The Stimulation of Superoxide Dismutase Enzyme Activity and Its Relation with the Pyrenophora teres f. teres Infection in Different Barley Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Inoculum
2.2. Plant Material
2.3. Setup of Treatments
2.4. Measurement of SOD Activity
2.5. Data Analysis
3. Results
3.1. Results of the SOD Activity Measurements
3.1.1. Results of the Treatment with Isolate H-618
3.1.2. Results of the Treatment with Isolate H-774
3.1.3. Results of the Treatment with Isolate H-949
3.2. Infection of Barley Cultivars Due to Inoculation with Different PTT Isolates
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afanasenko, O.; Mironenko, N.; Filatova, O.; Kopahnke, D.; Krämer, I.; Ordon, F. Genetics of host-pathogen interactions in the Pyrenophora teres f. teres (net form)—Barley (Hordeum vulgare) pathosystem. Eur. J. Plant Pathol. 2007, 117, 267–280. [Google Scholar] [CrossRef]
- Liu, Z.; Ellwood, S.R.; Oliver, R.P.; Friesen, T.L. Pyrenophora teres: Profile of an increasingly damaging barley pathogen. Mol. Plant Pathol. 2011, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Smedegård-Petersen, V. Pyrenophora teres f. maculata f. nov. and Pyrenophora teres f. teres on Barley in Denmark; Royal Veterinary and Agricultural University; Copenhagen, Denmark, 1971; pp. 124–144. [Google Scholar]
- Tomcsányi, A.; Szeőke, K.; Tóth, Á. TOMCSÁNYI, A., SZEŐKE, K., TÓTH, Á. (2006): Az őszi árpa védelme. Növényvédelem 2006, 42, 87–106. [Google Scholar]
- Mathre, D.E. Compendium of Barley Diseases; American Phytopathological Society: St. Paul, MN, USA, 1982. [Google Scholar]
- Steffenson, B.J. Reduction in Yield Loss Using Incomplete Resistance to Pyrenophora teres f. teres in Barley. Plant Dis. 1991, 75, 96. [Google Scholar] [CrossRef]
- Heitefuss, R. Defence reactions of plants to fungal pathogens: Principles and perspectives, using powdery mildew on cereals as an example. Naturwissenschaften 2001, 88, 273–283. [Google Scholar] [CrossRef]
- Able, A.J. Role of reactive oxygen species in the response of barley to necrotrophic pathogens. Protoplasma 2003, 221, 137–143. [Google Scholar] [CrossRef]
- Lehmann, S.; Serrano, M.; L’Haridon, F.; Tjamos, S.E.; Metraux, J.P. Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry 2015, 112, 54–62. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Groß, F.; Durner, J.; Gaupels, F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Lightfoot, D.J.; Mcgrann, G.R.D.; Able, A.J. The role of a cytosolic superoxide dismutase in barley–pathogen interactions. Mol. Plant Pathol. 2017, 18, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Dangl, J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005, 8, 397–403. [Google Scholar] [CrossRef]
- Kranner, I.; Roach, T.; Beckett, R.P.; Whitaker, C.; Minibayeva, F.V. Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J. Plant Physiol. 2010, 167, 805–811. [Google Scholar] [CrossRef]
- Tyagi, S.; Sharma, S.; Taneja, M.; Shumayla; Kumar, R.; Sembi, J.K.; Upadhyay, S.K. Superoxide dismutases in bread wheat (Triticum aestivum L.): Comprehensive characterization and expression analysis during development and, biotic and abiotic stresses. Agri Gene 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Berens, M.L.; Berry, H.M.; Mine, A.; Argueso, C.T.; Tsuda, K. Evolution of Hormone Signaling Networks in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 401–425. [Google Scholar] [CrossRef]
- Sies, H. On the history of oxidative stress: Concept and some aspects of current development. Curr. Opin. Toxicol. 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, D.; Chen, T.; Li, B.; Zhang, Z.; Qin, G.; Tian, S. Production, signaling, and scavenging mechanisms of reactive oxygen species in fruit–pathogen interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive Oxygen Species (ROS) Generation and Detoxifying in Plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Künstler, A.; Bacsó, R.; Gullner, G.; Hafez, Y.M.; Király, L. Staying alive—Is cell death dispensable for plant disease resistance during the hypersensitive response? Physiol. Mol. Plant Pathol. 2016, 93, 75–84. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Pucciariello, C.; Perata, P. New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant Cell Environ. 2017, 40, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Worth, C.; Rengel, Z. Using capillary electrophoresis to measure Cu/Zn superoxide dismutase concentration in leaves of wheat genotypes differing in tolerance to zinc deficiency. Plant Sci. 1999, 143, 231–239. [Google Scholar] [CrossRef]
- Takagi, D.; Takumi, S.; Hashiguchi, M.; Sejima, T.; Miyake, C. Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol. 2016, 171, 1626–1634. [Google Scholar] [CrossRef]
- Acar, O.; Türkan, I.; Özdemir, F. Superoxide dismutase and peroxidase activities in drought sensitive and resistant barley (Hordeum vulgare L.) varieties. Acta Physiol. Plant. 2001, 23, 351–356. [Google Scholar] [CrossRef]
- Lam, E.; Kato, N.; Lawton, M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature 2001, 411, 848–853. [Google Scholar] [CrossRef]
- Shetty, N.P.; Mehrabi, R.; Lütken, H.; Haldrup, A.; Kema, G.H.J.; Collinge, D.B.; Jørgensen, H.J.L. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol. 2007, 174, 637–647. [Google Scholar] [CrossRef]
- Ivanov, S.; Miteva, L.; Alexieva, V.; Karjin, H.; Karanov, E. Alterations in some oxidative parameters in susceptible and resistant wheat plants infected with Puccinia recondita f.sp. tritici. J. Plant Physiol. 2005, 162, 275–279. [Google Scholar] [CrossRef]
- Harrach, B.D.; Fodor, J.; Pogány, M.; Preuss, J.; Barna, B. Antioxidant, ethylene and membrane leakage responses to powdery mildew infection of near-isogenic barley lines with various types of resistance. Eur. J. Plant Pathol. 2008, 121, 21–33. [Google Scholar] [CrossRef]
- Asthir, B.; Koundal, A.; Bains, N.S.; Mann, S.K. Stimulation of antioxidative enzymes and polyamines during stripe rust disease of wheat. Biol. Plant. 2010, 54, 329–333. [Google Scholar] [CrossRef]
- Chung, W.H. Unraveling new functions of superoxide dismutase using yeast model system: Beyond its conventional role in superoxide radical scavenging. J. Microbiol. 2017, 55, 409–416. [Google Scholar] [CrossRef]
- Bowler, C.; Van Camp, W.; Van Montagu, M.; Inzé, D. Superoxide Dismutase in Plants. CRC. Crit. Rev. Plant Sci. 1994, 13, 199–218. [Google Scholar] [CrossRef]
- Monk, L.S.; Fagerstedt, K.V.; Crawford, R.M.M. Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiol. Plant. 1989, 76, 456–459. [Google Scholar] [CrossRef]
- Deng, B.; Du, W.; Liu, C.; Sun, W.; Tian, S.; Dong, H. Antioxidant response to drought, cold and nutrient stress in two ploidy levels of tobacco plants: Low resource requirement confers polytolerance in polyploids? Plant Growth Regul. 2012, 66, 37–47. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2. [Google Scholar] [CrossRef]
- Singh, R.; Rathore, D. Oxidative stress defence responses of wheat (Triticum aestivum L.) and chilli (Capsicum annum L.) cultivars grown under textile effluent fertilization. Plant Physiol. Biochem. 2018, 123, 342–358. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.L.; Luo, X.; Shah, J.; Schlauch, K.; et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 2013, 25, 3553–3569. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, S.; Feki, K.; Kamoun, Y.; Saïdi, M.N.; Jemli, S.; Ghorbel, M.; Alcon, C.; Brini, F. Highlight on the expression and the function of a novel MnSOD from diploid wheat (T. monococcum) in response to abiotic stress and heavy metal toxicity. Plant Physiol. Biochem. 2019, 142, 384–394. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Künstler, A.; Bacsó, R.; Hafez, Y.M.; Király, L. Reactive Oxygen Species and Plant Disease Resistance. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 269–303. ISBN 978-3-319-20421-5. [Google Scholar]
- El-Zahaby, H.M.; Hafez, Y.M.; Király, Z. Effect of Reactive Oxygen Species on Plant Pathogens in planta and on Disease Symptoms. Acta Phytopathol. Entomol. Hung. 2004, 39, 325–345. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; El-Shawy, E.S.A.A.; Hafez, Y.M.; Abdel-Dayem, S.M.A.; Chidya, R.C.G.; Saneoka, H.; Sabagh, A. El Nano-silver and non-traditional compounds mitigate the adverse effects of net blotch disease of barley in correlation with up-regulation of antioxidant enzymes. Pak. J. Bot. 2020, 52, 1065–1072. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, K.; Cséplő, M.; Kunos, V.; Búza, Z.; Bányai, J.; Seress, D.; Csorba, I.; Pál, M.; Vida, G.; Bakonyi, J. Investigation of net blotch resistance of barley and preliminary data on Hungarian pathotypes of Pyrenophora teres f. teres. In Proceedings of the Resistance Breeding: From Pathogen Epidemiology to Molecular Breeding; Brandsetter, A., Geppner, M., Eds.; Druck und Verlag: Sankt Pölten, Austria, 2019; pp. 27–29. [Google Scholar]
- Pál, M.; Kovács, V.; Vida, G.; Szalai, G.; Janda, T. Changes induced by powdery mildew in the salicylic acid and polyamine contents and the antioxidant enzyme activities of wheat lines. Eur. J. Plant Pathol. 2013, 135, 35–47. [Google Scholar] [CrossRef]
- Tekauz, A. A numerical scale to classify reactions of barley to Pyrenophora teres. Can. J. Plant Pathol. 1985, 7, 181–183. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Gawehn, K.; Grassl, M. Methods of Enzymatic Analysis. Verlag Chemie 1974, 1, 481–482. [Google Scholar]
- Zhang, C.; Bruins, M.E.; Yang, Z.Q.; Liu, S.T.; Rao, P.F. A new formula to calculate activity of superoxide dismutase in indirect assays. Anal. Biochem. 2016, 503, 65–67. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Bolwell, G.P. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef]
- Liu, X.; Williams, C.E.; Nemacheck, J.A.; Wang, H.; Subramanyam, S.; Zheng, C.; Chen, M.S. Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol. 2010, 152, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Jasso-Robles, F.I.; Gonzalez, M.E.; Pieckenstain, F.L.; Ramírez-García, J.M.; de la Luz Guerrero-González, M.; Jiménez-Bremont, J.F.; Rodríguez-Kessler, M. Decrease of Arabidopsis PAO activity entails increased RBOH activity, ROS content and altered responses to Pseudomonas. Plant Sci. 2020, 292, 110–372. [Google Scholar] [CrossRef]
- Pandey, C.; Großkinsky, D.K.; Westergaard, J.C.; Jørgensen, H.J.L.; Svensgaard, J.; Christensen, S.; Schulz, A.; Roitsch, T. Identification of a bio-signature for barley resistance against Pyrenophora teres infection based on physiological, molecular and sensor-based phenotyping. Plant Sci. 2021, 313, 111072. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, H.; Gajewska, E.; Karwowska, R.; Wielanek, M. Generation of superoxide anion and induction of superoxide dismutase and peroxidase in bean leaves infected with pathogenic fungi. Acta Biochim. Pol. 1996, 43, 679–685. [Google Scholar] [CrossRef]
- Gil-ad, N.L.; Bar-Nun, N.; Noy, T.; Mayer, A.M. Enzymes of Botrytis cinerea capable of breaking down hydrogen peroxide. FEMS Microbiol. Lett. 2000, 190, 121–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vanacker, H.; Harbinson, J.; Ruisch, J.; Carver, T.L.W.; Foyer, C.H. Antioxidant defences of the apoplast. Protoplasma 1998, 205, 129–140. [Google Scholar] [CrossRef]
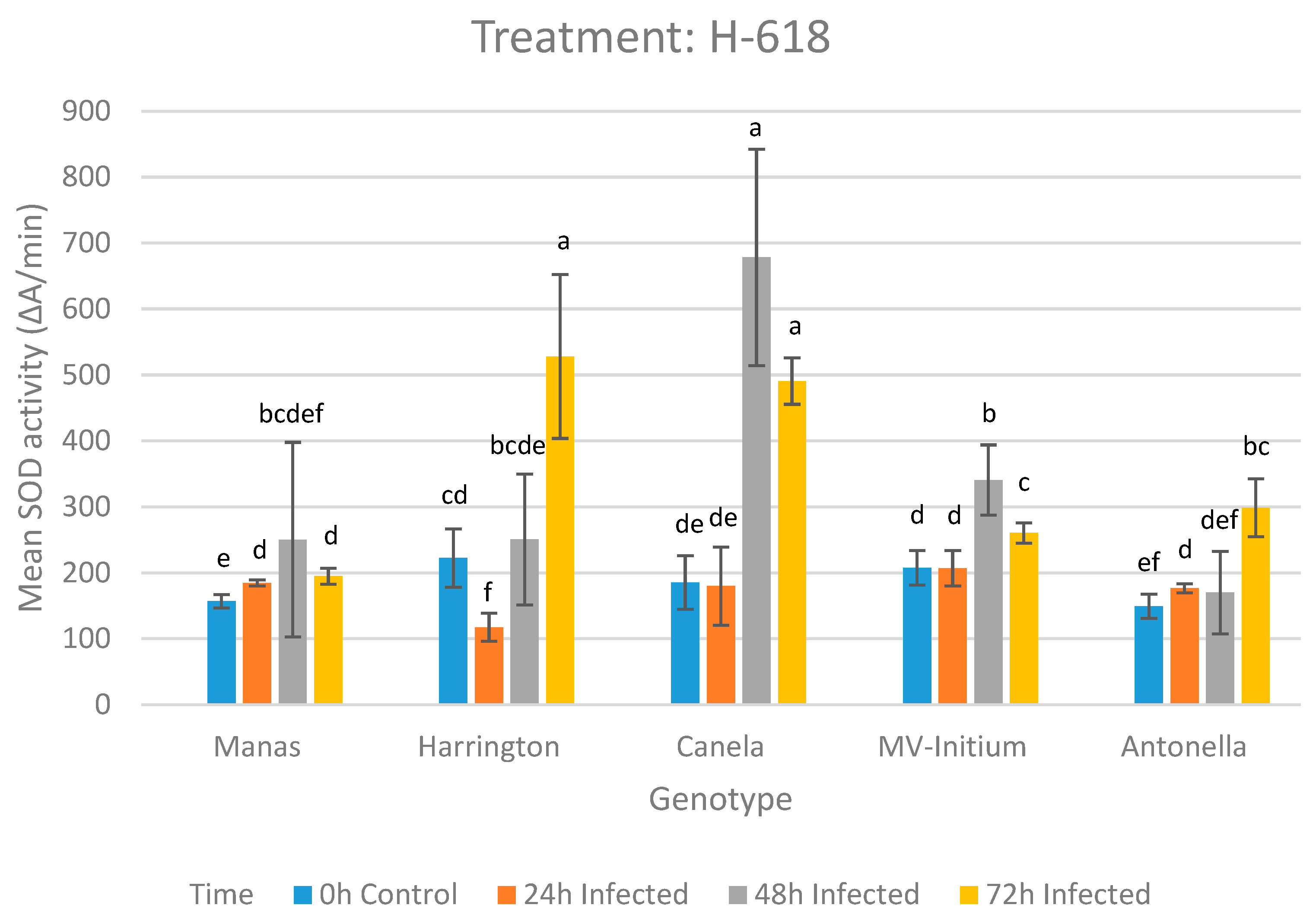
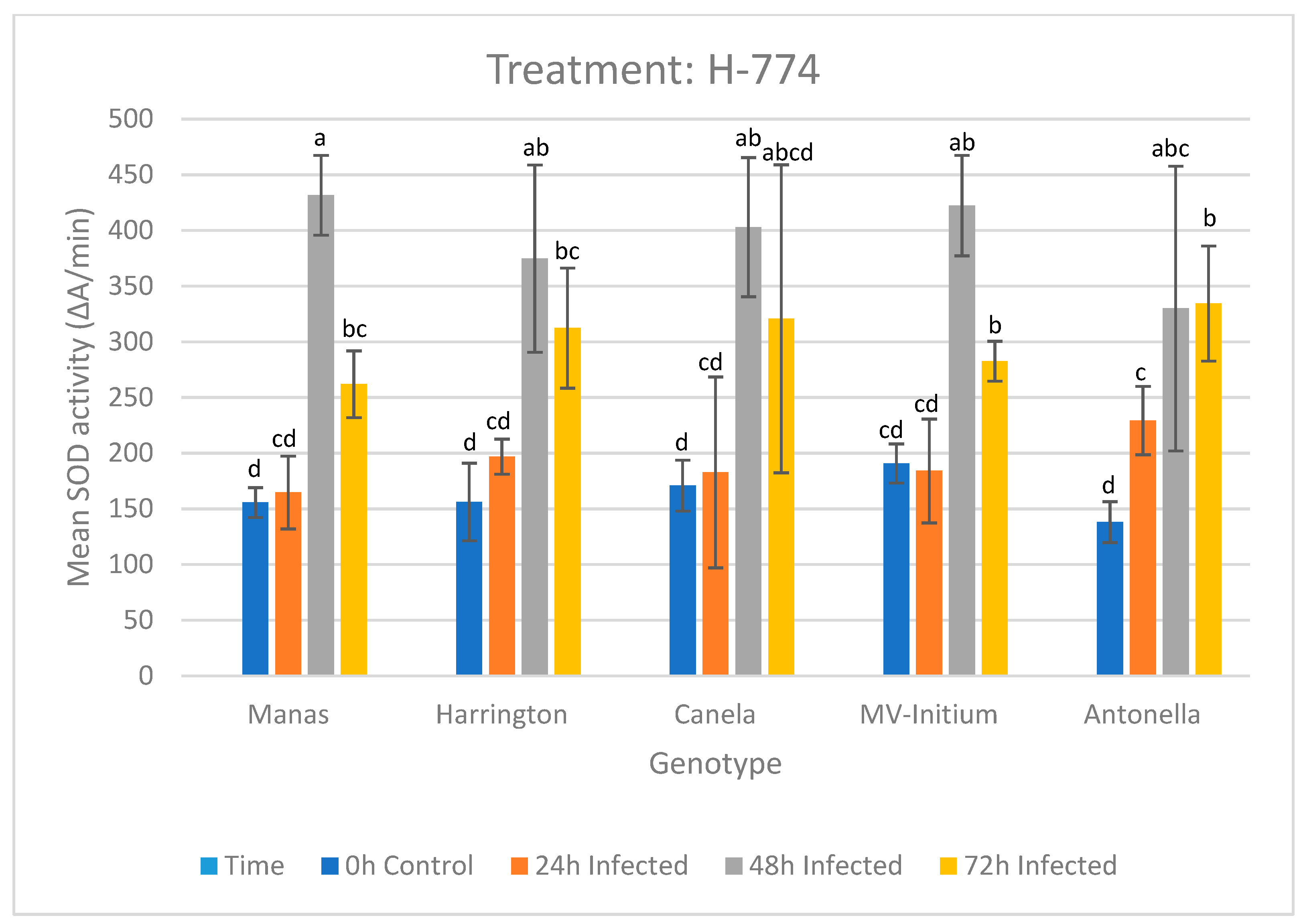

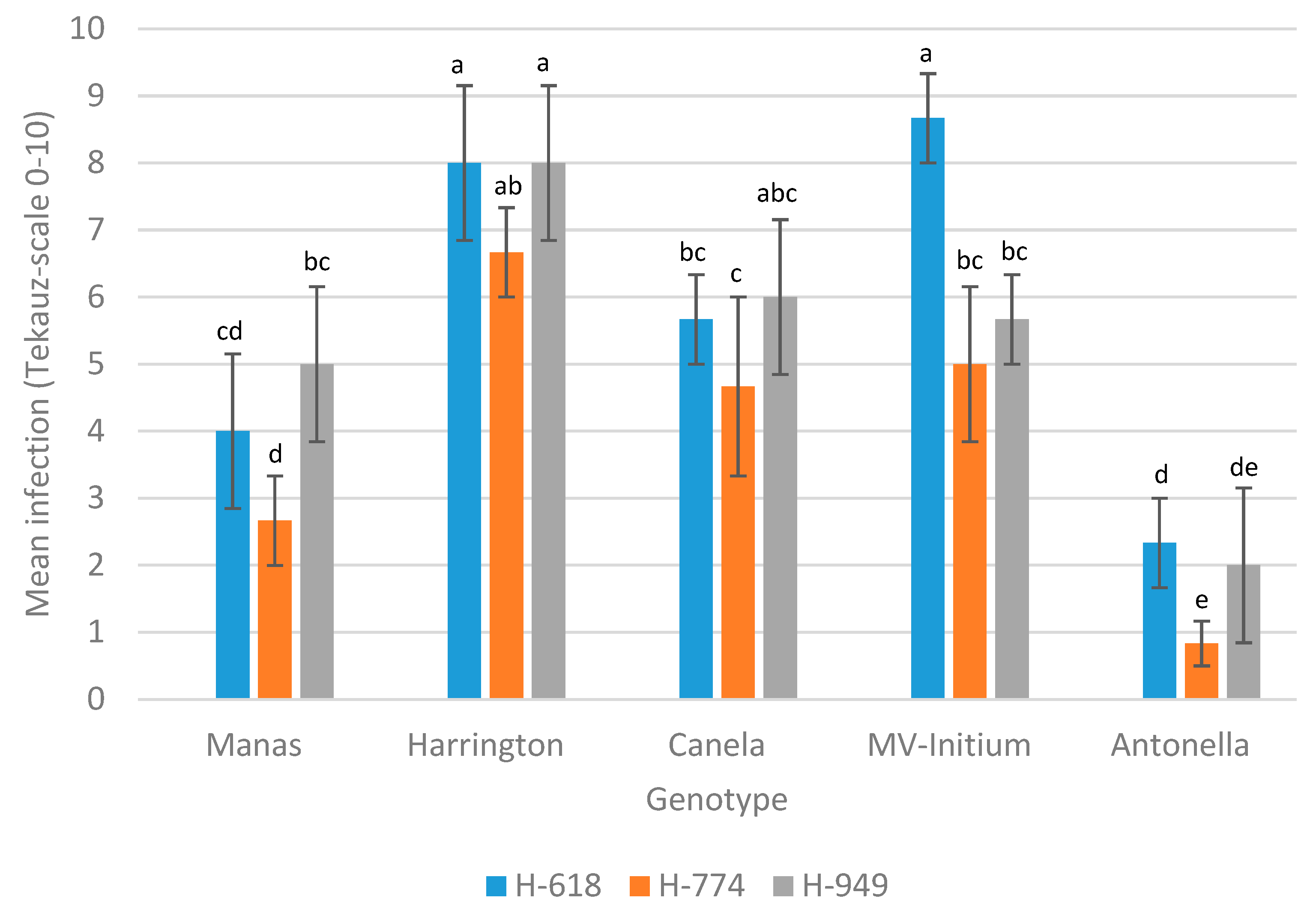
| 0 h | Manas | Harrington | Canela | MV-Initium | Antonella | |
| Manas | - | −65.707 * | −28.511 | −50.817 * | 7.638 | |
| Harrington | - | - | 37.196 | 14.889 | 73.345 * | |
| Canela | - | - | - | −22.306 | 36.149 | |
| MV-Initium | - | - | - | - | 58.455 * | |
| Antonella | - | - | - | - | - | |
| 24 h | Manas | Harrington | Canela | MV-Initium | Antonella | |
| Manas | - | 67.516 * | 4.814 | −21.997 | 8.355 | |
| Harrington | - | - | −62.702 * | −89.514 * | −59.160 * | |
| Canela | - | - | - | −26.812 | 3.541 | |
| MV-Initium | - | - | - | - | −30.353 | |
| Antonella | - | - | - | - | - | |
| 48 h | Manas | Harrington | Canela | MV-Initium | Antonella | |
| Manas | - | −0.468 | −427.996 * | −90.349 | 80.028 | |
| Harrington | - | - | −427.527 * | −89.880 | 80.497 | |
| Canela | - | - | - | 337.646 * | 508.025 * | |
| MV-Initium | - | - | - | - | 170.378 | |
| Antonella | - | - | - | - | - | |
| 72 h | Manas | Harrington | Canela | MV-Initium | Antonella | |
| Manas | - | −333.04 * | −295.857 * | −65.614 | −103.541 * | |
| Harrington | - | - | 37.183 | 267.425 * | 229.498 * | |
| Canela | - | - | - | 230.242 * | 192.315 * | |
| MV-Initium | - | - | - | - | −37.927 | |
| Antonella | - | - | - | - | - |
| 0 h | Manas | Harrington | Canela | MV-Initium | Antonella | |
| Manas | - | 66.309 | 11.583 | −13.354 | 80.508 | |
| Harrington | - | - | −54.726 | −79.663 | 14.199 | |
| Canela | - | - | - | 24.937 | 93.863 | |
| MV-Initium | - | - | - | - | −93.863 | |
| Antonella | - | - | - | - | - | |
| 7th day | Manas | Harrington | Canela | MV-Initium | Antonella | |
| Manas | - | 256.77 * | 712.713 * | 753.514 * | 371.11 * | |
| Harrington | - | - | 455.943 * | 496.744 * | 114.34 | |
| Canela | - | - | - | 40.8 | −341.602 * | |
| MV-Initium | - | - | - | - | −382.403 * | |
| Antonella | - | - | - | - | - | |
| 15th day | Manas | Harrington | Canela | MV-Initium | Antonella | |
| Manas | - | 10.84 | 464.567 * | 85.650 | 97.25 | |
| Harrington | - | - | 453.722 * | 74.805 | 86.405 | |
| Canela | - | - | - | −378.916 * | −367.317 * | |
| MV-Initium | - | - | - | - | 11.599 | |
| Antonella | - | - | - | - | - |
| Manas | Harrington | Canela | MV-Initium | Antonella | |
|---|---|---|---|---|---|
| Manas | - | −4.00 * | −1.66 * | −4.66 * | 1.66 * |
| Harrington | - | 2.33 * | −0.66 | 5.66 * | |
| Canela | - | −3.00 * | 3.33 * | ||
| MV-Initium | - | 6.33 * | |||
| Antonella | - |
| Manas | Harrington | Canela | MV-Initium | Antonella | |
|---|---|---|---|---|---|
| Manas | - | −4.00 * | −2.00* | −2.33 * | 1.83 * |
| Harrington | - | 2.00 * | 1.66 * | 5.83 * | |
| Canela | - | −0.33 | 3.83 * | ||
| MV-Initium | - | 4.16 * | |||
| Antonella | - |
| Manas | Harrington | Canela | MV-Initium | Antonella | |
|---|---|---|---|---|---|
| Manas | - | −3.00 * | −1.00 | −0.66 | 3.00 * |
| Harrington | - | 2.00 * | 2.33 * | 6.00 * | |
| Canela | - | 0.33 | 4.00 * | ||
| MV-Initium | - | 3.66 * | |||
| Antonella | - |
| Genotype | Isolate | H-618 Tekauz | H-774 Tekauz | H-949 Tekauz |
|---|---|---|---|---|
| Manas | H-618 SOD | 0.143 | - | - |
| H-774 SOD | - | 0.778 * | - | |
| H-949 SOD | - | - | −0.914 * | |
| Harrington | H-618 SOD | 0.662 | - | - |
| H-774 SOD | - | 0.938 * | - | |
| H-949 SOD | - | - | 0.997 * | |
| Canela | H-618 SOD | −0.787 * | - | - |
| H-774 SOD | - | 0.144 | - | |
| H-949 SOD | - | - | −0.956 * | |
| MV-Initium | H-618 SOD | −0.917 * | - | - |
| H-774 SOD | - | −0.584 | - | |
| H-949 SOD | - | - | −0.853 * | |
| Antonella | H-618 SOD | 0.999 * | - | - |
| H-774 SOD | - | 0.999 * | - | |
| H-949 SOD | - | - | 0.588 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunos, V.; Cséplő, M.; Seress, D.; Eser, A.; Kende, Z.; Uhrin, A.; Bányai, J.; Bakonyi, J.; Pál, M.; Mészáros, K. The Stimulation of Superoxide Dismutase Enzyme Activity and Its Relation with the Pyrenophora teres f. teres Infection in Different Barley Genotypes. Sustainability 2022, 14, 2597. https://doi.org/10.3390/su14052597
Kunos V, Cséplő M, Seress D, Eser A, Kende Z, Uhrin A, Bányai J, Bakonyi J, Pál M, Mészáros K. The Stimulation of Superoxide Dismutase Enzyme Activity and Its Relation with the Pyrenophora teres f. teres Infection in Different Barley Genotypes. Sustainability. 2022; 14(5):2597. https://doi.org/10.3390/su14052597
Chicago/Turabian StyleKunos, Viola, Mónika Cséplő, Diána Seress, Adnan Eser, Zoltán Kende, Andrea Uhrin, Judit Bányai, József Bakonyi, Magda Pál, and Klára Mészáros. 2022. "The Stimulation of Superoxide Dismutase Enzyme Activity and Its Relation with the Pyrenophora teres f. teres Infection in Different Barley Genotypes" Sustainability 14, no. 5: 2597. https://doi.org/10.3390/su14052597
APA StyleKunos, V., Cséplő, M., Seress, D., Eser, A., Kende, Z., Uhrin, A., Bányai, J., Bakonyi, J., Pál, M., & Mészáros, K. (2022). The Stimulation of Superoxide Dismutase Enzyme Activity and Its Relation with the Pyrenophora teres f. teres Infection in Different Barley Genotypes. Sustainability, 14(5), 2597. https://doi.org/10.3390/su14052597









