Studying the Physiological Reactions of C4 Grasses in Order to Select Them for Cultivation on Marginal Lands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
- Treatment 0—100% full dose of water and 180 NPK (90 N; 30 P; 60 K kg ha−1);
- Treatment I—100% full dose of water and 90 NPK (45 N; 15 P; 30 K kg ha−1);
- Treatment II—100% full dose of water and 270 NPK (135 N; 45 P; 90 K kg ha−1);
- Treatment III—85% dose of water and 180 NPK (90 N; 30 P; 60 K kg ha−1);
- Treatment IV—70% dose of water and 180 NPK (90 N; 30 P; 60 K kg ha−1).
2.2. Course of the Experiment
2.3. Physiological Parameters Measurements
2.4. Morphological Parameter Evaluation
2.5. Statistical Analysis
3. Results
3.1. Physiological Parameters
3.2. Morphological Parameters
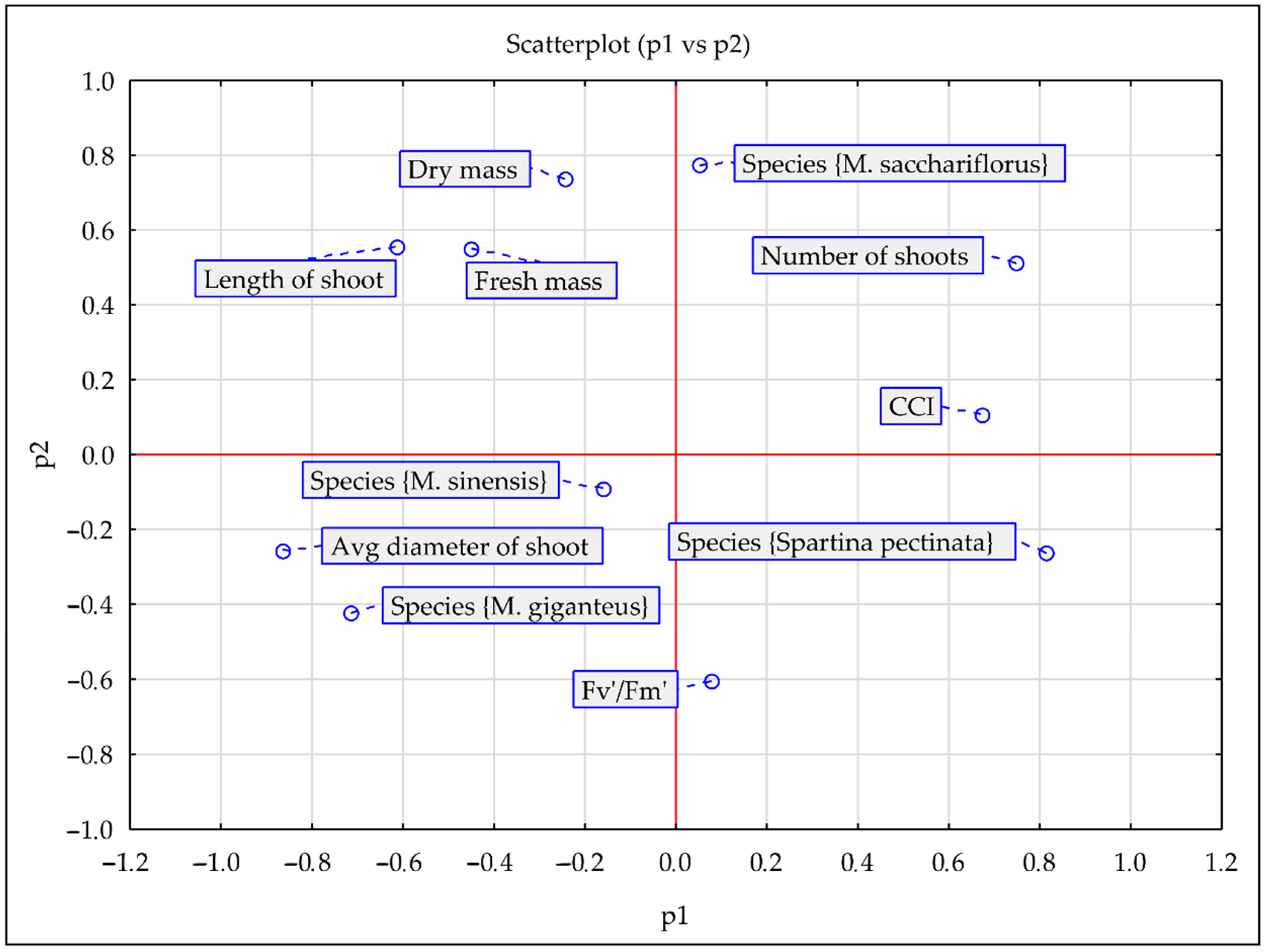
3.3. PCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What Is Sustainable Agriculture? A Systematic Review. Sustainability 2015, 7, 7833–7865. [Google Scholar] [CrossRef] [Green Version]
- Kajala, K.; Covshoff, S.; Karki, S.; Woodfield, H.; Tolley, B.J.; Jaqueline Dionora, M.A.; Mogul, R.T.; Mabilangan, A.E.; Danila, F.R.; Hibberd, J.M.; et al. Strategies for engineering a two-celled C 4 photosynthetic pathway into rice. J. Exp. Bot. 2011, 62, 3001–3010. [Google Scholar] [CrossRef] [PubMed]
- Helis, M.; Strzelczyk, M.; Golimowski, W.; Steinhoff-Wrześniewska, A.; Paszkiewicz-Jasińska, A.; Hawrot-Paw, M.; Koniuszy, A.; Hryniewicz, M. Biomass potential of the marginal land of the polish sudetes mountain range. Energies 2021, 14, 7156. [Google Scholar] [CrossRef]
- Strijker, D. Marginal lands in Europe—Causes of decline. Basic Appl. Ecol. 2005, 6, 99–106. [Google Scholar] [CrossRef]
- Skevas, T.; Swinton, S.M.; Hayden, N.J. What type of landowner would supply marginal land for energy crops? Biomass Bioenergy 2014, 67, 252–259. [Google Scholar] [CrossRef]
- Mellor, P.; Lord, R.A.; João, E.; Thomas, R.; Hursthouse, A. Identifying non-agricultural marginal lands as a route to sustainable bioenergy provision—A review and holistic definition. Renew. Sustain. Energy Rev. 2021, 135, 110220. [Google Scholar] [CrossRef]
- Wójcik-Leń, J.; Sobolewska-Mikulska, K. Issues related to marginal lands with reference to selected agricultural problematic areas. J. Water Land Dev. 2017, 35, 265–273. [Google Scholar] [CrossRef]
- Hryniewicz, M.; Strzelczyk, M.; Helis, M.; Paszkiewicz-Jasińska, A.; Steinhoff-Wrzesniewska, A.; Roman, K. Mathematical models use to yield prognosis of perennials on marginal land according to fertilisers doses. J. Water Land Dev. 2021, 51, 233–242. [Google Scholar] [CrossRef]
- Dubis, B.; Jankowski, K.J.; Załuski, D.; Bórawski, P.; Szempliński, W. Biomass production and energy balance of Miscanthus over a period of 11 years: A case study in a large-scale farm in Poland. GCB Bioenergy 2019, 11, 1187–1201. [Google Scholar] [CrossRef] [Green Version]
- Helios, W.; Kozak, M.; Malarz, W.; Kotecki, A. Effect of sewage sludge application on the growth, yield and chemical composition of prairie cordgrass (Spartina pectinata Link.). J. Elem. 2014, 19, 1021–1036. [Google Scholar] [CrossRef]
- Juliszewski, T.; Kwaśniewski, D.; Mudryk, K.; Wróbel, M. Ocena wybranych parametrów biomasy pozyskanej z plantacji drzew szybkorosnących. Inżynieria Rol. 2012, 16, 89–97. [Google Scholar]
- Krzyżaniak, M.; Stolarski, M.J.; Warmiński, K. Life cycle assessment of giant Miscanthus: Production on marginal soil with various fertilisation treatments. Energies 2020, 13, 1931. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Śnieg, M.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Short rotation coppices, grasses and other herbaceous crops: Productivity and yield energy value versus 26 genotypes. Biomass Bioenergy 2018, 119, 109–120. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Hastings, A.; Mos, M.; McCalmont, J.P.; Ashman, C.; Awty-Carroll, D.; Cerazy, J.; Chiang, Y.-C.; Cosentino, S.; Cracroft-Eley, W.; et al. Progress in upscaling Miscanthus biomass production for the European bio-economy with seed-based hybrids. GCB Bioenergy 2017, 9, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Dohleman, F.G.; Heaton, E.A.; Arundale, R.A.; Long, S.P. Seasonal dynamics of above- and below-ground biomass and nitrogen partitioning in Miscanthus × giganteus and Panicum virgatum across three growing seasons. GCB Bioenergy 2012, 4, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Thapa, S.; Voigt, T.; Rayburn, A.L.; Boe, A.; Lee, D.K. Phenotypic and Biomass Yield Variations in Natural Populations of Prairie Cordgrass (Spartina pectinata Link) in the USA. Bioenergy Res. 2015, 8, 1371–1383. [Google Scholar] [CrossRef]
- Mehmood, N.; Nayyar, H.; Gupta, D. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and an... Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- Taylor, S.H.; Ripley, B.S.; Woodward, F.I.; Osborne, C.P. Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment. Plant. Cell Environ. 2011, 34, 65–75. [Google Scholar] [CrossRef]
- Way, D.A.; Katul, G.G.; Manzoni, S.; Vico, G. Increasing water use efficiency along the C3 to C4 evolutionary pathway: A stomatal optimization perspective. J. Exp. Bot. 2014, 65, 3683–3693. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Schrama, M.; Vandecasteele, B.; Carvalho, S.; Muylle, H.; van der Putten, W.H. Effects of first- and second-generation bioenergy crops on soil processes and legacy effects on a subsequent crop. GCB Bioenergy 2016, 8, 136–147. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Sun, L.; Huang, Z.; He, L.; Zhang, W.; Chen, Z. Promotion of growth and Cu accumulation of bio-energy crop (Zea mays) by bacteria: Implications for energy plant biomass production and phytoremediation. J. Environ. Manag. 2012, 103, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Heshmat, K.; Asgari Lajayer, B.; Shakiba, M.R.; Astatkie, T. Assessment of physiological traits of common bean cultivars in response to water stress and molybdenum levels. J. Plant Nutr. 2020, 44, 366–372. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, J.; Soni, V.; Kalaji, H.M.; Elsheery, N.I. Waterlogging tolerance: A review on regulative morpho-physiological homeostasis of crop plants. J. Water L. Dev. 2021, 49, 16–28. [Google Scholar] [CrossRef]
- Silva, M.d.A.; Pincelli, R.P.; Barbosa, M.d.A. Water stress effects on chlorophyll fluorescence and chlorophyll content in sugarcane cultivars with contrasting tolerance. Biosci. J. 2018, 34, 75–87. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, X.; Lv, T.; Bai, M.; Wang, Z.; Niu, J. Effects of Water Stress on Fluorescence Parameters and Photosynthetic Characteristics of Drip Irrigation in Rice. Water 2020, 12, 289. [Google Scholar] [CrossRef] [Green Version]
- Khadem Moghadam, N.; Motesharezadeh, B.; Maali-Amiri, R.; Asgari Lajayer, B.; Astatkie, T. Effects of potassium and zinc on physiology and chlorophyll fluorescence of two cultivars of canola grown under salinity stress. Arab. J. Geosci. 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Horaczek, T.; Dąbrowski, P.; Kalaji, H.M.; Baczewska-Dąbrowska, A.H.; Pietkiewicz, S.; Stępień, W.; Gozdowski, D. JIP-test as a tool for early detection of the macronutrients deficiencin miscanthus plants. Photosynthetica 2020, 58, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by invivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Mastalerczuk, G.; Borawska-Jarmulowicz, B.; Kalaji, H.M.; Dąbrowski, P.; Paderewski, J. Gas-exchange parameters and morphological features of festulolium (Festulolium braunii K. Richert A. Camus) in response to nitrogen dosage. Photosynthetica 2017, 55, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Curran, P.J.; Dungan, J.L.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll content in slash pine. Tree Physiol. 1990, 7, 33–48. [Google Scholar] [CrossRef]
- Moran, J.A.; Mitchell, A.K.; Goodmanson, G.; Stockburger, K.A. Differentiation among effects of nitrogen fertilization treatments on conifer seedlings by foliar reflectance: A comparison of methods. Tree Physiol. 2000, 20, 1113–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaji, H.M.; Dąbrowski, P.; Cetner, M.D.; Samborska, I.A.; Łukasik, I.; Brestic, M.; Zivcak, M.; Tomasz, H.; Mojski, J.; Kociel, H.; et al. A comparison between different chlorophyll content meters under nutrient deficiency conditions. J. Plant Nutr. 2017, 40, 1024–1034. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Račková, L.; Paganová, V.; Swoczyna, T.; Rusinowski, S.; Sitko, K. Can chlorophyll-a fluorescence parameters be used as bio-indicators to distinguish between drought and salinity stress in Tilia cordata Mill? Environ. Exp. Bot. 2018, 152, 149–157. [Google Scholar] [CrossRef]
- Wu, Y.-w.; Li, Q.; Jin, R.; Chen, W.; Liu, X.-l.; Kong, F.-l.; Ke, Y.-p.; Shi, H.-c.; Yuan, J.-c. Effect of low-nitrogen stress on photosynthesis and chlorophyll fluorescence characteristics of maize cultivars with different low-nitrogen tolerances. J. Integr. Agric. 2019, 18, 1246–1256. [Google Scholar] [CrossRef]
- Faseela, P.; Sinisha, A.K.; Brestič, M.; Puthur, J.T. Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2019, 57, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Anjos, L.; Gaistardo, C.; Deckers, J.; Dondeyne, S.; Eberhardt, E.; Gerasimova, M.; Harms, B.; Jones, A.; Krasilnikov, P.; Reinsch, T.; et al. World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015. [Google Scholar]
- Borawska-Jarmulowicz, B.; Mastalerczuk, G.; Dąbrowski, P.; Kalaji, H.M.; Wytrazek, K. Improving tolerance in seedlings of some Polish varieties of Dactylis glomerata to water deficit by application of simulated drought during seed germination. Photosynthetica 2020, 58, 540–548. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik-Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of chlorophyll a fluorescence and plant gas exchange parameters as indicators of drought tolerance in perennial ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.H.; Langridge, J.L. Modeling plant and soil water dynamics in semi-arid ecosystems with limited site data. Ecol. Modell. 1996, 87, 153–167. [Google Scholar] [CrossRef]
- Valladares, F.; Pearcy, R.W. Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia. Plant Cell Environ. 1997, 20, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Shangguan, Z.P.; Shao, M.A.; Dyckmans, J. Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environ. Exp. Bot. 2000, 44, 141–149. [Google Scholar] [CrossRef]
- Kim, S.; Albrecht, K.; Sheaffer, C.; Lee, D.; Subramanian, S.; Owens, V. Biomass Production of Prairie Cordgrass (Spartina pectinata Link.) Using Urea and Kura Clover (Trifolium ambiguum Bieb.) as a Source of Nitrogen. Bioenergy Resour. 2020, 13, 1095–1107. [Google Scholar] [CrossRef]
- McDonald, A.J.S.; Davies, W.J. Keeping in Touch: Responses of the Whole Plant to Deficits in Water and Nitrogen Supply. In Advances in Botanical Research; Callow, J.A., Ed.; Academic Press Inc.: San Diego, CA, USA, 1996; Volume 22, pp. 229–300. [Google Scholar]
- Rascher, U.; Liebig, M.; Luttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2000, 23, 1397–1405. [Google Scholar] [CrossRef]
- Ciompi, S.; Gentili, E.; Guidi, L.; Soldatini, G.F. The effect of nitrogen deficiency on leaf gas exchange and chlorophyll fluorescence parameters in sunflower. Plant Sci. 1996, 118, 177–184. [Google Scholar] [CrossRef]
- Sugiharto, B.; Miyata, K.; Nakamoto, H.; Sasakawa, H.; Sugiyama, T. Regulation of expression of carbon-assimilating enzymes by nitrogen in maize leaf. Plant Physiol. 1990, 92, 963–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Yang, G.; Tan, C.; Zhao, C. Effects of nitrogen stress on the photosynthetic CO2 assimilation, chlorophyll fluorescence, and sugar-nitrogen ratio in corn. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, M.A.; Ramalho, J.D.C.; Dias, M.A. Effect of Nitrogen Supply on the Photosynthetic Performance of Leaves from Coffee Plants Exposed to Bright Light. J. Exp. Bot. 1993, 44, 893–899. [Google Scholar] [CrossRef]
- Khamis, S.; Lamaze, T.; Lemoine, Y.; Foyer, C. Adaptation of the photosynthetic apparatus in maize leaves as a result of nitrogen limitation: Relationships between electron transport and carbon assimilation. Plant Physiol. 1990, 94, 1436–1443. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Zhang, J. Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci. 2000, 151, 135–143. [Google Scholar] [CrossRef]
- Zlatev, Z.; Fernando, C.L. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir. J. Food Agric. 2012, 24, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.Z.; Bao, W.K.; Li, F.L.; Wu, N. Effects of water stress and nitrogen supply on leaf gas exchange and fluorescence parameters of Sophora davidii seedlings. Photosynthetica 2008, 46, 40–48. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J. Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. J. Exp. Bot. 1999, 50, 1199–1206. [Google Scholar] [CrossRef]
- Hura, T.; Grzesiak, S.; Hura, K.; Thiemt, E.; Tokarz, K.; Wȩdzony, M. Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: Accumulation of ferulic acid correlates with drought tolerance. Ann. Bot. 2007, 100, 767–775. [Google Scholar] [CrossRef] [Green Version]
- Paknejad, F.; Nasri, M.; Moghadam, H.R.T.; Zahedi, H.; Alahmadi, M.J. Effects of drought stress on chlorophyll fluorescence parameters, chlorophyll content and grain yield of wheat cultivars. J. Biol. Sci. 2007, 7, 841–847. [Google Scholar] [CrossRef]
- Bouchenak, F.; Henri, P.; Benrebiha, F.Z.; Rey, P. Differential responses to salinity of two Atriplex halimus populations in relation to organic solutes and antioxidant systems involving thiol reductases. J. Plant Physiol. 2012, 169, 1445–1453. [Google Scholar] [CrossRef]
- Clarke, A.K.; Hurry, V.M.; Gustafsson, P.; Öquist, G. Two functionally distinct forms of the photosystem II reaction-center protein D1 in the cyanobacterium Synechococcus sp. PCC 7942. Proc. Natl. Acad. Sci. USA 1993, 90, 11985–11989. [Google Scholar] [CrossRef] [Green Version]
- Fracheboud, Y.; Haldimann, P.; Leipner, J.; Stamp, P. Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J. Exp. Bot. 1999, 50, 1533–1540. [Google Scholar] [CrossRef]
- Coste, S.; Baraloto, C.; Leroy, C.; Marcon, É.; Renaud, A.; Richardson, A.D.; Roggy, J.C.; Schimann, H.; Uddling, J.; Hérault, B. Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: A calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann. For. Sci. 2010, 67, 607. [Google Scholar] [CrossRef] [Green Version]
- Boe, A.; Owens, V.; Gonzalez-Hernandez, J.; Stein, J.; Lee, D.K.; Koo, B.C. Morphology and biomass production of prairie cordgrass on marginal lands. GCB Bioenergy 2009, 1, 240–250. [Google Scholar] [CrossRef]
- Heaton, E.A.; Voigt, T.B.; Long, S.P. A quantitative review comparing the yields of two candidate C4 perennial biomass crops in relation to nitrogen, temperature and water. Biomass Bioenergy 2004, 27, 21–30. [Google Scholar] [CrossRef]
- Kering, M.K.; Biermacher, J.T.; Butler, T.J.; Mosali, J.; Guretzky, J.A. Biomass Yield and Nutrient Responses of Switchgrass to Phosphorus Application. Bioenergy Res. 2012, 5, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Arundale, R.A.; Dohleman, F.G.; Voigt, T.B.; Long, S.P. Nitrogen Fertilization Does Significantly Increase Yields of Stands of Miscanthus × giganteus and Panicum virgatum in Multiyear Trials in Illinois. Bioenergia. Res. 2014, 7, 408–416. [Google Scholar] [CrossRef]
- Guo, J.; Thapa, S.; Voigt, T.; Owens, V.; Boe, A.; Lee, D.K. Biomass Yield and Feedstock Quality of Prairie Cordgrass in Response to Seeding Rate, Row Spacing, and Nitrogen Fertilization. Agron. J. 2017, 109, 2474–2485. [Google Scholar] [CrossRef]
- Weijde Van der, T.; Huxley, L.M.; Hawkins, S.; Sembiring, E.H.; Farrar, K.; Dolstra, O.; Visser, R.G.F.; Trindade, L.M. Impact of drought stress on growth and quality of miscanthus for biofuel production. GCB Bioenergy 2017, 9, 770–782. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, S.; Patane, C.; Sanzone, E.; Copani, V.; Foti, S. Effects of soil water content and nitrogen supply on the productivity of Miscanthus × giganteus Greef et Deu. in a Mediterranean environment. Ind. Crops Prod. 2007, 25, 75–88. [Google Scholar] [CrossRef]


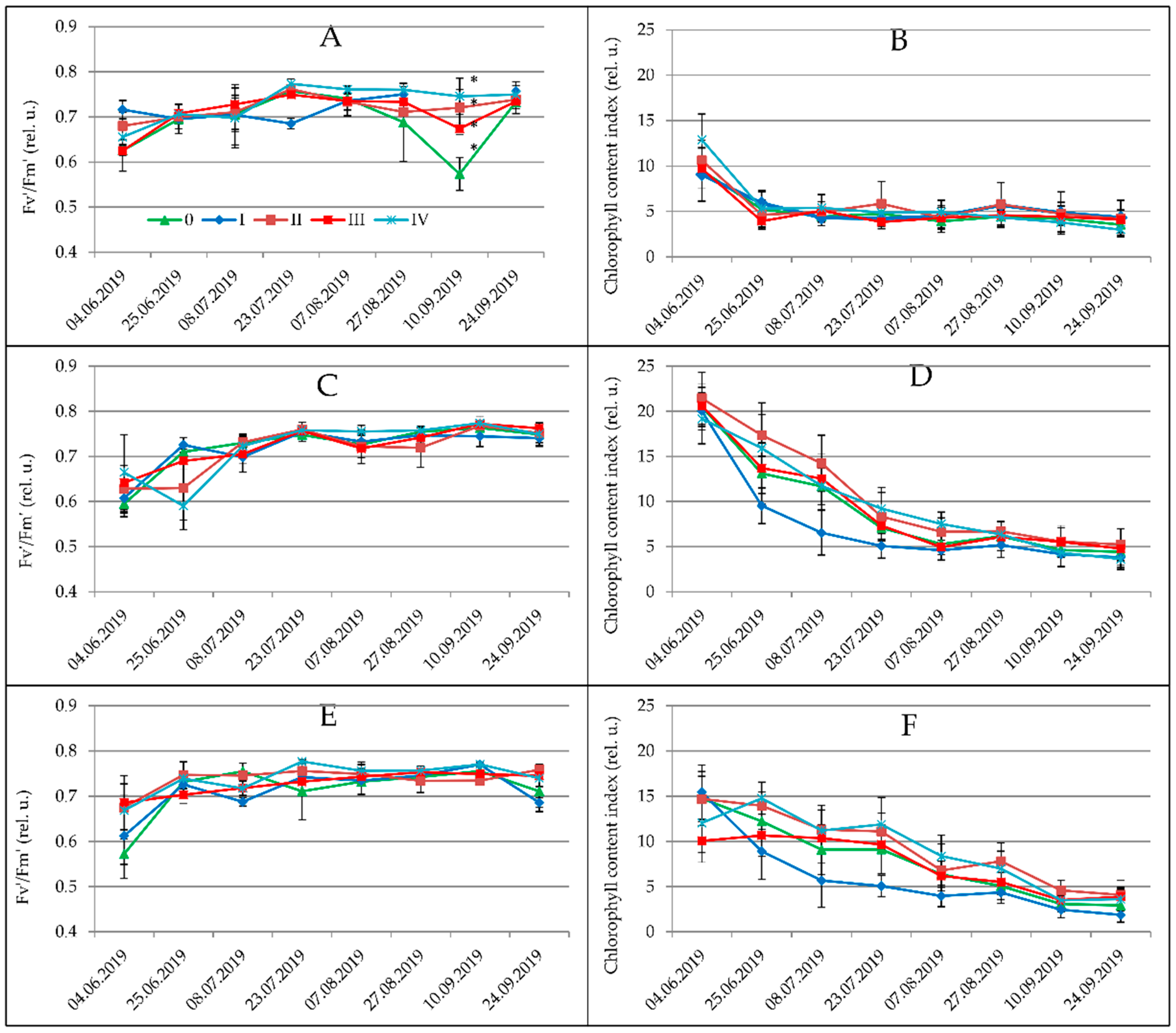



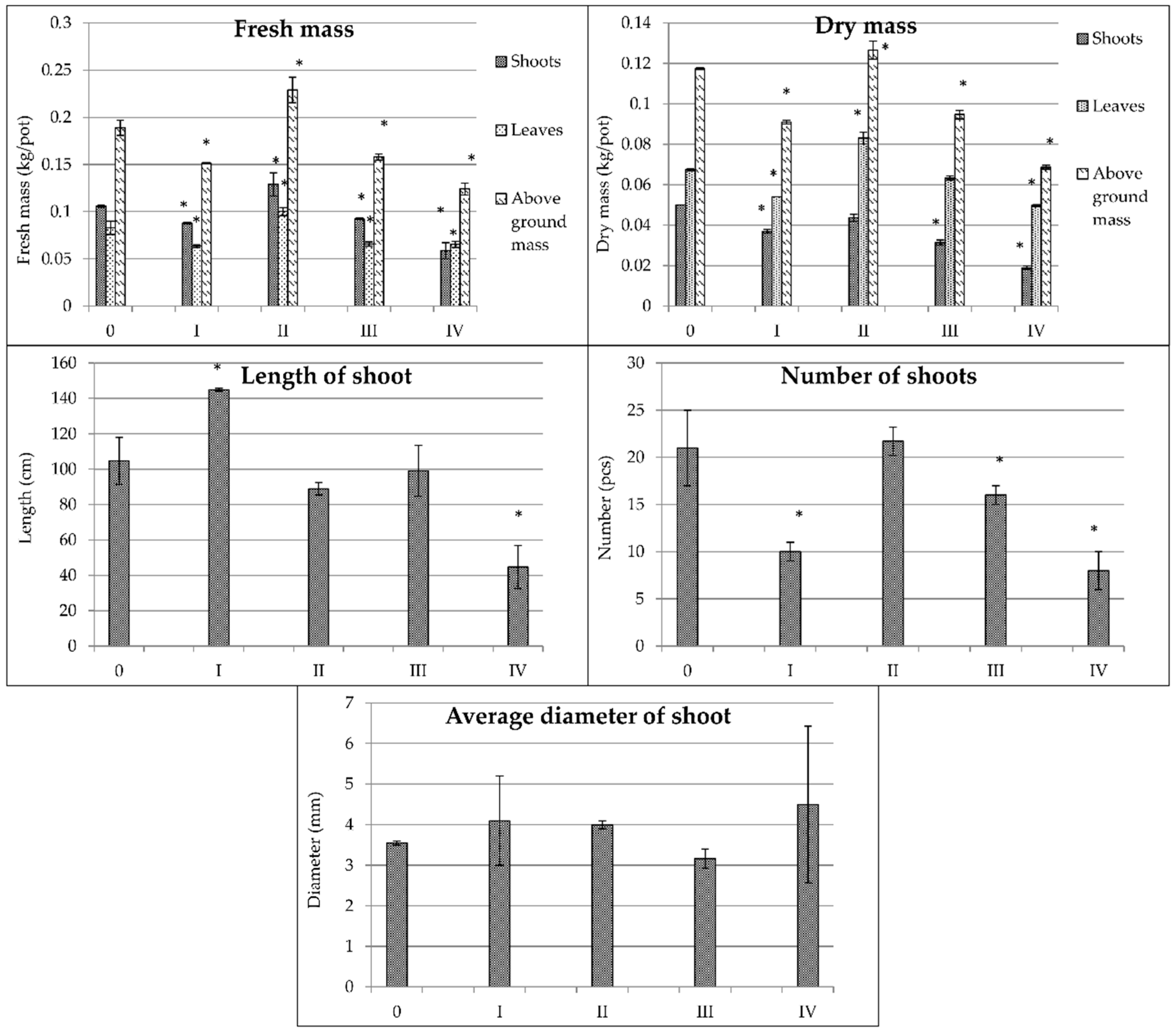
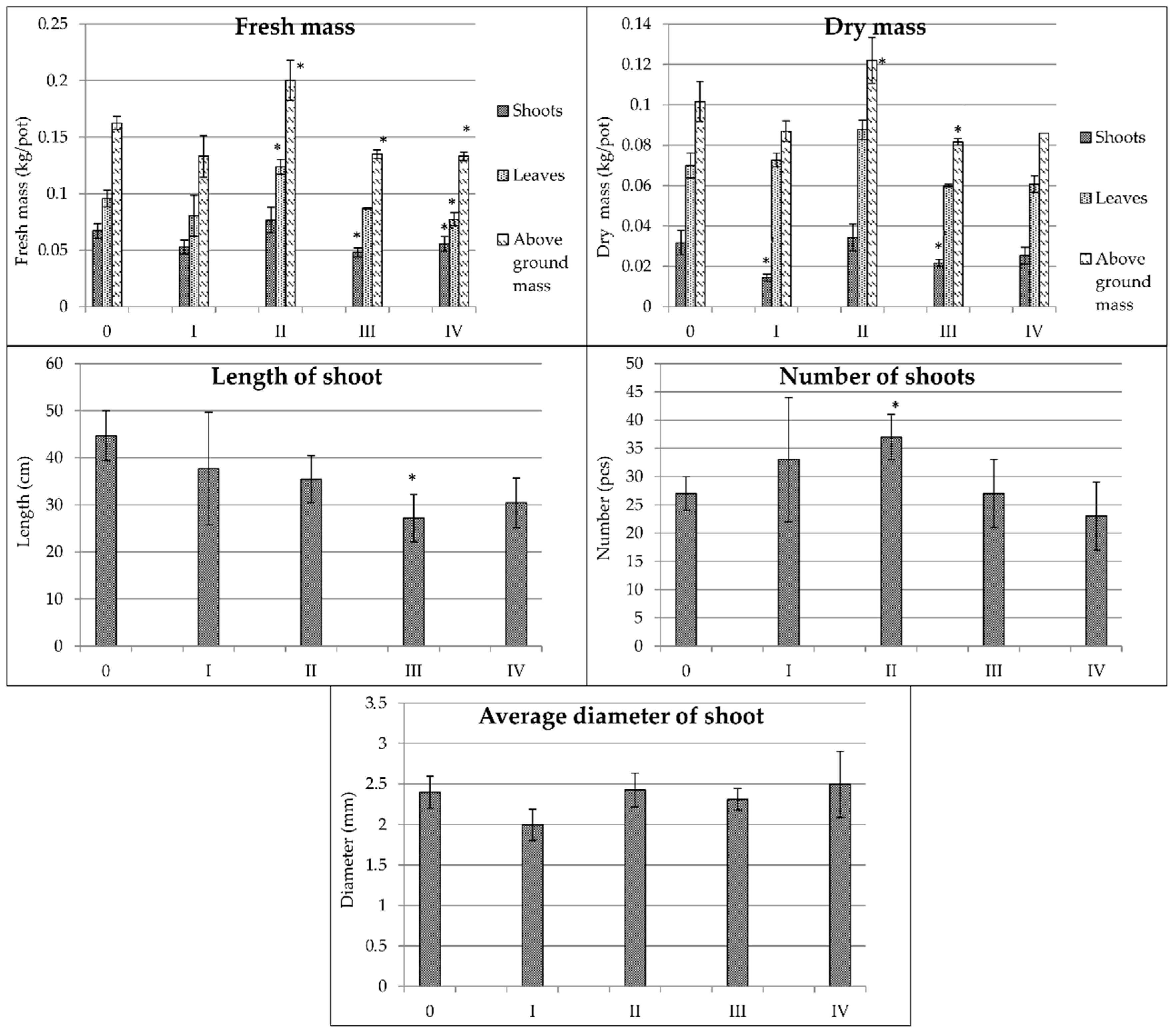
| Date of Measurement | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 June | 25 June | 8 July | 23 July | 7 August | 27 August | 10 September | 24 September | Mean | |
| Plant Species | |||||||||
| Spartina pectinata | 11.94 b | 12.11 c | 9.18 b,c | 9.42 b | 8.45 c | 7.67 b | 5.02 b | 5.57 a,b | 8.65 c |
| Miscanthus × giganteus | 16.10 c | 10.83 a,b | 9.86 c | 7.46 a | 6.25 b | 5.91 a | 4.14 a | 3.30 a | 8.02 b |
| Miscanthus sacchariflorus | 9.95 a | 8.50 a | 7.74 a | 6.57 a | 6.05 a,b | 6.50 a | 5.52 b | 7.52 b | 7.25 a |
| Miscanthus sinensis | 14.89 c | 10.25 b | 8.48 a,b | 7.11 a | 5.52 a | 5.71 a | 4.25 a | 3.82 a | 7.52 a,b |
| Water Regime and Fertilization Treatments | |||||||||
| 0 | 13.18 a,b | 9.38 b | 7.90 b | 6.77 b | 5.89 b | 5.55 a | 4.09 a,b | 4.67 a | 7.25 b |
| I | 11.23 a | 7.31 a | 5.90 a | 5.33 a | 4.79 a | 4.88 a | 3.89 a | 4.10 a | 5.90 a |
| II | 14.51 b | 12.30 c | 10.83 c | 8.97 c | 7.41 b | 7.27 b | 5.78 d | 6.13 a | 9.17 d |
| III | 13.15 a,b | 10.65 b | 9.54 c | 8.12 c | 7.12 b | 7.09 b | 5.08 c,d | 4.91 a | 8.18 c |
| IV | 13.87 b | 12.32 c | 9.82 c | 8.92 c | 7.61 b | 7.39 b | 4.83 b,c | 5.55 a | 8.79 c,d |
| Level of Plant | |||||||||
| upper (B) | 9.84 a | 6.56 a | 6.15 a | 5.92 a | 5.77 a | 6.05 a | 5.20 b | 5.25 a | 6.32 a |
| medium (D) | 17.11 c | 12.81 b | 10.77 c | 7.66 b | 6.82 b | 6.90 b | 5.29 b | 4.83 a | 9.05 b |
| lower (F) | 12.55 b | 11.87 b | 9.51 b | 9.37 c | 7.15 b | 6.41 a,b | 3.71 a | 5.15 a | 8.20 b |
| Date of Measurement | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 June | 25 June | 8 July | 23 July | 7 August | 27 August | 10 September | 24 September | Mean | |
| Plant Species | |||||||||
| Spartina pectinata | 0.712 b | 0.698 b | 0.742 b | 0.767 b | 0.768 d | 0.767 b | 0.781 b | 0.749 a | 0.748 c |
| Miscanthus × giganteus | 0.715 b | 0.645 a | 0.709 b | 0.761 b | 0.751 c | 0.738 a | 0.766 ab | 0.747 a | 0.729 b |
| Miscanthus sacchariflorus | 0.631 a | 0.702 b | 0.596 a | 0.711 a | 0.706 a | 0.737 a | 0.723 a | 0.720 a | 0.686 a |
| Miscanthus sinensis | 0.624 a | 0.700 b | 0.721 b | 0.750 b | 0.736 b | 0.742 a | 0.728 a | 0.738 a | 0.717 b |
| Water Regime and Fertilization Treatments | |||||||||
| 0 | 0.65 a | 0.673 a | 0.701 a | 0.743 a | 0.734 a | 0.744 a | 0.718 a | 0.734 a | 0.710 a |
| I | 0.691 a | 0.704 a | 0.698 a | 0.757 a | 0.739 a,b | 0.745 a | 0.761 a | 0.730 a | 0.721 a |
| II | 0.680 a | 0.691 a | 0.677 a | 0.733 a | 0.745 a,b | 0.746 a | 0.758 a | 0.738 a | 0.720 a |
| III | 0.671 a | 0.691 a | 0.693 a | 0.752 a | 0.738 a,b | 0.750 a | 0.757 a | 0.742 a | 0.722 a |
| IV | 0.669 a | 0.685 a | 0.716 a | 0.764 a | 0.763 b | 0.756 a | 0.769 a | 0.759 a | 0.719 a |
| Level of Plant | |||||||||
| upper (A) | 0.633 a | 0.701 a | 0.694 a | 0.741 a | 0.741 a | 0.749 a | 0.711 a | 0.726 a | 0.707 a |
| medium (C) | 0.671 a,b | 0.677 a | 0.691 a | 0.752 a | 0.739 a | 0.748 a | 0.765 b | 0.744 a | 0.721 b |
| lower (E) | 0.696 b | 0.694 a | 0.696 a | 0.746 a | 0.742 a | 0.746 a | 0.756 a,b | 0.739 a | 0.726 b |
| Fresh Mass (kg per Pot) | Dry Mass (kg per Pot) | Stem Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shoots | Leaves | Above-Ground Mass | Shoots/Leaves | Shoots | Leaves | Above-Ground Mass | Shoots/Leaves | Length (cm) | Number (Pieces) | Diameter (mm) | |
| Plant Species | |||||||||||
| Spartina pectinata | 0.060 a | 0.093 b | 0.153 a | 0.64 a | 0.025 a | 0.070 b | 0.096 a | 0.36 a | 35.1 a | 29.4 c | 2.33 a |
| Miscanthus × giganteus | 0.110 c | 0.073 a | 0.184 b | 1.51 c | 0.034 b | 0.064 a | 0.098 a | 0.53 b | 78.9 b | 8.7 a | 7.04 d |
| Miscanthus sacchariflorus | 0.101 b,c | 0.078 a | 0.179 b | 1.29 b | 0.050 c | 0.066 a | 0.119 b | 0.76 c | 101.9 c | 28.1 c | 3.22 b |
| Miscanthus sinensis | 0.095 b | 0.076 a | 0.171 b | 1.25 b | 0.036 b | 0.064 a | 0.100 a | 0.56 b | 96.5 c | 15.5 b | 3.80 c |
| Water Regime and Fertilization Treatments | |||||||||||
| 0 | 0.109 c | 0.084 b | 0.193 c | 1.30 c | 0.047 c | 0.069 c | 0.116 c | 0.68 d | 87.8 c | 21.4 b | 4.22 a |
| I | 0.095 b | 0.069 a | 0.164 b | 1.38 c | 0.036 b | 0.060 b | 0.097 b | 0.60 b | 105.1 d | 18.9 a | 4.14 a |
| II | 0.113 c | 0.106 c | 0.219 d | 1.07 b | 0.042 c | 0.086 d | 0.132 d | 0.49 a | 71.4 b | 25.5 b | 4.23 a |
| III | 0.076 a | 0.072 a | 0.148 a | 1.06 b | 0.030 a | 0.060 b | 0.090 b | 0.50 a | 71.7 b | 19.3 a | 4.03 a |
| IV | 0.067 a | 0.069 a | 0.135 a | 0.97 a | 0.027 a | 0.055 a | 0.082 a | 0.49 a | 54.4 a | 17.0 a | 3.88 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinhoff-Wrześniewska, A.; Dąbrowski, P.; Paszkiewicz-Jasińska, A.; Wróbel, B.; Strzelczyk, M.; Helis, M.; Kalaji, M.H. Studying the Physiological Reactions of C4 Grasses in Order to Select Them for Cultivation on Marginal Lands. Sustainability 2022, 14, 4512. https://doi.org/10.3390/su14084512
Steinhoff-Wrześniewska A, Dąbrowski P, Paszkiewicz-Jasińska A, Wróbel B, Strzelczyk M, Helis M, Kalaji MH. Studying the Physiological Reactions of C4 Grasses in Order to Select Them for Cultivation on Marginal Lands. Sustainability. 2022; 14(8):4512. https://doi.org/10.3390/su14084512
Chicago/Turabian StyleSteinhoff-Wrześniewska, Aleksandra, Piotr Dąbrowski, Anna Paszkiewicz-Jasińska, Barbara Wróbel, Maria Strzelczyk, Marek Helis, and Mohamed Hazem Kalaji. 2022. "Studying the Physiological Reactions of C4 Grasses in Order to Select Them for Cultivation on Marginal Lands" Sustainability 14, no. 8: 4512. https://doi.org/10.3390/su14084512
APA StyleSteinhoff-Wrześniewska, A., Dąbrowski, P., Paszkiewicz-Jasińska, A., Wróbel, B., Strzelczyk, M., Helis, M., & Kalaji, M. H. (2022). Studying the Physiological Reactions of C4 Grasses in Order to Select Them for Cultivation on Marginal Lands. Sustainability, 14(8), 4512. https://doi.org/10.3390/su14084512








