Optimization of the Preparation of Activated Carbon from Prickly Pear Seed Cake for the Removal of Lead and Cadmium Ions from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Prickly Pear Seed Cake Characterization
2.3. Preparation of Activated Carbon
2.4. Experimental Design and Analysis
2.5. Adsorption Capacity Evaluation
2.6. Characterization Methods
2.7. Adsorption Study on the Optimized Activated Carbon
2.8. Regeneration Study
3. Results
3.1. Characterization of Prickly Pear Seed Cake
3.2. Adsorption Capacity of the Newly Prepared Activated Carbon
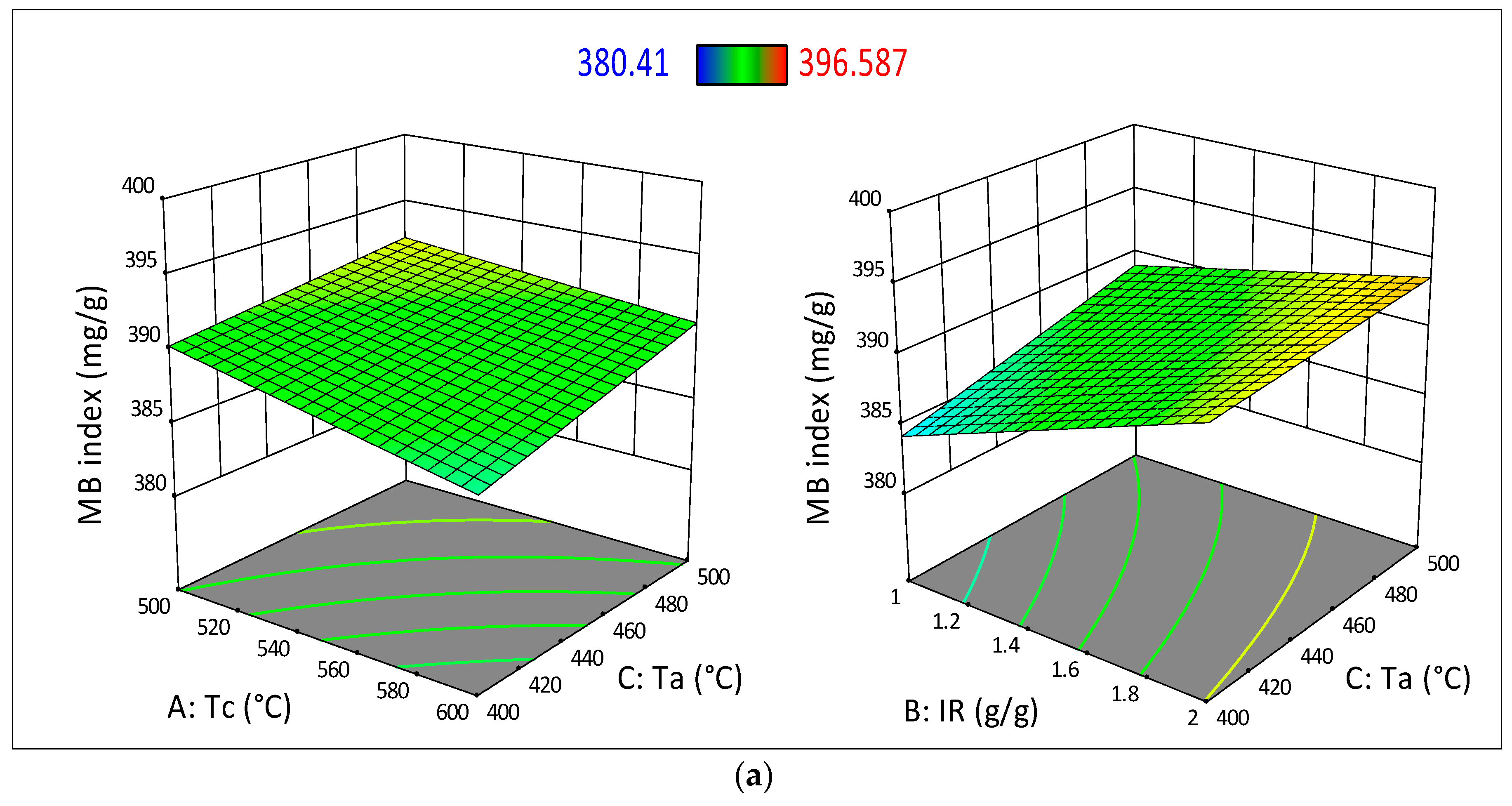
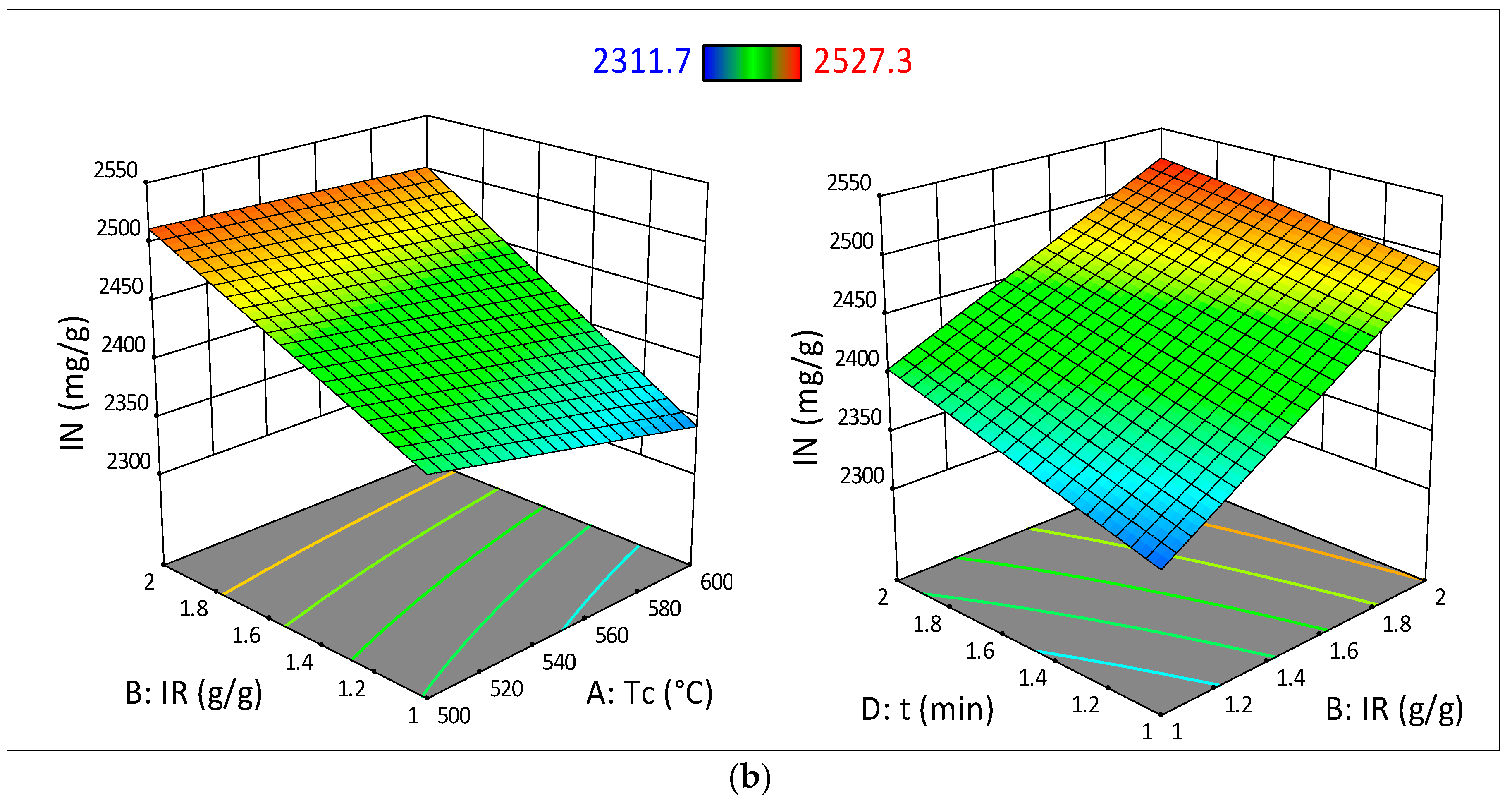
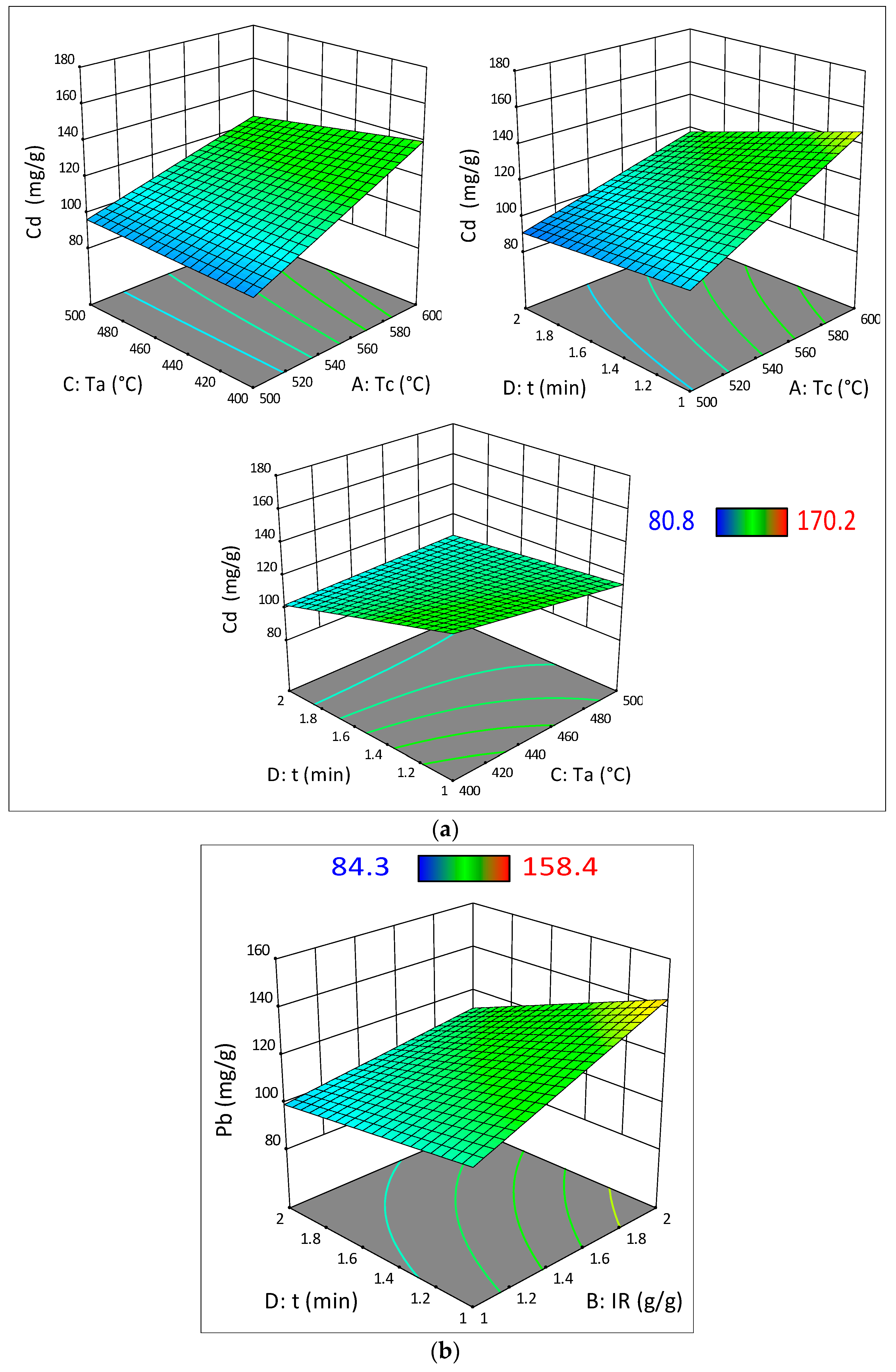
3.3. Analysis of Variance (ANOVA) and Surface Response Analysis
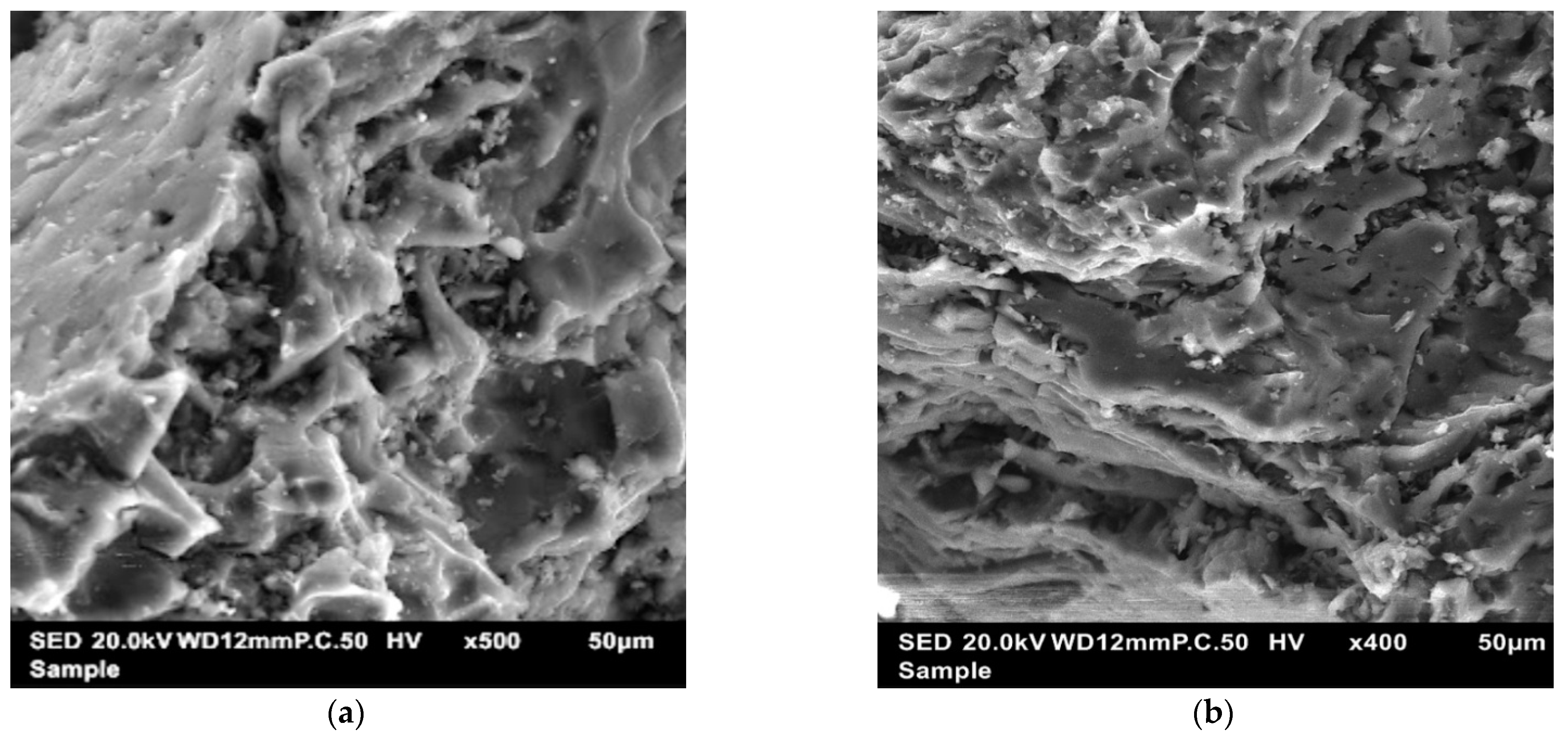
3.4. Characterization of Optimized Activated Carbon
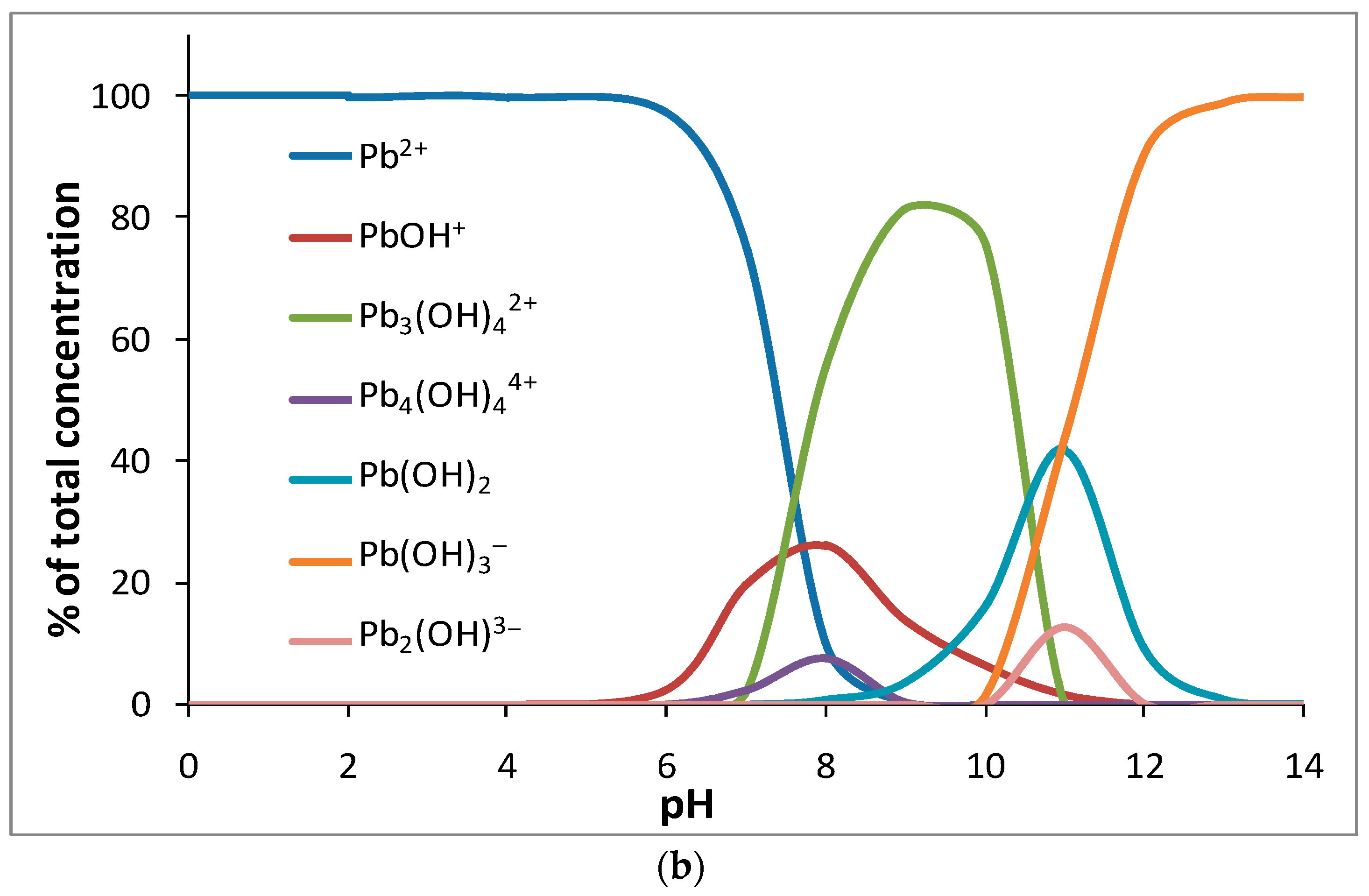
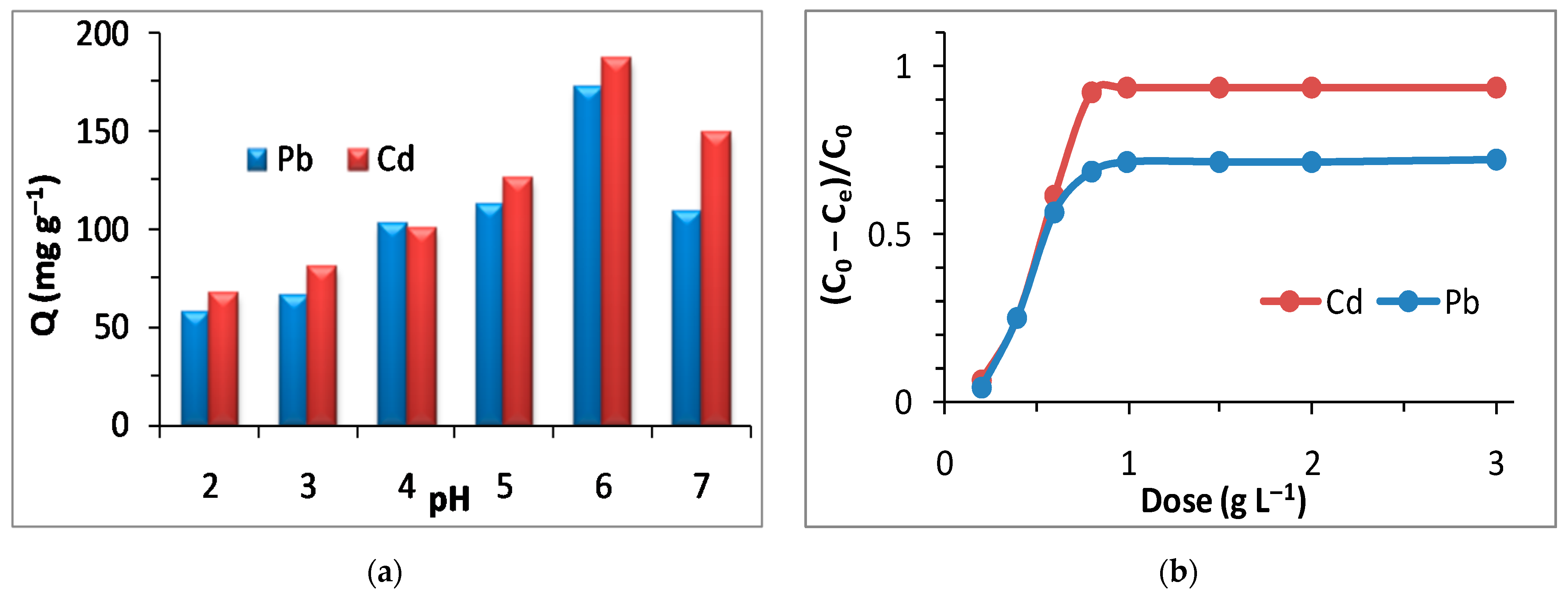
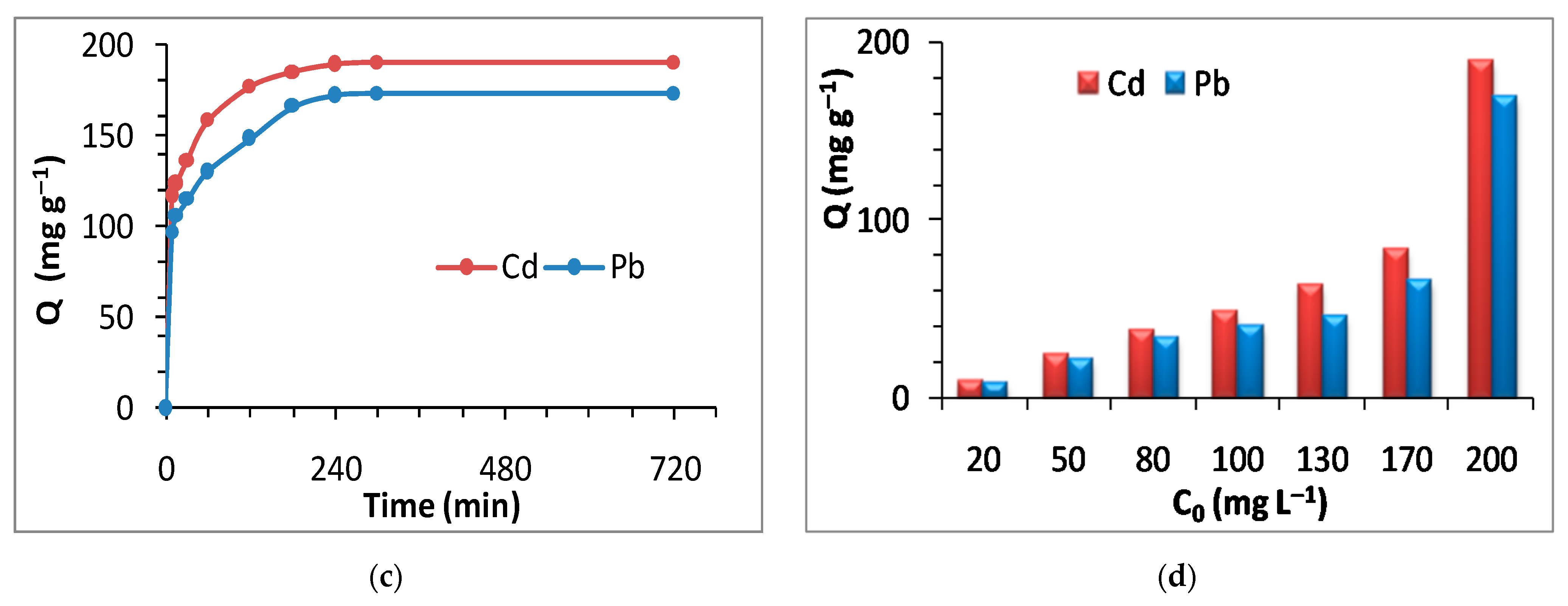
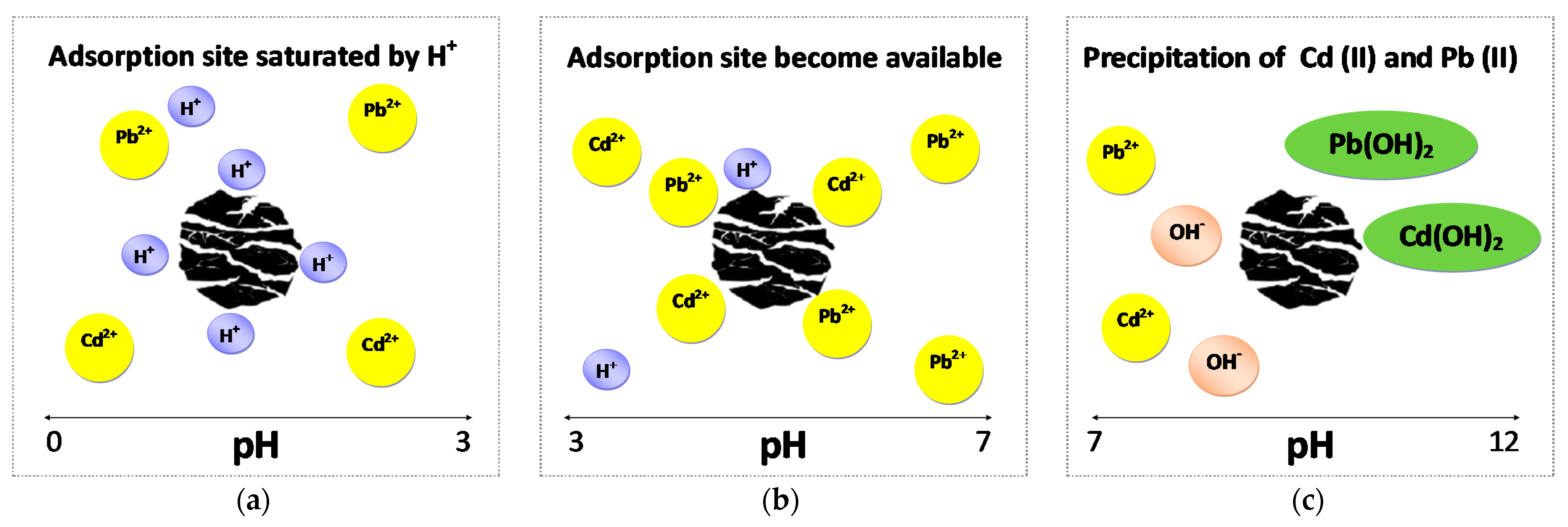
3.5. Adsorption Study: Effect of pH, Adsorbent Dose, Time, and Initial Metal Concentrations
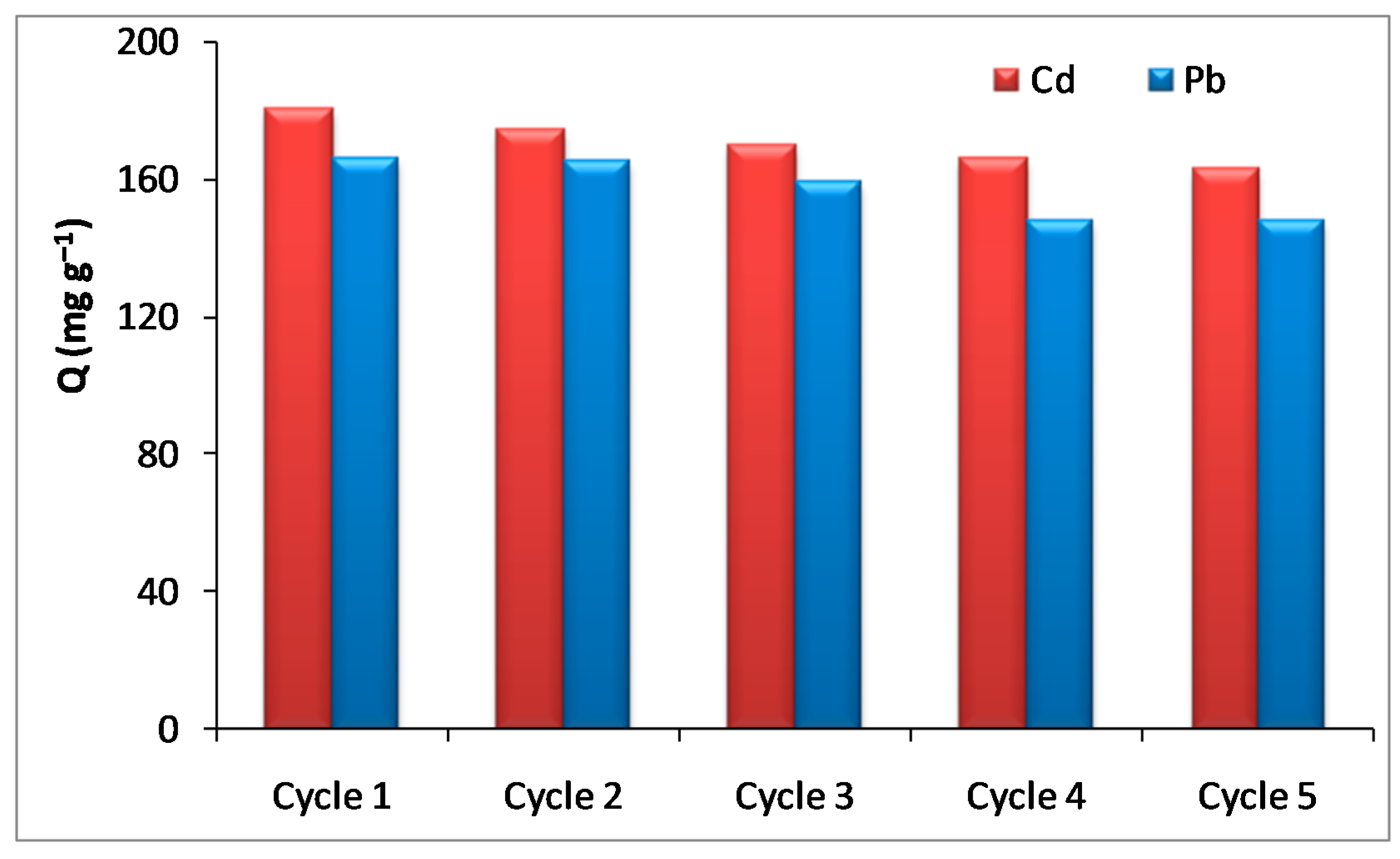
3.6. Regeneration Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Little, I.; Alorkpa, E.; Khan, V.; Povazhnyi, V.; Vasiliev, A. Efficient porous adsorbent for removal of cesium from contaminated water. J. Porous Mater. 2019, 26, 361–369. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef] [Green Version]
- Taleb, F.; Ben Mosbah, M.; Elaloui, E.; Moussaoui, Y. Adsorption of ibuprofen sodium salt onto Amberlite resin IRN-78: Kinetics, isotherm and thermodynamic investigations. Korean J. Chem. Eng. 2017, 34, 1141–1148. [Google Scholar] [CrossRef]
- Michail, C.; Koukou, V.; Martini, N.; Saatsakis, G.; Kalyvas, N.; Bakas, A.; Kandarakis, I.; Fountos, G.; Panayiotakis, G.; Valais, I. Luminescence Efficiency of Cadmium Tungstate. Crystals 2020, 10, 429. [Google Scholar] [CrossRef]
- Runtti, H.; Tuomikoski, S.; Kangas, T.; Lassi, U.; Kuokkanen, T.; Rämö, J. Chemically activated carbon residue from biomass gasification as a sorbent for iron(II), copper(II) and nickel(II) ions. J. Water Process Eng. 2014, 4, 2–24. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.H.; Zhang, W.; Shi, L.X.; Yi, K.X.; Yu, H.B.; Zhang, C.Y.; Li, S.Z.; Li, J.N. Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects. J. Environ. Manage. 2022, 302, 113995. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Tanhaei, B.; Sillanpää, M. Lead (II)-ion removal by ethylenediaminetetraacetic acid ligand functionalized magnetic chitosan–aluminum oxide–iron oxide nanoadsorbents and microadsorbents: Equilibrium, kinetics, and thermodynamics. J. Appl. Polym. Sci. 2017, 134, 44360. [Google Scholar] [CrossRef]
- Tepanosyan, G.; Sahakyan, L.; Belyaeva, O.; Asmaryan, S.; Saghatelyan, A. Continuous impact of mining activities on soil heavy metals levels and human health. Sci. Total Environ. 2018, 639, 900–909. [Google Scholar] [CrossRef]
- Munir, N.; Jahangeer, M.; Bouyahya, A.; El Omari, N.; Ghchime, R.; Balahbib, A.; Aboulaghras, S.; Mahmood, Z.; Akram, M.; Shah, S.M.A.; et al. Heavy Metal Contamination of Natural Foods Is a Serious Health Issue: A Review. Sustainability 2022, 14, 161. [Google Scholar] [CrossRef]
- Adam, A.M.; Saad, H.A.; Atta, A.A.; Alsawat, M.; Hegab, M.S.; Altalhi, T.A.; Refat, M.S. An Environmentally friendly method for removing Hg (II), Pb (II), Cd (II) and Sn (II) Heavy Metals from Wastewater Using Novel Metal—Carbon-Based Composites. Crystals 2021, 11, 882. [Google Scholar] [CrossRef]
- Sun, J.; Liu, L.; Yang, F. A WO3/PPy/ACF modified electrode in electrochemical system for simultaneous removal of heavy metal ion Cu2+ and organic acid. J. Hazard. Mater. 2020, 394, 122534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, C. Efficient removal of heavy metal ions based on the optimized dissolution-diffusion-flow forward osmosis process. Chem. Eng. J. 2018, 334, 1128–1134. [Google Scholar] [CrossRef]
- Corroto, C.; Iriel, A.; Cirelli, A.F.; Carrera, A.L.P. Constructed wetlands as an alternative for arsenic removal from reverse osmosis effluent. Sci. Total Environ. 2019, 691, 1242–1250. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Pan, S.Y.; Zhu, S.; Yu, Y.; Zheng, H. Performance evaluation and optimization of flocculation process for removing heavy metal. Chem. Eng. J. 2020, 385, 123911. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.L.; Zhou, J.X.; Li, C.Z.; Yang, Z.H.; Ruan, M.; Li, H.; Zhang, X.; Wu, Z.J.; Qin, X.L.; et al. Enhancement of heavy metals removal by microbial flocculant produced by Paenibacilluspolymyxa combined with an insufficient hydroxide precipitation. Chem. Eng. J. 2019, 374, 880–894. [Google Scholar] [CrossRef]
- Serrano, L.Z.; Lara, N.O.; Vera, R.R.; Cholico-González, D. Removal of Fe(III), Cd(II), and Zn(II) as Hydroxides by Precipitation–Flotation System. Sustainability 2021, 13, 11913. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Bilardi, S.; Moraci, N. Advancements in the use of filtration materials for the removal of heavy metals from multicontaminated solutions. Curr. Opin. Environ. Sci. Health 2021, 20, 100241. [Google Scholar] [CrossRef]
- Tavakoli, O.; Goodarzi, V.; Saeb, M.R.; Mahmoodi, N.M.; Borja, R. Competitive removal of heavy metal ions from squid oil under isothermal condition by CR11 chelate ion exchanger. J. Hazard. Mater. 2017, 334, 256–266. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Araissi, M.; Ayed, I.; Elaloui, E.; Moussaoui, Y. Removal of barium and strontium from aqueous solution using zeolite 4A. Water Sci. Technol. 2016, 7, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- El-Maguana, N.; Elhadiri, M.; Bouchdoug, M.; Benchanaa, A.J. Activated Carbon from Prickly Pear Seed Cake: Optimization of Preparation Conditions Using Experimental Design and Its Application in Dye Removal. Int. J. Chem. Eng. 2019, 12, 8621951. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Fawzan, F.F. Adsorption study of heavy metal ions from aqueous solution by nanoparticle of wild herbs. Egypt. J. Aquat. Res. 2018, 44, 187–194. [Google Scholar] [CrossRef]
- Lee, H.M.; Lee, B.H.; An, K.H.; Park, S.J.; Kim, B.J. Facile preparation of activated carbon with optimal pore range for high butane working capacity. Carbon Lett. 2020, 30, 297–305. [Google Scholar] [CrossRef]
- Wu, L.; Shang, Z.; Wang, H. Production of activated carbon from walnut shell by CO2 activation in a fluidized bed reactor and its adsorption performance of copper ion. J. Mater. Cycles Waste Manag. 2018, 20, 1676–1688. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs. commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef]
- Minh, T.D.; Lee, B.K. Effects of functionality and textural characteristics on the removal of Cd (II) by ammoniated and chlorinated nanoporous activated carbon. J. Mater. Cycles Waste Manag. 2017, 19, 1022–1035. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel 2014, 128, 162–169. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Kalsoom, T.; Yasmeen, S.; Kalsoom, A.; Raina, S.; Zhuang, Q.; Kong, J. Animal manure-derived biochars produced via fast pyrolysis for the removal of divalent copper from aqueous media. J. Environ. Manage. 2018, 213, 109–118. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Rasit, N.; Ooi, C.K.; Ma, N.L.; Ng, J.H.; Lam, W.H.; Chong, C.T.; Chase, H.A. Pyrolysis production of fruit peel biochar for potential use in treatment of palm oil mill effluent. J. Environ. Manage. 2018, 213, 400–408. [Google Scholar] [CrossRef]
- Dhelipan, M.; Arunchander, A.; Sahu, A.K.; Kalpana, D. Activated carbon from orange peels as supercapacitor electrode and catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Saudi Chem. Soc. 2017, 21, 487–494. [Google Scholar] [CrossRef]
- Khadhri, N.; Saad, M.K.; Ben Mosbah, M.; Moussaoui, Y. Batch and continuous column adsorption of indigo carmine onto activated carbon derived from date palm petiole. J. Environ. Chem. Eng. 2019, 7, 102775. [Google Scholar] [CrossRef]
- Elhleli, H.; Mannai, F.; Ben Mosbah, M.; Khiari, R.; Moussaoui, Y. Biocarbon Derived from Opuntia ficus indica for p-Nitrophenol Retention. Processes 2020, 8, 10. [Google Scholar] [CrossRef]
- Saad, M.K.; Khiari, R.; Elaloui, E.; Moussaoui, Y. Adsorption of anthracene using activated carbon and Posidonia oceanica. Arab. J. Chem. 2014, 7, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.F.; Mörsel, J.-T. Oil cactus pear (Opuntia ficus L.). Food Chem. 2003, 82, 339–345. [Google Scholar] [CrossRef]
- Habibi, Y.; Heux, L.; Mahrouz, M.; Vignon, M.R. Morphological and structural study of seed pericarp of Opuntia ficus-indica prickly pear fruits. Carbohydr. Polym. 2008, 72, 102–112. [Google Scholar] [CrossRef]
- Amari, A.; Alalwan, B.; Eldirderi, M.M.; Mnif, W.; Rebah, F.B. Cactus material-based adsorbents for the removal of heavy metals and dyes: A review. Mater. Res. Express. 2019, 7, 012002. [Google Scholar] [CrossRef]
- Liosis, C.; Papadopoulou, A.; Karvelas, E.; Karakasidis, T.E.; Sarris, I.E. Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review. Materials 2021, 14, 7500. [Google Scholar] [CrossRef]
- Shimada, M.; Iida, T.; Kawarada, K. Pore structure and adsorption properties of activated carbon prepared from granular molded waste paper. J. Mater. Cycles Waste Manag. 2004, 6, 111–118. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, S.; Miao, M.; Liu, Y.; Gu, Z.; Jin, Y. Cost-effective preparation of metal-free electrocatalysts by phosphoric acid activation of lignocellulosic materials for oxygen reduction reaction. Int. J. Hydrogen Energy 2019, 44, 2811–2822. [Google Scholar] [CrossRef]
- Yang, H.M.; Zhang, D.H.; Chen, Y.; Ran, M.J.; Gu, J.C. Study on the application of KOH to produce activated carbon to realize the utilization of distiller’s grains. IOP Conf. Ser. Earth Environ. Sci. 2017, 69, 012051. [Google Scholar] [CrossRef] [Green Version]
- Üner, O.; Geçgel, Ü.; Bayrak, Y. Preparation and characterization of mesoporous activated carbons from waste watermelon rind by using the chemical activation method with zinc chloride. Arab. J. Chem. 2019, 12, 3621–3627. [Google Scholar] [CrossRef] [Green Version]
- Saad, S.; Dávila, I.; Mannai, F.; Labidi, J.; Moussaoui, Y. Effect of the autohydrolysis treatment on the integral revalorisation of Ziziphus lotus. Biomass Convers. Biorefin. 2022, 1–13. [Google Scholar] [CrossRef]
- M’barek, I.; Slimi, H.; AlSukaibi, A.K.D.; Alimi, F.; Lajimi, R.H.; Mechi, L.; ben Salem, R.; Moussaoui, Y. Cellulose from Tamarixaphylla’s stem via acetocell for cadmium adsorption. Arab. J. Chem. 2022, 15, 103679. [Google Scholar] [CrossRef]
- El Azizi, C.; Hammi, H.; Chaouch, M.A.; Majdoub, H.; Mnif, A. Use of Tunisian Opuntia ficus-indica Cladodes as a Low Cost Renewable Admixture in Cement Mortar Preparations. Chem. Africa 2019, 2, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Mannai, F.; Ammar, M.; Yanez, J.G.; Elaloui, E.; Moussaoui, Y. Cellulose fiber from Tunisian Barbary Fig “Opuntia ficusindica” for papermaking. Cellulose 2016, 23, 2061–2072. [Google Scholar] [CrossRef]
- Ferhi, F.; Das, S.; Elaloui, E.; Moussaoui, Y.; Yanez, J.G. Chemical characterisation and suitability for papermaking applications studied on four species naturally growing in Tunisia. Ind. Crops Prod. 2014, 61, 180–185. [Google Scholar] [CrossRef]
- Wise, L.; Murphy, E.; Addieco, M.A.A. Cloriteholocellulose: Its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Paper Trade J. 1946, 122, 35–43. [Google Scholar]
- Karoui, S.; Ben Arfi, R.; Mougin, K.; Ghorbal, A.; Assadi, A.A.; Amrane, A. Synthesis of novel biocomposite powder for simultaneous removal of hazardous ciprofloxacin and methylene blue: Central composite design, kinetic and isotherm studies using Brouers-Sotolongo family models. J. Hazard. Mater. 2020, 387, 121675. [Google Scholar] [CrossRef]
- Machrouhi, A.; Alilou, H.; Farnane, M.; El Hamidi, S.; Sadiq, M.; Abdennouri, M.; Tounsadi, H.; Barka, N. Statistical optimization of activated carbon from Thapsiatranstagana stems and dyes removal efficiency using central composite design. J. Sci. Adv. Mater. Devices 2019, 4, 544–553. [Google Scholar] [CrossRef]
- Tang, X.; Ran, G.; Li, J.; Zhang, Z.; Xiang, C. Extremely efficient and rapidly adsorb methylene blue using porous adsorbent prepared from waste paper: Kinetics and equilibrium studies. J. Hazard. Mater. 2021, 402, 123579. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G.; Hayes, R.; Drikas, M. Granular activated carbon: Importance of surface properties in the adsorption of naturally occurring organics. Colloids Surf. A Physicochem. Eng. Asp. 1993, 78, 65–71. [Google Scholar] [CrossRef]
- Masmoudi, M.; Baccouche, A.; Borchani, M.; Besbes, S.; Blecker, C.; Attia, H. Physico-chemical and antioxidant properties of oils and by-products obtained by cold press-extraction of Tunisian Opuntia spp. seeds. Appl. Food Res. 2021, 1, 100024. [Google Scholar] [CrossRef]
- Moussaoui, Y.; Ferhi, F.; Elaloui, E.; Bensalem, R.; Belgacem, M.N. Utilization of Astragalus armatus Roots in papermaking applications. BioResources 2011, 6, 4969–4978. [Google Scholar]
- Yang, Y.; Cannon, F.S. Biomass activated carbon derived from pine sawdust with steam bursting pretreatment; perfluorooctanoic acid and methylene blue adsorption. Bioresour. Technol. 2022, 344, 126161. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Chen, D.; Huang, L.; Zhang, Y.; Zhang, Y.; Wang, Q.; Xiao, G.; Zhang, W.; Lei, H.; Ruan, R. Activated carbon from lignocellulosic biomass as catalyst: A review of the applications in fast pyrolysis process. J. Anal. Appl. Pyrolysis 2021, 158, 105246. [Google Scholar] [CrossRef]
- Ibrahim, M.; Souleiman, M.; Salloum, A. Methylene blue dye adsorption onto activated carbon developed from Calicotomevillosa via H3PO4 activation. Biomass Conv. Bioref. 2021, 1–14. [Google Scholar] [CrossRef]
- Khalili, S.; Khoshandam, B.; Jahanshahi, M. Optimization of production conditions for synthesis of chemically activated carbon produced from pine cone using response surface methodology for CO2 adsorption. RSC Adv. 2015, 5, 94115–94129. [Google Scholar] [CrossRef]
- Saad, M.K.; Mnasri, N.; Mhamdi, M.; Chafik, T.; Elaloui, E.; Moussaoui, Y. Removal of methylene blue onto mineral matrices. Desalination Water Treat. 2015, 56, 2773–2780. [Google Scholar] [CrossRef]
- Kathiravan, D.; Huang, B.R.; Saravanan, A. Surface modified highly porous egg-shell membrane derived granular activated carbon coated on paper substrate and its humidity sensing properties. Mater. Chem. Phys. 2022, 277, 125486. [Google Scholar] [CrossRef]
- Al-Onazi, W.A.; Ali, M.H.H.; Al-Garni, T. Using pomegranate peel and date pit activated carbon for the removal of cadmium and lead ions from aqueous solution. J. Chem. 2021, 2021, 5514118. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Adsorption of toxic metal ions onto activated carbon: Study of sorption behaviour through characterization and kinetics. Chem. Eng. Processing Process Intensif. 2008, 47, 1269–1280. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Kavand, M.; Eslami, P.; Razeh, L. The adsorption of cadmium and lead ions from the synthesis wastewater with the activated carbon: Optimization of the single and binary systems. J. Water Process. Eng. 2020, 34, 101151. [Google Scholar] [CrossRef]
- Üçer, A.; Uyanik, A.; Aygün, Ş. Adsorption of Cu (II), Cd (II), Zn (II), Mn (II) and Fe (III) ions by tannic acid immobilised activated carbon. Sep. Purif. Technol. 2006, 47, 113–118. [Google Scholar] [CrossRef]
- Yang, X.; Wanc, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wangh, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–662. [Google Scholar] [CrossRef]
- Enshirah, D.; Awatif, A. Regeneration of spent activated carbon obtained from home filtration system and applying it for heavy metals adsorption. J. Environ. Chem. Eng. 2017, 5, 3091–3099. [Google Scholar] [CrossRef]

| Biomass | Ash | Lignin | Holocellulose | Cellulose |
|---|---|---|---|---|
| Prickly pear seed cake (this work) | 1.2 | 19.2 | 59.4 | 34.2 |
| Prickly pear seed cake [53] | 1.5 | 18.7 | 57.1 | 31.0 |
| Opuntia ficus-indica [46] | 5.5 | 4.8 | 64.5 | 53.6 |
| Astragalus armatus [54] | 3.0 | 16.7 | 54.0 | 35.0 |
| Pituranthos chloranthus [47] | 5.0 | 17.6 | 61.9 | 46.6 |
| Retama raetam [47] | 3.5 | 20.5 | 58.7 | 36.0 |
| Tamarix aphylla [44] | 3.5 | 30.0 | 50.0 | 39.0 |
| Scheme | Coded Values | Actual Values | Responses (mg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tc | IR | Ta | t | Tc | IR | Ta | t | MB Index (mg g−1) | IN(mg g−1) | Cd Removal (mg g−1) | Pb Removal (mg g−1) | |
| 1 | −1 | −1 | −1 | −1 | 500 | 1 | 400 | 1 | 387.366 | 2348.4 | 100.6 | 93.4 |
| 2 | 1 | −1 | −1 | −1 | 600 | 1 | 400 | 1 | 383.145 | 2322.6 | 151.6 | 108.6 |
| 3 | −1 | 1 | −1 | −1 | 500 | 2 | 400 | 1 | 393.541 | 2496.6 | 105.0 | 131.5 |
| 4 | 1 | 1 | −1 | −1 | 600 | 2 | 400 | 1 | 389.132 | 2489.2 | 170.2 | 158.4 |
| 5 | −1 | −1 | 1 | −1 | 500 | 1 | 500 | 1 | 390.212 | 2355.9 | 93.4 | 107.2 |
| 6 | 1 | −1 | 1 | −1 | 600 | 1 | 500 | 1 | 387.112 | 2311.7 | 131.6 | 129.4 |
| 7 | −1 | 1 | 1 | −1 | 500 | 2 | 500 | 1 | 394.055 | 2499.4 | 99.4 | 136.3 |
| 8 | 1 | 1 | 1 | −1 | 600 | 2 | 500 | 1 | 392.874 | 2481.3 | 136.4 | 148.6 |
| 9 | −1 | −1 | −1 | 1 | 500 | 1 | 400 | 2 | 385.985 | 2462.6 | 90.2 | 92.6 |
| 10 | 1 | −1 | −1 | 1 | 600 | 1 | 400 | 2 | 380.41 | 2332.2 | 118.8 | 119.6 |
| 11 | −1 | 1 | −1 | 1 | 500 | 2 | 400 | 2 | 394.312 | 2525.3 | 80.8 | 108.9 |
| 12 | 1 | 1 | −1 | 1 | 600 | 2 | 400 | 2 | 391.745 | 2517.7 | 119.8 | 122.8 |
| 13 | −1 | −1 | 1 | 1 | 500 | 1 | 500 | 2 | 388.477 | 2421.9 | 85.6 | 84.3 |
| 14 | 1 | −1 | 1 | 1 | 600 | 1 | 500 | 2 | 390.332 | 2403.8 | 116.4 | 101.6 |
| 15 | −1 | 1 | 1 | 1 | 500 | 2 | 500 | 2 | 396.587 | 2527.3 | 107.4 | 98.6 |
| 16 | 1 | 1 | 1 | 1 | 600 | 2 | 500 | 2 | 391.312 | 2522.3 | 117.0 | 118.6 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value Prob>F | |
|---|---|---|---|---|---|---|
| MB index | ||||||
| Model | 252.94 | 5 | 50.59 | 21.16 | <0.0001 | Significant |
| Tc | 37.43 | 1 | 37.43 | 15.66 | 0.0027 | |
| IR | 159.51 | 1 | 159.51 | 66.72 | <0.0001 | |
| Ta | 40.08 | 1 | 40.08 | 16.77 | 0.0022 | |
| (Tc_Ta) | 5.14 | 1 | 5.14 | 2.15 | 0.1732 | |
| (IR_Ta) | 10.77 | 1 | 10.77 | 4.51 | 0.0597 | |
| Residual | 23.91 | 10 | 2.39 | |||
| Cor Total | 276.85 | 15 | ||||
| R2 = 0.9136; R2adj = 0.870 | ||||||
| R2pred = 0.778; Std. Dev. = 1.55 | ||||||
| IN | ||||||
| Model | 93,695.34 | 5 | 18,739.07 | 43.40 | <0.0001 | Significant |
| Tc | 4115.22 | 1 | 4115.22 | 9.53 | 0.0115 | |
| IR | 75,625.00 | 1 | 75,625.00 | 175.15 | <0.0001 | |
| t | 10,404.00 | 1 | 10,404.00 | 24.10 | 0.0006 | |
| (Tc_IR) | 2034.01 | 1 | 2034.01 | 4.71 | 0.0551 | |
| (IR_t) | 1517.10 | 1 | 1517.10 | 3.51 | 0.0903 | |
| Residual | 4317.84 | 10 | 431.78 | |||
| Cor Total | 98,013.18 | 15 | ||||
| R2 = 0.955; R2adj = 0.933 | ||||||
| R2pred = 0.887; Std. Dev. = 20.78 | ||||||
| Cdremoval | ||||||
| Model | 8365.47 | 6 | 1394.25 | 24.77 | <0.0001 | Significant |
| Tc | 5602.52 | 1 | 5602.52 | 99.54 | <0.0001 | |
| Ta | 155.00 | 1 | 155.00 | 2.75 | 0.1314 | |
| t | 1447.80 | 1 | 1447.80 | 25.72 | 0.0007 | |
| (Tc_Ta) | 290.70 | 1 | 290.70 | 5.16 | 0.0492 | |
| (Tc_t) | 434.72 | 1 | 434.72 | 7.72 | 0.0214 | |
| (Ta_t) | 434.72 | 1 | 434.72 | 7.72 | 0.0214 | |
| Residual | 506.56 | 9 | 56.28 | |||
| Cor Total | 8872.04 | 15 | ||||
| R2 = 0.942; R2adj = 0.904 | ||||||
| R2pred = 0.819; Std. Dev. = 7.50 | ||||||
| Pbremoval | ||||||
| Model | 5869.64 | 4 | 1467.41 | 25.08 | <0.0001 | Significant |
| Tc | 1497.69 | 1 | 1497.69 | 25.60 | 0.0004 | |
| IR | 2185.56 | 1 | 2185.56 | 37.36 | <0.0001 | |
| t | 1730.56 | 1 | 1730.56 | 29.58 | 0.0002 | |
| (IR_t) | 455.82 | 1 | 455.82 | 7.79 | 0.0175 | |
| Residual | 643.48 | 11 | 58.50 | |||
| Cor Total | 6513.11 | 15 | ||||
| R2 = 0.901; R2adj = 0.865 | ||||||
| R2pred = 0.791; Std. Dev. = 7.65 | ||||||
| Activated Carbon | AC1 | AC2 |
|---|---|---|
| Carboxylic groups (mmol g−1) | 10.77 | 15.69 |
| Lactonic groups (mmol g−1) | 1.19 | 1.99 |
| Phenolic groups (mmol g−1) | 1.74 | 5.22 |
| Total basic groups (mmol g−1) | 10.66 | 14 |
| pHPZC | 6.8 | 5.8 |
| SBET (m2 g−1) | 551 | 404 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhahri, R.; Yılmaz, M.; Mechi, L.; Alsukaibi, A.K.D.; Alimi, F.; ben Salem, R.; Moussaoui, Y. Optimization of the Preparation of Activated Carbon from Prickly Pear Seed Cake for the Removal of Lead and Cadmium Ions from Aqueous Solution. Sustainability 2022, 14, 3245. https://doi.org/10.3390/su14063245
Dhahri R, Yılmaz M, Mechi L, Alsukaibi AKD, Alimi F, ben Salem R, Moussaoui Y. Optimization of the Preparation of Activated Carbon from Prickly Pear Seed Cake for the Removal of Lead and Cadmium Ions from Aqueous Solution. Sustainability. 2022; 14(6):3245. https://doi.org/10.3390/su14063245
Chicago/Turabian StyleDhahri, Rimene, Murat Yılmaz, Lassaad Mechi, Abdulmohsen Khalaf Dhahi Alsukaibi, Fathi Alimi, Ridha ben Salem, and Younes Moussaoui. 2022. "Optimization of the Preparation of Activated Carbon from Prickly Pear Seed Cake for the Removal of Lead and Cadmium Ions from Aqueous Solution" Sustainability 14, no. 6: 3245. https://doi.org/10.3390/su14063245
APA StyleDhahri, R., Yılmaz, M., Mechi, L., Alsukaibi, A. K. D., Alimi, F., ben Salem, R., & Moussaoui, Y. (2022). Optimization of the Preparation of Activated Carbon from Prickly Pear Seed Cake for the Removal of Lead and Cadmium Ions from Aqueous Solution. Sustainability, 14(6), 3245. https://doi.org/10.3390/su14063245







