A Green Analytical Methodology for Detecting Adulteration in Automotive Urea-SCR Products Using Microfluidic-Paper Analytical Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents Preparation
2.2. Design and Fabrication Method of the µPAD
2.3. Analysis Procedure and Data Acquisition
3. Results
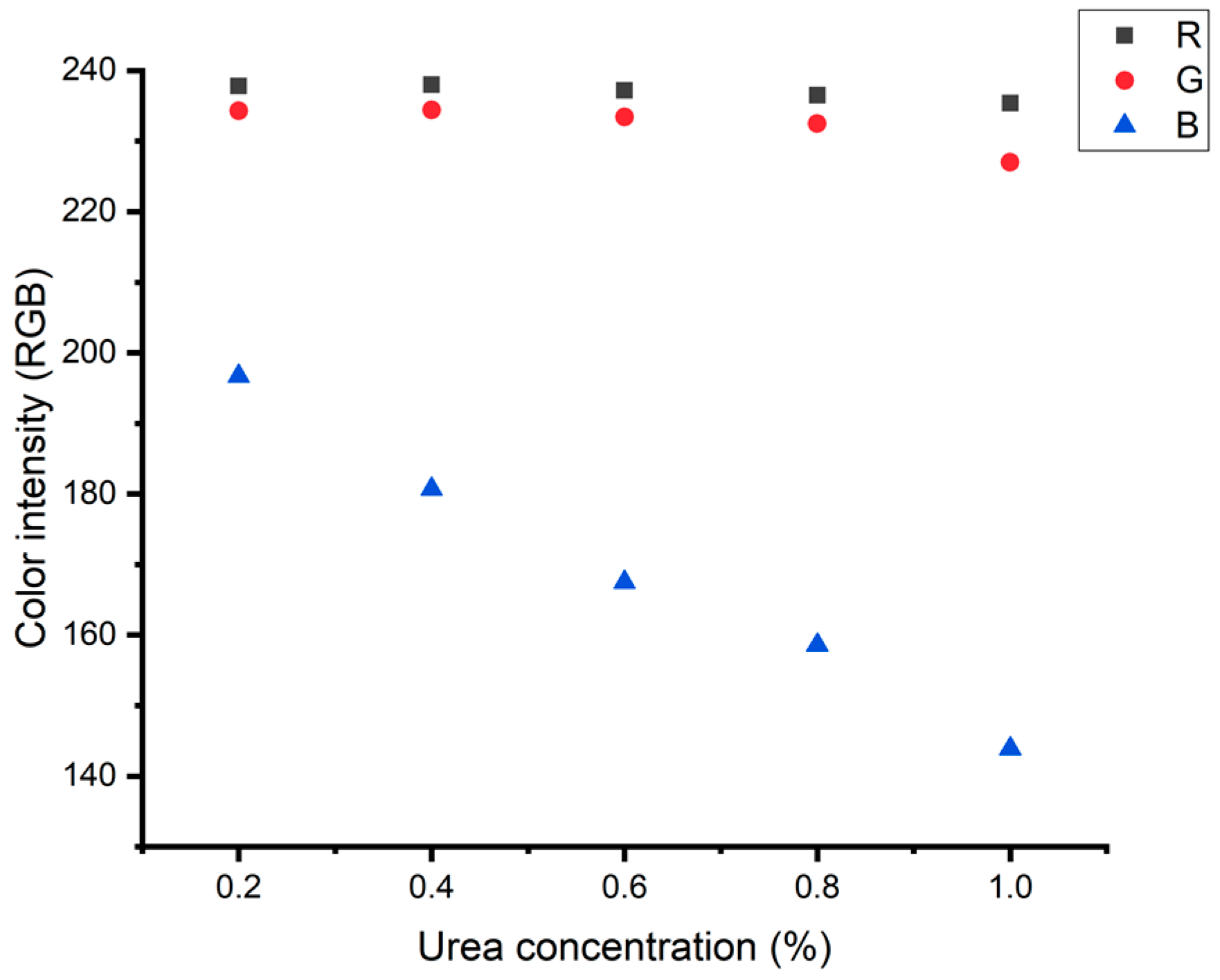
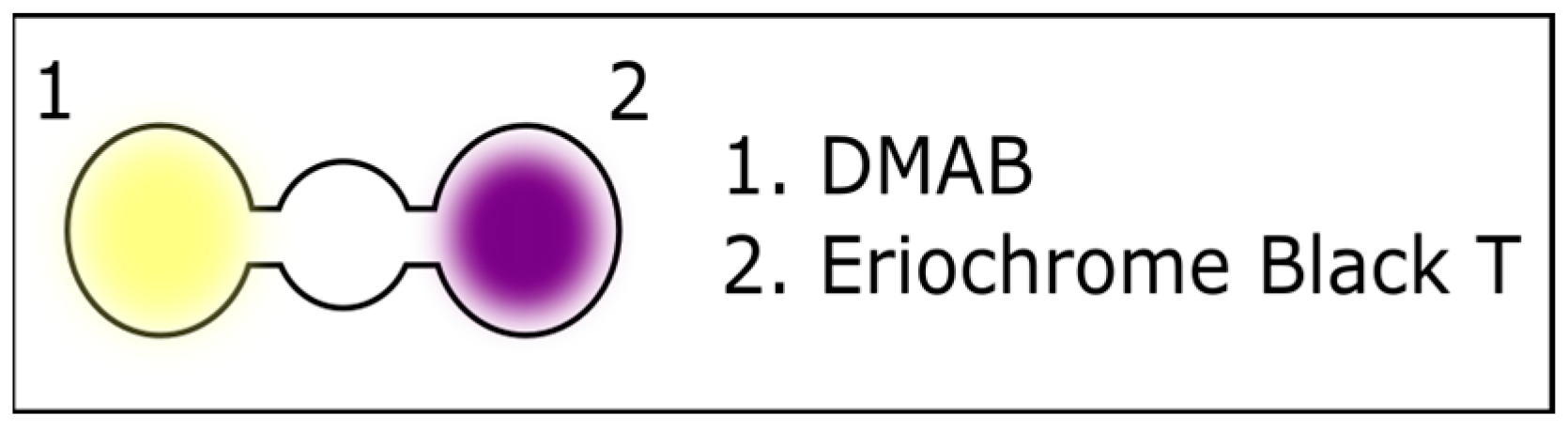
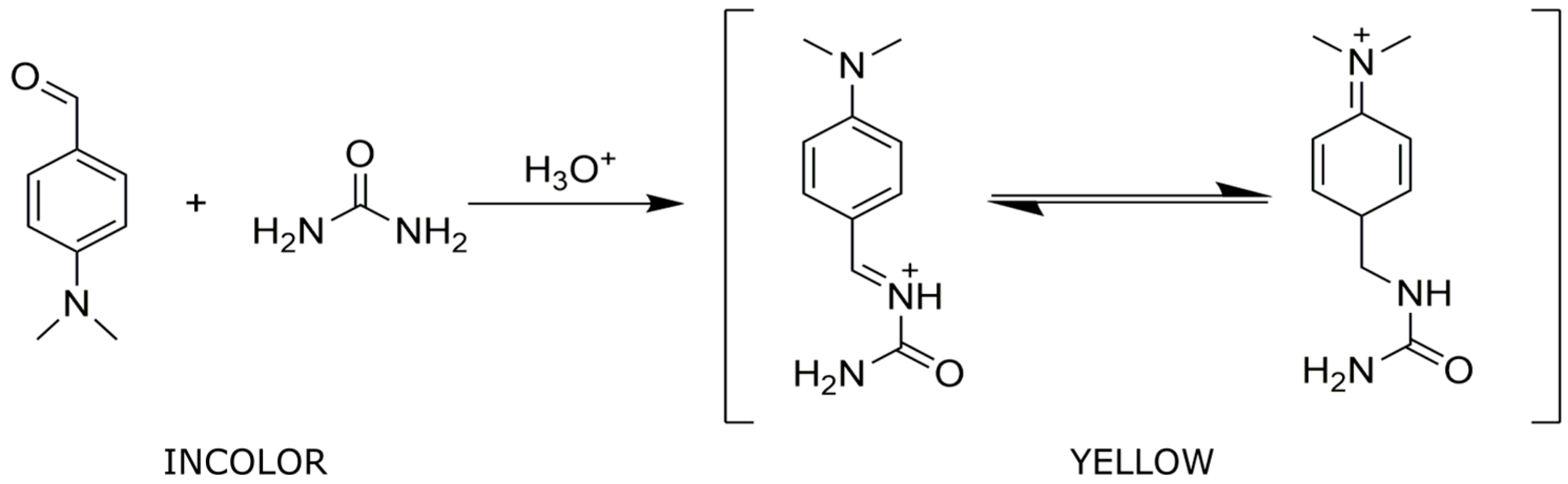
3.1. Optimization of the Spot Test Reaction for Urea Detection
3.2. Optimization of the Spot Test Reaction for Water Hardness Detection
3.3. Analytical Parameters Using the Paper-Based Analytical Device
3.4. Accuracy Evaluation and Sample Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sorrels, J.L.; Randall, D.D.; Schaffner, K.S.; Fry, C.R. Selective Catalytic Reduction: Chapter 2, Section 4. In Air Pollution Control Cost Manual; EPA: Durham, NC, USA, 2019. Available online: https://www.epa.gov/economic-and-cost-analysis-air-pollution-regulations/cost-reports-and-guidance-air-pollution (accessed on 11 January 2022).
- ABNT (Brazilian Association of Technical Standards). Diesel Engines—NOx Reduction Agent AUS 32 Part 1: Quality Requirements; ABNT: Rio de Janeiro, Brazil, 2011; NBR ISO 22241-1. [Google Scholar]
- DE Verband der Automobilindustrie. Adblue Brand List and Licenses List. January 2022. Available online: https://en.vda.de/en/topics/innovation-and-technology/ad-blue/AdBlue-brand-list-and-licensees-list.html (accessed on 13 January 2022).
- Sorrels, J.L.; Randall, D.D.; Schaffner, K.S.; Fry, C.R. Chapter 2—Selective Catalytic Reduction. In Econ. Cost Anal. Air Pollut. Regul.; 2016. Available online: https://www.epa.gov/economic-and-cost-analysis-air-pollution-regulations/chapter-2-selective-catalytic-reduction (accessed on 11 January 2022).
- Fojtíková, P.; Kahoun, D.; Boček, J.; Burian, M.; Kupka, R.; Schwarz, J. Tracking AdBlue properties during tests of selective catalytic reduction (SCR) systems—The suitability of various analytical methods for urea content determination. Int. J. Energy Res. 2020, 44, 2549–2559. [Google Scholar] [CrossRef]
- Gurusamy, P.; Gandhi, U.; Mangalanathan, U.; Marx, K.L.R. Electrical Conductivity Based Quality Measurement of Diesel Exhaust Fluid. J. Anal. Chem. 2018, 73, 1128–1135. [Google Scholar] [CrossRef]
- Gurusamy, P.; Gandhi, U.; Mangalanathan, U. A study on optical characteristics and quality monitoring of diesel exhaust fluid. Optik 2018, 155, 31–35. [Google Scholar] [CrossRef]
- Pan, R.; Jeffries, J.B.; Dreier, T.; Schulz, C. Measurements of liquid film thickness, concentration, and temperature of aqueous urea solution by NIR absorption spectroscopy. Appl. Phys. B 2016, 122, 2–10. [Google Scholar] [CrossRef]
- França, H.H.; Silva, N.C.; Honorato, F.A. Evaluation of diesel exhaust fluid (DEF) using near-infrared spectroscopy and multivariate calibration. Microchem. J. 2019, 150, 1–5. [Google Scholar] [CrossRef]
- Yassine, M.M.; Dabek-Zlotorzynska, E.; Celo, V. Development of a hydrophilic interaction liquid chromatography-mass spectrometry method for detection and quantification of urea thermal decomposition by-products in emission from diesel engine employing selective catalytic reduction technology. J. Chromatogr. A 2012, 1229, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.Q.; Yu, K.X.; Gong, Y.X. Rapid and quantitative determination of urea in milk by reaction headspace gas chromatography. Microchem. J. 2019, 147, 838–841. [Google Scholar] [CrossRef]
- Polcar, A.; Cupera, J.; Kumbár, V.; Dostál, P.; Votava, J. Influence of urea concentration of refractive index of adblue fluid evaluated by regression analysis. Acta Agric. Silvic. Mendelianae Brun 2016, 64, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Paula, W.F.; Demarchi, P.H.; Teste, A. Blucher Engineering Proceedings. In Proceedings of the XXIV Simpósio International de Engenharia Automotiva—SIMEA, São Paulo, Brazil, 5–6 September 2016; Blucher: São Paulo, Brazil, 2016; Volume 3, pp. 742–759. [Google Scholar]
- da Silva, V.A.O.P.; de Freitas, R.C.; de Oliveira, P.R.; Moreira, R.C.; Marcolino-Júnior, L.H.; Bergamini, M.F.; Coltro, W.K.T.; Janegitz, B.C. Microfluidic paper-based device integrated with smartphone for point-of-use colorimetric monitoring of water quality index. Meas. J. Int. Meas. Confed. 2020, 164, 108085. [Google Scholar] [CrossRef]
- Petruci, J.F.S.; Cardoso, A.A. Sensitive luminescent paper-based sensor for the determination of gaseous hydrogen sulfide. Anal. Methods 2015, 7, 2687–2692. [Google Scholar] [CrossRef]
- Fernandes, G.M.; Sousa, J.; Petruci, J.F.S.; Batista, A.D. Paper-based analytical device for colorimetric detection of Cu2+ in Brazilian sugarcane spirits by digital image treatment. Microchem. J. 2020, 159, 105463. [Google Scholar] [CrossRef]
- Capitán-Vallvey, L.F.; López-Ruiz, N.; Martínez-Olmos, A.; Erenas, M.M.; Palma, A.J. Recent developments in computer vision-based analytical chemistry: A tutorial review. Anal. Chim. Acta 2015, 899, 23–56. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; Petruci, J.F.d.S.; Batista, A.D. Novel approaches for colorimetric measurements in analytical chemistry—A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.D.; Rivas, B.L. Determination of urea using p-N,N-dimethylaminobenzaldehyde: Solvent effect and interference of chitosan. J. Chil. Chem. Soc. 2017, 62, 3538–3542. [Google Scholar] [CrossRef] [Green Version]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- ICH. Harmonised Tripartite Guidelines. Validation of Analytical Procedures: Text and Methodology Q2(R1). 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 11 January 2022).

| Urea | Water Hardness | |
|---|---|---|
| Linear range | 0.2–1.0% | 0.1–3.5 mM |
| Color channel | Channel B (RGB) | Channel V (HSV) |
| Calibration curve | y = −63.8x + 207.8 | y = 0.035x + 0.611 |
| R2 | 0.992 | 0.993 |
| Repeatability (RSD) (n = 9) | 1.5% (0.2% urea) | 1.7% (2.5 mM) |
| Limit of Detection | 0.2% | 0.02 mM |
| Urea | Water Hardness | |||||
|---|---|---|---|---|---|---|
| Added (% m/v) | Found (% m/v) | Recovery (%) | Added (mM) | Found (mM) | Recovery (%) | |
| Sample 1 | 0.2 | 0.19 ± 0.02 | 95.1 ± 8.6 | 0.1 | 0.122 | 122.2 ± 1.6 |
| Sample 2 | 0.5 | 0.58 ± 0.03 | 115.3 ± 5.1 | 2.5 | 2.7 | 107.9 ± 6.5 |
| Sample 3 | 1.0 | 1.09 ± 0.03 | 109.4 ± 2.7 | 3.5 | 3.2 | 91.5 ± 2.1 |
| Urea | Water Hardness | |
|---|---|---|
| Sample 1 | 30.1 ± 0.5% | Not detected |
| Sample 2 | 32.6 ± 2.0% | Not detected |
| Sample 3 | 33.3 ± 1.5% | Not detected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Souza, D.; Fernandes, G.M.; Dias, B.C.; Stefanelli Junior, J.R.; Sequinel, R.; da Silveira Petruci, J.F. A Green Analytical Methodology for Detecting Adulteration in Automotive Urea-SCR Products Using Microfluidic-Paper Analytical Devices. Sustainability 2022, 14, 3363. https://doi.org/10.3390/su14063363
da Silva Souza D, Fernandes GM, Dias BC, Stefanelli Junior JR, Sequinel R, da Silveira Petruci JF. A Green Analytical Methodology for Detecting Adulteration in Automotive Urea-SCR Products Using Microfluidic-Paper Analytical Devices. Sustainability. 2022; 14(6):3363. https://doi.org/10.3390/su14063363
Chicago/Turabian Styleda Silva Souza, Danielle, Gabriel Martins Fernandes, Barbara Cristina Dias, José Roberto Stefanelli Junior, Rodrigo Sequinel, and João Flávio da Silveira Petruci. 2022. "A Green Analytical Methodology for Detecting Adulteration in Automotive Urea-SCR Products Using Microfluidic-Paper Analytical Devices" Sustainability 14, no. 6: 3363. https://doi.org/10.3390/su14063363
APA Styleda Silva Souza, D., Fernandes, G. M., Dias, B. C., Stefanelli Junior, J. R., Sequinel, R., & da Silveira Petruci, J. F. (2022). A Green Analytical Methodology for Detecting Adulteration in Automotive Urea-SCR Products Using Microfluidic-Paper Analytical Devices. Sustainability, 14(6), 3363. https://doi.org/10.3390/su14063363







