations, while the FD index and soil factors showed lower correlations.

Table 2.
Direct, indirect, and total standardized effects on soil respiration, based on the structural equation model.
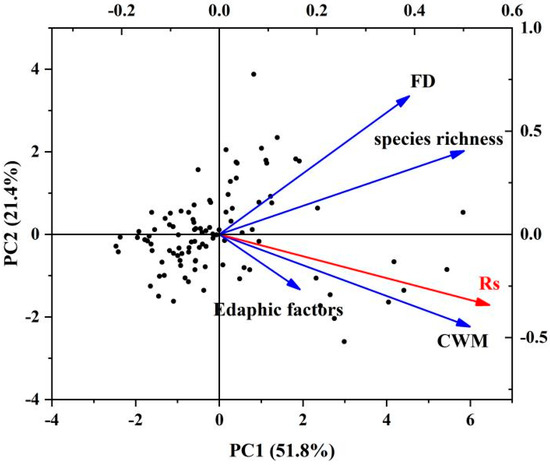
Figure 6.
Principal component analysis of functional diversity index, trait weight mean of community traits, soil factors, species richness, and soil respiration.
4. Discussion
4.1. Average Weight of Community Traits Can Better Explain the Change of Rs
In this study, an SEM was used to evaluate the relative contributions of FD, environmental factors, and plant factors to Rs. In the optimal SEM to explain the relationship between diversity and Rs, the CMW was significantly correlated with Rs, while the FD index was not correlated with Rs, and the total effect of the CMW (0.815) was higher than that of the FD index (−0.007) (Figure 5; Table 2). The CMW was significantly better than the FD index in explaining the change of Rs. Among the factors, the correlation path coefficient between the CMW and Rs was the largest, indicating that the CMW had a greater impact on Rs. The CMW was more suitable to explain the changes of Rs in arid areas than the FD index, and PCA showed that Rs presented a greater correlation with CWMs than Rs did with the FD index. Therefore, this study concluded that CWMs were more suitable than FD indices to explain the variation in Rs across arid regions. Rs was also a key factor affecting important ecosystem functions (such as climate regulation, nutrient cycling, and vegetation productivity) [8]. The results also indicated that the CMW could better explain ecosystem functions. Previous studies have shown that the CMW can well explain ecosystem functions [36,37,38,39]. In particular, the functional trait value of the dominant species was found to drive ecosystem function [40,41], which was consistent with the present study. In addition, some studies have suggested that FD may be better in evaluating the response of communities to environmental changes and in predicting ecosystem functional changes [42,43]. The inconsistent results in regard to the relationship between diversity and the ecosystem function of different community types may be due to the differences in methods used [44]. Inconsistencies may also be caused by different ecosystem types and succession stages. In addition, the mean value of Rs may explain the function of soil ecosystems to a certain extent. Rs is an important process of ecosystem function [17]. It can be proposed that the CWM can not only explain the ecosystem function well, but also explain Rs to a certain extent.
4.2. Biomass Ratio Effect Was More Suitable for Explaining the Changes of Rs in the Arid Desert Area than the Niche Complementarity Effect
Research has shown that when there are large differences in functional traits among woody plants in a forest, species have different strategies for acquiring and using resources, thereby reducing the overlapping of ecological niches. The niche complementarity effect reduces the competition for resources among species and maximizes the use of limited resources such as light, promoting the growth of many species in different niches and improving ecosystem functions [12]. In this case, when the niche complementarity hypothesis dominates, this hypothesis is usually characterized by diversity indices (e.g., species diversity and FD). In contrast, in communities with habitat filtration and diffusion constraint effects, niche complementarity is not significant, which magnifies the resource competition among species, and the dominant species can use these resources to a greater extent, thus occupying the dominant position. Therefore, the dominant species has a greater impact on the community, and the ecosystem processes mainly depend on the functional traits of the dominant species in the community. In this case, the biomass ratio effect is more important [36]. Therefore, when the biomass ratio hypothesis is dominant, CWM as the value of functional traits in dominant species might be the most important factor for explaining ecosystem function [20,21]. As an important component of ecosystem function and a key ecosystem process [17], Rs is also indirectly affected by the two hypotheses when they affect ecosystem function. The ability of CWM to interpret Rs changes in this study was significantly superior to that of FD (Figure 5). In the best SEM explaining the relationship between diversity and Rs, the dominant species CWM was significantly correlated to Rs (Table 1). This indicated that the CWM of dominant species had a significant effect on Rs, so the biomass ratio hypothesis was more suitable for explaining the changes of Rs in this research area.
4.3. Impacts of Environmental Factors on the Relationship between Biodiversity and Rs
In this study, an SEM was used to evaluate the relative effects of the FD index, CWM, soil factors, and species richness on Rs. The total effect of species richness on Rs was 0.543, second only to the effect of CWM on Rs. Therefore, species richness is an important factor determining ecosystem function [45]. Previous studies have shown that ecosystem function changes rapidly with changes in species richness [46]. Rs is an important process of ecosystem function [47]. When ecological function changes with species richness, Rs will also change. Therefore, the change of species richness also affects the change of Rs. Plant species richness may also affect Rs by changing plant productivity [48].
The total effect of soil factors on Rs was 0.025, which had an impact on the change in Rs. Among the soil factors, soil pH and SWC were significantly correlated with Rs (p < 0.05). Studies have shown that environmental factors have different effects on Rs under different vegetation conditions in environments with relatively limited resources. In areas with higher biomass, plant root biomass will also be higher, resulting in more efficient water uptake and a higher Rs rate [49]. The influence of soil moisture on Rs is complex. The direction and degree of the influence of soil moisture on Rs vary greatly in different ecosystems. When the SWC is sufficient and does not become a limiting factor, Rs is positively correlated with soil temperature. In arid and semi-arid areas, where water is the limiting factor, Rs has been found to be jointly affected by SWC and soil temperature. This is inconsistent with the results of this paper. This may be because the response intensity of Rs to soil temperature in arid areas is affected by SWC [50]. Rs may also be affected by the lack of energy released by the surrounding environment and the supply of basic materials, so that the response sensitivity of Rs to soil temperature is not high [51]. Therefore, through the impact of species richness and SWC on Rs, it could be shown that the relationship between biodiversity and Rs was affected by the joint action of biotic and abiotic factors. Some studies have also suggested that biodiversity is not the only factor influencing ecosystem function, but that ecosystem function is also affected by the joint influence of environmental factors and other biotic factors [49]. This was consistent with the results of this paper.
In summary, this study discusses the effect of functional diversity on RS and the effects of environmental and biotic factors on the relationship between biodiversity and soil respiration in arid desert ecosystems, and future studies on the relationship between biodiversity and RS should expand the scope of research to explore the main factors affecting the relationship between biodiversity and RS and the effect of FD on RS under different ecosystems.
5. Conclusions
In this study, an SEM was used to analyze the relationships between various factors and Rs, and PCA was used to verify the relationship between various factors and Rs. Among them, the mean weight of plant traits had a positive correlation with Rs, and the FD index had no significant effect on Rs. The results showed that compared with the FD index, the CMW had a stronger impact on Rs. The findings suggested that the effect of the biomass ratio hypothesis on Rs was greater than that of the niche complementarity hypothesis. Therefore, this method could be used to evaluate the change traits of community dynamics and Rs in the future. Species richness and soil factors had different effects on Rs. Therefore, biotic factors and environmental factors also affected the relationship between diversity and Rs. The results of this study provide a scientific basis for better understanding the complex relationship between biodiversity and Rs in arid desert ecosystems.
Author Contributions
Conceptualization, G.L., F.L. and J.W.; formal analysis, F.L., J.W. and Y.S.; investigation, F.L. and J.W.; writing—original draft, F.L.; writing—review and editing, G.L., F.L. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (no. 31560131); Xinjiang Uygur Autonomous Region Graduate Research and Innovation Project XJ2019G020; and Xinjiang Uygur Autonomous Region Graduate Research and Innovation Project XJ2021G040.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Jinlong Wang for data analysis and Yulin Shu for manuscript editing. We are also very grateful to the journal’s anonymous reviewers and editors for their constructive comments regarding the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Liu, S.H.; Fang J, Y. Effect factors of soil respiration and the temperature’s effects on soil respiration in the global scale. Acta Ecol. Sin. 1997, 17, 19–26. [Google Scholar]
- Kuzyakov, Y.V.; Larionova, A.A. Contribution of rhizomicro- bial and root respiration to the CO2 emission from soil (A review). Eurasian Soil Sci. 2006, 39, 753–764. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Y.; Xu, Z.; Wu, Q. Soil respiration in a subtropical forest of southwestern China: Components, patterns and controls. PLoS ONE 2018, 13, e0204341. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Fang, L.; Bian, Z.F.; Wang, Q.; Yu, Y.C. A review of the composition of soil carbon pool. Acta Ecol. Sin. 2014, 34, 4829–4838. [Google Scholar]
- Hibbard, K.A.; Law, B.E.; Reichstein, M.; Sulzman, J. An analysis of soil respiration across northern hemisphere temperate ecosystems. Biogeochemistry 2005, 73, 29–70. [Google Scholar] [CrossRef]
- Han, G.X.; Zhou, G.S.; Xu, Z.Z. Reearch and prospects for soil respiration of farmland ecosystems China. Acta Phytoecol. Sin. 2008, 32, 719–733. [Google Scholar]
- Liu, Y.R.; Delgado-Baquerizo, M.; Wang, J.T.; Hu, H.W.; Yang, Z.; He, J.Z. New insights into the role of microbial community composition in driving soil respiration rates. Soil Biol. Biochem. 2018, 118, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Liira, J.; Schmidt, T.; Aavik, T.; Arens, P.; Augenstein, I.; Bailey, D.; Billeter, R.; Bukáček, R.; Burel, F.; De Blust, G.; et al. Plant functional group composition and large-scale species richness in European agricultural landscapes. J. Veg. Sci. 2008, 19, 3–14. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.S.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field—Methodology matters! Funct. Ecol. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive la difference: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt-Römermann, M.; Römermann, C.; Sperlich, S.; Schmidt, W. Explaining grassland biomass—The contribution of climate, species and functional diversity depends on fertilization and mowing frequency. J. Appl. Ecol. 2011, 48, 1088–1907. [Google Scholar] [CrossRef] [Green Version]
- Roscher, C.; Schumacher, J.; Gubsch, M.; Lipowsky, A.; Weigelt, A.; Buchmann, N.; Schmid, B.; Schulze, E.D. plant functional traits to explain diversity–productivity relationships. PLoS ONE 2012, 7, e36760. [Google Scholar] [CrossRef]
- Huang, X.; Su, J.; Li, S.; Liu, W.; Lang, X. Functional diversity drives ecosystem multifunctionality in a Pinus yunnanensis natural secondary forest. Sci. Rep. 2019, 9, 6979. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.J.; Yu, Q.H.; Wang, S.L.; Lü, L. Changes in permafrost environments along the Qinghai–Tibet engineering corridor induced by anthropogenic activities and climate warming. Cold Reg. Sci. Technol. 2008, 53, 317–333. [Google Scholar] [CrossRef]
- Tilman, D. Distinguishing between the effects of species diversity and species composition. Oikos 1997, 80, 185. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Tobner, C.M.; Paquette, A.; Gravel, D.; Reich, P.B.; Williams, L.J.; Messier, C. Functional identity is the main driver of diversity effects in young tree communities. Ecol. Lett. 2016, 19, 638–647. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.R.; Chang, S.X.; Cheng, J.Y.; Liu, X.Y. Community-weighted mean of leaf traits and divergence of wood traits predict aboveground biomass in secondary subtropical forests. Sci. Total Environ. 2017, 574, 654–662. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lv, G.; Qin, L.; He, J.; Xu, M. Preliminary study of contrast integration of photosynthesis and soil respiration properties of gossypium and phragmites australis community in Ebinur Lake area. Xinjiang Agric. Sci. 2012, 49, 1509–1518. [Google Scholar]
- Wang, J.; Teng, D.; He, X.; Qin, L.; Yang, X.; Lv, G. Spatial non-stationarity effects of driving factors on soil respiration in an arid desert region. Catena 2021, 207, 105617. [Google Scholar] [CrossRef]
- Zhang, X.N.; Lü, G.H.; Yang, X.D.; Gong, L.; Qin, L.; He, X.M.; Liu, H.Q. Responses of desert plant diversity, community and interspecific association to soil salinity gradient. Acta Ecol. Sin. 2013, 33, 5714–5722. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, W.; Wang, J.; Wang, Y.; Li, J.; Yuan, D.; Fan, Y.; Wei, X. Leaf functional traits of oasis plants in extremely arid areas and its response to soil water and salt factors. J. Beijing For. Univ. 2019, 41, 1000–1522. [Google Scholar]
- Jiang, Y.; Zhang, B.; Wang, W.; Li, B.; Wu, Z.; Chu, C. Topography and plant community structure contribute to spatial heterogeneity of soil respiration in a subtropical forest. Sci. Total Environ. 2020, 733, 139287. [Google Scholar] [CrossRef]
- Yang, X.D.; Ali, A.; Xu, Y.L.; Jiang, L.M.; Lv, G.H. Soil moisture and salinity as main drivers of soil respiration across natural xeromorphic vegetation and agricultural lands in an arid desert region. Catena 2019, 177, 126–133. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Dong, X.J.; Xu, B.X.; Chen, Y.L.; Zhao, Y.; Gao, Y.H.; Hu, Y.G.; Huang, L. Soil respiration sensitivities to water and temperature in a revegetated desert. J. Geophys. Res. Biogeosci. 2015, 120, 773–787. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Norman, R.J.; Edberg, J.C.; Stucki, J.W. Determination of Nitrate in Soil Extracts by Dual-wavelength Ultraviolet Spectrophotometry 1. Soil Sci. Soc. Am. J. 1985, 49, 1182–1185. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.; Chang, S.X.; Peng, C.; Xiao, W.; Zhang, J.; Xiang, W.; Li, Y.; Wang, W. Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a Moso bamboo forest. Agric. For. Meteorol. 2019, 268, 48–54. [Google Scholar] [CrossRef]
- Fox, J.; Monette, G. Generalized Collinearity Diagnostics. Publ. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Fox, J. Applied Regression Analysis and Generalized Linear Models, 2nd ed.; Sage Publications: Thousand Oaks, FL, USA, 2008. [Google Scholar]
- Hoyle, R.H. Handbook of structural equation modeling. Struct. Equ. Modeling 2012, 20, 354–360. [Google Scholar]
- Edwards, J.R.; Lambert, L.S. Methods for integrating moderation and mediation: A general analytical framework using moderated path analysis. Psychol. Methods 2007, 12, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finegan, B.; Peña-Claros, M.; de Oliveira, A.; Ascarrunz, N.; Bret-Harte, M.S.; Carreño-Rocabado, G.; Casanoves, F.; Díaz, S.; Eguiguren Velepucha, P.; Fernandez, F. Does functional trait diversity predict above-ground biomass and productivity of tropical forests? Testing three alternative hypotheses. J. Ecol. 2015, 103, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Kunstler, G.; Falster, D.; Coomes, D.A.; Hui, F.; Kooyman, R.M.; Laughlin, D.C.; Poorter, L.; Vanderwel, M.; Vieilledent, G.; Wright, S.J.; et al. Plant functional traits have globally consistent effects on competition. Nature 2016, 529, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Prado-Junior, J.A.; Schiavini, I.; Vale, V.S.; Arantes, C.S.; van der Sande, M.T.; Lohbeck, M.; Poorter, L. Conservative species drive biomass productivity in tropical dry forests. J. Ecol. 2016, 104, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Li, M.H.; Zimmermann, N.E.; Li, S.P.; Li, H.; Ren, H.; Sun, H.; Han, X.; Jiang, Y.; Jiang, L. Plant functional diversity modulates global environmental change effects on grassland productivity. J. Ecol. 2018, 106, 1941–1951. [Google Scholar] [CrossRef]
- Orwin, K.H.; Buckland, S.M.; Johnson, D.; Turner, B.L.; Smart, S.; Oakley, S.; Bardgett, R.D. Linkages of plant traits to soil properties and the functioning of temperate grassland. J. Ecol. 2010, 98, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Laughlin D, C. Applying trait-based models to achieve functional targets for theory-driven ecological restoration. Ecol. Lett. 2014, 17, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Tolonen, K.E.; Leinonen, K.; Marttila, H.; Erkinaro, J.; Heino, J. Environmental predictability of taxonomic and functional community composition in high-latitude streams. Freshw. Biol. 2017, 62, 1–16. [Google Scholar] [CrossRef]
- Göthe, E.; Baattrup-Pedersen, A.; Wiberg-Larsen, P.; Graeber, D.; Kristensen, E.A.; Friberg, N. Environmental and spatial controls of taxonomic versus trait composition of stream biota. Freshw. Biol. 2017, 62, 397–413. [Google Scholar] [CrossRef]
- Dănescu, A.; Albrecht, A.T.; Bauhus, J. Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia 2016, 182, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Schielzeth, H.; Barnes, A.D.; Barry, K.E.; Bonn, A.; Brose, U.; Bruelheide, H.; Buchmann, N.; Buscot, F.; Ebeling, A.; et al. A multitrophic perspective on biodiversity-ecosystem functioning research. Adv. Ecol. Res. 2019, 61, 1–54. [Google Scholar] [PubMed]
- Maureaud, A.; Andersen, K.H.; Zhang, L.; Lindegren, M. Trait-based food web model reveals the underlying mechanisms of biodiversity–ecosystem functioning relationships. J. Anim. Ecol. 2020, 89, 1497–1510. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, S.R. Plant decomposition and soil respiration in terrestrial ecosystems. Bot. Rev. 1977, 43, 449–528. [Google Scholar] [CrossRef]
- Dias, A.; Ruijven, J.; Berendse, F. Plant species richness regulates soil respiration through changes in productivity. Oecologia 2010, 163, 805–813. [Google Scholar] [CrossRef] [Green Version]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Bongers, F. Biomass is the main driver of changes in ecosystem process rates during tropical forest succession. Ecology 2015, 96, 1242–1252. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, S.; Chen, Y.F.; Wu, C.Z.; Fan, H.; Lin, Y.; Li, J. Soil respiration characteristics and influencing factors of Castanopsis eyrei forest in different forest ages in Wuyi Mountain. Acta Ecol. Sin. 2021, 41, 2326–2338. [Google Scholar]
- Atkin, O.K.; Edwards, E.J.; Loveys, B.R. Response of root respiration to change in temperature and its relevance to global warming. New Phytol. 2000, 147, 141–154. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).