Bioremediation of 27 Micropollutants by Symbiotic Microorganisms of Wetland Macrophytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of a Bioremediation Experiment
2.2. Microorganisms
3. Results
3.1. General Parameters and Macronutrients
3.2. Removal of Micropollutants
- Carbamazepine, which is a poorly biodegradable compound, and its metabolites can build back to the parent compound. Therefore, removal is not assumed in conventional WWTPs [51], while in the presented experiments, this compound was removed up to 80%.
- Fluorosurfactants, in this case, PFOA and PFOS, are generally persistent compounds that tend to accumulate in the surrounding media [61] and, in the present study, were removed up to 66% (PFOA) and 27% (PFOS).
3.3. Microbial Composition
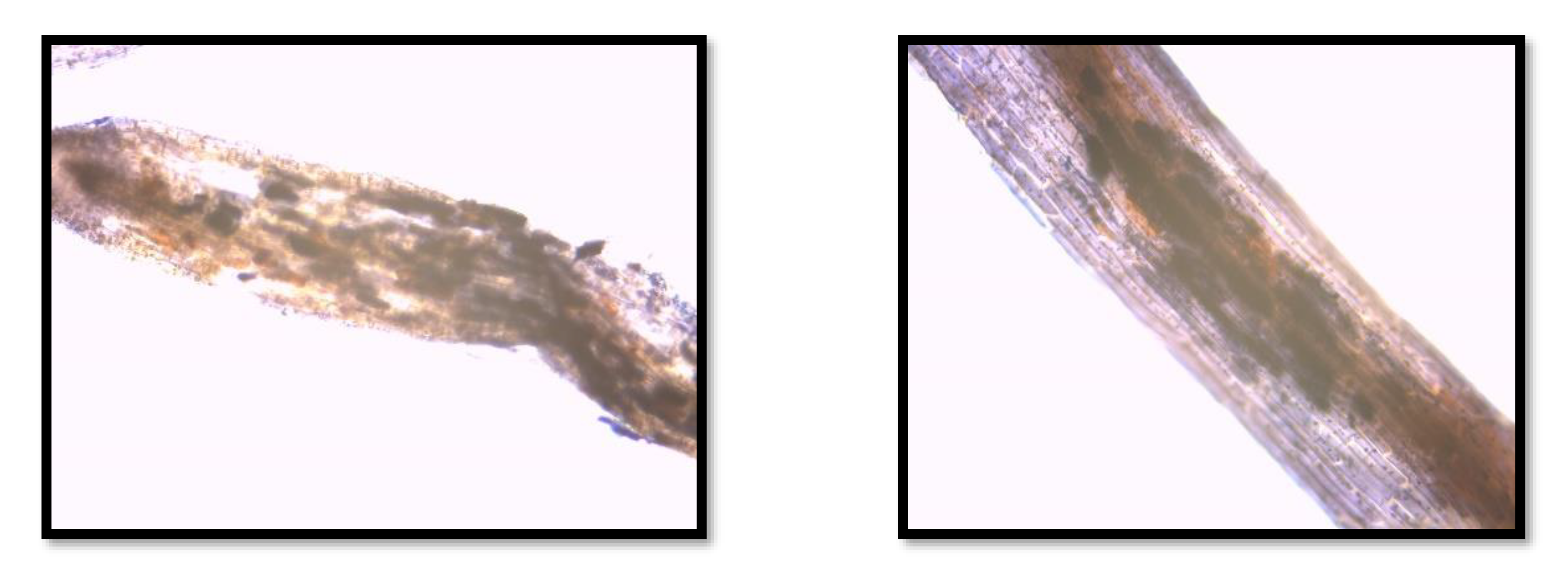
3.4. Colonization of the Roots by AMF
4. Conclusions
- Compared to our previous phytoremediation experiments, the currently described bioremediation experiment in semi-hydroponic conditions showed improved MP removal, which we believe was due to the additional aeration, recirculation of the liquid medium, and commercially bought hydroponic solutions, which favor the growth conditions of the plants and, therefore, enhance the development of the rhizosphere and consequent removal of MPs.
- The most efficient bioremediative system was the system with Iris pseudacorus, which removed 22 out of 27 of the MPs with more than 80% efficiency.
- Compounds, which are not well-removed in other bioremediation experiments, were removed here, with more than 90% efficiency (e.g., beta-blockers, carbendazim, cyclophosphamide, and DEET).
- Generally persistent compounds were removed with high efficiency (metoprolol up to 91%, lidocaine up to 84%, and TCIPP up to 90%).
- Possible ongoing nitrification likely enhanced the bioremediative process, as many of the MPs are degraded by nitrifying bacteria.
- Lythrum salicaria had the lowest efficiency for removing MPs (contrary to previous phytoremediation experiments). This is probably due to its weak physiological status after the winter season.
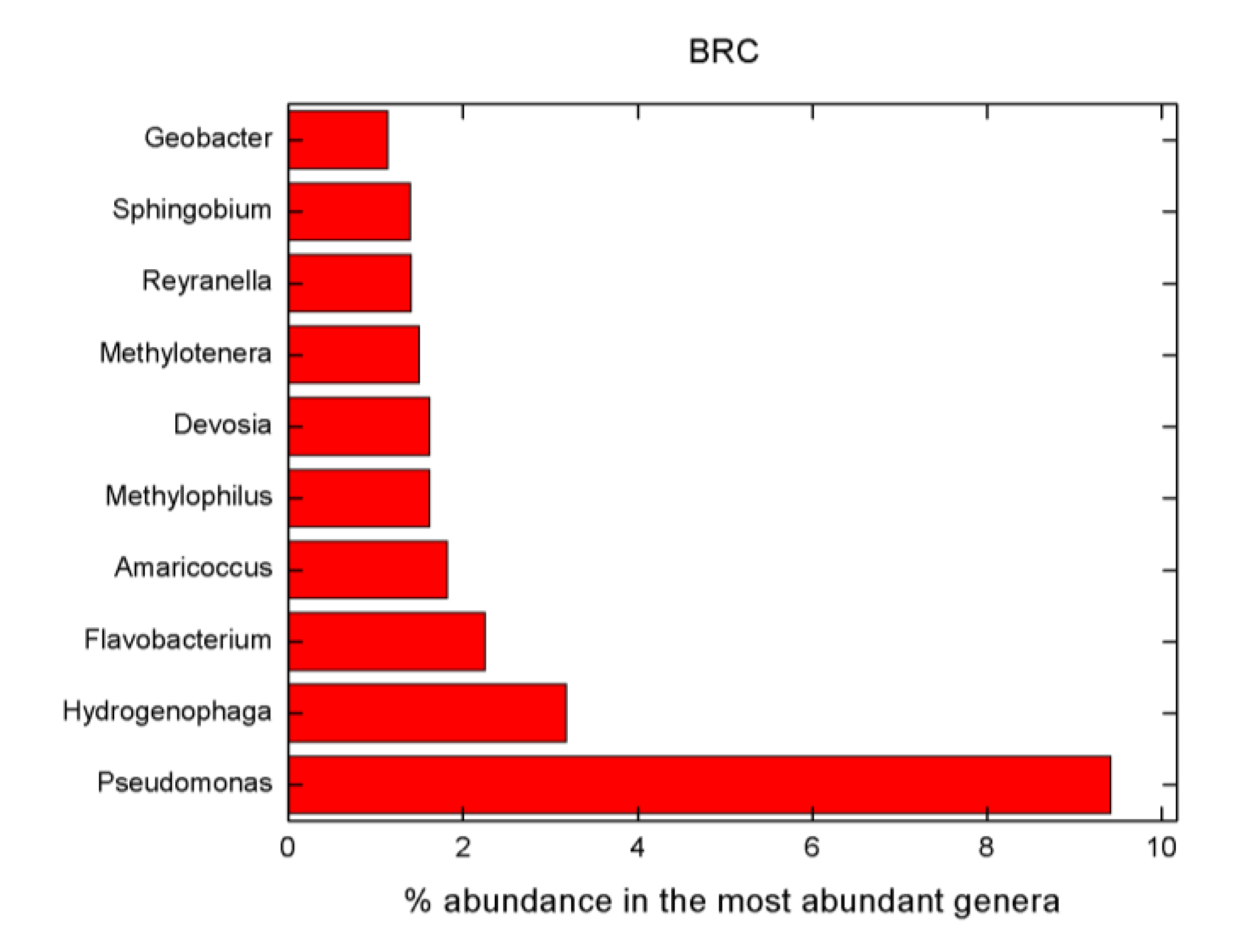
- Pseudomonas, Flavobacterium, Variovorax, Methylotenera, Reyranella, Amaricoccus and Hydrogenophaga belong to genera that are known to be potential MP degraders. High abundances of these organisms were also found in our samples.
- A colonization of the plant roots by AMF was established. This information is valuable, as AMF contribute to phyto- and bioremediation. The macrophyte with the highest colonization was Iris pseudacorus (56%).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Falås, P.; Wick, A.; Castronovo, S.; Habermacher, J.; Ternes, T.A.; Joss, A. Tracing the limits of organic micropollutant removal in biological wastewater treatment. Water Res. 2016, 95, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Cirja, M.; Ivashechkin, P.; Schäffer, A.; Corvini, P.F.X. Factors affecting the removal of organic micropollutants from wastewater in conventional treatment plants (CTP) and membrane bioreactors (MBR). Rev. Environ. Sci. Biotechnol. 2008, 7, 61–78. [Google Scholar] [CrossRef]
- Xue, P.; Zhao, Y.; Zhao, D.; Chi, M.; Yin, Y.; Xuan, Y.; Wang, X. Mutagenicity, health risk, and disease burden of exposure to organic micropollutants in water from a drinking water treatment plant in the Yangtze River Delta, China. Ecotoxicol. Environ. Saf. 2021, 221, 112421. [Google Scholar] [CrossRef]
- Anderson, J.C.; Joudan, S.; Shoichet, E.; Cuscito, L.D.; Alipio, A.E.C.; Donaldson, C.S.; Khan, S.; Goltz, D.M.; Rudy, M.D.; Frank, R.A.; et al. Reducing nutrients, organic micropollutants, antibiotic resistance, and toxicity in rural wastewater effluent with subsurface filtration treatment technology. Ecol. Eng. 2015, 84, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- EC. Commission Implementing Decision (EU). Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to DIRECTIVE 2008/105/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015D0495&from=PT (accessed on 1 March 2022).
- EC. Commission Implementing Decision (EU). Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Commission Implementing Decision (EU) 2015/495. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0840&rid=7 (accessed on 1 March 2022).
- EC. Report from the Commission to the European Parliament and the Council. 2019. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:52020DC0016 (accessed on 1 March 2022).
- Kharel, S.; Stapf, M.; Miehe, U.; Ekblad, M.; Cimbritz, M.; Falås, P.; Nilsson, J.; Sehlén, R.; Bregendahl, J.; Bester, K. Removal of pharmaceutical metabolites in wastewater ozonation including their fate in different post-treatments. Sci. Total Environ. 2021, 759, 143989. [Google Scholar] [CrossRef] [PubMed]
- Trapido, M.; Epold, I.; Bolobajev, J.; Dulova, N. Emerging micropollutants in water/wastewater: Growing demand on removal technologies. Environ. Sci. Pollut. Res. 2014, 21, 12217–12222. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; al Aukidy, M.; Zambello, E. What have we learned from worldwide experiences on the management and treatment of hospital effluent? An overview and a discussion on perspectives. Sci. Total Environ. 2015, 514, 467–491. [Google Scholar] [CrossRef]
- EU Delivering on the UN 2030 Agenda Sustainable Development in Europe and the World #SustainableEurope #EU4SDGs #2030isNow n.d. Available online: https://ec.europa.eu/info/sites/default/files/factsheet-eu-delivering-2030-agenda-sustainable-development_en.pdf (accessed on 1 March 2022).
- Masi, F.; Rizzo, A.; Regelsberger, M. The role of constructed wetlands in a new circular economy, resource oriented, and ecosystem services paradigm. J. Environ. Manag. 2018, 216, 1–10. [Google Scholar] [CrossRef]
- Hsu, C.B.; Hsieh, H.L.; Yang, L.; Wu, S.H.; Chang, J.S.; Hsiao, S.C.; Su, H.C.; Yeh, S.C.; Yeh, C.H.; Ho, Y.S.; et al. Biodiversity of constructed wetlands for wastewater treatment. Ecol. Eng. 2011, 37, 1533–1545. [Google Scholar] [CrossRef]
- Knapp, S.; Schmauck, S.; Zehnsdorf, A. Biodiversity impact of green roofs and constructed wetlands as progressive eco-technologies in urban areas. Sustainability 2019, 11, 5846. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Langenhoff, A.; Bruning, H.; Rijnaarts, H. Sorption of micropollutants on selected constructed wetland support matrices. Chemosphere 2021, 275, 130050. [Google Scholar] [CrossRef] [PubMed]
- Reyes Contreras, C.; López, D.; Leiva, A.M.; Domínguez, C.; Bayona, J.M.; Vidal, G. Removal of organic micropollutants in wastewater treated by activated sludge and constructed wetlands: A comparative study. Water 2019, 11, 2515. [Google Scholar] [CrossRef] [Green Version]
- Ruppelt, J.P.; Pinnekamp, J.; Tondera, K. Elimination of micropollutants in four test-scale constructed wetlands treating combined sewer overflow: Influence of filtration layer height and feeding regime. Water Res. 2020, 169, 115214. [Google Scholar] [CrossRef] [PubMed]
- Venditti, S.; Brunhoferova, H.; Hansen, J. Behaviour of 27 selected emerging contaminants in vertical flow constructed wetlands as post-treatment for municipal wastewater. Sci. Total Environ. 2022, 819, 153234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jing, R.; Feng, X.; Dai, Y.; Tao, R.; Vymazal, J.; Cai, N.; Yalng, Y. Removal of acidic pharmaceuticals by small-scale constructed wetlands using different design configurations. Sci. Total Environ. 2018, 639, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Brunhoferova, H.; Venditti, S.; Schlienz, M.; Hansen, J. Removal of 27 micropollutants by selected wetland macrophytes in hydroponic conditions. Chemosphere 2021, 281, 130980. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Mülletr, R.; Moormalnn, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, L.; Sunyer-Caldú, A.; Uggetti, E.; Díez-Montero, R.; Díaz-Cruz, M.S.; García, J.; García-Galán, M.J. Bioremediation of emerging micropollutants in irrigation water. The alternative of microalgae-based treatments. J. Environ. Manag. 2020, 274, 111081. [Google Scholar] [CrossRef] [PubMed]
- Madadi, R.; Bester, K. Fungi and biochar applications in bioremediation of organic micropollutants from aquatic media. Mar. Pollut. Bull. 2021, 166, 112247. [Google Scholar] [CrossRef]
- Pop, C.E.; Draga, S.; Măciucă, R.; Niță, R.; Crăciun, N.; Wolff, R. Bisphenol A effects in aqueous environment on lemna minor. Processes 2021, 9, 1512. [Google Scholar] [CrossRef]
- Segura, A.; Ramos, J.L. Plant-bacteria interactions in the removal of pollutants. Curr. Opin. Biotechnol. 2013, 24, 467–473. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Chandra, H.; Kalra, S.J.S.; Mishra, P.; Khan, H.; Yadav, P. Plant–microbe interaction in aquatic system and their role in the management of water quality: A review. Appl. Water Sci. 2017, 7, 1079–1090. [Google Scholar] [CrossRef] [Green Version]
- Adrados, B.; Sánchez, O.; Arias, C.A.; Becares, E.; Garrido, L.; Mas, J.; Brix, H.; Morató, J. Microbial communities from different types of natural wastewater treatment systems: Vertical and horizontal flow constructed wetlands and biofilters. Water Res. 2014, 55, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Xiang, W.; Xiao, X.; Xue, J. Purification effect and microorganisms diversity in an Acorus calamus constructed wetland on petroleum-containing wastewater. Environ. Pollut. Bioavailab. 2020, 32, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Nitz, H.; Duarte, M.; Jauregui, R.; Pieper, D.H.; Müller, J.A.; Kästner, M. Identification of benzene-degrading Proteobacteria in a constructed wetland by employing in situ microcosms and RNA-stable isotope probing. Appl. Microbiol. Biotechnol. 2020, 104, 1809–1820. [Google Scholar] [CrossRef]
- Qin, P.; Lu, S.; Liu, X.; Wang, G.; Zhang, Y.; Li, D.; Radl, V. Removal of tri-(2-chloroisopropyl) phosphate (TCPP) by three types of constructed wetlands. Sci. Total Environ. 2020, 749, 141668. [Google Scholar] [CrossRef]
- Sauvêtre, A.; Węgrzyn, A.; Yang, L.; Vestergaard, G.; Miksch, K.; Schröder, P.; Radl, V. Enrichment of endophytic Actinobacteria in roots and rhizomes of Miscanthus × giganteus plants exposed to diclofenac and sulfamethoxazole. Environ. Sci. Pollut. Res. 2020, 27, 11892–11904. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Khalvati, M.; Bartha, B.; Dupigny, A.; Schröder, P. Arbuscular mycorrhizal association is beneficial for growth and detoxification of xenobiotics of barley under drought stress. J. Soils Sediments 2010, 10, 54–64. [Google Scholar] [CrossRef]
- Rajtor, M.; Piotrowska-Seget, Z. Prospects for arbuscular mycorrhizal fungi (AMF) to assist in phytoremediation of soil hydrocarbon contaminants. Chemosphere 2016, 162, 105–116. [Google Scholar] [CrossRef]
- Webster, R. Soil Sampling and Methods of Analysis—Edited by M.R. Carter & E.G. Gregorich. Eur. J. Soil Sci. 2008, 59. [Google Scholar] [CrossRef]
- Vallino, M.; Fiorilli, V.; Bonfante, P. Rice flooding negatively impacts root branching and arbuscular mycorrhizal colonization, but not fungal viability. Plant Cell Environ. 2014, 37, 557–572. [Google Scholar] [CrossRef]
- Daniels, B.A.; Trappe, J.M. Factors Affecting Spore Germination of the Vesicular-Arbuscular Mycorrhizal Fungus, Glomus epigaeus. Mycologia 1980, 72, 457–471. [Google Scholar] [CrossRef]
- Le Tacon, F.; Skinner, F.A.; Mosse, B. Spore germination and hyphal growth of a vesicular–arbuscular mycorrhizal fungus, Glomus mosseae (Gerdemann and Trappe), under decreased oxygen and increased carbon dioxide concentrations. Can. J. Microbiol. 1983, 29, 1280–1285. [Google Scholar] [CrossRef]
- Hamel, C.; Dalpe´, Y. Arbuscular mycorrhizae. In Soil Sampling and Methods of Analysis, 2nd ed.; Taylor & Francis: Oxfordshire, UK, 2007. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hydroponic Nutrient Solutions n.d. Available online: https://www.smart-fertilizer.com/articles/hydroponic-nutrient-solutions/ (accessed on 11 January 2022).
- Bozorg-Haddad, O.; Delpasand, M.; Loáiciga, H.A. Water quality, hygiene, and health. In Economical, Political, and Social Issues in Water Resources; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Margot, J.; Lochmatter, S.; Barry, D.A.; Holliger, C. Role of ammonia-oxidizing bacteria in micropollutant removal from wastewater with aerobic granular sludge. Water Sci. Technol. 2016, 73, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, B.; Liu, C. Removal mechanisms of β-blockers by anaerobic digestion in a UASB reactor with carbon feeding. Bioresour. Technol. Rep. 2020, 11, 100531. [Google Scholar] [CrossRef]
- Herzog, B.; Huber, B.; Lemmer, H.; Horn, H.; Müller, E. Analysis and in situ characterization of activated sludge communities capable of benzotriazole biodegradation. Environ. Sci. Eur. 2013, 25, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sbardella, L.; Comas, J.; Fenu, A.; Rodriguez-Roda, I.; Weemaes, M. Advanced biological activated carbon filter for removing pharmaceutically active compounds from treated wastewater. Sci. Total Environ. 2018, 636, 519–529. [Google Scholar] [CrossRef]
- Li, Y.; Chi, M.M.; Ge, X.Z. Identification of a novel hydrolase encoded by hy-1 from Bacillus amyloliquefaciens for bioremediation of carbendazim contaminated soil and food. Int. J. Agric. Biol. 2019, 12, 218–224. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.G.; Wei, X.D.; Liu, Y.S.; Liu, S.S.; Hu, L.X.; He, L.-Y.; Chen, Z.-F.; Chen, F.-R.; Yang, Y.-Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Effect of flow configuration and plant species. Sci. Total Environ. 2016, 571, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Castellet-Rovira, F.; Lucas, D.; Villagrasa, M.; Rodríguez-Mozaz, S.; Barceló, D.; Sarrà, M. Stropharia rugosoannulata and Gymnopilus luteofolius: Promising fungal species for pharmaceutical biodegradation in contaminated water. J. Environ. Manage. 2018, 207, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Shreve, M.J.; Brockman, A.; Hartleb, M.; Prebihalo, S.; Dorman, F.L.; Brennan, R.A. The white-rot fungus Trametes versicolor reduces the estrogenic activity of a mixture of emerging contaminants in wastewater treatment plant effluent. Int. Biodeter. Biodegr. 2016, 109, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Ávila, C.; García-Galán, M.J.; Uggetti, E.; Montemurro, N.; García-Vara, M.; Pérez, S.; García, J.; Postigo, C. Boosting pharmaceutical removal through aeration in constructed wetlands. J. Hazard. Mater. 2021, 412, 125231. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Torán, J.; López-García, E.; Barbieri, M.V.; Postigo, C.; de Alda, M.L.; Caminal, G.; Sarrà, M.; Blánquez, P. Fungal bioremediation of diuron-contaminated waters: Evaluation of its degradation and the effect of amendable factors on its removal in a trickle-bed reactor under non-sterile conditions. Sci. Total Environ. 2020, 743, 140628. [Google Scholar] [CrossRef]
- Makut, M.; Bello, A. Assessment of the Biodegradation of Herbicides by Bacteria Isolated from the Soil. Asian J. Biotechnol. Bioresour. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Dwivedi, S.; Singh, B.R.; Al-Khedhairy, A.A.; Musarrat, J. Biodegradation of isoproturon using a novel Pseudomonas aeruginosa strain JS-11 as a multi-functional bioinoculant of environmental significance. J. Hazard. Mater. 2011, 185, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Frková, Z.; Johansen, A.; de Jonge, L.W.; Olsen, P.; Gosewinkel, U.; Bester, K. Degradation and enantiomeric fractionation of mecoprop in soil previously exposed to phenoxy acid herbicides–New insights for bioremediation. Sci. Total Environ. 2016, 569, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- González-Barreiro, O.; Rioboo, C.; Herrero, C.; Cid, A. Removal of triazine herbicides from freshwater systems using photosynthetic microorganisms. Environ. Pollut. 2006, 144, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, V.C.; De Gerónimo, E.; Marino, D.; Primost, J.; Carriquiriborde, P.; Costa, J.L. Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere 2013, 93, 1866–1873. [Google Scholar] [CrossRef]
- Wang, S.; Seiwert, B.; Kästner, M.; Miltner, A.; Schäffer, A.; Reemtsma, T.; Yang, Q.; Nowalk, K.M. (Bio)degradation of glyphosate in water-sediment microcosms—A stable isotope co-labeling approach. Water Res. 2016, 99, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Costanza, J.; Arshadi, M.; Abriola, L.M.; Pennell, K.D. Accumulation of PFOA and PFOS at the Air-Water Interface. Environ. Sci. Technol. 2019, 6, 487–491. [Google Scholar] [CrossRef]
- Dotro, G.; Langergraber, G.; Molle, P.; Nivala, J.; Puigagut, J.; Stein, O.; von Sperling, M. Treatment Wetlands. Water Intell. Online 2017, 16. [Google Scholar] [CrossRef] [Green Version]
- Sossalla, N.A.; Nivala, J.; Escher, B.I.; Reemtsma, T.; Schlichting, R.; van Afferden, M.; Müller, R. Resilience of micropollutant and biological effect removal in an aerated horizontal flow treatment wetland. Water 2020, 12, 3050. [Google Scholar] [CrossRef]
- Naghipour, D.; Amouei, A.; Taher Ghasemi, K.; Taghavi, K. Removal of metoprolol from aqueous solutions by the activated carbon prepared from pine cones. Environ. Health Eng. Manag. 2019, 6, 81–88. [Google Scholar] [CrossRef]
- Martínez-Orgániz, Á.; Bravo, J.E.B.; Llompart, M.; Dagnac, T.; Pablo Lamas, J.; Vázquez, L.; Sampedro-Rosas, L. Emerging pollutants and antibiotics removed by conventional activated sludge followed by ultraviolet radiation in a municipal wastewater treatment plant in mexico. Water Qual. Res. J. 2021, 56, 167–179. [Google Scholar] [CrossRef]
- Kim, U.J.; Oh, J.K.; Kannan, K. Occurrence, Removal, and Environmental Emission of Organophosphate Flame Retardants/Plasticizers in a Wastewater Treatment Plant in New York State. Environ. Sci. Technol. 2017, 51, 7872–7880. [Google Scholar] [CrossRef]
- Salgado, I.; Cárcamo, H.; Carballo, M.E.; Cruz, M.; del Carmen Durán, M. Domestic wastewater treatment by constructed wetlands enhanced with bioremediating rhizobacteria. Environ. Sci. Pollut. Res. 2017, 25, 20391–20398. [Google Scholar] [CrossRef]
- Yang, C.W.; Liu, C.; Chang, B.V. Biodegradation of amoxicillin, tetracyclines and sulfonamides in wastewater sludge. Water 2020, 12, 2147. [Google Scholar] [CrossRef]
- Sauvêtre, A.; Schröder, P. Uptake of carbamazepine by rhizomes and endophytic bacteria of Phragmites australis. Front. Plant Sci. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Maszenan, A.M.; Seviour, R.J.; Patel, B.K.C. Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Alexandrino, D.A.M.; Mucha, A.P.; Almeida, C.M.; Gao, W.; Jia, Z.; Carvalho, M.F. Biodegradation of the veterinary antibiotics enrofloxacin and ceftiofur and associated microbial community dynamics. Sci. Total Environ. 2017, 581, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fan, Y.; Cun, D.; Song, X.; Dai, Y.; Wang, F.; Wu, C.; Liang, W. Treatment performance and microbial response to dibutyl phthalate contaminated wastewater in vertical flow constructed wetland mesocosms. Chemosphere 2020, 246, 125635. [Google Scholar] [CrossRef] [PubMed]
- Kuyper, T.W. Book Review; R.L. Peterson, H.B. Massicotte and L.H. Melville. Mycorrhizas: Anatomy and Cell Biology. Mycopathologia 2005, 159. [Google Scholar] [CrossRef]
- Bucking, H.; Liepold, E.; Ambilwade, P. The role of the mycorrhizal symbiosis in nutrient uptake of plants and the regulatory mechanisms underlying these transport processes. In Plant Science; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhang, S.; Shan, X.Q.; Chen, B.D.; Zhu, Y.G.; Bell, J.N.B. Effect of arbuscular mycorrhizal fungus (Glomus caledonium) on the accumulation and metabolism of atrazine in maize (Zea mays L.) and atrazine dissipation in soil. Environ. Pollut. 2007, 146, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Joner, E.J.; Leyval, C. Phytoremediation of organic pollutants using mycorrhizal plants: A new aspect of rhizosphere interactions. Sustain. Agric. 2009, 23, 495–502. [Google Scholar] [CrossRef] [Green Version]
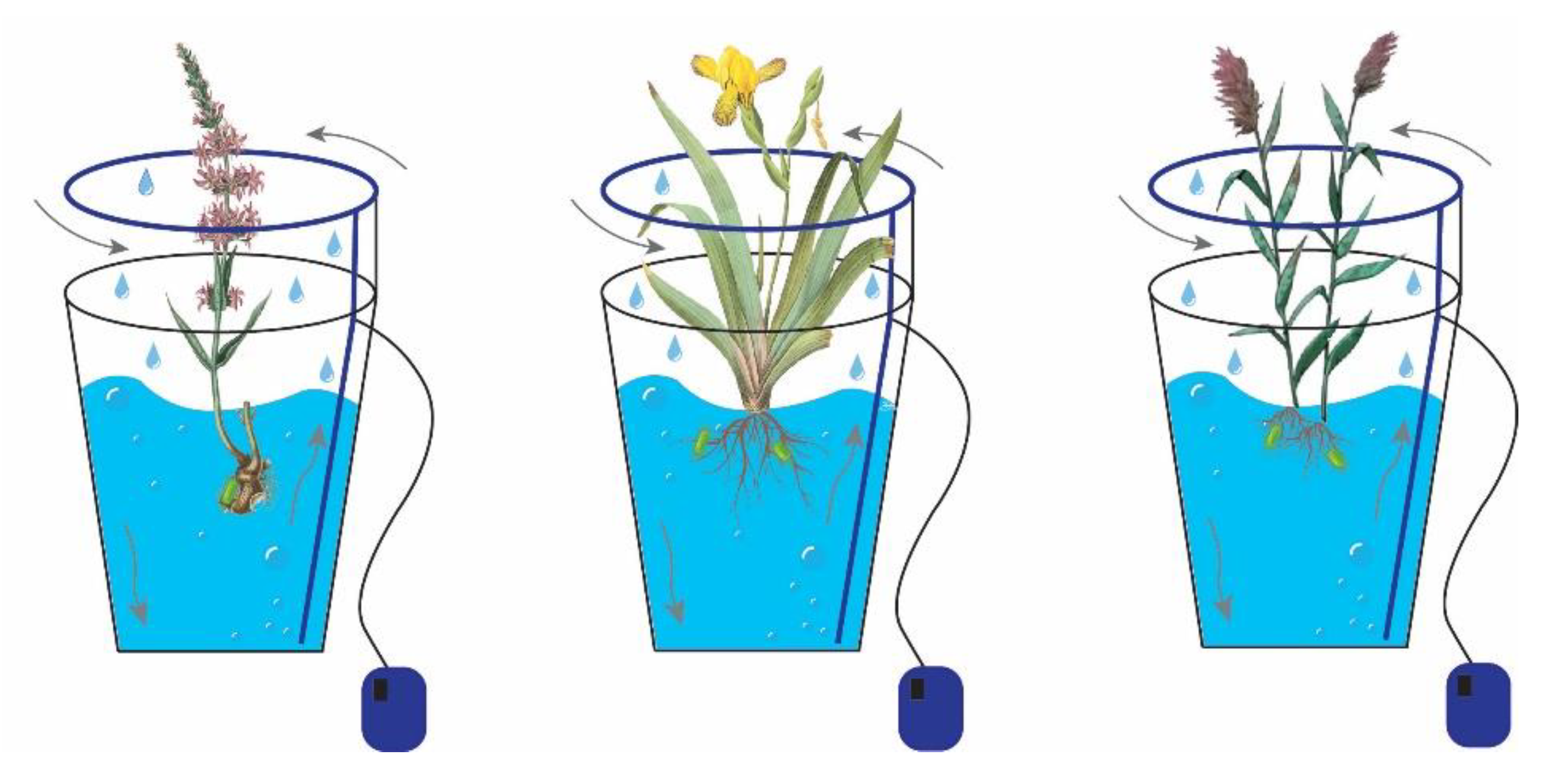
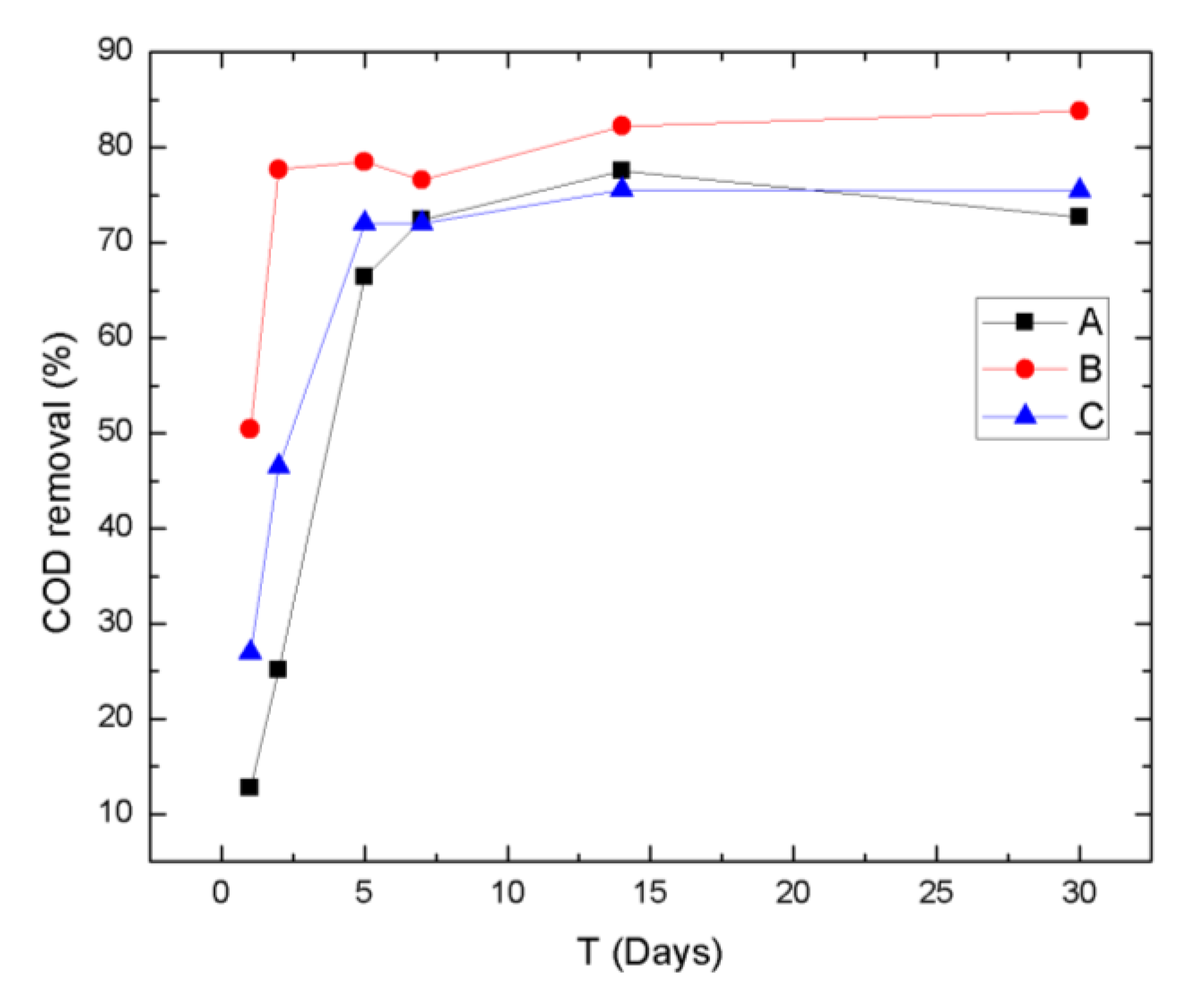

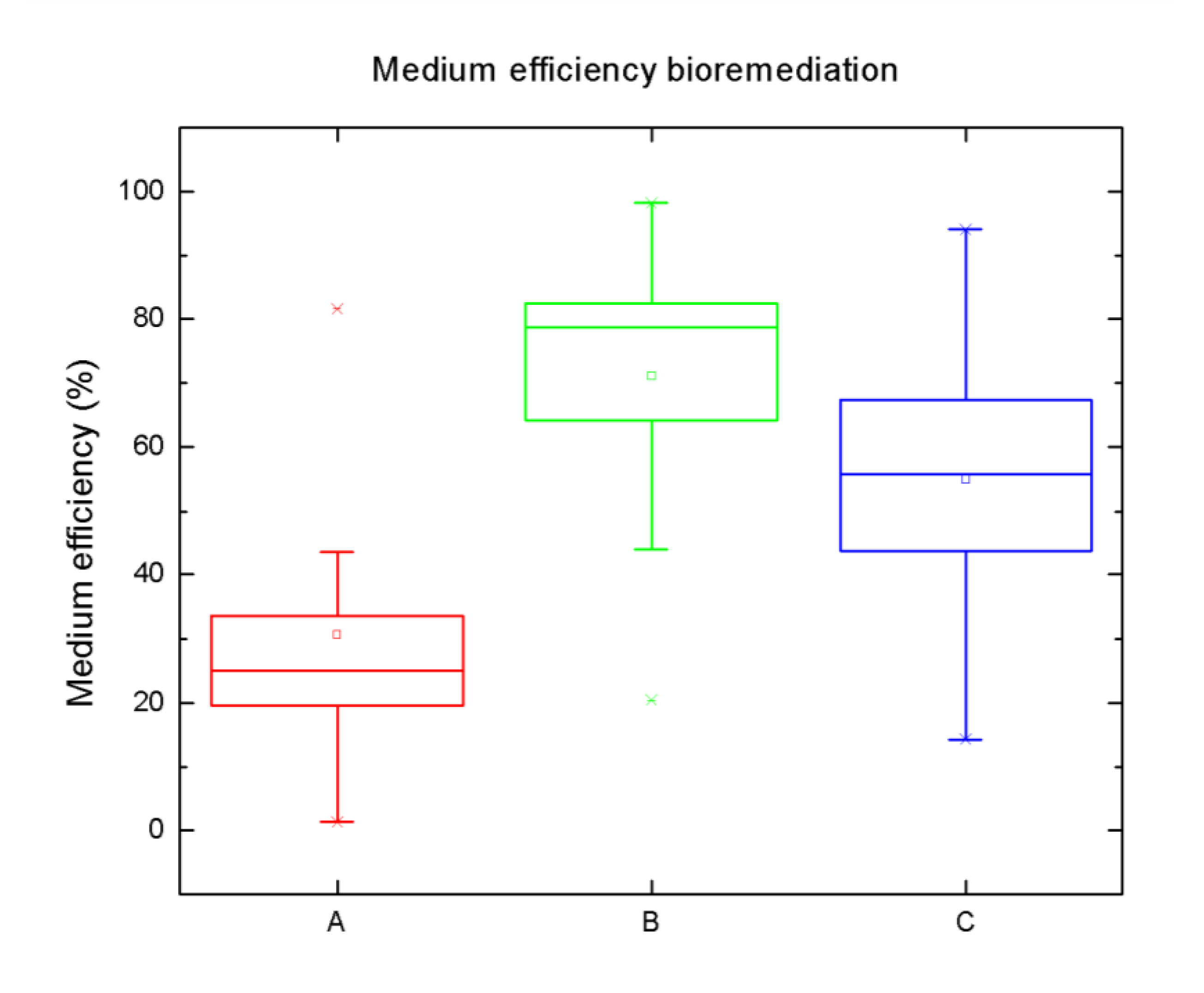
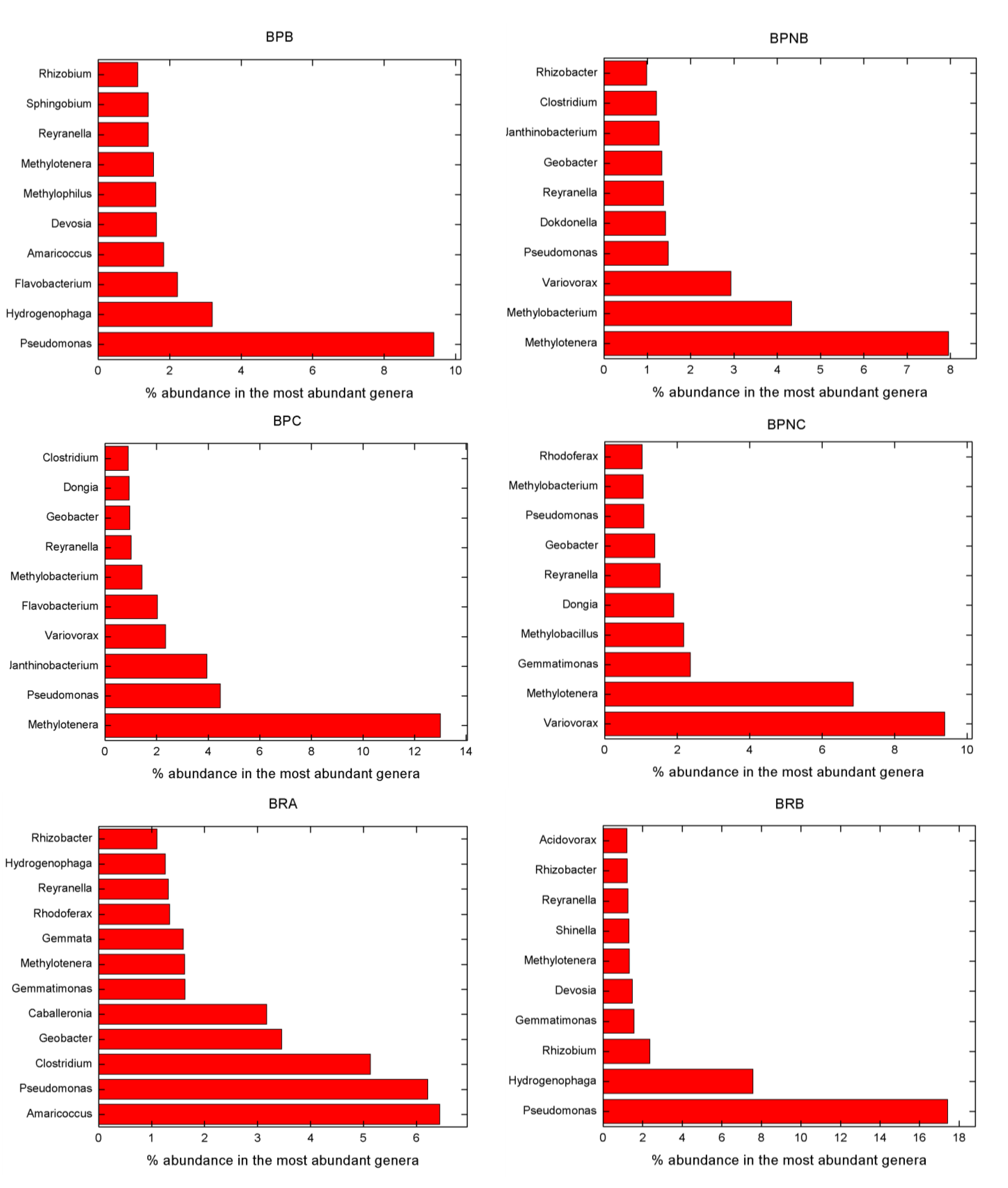
| Application | Compound | CAS Number | Therapeutic Group/Use |
|---|---|---|---|
| Pharmaceuticals and metabolites | Atenolol | 29122-68-7 | Beta Blocker |
| Bezafibrate | 41859-67-0 | Lipid regulator | |
| Carbamazepine | 298-46-4 | Psychiatric drug | |
| Clarithromycin | 81103-11-9 | Antibiotic | |
| Ciprofloxacin | 85721-33-1 | Antibiotic | |
| Cyclophosphamide | 50-18-0 | Cytostatic | |
| Diclofenac | 15307-86-5 | Analgesic/anti-inflammatories | |
| Erythromycin A | 114-07-8 | Antibiotic | |
| Ketoprofen | 22071-15-4 | Analgesic/anti-inflammatories | |
| Lidocaine | 137-58-6 | Anaesthetic | |
| Metoprolol | 51384-51-1 | Beta Blocker | |
| Propranolol | 525-66-6 | Beta Blocker | |
| N4-acetylsulfamethoxazole | 21312-10-7 | Metabolite of Sulfamethoxazole | |
| Sulfamethoxazole | 723-46-6 | Antibiotic | |
| Pesticides/Herbicides | Carbendazim | 10605-21-7 | Fungicide |
| DEET | 134-62-3 | Insect repellent | |
| Diuron | 330-54-1 | Herbicide | |
| Isoproturon | 34123-59-6 | Herbicide | |
| Terbutryn | 886-50-0 | Herbicide | |
| Mecoprop (MCPP) | 7085-19-0 | Herbicide | |
| Tolyltriazole | 29385-43-1 | Fertilizer | |
| Glyphosate | 1071-83-6 | Herbicide | |
| Aminomethylphosphonic acid (AMPA) | 1066-51-9 | Degradation product | |
| Fluorosurfactants | Perfluorooctanesulfonic acid (PFOS) | 1763-23-1 | Surfactant |
| Perfluorooctanoic acid (PFOA) | 335-67-1 | Surfactant | |
| Corrosion inhibitor | Benzotriazole | 95-14-7 | Corrosion inhibitor/Antiviral |
| Flame retardant | Tris(2-chloroisopropyl)phosphate (TCPP) | 13674-84-5 | Flame retardant |
| Compound | Achieved Removal in Current Study (%) | Achieved Removal in Previous Studies | Reference |
|---|---|---|---|
| atenolol | 98.8 | 80% | [46] |
| benzotriazole | 93 | complete removal, however conditioned by low concentration of the compound | [47] |
| bezafibrate | 99.9 | contribution of the biofilm to removal of 25% | [48] |
| carbendazim | 99.3 | 41.8% | [49] |
| ciprofloxacin | 99.5 | contribution of the biofilm to removal of 22% | [48] |
| clarithromycin | 99.4 | 75.8–98.6% | [50] |
| cyclophosphamide | 91.8 | >20% | [51] |
| DEET | 99.6 | no significant removal | [52] |
| diclofenac | 99.7 | 97 ± 4% | [53] |
| diuron | 99.7 | 83% | [54] |
| erythromycin | 98.3 | 75.8–98.6% | [50] |
| glyphosate | 99.2 | 82.6% | [55] |
| isoproturon | 99.6 | complete removal | [56] |
| ketoprofen | 99.9 | complete removal | [53] |
| MCPP | 99.5 | 99% | [57] |
| metoprolol | 91 | 60% | [46] |
| propranolol | 98.9 | 60% | [46] |
| sufamethoxazole | 90.5 | 75.8–98.6% | [50] |
| N-acetyl-sulfamethoxazole | 99.5 | no information founded | |
| TCIPP | 89.9 | 60% | [32] |
| terbutryn | 99.6 | complete removal | [58] |
| tolyltriazole | 95.7 | complete removal | [47] |
| Sample | Pseudomonas | Flavobacterium | Variovorax | Methylotenera | Reyranella | Amaricoccus | Hydrogenophaga |
|---|---|---|---|---|---|---|---|
| % | |||||||
| BPB | 9.39 | 2.22 | 0 | 1.56 | 1.41 | 1.84 | 3.2 |
| BPNB | 1.48 | 0 | 2.93 | 7.96 | 1.38 | 0 | 0 |
| BPC | 4.47 | 2.03 | 2.36 | 13.01 | 1.02 | 0 | 0 |
| BPNC | 1.09 | 0 | 9.39 | 6.86 | 1.54 | 0 | 0 |
| BRA | 6.22 | 0 | 0 | 1.62 | 1.31 | 6.44 | 1.26 |
| BRB | 17.43 | 2.61 | 0 | 1.34 | 1.26 | 0 | 7.58 |
| BRC | 9.42 | 2.26 | 0 | 1.5 | 1.41 | 1.82 | 3.19 |
| Sample | Colonization by AMF (%) |
|---|---|
| Phragmites before bior. exp. | 34 |
| Iris before bior. exp. | 56 |
| Lythrum before bior. exp. | 36 |
| Phragmites after bior. exp. | 10 |
| Iris after bior. exp. | 15 |
| Lythrum after bior. exp. | 10 |
| Phragmites after bior. exp. new roots | 0 |
| Iris after bior. exp. new roots | 0 |
| Lythrum after bior. exp. new roots | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunhoferova, H.; Venditti, S.; Laczny, C.C.; Lebrun, L.; Hansen, J. Bioremediation of 27 Micropollutants by Symbiotic Microorganisms of Wetland Macrophytes. Sustainability 2022, 14, 3944. https://doi.org/10.3390/su14073944
Brunhoferova H, Venditti S, Laczny CC, Lebrun L, Hansen J. Bioremediation of 27 Micropollutants by Symbiotic Microorganisms of Wetland Macrophytes. Sustainability. 2022; 14(7):3944. https://doi.org/10.3390/su14073944
Chicago/Turabian StyleBrunhoferova, Hana, Silvia Venditti, Cédric C. Laczny, Laura Lebrun, and Joachim Hansen. 2022. "Bioremediation of 27 Micropollutants by Symbiotic Microorganisms of Wetland Macrophytes" Sustainability 14, no. 7: 3944. https://doi.org/10.3390/su14073944
APA StyleBrunhoferova, H., Venditti, S., Laczny, C. C., Lebrun, L., & Hansen, J. (2022). Bioremediation of 27 Micropollutants by Symbiotic Microorganisms of Wetland Macrophytes. Sustainability, 14(7), 3944. https://doi.org/10.3390/su14073944









