Analysis of Lanthanum and Cobalt Leaching Aimed at Effective Recycling Strategies of Solid Oxide Cells
Abstract
:1. Introduction
2. Materials and Methods
- (a)
- Literature survey and selection: firstly, the scientific literature was surveyed running a comprehensive search on Scopus, Science Direct and Web of Science databases. The adopted keywords were ‘lanthanum’ AND ‘cobalt’ AND ‘leaching’ AND ‘hydrometallurgical’ AND ‘recovery’ AND ‘waste’. Secondly, a screening of the results based on the publication language and type of reference was made, selecting only research papers and reviews in English. Thirdly, a chronological filter was applied to exclude the references published before 2000. The abstracts of the resulting papers were analyzed to understand whether they fall under the scope of this study. More studies, compliant with the above-mentioned criteria, were added from the references cited by the selected review papers.
- (b)
- Literature classification: the articles selected in the previous phase were classified according to the type of metal they focus on. Another classification was made considering the type of solvent used for the leaching process, with a further distinction based on its nature (inorganic or organic).
- (c)
- Evaluation of the process approach: after the literature selection, it was observed that the word “green” was present in the title, abstract or among the keywords, despite being not strictly connected to the aim of the research. In many other papers, the word “green” and expressions like “environmentally friendly” and “sustainable” (referred to as the processes) occurred in the text. However, the criteria that lead to define a process “green” were not specified. To provide a clear and unambiguous classification, in this review, a process is classified “green” if it is compliant with at least three principles of Green Chemistry [18].
- 3.
- Less Hazardous Chemical Syntheses: the toxicity to human health and the environment was evaluated by consulting the safety data sheet of each solvent.
- 4.
- Designing Safer Chemicals: to evaluate the safety of a chemical agent, its concentration was evaluated. First, considering all the references, the overall median value of the concentration of the bleaching agent was determined. The overall median value was then compared to the specific median value calculated for each solvent. If the specific median concentration was lower or equal to the overall median value, the solvent was considered a safer chemical.
- 5.
- Safer Solvents and Auxiliaries: a solvent was considered compliant with this principle if in at least 60% of the processes proposed by the selected references it was used without the addition of an auxiliary agent.
- 10.
- Design for Degradation: the products obtained at the end of the leaching process were analyzed. In the case of inorganic solvents, the released products (NOx, Cl2, or SO3) and the leachate obtained were considered a threat to the environment.
- 12.
- Inherently Safer Chemistry for Accident Prevention: the potential for chemical accidents (releases, explosions, and fires) of all the solvents was evaluated from their safety data sheet.
3. Results and Discussion
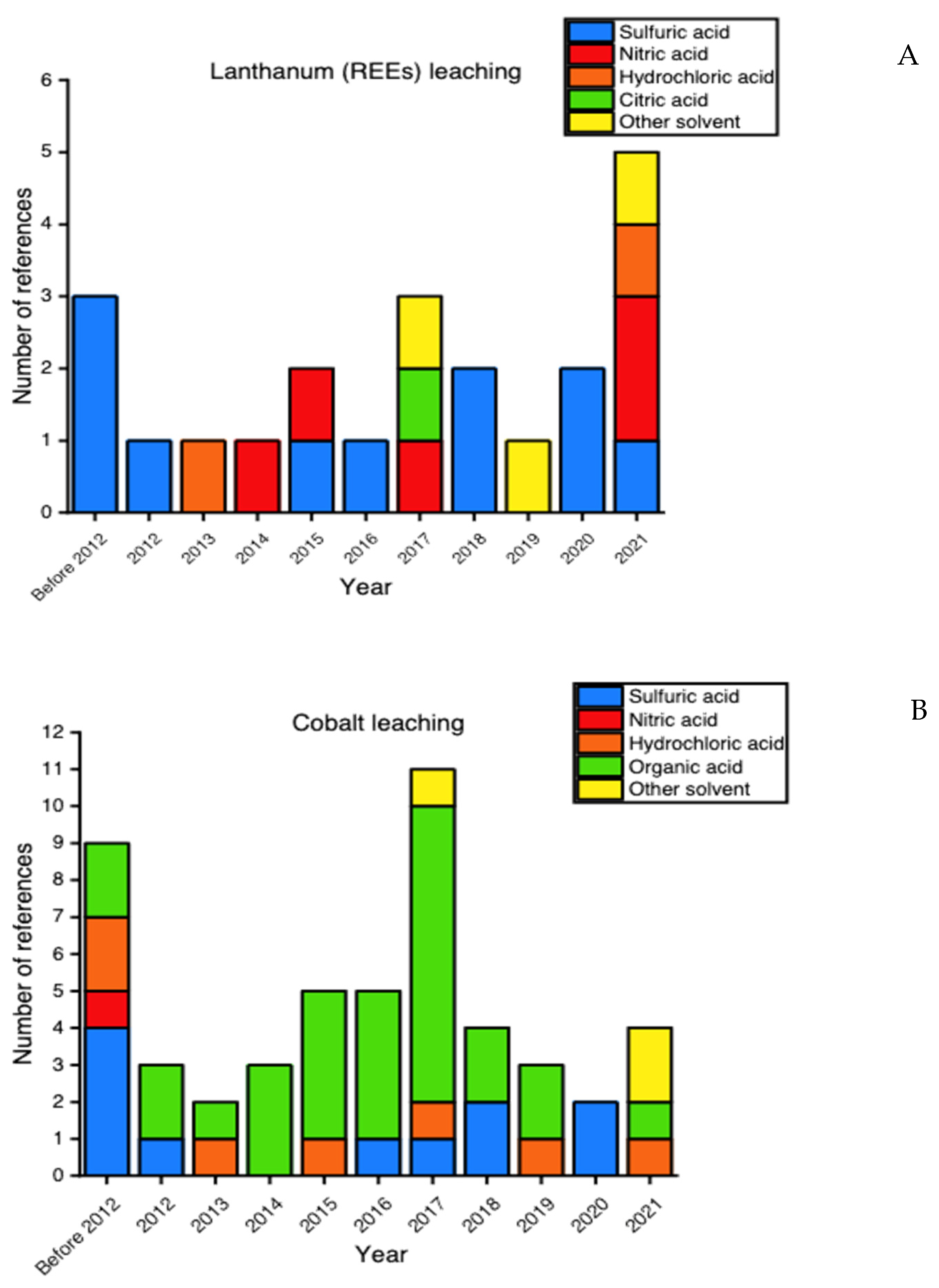
3.1. Literature Selection and Classification
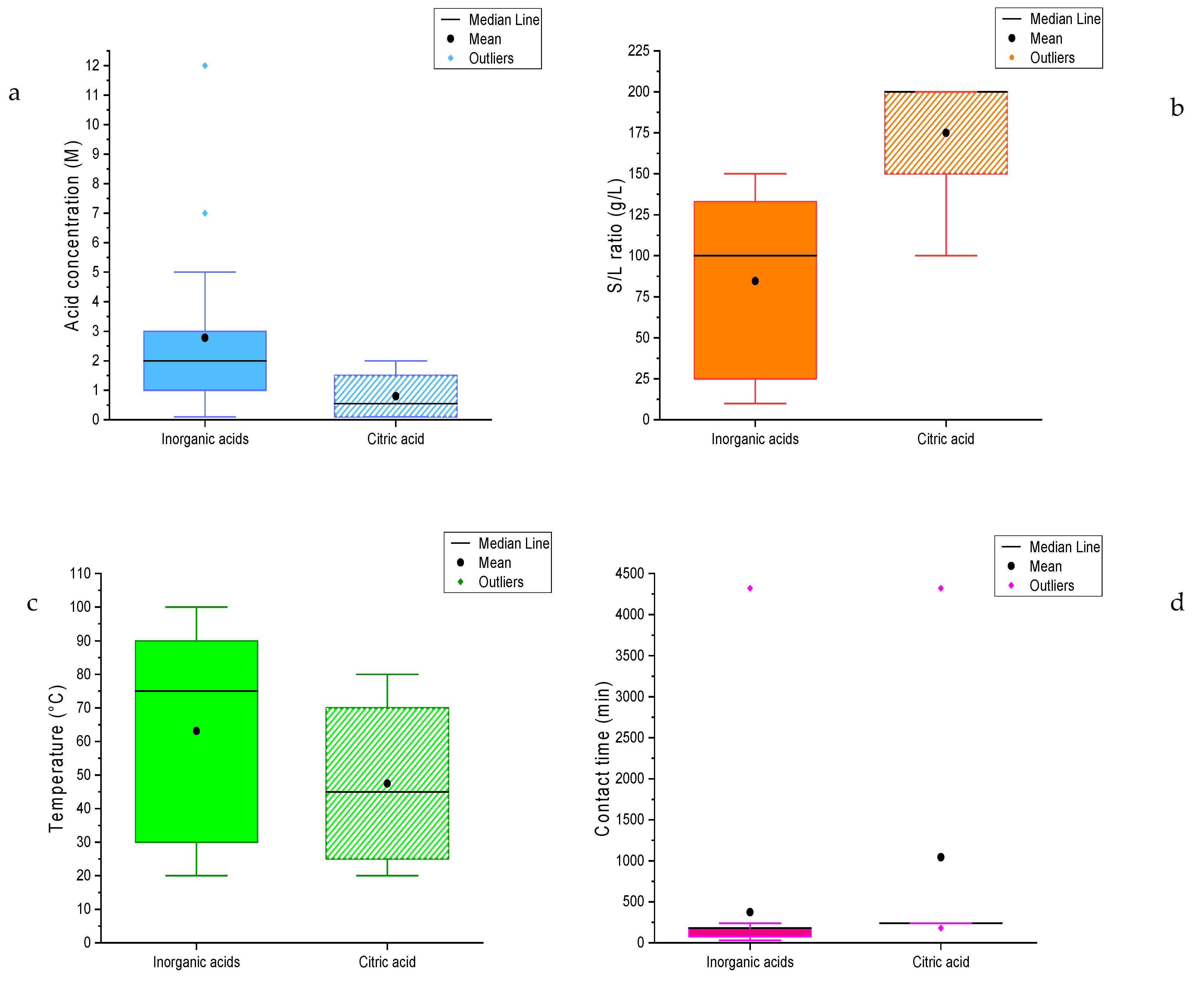
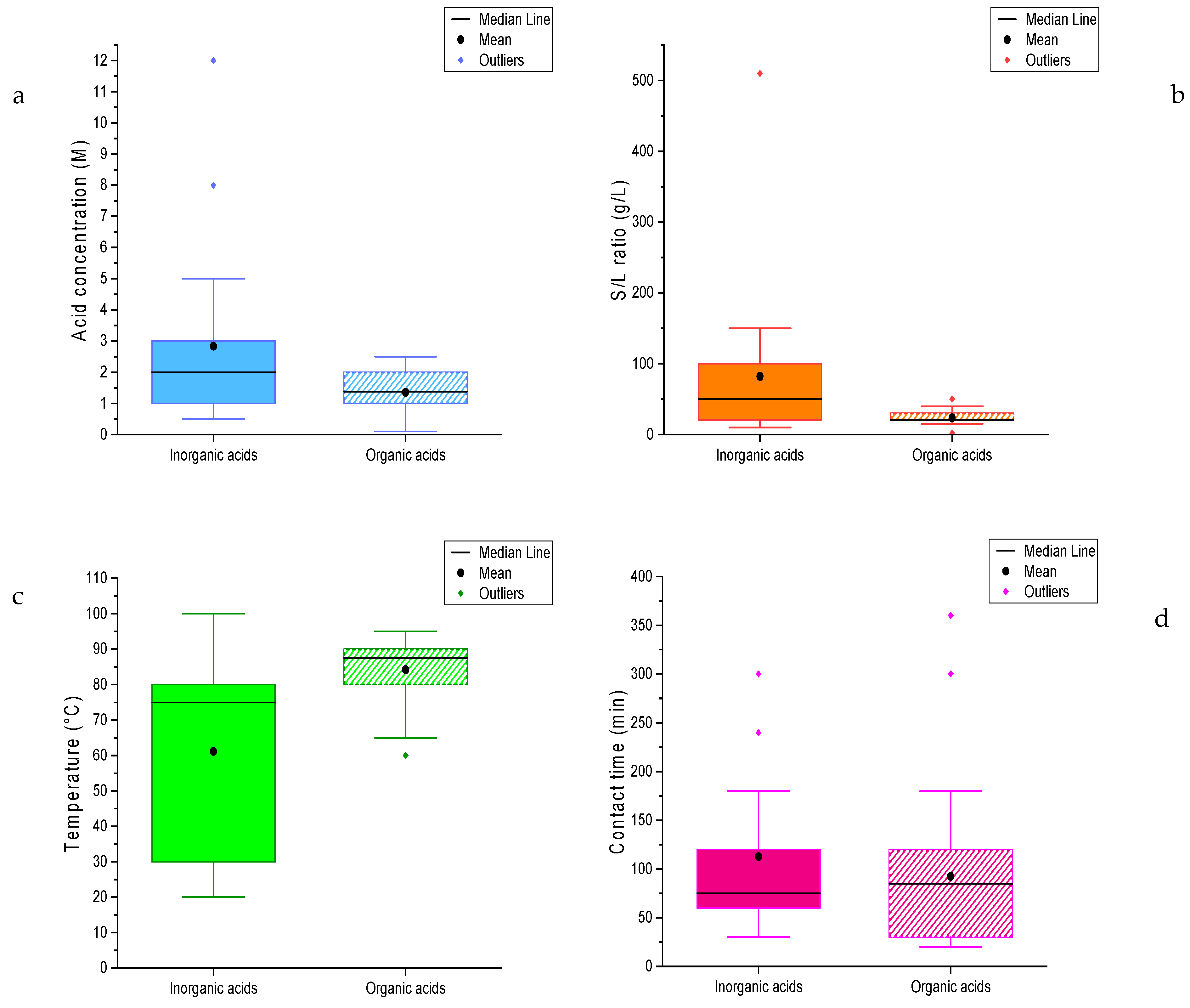
3.2. Experimental Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Férriz, A.M.; Bernad, A.; Mori, M.; Fiorot, S. End-of-Life of Fuel Cell and Hydrogen Products: A State of the Art. Int. J. Hydrog. Energy 2019, 44, 12872–12879. [Google Scholar] [CrossRef]
- Mori, M.; Stropnik, R.; Sekavčnik, M.; Lotrič, A. Criticality and Life-Cycle Assessment of Materials Used in Fuel-Cell and Hydrogen Technologies. Sustainability 2021, 13, 3565. [Google Scholar] [CrossRef]
- Strazza, C.; del Borghi, A.; Costamagna, P.; Gallo, M.; Brignole, E.; Girdinio, P. Life Cycle Assessment and Life Cycle Costing of a SOFC System for Distributed Power Generation. Energy Convers. Manag. 2015, 100, 64–77. [Google Scholar] [CrossRef]
- Malik, V.; Srivastava, S.; Bhatnagar, M.K.; Vishnoi, M. Comparative Study and Analysis between Solid Oxide Fuel Cells (SOFC) and Proton Exchange Membrane (PEM) Fuel Cell-A Review. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 47, pp. 2270–2275. [Google Scholar]
- Ferraris, M.; De La Pierre, S.; Sabato, A.G.; Smeacetto, F.; Javed, H.; Walter, C.; Malzbender, J. Torsional shear strength behavior of advanced glass-ceramic sealants for SOFC/SOEC applications. J. Eur. Ceram. Soc. 2020, 40, 4067–4075. [Google Scholar] [CrossRef]
- Solid Oxide Fuel Cell Market Size, Share & COVID-19 Impact Analysis, By Application (Stationary, Transport, Portable), By End-User (Commercial, Data Centers, Military & Defense, and Others), and Regional Forecast, 2021–2028. Available online: https://www.fortunebusinessinsights.com/industry-reports/solid-oxide-fuel-cell-market-101306 (accessed on 17 December 2021).
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in Material Selection for Solid Oxide Fuel Cell Technology: A Review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials for Strategic Technologies and Sectors in the EU—A Foresight Study; The reuse policy of European Commission documents is regulated by Decision 2011/833/EU (OJ L 330, 14.12.2011, p. 39); European Commission: Bruxelles, Belgium, 2020. [Google Scholar]
- Zhang, L.; Xu, Z. A Review of Current Progress of Recycling Technologies for Metals from Waste Electrical and Electronic Equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Ali Kucuker, M.; Kuchta, K.; et al. Recent Advances on Hydrometallurgical Recovery of Critical and Precious Elements from End of Life Electronic Wastes-a Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef] [Green Version]
- Abhilash, S.S.; Meshram, P.; Pandey, B.D. Metallurgical Processes for the Recovery and Recycling of Lanthanum from Various Resources-A Review. Hydrometallurgy 2016, 160, 47–59. [Google Scholar]
- Sposato, C.; Catizzone, E.; Blasi, A.; Forte, M.; Romanelli, A.; Morgana, M.; Braccio, G.; Giordano, G.; Migliori, M. Towards the Circular Economy of Rare Earth Elements: Lanthanum Leaching from Spent FCC Catalyst by Acids. Processes 2021, 9, 1369. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Stopic, S.; Friedrich, B.; Ten rio, J.A.S.; Espinosa, D.C.R. Cobalt Recovery from Li-Ion Battery Recycling: A Critical Review. Metals 2021, 11, 1999. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Santoro, L.; Tshipeng, S.; Pirard, E.; Bouzahzah, H.; Kaniki, A.; Herrington, R. Mineralogical Reconciliation of Cobalt Recovery from the Acid Leaching of Oxide Ores from Five Deposits in Katanga (DRC). Miner. Eng. 2019, 137, 277–289. [Google Scholar] [CrossRef]
- Lotrič, A.; Sekavčnik, M.; Kuštrin, I.; Mori, M. Life-Cycle Assessment of Hydrogen Technologies with the Focus on EU Critical Raw Materials and End-of-Life Strategies. Int. J. Hydrog. Energy 2021, 46, 10143–10160. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. End of Life of Fuel Cells and Hydrogen Products: From Technologies to Strategies. Int. J. Hydrog. Energy 2019, 44, 20965–20977. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner John, C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Kozlowskaya, I.Y.; Martsul’, V.N. Acid Leaching of Lanthanum from Spent Cracking Catalyst. Russ. J. Appl. Chem. 2014, 87, 1817–1822. [Google Scholar] [CrossRef]
- Vargas, S.J.R.; Schaeffer, N.; Souza, J.C.; da Silva, L.H.M.; Hespanhol, M.C. Green Separation of Lanthanum, Cerium and Nickel from Waste Nickel Metal Hydride Battery. Waste Manag. 2021, 125, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Shuai, W.; Wang, X.; Liu, Y. Extraction of Rare Earth Elements from a Contaminated Cropland Soil Using Nitric Acid, Citric Acid, and EDTA. Environ. Technol. 2017, 38, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Petrus, H.T.B.M.; Wijaya, A.; Putra, A.D.P.; Iskandar, Y.; Bratakusuma, D.; Mufakhir, F.R.; Astuti, W.; Wiratni. Effect of Temperature and Acid Concentration on Lanthanum Extraction from Spent Catalyst Using Organic Acid. In IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2019; Volume 478. [Google Scholar]
- Innocenzi, V.; Ferella, F.; de Michelis, I.; Vegliò, F. Treatment of Fluid Catalytic Cracking Spent Catalysts to Recover Lanthanum and Cerium: Comparison between Selective Precipitation and Solvent Extraction. J. Ind. Eng. Chem. 2015, 24, 92–97. [Google Scholar] [CrossRef]
- Zhi, H.; Ni, S.; Su, X.; Xie, W.; Zhang, H.; Sun, X. Separation and Recovery of Rare Earth from Waste Nickel-Metal Hydride Batteries by Phosphate Based Extraction-Precipitation. J. Rare Earths 2021. [Google Scholar] [CrossRef]
- Porvali, A.; Wilson, B.P.; Lundström, M. Lanthanide-Alkali Double Sulfate Precipitation from Strong Sulfuric Acid NiMH Battery Waste Leachate. Waste Manag. 2018, 71, 381–389. [Google Scholar] [CrossRef]
- Lie, J.; Lin, Y.C.; Liu, J.C. Process Intensification for Valuable Metals Leaching from Spent NiMH Batteries. Chem. Eng. Process. Process Intensif. 2021, 167, 108507. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Dutra, A.J.B. Separation of Nickel(II), Cobalt(II) and Lanthanides from Spent Ni-MH Batteries by Hydrochloric Acid Leaching, Solvent Extraction and Precipitation. Hydrometallurgy 2013, 133, 37–43. [Google Scholar] [CrossRef]
- Innocenzi, V.; Vegliò, F. Recovery of Rare Earths and Base Metals from Spent Nickel-Metal Hydride Batteries by Sequential Sulphuric Acid Leaching and Selective Precipitations. J. Power Sources 2012, 211, 184–191. [Google Scholar] [CrossRef]
- Ahn, N.K.; Shim, H.W.; Kim, D.W.; Swain, B. Valorization of Waste NiMH Battery through Recovery of Critical Rare Earth Metal: A Simple Recycling Process for the Circular Economy. Waste Manag. 2020, 104, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Sobianowska-Turek, A. Hydrometallurgical Recovery of Metals: Ce, La, Co, Fe, Mn, Ni and Zn from the Stream of Used Ni-MH Cells. Waste Manag. 2018, 77, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Process Optimization and Kinetics for Leaching of Rare Earth Metals from the Spent Ni-Metal Hydride Batteries. Waste Manag. 2016, 51, 196–203. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Bernardes, A.M.; Tenório, J.A.S. Spent NiMH Batteries-The Role of Selective Precipitation in the Recovery of Valuable Metals. J. Power Sources 2009, 193, 914–923. [Google Scholar] [CrossRef]
- Pietrelli, L.; Bellomo, B.; Fontana, D.; Montereali, M.R. Rare Earths Recovery from NiMH Spent Batteries. Hydrometallurgy 2002, 66, 135–139. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Ju, Z.; Wu, F. Recovery of Ni, Co and Rare Earths from Spent Ni-Metal Hydride Batteries and Preparation of Spherical Ni(OH)2. Hydrometallurgy 2009, 100, 41–46. [Google Scholar] [CrossRef]
- Contestabile, M.; Panero, S.; Scrosati, B. A Laboratory-Scale Lithium-Ion Battery Recycling Process. J. Power Sources 2001, 92, 65–69. [Google Scholar] [CrossRef]
- Tan, Q.; Li, J. Recycling Metals from Wastes: A Novel Application of Mechanochemistry. Environ. Sci. Technol. 2015, 49, 5849–5861. [Google Scholar] [CrossRef] [PubMed]
- Petranikova, M.; Ebin, B.; Tunsu, C. Selective Recovery of Cobalt from the Secondary Streams after NiMH Batteries Processing Using Cyanex 301. Waste Manag. 2019, 83, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Ultrasonic-Assisted Leaching Process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Wang, R.C.; Lin, Y.C.; Wu, S.H. A Novel Recovery Process of Metal Values from the Cathode Active Materials of the Lithium-Ion Secondary Batteries. Hydrometallurgy 2009, 99, 194–201. [Google Scholar] [CrossRef]
- Guzolu, J.S.; Gharabaghi, M.; Mobin, M.; Alilo, H. Extraction of Li and Co from Li-Ion Batteries by Chemical Methods. J. Inst. Eng. (India) Ser. D 2017, 98, 43–48. [Google Scholar] [CrossRef]
- Biswas, S.; Chakraborty, S.; Chaudhuri, M.G.; Banerjee, P.C.; Mukherjee, S.; Dey, R. Optimization of Process Parameters and Dissolution Kinetics of Nickel and Cobalt from Lateritic Chromite Overburden Using Organic Acids. J. Chem. Technol. Biotechnol. 2014, 89, 1491–1500. [Google Scholar] [CrossRef]
- Verma, A.; Corbin, D.R.; Shiflett, M.B. Lithium and Cobalt Recovery for Lithium-Ion Battery Recycle Using an Improved Oxalate Process with Hydrogen Peroxide. Hydrometallurgy 2021, 203, 105694. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Organic Oxalate as Leachant and Precipitant for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Shen, B. Novel Approach to Recover Cobalt and Lithium from Spent Lithium-Ion Battery Using Oxalic Acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using Lactic Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-Acid-Assisted Recovery of Cobalt and Lithium from Spent Li-Ion Batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic Acid-Based Leaching System: A Sustainable Process for Recovery of Valuable Metals from Spent Li-Ion Batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of Metals from Spent Lithium-Ion Batteries with Organic Acids as Leaching Reagents and Environmental Assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of Mild Organic Acid Reagents to Recover the Co and Li from Spent Li-Ion Batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadzadeh, R.; Rashchi, F.; Vahidi, E. Recovery of Lithium and Cobalt from Spent Lithium-Ion Batteries Using Organic Acids: Process Optimization and Kinetic Aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Long, H.L.; Zhou, L.; Wu, Z.S.; Zhou, X.; You, L.; Yang, Y.; Liu, J.W. Leaching Procedure and Kinetic Studies of Cobalt in Cathode Materials from Spent Lithium Ion Batteries Using Organic Citric Acid as Leachant. Int. J. Environ. Res 2016, 10, 159–168. [Google Scholar]
- Chen, X.; Luo, C.; Zhang, J.; Kong, J.; Zhou, T. Sustainable Recovery of Metals from Spent Lithium-Ion Batteries: A Green Process. ACS Sustain. Chem. Eng. 2015, 3, 3104–3113. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of Cobalt and Lithium from Spent Lithium Ion Batteries Using Organic Citric Acid as Leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Chen, X.; Zhou, T.; Zhang, J.; Xu, B. A Sustainable Process for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Waste Manag. Res. 2016, 34, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Santana, I.L.; Moreira, T.F.M.; Lelis, M.F.F.; Freitas, M.B.J.G. Photocatalytic Properties of Co3O4/LiCoO2 Recycled from Spent Lithium-Ion Batteries Using Citric Acid as Leaching Agent. Mater. Chem. Phys. 2017, 190, 38–44. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Manjanna, J.; Pai, K.; Vadavi, R.; Keny, S.J.; Tripathi, V.S. Recovery of Valuable Metal Ions from the Spent Lithium-Ion Battery Using Aqueous Mixture of Mild Organic Acids as Alternative to Mineral Acids. Hydrometallurgy 2015, 151, 73–77. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, T. Hydrometallurgical Process for the Recovery of Metal Values from Spent Lithium-Ion Batteries in Citric Acid Media. Waste Manag. Res. 2014, 32, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.W.; Xi, X.L.; Zhang, Z.Z.; Huang, Z.Q.; Chen, J.P. Hydrometallurgical Treatment for Mixed Waste Battery Material. In IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2017; Volume 170. [Google Scholar]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating Organic Acids as Alternative Leaching Reagents for Metal Recovery from Lithium Ion Batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Anwani, S.; Methekar, R.; Ramadesigan, V. Life Cycle Assessment and Economic Analysis of Acidic Leaching and Baking Routes for the Production of Cobalt Oxalate from Spent Lithium-Ion Batteries. J. Mater. Cycles Waste Manag. 2020, 22, 2092–2106. [Google Scholar] [CrossRef]
- Pietrelli, L.; Bellomo, B.; Fontana, D.; Montereali, M. Characterization and Leaching of NiCd and NiMH Spent Batteries for the Recovery of Metals. In Waste Management; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; Volume 25, pp. 221–226. [Google Scholar]
- Aaltonen, M.; Peng, C.; Wilson, B.P.; Lundström, M. Leaching of Metals from Spent Lithium-Ion Batteries. Recycling 2017, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.S.; Ho, H.J. Leaching Behavior Analysis of Valuable Metals from Lithium-Ion Batteries Cathode Material. Key Eng. Mater. 2018, 775, 419–426. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical Processing of Spent Lithium Ion Batteries (LIBs) in the Presence of a Reducing Agent with Emphasis on Kinetics of Leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Chen, R.; Wu, F.; Chen, S.; Zhang, X. Environmental Friendly Leaching Reagent for Cobalt and Lithium Recovery from Spent Lithium-Ion Batteries. Waste Manag. 2010, 30, 2615–2621. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Mu, Y.Y.; Song, X.F.; Yu, J.G. Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Using l -Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.; Santhosh, G.; Manjanna, J. Dissolution of Cathode Active Material of Spent Li-Ion Batteries Using Tartaric Acid and Ascorbic Acid Mixture to Recover Co. Hydrometallurgy 2016, 161, 54–57. [Google Scholar] [CrossRef]
- Cheng, Q. Effect of Different Reductants on Leaching Lithium and Cobalt from Lithium Ion Batteries in Tartaric Acid Solution. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2018; Volume 192. [Google Scholar]
- Chen, X.; Kang, D.; Cao, L.; Li, J.; Zhou, T.; Ma, H. Separation and Recovery of Valuable Metals from Spent Lithium Ion Batteries: Simultaneous Recovery of Li and Co in a Single Step. Sep. Purif. Technol. 2019, 210, 690–697. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Song, J.; Cao, H.; Lin, X.; Zhang, X.; Zheng, X.; Zhang, Y.; Sun, Z. Selective Recovery of Valuable Metals from Spent Lithium-Ion Batteries–Process Development and Kinetics Evaluation. J. Clean. Prod. 2018, 178, 833–845. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.-I. Reductive Leaching of Cathodic Active Materials from Lithium Ion Battery Wastes. Hydrometallurgy 2003, 68, 5–10. [Google Scholar] [CrossRef]
- Pinna, E.G.; Ruiz, M.C.; Ojeda, M.W.; Rodriguez, M.H. Cathodes of Spent Li-Ion Batteries: Dissolution with Phosphoric Acid and Recovery of Lithium and Cobalt from Leach Liquors. Hydrometallurgy 2017, 167, 66–71. [Google Scholar] [CrossRef]
- Suarez, D.S.; Pinna, E.G.; Rosales, G.D.; Rodriguez, M.H. Synthesis of Lithium Fluoride from Spent Lithium Ion Batteries. Minerals 2017, 7, 81. [Google Scholar] [CrossRef] [Green Version]

| Solvent (Concentration) | Reducing Agent | S/L (g/L) | T (°C) | Contact Time (h) | Recovery Efficiency % | Reference | |
|---|---|---|---|---|---|---|---|
| Recovery of lanthanum | |||||||
| Spent cracking catalyst | Nitric acid (7 M) | - | 100 | 90 | 3 | 99% La | [19] |
| Spent cracking catalyst | Nitric acid (5 M) | - | 10 | 80 | 3 | ≈100% La | [12] |
| Spent NiMH batteries | Nitric acid (4 M) | - | 83.3 | 70 | ≈30% La | [20] | |
| Cropland soil | Nitric acid (0.1 M) | - | 100 | 20 | 72 | 62.67% La | [21] |
| Spent catalyst | Citric acid (0.1 M) | - | 200 | 30 | 4 | 100% La | [22] |
| Spent catalyst | Citric acid (1 M) | - | 200 | 60 | 5 | 100% La | [22] |
| Spent catalyst | Citric acid (2 M) | - | 200 | 80 | 6 | 100% La | [22] |
| Cropland soil | Citric acid (0.1 M) | - | 100 | 20 | 72 | 66.59% La | [21] |
| Cropland soil | EDTA (0.1 M) | - | 100 | 20 | 72 | 79.6% La | [21] |
| Spent cracking catalyst | Sulfuric acid (2 M) | - | 150 | 25 | 3 | 61.2% La | [23] |
| Spent cracking catalyst | Sulfuric acid (2 M) | - | 150 | 80 | 3 | 89% La | [23] |
| Spent NiMH batteries | Sulfuric acid (2 M) | - | 100 | 4 | 99.9% La | [24] | |
| Spent NiMH batteries | First stage: Sulfuric acid (2 M) Second stage: H2O | - | 96.2 | First stage: 30 Second stage: RT | First stage: 3 Second stage: 1.5 | 80.1% La | [25] |
| WEEE | Sulfuric acid (1 M) | - | 50 | 80 | 1 | 60% La | [9] |
| Recovery of lanthanum and cobalt | |||||||
| Spent NiMH batteries | HCl (1 M) | - | 20 | 45 | 1 | 94.61% Co 99.14% La | [26] |
| Spent NiMH batteries | HCl (0.5 M) | - | 20 | 100 | 0.5 | 99.28% Co 97.02% La | [26] |
| Spent NiMH batteries | HCl (1 M) | - | 20 | 65 | 0.5 | 94.13% Co 95.57% La | [26] |
| Spent NiMH batteries | HCl (12 M) | - | 150 | 40 | 1.67 | [27] | |
| Spent NiMH batteries | Sulfuric acid (3 M) | - | 150 | First stage: 80 Second stage: RT | First stage: 3 Second stage: 1 | 99% La 76% Co | [28] |
| Spent NiMH batteries | Sulfuric acid (1 M) | - | 25 | 90 | 4 | ≈100% Co ≈90% La | [29] |
| Spent NiMH batteries | Sulfuric acid (3 M) | - | 100 | 25 | 1.25 | 88.7% La 79.4% Co | [30] |
| Spent NiMH batteries | Sulfuric acid (2 M) | - | 100 | 75 | 2 | 97.8% Co 69.5% La | [31] |
| Spent NiMH batteries | Sulfuric acid (2 M) | - | 50 | 90 | 4 | 92.31% Co 84% La | [32] |
| Spent NiMH batteries | Sulfuric acid (2 M) | - | 100 | 20 | 2 | 100% Co 0% La | [33] |
| Spent NiMH batteries | Sulfuric acid (3 M) | 133 | 95 | 4 | >90% Co <5% RE | [34] | |
| Recovery of cobalt | |||||||
| Spent lithium-ion batteries | HCl (4 M) | - | 100 | 80 | 1 | [35] | |
| Spent lithium-ion batteries | HCl (1 M) | - | 10 | RT | 2 | 90% Co | [36] |
| Spent NiMH batteries | HCl (8 M) | - | 510 | 30 | >0.5 | >90% Co | [37] |
| Spent lithium-ion batteries | HCl (2 M) | - | 25 | 60 | 5 | 77% Co | [38] |
| Spent lithium-ion batteries | HCl (4 M) | - | 20 | 80 | 1 | 99.5% Co | [39] |
| Spent lithium-ion batteries | HCl (5 M) | - | 10 | 95 | 1.17 | 99.74% Co | [40] |
| Lateritic chromite overburden | Oxalic acid (0.15 M) | - | 20 | 80 | 3 | 44.33% Co | [41] |
| Spent lithium-ion batteries | Oxalic acid (0.23 M) | H2O2 (0.46 M) | 15 | 65 | 1 | [42] | |
| Spent lithium-ion batteries | Oxalic acid (0.46 M) | - | 15 | 90 | 1 | [42] | |
| Spent lithium-ion batteries | Oxalic acid (1 M) | - | 50 | 80 | 2 | 98% Co | [43] |
| Spent lithium-ion batteries | Oxalic acid (1 M) | - | 15 | 95 | 2.5 | 97% Co | [44] |
| Spent lithium-ion batteries | Lactic acid (1.5 M) | H2O2 (0.5 v%) | 20 | 70 | 0,33 | 98.9% Co | [45] |
| Spent lithium-ion batteries | Ascobic acid (1.25 M) | Ascorbic acid (1.25 M) | 25 | 70 | 0,33 | 94.8% Co | [46] |
| Spent lithium-ion batteries | Succinic acid (1.5 M) | H2O2 (4 v%) | 15 | 70 | 0.67 | 96% Co | [47] |
| Spent lithium-ion batteries | L-Aspartic acid (1.5 M) | H2O2 (4 v%) | 10 | 90 | 2 | 60% Co | [48] |
| Spent lithium-ion batteries | Iminodiacetic acid (1 M) | Ascorbic acid (0.02 M) | 3 | 80 | 6 | 91% Co | [49] |
| Spent lithium-ion batteries | Maleic acid (1 M) | Ascorbic acid (0.02 M) | 4 | 80 | 6 | 97% Co | [49] |
| Spent lithium-ion batteries | Citric acid (2 M) | H2O2 (1.25 v%) | 30 | 60 | 2 or 5 | 81.5% Co after 2 h 96.46% Co after 5 h | [50] |
| Spent lithium-ion batteries | Citric acid (1.25 M) | H2O2 (1 v%) | 20 | 90 | 0.5 | 92% Co | [48] |
| Spent lithium-ion batteries | Citric acid (2 M) | H2O2 (1.03 v%) | 30 | 80 | 2 | 84.04% Co | [50] |
| Spent lithium-ion batteries | Citric acid | H2O2 (1 v%) | 15 | 90 | 5 | 99.07% | [51] |
| Spent lithium-ion batteries | Citric acid (1.5 M) | Tea waste (0.4 g/g) | 30 | 90 | 2 | 96% Co | [52] |
| Spent lithium-ion batteries | Citric acid (1.5 M) | Phytolacca Americana (0.4 g/g) | 40 | 80 | 2 | 83% Co | [52] |
| Spent lithium-ion batteries | Citric acid (2 M) | H2O2 (0.6 g/g) | 50 | 70 | 1.33 | 98% Co | [52] |
| Spent lithium-ion batteries | Citric acid (1.25 M) | H2O2 (1 v%) | 20 | 90 | 0.5 | 90% Co | [53] |
| Spent lithium-ion batteries | Citric acid (1.25 M) | H2O2 (1 v%) | 16.7 | 90 | 0.58 | 90.2% Co | [54] |
| Spent lithium-ion batteries | Citric acid (2 M) | H2O2 (0.55 M) | 25 | 60 | 5 | 96% Co | [38] |
| Spent lithium-ion batteries | Citric acid (2 M) | H2O2 (3 v%) | 20 | 80 | 1.5 | [55] | |
| Spent lithium-ion batteries | Citric acid (0.1 M) | Ascorbic acid (0.02 M) | 5 | 80 | 6 | 80% Co | [56] |
| Spent lithium-ion batteries | Citric acid (2 M) | H2O2 (2 v%) | 33.3 | 80 | 1.5 | 95% Co | [57] |
| Spent lithium-ion batteries | Citric acid (2.5 M) | H2O2 (5 v%) | 25 | 85 | 2 | 85.1% Co | [58] |
| Spent lithium-ion batteries | Citric acid (1.25 M) | - | 20 | 80 | 2 | 62% Co | [59] |
| Spent lithium-ion batteries | Citric acid (1.25 M) | H2O2 (2 v%) | 20 | 80 | 0.33 | 74% Co | [59] |
| Spent lithium-ion batteries | Citric acid (1.25 M) | H2O2 (2 v%) | 20 | 95 | 0.5 | 91% Co | [59] |
| Spent lithium-ion batteries | Citric acid (1.5 M) | H2O2 (2 v%) | 20 | 95 | 0.5 | 95% Co | [59] |
| Spent lithium-ion batteries | Citric acid (1 M) | - | 20 | 95 | 1.5 | 95% Co | [59] |
| Spent lithium-ion batteries | Sulfuric acid (1 M) | H2O2 (4 v%) | 20 | 95 | 1 | 85.4% Co | [60] |
| Spent NiMH batteries | Sulfuric acid (2 M) | - | 100 | 20 | 1.17 | 100% Co | [61] |
| Spent lithium-ion batteries | Sulfuric acid (2 M) | H2O2 (0.55 M) | 25 | 40 | 5 | 48% Co | [38] |
| Spent lithium-ion batteries | Sulfuric acid (2.5 M) | H2O2 (5 v%) | 25 | 85 | 3 | 82.9% Co | [62] |
| Spent lithium-ion batteries | Sulfuric acid (2 M) | H2O2 (2 v%) | 100 | 70 | 5 | 96.7% Co | [62] |
| Spent lithium-ion batteries | Sulfuric acid (2 M) | D-glucose (12% g/g scraps) | 100 | 80 | 5 | 93.1% Co | [62] |
| Spent lithium-ion batteries | Sulfuric acid (2 M) | Ascorbic acid (10% g/g scraps) | 100 | 80 | 5 | 100.7% Co | [62] |
| Spent lithium-ion batteries | Sulfuric acid (2 M) | H2O2 (10 v%) | 33.3 | 70 | 2 | 98.46% | [63] |
| Spent lithium-ion batteries | Sulfuric acid (1 M) | NaHSO3 (0.075 M) | 20 | 95 | 4 | 91.6% Co | [64] |
| Spent lithium-ion batteries | DL-Malic acid (1.5 M) | H2O2 (2 v%) | 20 | 90 | 0.67 | 93% Co | [48] |
| Spent lithium-ion batteries | DL-Malic acid (2 M) | H2O2 (2 v%) | 30 | 80 | 2 | 84.02% Co | [50] |
| Spent lithium-ion batteries | DL-Malic acid (1.25 M) | H2O2 (2 v%) | 20 | 95 | 0.33 | 91% Co | [59] |
| Spent lithium-ion batteries | DL-Malic acid (1.5 M) | H2O2 (2 v%) | 20 | 90 | 0.33 | 73% Co | [59] |
| Spent lithium-ion batteries | DL-Malic acid (1 M) | H2O2 (2 v%) | 20 | 95 | 0.5 | 98% Co | [59] |
| Spent lithium-ion batteries | DL-Malic acid (1.5 M) | H2O2 (2 v%) | 20 | 95 | 0.5 | 98% Co | [59] |
| Spent lithium-ion batteries | DL-Malic acid (1.5 M) | H2O2 (2 v%) | 20 | 90 | 0.67 | 93% Co | [65] |
| Spent lithium-ion batteries | L-tartaric acid (2 M) | H2O2 (4 v%) | 17 | 70 | 0.5 | 98.64% | [66] |
| Spent lithium-ion batteries | Tartaric acid (0.4 M) | Ascorbic acid (0.02 M) | 2 | 80 | 5 | >95% Co | [67] |
| Spent lithium-ion batteries | Tartaric acid (1 M) | Glucose (20 g/L) | 10 | 80 | 1.5 | 46.6% Co | [68] |
| Spent lithium-ion batteries | Tartaric acid (1 M) | Ascorbic acid (30 g/L) | 10 | 80 | 1.5 | 47.3% Co | [68] |
| Spent lithium-ion batteries | Tartaric acid (1 M) | H2O2 (10 v%) | 10 | 80 | 1.5 | 53.2% Co | [68] |
| Spent lithium-ion batteries | Tartaric acid (0.6 M) | H2O2 (3 v%) | 33.3 | 80 | 0.5 | 97% Co | [69] |
| Spent lithium-ion batteries | Formic acid (2 M) | H2O2 (6 v%) | 50 | 60 | 0.19 | 90.49% Co | [70] |
| Spent lithium-ion batteries | Acetic acid (3.5 M) | H2O2 (4 v%) | 40 | 60 | 1 | 93.62% Co | [71] |
| Spent lithium-ion batteries | Nitric acid (1 M) | H2O2 (1.7 v%) | 20 | 75 | 0.5 | >95% Co | [72] |
| Spent lithium-ion batteries | Phosphoric acid (2 M) | H2O2 (2 v%) | 8 | 90 | 1 | 99% Co | [73] |
| Spent lithium-ion batteries | Hydrogen fluoride (15 v%) | - | 20 | 75 | 2 | 58% Co | [74] |
| Leaching Acid Agent | ||||||
|---|---|---|---|---|---|---|
| No. Green Chemistry Principle | Nitric | Hydrochloric | Sulfuric | Citric | DL-Malic | Oxalic |
| 3. Less Hazardous Chemical Syntheses | ● | ● | ● | ● | ● | ● |
| 4. Designing Safer Chemicals | ● | ● | ● | ● | ● | ● |
| 5. Safer Solvents and Auxiliaries | ● | ● | ● | ● | ● | ● |
| 10. Design for Degradation | ● | ● | ● | ● | ● | ● |
| 12. Inherently Safer Chemistry for Accident Prevention | ● | ● | ● | ● | ● | ● |
| Median Value | ||||
|---|---|---|---|---|
| Element of Interest | Acid Concentration | S/L Ratio | Temperature | Contact Time |
| Lanthanum | 2 M | 100 g/L | 70 °C | 180 min |
| Cobalt | 1.5 M | 20 g/L | 80 °C | 80 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetto Mas, A.; Fiore, S.; Fiorilli, S.; Smeacetto, F.; Santarelli, M.; Schiavi, I. Analysis of Lanthanum and Cobalt Leaching Aimed at Effective Recycling Strategies of Solid Oxide Cells. Sustainability 2022, 14, 3335. https://doi.org/10.3390/su14063335
Benedetto Mas A, Fiore S, Fiorilli S, Smeacetto F, Santarelli M, Schiavi I. Analysis of Lanthanum and Cobalt Leaching Aimed at Effective Recycling Strategies of Solid Oxide Cells. Sustainability. 2022; 14(6):3335. https://doi.org/10.3390/su14063335
Chicago/Turabian StyleBenedetto Mas, Alice, Silvia Fiore, Sonia Fiorilli, Federico Smeacetto, Massimo Santarelli, and Ilaria Schiavi. 2022. "Analysis of Lanthanum and Cobalt Leaching Aimed at Effective Recycling Strategies of Solid Oxide Cells" Sustainability 14, no. 6: 3335. https://doi.org/10.3390/su14063335
APA StyleBenedetto Mas, A., Fiore, S., Fiorilli, S., Smeacetto, F., Santarelli, M., & Schiavi, I. (2022). Analysis of Lanthanum and Cobalt Leaching Aimed at Effective Recycling Strategies of Solid Oxide Cells. Sustainability, 14(6), 3335. https://doi.org/10.3390/su14063335










