Abstract
This paper follows the transition from ethnobotany to a deeper scientific understanding of the food and medicinal properties of African agroforestry tree products as inputs into the start of domestication activities. It progresses on to the integration of these indigenous trees as new crops within diversified farming systems for multiple social, economic and environmental benefits. From its advent in the 1990s, the domestication of indigenous food and non-food tree species has become a global programme with a strong African focus. This review of progress in the third decade is restricted to progress in Africa, where multi-disciplinary research on over 59 species has been reported in 759 research papers in 318 science publications by scientists from over 833 research teams in 70 countries around the world (532 in Africa). The review spans 23 research topics presenting the recent research literature for tree species of high priority across the continent, as well as that in each of the four main ecological regions: the humid zone of West and Central Africa; the Sahel and North Africa; the East African highlands and drylands; and the woody savannas of Southern Africa. The main areas of growth have been the nutritional/medicinal value of non-timber forest products; the evaluation of the state of natural resources and their importance to local people; and the characterization of useful traits. However, the testing of putative cultivars; the implementation of participatory principles; the protection of traditional knowledge and intellectual property rights; and the selection of elite trees and ideotypes remain under-researched. To the probable detriment of the upscaling and impact in tropical agriculture, there has been, at the international level, a move away from decentralized, community-based tree domestication towards a laboratory-based, centralized approach. However, the rapid uptake of research by university departments and national agricultural research centres in Africa indicates a recognition of the importance of the indigenous crops for both the livelihoods of rural communities and the revitalization and enhanced outputs from agriculture in Africa, especially in West Africa. Thus, on a continental scale, there has been an uptake of research with policy relevance for the integration of indigenous trees in agroecosystems and their importance for the attainment of the UN Sustainable Development Goals. To progress this in the fourth decade, there will need to be a dedicated Centre in Africa to test and develop cultivars of indigenous crops. Finally, this review underpins a holistic approach to mitigating climate change, as well as other big global issues such as hunger, poverty and loss of wildlife habitat by reaping the benefits, or ‘profits’, from investment in the five forms of Capital, described as ‘land maxing’. However, policy and decision makers are not yet recognizing the potential for holistic and transformational adoption of these new indigenous food crop opportunities for African agriculture. Is ‘political will’ the missing sixth capital for sustainable development?
1. Introduction
The idea of domesticating traditionally important indigenous trees as new crops in the tropics for their useful and marketable food and non-food products originated in the 1980s [1]. This idea, which includes both cultivation and genetic improvement, was taken up by the World Agroforestry Centre (ICRAF) in 1993, following a conference in Edinburgh in 1992 aimed at ‘Rebuilding Tropical Forest Resources’ and the advent of Woody Plant or Really Green Revolution [2]. Since then, it has become a pan-tropical programme expanding across the first two decades in both the number of candidate species for domestication and the range of research topics involved in its multidisciplinary approach [3,4,5,6,7,8,9,10] targeting 14 of the 17 UN Sustainable Development Goals [11,12]. Non-timber forest products are currently believed to be used by about 44% of the global population [13].
Tens of thousands of tree species around the tropics and sub-tropics produce both edible and medical products which have been important in the day-to-day lives of local people. With the expansion of agriculture to feed a growing human population, many of these species were cleared from the land to make way for a small number of staple food crops, often with little regard either for their traditional and cultural significance or their diverse and highly nutritious fruits, nuts and leaves. Over the first two decades of their domestication (1992–2002; 2002–2012), research sought to develop techniques, skills and strategies capturing the many potential social and economic benefits that the cultivation of these products could deliver through community agroforestry projects and expanded marketing and trade. In addition, research and development projects have also been initiated to process and add value to the products both locally and internationally. Trees, especially indigenous trees, play an important role in the capture of environmental, social and economic benefits/profits from agriculture, described as ‘Land Maxing’ [14], by investing in the five forms of capital (natural, human, social, physical and financial) and so converting the so-called ‘inevitable trade-offs’ to sustainable policy ‘trade-ons’ [15].
The highlights of the first two decades [16] were as follows:
- Greater understanding of ethnobotany, traditional knowledge of indigenous species and their deliberate cultivation by farmers;
- The application of vegetative propagation techniques in community-based village nurseries using marcotting/grafting to capture mature traits of individual trees and stem cuttings for the multiplication of elite trees as putative cultivars;
- The development of appropriate nursery techniques for the sexual and asexual propagation of candidate species in remote villages;
- The development of participatory priority setting for the selection of candidate species for domestication and integration into agroforestry systems. Some 50 local species were identified for further research and development around the tropics and sub-tropics;
- The establishment of Rural Resource Centres to provide: (i) knowledge and skills for the participatory domestication of local species using appropriate, low-technology techniques adapted for use in remote locations with minimal infrastructure in ways that allow local community members to benefit from their own initiatives and (ii) the capacity to develop community engagement and infrastructure to maximize environmental, social and economic benefits;
- The quantification at the village level of 3- to 10-fold continuous tree-to-tree intraspecific variation in community farmland to understand the range of genetic variation available to local communities and its accordance with local knowledge. Subsequently, this knowledge was formulated to identify market-specific ideotypes. Primarily, it was morphological variation that was investigated, but this was later expanded into nutritional and other biochemical traits;
- The start of the evaluation of genetic variation using molecular technologies;
- The examination of communal, socio-economic, legal and political issues affecting both the adoption and impact of domesticating indigenous food and non-food tree species and the marketing of their Agroforestry Tree Products (AFTPs).
At the end of the second decade [16], it was envisioned that the developments in the third decade would include the following:
- Improved capture of ontogenetically mature phenotypes by better understanding of the physiology of grafting and marcotting;
- Analysis of variations in nutraceutical, pharmaceutical and other ingredients of AFTPs to meet the needs of new market opportunities and the identification of market-oriented ideotypes;
- Greater investment in postharvest processing to expand local, regional and global trade opportunities;
- Investigation of reproductive biology for increased use of controlled breeding in centralized tree domestication research;
- Upscaled tree domestication, especially in Africa, focusing on species with impact on income generation and nutrition;
- Impact analysis based on well-defined criteria and indicators;
- A better understanding of the role of domesticated agroforestry trees in the achievement of sustainable multifunctional farming systems and wider local and regional marketing;
- Expanded opportunities for successful marketing of AFTPs, including public–private partnerships in commercial markets;
- Recognition of intellectual property rights to protect poor farmers and local communities from unscrupulous entrepreneurs;
- Enhance policy support for the upscaling of intensified agroforestry systems.
This review examines progress made in Africa over the third decade (2012–2021), as reported in the scientific literature. Thus, it presents an update on reports of the work done in the first two decades, rather than a state-of-the-art review across the three decades.
Progress in the Third Decade
We evaluated the published literature, both by region/agroecological zone and by research topic/discipline and from a pan-African policy perspective, using Internet databases such as Google Scholar and ResearchGate, as well as our personal knowledge and networks. A similar approach in the first and second decades [16] examined 16 research topics and over 50 species from around the world. In addition to a set of more general pan-African research papers, the present review is focussed on four African regions (Humid West and Central Africa, Sahel and North Africa, the Highlands and Drylands of East Africa and the Savanna and Miombo Woodlands of Southern Africa). These papers were then partitioned into a set of 23 research topics (Table 1).

Table 1.
The list of 23 research topics used to characterize the scientific literature relating to the domestication of indigenous African food and non-food tree species.
2. Overview of the Literature
2.1. Regional
We reviewed a total of 759 articles published between 2012 and 2021 from 4 Regions of Africa, recognizing some overlap between socio-political boundaries and broad ecological zones. Out of these publications, 39% originated from Humid West and Central Africa (HWCA), 20% from Sahelian West and North Africa (SWNA), 9% from Highland and Dry East Africa (HDEA) and 15% from the Savannah and Miombo Woodlands of Southern Africa (SMWSA). A total of 17% were of pan-African (PA) importance. In total, this is more than double (125%) the total number of African papers published over the first two decades combined (Figure 1).
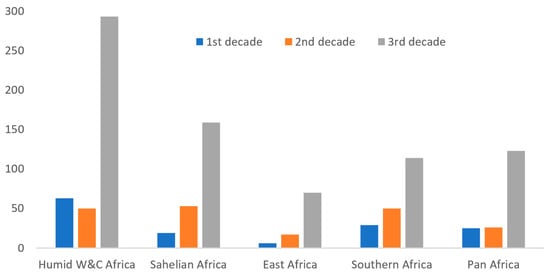
Figure 1.
The total number of research publications relating to the domestication of indigenous trees in Africa, by decade and region.
2.2. Research Topics
When the 759 publications were divided by research topic, those from most topics had greatly increased in number, with some new topics also being very well represented, especially: (i) the nutritional/medicinal value of NTFPs (see: [17]); (ii) the evaluation of the state of natural resources and their genetic diversity, governance and management; (iii) the ethnobotany of candidate species; and (iv) the characterization of useful traits (Figure 2). However, topics that were not represented more frequently were: (i) the testing of putative cultivars, (ii) the implementation of participatory principles, (iii) protection of traditional knowledge and intellectual property rights and (iv) the selection of elite trees and ideotypes (Figure 2). At the pan-African level, three topics were well represented. These were (i) the domestication concept and strategy, (ii) the potential impact of cultivation and (iii) the policy relevance and implications for Sustainable Development Goals (Figure 2).
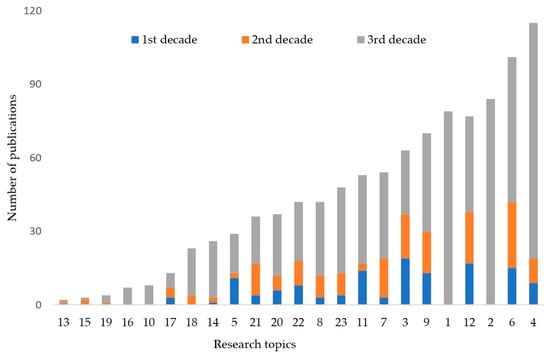
Figure 2.
The total number of research publications relating to the domestication of indigenous trees in Africa by research topic (see Table 1) and decade.
Impressively, in addition to this growth in the number of publications relating to tree domestication, there was a huge increase in both the number of research teams in universities and research institutes conducting this work (833 in total from 70 countries [532 from 34 countries in Africa; 182 as research partners from 17 countries in Europe; 37 from 11 countries in Asia; 66 from five countries in the Americas; and 16 from 2 countries in Oceania]; see Supplementary Materials Table S1). Interestingly, this growth was also associated with multidisciplinary partnerships and a greatly increased range and number of science journals publishing these papers (290 journals and 28 books—see Supplementary Materials Table S2).
2.3. Species
In the literature for tree domestication during the third decade, 59 African species were identified (Table 2), generally with different species in each Region, although a few dry zone species were reported from other regions, notably baobab (Adansonia digitata) being important in three regions. A wider range of ethnobotanically important species were also discussed, indicating the potential for further growth in the future.

Table 2.
African tree and other woody species producing edible products that have been identified as prime candidates for domestication in the literature over the third decade (species in bold have pan-African priority in the region).
3. Advances in Domestication Technologies and Their Application
The first and second decades of agroforestry tree domestication were strongly based on the decentralized concept of participatory domestication [18] and a bottom-up philosophy for very rapid progress arising from the vegetative propagation of elite trees selected at the village level. The foundation of this community-based approach was the development of Rural Resource Centres by ICRAF [18] to assist participating communities acquire the skills, knowledge and understanding of long-known, simple horticultural techniques such as grafting, air layering (marcotting) and the rooting of cuttings, which can be implemented by local people in remote villages without access to financial capital, and utility services such as electricity and piped water. It is, however, important to recognize here that there is an inadequate resource of people nationally, regionally and internationally with the appropriate skills in vegetative propagation to meet the scale of the need for these techniques if participatory domestication is to be scaled up across Africa.
Participatory domestication provides the basis for the development of a self-help strategy for community engagement [19,20], leading to the development of ‘socially-modified crops’ [14,15]. It is focussed on the intensification of agroforestry for multiple environmental, social and economic benefits as part of the three-step approach to rapidly reducing hunger by closing the Yield Gaps in staple food crops while also addressing malnutrition, poverty and social injustice [15,21,22]. This has important policy implications for African people and for the planet (see Section 10 below).
In the third decade, there has been reduced research activity in Participatory Domestication led by the World Agroforestry Centre (ICRAF) due primarily to a loss of donor funds. This is less a loss of support for the concept and more a need to make funds available to other causes. Meanwhile a new source of funding became available for a more centralized top-down biotechnological programme focussed on a laboratory approach to crop improvement by genetic characterization and tree breeding [23]. It is based on the acquisition of knowledge of the mechanisms of genetic variation in modern science laboratories, again led by the ICRAF [24]. Interestingly, in parallel with this change in direction by the ICRAF, there has been a substantial increase in domestication-related research in African universities and research institutes (Supplementary Materials Tables S1 and S2), most of it in support of local livelihoods and land uses. It must be hoped that decentralized tree domestication has not succumbed to the prevailing view that academic advancement is more important than real-life impact [25]. Indeed. It is important to recognize that a participatory approach to domestication with benefits flowing to local communities is essential when the traits being selected are rooted in Traditional Knowledge [18].
While the centralised approach is relatively easy to coordinate, its results do not always filter down to small-scale farmers, who encounter high transaction costs when receiving external farm inputs, such as tree planting material and information on the management of cultivars [23]. Thus, the centralised approach does not promote local development based on community decision making about which species to prioritise for cultivation; the development of efficient farm management methods in a smallholder context; or the resolution of social, economic and political barriers faced by smallholder farmers [23]. Thus, perhaps to the detriment of short-term upscaling and impact, tree domestication studies in the third decade have moved towards a much longer-term strategy than the rapid and highly successful decentralized community approach. This shift in strategy will require a means to disseminate improved germplasm to farmers lacking the financial resources to purchase the planting material [26]. In addition to some indigenous fruit and nut tree species, this centralized domestication programme additionally includes over 50 annual orphan crops (cereals, legumes, etc.) as important understorey additions to agroforestry systems. This is aimed at furthering diversification of farming systems and diets [27]; however, their successful integration into complex agroecosystems may require selection for higher yields in shady environments.
It is important to emphasise here that these two different strategies are not mutually exclusive. Historically, tree crops have been domesticated from the wild over millennia using decentralised horticultural approaches based on techniques of vegetative propagation to create cultivars that capture the unique characteristics of naturally occurring elite individuals. Over recent decades, centralised tree breeding programmes between these cultivars and wild relatives has led to new generations of selected varieties.
It is clear from the above summaries of these two strategies that they lie at opposite ends of a domestication strategy spectrum (Figure 3) and that they have different merits relevant to the domestication of indigenous trees and to needs of tropical farmers in the short- and long-term. A number of review papers have been written about the merits of both approaches and their use in recent years (Table 3), including the concept of a systems approach [28]. So, we will not replicate the information here. However, to assist development agencies and donors to understand their differences and likely impacts, a detailed cost–benefit analysis would be helpful.
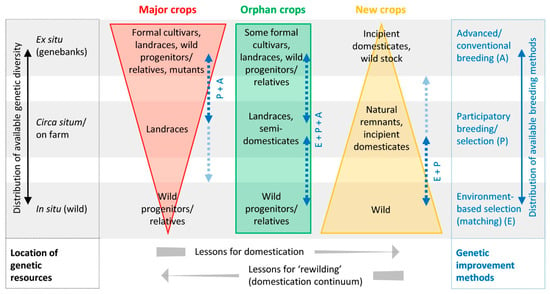
Figure 3.
The spectrum of domestication strategies forming the Domestication Syndrome: Centralized for major food crops and decentralized for new tree crops (Reprinted with a permision from ref. [26]. 2019 John Wiley and Sonswith under License Number 5250250091483).

Table 3.
Sources of information about different domestication strategies and their use.
Finally, the recently republished tree domestication strategy for indigenous fruit trees [35] presents overarching principles for the establishment of three interlinked tree populations: gene resource population, selection resource population and production resource population. The strategy includes ways in which science-based principles for low-technology, appropriate methods of vegetative propagation can be used in remote area without access to electricity and piped water and yet maintain the genetic diversity of the preferred traits of the elite mother tree [36,37]. This is the basis of the concept of decentralized domestication.
4. Pan-African Priority Species
In this section, we review the progress made to domesticate those species which for ecological reasons are found naturally across two or more socio-political regions in the continent of Africa. It is important to recognize here that these species are much more than ‘Famine Foods’ [38,39] and are highly appreciated by local people for many different reasons.
4.1. Adansonia digitata (Baobab)
4.1.1. Ethnobotany, Uses and Natural Resources
Adansonia digitata is a large, deciduous, drought-tolerant tree reaching 18–25 m in height and with a trunk up to 10 m in diameter [40,41]. It is the only species of its genus in Africa and is distributed across the drylands stretching from Senegal to Sudan and from Ethiopia to Natal. It provides a wide variety of non-timber forest products (NTFPs), although these are least used in southern Africa [40,41,42].
In addition to its important edible fruits, baobab has been declared to be one of Africa’s most important leafy vegetables [43], as seen regarding the livelihoods of local communities in Mali [44]. Consequently, it has been identified as a high-priority species for domestication across Africa. Baobab products—leaf, fruit pulp and seed kernels—are widely marketed and have been approved for trade as ‘novel food’ in the European Commission. Baobab is adaptable to adverse climatic conditions, suggesting its potential to contribute to climate-resilient strategies in Africa. Every part of the tree is used: roots, bark, wood, leaves, flowers, capsules, gum, seeds and fruits for food, fibre and medicine. Its edible fruits and leaves are rich in vitamins, making them important for nutrition. The bark fibres are also important products for multiple everyday uses. Across the continent, over 300 uses of the tree have been recorded. Its medicinal products treat up to more than 20 diseases. These are used for subsistence and/or sold by local communities to generate income, making a significant contribution towards poverty alleviation in rural areas.
In Sudan, an ethnobotanical survey found 25 different uses for baobab products [45,46] with preferences for sweet leaves and large fruits with a high yield of white fruit pulp, as opposed to acidic and/or slimy pulp and bitter leaves. Rashford [43] has reviewed leaf palatability, nutritional content and seasonal consumption in Sub-Sahara Africa, with wide variation in consumers preferences [47]. In Burkina Faso, both gender-related and village-related differences in use and management have been recognized, indicating the need to seek locally appropriate management systems [45]. The identified traits were also considered to be important indicators of individual candidate trees with potential for domestication. In Kenya, baobab is recognised as being important for nutritional diversity in the staple diet. Fruit pulp is also consumed as snacks, primarily fresh without any processing [48]. However, about 60% of respondents stored fruits for later consumption when nutritious food was scarce. Households also sell baobab fruits to augment their income and thus to purchase other foods (45%) and to pay for healthcare (13%) and education (23%). In Sudan, the sale of baobab products could be both negatively and positively affected by different internal and external factors, but those that lift households out of poverty are beneficial to rural development [49,50]. This requires appropriate institutional, technical and financial support.
In Kenya, when a multi-stakeholder approach was taken to the initiation of a community-based development enterprise for both baobab kernel oil and powdered fruit pulp, the target impacts were to benefit community livelihoods and their capacity to manage and utilize the resource, as well as to enhance consumption and food security [39].
To promote the conservation and domestication of baobab across Africa, Gebauer et al. [51] comprehensively reviewed its ecology, diversity and current utilization and presented ideas to encourage further studies and investment in the species. These include studies of its phenotypic and genetic variation, population status/uses, socioeconomics of management/processing and trade, nutritional value, horticultural production, ecophysiology and its root system. In addition, domestication-related studies have considered the potential to cultivate young baobab trees in a leaf production system [43].
4.1.2. Genetic Variation and Domestication
Phenotypic Characterization and Provenance Variation
A number of studies this decade have sought to gain a deeper understanding of the variation in baobab. The first made a proximate analysis of 178 fruit samples from 11 Sahelian West Africa sites in Burkina Faso, Mali and Niger [52] and evaluated the variability in fruit morphology, as well as sugar and vitamin C content.
Like most such studies, there was a high level of intraspecific variation between individual trees, but there was also a linear relationship between rainfall and vitamin C content across these sites, with vitamin content being higher in sites with lower precipitation. A negative relationship was found between fruit size and both latitude and longitude, while there was a positive correlation between sugar content and latitude and longitude. The authors, however, concluded that the levels of vitamin C and sugar within site variation merited a more detailed study to identify superior trees. A somewhat similar study of 10 provenances in Mali [53] confirmed some of these correlations. The study also found that pulp iron content was positively correlated with topsoil sodicity and base saturation, while pulp vitamin C content correlated positively with topsoil sand fraction, water and base saturation. Furthermore, significant negative correlations were found between rainfall and pulp vitamin C content and between mean annual temperature and fruit and pulp weight and pulp fraction, suggesting that these traits are influenced by the environment [53].
In a pan-African study of 17 baobab provenances from Kenya, Tanzania, Zambia, Zimbabwe, Malawi and Mali, significant variation was found in the pulp moisture, protein, fibre, ash and elemental content among provenances [54]. However, there were differences at the national level in most traits, indicating opportunities for selection, both for germplasm conservation and for domestication. Two follow-up studies in Kenya found tree-to-tree variation in a wide range of nutritional parameters. In the first, trees from coastal populations with larger fruits produced higher fruit yields than those inland [55]. Some elite trees were selected with high fruit weight, high pulp proportion and intermediate or sweet-tasting fruit pulp in the two regions. The second study in six populations confirmed the importance of intraspecific variation between individual trees at the population level [56], especially in iron, zinc and vitamin C. Despite the correlations reported above between fruit traits and the local environment, these authors suggest that genetics play a stronger role in nutritional variation than the environment. Thus, these results further illustrate the importance of tree-to-tree variation in the domestication and cultivation of this species.
In Ghana, it was concluded from studies on 14 morphological traits that the diversity of genetic variation makes its domestication highly appropriate [57]. To further determine the potential to domesticate baobab for its nutritional qualities, a study has examined the fatty acid composition of seed oil in provenances from across the African continent [58]. This identified significantly different mean provenance variation, with the following overall means: saturated fatty acids (17–22%), monounsaturated fatty acids (32–38%) and polyunsaturated fatty acids (22–26%). Palmitic acid, oleic acid and linoleic acid were the most abundant of each of these fatty acids, respectively. In parallel with this, the nutritional content of leaves showed high variability across genotypes from 36 populations, which could be placed in 4 variance clusters [59]. These authors recommended tree breeding between divergent populations to develop genotypes with higher nutritional values, as well as greater tolerance of biotic and abiotic stress.
Together, the above studies illustrate the potential to select elite individual baobab trees for domestication and expanded cultivation in dryland Africa [51] and that further research on tree genetics is needed to complement ethnobotanical knowledge [46] to ensure that the cultivated trees meet the needs of the people.
To aid in this research, germplasm conservation and domestication programmes, baobab ‘descriptors’, have been published [60]. These include ‘passport’ descriptors defining the parameters that should be observed when the accession is collected; ‘management’ descriptors for accessions in the genebank; ‘environment and site’ descriptors, which are site-specific parameters for characterization and evaluation trials; and ‘site’ descriptors for germplasm collecting. Studies have found that descriptors to discriminate between phenotypes were equally expressed across all environments.
Genetic Variation
Unlike other species in the genus Adansonia, baobab (A. digitata) is tetraploid. However, a study of the variation in floral and pollen characters and chromosome number in specimens from Africa has identified a new diploid species, which has been found to co-exist with baobab below 800 m altitudes in Africa but is reported to extend up to 1500 m [61]. It has been called Adansonia kilima sp. nov. and is superficially similar to A. digitata, but the species can be differentiated on the basis of floral morphology, pollen and chromosome number. However, by using a combination of phylogenetic analyses and statistical comparisons of various traits such as flowers, stomata and chromosome counts, Cron et al. [62] found that A. kilima is neither cytologically nor morphologically distinct, and the authors have thus reduced the proposed species to synonymy with A. digitata.
Six studies have sought to gain a better understanding of the genetic variation underlying the morphological and nutritional variation of baobab. The first was a seedling provenance trial to assist a breeding programme for leaf production in an irrigated hedge system in Mali. It involved 14 provenances—10 from Mali, 2 from Niger and 2 from Burkina Faso. Highly significant differences in leaf production were found among provenances and families within provenances, with moderate to low heritability estimates for leaf productivity [63]. Strong correlations between diameter and leaf production indicated the potential to use diameter as a predictor for leaf production vis à vis its seasonality. Somewhat similar provenance trials with seedlings were also conducted in Malawi [64,65,66]. In general, West African provenances grew faster than those from East Africa but with weak correlations between leaf morphological traits and climate, although the leaflet number was significantly greater in drier areas. The Malawi trial showed that the mainland populations were genetically distant from the one on the isolated Likoma Island. A study in Malawi also sought to determine whether the morphometric traits in fruit and seed characteristics within and between provenances could delineate populations from different zones into landraces. The results found that single seed weight showed pronounced evidence of divergence of populations into ecotypes [67].
The other three studies used microsatellites to assess genetic diversity, the first in seven coastal and inland Kenyan populations [68], the second in populations of the Nuba Mountains of Sudan [69] and the third in Malawi [67]. All these genetic studies also found high morphological diversity in fruits and/or leaves. Interestingly, there was evidence that some baobab morphotypes retained leaves during the dry season [70]. Within the high diversity in the fruit length, width and length/width ratio along a climate gradient from the Blue Nile to North Kordofan in Sudan, clavate and crescent shapes were found only in North Kordofan, while a rhomboid shape was only found in the Blue Nile [71]. In Kenya, the genetic diversity was high, with little difference between coastal and inland sites, despite limited geneflow between populations. Likewise, there was little difference in genetic diversity between sites in Sudan, although two distinct gene pools were observed, with the one close to a main road being more diverse, suggesting an influence of human intervention [72]. In Malawi, moderate genetic differentiation was observed among populations indicating the presence of a large number of common alleles, resulting in a homogenisation effect. The authors attributed the low genetic variation found to marginalized populations, anthropogenic factors and founder effects.
A physiological study to examine geographical variation in drought tolerance using plants from Mali and Malawi found that baobab responded to water stress by leaf shedding and allocating more biomass to the root system, while regulating photosynthesis and transpiration by stomatal control [73]. Water stored in the taproot was used to protect old leaves and for the formation of new leaves and roots, with a significant correlation between taproot water content stomatal closure. Interestingly, the two populations had different drought strategies, suggesting that selection for drought tolerance may be possible.
Domestication
In Niger, farmers have chosen a baobab-based agroforestry programme that suits their livelihood needs for its potential to improve their household food security and income [74]. A study of seed germination to enhance the performance of seeds of A. digitata in Nigeria found that a soak in 98% sulphuric acid for an hour prior to sowing improved establishment [75]. However, in southern Africa, domestication began with the selection of clonal propagation, especially in Zambia and Malawi [65]. To take advantage of the high intraspecific phenotypic and genetic variation within and between populations, vegetative propagation techniques have many advantages. One study reviewing the literature on the use of vegetative propagation in baobab reported only 30% rooting success with IBA hormone [76] and recommended the need for further studies, especially for the rooting of cuttings and marcotting. Anjarwalla et al. [77] and Jenya et al. [40], however, reviewed ‘top cleft’ and ‘side veneer’ grafting techniques in Kenya and Malawi. Top cleft grafting showed slightly more survival success than side veneer (71% vs. 55%). Using 2-year-old rootstock was slightly more successful than 1-year old ones. The study in Malawi also found that top cleft grafts had higher success rates than side veneer grafts. The results indicate that baobab is easily amenable to grafting when done at the right time with the correct size of scions—the same is probably true for the rooting of cuttings [37]. Both studies recommended grafting for use in domestication programmes. Nevertheless, when using grafting, attention must be paid to the risks of graft incompatibility and to the common situation that shoot growth from the rootstock can replace the intended growth from the selected scion. Marcotts and rooted cuttings on their own roots avoid these risks [37].
4.1.3. Commercialization of Products
As discussed earlier, nutritious baobab products are very important locally across dry Africa and have potential markets as lifestyle food products in Europe and in the USA [78]. Indeed, thanks to processing and packaging, they have already gained access to international markets [43] with several hundred formulations in European markets, following approval for trade by the European Commission. In Kenya, women have been the main actors in the baobab value chain networks, principally trading unprocessed pulp known locally as ‘mabuyu’, made out of baobab-pulp-covered seeds, sugar and food colour [79]. This candy increases in value from <1 USD/kg paid to fruit collectors up to USD 1.5 paid by the consumer. This is an important source of extra income for rural households, especially in the dry season, suggesting that there is great potential to increase market income through the domestication of baobab trees.
To enter international markets, food products have to meet food safety standards associated with processing and product quality. In Malawi, a baobab-processing sector has emerged to supply both food and non-food products to local consumers, based on small-scale, informal enterprises. Studies by Darr et al. [78] have shown the importance of value-addition through approved standards of processing and packaging. Baobab fruits may have other potential uses. For example, the transesterification of baobab oil has been carried out to assess the potential of baobab kernel oil for use as biodiesel [80]. The results show that important fuel properties of biodiesel from baobab oil met both the European and American (EN and ASTM) standards. Baobab is one of two species examined to evaluate concerns about ecological sustainability and inequality arising from the increased formalization of trade in natural products [81]. The study concludes that better informed and more respectful of local knowledge is needed to ensure respect for customary laws.
Marketing Concerns
Concerns have been raised that developing international markets for African indigenous food products could damage local supply, environmental degradation and loss of local dietary diversity. These concerns for subsistence users of baobab were greatest in West Africa, where there is a high dependence on these products, unlike southern Africa, where baobab has been reported to be ‘underutilized’ and where commercialization could help reduce poverty [42]. This concern was the subject of a conference “Bridging the gap between increasing knowledge and decreasing resources” in the Czech Republic in 2014, which examined these issues [82]. The meeting concluded that research should “address the improvement of the long-term food security and nutrition of local communities in the target regions by (i) ensuring the availability of and access to baobab products with high nutritional value, (ii) increasing the use of baobab products in daily diets, and (iii) raising incomes from selling raw and processed baobab products of high nutritional value”. These objectives concur strongly with the initiatives to domesticate indigenous food trees as new crops for subsistence farmers [3,14]. In addition, Chivandi et al. [83] have identified the need for research to focus on how to tap into health benefits of indigenous tree products for people and animals and to develop new products such as natural sweeteners and pectins for industrial use. They also emphasise the environmental and livelihood benefits arising from increased community-based sustainable utilisation and conservation of these tree species.
Concerns have also been raised about increased grazing pressure on natural regeneration. Studies have ascertained that baobab populations do decline under heavy livestock grazing, but that they are sustainable under moderate grazing pressures [41], as seed production is substantial [84]. However, the predation of fruits by baboons also leads to population decline, as does drought due to failure of the ‘rains’. These authors did conclude, however, that planting and protection from livestock is required to overcome recruitment bottlenecks.
4.2. Sclerocarya birrea (Marula)
4.2.1. Ethnobotany, Uses and Natural Resources
There are three sub-species of Sclerocarya birrea in Africa: subsp. birrea, subsp. caffra and subsp. multifoliolata. Their natural distribution spans drylands in 29 African countries, but they primarily grow in different areas in Africa. Subsp. birrea is found in the Sahel and northern Africa, and subsp. caffra is found in the Miombo Woodlands of southern Africa. In East Africa, both subspecies are found, while in Tanzania alone subsp. multifoliolata is native, but it can also be found together with the other two subspecies. Under good conditions, the tree can grow up to 15–20 m, with a mean diameter of 80–100 cm at maturity [85]. Generally, S. birrea occurs in areas receiving 200–1600 mm rainfall per annum on sandy to loamy soils. However, in Malawi, S. birrea thrives mainly in hot, dry areas at altitudes of 500–1000 m with a mean annual rainfall of 900–1000 mm and mean annual temperature of 22 to 23 °C. The species also thrives on hydromorphic soils with limited drainage, which are seasonally waterlogged [85].
To understand the population stability of marula in the Bushbuck Ridge area of South Africa, tree density and size–class distribution profiles were evaluated in areas with different land uses. Tree density was highest in homegardens due to discarded seeds from household beer making and lowest in crop fields [86]. Female tree densities were relatively constant across all land-use types, suggesting that the combination of cultural and economic values can conserve species, although harvesting them for fuel wood in rangelands may pose a threat. The authors consider that such findings may be relevant to other social-ecological situations where natural resources are important for sustaining livelihoods.
A mature tree of S. birrea can produce as many as 91,000 fruits. These can be eaten fresh or fermented to make beer [85], while the edible kernels are rich in a highly nutritious oil. The leaves and the bark of S. birrea also have medicinal uses, and the wood can be carved into utilitarian items such as spoons and plates or decorative animal figures [85]. In addition, the species is a browse for livestock and provides shade for cereal crops.
Within many African communities, the fruits and kernels in particular, have great cultural and ceremonial value [85]. Nevertheless, the natural resource is under considerable pressure due to the extensive clearing of woodlands for settlement and agriculture, which threaten the ecosystem services and genetic diversity of the species [85]. Despite this, a survey in southern Africa found that local communities had positive attitudes towards the conservation of marula trees and were also keen to participate in their domestication [87]. It was also clear that there is a need for more information about local management strategies [88], including its vulnerability to wildlife pressures from fruit and seed predation, especially from elephants [89]. An ethnobotanical survey in Namibia found that 87% of the population had indigenous knowledge passed down from their parents, with a majority indicating that the species was in a decline not being addressed by any management [90].
In Zimbabwe, a study compared the distribution and stem densities of marula in natural vegetation and on farms and found that there were nearly twice as many trees in farms (7 vs. 4 stems/ha), with female trees outnumbering males (55 vs. 34%) [91]. The author concluded that this is the result of farmer protection of fruit producing female trees and advocated the need for further work to domesticate this species. Another study examined the harvesting and utilization of the species by smallholder communities [92,93] and found that 49% of households consumed ripe fruits raw, while 76% also made a traditional fermented wine/beer and 54% consumed the kernels. The utilization of these products is a major source of income for local people meeting the economic needs of poor communities in rural areas while also contributing to food and nutritional security [85]. Indeed, the larger fruited S. birrea subspecies caffra is more commonly cultivated as homestead trees in southern Africa than is subspecies birrea in the Sahel, due to its tendency to have larger fruits.
4.2.2. Genetic Variation and Domestication
Marula was one of the priority tree species identified for domestication by the ICRAF and local farmers in the 1990s. When 21 provenances (20 of S. birrea ssp. caffra and 1 of S. birrea ssp. birrea) from Malawi, Zambia, Zimbabwe, Namibia, Botswana, Tanzania, Mozambique and Mali were planted in Mangochi, Malawi, there were significant differences—up to threefold—between the populations for most vegetative growth traits [94]. Studies in Namibia and South Africa found some evidence that farmers have initiated domestication by selecting desirable trees in the lands [95]. The Marracuene population from Mozambique was ranked top for height, diameter at breast height, crown width and crown depth. After 7 years, only 8 populations had fruits, of which the Marracuene population had the greatest fruit yield with considerable tree-to-tree variation in fruit production ranging from 1 to 1228 fruits per tree [94]. A follow-up study then found significant variation among the provenances in the number of fruits, fruit weight, pulp weight, seed weight, fruit length and diameter [85], with no correlation between the number of fruits and other fruit traits. The authors recommended that studies should investigate fruit taste and other quality traits. A weak relationship between fruiting and vegetative growth suggests that growth should not be used as a predictor of fruiting [96]. Likewise, only moderate correlations have been reported between fruit yield and crown growth. Thus, more work is needed to understand any possible relationships between vegetative growth and fruiting. Relevant here may be the recently reported provenance differences in their susceptibility to leaf predation by insect pests in Malawi [97].
Genetic variation, especially tree-to-tree, in many parameters has important implications for domestication of this species [36,95,98]. In a follow-up provenance study five years later [99], phenological traits differed between provenances, although with some overlap. Like many indigenous fruit and nut species in Africa, marula is a dioecious species with male and female trees. The earliest flowering was in the eastern provenances (Mozambique and Swaziland) and later in others. The early flowering genotypes fruited and matured between August and January, while the later provenances fruited from September to May, with male trees flowering slightly earlier than females, but some trees classified as female in one year were found to have some male flowers in the next season. Fruit yields also differed between years (2016/2017 versus 2017/2018), indicating probable environmental influences on phenology. These results suggest a need for more detailed phenological studies. This is especially the case in the Sahel, where the flowering season in Senegal (February to March) is only a few months after that in the southern hemisphere, with fruiting from April to July [100]. However, at the most southerly site (Amaly), there was a small amount of flowering from October to November. Nevertheless, marked differences were observed between and within sites for the leafing and flowering phases and a significant difference within and between species [100]. Marula nuts can contain up to four oil-rich kernels, but in Burkina Faso, the number ranged from one to three [101], as has also been reported from southern Africa, perhaps due to poor pollination success [102].
While provenances are grown from seed, horticultural cultivars are vegetative propagules. When growth of seedings was compared with that of stem cuttings, survival was greatest in cuttings, although seedlings grew faster [103]. The author recommended the use of cuttings because of their better survival, but of course, cuttings from selected elite trees are also genetically superior and identical for whatever trait(s) have been selected [37]. Likewise, marula is one of the species included in the African Orphan Crops Consortium initiative for genomic studies aimed at the breeding of high yielding, nutritious and climate-resilient crop varieties, discussed below [24].
4.2.3. Commercialization of Products
Like many other indigenous tree food products, S. birrea has nutritious fruits containing high levels of vitamins and oil rich kernels with unique fatty acid profiles, etc. [104] with dietary significance for humans [105], as well as a supplement for livestock feeds [106]. For example, a study in the Sudano-Sahelian zone in the north of Cameroon gave the products of S. birrea subspecies birrea the third highest total use value of the 50 woody species documented [107]. There is evidence in southern Africa that the processing of fruits and kernels is common and, in some places, highly advanced. In South Africa and Botswana, there is commercial processing of the fruits for juice, jams, snacks and liquors. In addition to the traditional uses of marula, there are numerous potential new commercial uses, such as the ‘Amarula’ liqueur [108], biodiesel [109], vinegar [110], jams, jellies, yoghurt and ice cream [111]. In addition, there are health and medicinal uses [112,113], as phytochemical analysis in Nigeria has shown that the stems, roots and leaves contain alkaloids, tannins, phenols and flavonoids with medicinal properties [114]. This rich phytochemical content varies between sites. Additionally, marula oil has many food and domestic uses as it is 10 times more stable to oxidation than olive oil and has free radical scavenging properties. These are traits conferring its use in cosmetics [94].
With extensive tree-to-tree variation in so many traits, it is clear that when domesticating marula and many other indigenous fruit and nut trees, there is great potential to identify ideotypes—ideal trait combinations—to meet specific market and/or industry needs by developing a hierarchy of different ideotypes to meet different market opportunities [115]. Individual trees matching a selected ideotype can then be propagated vegetatively to form elite cultivars to meet market needs.
4.3. Vitellaria paradoxa (Shea Nut)
4.3.1. Ethnobotany, Uses and Natural Resources
The shea nut tree (Vitellaria paradoxa) occurs from the Sahel to the drylands of central East Africa. It is one of the best known among many indigenous trees of Africa as the oil from its nuts is the source of shea butter that is widely used in the confectionary and cosmetic industries. Shea butter production as a source of edible fat from agroforestry parklands is second only to cocoa butter as a source of stearic acid in the multi-billion dollar chocolate and cosmetic industries [116]. Shea is also famous as one of the dominant species in the Sahelian parklands which are densely populated by both people and livestock [117]. In Burkina Faso, V. paradoxa, Tamarindus indica and Adansonia digitata were ranked the most important medicinal plants [118], while the same species are also the source of important traditional foods with high nutritional value [119].
In addition to its products and their role in livelihoods, V. paradoxa parklands are recognized for their contribution to environmental services. These roles were studied in Benin where soil and land-use variables of farmland in particular strongly affected the yield of shea fruits [120]. Interestingly, in Benin, a survey found that the parklands contained a good distribution of tree diameter classes, showing that shea agroforests have a stable and regenerating population with opportunity for improved management of juveniles to guarantee the stability of the system [121]. There is, however, variation in fruit yields between years, as well as strong variation in the production of individual trees. These parklands provide some resilience to local climate change, involving a complex set of factors influencing the density and age of individual trees and their impacts on the production of both staple crops and tree crops [122]. Understanding the interactions between trees and crops across different ecological conditions would assist the development of successful adaptation strategies to combat climate change in West Africa. For example, shea and other local species have also been reported to be excellent for carbon storage and thus for the mitigation of climate change and as a source of wood fuel [123]. It is clear, therefore, that V. paradoxa has an important potential role in the restoration of degraded farmland. This could be enhanced by domestication. For example, in Burkina Faso, about 51% of land was found to be favourable for its cultivation [124]. Interestingly, male and female farmers in Burkina Faso have been found to hold overlapping areas of knowledge about shea uses, yields and shea nut characteristics and recognize the importance of the same management practices and principles guiding the selection and conservation of shea trees in cultivated fields [125]. This case study from Burkina Faso indicates the importance of intra-household knowledge sharing to the achievement of resilient agroforestry practices.
In the context of climate change, a modelling study has concluded that habitat protection and restoration could be achieved in Benin by the domestication of the species to both conserve V. paradoxa and support farmers’ livelihoods [126].
4.3.2. Genetic Variation and Domestication
Shea nuts are especially important for women’s incomes, but in some areas, productivity is in decline. Two actions which can reverse this decline are the removal of parasite-infested trees, heavy pruning of old trees and grafting scions from selected trees on to young rootstocks [127,128]. In this respect, participatory surveys of local knowledge have found that local people in Burkina Faso recognize at least 25 ethno-varieties [129], based on locally agreed criteria, such as nut size, characteristics and folk names, which relate to 11 primary fruit and nut variants of the shea tree in the area [129] providing an excellent starting point for domestication. When 28 ethno-varieties from three farming systems were sampled in Uganda and analysed using microsatellites, 86% of the genetic variation was found to occur within individual trees with high gene flow [130], although three distinct populations were identified. Using a set of 12 quantitative traits, a study of 220 elite trees in Côte d’Ivoire identified three agro-morphological clusters which did not completely overlap with their geographic origins. One cluster was considered to be especially relevant for the development of high-yielding grafted plants [131]. In Benin, the shea parklands of Bembèrèkè were found to have considerable morphotype diversity with the widespread small fruit type (‘Yanki’) said to produce high fruit and butter yields [132].
A study in Burkina Faso to assess the interannual variation in fruit production along a climatic gradient found that fruit production was strongly and positively related to tree size and that at the individual tree level variation in fruit production between years was greater in drier areas, with some variation between climate zones [133]. Conversely, in addition to the livelihood benefits that can be derived from the domestication of indigenous fruit and nut species for cultivation in agroforestry parklands in the Sahel, there are important environmental benefits from revegetation and land rehabilitation.
Importantly, V. paradoxa has important physico-chemical properties relating to food and medicinal uses [134]. For example, one study of the medicinal properties of shea nuts, found that the extract of defatted kernels and its constituents contained potential antioxidant, anti-inflammatory, skin-whitening, chemopreventive, and anticancer agents [135].
Although early studies of vegetative propagation of shea trees found that it is not an easy species, a recent study of grafting on two-year-old rootstocks in Côte d’Ivoire found that terminal slit grafting resulted in the greatest success (90%), with recovery in 21 days and good vegetative growth (1.63 twigs; 5.59 leaves; and 3.28 cm in height per month) recorded in the grafted plants [136]. This opens the way for the development of elite cultivars.
However, good fruit production in agroforestry systems depends on the successful pollination of many flowers. A study of pollination success in Burkina Faso examined the effects of local and landscape habitat diversity and found that the loss of biodiversity can lead to pollen limitation [137], indicating that shea yields in parklands are likely to benefit from the retention of a diverse array of other tree and shrub species.
With all these different selection opportunities, it is clear that the domestication of shea trees could be based on a range of different ideotypes [115] appropriate either for domestic use or for income generation in local and regional markets/industries.
4.3.3. Commercialization of Products
The development of a value chain can result in social issues such as gender inequality. The international trade in shea has been opaque to the consumers of its edible products about the role of women involved in the production, harvesting and post-harvest processing of shea nuts [116]. In an attempt to address this, a 3D animation has been produced to indicate opportunities to improve the extraction yields, to lower production costs and to improve the marketable product. Furthermore, to raise awareness of these gender issues, Ingram et al. [138] have examined examples from the shea and cocoa industries to illustrate the opportunity to effect changes in agroforestry systems.
4.3.4. Post-Harvest Storage
The expansion of shea production raises post-harvest issues. For example, the storage of shea fruits and nuts is often associated with their damage by a wide range of insects (mites, ants and weevils), threatening food security, self-sufficiency and farmer income. These risks increase with longer periods in storage. To minimize these losses, care should be taken to maximise product quality, ensure proper drying prior to storage and to promote general hygiene [139]. To minimise damage during the shelling of nuts and to maximise increase in the efficiency of the process, a special shea nut shelling machine has been developed [140] with high shelling, recovery and cleaning efficiencies (96%, 83% and 70%, respectively).
4.3.5. Impacts and Multiple Benefits
The potential of V. paradoxa to sequester carbon in standing biomass for the mitigation of climate change is one of its multiple benefits. However, one must also recognize that in the preparation of cosmetic products from shea nut oils, there are carbon emissions arising from post-harvest operations and the use of shea products for fuel. One study has examined ways to minimise these emissions by more efficient use of the co-products, and found that substantial savings can be made over the relatively inefficient traditional processes [50]. Furthermore, there is an opportunity to use ‘green chemistry’ to produce a wide range of new products from shea butter as part of a new global approach to the industrial development of sustainably produced and healthy edible vegetable oils and fats [141]. Using a modelling approach to this kind of development, Seghieri [142] has simulated the intensification of shea parklands’ production to predict their economic, agronomic and environmental performance.
4.4. Allanblackia Species (Tallow Tree)
4.4.1. Ethnobotany, Uses and Natural Resources
Three Allanblackia species have been of special interest: A. parviflora in Ghana, A. floribunda in Cameroon and Nigeria and A. stuhlmannii in Tanzania. The oil-rich kernels of Allanblackia species have traditionally been used by local people for their edible oils and medicinal properties. Allanblackia trees are said to be dioecious with separate male to female trees. The ratio of male:female has been found to vary between farmland of different age ranging from 1:0.5 to 1:2 on recent and mature farmland, respectively [143]. The predominance of female trees in farmland was thought to be the result of the selective felling of non-productive male trees, with obvious implications for domestication. Towards the end of the second decade, their domestication became a major Public–Private Partnership agroforestry initiative due to commercial interest in their unique fatty acid profiles [16]. This has led to enhanced interest in its cultivation as a new crop.
4.4.2. Genetic Variation and Domestication
The focus of genetic selection has been the unique fatty acid profile of Allanblackia kernels, especially their stearic and oleic acid contents. This has led to studies on their chemistry to better understand the health benefits of Allanblackia oils. For example, Crockett [144] has emphasised the need for a better authentication of the oils to determine their safety for human consumption to prevent heart disease. A study using High-Performance Thin-Layer Chromatography found the presence in seed oils of phytochemicals probably from the seed shells/husks [145]. Such secondary metabolites may be important in future research. In a parallel study, [146] examined oil yields finding large tree-to-tree variation of importance for domestication and no suggestion that oil yield was affected by soil properties. Nevertheless, the highest oil yields were found in trees from wet evergreen forests. In southern Nigeria, little variation in the mean fruit length, width or weight was identified between three provenances, although high within-provenance variation was observed [147].
Domestication has been constrained by a number of issues arising from: (a) difficult propagation by seed and by vegetative propagules; slow vegetative growth and plagiotropism; and (b) farmer participation in both cultivation and business development [148]. Seeking to resolve some of the seed germination problems, sectioned and stratified seeds were tested for their germination capacity [149]. Proximal sections germinated best, while distal sections only produced seed roots.
Allanblackia trees have been found to be difficult to propagate vegetatively, being slow to root as stem cuttings and typically producing only one or two roots. In addition, due to the weeping branching habit of the trees, branch cuttings tend to develop with plagiotropism. However, fertilizer applications to stockplants enhanced both stockplant growth and the subsequent rooting of cuttings [150]. In accordance with the principles for robust propagation by cuttings [37], more recent studies have found that the light environment of the stockplants affects subsequent rooting success [151]. However, the optimum irradiance has not yet been identified, nor has the importance of light quality been determined. When air-layering was tested on shoots from stumps of different heights, those from short stumps rooted best [149].
Obviously, when developing clonal stands of selected trees, it is important to ensure the optimum sex ratio. In a comparative study of flowering in seedlings, stecklings and grafted plants, grafted mature trees started to flower after two years, while faster-growing juvenile seedlings were yet to flower after 8 years [152].
Despite the Public–Private Partnership approach developed for the domestication of Allanblackia species the proposed pathways conformed to those of decentralised participatory domestication and the development of multifunctional agriculture [153] aimed at integrated rural development and the reconciliation of biodiversity and livelihood concerns to achieve societal goals [148]. Thus, like the domestication of other West African indigenous fruits and nuts, a community approach has been promoted for Allanblackia [154], in which communities are encouraged to develop their own village nurseries and to expand with satellite nurseries.
4.4.3. Commercialization of Products
The prime focus of the commercialization has been its potential in the manufacture of margarine for international markets. However, as with domestication, a localized approach to marketing was promoted through small- and medium-term enterprise development for the Allanblackia product trade [155]. This initiative has resulted in the export of nuts for processing in Europe.
4.5. Tamarindus indica (Tamarind)
4.5.1. Ethnobotany, Uses and Natural Resources
A national-scale analysis of the traditional use of indigenous plants in Burkina Faso found tamarind (Tamarindus indica) to be one of the top three most important medicinal species [118] worthy of development. In sub-Sahara Africa, this species is traditionally used to build resilience into the farming system in terms of food security, income generation and ecosystem stability [156]. Indigenous fruits are one of the most important groups of plants for consumption by local people, with T. indica among those of greatest importance for their nutritional value across the continent [104,105,107]. Nevertheless, there is a need for much more information about the nutritional composition of tamarind.
Approximately half of the population in one study in Uganda [157] had T. indica in their compounds or homegardens, about 50:50 self-propagated or planted, mostly intercropped with other crops or trees. In Senegal, the effects of climate change on the species distribution and density around villages were recorded and modelled for future climates. The results showed a decreasing south to north gradient in tree density, suggesting that, under climate change, the area for future cultivation will decline. T. indica has been reported to be one of a number of indigenous fruit tree species with potential to be used in small-scale local industries in northern Nigeria to promote food security and rural development, especially if some of the current constraints such as inadequate electricity supply and access to capital can be removed [158]. As climatic conditions affect flowering and fruiting [159], there are concerns about the impact of climate change. However, using three different climate models to assess future impacts of climate change, it was concluded that a decline in precipitation will convert the currently poorly suitable sub-humid humid zone into highly suitable zones, while an increase in rainfall would convert the currently highly suitable semi-arid and subhumid dry zones into poorly suitable areas [160]. An increase in aridity would therefore extend the potential geographic range of tamarind in Benin. In addition, genetic conservation appears to be important in the northwest basin to avoid future inbreeding depression.
4.5.2. Genetic Variation and Domestication
Tamarind is an important smallholder crop in the Sahelian countries, making it highly appropriate for local domestication based on the most interesting phenotypes/genotypes possessing several desired traits. Plotting the frequency distribution of the different fruit traits indicated that farmers have already achieve stage one of domestication, demonstrating the use of this technique to assess the level of farmer domestication.
In Mali, a field trial tested different provenances from four contrasting agro-ecological zones and found that elite trees of tamarind contain the best combination for the most desirable fruit traits. There was good correspondence between these data and farmers’ selection criteria, with trees from the driest areas producing smaller fruits and less pulp [161]. Although the study found evidence that the environment influenced both morphological and nutritional traits of tamarind fruits, marked genetic variation was also evident. As part of a first step towards domestication, this study characterized tree-to-tree variation in pod morphology, sweetness and tartaric acid content in four provenances. Although most trees produced sour fruits with high tartaric acid content, some sweet tamarinds were identified, especially those from Hombori. Importantly, a few trees in each provenance were found to combine a number of desirable fruit traits and so to represent elite trees—or an ideotype—appropriate for local domestication.
4.5.3. Commercialization of Products
Although having a sharp, sour taste, the pulp of tamarind fruits is used industrially to make sauces, syrups, jams, chutney and beverages [162], with greater potential for its oils. In addition, its leaves, bark and roots are used as medicinal products, fuelwood and fodder. In Uganda, T. indica is greatly valued by the majority of the population as food, medicinal, cultural, social, environmental amelioration and income generation purposes [157].
4.6. Zizyphus species (Ber)
4.6.1. Ethnobotany, Uses and Natural Resources
There are many Ziziphus species, several of which are indigenous to Africa. Here, we discuss three species: Z. mauritiana, Z. mucronata and Z. spina-christi. They are all indigenous to dry, low-rainfall and high-temperature areas. They produce edible fruits which are used in local cuisine, as well as to make alcoholic and non-alcoholic drinks, jams, etc. The trees have large spines and are also used to make hedges to exclude livestock.
Z. mauritiana fruits are harvested in large quantities in the Zambesi basin of Zimbabwe [163]. These fruits are marketed both locally and to neighbouring countries, although many households in this area are food-insecure and dependent on Food Aid. The authors of this study concluded that the cultivation of this species should be encouraged, both for food and for income generation. In an ethnobotanical study in the north of Cameroon, Z. mauritiana was one of the 50 species considered to have high use value with respect to its multiple uses and ethnobotanical heritage and that the species is either conserved or planted in farming systems [107,164]. Likewise, a pan-African multi-species study reported that Z. mauritiana had great potential for its high nutritional quality and potential for income generation [104]. This study called for more data to fully evaluate the potential of this species in Sub-Saharan Africa. One study with this objective has been conducted in the Sahelian and Sudanian ecozones of Mali to examine the relationships in a number of dry zone species between growth and fuelwood properties with mean annual rainfall and geographical coordinates [165], but Z. mauritiana was not one that was recommended as having good growth:fuelwood properties. In northern Namibia, however, the roots of Z. mucronata are recognized for their medicinal properties and are used to treat diarrhoea in cattle [90].
4.6.2. Genetic Variation and Domestication
Tree breeding and genetic studies in Z. mauritiana have been aimed at improving both productivity and drought adaptation in the Sahel. This has focussed on combining the heavy-fruiting characteristics of Asian germplasm with the greater pest and drought tolerance of local germplasm [166].
4.6.3. Commercialization of Products
Adam, Pretzsch [49] found that the fruits of Ziziphus spina-christi, like Z. mauritiana and Z. mucronate, are sold in Sudan within a subsistence strategy not always with positive benefits. They concluded that further research was needed to determine the potential to develop approaches that ensure strategies that lift people out of poverty, together with appropriate institutional, technical and financial support [50]. However, another study in Sudan has examined the role of home gardens and forest ecosystems for the domestication and conservation of Z. spina-christi [167]. It assessed the diversities of morphological traits and amplified fragment length polymorphisms with regard to variation within and among locations and sites. It appeared that both fruit size and genetic diversity were greater in homegardens than in forests, perhaps due to the human selection of germplasm. This suggests that homegarden populations have undergone some domestication and are probably a source of desirable germplasm.
4.7. Prunus africana (Pygeum)
4.7.1. Ethnobotany, Uses and Natural Resources
Prunus africana is found in many highland areas of Africa, often in isolated populations. Extracts from its bark are used in the pharmaceutical industry as a major source of compounds to treat benign prostate hyperplasia (prostate enlargement) in men [168]. This, especially in Cameroon, Kenya and southern Africa, has led to unsustainable overexploitation, which is threatening the existence of the species.
4.7.2. Genetic Variation and Domestication
The over exploitation of Prunus africana has led to calls for its integration into agroforestry systems and its domestication to improve the source of these medicinal products to supply the pharmacological industry. To this end, seedling and vegetative propagation studies have been implemented in parallel with studies aimed at reducing the misuse of the products, domestication and conservation of the genetic resource and the improvement of its wise use locally and internationally [169,170]. Low-technology propagation of cuttings in a non-mist propagator have been found to be enhanced by the use of endomycorrhizal fungi in the rooting medium [171]. Meanwhile, Komakech et al. [172] have developed in vitro culture methods to mass produce planting stock of Prunus africana as an alternative to seed germination and the rooting of cuttings [172]. Shooting and rooting media were designed for optimal survival, and the parameters for photosynthesis, growth physiology and genetic fidelity were tested for uniformity with the mother tree. In support of its domestication and conservation, genomic and cytological studies have examined this unique African member of the Prunus genus [173].
The World Agroforestry Centre has also led a pan-African programme with multiple partners to implement the conservation, domestication and cultivation of pygeum by farmers using agroforestry systems [174]. This has placed great emphasis on the role of farmers, the industry and policy makers to ensure much more sustainable production, harvesting and bark processing. As part of this initiative, a study of the role of mycorrhizal fungi on the growth and phytochemical content of the bark has for the first time found that the associations between mycorrhizal fungi and the roots of pygeum have positive effects on the growth of seedlings [175].
5. Humid Lowlands of West and Central Africa
5.1. The State of Natural Resources and Their Governance and Management
The humid West and Central Africa Region spans from the Congo Basin westwards along the coast of the Gulf of Guinea, up to Guinea. It has the greatest share of the 15,387 vascular plant species found in Africa, with more than 30% being endemic [176]. This equates to 5–7% of the world’s trees flora, with five of its countries appearing as the botanically best explored on the continent. These trees produce a large number of Non-Timber Forest Products (NTFPs)—most of which are neglected, underutilized and poorly documented [6,177,178,179,180,181,182,183,184,185,186,187,188,189,190]. According to Ingram [191], 951 and 706 species are exploited for NTFPs in the Democratic Republic of Congo and Cameroon, respectively, 35% of which are traded locally. The access and use of these NTFPs are increasingly constrained by deforestation, destruction of their natural habitats and by overharvesting. Indeed, the recovery and survival after harvesting of bark- and root-based products from Afrostyrax lepidophyllus, Annickia chlorantha, Garcinia kola, Garcinia lucida, Prunus africana, Pycnanthus angolensis and Scorodophloeus zenkeri are affected by: (i) the ecological conditions where they grow, (ii) the anatomical characteristics of the targeted tissue and (iii) the technique, intensity and frequency of exploitation [192,193,194,195,196]. Unsustainable trade also threatens Garcinia kola [197], Gnidia glauca [198], Afrostyrax lepidophyllus, Baillonnela toxisperma, Irvingia spp. and Pentaclethra macrophylla [199,200].
5.1.1. Governance Issues
In many countries of the region, including Cameroon, the Democratic Republic of Congo, Nigeria, Benin, Cote d’Ivoire and Ghana, the economic, social and cultural importance of NTFPs are increasingly being recognized in national and regional policy and in regulatory frameworks. This is due to the adoption by these nations of a government strategy promoting NTFPs for poverty alleviation and the livelihoods support to poor and vulnerable forest-dependent communities. However, an analysis of the new forestry policy in Cameroon has highlighted some incoherence and dual ownership for the land and trees which, if strictly enforced, could be a disincentive for on-farm tree planting [201,202,203]. The governance of NTFPs harvesting is generally regulated by both customary and statutory systems involving different institutional arrangements governing access and trading [182,204,205,206].
As a result, the concept of privately owned tree crops producing Agroforestry Tree Products (AFTPs) has been proposed to distinguish them from common-property forest resources [207]. This is important, as these tree products are the backbone of most rural economies and contribute to the enhancement of the socio-economic welfare of forest-dependent communities [180,187,189,191,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224]. Furthermore, studies have demonstrated that the sustainable exploitation, commercialization and management of NTFPs/AFTPs could contribute immensely to the attainment of important Sustainable Development Goals (SDGs) such as SDG 2 (zero hunger), SDG 3 (good health and wellbeing), SDG 13 (climate action) and SDG 15 (life on land) [21,225,226,227]. Moreover, Leakey [12,22] has said that 14 out of 17 SDGs could be directly and indirectly achieved through the agricultural production of NTFPs as agroforestry tree products.
5.1.2. Management Issues
Many studies over the last decade have demonstrated farmers’ interest and willingness to domesticate and cultivate local tree species in humid West and Central Africa [147,183,212,228,229,230,231,232,233,234,235]. Moreover, many of these species can be found in farmers’ fields, where they provide products and services useful for the livelihood and resilience of farmers [236,237,238,239,240,241].
In the first two decades (1992–2002 and 2003–2012), much of the groundwork for the participatory/decentralized approach to tree domestication was conducted in Cameroon. This involved the identification of candidate species by stakeholders; the documentation of associated local knowledge about products’ variability and consumer preferences; incentives and disincentives for adoption; and the development of simple, appropriate and affordable horticultural propagation techniques to capture the genetic traits of individual elite trees [3,37]. This work has continued in Cameroon and expanded to other countries during the third decade (2012–2021), as reported here.
5.2. Ethnobotany of Food and Medicinal Species for Cultivation
Several ethnobotanical studies undertaken in West and Central Africa have further documented traditional knowledge associated with the use, conservation and management of indigenous tree species identified as ecologically, socially and/or economically important for society, as follows:
- Local management practices and socioeconomic factors affecting farmers’ strategies for cultivating species were documented on candidate species selected for domestication during the first two decades: Irvingia gabonensis [233], Dacryodes edulis [242] and Garcinia kola [196,243,244]. Farmers have developed a set of quantitative and qualitative criteria to segregate these species and recognize them as “locally acknowledged morphotypes (LAM)” or ethnovarieties. Market integration and other economic opportunities were found to be the most important factors influencing farmers’ decision to maintain and intensify the cultivation of preferred ethnovarieties in their farms. Moreover, social networks for seed and planting material exchange, including ethnically and gender-differentiated uses were found to contribute to gene flow and the conservation of high genetic diversity despite the selection, urbanization and agricultural intensification associated with the domestication process [245]. Furthermore. perceptions of and preferences for fruit traits linked to intraspecifc tree diversity vary according to interethnic and rural–urban differences, which need to be considered in future domestication activities [246].
- Local use and socioeconomic importance were documented, as well as the pressure on natural stands due to overexploitation and unsustainable management of food, medicine and other services from Baillonella toxisperma, a multipurpose food, medicinal and timber species in Cameroon [247]; Garcinia lucida, a medicinal species in Cameroon [248]; Xylopia aethiopica, a tree species used as food, spice and medicine in Benin and other Central and West African countries [249]; Gnidia glauca, a medicinal tree species in Cameroon [198]; Cola spp., a group of food, medicinal and ritual species in Benin [250,251]; and Canarium schweinfurthii, a multipurpose tree species used for food, ritual and medicinal purposes [252,253,254,255]. This has provided evidence of unsustainable exploitation and has led to recommendations for the domestication of these species to create an alternative source of products.
- Many species locally threatened by deforestation, land use change and unsustainable exploitation for food, medicine, fibre and other services, have little-known resource bases, habitats and unrecorded local knowledge [192,227,256]. The key challenges to sustainability that were identified were: unfavourable cultural beliefs [233,249], indiscriminate and illegal logging, low accessibility and low acceptability, as well as inadequate research on their cultivation [257]. Criteria were designed and used to assess the sustainability of wild harvesting based on biological type, part used, habitat type and status, popularity and stand structure. Overexploited and threatened species were classified as vulnerable and have been recommended for domestication [192,198,227,256].
- Indigenous fruits are consumed by the members of the majority of households in Cameroon, as they are recognized for their nutritious value [258,259,260]. In addition, the study examined the relationship between food insecurity and deforestation and found that rural people in the deforested agricultural areas close to towns and cities had the greatest food insecurity due to the absence of nutritious forest foods [261]. The consumption of wild forest food is not associated with any stigma of poverty [262]. The authors suggested that the culinary uses of indigenous foods should be widely promoted to improve food security [262].
Findings from the above research have confirmed the importance of ethnobotanical studies as an inclusive and participatory step towards an appropriate and contextualised domestication strategy that takes into account the needs and constraints of the local situation. By recognizing the opportunities and benefits for local households, these research outputs underpin the concept of domesticating ‘socially modified crops’ [14,29].
5.3. Commercial Value of Non-Timber Forest Products in Local Markets
NTFPs are known as an important source of income for many actors involved in their value chains, and the commercial value of these tree products is one of the important criteria when selecting candidate species for domestication. Income generation is an incentive for farmers to adopt the cultivation and domestication of useful and marketable species [181,249,263]. This leads to a ‘win-win’ situation combining poverty reduction with biodiversity conservation to achieve both forest conservation and income generation for rural people [3,22,264].
During this third decade, value chains of some economically important NTFPs have been explored across West and Central Africa with the aim of guiding policy and institutional reforms that could better support their contribution to rural livelihoods. In some areas of Cameroon, for example, more than 80% of households rely on forest-dependent livelihood activities. The annual economic value of 16 food-based NTFPs involving 283,000 people has been estimated at USD 64.7 million, with an added value of USD 13 million or 0.2% of the national GDP [218,265]. The contribution of NTFP income to rural livelihoods depends on many ecological, social and economic variables, including the availability and accessibility to forest resources and to markets, as well as gender [179,187,190,213,214,266,267,268]. The distribution and use of revenues vary according to gender and wealth, with harvesters in remote areas earning less than traders and processors in more accessible sites. Women typically dominate the value chains and generate income from their interventions into the NTFPs sector [224,264,269]. The financial contribution of NTFPs to the household economy remains relatively low, but the products are highly valued for their cultural importance and food security benefits [270,271]. Importantly, however, poor institutional capacity to control and enforce existing regulations increases the transaction costs, while corruption negatively affects the conservation status of NTFP species, as well as the financial benefits at all levels of the value chain [138,265,272].
The third decade has seen an explosion of action-research on ways to improve the commercialization of NTFPs and agroforestry tree products (AFTPs) for smallholder producers. For example, in Cameroon, research aimed at improving the commercialization of Cola spp. has shown that collective action and group sales at the producer and trader levels could lead to improved market information, increased product quality and quantity, reduced transaction costs, enhanced negotiating abilities and strengthened social bonds [273]. Strangely, according to Gyau et al. [274], the perceived usefulness is mainly related to economic benefits of group activities and did not influence farmers’ intentions to participate in kola group sales in Cameroon, suggesting that extension messages should rather emphasise the social benefits of collective action to increase farmers’ participation. Likewise, Cosyns et al. [275] demonstrated that project interventions promoting commercialization of Ricinodendron heudelotii kernels in southern Cameroon allowed participating households to increase their social assets significantly, whether it be in terms of linking, bridging or bonding capital. This improvement was nevertheless mutually reinforced by a positive change in other farmer livelihood assets, in particular financial capital. Facheux et al. [276], comparing three different types of interventions (group sales, facilitating a village-level stabilisation fund to allow for off-season sales, provision of storage methods) in the market chains for R. heudelotii and Cola spp. in Cameroon, concluded that the three modes are complementary. Nevertheless, group sales, because they do not involve the use of new technology or credit facilities, can serve as a good starting point for helping producer groups achieve quick and meaningful gains as a prelude to introducing other post-harvest and marketing interventions. The success of group sales depends greatly on the creation of some form of partnership or strategic alliances between producers and traders. This stimulated an analysis of the case of producer groups and traders in the NTFP value chains in Cameroon [277]. They found that, despite low levels of satisfaction of the outcome of the relationship and low levels of trust between producers and traders, both parties were committed to continue the alliance, possibly for other strategic reasons such as reducing transaction costs.
In addition to collective marketing, facilitating processing and reducing post-harvest losses can significantly improve income from several NTFPs, but these aspects remain largely understudied. One exception is the work on a njansang (Ricinodendron heudelotii) extraction machine [278]. Manual extraction of njansang kernels is time-consuming and tedious, so the authors examined the feasibility of introducing a njansang extraction machine to mechanize the process. However, the authors argue that such technology development should follow a participatory and iterative approach involving all stakeholders to make sure the equipment meets the needs of the end-users.
To improve marketing processes and the commercial success from the trade of NTFPs in West and Central Africa, there is also need for the promotion of an enabling business environment through a sound and contextualized regulatory framework that takes into consideration: (i) the interests of all stakeholders involved and the development of local and export markets and (ii) equitable benefit sharing along the NTFP value chains, while being consistent with the sustainable use, management and domestication of these underutilized species [269,270,271,279].
The observed trend of farmers venturing in the cultivation of NTFP species, such as I. gabonensis in Cameroon, shows their commitment to the long-term availability of the resources [280]. However, despite some policy and institutional reforms in the NTFP value chain, more effort is needed to implement them for improving the business environment and the sustainability of the NTFP subsector [237,271,281,282,283]. In addition, internationally there is interest in increasing the trade in ‘green’ market products, usually labelled ‘organic’, ‘fair trade’, ‘bio’ or ‘deforestation-free’, with the objective of reducing deforestation and mitigating climate change. According to Leakey and Van Damme [284], this crucially needs to be extended to the many poor, hungry and marginalized smallholder farmers in developing countries. In this context, agroforestry tree domestication is important for the enhancement of economic returns as value chains from local to global become more sophisticated and demand higher quality, greater uniformity and a regular and continuous supply.
5.4. Priority Setting for Domestication/Commercialization
The top five priority species identified as candidates for domestication in West and Central Africa in the 1990s were Irvingia gabonensis/wombulu, Dacryodes edulis (safou) Chrysophylum albidum, Ricinodendron heudelotii and Garcinia kola [3]. Subsequently, the list was updated based on context specificity and emerging markets, including two heavily exploited medicinal species—Prunus africana and Pausinystalia johimbe [16]. For the originally identified priority species, progress during the third decade focussed on contextualising information on producers’ and consumers’ preferences with a view to guiding the identification of ethnovarieties that meet specific market and consumers’ niches [233,242]. In addition, some of the original group of priority species for Central African countries were extended to neighbouring countries. These include G. kola and Cola spp. in Benin, Sierra Leone and Nigeria [244,250,251,285,286,287] and I. gabonensis and Ricinodendron heudelotii in Benin and Côte d’Ivoire [233,256,288]. In Nigeria, Cola pachycarpa has been selected among the three botanically identical monkey cola species (Cola lateritia, C. lepidota and C. pachycarpa) for domestication programs, largely based on the sweetness of their edible fruit [285]. In addition, more species have been prioritized for domestication across the region. They include: Annonidium mannii, Afrostyrax lepidophyllus, Baillonnella toxisperma, Canarium schweinfurthii, Gambeya africanum, Garcinia lucida, Gnidia glauca, Pentaclethra macrophylla, Trichoscypha acuminata, Tetrapleura tetraptera and Scorodophloeus zenkeri and Synsepalum dulcificum. These newly identified priority species should be considered in domestication programmes to meet the needs of forest and biodiversity conservation, markets and livelihoods of rural communities.
5.5. Characterization of Useful Traits
The genetic variation of individual trees within and between populations for more than 70% of candidate tree species for domestication has been assessed using morphological, biochemical and molecular markers. This is an essential step in the domestication process that allows the identification of elite trees and populations and confers the opportunity for the selection and development of genetically superior putative cultivars to enhance income and livelihoods [289]. In studies from earlier decades, the intraspecific variation was examined in priority species such as I. gabonensis [16]. This has now been extended to include sensory evaluation, as this determines consumers’ preferences [290]. The study found that desirable flesh attributes were flavour, juiciness, sweetness, taste and texture, while the preferred kernel traits were colour, texture and flavour.
Significant progress has been made during the third decade in the intraspecific characterization of Dacryodes edulis in Cameroon and Gabon [242,245,246,291,292,293,294]; Irvingia spp. in Benin and Nigeria [233,295,296,297]; G. kola in Nigeria [298,299,300,301,302], Cote d’Ivoire [303] and Benin [244,304,305]; Cola spp. in Nigeria [306] and in Benin [287,304]; Allanblackia floribunda in Nigeria [147]; Chrysophyllum albidum in Benin and Nigeria [307,308]; Penthacletra macrophylla and Trichoscypha acuminata in Cameroon [234,309]; and Strychnos spinosa in Benin [310].
In Ghana, most of the low-kernel-oil-yielding trees of A. parviflora were identified in the semideciduous forest zone, while the wet evergreen forest zone described as having very high-kernel-oil-yielding trees was recommended for selection in the domestication process [146].
5.6. Assessments of Genetic Variation
5.6.1. Provenance Studies
Based on provenance studies with Dacryodes edulis, an approach of controlled cross-pollination combining the male parent, the hermaphroditic male type and the status of female parent was used to identify six best combinations from Boumnyebel and Makenene provenances. They were characterised by a high fruit setting rate (>70%), fruiting index (>50%) and a low rate of fruit dropping after fruit set (<20%). Though the breeding methodology did not improve fruit size as expected, it was recommended for controlling early fruit drop, known as the most important agronomic challenge affecting Dacryodes edulis production [291].
5.6.2. Molecular Studies
The most used molecular markers for genetic characterization of species under domestication in West and Central Africa are the microsatellites. In D. edulis, six microsatellite markers were used for niche characterization, gene flow and genetic structuration in selected Cameroonian and Gabonese populations and accessions [291,311,312,313]. However, these markers failed to segregate clusters and ethnovarietal groups due to the outcrossing reproduction systems of the species added to the social networks, which strengthened gene flow and contributed to an unexpected high level of genetic diversity conservation within and among populations, albeit affected by the selection intensity placed on preferred traits [242,245,314]. Recommendations for next steps in genetic studies include the isolation and characterization of more DNA markers in D. edulis and their use for early selection in progeny trials and advanced studies such as gene discovery, trait-linked marker-assisted selection and gene mapping [291,312,314].
The development of the domestication programme for many candidate species is constrained by the taxonomic confusion amongst existing varieties or species. In Irvingia spp., the morphological, agronomical, biochemical, ecological and molecular characterization undertaken in Benin, Nigeria, was mainly aimed at the taxonomic delimitation between the so-called “bitter” and “sweet” varieties for bush mangoes [295,296,315,316]. In the same way, in Cameroon, more than eight species belonging to the Irvingiaceae, including five from the Irvingia genus, were found to be grouped under the common name of “bush mango” and used by the Baka indigenous people [200]. Furthermore, there is a need for the characterization of all species grouped under the name ‘bush mango’ within West and Central Africa to clarify their identity and to ensure the accurate association of knowledge with genetic resources in domestication programmes. Molecular techniques have been used to address the issue. Amplified Fragment Length Polymorphisms of DNA combined with chloroplastic microsatellites were therefore used but failed to consistently differentiate between bitter and sweet bush mangoes from both Nigeria and Benin, indicating the need for exploring more discriminating markers to segregate the species and their genetically diverse varieties [296].
Molecular techniques involving inter-primer binding site retrotransposons markers were successfully used for genetic differentiation within the three Gnetum species, namely, G. africanum, G. buchholzianum and G. latispicum, and could be recommended for conservation, taxonomy and evolution studies of indigenous species [317]. Interestingly, consumers’ preferences for the bitter taste of Gnetum leaf-based meals have been found to vary between Nigeria and Cameroon. Sensory attributes should therefore be assessed when selecting vines of Gnetum sp. for domestication [318].
In addition, G. kola and Cola spp. have been characterized in Nigeria and Benin using Random Amplified Polymorphic DNA [300,306] and microsatellites markers, respectively [244]. The knowledge of genetic diversity within and among studied populations in these countries provide important information for the assessment of the domestication strategies and their influence on the conservation of the genetic resources of the targeted species [244,245,312].
5.7. Nutritional Value of Non-Timber Forest Products
The knowledge on the composition, nutritional and medicinal/therapeutic value of fruits, leaves, barks, roots and other products from candidate species for domestication is critical when promoting their domestication, use and developing appropriate value-adding technologies.
Biochemical and nutritional characterization were used for Cola nitida in Ghana for the selection of highly nutritious varieties [319] and in Nigeria to identify important micronutrient profiles in collections of Irvingia spp. [320], as well as for the selection of nutritionally superior trees of Tetrapleura tetraptera [321]. A wide variation in nutrients (moisture, ash, protein and β-carotene), minerals (zinc, potassium, phosphorus, sodium and copper) as well as bioactive compound such as plant sterols (γ- and β-sitosterol, ergosterol and campesterol), vitamin E, caffeine and squalene were observed among 11 accessions of D. edulis from southeastern Nigeria, suggesting a huge potential for genetic selection for nutritional, therapeutic and commercial exploitation at national and international levels [293]. In Côte d’Ivoire, two varieties of safou, namely, D. edulis var edulis and D. edulis var parvicarpa were identified based on their morphological and chemical fruit traits, with D. edulis var. edulis showing higher content in minerals such as calcium, potassium, zinc, iron, magnesium and manganese and therefore recommended as a potential source for essential nutrients and cosmetic products for Ivorian populations [322]. Based on nutritional traits, Tetrapleura tetraptera accessions were structured into four clusters, from which five accessions were identified using multiple traits performance as superiors and recommended for broadening the genetic base of the germplasm for the domestication and varietal development [321].
In addition to their protein, ash, fat and crude fibre content, the peel and pulp of I. wombulu from Nigeria and Côte d’Ivoire contain appreciable amounts of minor nutrients and micro-nutrients. These include essential and non-essential amino acids, such as lysine and methionine, and prominent essential oils (δ-αtocopherol, β-tocopherol and stigmasterol). They could therefore provide supplements for the food, pharmaceutical and cosmetic industries [220,323]. The ascorbic acid concentration in the fruit mesocarp of the closely related I. gabonensis is higher than some common sources of vitamin C [324,325,326]. Furthermore, based on its high saponification value, substantial contents of lauric and myristic acid, triacylglycerol and phenolic compounds, its kernel oils have been recommended for food additives, as flavour ingredients or for processing as margarine, oil creams, cooking oils, defoaming agents, cosmetics and pharmaceutical products [327,328,329]. Other parts of Irvingia spp. trees, such as the leaves, seed coats and fruit peel, are utilizable sources of food nutrients and phytochemicals [330]. With appropriate fermentation, kernel paste has been found to reduce the antinutrient and increase the essential nutrient contents of the food-thickening products made from processed kernels [331]. Thus, the analysis of the hydrocolloids contributing to the food-thickening property of the kernels have shown enhanced nutritive value [332].
Like the Irvingia species, bioactive compounds such as polyphenols, flavonoids, alkaloids, terpenes and sterols have been found in D. edulis cake after extracting oil from the fruit pulp and the kernels [333]. D. edulis fruit pulp powder has been used for the flavouring and nutritional fortification of rice-based biscuits with protein, essential amino acids and minerals [334].
In Cameroon, Baillonella toxisperma, Pentaclethra macrophylla and Trichoscypha abut have been reported to be excellent sources of bioactive compounds such as flavonoids, polyphenols, carotene, vitamin C and essential minerals. Their daily consumption has therefore been recommended for protection against ailments and oxidative stress [335] and to meet the nutritional needs of women. Similarly, in Gabon, substantive amounts of bioactive compounds have been found in widely consumed fruits such as Panda oleosa, Afrostyrax lepidophyllus, Gambeya lacourtiana, Pseudospondias longifolia and Poga oleosa. Similarly, studies in Nigeria have examined the phytochemical and physical chemical properties of Chrysophyllum albidum oil with regard to its nutritional and medicinal uses [336].
While the above studies have helped to document the nutritional value of some indigenous tree species, their contribution to household nutrition and food security is poorly known. According to Tata-Ngome et al. [261], this is because information on household consumption patterns and the use of indigenous tree products is lacking; hence, they are not properly captured in food and nutrition surveys with implications for policies and strategies concerning food security and health concerns.
5.8. Medicinal Value of Wild Non-Timber Forest Products
In addition to providing essential nutrients, many tree products have medicinal properties [222,337,338,339,340]. For example, the seeds of I. gabonensis show antimicrobial and antioxidant activities [341,342], as well as the ability to improve liver and kidney functions. Furthermore, there are reports of suitability for the management and control of body weight, with benefits on Type 2 diabetes by lowering blood glucose level and ameliorating the lipid profile [343,344,345,346,347], as well as its seed extract having a nephrocurative effect on acetaminophen-induced kidney damage due to their flavonoid content [348]. Additionally, studies on I. gabonensis in Nigeria have revealed biochemical properties conferring higher antioxidant activity and potentially enhancing their range of medicinal, food or cosmetic uses [341], creating new market opportunities.
The fruit peel and pulp of I. wombulu also have antifungal properties effective on common fungal pathogens and so have been recommended for use as a natural and eco-friendly fungicide [349].
The polyphenol content of D. edulis fruits also has high therapeutic value as a potent functional food and/or nutraceutical in the treatment and management of diabetes and its complications [350], while its antimicrobial properties are effective on some common human pathogens such as Staphylococcus aureus, Klebsiella pneumonia, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa and Proteus vulgaris.
Likewise, the bioactive constituents of C. schweinfurthii have antimalarial, anticancer, antioxidant, antimicrobial and antibacterial, anti-diabetic, analgesic activities as well as having nephroprotective, growth promoting, food preserving and anthelminthic and termiticidal properties [252].
The oil extracts from G. kola seeds were recommended for applications in food, cosmetics and pharmaceutical industries based on its flavonoids, phenolic, minerals and other phytochemicals contents reported as having antioxidant, antimicrobial, anti-inflammatory, hypocholesterolemic, antiviral, anti-diarrheal, antiproliferative, antiandrogenic and anticoronary activities [351].
Meanwhile, the seed oils of Allanblackia spp. used for vegetable-based dairy products such as ice cream and spreads have been found to provide health advantages for people prone to cardiovascular disease or with hypercholesterolemia [144].
The increased medicinal and nutritional value of products from agroforestry trees can be expected to motivate farmers to adopt agroforestry systems. However, the application of the existing useful nutritional, medicinal and therapeutical knowledge through development of efficient community-based enterprises is constrained by issues of intellectual property rights over Traditional Knowledge. This poses issues of political will and financial investment.
5.9. Selection of Elite Trees and Ideotypes
Most studies on genetic variation during the third decade have been focussed on assessing the genetic and social consequences of the morphological diversity observed in preferred fruits traits among trees within and between populations, such as in D. edulis [245,314].
Five plus-trees of Cola acuminata were selected for use in cultivar development based on the morphological characterisation of 1204 fruits and kernels from three populations in the centre region of Cameroon [180]. Finally, by using 10 fruits and kernel traits from 152 I. gabonensis and 293 D. edulis trees, [352] have shown that farmers in Nigeria have selected and domesticated I. gabonensis (stage 2 of domestication, with a 44% relative gain in flesh depth) while those from Cameroon have domesticated D. edulis (stage 2 and 3 of the domestication with 67% relative gain in flesh depth). Interestingly, the domestication process also contributed to increased genetic diversity [352] for both species, supporting the concept that the participatory domestication approach provides a steppingstone for an enhanced integration and conservation of genetic diversity of candidate species into the farming systems [18].
Based on 9 tree and fruit traits, 41 robust trees including promising individuals with end-users’ preferred traits (high fruit mass and pulp-dense genotype) were selected from 203 accessions of Synsepalum dulcifcum, a shrub known as a miracle multipurpose natural sweetener in the Dahomey Gap and Upper Guinea of West Africa [353]. By further finetuning their participatory breeding approach to take into account farmers’ and end-users’ (consumers and processing companies) preferred traits, the authors observed high similarity in the preference for breeding traits (fruit size and miraculin content) across agroecological niches and actors’ categories, suggesting opportunities for regional breeding programmes and the targeted ex situ conservation of the species [354,355].
5.10. Nursery Development and Improved Planting Stock
Seedling germination in tree nurseries is the starting point of many domestication activities and has been further studied in many species. For example, in Benin, fresh seeds of Irvingia gabonensis and Irvingia wombulu were found to germinate optimally without any pretreatment, even under the climatic conditions of the savanna lowlands of the Dahomey Gap (Vihotogbé et al., 2014 [315]). Interestingly, the early growth of both I. gabonensis and Chrysophylum albidum seedlings was better under full sun and unshaded agroforestry systems [356].
In some species, such as those of Allanbackia and Garcinia, seed germination is recognized to be difficult due to seed dormancy systems. However, significant progress has been made in Allanblackia parviflora by using proximal and longitudinal sections and by stratifying whole seeds at warm temperature to improve imbibition [149]. Scarification to break seed dormancy was achieved in C. schweinfurthii by physically cracking, removing or burning the seedcoat, to facilitate domestication by farmers [357]. Likewise, the germination of G. kola seeds has been improved by mechanical scarification, storage at room temperature and pretreatment with gibberellic acid (GA3) [358]. GA3 has also been found to promote the germination of Prunus africana seeds treated with sodium nitrate [169]. Benefits in seedling growth were observed when G. kola and Prunus africana seedlings were inoculated with Arbuscular Mycorrhizal Fungi [175].
The technologically accessible and financially affordable horticultural techniques developed in the first decade [37] have been extended to other species and locations in the third decade. For example, in the humid savanna highlands of Cameroon, the training, technical skills and backstopping knowledge received by small-scale nursery operators were recognized and expanded for the production of the high-quality tree seedlings needed for the successful reforestation and tree domestication initiatives [228,359]. This concept was also expanded through the Rural Resource Centres developed and promoted within the region as a pathway for upscaling multifunctional agriculture and promoting sustainable natural resource management in the Congo Basin [183]. In another example, the domestication of candidate species in Ghana was moved to village satellite nurseries and away from a central nursery to ease access to communities [154].
With regard to pests of indigenous fruit tree species, D. edulis is damaged by four psyllid species: Pseudophacopteron serrifer, P. tamessei, P. eastopi and P. pusillum. A recent study has determined the reproductive cycles of the latter two species, which lay eggs on different parts of their host [360].
5.11. Vegetative Propagation
The successful vegetative propagation through leafy and leafless stem cuttings as well as air layering or marcotting have been reported for Allanblackia spp. Cola spp. and Garcinia spp. and many other tree candidates for domestication in WCA [149,150,232,361,362,363,364]. For example, these studies suggest that Garcinia spp., initially considered as difficult to regenerate, could be vegetatively propagated using stem cuttings and grafting [31,363,364,365]. In Côte d’Ivoire, leafy greenwood and softwood stem cuttings of G. kola presented good ability to shooting and rooting in non-mist propagators while leafless hardwood stem cuttings were only able to shoot even without application of exogenous hormones [363,364]. In the Democratic Republic of Congo, leafy stem cuttings of C. acuminata with 25 cm2 leaves rooted well (81%) when treated with the auxin Indol-butyric acid [366]. In the difficult to propagate A. floribunda, like Cola anomala, rooting was significantly improved with an application of nitrogen-based fertilisers [150,362]. The leafy stem cutting production and subsequent rooting speed were significantly improved by appropriate light management for A. foribunda stockplants, confirming previous findings on the importance of the pre-severance light environment for successful stockplant management [151].
The rooting of cuttings from mature trees is generally recognized as being difficult, in contrast to that of juvenile cuttings. However, the good rooting of mature cuttings of G. kola and Annona muricata enabled them to be categorized as easy to root species, while Anonidum mannii, Gambeya albida and Irvingia gabonensis were rated moderately easy [367,368]. In contrast, Triplochiton scleroxylon, which is easy to propagate by juvenile cuttings [37], was difficult to propagate from mature cuttings [367]. The propagation of Ricinodendron heudelotii and Gnetum spp. through leafy stem cuttings was significantly improved during this third decade by pretreating cuttings with nutrient-based solutions enriched with exogenous auxins and/or honey [369,370,371]. It was observed that exogenous auxins and specifically Indole-butyric acid pretreatment can promote the synthesis and accumulation of phenolic compounds and enzymes needed for root differentiation [372].
The success of air layering/marcotting, which is used to capture the phenotype of a mature individual tree, was affected by the position, orientation and diameter of the marcotted branches and the marcotting season and rooting substrate, with orthotropic and upper branches of Cola acuminata, G. kola and D. edulis and lower pollarded stems of A. parviflora showing the best rooting [149,373,374]. On the other hand, marcotts of P. macrophylla and C. lepidota in the Nigerian savanna rooted well following an application of auxin when treated during the rainy season [375,376], while in Gabon, Santiria trimera marcots formed roots independent of the type of substrate used [377].
In recent work by the ICRAF, it was observed that G. kola could be successfully propagated using the side tongue, top cleft, side veneer and whip-and-tongue grafting methods, while the top cleft grafting method gave the highest percentage of success for Irvingia gabonensis, Cola nitida, Ricinodendron heudelotii and Monodora myristica [378]. The authors also demonstrated that cross-species rootstock compatibility was successful between I. gabonensis and I. wombulu. These results are important for guiding appropriate propagation approaches in the domestication processes of these priority species of the WCA.
With the above success in vegetative propagation, it should be easy to capture the huge tree-to-tree variation found in indigenous fruits and nut trees across their natural range and thus improve their production quantitatively and qualitatively on farms [12]. This does not, however, offset the need for seed-based regeneration, which is important to maintain and broaden the genetic base and adaptability of domesticated species. Moreover, comparative studies on the growth, flowering and fruiting of trees grown from vegetative and seed-based planting material have shown the fast growth, biomass accumulation and carbon sequestration benefits of D. edulis trees from stecklings and grafts as compared to seedlings [379,380], while A. floribunda trees from seedlings grew faster and had greater yields than those from vegetatively propagated materials [152]. Nevertheless, grafts and marcots have the huge advantage of retaining reproductive maturity and hence early flowering and fruiting [37].
Interestingly, CO2 sequestration has been found to be greater in plants derived from rooted cuttings and marcots than in seedlings, offering additional income-generating opportunities from Payments for Ecosystem Services to counter climate change [380].
5.12. Post-Harvest Issues
Most indigenous fruits are consumed fresh, and many have a short shelf-life, affecting product quality and marketability. For example, the ripe, oil-rich fresh fruits of D. edulis can only be stored for a few days before softening and rotting. This negatively affects transportability to the market, as well as the fruits’ organoleptic and nutritional attributes. To date, little has been done to find ways of increasing shelf-life. However, storage at 18 °C has been reported to preserve fruit texture [381,382]. Nuts generally have a longer shelf-life if kept dry. The shelf-life of bitter kola (G. kola) nuts in Nigeria has been extended from two to eight weeks by storage in sandy soils and jute bags [383].
Many priority species for domestication produce oily and edible kernels, the extraction of which is usually performed manually and characterized by being labour-intensive and time consuming. Efforts have been made to mechanise the kernel extraction from Ricinodendon heudelotii and I. gabonensis nuts. The mechanized kernel extractor for R. heudelotii has cut the time required from 61 min to 2 min, but the damage to the kernels is extensive [278]. Steps are being taken to improve this technology [384,385]. For I. gabonensis, a link between moisture content and nut cracking has improved the physical and mechanical factors influencing efficient and economical kernel extraction [386]. In a different case, a kernel/juice extraction machine for I. gabonensis had a kernel extraction efficiency of 66% without kernel damage and juice extraction efficiency of 69%. This juice and seed extractor is appropriate for use by low-income rural women [387], thus increasing women’s economic empowerment and reducing the socio-economic gender gaps in Africa. In Côte d’Ivoire, well processed Irvingia wombolu and unfermented seeds free of smell fetch higher prices off-season (145 CFA for 12 seeds) than they do at harvest time (110 CFA for 15 seeds) [220].
In addition, post-harvest product processing can be important, as it may affect not only the nutritional value but also market acceptability of NTFPs from indigenous food trees and hence their value and utilization of indigenous fruit trees. For example, a recipe for an instant dry soup mix powder of I. gabonensis, called “ogbono” in Nigeria, has been reported to increase acceptability while being easy to cook [388].
5.13. Enterprise Development
Non-timber forest products (NTFPs) are mostly considered as common property from forests and woodlands, nonetheless, often with legal restrictions on harvesting and commerce. However, there are complex issues around the practicality of compliance [201]. It has been proposed that when indigenous forest trees are cultivated on farms, they should be seen as privately owned crops and be recognized to produce Agroforestry Tree Products (AFTPs), hence freed from these legal restrictions [16,207]. This is important for both trade and value-adding enterprise development, both of which create the incentive for domestication to improve livelihoods and to ensure the sustainable use of natural resources. To date, food, pharmaceutical and cosmetic applications of many NTFPs are well documented, but there has been little enterprise development. Among the few reported cases, dried fruit, jams, juices and other drinks are being produced from several indigenous fruits in Nigeria [158], such as C. albidum, I. gabonensis and Spondias mombin. Simple procedures suitable for small-scale commercial production are being applied, including osmotic dehydration, open kettle jam-making process and mechanical juice extraction followed by hot water pasteurization. However, these processes are often constrained by inadequate electricity supply, inappropriate technology, poor capacity for production and food safety and lack of working capital [158,389]. Bannor, Ros-Tonen [390] have identified some positive influences of domestication on entrepreneurism, including high-quality and uniform raw products, market information, appropriate knowledge and personal commitment.
5.14. Upscaling and Capacity Building—The Role of Rural Resource Centres
In the humid lowlands of West and Central Africa, a community-based extension approach involving relay organisations and Rural Resource Centres has been at the forefront of technology extension and upscaling for three decades [19,20,21,391]. It has successfully promoted capacity building and is reaching increased numbers of farmers disseminating new knowledge and skills. These range from the identification and selection of candidate species, nursery development, vegetative propagation techniques and agroforestry-tailored skills to the needs of stakeholders at each level of the domestication process. Essougong et al. [32] reported that, on average, Rural Resource Centres organized 40 training sessions annually, reaching 1444 farmers in Cameroon. It was observed that a single Relay Organization was supporting on average 100–240 farmers to acquire essential domestication skills, such as the vegetative propagation techniques, as well as tree planting, management and harvesting techniques [223]. To enhance organisational and financial sustainability of this extension and capacity building approach, other activities include social networking between Centres as well as their private and institutional clients, development partners, local research teams and municipalities [34].
As a farmer-centred approach to extension, Rural Resource Centres have been identified as an excellent source of agricultural information [32]. The model has been awarded the distinction of being one of the top 20 innovations benefitting smallholder farmers [20]. For example, in 2011–2013, Rural Resource Centres in Cameroon, Nigeria and the Democratic Republic of Congo actively engaged over 5300 farming households. This resulted in more than 315 small-scale village nurseries producing around 6.3 million agroforestry trees—including selected putative cultivars of priority indigenous fruit and nut trees [19,20,392].
To build on this success and expand the scale of Rural Resource Centres both in the region and across Africa will require capacity building in the ‘grassroots’ relay/implementing organisations, fund raising and wider ownership of the approach to deliver their skills and services [19,20].
6. Sahel and North Africa
6.1. The State of Natural Resources and Their Governance and Management
The Sahel and North Africa region is characterised by extensive parklands, which may contain over 40 indigenous multifunctional tree species [117,128], with lower species richness at farm-field level than in the fallows [393]. The species composition and density vary depending on the agro-ecological zone (humid, sub-humid and semi-arid) and farmers’ preferences for their products and services [128]. Nevertheless, the dominant species include Vitellaria paradoxa, Parkia biglobosa, Adansonia digitata, Faidherbia albida, Balanites aegyptiaca, Acacias (Acacia raddiana and A. senegal) and Lannea microcarpa [117,128,393]. Farmers have been using these trees for millennia for food, fodder, medicine, income and regulatory ecosystem services—e.g., soil fertility improvement, water regulation and micro-climate buffering [128,394,395].
The governance and management of tree resources in the Sahel and North Africa is mostly influenced by land tenure, gender and ethnicity [396]. Tree resources belong to land owners such that land users are not usually allowed to plant or harvest them on rented land [128]. Furthermore, since women do not own land [128,396], they are disproportionally disadvantaged in the management of fruit trees, and their user rights are limited [396]. Women are only allowed to manage tree species of subsistence value such as shea nut (Vitellaria paradoxa), bread fruit (Artocarpus altilis) and oil bean tree (Pentaclethra macrophylla) [397]. However, men usually take over whenever “by products” of such tree species become valuable [396]. These practices emphasize the importance of recognizing social and cultural dynamics when upscaling agroforestry programmes and promoting extension services in the Sahel and North Africa. Ecological conditions also influence the governance and management of tree resources in this semi-arid region, where farmers typically integrate livestock within agroforestry practices more than their counterparts in sub-humid areas [398]. This variation probably reflects the farmers’ long-term experience with these ecological conditions of their respective areas and the perceived benefits from such practices.
At the field level, the management of trees generally varies depending on the species and desired outcomes. For instance, shrubs are repeatedly coppiced at the onset of the rainy season to prepare the fields for sowing, while fodder trees are pruned in the dry season to provide livestock fodder during the lean season. Old fruit trees such as P. biglobosa and V. paradoxa are typically pruned to rejuvenate their fruit production [393]. However, A. digitata, L. microcarpa and V. paradoxa are usually pruned annually for fruit or leaf harvesting [120,393]. Additionally, agroforestry trees are pruned to reduce the shading of associated field crops and/or to increase the yield of cereal crops [128]. These traditional management systems provide springboards for improved agroforestry programmes in the region.
Despite the importance of this long history and its associated good management practices, the rich forest biodiversity in the region is threatened by changes in land use and climate change. The two factors are implicated in habitat fragmentation and/or the contraction of suitable habitat of indigenous trees such as tamarind (Tamarindus indica) [160], Detarium microcarpum [399], V. paradoxa [124], and P. biglobosa [400]. This is threatening millions of livelihoods that depend on these natural resources. This is one of the many reasons the areas highlighted in the third decade (2012–2021) are important for the initiation and upscaling of agroforestry tree programmes to ensure sustained and improved livelihoods in the region.
6.2. Ethnobotany of Food and Medicinal Species Candidates for Cultivation
In Burkina Faso, baobab is known to be harvested for 25 uses, mostly centred on the nutritional value of its fruit [45], while, in Benin, Moringa oleifera is valued as a leafy food and fodder and as a medicine [401]. The nuts, bark and leaves of Carapa procera, however, are highly valued for cosmetic and therapeutic purposes in Mali [402]. On the other hand, Garcinia kola is one of popular plant species for treating male infertility in Benin [403].
Local knowledge and preferences are especially important when identifying ethnovarieties and plant traits of importance when engaging with farmers to domesticate wild trees [129,404]. In Benin, for example, large fruits with many good quality seeds are preferred in P. biglobosa [404]. These preferred traits within use categories are crucial when ranking farmers’ preferences to guide species selection [118,119,405]. Accordingly, in eastern Burkina Faso, A. digitata, Bombax costatum, P. biglobosa and V. paradoxa have been identified as the most important out of 25 species for health-related uses [119], although more generally, Khaya senegalensis, A. digitata, Diospyros mespiliformis and T. indica were ranked highly in a nationwide assessment of 1033 plants for traditional medicine, human nutrition and animal fodder [118].
On the other hand, C. procera has been identified as the most preferred oil species by women in Burkina Faso [405], while, in the Sudanian zone of Benin, the fruits, leaves and bark of Strychnos spinosa were highly valued for 20 different uses, including food and medicine [406], varying by climate zone, ethnic group and gender. Likewise, 38 indigenous edible fruit species including 25 trees and 13 shrubs have been identified in the Sahelian areas of Cameroon [157]. The species are used in various ways, such as salads or cooked sauce from the leaves of Ficus spp., Vitex spp., Ziziphus spp. and Lannea spp.; the pulp of baobab and Ziziphus mauritiana is used for preparing refreshing juice and biscuits; while the seed of Balanites aegyptiaca may be boiled and eaten like peanuts or used for extracting oil [157]. Likewise, the income-generating food and medicinal products of Xylopia aethiopica trees are greatly appreciated in southern Benin, as in many other parts of Africa, often leading to over-exploitation. This raises the need to sensitize local people to the opportunities for domestication and species conservation [249]. Taken together, these examples illustrate the crucial role of ethnobotanical studies in agroforestry programmes by helping to identify priority species for specific local needs and knowledge gaps for conservation and sustainable use of such resources. Indeed, demand-driven research that considers the role of traditional ecological knowledge in light of the changing socio-economic and environmental conditions may contribute to the formulation and implementation of viable policy-relevant strategies [407].
6.3. Commercial Value of Non-Timber Forest Products in Local Markets
Non-timber forest products (NTFPs) from trees are very important in the economies of rural households in the Sahel and North Africa [128,264,408] and to provide commercial incentives for farmers to adopt agroforestry trees [409]. For instance, shea products have a strong economic potential, where a collector may earn about USD 300.00 annually from sales of edible shea caterpillars (Cirina butyrospermii) in Burkina Faso, Mali and Togo, while in Burkina Faso, shea butter contributes to nearly 90% of total fat and oil consumed in rural households and 25% in urban households [128], indicating a strong commercial value in local markets for these products. Through a local market value chain analysis of Carapa procera in Mali, Dembélé et al. [410] have shown that poverty levels of traders may improve by an average of 63% as they can earn an annual income of USD 221.53 against a poverty threshold of USD 350. In Benin, the African fan palm (Borassus aethiopium) has potential to uplift the welfare of women who are particularly specialized in hypocotyl and fruit collection for commercial use as food [411]. Moreover, baobab leaves are one of the commercially most important leafy vegetables in West Africa [43].
Other studies have reported on the commercial importance of NTFPs for their industrial or cosmetic potential uses. Notably, an ethanolic extract of M. oleifera leaves can be used to extend the shelf-life of cream cheese [412].
6.4. Priority Setting for Domestication/Commercialization
To date, priority setting for the domestication of indigenous fruit trees in the region has been done mostly on three fronts: (i) climate change and habitat suitability, e.g., [124,160,400,413]; (ii) farmers’ preferences and prioritisation (e.g., [74,118,129,211,285,286,405,406,414,415]); and (iii) combined literature review and prioritization schemes [416].
For instance, black plum (Vitex doniana) is a highly valued multipurpose tree in Benin. Currently, the species can potentially be cultivated in about 85% of the country, but this area is expected to increase by 3–12% under future climatic conditions [413]. In contrast, nearly 51% of Burkina Faso is presently suitable for the cultivation of V. paradoxa, and that the area may decline by 13% by 2070 [124]. On the other hand, Detarium microcarpum is currently cultivated in only two ecological zones (Sudanian and Sudano-Guinean) of the three zones in Benin [399].
Prioritising tree species preferred by farmers is considered to be instrumental for the success of domestication programmes [417], since it empowers farmers to simultaneously improve the production of both their crops and woody multipurpose species, as for example with baobab in Niger [74]. In Sierra Leone, Heritiera utilis, Garcinia kola and Beilschmiedia mannii were highly appreciated by the local farmers through a rigorous national re-prioritisation process that employed a combination of focus-group data, field observations, a market survey and a ranking exercise [286]. In Benin, P. biglobosa fruits containing many large, good quality seeds were selected by farmers for domestication [404], while in Burkina Faso, T. indica, V. paradoxa and A. digitata have been ranked as the most preferred medicinal plants [118], although women selected C. procera for its oil [405]. This may be indicative of how socio-economic factors including gender may influence the choices and decisions of farmers with regard to agroforestry tree cultivation and domestication, which ought to be considered in agroforestry programmes. Moreover, Moringa oleifera appears to be a promising tree for domestication in Benin with an adoption rate of up to 97% in the southern part of the country, where row planting of species was found to be preferred [414].
Lastly, 10 top-priority indigenous fruit trees have been earmarked for cultivation and commercialization in Benin for their economic potential [416]. These are Vitellaria paradoxa, Parkia biglobosa, Adansonia digitata, Irvingia gabonensis, Blighia sapida, Tamarindus indica, Dalium guineense, Vitex doniana, Borassus aethiopum and Garcinia kola.
In the deciduous forest area of Ghana and the Guineo-Congolian zone of Benin, the small tree/shrub Synsepalum dulcificum (miracle berry) has been accorded high priority for with regional potential for plant breeding and commercialization [355].
6.5. Characterization of Useful Traits
As reported for baobab in the Pan Africa section, the quantification of useful traits, including the nutrient content of fruits and leaves of trees is one of the most important steps in identifying superior planting material for domestication [53]. The influence of environmental factors (e.g., soil and climate) on nutritional traits and fruit morphology has been widely reported for indigenous fruits in dry Africa [54,161,417,418]. For example, significant morphological variation has been reported for Annona senegalensis between Ekiti and Osun States in northern Nigeria [417], as well as in Benin [418], where about 42% of the phenotypic variability between climate zones has been attributed to differences in mean annual rainfall and the mean temperature of the warmest quarter [418].
In Côte d’Ivoire, elite shea trees with strong trunks, small leaves and a high number of fruits per tree have been identified as favourable grafting stock [131]. Variation in phenological traits have also been reported for Sclerocarya birrea in Senegal, including marked differences in leafing and flowering within the species [100].
In Benin, S. spinosa has been characterised for trunk (dbh) and fruit traits (fruit mass, pulp mass, seed number, ratio) across seven populations in two climatic zones (Sudano-Guinean vs. Sudanian zones) [310]. High significant variations among populations in the traits studied were found, with a within-population variation accounting for 53–90% of the total variation, suggesting a high potential for the selection for domestication [310]. The study found tree populations from Bassila (Sudano-Guinean Zone) as having useful traits for domestication. Similarly, morphotypes 1 and 2 of B. aethiopum showed high performance for fruit (fruit length, dry weight and shape index) and seed traits (number of seeds), and these might be good candidates for selection and breeding programmes in Benin [419]. So, too, have high values for fruit, seed and leaflet traits of Afzelia africana from the humid areas of Benin shown best performance under optimal climatic and anthropogenic disturbance conditions [420].
While these few studies provide an insight into the depth of the trait variations important for elite tree selection and domestication, they also underscore the importance of considering local environmental factors for agroforestry programmes in different sites [161].
6.6. Assessment of Genetic Variation and Its Characterization
Genetic variability assessment is one of the fundamental steps towards domestication of wild tree species, since it would help strengthen breeding and conservation programmes [421]. This is crucial for domestication and sustainable management, particularly in the context of rapid environmental changes [400]. Notable examples in the third decade include Prosopis africana [422], A. spinosa [423] and P. biglobosa [400,421].
6.6.1. Provenance Studies
Weber, Montes [422] analysed growth from a nursery test of 28 provenances and 275 progenies nursery test of Prosopis africana at 8 months relative to field performance at 11 and 13 years to investigate clines in the growth and survival of Prosopis africana from sites in Burkina Faso and Niger across the Sahelian and Sudanian ecozones. The study found that the root/shoot ratio had the highest heritability of growth variables in the nursery and that larger root/shoot ratios equated with better field growth and survival in dry locations [422]. The authors have suggested that investment in underground tissues indicates mean annual rainfall is a good predictor of field performance under dry conditions. Thus, Prosopis fricana provenances from the drier locations have been recommended for agroforestry projects in rural communities [422].
In Morocco, the morphological diversity between 122 argan tress (A. spinosa) has been characterised using 30 quantitative traits over a period of three years in an in situ preserved population [423]. Interestingly, vigour traits (leaf and shoot sizes) were positively correlated with fruit traits, while broad-sense heritability estimates were high for clustered leaf traits, thorn numbers and for fruit, stone and kernel traits. Thus, with regard to the selection of elite trees for breeding programmes, genotypes have been grouped into five relatively homogenous clusters, with a focus on traits related to fruit, stone, kernel morphologies and leaf size, shoot length and glomeruli density [423].
In Senegalia senegal, provenance studies investigating growth and gum arabic yield found genotype-specific variation, suggesting a need to investigate the effect of soil pH, the physical and chemical properties of soils and management to clarify traits underlying drought tolerance and gum yield [424].
6.6.2. Molecular Studies
To enhance selection towards conservation, breeding and sustainable utilization of P. biglobosa in Nigeria, [421] assessed 16 accessions for their variability based on DNA profiling and seed protein. Interestingly, a genetically distinct accession with a unique seed protein marker has been selected for potential exploitation in breeding programmes. Due to the narrow genetic base of the accessed accessions, the authors have recommended the intensification of germplasm screening across the different agroecological zones of Nigeria and their characterization using specific markers [421].
Land-use change is one of the major threats to biodiversity and genetic variation in agro-ecosystems undermining the capacity of ecosystems to provide goods and ecosystem services [400,425]. It can also lead to habitat fragmentation [400]. In Burkina Faso, a study was conducted on the influence of habitat fragmentation on patterns of genetic diversity and gene dispersal of P. biglobosa in 2475 genotypes from 4 mature tree populations using 10 highly polymorphic nuclear microsatellites [400]. The study found high genetic diversity across the four populations—with similar genetic diversity and inbreeding between adults with a low level of selfing. This result seems to concur with other studies, indicating long-distance pollen transfer between P. biglobosa populations, facilitated mainly by bats and honey bees [426]. Notably, stem diameter had a pronounced effect on male reproductive success in the three populations, with the highest male reproductive success recorded in trees with a dbh of 60–75 cm [400]. This implies that fragmentation under the current conditions does not pose serious limitations to gene flow in P. biglobosa [400]. Nevertheless, it is important to safeguard high plant diversity and pollination services for posterity [137].
6.7. Nutritional Value of Non-Timber Forest Products
Nutritionally, a recent review of the carbohydrates, proteins, fats, fibres, ash and dry matter of 98 indigenous food tree species from sub-Saharan Africa has provided a valuable overview of the potential to domesticate these species for their nutritional benefits in the region [427]. The candidate species include A. digitata, Afzelia africana, B. aegyptiaca, D. microcarpum, S. birrea, T. indica and V. paradoxa. Fruits, seeds and leaves differ in their fibre and carbohydrate contents, but generally, seeds have comparably high contents of fat, protein and dry matter, while leaves are good sources of protein and ash. Nonetheless, indigenous food trees are good sources of carbohydrates, proteins, fats, ash and dry matter [427], potentially important for the alleviation of food and nutrition insecurity facing the region and for the attainment of Goal 2 (End hunger and improve nutrition) of the UN Sustainable Development Goals and the 2063 African Agenda.
6.8. Medicinal of Non-Timber Forest Products
In addition to food and nutritional security, the importance of non-timber forest products in the region cannot be overemphasized as they contribute to health and income generation. Despite the wide appreciation of the medicinal and food products of many tree species in dry Africa, such as Parkia biglobosa [428], there is lack of knowledge about their use to reduce poverty and improve nutrition [427]. One explanation for this could be the lack of data to inform decision makers at different levels. To address this, attempts have been made to assess and quantify medicinal values of a number of indigenous fruit trees, especially in humid West Africa and in southern Africa, as reported in other sections of this review. In the Sahel and North Africa, studies have examined the presence and amounts of different phytochemicals (notably alkaloids, tannin, phenol and flavonoids) in the roots, stems and leaves of Sclerocarya birrea and Sterculia setigera in northern Nigeria [114]. Apart from the diversity of phytochemicals found in the two species, the study also found that the quantitative variation in this phytochemical composition varied between sites (Kem and Yola, both in the Adamawa State), probably due to differences in environmental conditions in the States [114]. These results indicate the medicinal potential of these species.
Studies on the numerous pharmacological properties of Strychnos spinosa (e.g., antimicrobial, antiplasmodial, anti-trypanosomal, anti-leishmanial, anti-nematicidal, anti-inflammatory, anti-oxidant and antidiabetic) have indicated a need for more clinical trials to assess the effects of its bioactive components [429].
In Nigeria, Ewere et al. [340] have shown that I. gabonensis may be used to treat inflammation and haematological abnormalities following revelations of the protective effect of ethanol stem bark extracts of the species against sodium-arsenite-induced inflammation and haematological derangements in Wistar rats, while extracts of Annona muricata could become an alternative option for treating haemorrhagic anaemia [430]. Similarly, the Ethiopian pepper (Xylopia aethiopica) has shown to possess potential pharmacological benefits following a literature study [431].
6.9. Selection of Elite Trees and Ideotypes
The selection of the right tree species is crucial for the success of agroforestry programmes to deliver the expected potential environmental, social and economic benefits [14,15,289,425]. Like prioritisation, it ensures that conservation efforts including resources are focussed on the species and candidate trees with the greatest potential to meet the needs of farmers through the propagation of elite individual trees.
In Morocco, 12 promising genotypes of A. spinosa have been selected from a group of 52 candidate genotypes based on fruit density and kernel yield to address the specific environmental conditions in the country [432]. Although it is not known how well the selected elite trees and ideotypes will perform under a wider set of field conditions, the study provides useful information for consideration under different conditions prior to the commencement of full scale agroforestry programmes [433].
6.10. Nursery Developments and Improved Planting Stock
Despite the alternative of vegetative propagation, insufficient, low quality and inefficient seed supply system are challenges to smallholder tree-planting farmers, posing a serious constraint to the adoption of agroforestry systems based on indigenous species [425]. However, in baobab, through the combination of provenance studies [64] and field trials with poultry and cow manure [434], good field performance has been achieved.
6.11. Vegetative Propagation
Vegetative propagation is used to capture elite phenotypes as putative cultivars and has been evaluated in a number of important tree species in the region, including Prosopis africana [433], Argania spinosa [435,436], B. aegyptiaca [437,438], A. digitata [76] and V. paradoxa [136]. For instance, in Niger, Prosopis africana has been found to be amenable to air layering with no significant differences between the diameter classes and between the positions, although the success rate was low (28% after 4 months) [439]. Air layering has also been successful in B. aegyptiaca in Niger—93% after 2 months, with 83% survival [437]—while in Morocco, A. spinosa has been successfully propagated vegetatively by leafy semi-hardwood stem cuttings in studies examining the effects of nutrient solutions, auxins, cutting position and genotypes [435,436]. Basal cuttings treated with Hoagland solution had the best rooting (64%) with the most roots (45), allowing the selected elite trees to be successfully propagated. Similarly, terminal slit grafting has been recommended as the best method for shea trees in nurseries for use by farmers in Côte d’Ivoire based on 2-year old rootstocks that showed promising results such as high recovery rate (90%) and high mean values of growth traits [136].
Together with results in baobab and shea, these studies indicate the potential to develop putative cultivars of indigenous tree species in the Region, opening the opportunity to use participatory approaches to domesticate and upscale the availability of elite trees [19,20,425], with a shorter period to fruit production [102]. This would reduce the investment costs that constrain farmer adoption of agroforestry technologies with indigenous trees [241].
6.12. Post-Harvest Issues
Characteristically almost all work on smallholdings is done manually and is thus labour demanding and tedious [140]; this limits the potential outputs and economic benefits from agroforestry in the region [409]. It is therefore important to address post-harvest storage, drying, shelling, and pulp and seed extraction so as to reduce wastage and ultimately maximise benefits, especially in nut producing species like Vitellaria paradoxa [139]. Similarly, the post-harvest processing of young shoots and leaves of Moringa oleifera and M. peregrine by drying them under partial shade, as opposed to direct sun, results in higher amino acid and mineral contents [440].
6.13. Upscaling and Capacity Building
There are a multitude of challenges and barriers to agroforestry adoption which affect upscaling of agroforestry systems in the Sahel and North Africa. These include institutional, cultural, financial, political, technical and knowledge-based issues [425,441]. The recent re-publication of a tree domestication strategy [35] addresses these issues, providing an opportunity for upscaling and capacity building of agroforestry. The strategy also presents principles for the establishment of three-interlinked tree populations: gene resource population, selection resource population, and production resource population to meet the production, social, economic and environmental intensification needs of agroforestry.
The challenge now, however, is to scale up the application of tree domestication within agroforestry for meaningful social, economic and environmental impact at different scales (national, regional, global) [35]. The key to adoption partly lies in addressing the site-specific needs which are driven by different preferences and aspirations of local communities. Indeed, membership to a tree farmer group, awareness of farmers about socio-ecological benefits of agroforestry, and effective provision of extension services positively affect adoption [409,442,443]. Accordingly, success involves meeting farmers’ choices, with appropriate technological designs and implementation [409] through participatory approaches which form the core of the tree domestication strategy [35]. In this regard, it is widely recognised that farmer institutions can play a crucial role in upscaling and capacity building of agroforestry programmes [409,442,443]. For example, by drawing data from 1080 rural households in Burkina Faso, Mali, Niger and Senegal, Binam, Place [442] found that farmers belonging to well-structured formal and informal institutions demonstrated a better collaborative attitude towards tree regeneration on-farm and developed plans for good management and protection of natural resources.
Given that awareness of the benefits of agroforestry and technical support are some of the positive determinants of farmers’ adoption of agroforestry [409,443], farmer institutions such as Rural Resource Centres [19,20,444] that have been comprehensively discussed earlier in this review will be essential for the design and effective implementation of the tree domestication strategy towards upscaling and capacity building. Among other things, farmer institutions may serve as platforms for farmer-to-farmer exchange of knowledge, skills and benefits of agroforestry. They may also serve as entry points for introduction of new agroforestry technologies and associated technical support. It is also recognised that addressing key barriers to both the supply and demand of tropical tree foods [441] will make agroforestry have more meaningful impact. To achieve this, and to make the tree domestication strategy more dynamic, more research on producers’ and consumers’ behaviour and preferences ought to be considered [27].
7. Highlands and Drylands of East Africa
7.1. State of Natural Resources and Their Governance and Management
The highlands and drylands of Kenya, Uganda, Tanzania, Burundi, Ethiopia, Eritrea, Sudan, Somalia and Djibouti have a broad agroforestry base where farmers and local communities recognize trees as important components of a sustainable and resilient livelihood system. For example, the farming and adjacent forest landscape of the Ethiopian Highlands near to Mt. Duro (Oromia Region) is rich in useful species diversity (43 genera, 27 families) with predominance of the Fabaceae family [445]. The increasing population pressure resulting from encroaching farmland is, however, reducing species diversity of the natural forest emphasizing the need to train farmers in technologies that both conserve the forest biodiversity and increase farm productivity [445]. Nearby, in Basona Worena District the predominant tree species are eucalypts for timber, Olea africana for fodder and fuelwood, Croton macrostachyus for shade and erosion control in coffee plantations and nitrogen-fixing species like Sesbania sesban, Cupressus lusitanica, and Acacia abyssinica [446]. These trees are appreciated for restoration activities due to their hardiness, multiple uses, and positive impacts on soil fertility, landscape, and livelihoods. By contrast, in the highlands of Uganda, the agroforestry activities of local communities have a stronger focus on tree planting near homesteads [447]. This study indicated that assurance of economic returns incentivized community nurturing of natural regeneration of trees, emphasizing that economic benefits to different user groups are of paramount importance for the promotion of agroforestry practices.
Apart from traditional uses such as fuelwood, timber, fodder, and fruits, the drylands of East Africa are bestowed with some species producing products such as gum arabic, a resin produced from trees or shrubs of Acacia senegal and Acacia seyal particularly in South Sudan, Eritrea, Somalia, and northern Kenya. It is gathered by pastoralists in the wild or traditionally cultivated in gum gardens [448]. Other important gums include frankincense and myrrh, obtained in the dry savannahs of Ethiopia, Eritrea, Dijbouti and Somalia from various species of the genera Boswellia and Commiphora.
In this dryland environment, the thorny Balanites aegyptiaca or desert date, has been shown to be both a good intercrop with sorghum and as a candidate for dry land restoration in northern Ethiopia. In the semi-arid drylands of Southeastern Ethiopia, Tamarindus indica based agroforestry was studied for fruit yield predictability and it was found that on-farm trees gave higher yields than those in bush land [449]. Dobera glabra, was also found to be a popular fruit tree species in this region. Meanwhile, in the semi-arid region of East Shewa the richness of tree species was relatively low as expected for a dryland area. However, tree species abundance and composition were similar between the croplands and homesteads with a native:exotic ratio of 75:25%. The retention of local species in the studied area was indicative of prevalence of farmer managed natural regeneration and led to a positive correlation with the number of trees, but not with species richness due to the planting of fewer tree species on larger holdings [450]. The authors, nevertheless, suggested that tree planting and managed natural regeneration are key to enhanced species richness and tree cover. Other factors influencing the choice of agroforestry species in southern Ethiopia are the ease of road access and farm size. Both had a negative effect on native tree populations due to their replacement by exotic fast-growing trees like Eucalyptus and other non-indigenous annual cash crops. Abebe et al. [451] found a high richness for indigenous species in the home gardens of Sidama zone in Southern Ethiopia with prominence of Coffea arabica-based home gardening system. However, commercial cash-crop oriented home gardening system (enset-coffee-maize-chat type and enset-coffee-maize-chat-Pineapple type) had a lesser tree density than the subsistence one (enset-coffee-maize-sweet potato type and enset-coffee-maize type). Thus, commercialization of home gardens threatens the species richness and diversity in the area, compromising the long-term sustainability of the landscape [451].
A critical review of wild edible species of Ethiopia found a lack of scientifically based information about a key range of genetic variables important for tree domestication which has resulted in a failure to capture their full potential in terms of quality, production, and marketing [452].
In the Sudanese savannas characterized by low rainfall and semidesert landscape, agroforestry practices have been reported to enhance farmer income generation from non-timber forest products. This has a high potential for poverty alleviation influenced by: (i) the biological product; (ii) trade and marketing networks, and (iii) the level of labour and capital inputs [50]. However, the authors urged caution with regard to the generalization of their findings due to regional differences.
7.2. Ethnobotany of Food and Medicinal Species for Cultivation
In the highlands and drylands trees produce a very wide range of useful food and non-food products. The most commonly used food products are fruit, followed by leaves and then occasionally roots, with many species having multiple edible parts (Adansonia digitata, Balanites aegyptiaca, Grewia spp. etc.). In southern Ethiopia, Cordia africana, Millettia ferruginea, Erythrina brucei and Olea capensis are the major indigenous multipurpose tree species used in agroforestry systems, while in the north of the country, Croton macrostachyus, Vernonia amygdalina, Faidherbia albida, Acacia nilotica, Acacia seyal and Grewia bicolour are common. Whereas, in the central highlands, Albizia gummifera, Cordia africana, Croton macrostachyus, Ficus vasta and Vernonia amygdalina are popular [453] with potential for greater utilization [454].
Local communities have good knowledge about the uses of these indigenous non-timber forest products which support livelihoods and nutrition of small holders in dryland with region-specific preferences. Importance of indigenous fruit trees for food and nutritional security and the livelihoods of local populations across Africa have been amply justified by many studies [455]. In the arid lowlands of Ethiopia, 52 wild edible fruits are consumed, notably those from Celtis africana, Ziziphus spina-christi, Balanites aegyptiaca and Tamarindus indica prioritized for conservation and domestication; as well as Opuntia ficus-indica, Carissa edulis and Ximenia americana in the Amhara Region and Syzygium guineense and Carissa edulis in the Oromia Region [456]. Likewise, in the Nuba Region of Sudan, the products of 14 species including Adansonia digitata, Ziziphus spina-christi and Balanites aegyptiaca contribute to the livelihoods of fruit collectors and traders [49]. However, their financial returns are constrained by a lack of market organization government taxation policies and road connectivity. An ethnobotanical survey of 300 households in this area subsequently indicated the local importance of cultural practices associated with the harvesting and widespread use of tree products vis à vis their conservation and sustainable use for future generations [457].
In addition to food, tree products have important medicinal uses. For example, in Burundi, Tanzania, and Uganda, >80% of the population use traditional medicines particularly in the rural areas [458,459], while in the Mabira Central Forest Reserve of Uganda a total of 190 species with 68 perennial trees from 152 genera in 61 families are recognized for their medicinal importance [460] Ninety four percent of these species were from forests. Likewise in the North Shewa Zone of Oromia Region of Ethiopia 73 medicinal plants of which 14 were trees, came from 42 families [459]. A major concern identified by these studies is the preservation and transmission of this knowledge to future generations and the need for the documentation of traditional knowledge about their cultural uses and processing before it is lost.
7.3. Commercial Value of Non-Timber Forest Products in Local Markets
The East African drylands, especially in the Horn of Africa, are also well known for their commercially-important tree gums and resins, and to a lesser extent their use as fodder and for craft wood. Here local communities are especially engaged in harvesting resins from Boswellia spp. and Commiphora spp. for their pharmaceutical and industrial applications [461]. Despite the industrial value of these species, the management practices, harvesting and market value chains are quite primitive and underdeveloped and there are concerns for protection against overgrazing, and the need for assisted natural regeneration, training and congenial government policies.
As most of these dry zone tree species are insect pollinated, beekeeping is also a major activity in the region and Ethiopia, Kenya and Tanzania are major producers and exporters of honey and beeswax [448].
7.4. Priority Setting for Domestication/Commercialization
Many of the important indigenous trees of East Africa with potential for domestication as new crops are also found in other regions of Africa and progress with them in the 3rd decade has been reported above in the Pan Africa section.
Prioritization is needed to maximise returns on investment without compromising the useful biodiversity on farming landscape and was implemented in earlier decades of this domestication initiative [16]. Recent updates for Uganda have added Ficus natalensis, Markhamia lutea, and Albizia coriaria [447] while Tamarindus indica, Vitex doniana, Vitex mombassae and Sclerocarya birrea have been listed on abundance and utilization as community favourites for the drylands of Kenya, Uganda, and Tanzania [105].
In addition, the African Orphan Crops Consortium (http://africanorphancrops.org/meet-the-crops/, accessed on 1 January 2022) has listed 53 trees among its 101 African underutilized crops for genetic characterization [24].
7.5. Characterization of Trees
Characterization of trees for different traits, phenology, and nutrients is a critical step for the capture and improvement of their useful genetic diversity. This directly helps in the selection of domestication traits for greater marketability, enhanced consumption, nutritional value and for greater ease of management and maintenance in the field [30]. In the semi-arid drylands of Ethiopia, Tamarindus indica is a species maintained for its commercial value on farm as well as in bushlands. In general trees on farm were found to be larger and to produce more fruits, but the trees were less numerous [449] indicating active management by the farmers. This was also very evident from positive correlation between total yield and production related traits (e.g., dbh), as well as better yield predictability.
In addition to foods, another trait dependent economic activity that can support livelihoods of local communities is wood carving. Wood density and colour are important characteristics of species suitable for carving purposes. Based on these traits, Dalbergia melanoxylon (ebony), Combretum schumanii (Mkongolo), Cordia sinensis and Olea africana (African olive) and Brachylaena huillens are the slow growing species preferred by wood carvers in Kenya [462]. Like B. huillensis and D. melanoxylonare the continued existence of many of these slow growing species is under threat thus placing the people’s livelihoods in jeopardy. Consequently, a study has examined the potential of other species and found Azadrachta indica (mkilifi), Terminalia prunoides (mutoo), Afzelia quazensis (mbambakofi), Brachystegia spiciformis (mrithi) and Manilkara sansibarika (mgambo) to be alternatives for the carving industry.
7.6. Assessment of Genetic Variation
DNA Markers can now be used to characterize genetic variation, however to date, many of the indigenous trees of East Africa have not yet been characterized in this way [24]. Studies in A. digitata [67,68,72] have been reported in the Pan Africa section of this review.
7.7. Nutritional Value of Non-Timber Forest Products
Agroforestry can provide healthy, local, and affordable solutions for increasing vegetable and fruit uptake in a sustainable manner. Apart from energy they provide micronutrients to counteract the major micronutrient deficiencies in vitamin A, iron, and zinc prevalent in sub-Saharan Africa as indicated by 91% of the world’s Hidden Hunger Index affected pre-school children being in Africa [463]. Indigenous fruits like Adansonia digitata, Sclerocarya birrea and Grewia tenax are rich in vitamin C, vitamin A and iron respectively [455]. Thus, a child eating 10 g of A. digitata pulp would get 100% of its vitamin C requirement. Likewise, the consumption of 40–100 g of G. tenax berries meets nearly 100% per cent of the daily iron requirement of a child less than eight years old.
In the Lake Victoria basin of eastern Uganda, fruits of Saba comorensis are rich in β-carotene, vitamins C, and minerals like potassium, magnesium, and calcium. Being rich in vitamin C, zinc, and iron, it has a potential to address the issue of stunted growth and hidden hunger [464]. Due to climate change and unpredictable crop yields, this species is enhancing livelihoods and the food and nutritional security of local communities.
The African Orphan Crops Consortium has published a synopsis of the nutritional contents of some of its candidate trees and crops [24].
7.8. Medicinal Value of Non-Timber Forest Products
Many east African dryland tree species have medicinal properties [17]. A study of locally-consumed wild plants from Nakisunga sub-county, Uganda, many of which were herbaceous, found them to be worthy of further scientific study of their nutrient quality and conservation measures devised for their sustainable production with regard to their efficacy to relieve the effects of HIV-AIDS [465]. A woody vine, Telfairia pedata, producing oyster nuts with health and nutritional benefits is also recognized as having potential for domestication in Tanzania [466].
7.9. Nursery Development and Improved Planting Stock
The development of procedures and methods for production, procurement, processing, distribution, of seeds and the successful planting of seedlings and clonal propagules (cuttings, grafts, etc.) is fundamental to tree domestication. Key biological traits and management practices include understanding germination behavior, rooting and shooting behavior, scion and root stock compatibility, responsiveness to management and associated cultural practices.
Initial efforts to domesticate and popularize Markhamia lutea an indigenous tree species in the Kenya Highlands indicated its great potential in agroforestry for erosion control, shade, shelter, soil amelioration and as a source of fuel and easy to work but durable wood [467]. Seeds collected from Kakamega Forest had 90–100% germination grew better than those from Siyaya and Teso. The demand for seeds of this species from the Kenyan Forest Seed Centre has been very high. Early growth has shown some provenance variation, but nursery management is equally important.
Regeneration studies with Allanblackia stuhlmannii, an increasingly important fruit tree (see Pan Africa section) from the Usambara Mountains in Tanzania has indicated that, despite its reputation as being difficult to germinate, overall germination was around 75–80% [468]. Early seedling growth also showed good survival.
7.10. Vegetative Propagation
Propagation poses a major challenge for successful domestication and adoption of many tropical tree species. Like most long-lived trees the endangered medicinal tree species of the African Highlands, Prunus africana, has very long regeneration cycle as well as recalcitrant seed germination. The species is overexploited due to its pharmaceutical value and needs an immediate attention for upscaling production by on-farm adoption. While stem cuttings are easily rooted in village nurseries [171], in-vitro culture methods have been developed for mass production under centralized laboratory conditions [172].
7.11. Trade and Law
One of the major factors affecting sustainable utilization of forest produce by local communities is the policy and law governing access and use of forests and natural resources. Although wild edible plants do have an important role to play in resilience of dependent communities, a lot needs to be achieved regarding the governance and laws for balancing the exploitation and replenishment of forests. While many farmers in East Africa cultivate and harvest tree products there seems to be a lack of clarity about the law at least in the arid and semi-arid Tharaka County in Kenya, where foresters who are government appointed custodians of natural forests were unclear about the laws dealing with access and exploitation of forest produce [469]. Similarly, some of the farmers were also unclear about rules, fees and process of obtaining licenses, possibly associated with unscrupulous practices such as corruption and bribes. This uncertainty poses constraints to the equitable access of local communities to the natural resources and the development of value-added products. As in other parts of Africa, the recognition of agroforestry trees and products as farm crops may minimize this problem [207].
In Kenya, the trees-on-farms legislation under Farm Forestry Rules of 2009, aim to promote at least 10% forest cover on agricultural landholdings to combat climate change. Despite poor implementation of this law, Chisika et al. [470] found relatively good tree cover on farms, but they attributed this mainly to other legal instruments affecting farm forestry.
7.12. Local Impacts of Cultivation and Marketing of Tree Products
Local home gardens are known to be rich in species diversity and are important for on-farm conservation of plant genetic resources. Such complex and dynamic agro-ecosystems have potential to reduce crop failures, are resource efficient, provide ecological services, and support livelihood by providing nutrition and income generation. All of these make home gardens climate resilient systems of crop production.
In the semi-arid zone of Sudan, ‘Jubraka’ is a farming system in which home gardens are important source of food during periods hunger and food scarcity, especially before onset of rainy season. A study of 61 Jubrakas in four villages of the Nuba Mountains, South-Kordofan Province, Sudan, identified a total of 110 species from 35 plant families of which perennials accounted for 57%, including 12 indigenous fruit trees [69]. Generally, it was found that commercialization actually increased the species richness of these home gardens.
Another study in Sudan evaluated the suitability of manufacturing juice and candy from the highly nutritious fruits of A. garckeana. The results indicated that the products had high sensory and energy benefits [471].
8. Savannah and Miombo Woodlands of Southern Africa
8.1. The State of Natural Resources and Their Governance and Management
The Miombo Woodlands contain about 75 indigenous fruit trees, which have provided highly nutritious edible fruits for hunting-gathering communities [16,472] long before settled agriculture [473]. They have supplemented the diet of rural families by providing essential micronutrients, antioxidants, polyphenols, and health benefits, especially for mothers and children. They also serve as an alternative for income (cash and barter), especially during times of food scarcity and economic hardship [16,78,473,474,475,476,477], as such they play an important role in reducing the vulnerability of rural households to hunger and poverty in southern Africa [472]. In Zimbabwe, indigenous fruit trees can reduce this vulnerability by 33% during critical periods in the cropping season [473], also providing a coping strategy for farmers in Zambia and Malawi during periods of drought.
Against this background, tree planting in the Southern African region has historically involved developing commercial timber plantations of Eucalyptus, Pinus and Acacia species [478], or exotic fruits [479]. However, recently, tree planting initiatives in the region have been expanded to include the planting of agroforestry species, indigenous fruit trees, and to a limited extent, indigenous commercial (timber and food) species [478].
Many of Africa’s indigenous fruit and nut trees have great potential for domestication and the ‘rebooting’ of tropical agriculture [22]. All of the ten species identified to have untapped potential for food and nutrition security by Omotayo and Aremu [257] occur naturally in southern Africa. In Zimbabwe, about 99% of homesteads in semi-arid areas contained trees (each with around 4–18 trees) primarily planted for fruits and shade. However, fuelwood, traditional medicines, windbreaks and homestead decoration were considered secondary benefits [480]. In recent times, farming and non-farming households with access to these nutrient-rich indigenous fruits had managed to live above the poverty line throughout the year through sales of fruits when agricultural labour demands were high [473]. With the worsening food crisis, high levels of population growth, and increasing rural and urban poverty levels in southern Africa, there is growing recognition of the importance of indigenous fruit trees.
As reported in the second decade of tree domestication the food production capacity in the Southern Africa region is reaching its limit due to the over-cultivation of the fragile soils and consequent loss of soil fertility and health [16]. The periodic droughts in the regions have exacerbated this situation and have led to greater exploitation and loss of wild indigenous fruit trees in some areas. Moreover, in countries such as Malawi, Zambia and Zimbabwe, land clearance for maize (Zea mays) and other staples has severely reduced the availability of indigenous fruits and nuts. Over the last three decades, to minimise this worsening situation, the domestication of indigenous fruit trees has been adopted to incentivize the integration of indigenous fruit trees into farming systems for cultivation and sustained use.
The exploitation of certain indigenous fruit trees as a wild-harvested food source and for medicinal purposes in certain parts of southern Africa, especially rural areas, is well-documented [481]. Wild fruit trees are severely prone to over-exploitation and extinction as forests recede due to deforestation and the fact that they are being cut down for medicinal purposes without replacement [472]. The commercialization of harvesting and trade of some species such as Uapaca kirkiana and Ziziphus mauritiana from natural populations can result in genetic erosion and reduction of the regenerative capacity of the species as the seeds are being removed from their natural environment [23,478]. As a result, indigenous fruit trees are scattered, populations are small and fragmented, and in some cases, they have completely disappeared from the landscape [478]. This is one of the key reasons for promoting domestication and cultivation. The genetic makeup of some species may have been altered by farmers retaining desirable trees and felling others [478] as part of domestication.
The growing popularity of indigenous fruit trees in rural communities for their food and non-food products in southern Africa has led to domestication and commercialization programmes in selected priority species to improve the quality of their products [478]. This Agroforestry Tree Domestication Programme started in Southern Africa in the early 1990s, led by the World Agroforestry Centre (ICRAF) and continues today [16,85,482]. It provides local communities with a sustainable means to cultivate indigenous fruits and nuts, diversify farming systems and enhance how these species promote food and nutritional security while increasing household incomes can be used to pay school fees, improve homesteads and gain access to better healthcare [96].
In southern Africa, the participatory, more decentralised domestication approach to domestication has been gaining attention in the last decade due to the failure of top-down approaches and the complexity of natural resources management, conservation, and utilisation within rural communities [473,478]. The approach was developed through close collaboration between scientists and farmers, where the farmers and local communities are involved in selecting species [478]. The farmers utilise the participatory domestication approach on their farms and tree nurseries, where the focus is initially on satisfying the immediate domestic needs of households for tree foods and then expands by producing planting material for sale to other farmers and by commercializing tree products).
8.2. Ethnobotany of Food and Medicinal Species for Cultivation
The traditional use of indigenous food plants has been widespread in southern Africa. For example, in northern Namibia, 87% of people between 18 and 98 years old reported that they had learned about using these species from their parents. However, 56% indicated that their use was declining, with only limited attention to improving management practices. Four species (Berchemia discolor, Hyphaene petersiana, Sclerocarya birrea and Diospyros mespiliformis) were especially important for treating toothache diarrhoea, cough, tonsillitis, burns, skin allergy, stomach ache, snake bite, and constipation [90]. In Central Sekhukhuneland in South Africa, 44% of species were used for food, while 46% were medicinal, and 40% had various craft uses [483]. Using a Species Popularity Index, this study revealed a need for a greater systematic recording of indigenous plants. Likewise, medicinal plants are still widely used in rural areas of Mpumalanga, South Africa, especially for urinogenital disorders, while Combretum collinum is important for respiratory diseases [484].
Azanza garckeana is one of the priority species for domestication in southern Africa. It is a popular multipurpose fruit tree that is widely recognized across Africa for its phytochemical properties and so for its nutritional and medicinal uses [485,486,487,488,489,490,491,492]. A total of 22 traditional medicinal uses of A. garckeana are documented in the literature, ranging from asthma, abscesses, anaemia, diabetes and epilepsy to malaria, chest and liver problems and sexually transmitted diseases [489]. Likewise, other ethnobotanically important species with potential for domestication, like Vangueria infausta and Dovyalis caffra, have nutritional and medicinal properties [493,494].
8.3. Commercial Value of NTFPs in Local Markets
Due to a lack of knowledge and the bias of research and development in large-scale agriculture, the indigenous fruit trees of southern Africa remain relatively unknown in the global market [112]. Thus, they do not contribute much to southern Africa’s regional and export trade as they are generally considered to have little commercial potential. As a result, they are mostly sold in informal markets. For example, in Eswatini, indigenous fruits occur in abundance during the summer and autumn seasons. Some fruits are sold raw in informal local markets or processed into value-added products at a small-scale subsistence level for economic gain [472]. In the Miombo Woodlands, A. garckeana and Strychnos spinosa are popular multipurpose fruit trees characterised by edible and nutritious fruits with medicinal uses. These fruits have great social and economic importance locally and in the other parts of Africa where they are indigenous [491]. They generate substantial income for the household in local markets [489,490]. Indigenous fruits can be processed into value-added products to improve digestion, metabolism, and preservation [476]. Examples of value addition include fermentation, which can add value to the underutilised indigenous fruits [472].
Since the start of the indigenous fruit tree programme fruit markets in southern Africa have been largely informal, small, and volatile. These markets encounter numerous challenges, including a short season, seasonal gluts; bulky and perishable products; limited and conflicting market knowledge, lack of marketing networks and associations, inadequate policies, and inadequate processing and storage methods [16].
Even though the indigenous fruit trees are not generally considered to have commercial potential in southern Africa, some fruits such as Uapaca kirkiana, Sclerocarya birrea and Z. mauritiana are being used commercially to make alcoholic [94,495]. This commercialization of indigenous fruits has implications for sustainable harvesting and the protection of the natural resource.
8.4. Priority Setting for Domestication/Commercialization
Since the start of tree domestication in southern Africa, the list of priority species has been extended from Sclerocarya birrea, Uapaca kirkiana, Strychnos cocculoides, Vangueria infausta, Parinari curatellifolia, Ziziphus mauritiana and Adansonia digitata to include Sizygium cordatum (Gaertner) and Vitex species [85,96,482]. Two of these species—S. birrea and A. digitata—are also priority species in other African Regions, so recent research om them is reported in the Pan-Africa Section of this paper. Strychnos spinosa, an important tree for domestication in southern Africa, is also important in West Africa [406]. Likewise, A. garckeana, one of the original group of priority species in southern Africa, is a small, semi-deciduous tree, producing fruits with rough and hairy skins and is semi-domesticated in Nigeria and Benin, where communities grow the species in homegardens and crop fields [489,490] to support their nutrition, health and income security.
The prioritisation of indigenous fruit trees differs across the Region. For example, based on ethnobotanical surveys, A. garkeana and Flacourtia indica are high priority in Malawi; Vitex species and Tamarindus indica in Tanzania; Anizophyllea boehmii in Zambia; and A. garkeana and Z. mauritiana in Zimbabwe [481]. In another survey, A. digitata L., P. curatellifolia, S. birrea, S. cocculoides and U. kirkiana were perceived as the most important species for domestication in Malawi, Tanzania and Zimbabwe [481].
8.5. Characterization of Useful Traits
Using simple linear regressions, a study in South Africa sought relationships between diameter at breast height (dbh) and total fresh fruit biomass in Strychnos madagascariensis and Strychnos spinosa. It found that dbh explained 99.9% of the variation in fruit biomass; thus, it provided useful predictions of fruit yield in the savannah woodlands of southern Africa [496]. Interestingly, a study of S. spinosa in Benin (West Africa) across seven populations also found highly significant differences among populations in nine traits. In addition, within-population variation accounted for 53–90% of the total variation indicating a high potential for selection for domestication purposes [310].
8.6. Assessment of Genetic Variation
To initiate a domestication programme on U. kirkiana in southern Africa, a provenance trial was established in 1997 at Nauko in Malawi. It contained six provenances from Malawi and one from Mozambique. The provenance trial showed significant differences between provenances in the sex ratio of flowers [482]. Phalombe provenance had a male:female ratio of about 1, while other provenances contained more male trees [482]. The Phalombe provenance also produced the most fruits per tree, perhaps because of better pollination. The authors recommended further studies to investigate variation in desirable fruits traits such as fruit size, sweetness and pulp ratio for domestication.
8.7. Nutritional Value of Non-Timber Forest Products
Uapaca kirkiana produces nutritious fruits with many foods and non-food uses due to its phytochemical content [83,104], making it important to address malnutrition. A recent analytical study in support of traditional knowledge found that U. kirkiana fruit is a good source of micronutrients (Fe, Cu, Zn), phenolic compounds, vitamin C and bioactive compounds [497,498,499]. Therefore, it is recommended that these functional tree foods be incorporated into diets for their nutritional and health benefits, such as additives and as probiotic jams, especially for women and children in sub-Saharan Africa.
Likewise, A. garckeana has great potential as a high-value nutraceutical due to its importance as a source of several bioactive compounds such as alkaloids, flavonoids and tannins, which can be used as dietary supplements or functional foods [489]. Therefore, the fruits of A. garckeana are an important resource for dietary health across the region [488,489,490,491]. In addition to its micronutrient content, A. garckeana can also reduce protein-energy malnutrition, which has plagued the residents of sub-Saharan Africa [490].
8.8. Medicinal Value of Non-Timber Forest Products
The bark, fruits, leaves, roots and stems of A. garckeana are reported to possess diverse medicinal properties and uses [113,489,490], with numerous reports about its phytochemical properties [485,487,488,491,492]. Furthermore, there are reports of its antimicrobial activity associated with betulinic acid [486] with potential in the beverage, pharmaceutical and cosmetic industries. Dovyalis caffra is another species with medicinal and veterinary potential associated with pain relief and rheumatism in humans. These properties have been tested and suggested for further study to evaluate its activity against bacteria and parasites [493]. Across much of dry Africa, the pharmacological benefits of Parkia biglobosa have been associated with its properties to treat malaria, helminthic worms, bacteria, diabetes, hypersensitivity, inflammation, cancer, sleeping sickness and for its antioxidant properties [428]. Strychnos spinosa is another indigenous fruit exhibiting various health-promoting benefits against a variety of pathogens due to its diverse nutritional and phytochemical constituents [500].
8.9. Selection of Elite Trees and Ideotypes
In the dioecious species U. kirkiana, a study to integrate ecological knowledge and scientific principles in selecting superior Uapaca kirkiana phenotypes in Malawi and Zambia involved focus-group discussions with fruit collectors and roadside marketers. Following these discussions, superior female trees of U. kirkiana on cultivated land and forest reserves were identified in Malawi and Zambia based on fruit load, size and sweetness [473]. The local communities retained the identity of these elite individuals. Likewise in Zimbabwe, U. kirkiana tree selections focussed on fruit size, and total soluble solutes as farmers indicated the importance of taste [501].
8.10. Nursery Developments and Improved Planting Stock
The domestication of indigenous fruit trees may encounter similar institutional barriers to conventional trees species [478]. These include the lack of investment in tree seed centre activities. For example, seed centres in Zambia and Mozambique are experiencing shortages in human capacity, equipment and funding and failing to meet domestic seed demands. Private forest companies and tree growers are relying on seed imports [478].
The physiological quality of the seed in terms of purity and viability is often compromised as most seed centres have dilapidated seed testing and cleaning equipment. In contrast, others like those in Zimbabwe face frequent power cuts resulting in loss of seed viability [478]. In addition, in contrast to South Africa where the benefits are internalized within the private sector that drives tree breeding initiatives and programmes with support from the government, tree breeding research in countries such as Malawi, Zambia and Zimbabwe is undertaken by government institutions. The outputs of this research are then deployed for planting as improved tree germplasm [478]. Thus, individual countries are at different levels of sophistication regarding their breeding initiatives and programmes. A lack of research investments has hampered some countries’ capacity to produce enough seed to sustain planting programmes [478]. The concept of a participatory tree domestication programme for agroforestry with indigenous fruit trees [18] circumvent this problem and empower farmers to meet their own needs for improved planting stock.
Marunda et al. [478] note that planting stock is inadequate despite the effort to domesticate indigenous fruit trees. Thus, the demand for fruits is inevitably still being met from dwindling natural populations. Ofori, Gyau [23] concur, indicating that it is important to minimise losses in underlying genetic diversity. The strategy embraced for indigenous fruit trees is designed to minimize these losses. Nevertheless, depending on the domestication method used, such losses are a concern.
8.11. Vegetative Propagation
Despite a general lack of knowledge about the vegetative propagation of indigenous fruit trees in the Region, the techniques and principles developed in earlier decades [16,37] are being applied to priority species across southern Africa. Meanwhile, in South Africa, studies with stem cuttings have been successfully initiated in other species, such as Brackenridgea zanguebarica Oliv. [502] and Cyclopia subternata [503]. Vegetative propagation using cuttings, grafting and layering can capture superior fruit and tree traits in mature tissues. By retaining sexual maturity in the new clonal material small plants can flower and fruit within a few years of propagation [16]. Interestingly, a study of the fruits from natural seedlings versus vegetatively propagated plants found that vegetative propagules (grafts and marcots) of U. kirkiana produced larger fruits in November, while in December, those from natural stands were larger [504]. Seeds from grafts germinated rapidly and grew faster than those from natural stands. Thus, grafting and marcotting was recommended as a viable alternative source of seed.
8.12. Community Action
A study in Malawi investigated farmers’ attitudes towards tree planting on their farms and found that farmers who had planted trees in the last five years were more positive than those who had not. Furthermore, membership in a farmer group had a significant influence on their behaviour. Nevertheless, households generally considered that buying food and agricultural inputs and investing in their children’s education were more important than planting trees. The authors concluded that these attitudes indicate that poverty is a barrier to tree planting [505]. However, more generally in southern Africa, the fruits of Miombo tree species on farms are recognised both as a safety-net and a source of income and employment, indicating the importance of wider cultivation in the region for both livelihoods and the alleviation of environmental issues such as climate change [506].
8.13. Post-Harvest Issues
Damage to indigenous fruit trees and their fruits from tree climbing and stick throwing continue to be a problem, causing post-harvest losses. Vangueria infausta is an important indigenous fruit of southern Africa widely consumed by rural communities. However, its greater use is constrained by knowledge of postharvest practices. Consequently, studies have examined factors affecting consumer appeal, nutritional benefits and shelf-life [494]. In addition, a study to determine the effect of harvesting time on physicochemical properties and selected nutritional composition of V. infausta fruits found that the best harvest time was January, when fruits were most attractive to consumers; however, further work is required on the physicochemical properties and nutritional value [494]. However, some processing technologies have been developed to add value in quality, mineral bioaccessibility, shelf-life and postharvest handling to IFT fruits [497].
8.14. Enterprise Development
The past failures to domesticate underutilised fruit trees have resulted in poor global recognition and limited integration into formal markets, supply chains and retail outlets. Many of these products are mostly sold fresh by street vendors rather than by shopkeepers [78]. Given the importance of indigenous fruit trees to the human diet, food and nutrition security, livelihoods and gender equality, there have been many calls to domesticate them as farm crops [112,472,507].
In support of the domestication of U. kirkiana, a market study in Zambia during the last decade proposed the need to develop policy interventions to expand the fruit processing sector, promote agribusiness linkages, and increase knowledge to support improved marketing. A more recent survey has found a high informal market demand for the fresh and secondary products of the U. kirkiana fruit in Zambia, principally for jam, butter and syrup, potentially expanding [508].
A study in South Africa has investigated the use of flour from the fruit peel of Parinari curatellifolia to fortify the nutritional and antioxidant properties of biscuits [509]. Despite mixed results, the authors recommended the use of peel flour to enhance the nutritional and other properties of bakery products.
8.15. Trade and the Law
Since the start of the tree domestication initiative, there has been concern that the commercialization of IFTs may directly conflict with traditional norms, conservation biology and national conservation laws and regulations. Like all non-timber forest products (NTFPs), many laws and policies (e.g., those relating to natural resources, agriculture, forestry, environmental laws, land tenure and resource rights and economic and financial measures such as trade and taxation) restrict the allowable management, use and commercialization of indigenous fruit trees [16]. Thus, there are often serious difficulties in cases where both customary and statutory regimes exist. As traditional governance systems are generally better placed as the first line of contact given their proximity to the resource base, there may be the need for the State to act as a complementary layer of governance [81]. This should avoid conflict and confusion between the two regimes. One possible solution is to recognise domesticated indigenous trees as crops owned by the farmer—making common-property NTFPs distinct from privately owned agroforestry tree products [207].
8.16. Local Impacts of Cultivation and Marketing
Agroforestry, the combination of crops with trees, harnesses the role of trees to create niches essential for a sustainable and functional agroecosystem [25,510]. Within this framework, the domestication of indigenous trees diversifies and intensifies productive farming systems with crops producing traditionally useful and marketable products [14]. The colonization of these niches can also encompass wild indigenous vegetables, often shrubs and herbs. In South Africa, several research institutions are at the forefront of research on these species to boost food and nutrition security [495]. The Agricultural Research Council of South Africa is one such institution and focuses on water use efficiency, plant nutrition, cultivation practices (planting methodology, spacing and harvesting, allelopathic effects, agronomic traits) and promoting wild vegetables [511,512,513]. This aligns with wider initiatives with ‘orphan’ crops across the continent [495,514].
However, further research is needed to understand the socioecological context that explains tree survival when developing diversified homestead tree plantings [480]. The diversification of farming systems with new domesticated crops requires that cultivated plots are properly managed and secured. This has not always been the case for many trees, such as marula (Sclerocarya caffra), kei apple (Dovyalis caffra) and monkey orange (Strychnos spp.), as few mixed species cultivation trials have been undertaken. Consequently, rural populations can encounter challenges with regard to the cultivation of indigenous species, such as fragile and degraded soils [112].
9. Adoption, Outcomes and Impact
ICRAF’s dominance in tree domestication research in early years has been superseded by a multitude of National Agricultural Research Systems and University Departments. However, these institutions are less well placed to conduct the Development Research needed to underpin a scaling-up of the overall initiative across Africa (and the rest of the tropics/sub-tropics), leaving a gap that needs to be filled by a new coordinating institution or network.
The third decade of tree domestication has been a period of consolidation with an expansion of both the concept and interest in indigenous species with more players across a broad spectrum of disciplines, especially phytochemical analysis and understanding of the baseline ethnobotany and state of the natural resource. Of special significance is the adoption by African academics from a very wide range of disciplines, universities/institutes and countries in published research on an increased number of topics (Supplementary Materials Tables S1 and S2). Significantly, this research on the multi-disciplinary interface of the Domestication Syndrome (the techniques and strategies that allow the capture and use of a range of desirable traits) has often been conducted in partnership with scientists from Europe, the USA and many other countries outside Africa. Consequently, probably the most important outcome has been a greatly improved knowledge of over 40 of Africa’s indigenous tree species. This knowledge spans from their ethnobotany, through characterization and propagation, to their phytochemistry and genetics.
The most important knowledge gap, however, is in the specific performance of putative cultivars in field trials and in farmers’ fields. Consequently, progress made in the second decade to identify livelihood and agroecological impacts has not advanced much in the third decade. In part, this is due to reduced donor funding of participatory domestication at the community level. Interestingly, however, the continuation of tree domestication activities in hundreds of rural communities in Cameroon, even without significant new funding or technical backstopping, indicates the resilience of this grassroots approach to Rural Development. This is in marked contrast to the persistence of conventional top-down Development programmes. Encouragingly, the merits of Rural Resource Centres as the community hubs for agroforestry have expanded greatly within the African continent, especially in Ethiopia, Rwanda, Kenya, Mali, Burkina Faso and Chad. Whilst the principles behind the Rural Resource Centres are common, their institutional make-up is driven by the capacities and resources available but also determined by the needs of the farmer community and other stakeholders, as well as their environment.
The importance of an enabling socio-political context in the success of such bottom-up approaches is illustrated by recent developments in Cameroon. The political unrest since 2016 in the Northwestern and Southwestern regions of Cameroon, exactly the areas where participatory tree domestication was spreading fast, has brutally ended the dissemination of tree cultivation by Rural Resource Centres to neighbouring villages. Unfortunately, those Centres which were located in conflict hotspots were destroyed, and promotors fled their village for fear of repression. Furthermore, technical backstopping and monitoring became impossible for security reasons.
10. Policy Relevance
There is strong evidence that high-value, non-timber forest products with potential in agroforestry systems for the production of food and medicines can have significant impacts on poverty alleviation, well-being, food security and also provide ecosystem services for the mitigation of climate change [515]. Much has been written this decade about the need for enabling policies to support the reform of tropical agriculture, especially for hundreds of millions of very poor smallholder farmers in Africa locked in subsistence agriculture by current adverse policies [25]. Importantly, this can be achieved through the use of indigenous technologies [516] and new crops developed from indigenous species.
By diversifying these farming systems with new indigenous tree crops producing traditional African foods it is possible to restore and rehabilitate highly degraded natural capital [517], diversify diets, habitats for biodiversity and local markets [27]. Interestingly, new market opportunities are emerging from studies of the unique phytochemistry of indigenous tree products. For example, in addition to high nutritional value [330] and post-harvest processing [385], studies have examined: the potential to select for functional foods [144], medicinals [489], bactericidal properties [493], fermented products [110,331], biodiesel [109], insecticides [518], paint formulation [519] and aluminium corrosion inhibitors [520]. Identifying candidate trees to select for all these potential commercial uses can be greatly enhanced by the examination of the intraspecific tree-to-tree variation at different sites with the purpose of formulating a selection ideotype [115].
Simultaneously, this approach to the domestication of indigenous trees and the commercialization of their products can catalyse a highly adaptable and generic model for the rehabilitation of degraded farmland, so stimulating increased productivity of staple food crops by better land husbandry and rehabilitated agroecosystems [22,510,521]. If scaled up significantly, this approach to domestication could be a catalyst for the conversion of the so-called inevitable ‘Trade-offs’ to ‘Trade-ons’ that maximize environmental, social and economic benefits, or ‘profits’, from investment in the five Capitals (natural, human, social, physical and financial) [14,15,22]. Such policy change offers opportunities to trigger a transformational quantum leap towards the development of African economy conferring a better life for millions of the world’s poorest people—especially in Africa [25]. With the development of new local industries based on the selection of unique properties of a wide range of elite indigenous trees, this would rebalance the global economy and social divide between the very rich and the very poor [25,289]. It may also help to restore wildlife habitats by converting degraded land into perennial vegetation for improved wildlife habitat and for carbon sequestration to address climate change, and thus reverse the current pressures on planetary health [22]. There is, however, a big constraint to achieving all this. It is the lack of well-replicated, randomized impact studies to convince donors and development agencies of the importance of these approaches. Unfortunately, donors are very reticent to fund such studies at the start of development programmes.
In the wake of COP26, the benefits of endogenous (home-grown) incentives to combat deforestation/land degradation and associated climate change, using agricultural and landuse practices such as ‘land maxing’ with indigenous tree species could reverse the current situation, where 90% of the change in forest cover is attributable to agriculture [14]. It seems clear, however, that ‘political will’ by the international community is the missing sixth capital for sustainable development [22].
Policies are now needed to implement a continent-wide tree domestication programme on a scale which will deliver multiple impacts. This will require the up- and outscaling of research and then the implementation of a massive development programme. It is clear from experience in Humid West Africa that for successful and rapid impact, a development programme should be based at the grassroots community level using the tried and tested decentralized Participatory Domestication strategies, philosophy and techniques developed in Cameroon [21]. This can then be supported by a longer-term upstream centralized programme of tree breeding and biotechnology. This will then require finding ways to disseminate the new germplasm from centralized breeding programmes to the network of community level Rural Resource Centres.
11. Towards the Fourth Decade
Much has been achieved during the third decade, especially with regard to the uptake and expansion of the overall concepts of agroforestry tree domestication across a wide range of academic institutions in Africa and partnerships around the world, as well as across an increased number of species. However, research publications in the third decade have not spanned the 23 topics examined by this review equally (Figure 3) and no single species has had a research agenda spanning all topics.
Notably, the weakly researched topics (less than five publications) have been: priority setting (Topic 5), although this was a key topic in the first and second decades; elite tree selection and identification of ideotypes (10); testing of putative cultivars (13); implementation of participatory principles (15), again a key topic in earlier decades; new techniques and skills (16); nursery developments and improved planting stock (17); protection of traditional knowledge and intellectual property rights (19); and the impacts of cultivation and diversification of farming systems (21). Only 6 topics had more than 30 publications.
Consequently, these and many areas of new research and development identified for the third decade are still in need of adoption or expansion in the fourth decade (Table 4). In addition, and within the concept of ‘re-booting’ tropical agriculture and making African lives matter [22,25], there is especially a need to examine how closing the yield gap in staple food crops using tree domestication and AFTP commercialization [522] can: (i) reduce the area needed for staple food crop production and (ii) create more climate-friendly and wildlife-friendly land use on formerly degraded farmland. This should include an examination of the different patterns of domesticated indigenous tree crops in different combinations of tree species × configuration × density to maximise the win-win-win benefits [22], which are likely to be critical for up- and outscaling in the fourth decade.

Table 4.
Areas of new research identified for the third decade [16] and their current situation for the fourth decade.
In the fourth decade, we specifically recommend that the current interest in the phytochemistry of indigenous tree species for enhanced nutrition and their medicinal properties [17] should be expanded to explore the intraspecific tree-to-tree variation at different sites to identify traits appropriate for market-oriented ideotype selection [115].
In addition to the requirements of Table 4, the prime need for the fourth decade is progress towards up- and out-scaling at the national level. This will require a very considerable expansion of capacity building for NGOs, CBOs and community groups at the local/national level. In addition, there will be a need for a dedicated Centre to test, develop and promote selected cultivars of indigenous crops. This should build on the successes of Rural Resource Centres in Cameroon and their truly bottom-up approach to the dissemination of skills, techniques and strategies [19]—especially in participatory domestication, vegetative propagation and the field testing of putative cultivars. Furthermore, to maximise win-win benefits in view of the small size of land holdings in Africa, studies to establish appropriate planting densities for new tree crops are likely to be critical for up and outscaling in the fourth decade.
12. Conclusions
It is clear from this review that there is a growing acceptance of the critical role that indigenous fruit trees can play in: (i) breaking the cycles of poverty and malnutrition, (ii) improving the quality of life for millions of rural people in the continent and (iii) in transitioning to climate-resilient strategies in Africa. This has been achieved through the transition from ethnobotany into mainstream agriculture via a deeper scientific understanding of the food and medicinal properties of the properties of these indigenous African trees and their progress towards domestication as new crops in farming systems delivering multiple social, economic and environmental benefits for ‘land maxing’ [14]. Thus, the groundwork for a quantum leap towards a more productive, sustainable and multifunctional approach to tropical agriculture, in line with the UN World Health Organization’s “One Health” concept, is well advanced and the priority now for the next decade is to put what has been achieved into practice on a greatly increased scale by embracing the local capacity for the selection and domestication of indigenous trees producing high-quality products in agroforestry systems for the betterment of rural people throughout Africa [11,14,15].
To build on these outcomes from the third decade, there is need to support the skills, techniques and philosophy behind the locally appropriate strategy of domesticating a range of different trees at the community ‘grassroots’ level as a source of numerous social, economic and environmental benefits for local communities. Then, through the processing and value addition of tree products grown on-farm opportunities for income generation and local employment can multiply the livelihood benefits, especially for women and children. However, more work needs to be done to capture the opportunities presented by the genetic potential of individual elite trees and move these initiatives towards a decentralised, continent-wide, tree domestication development programme on the scale needed to meaningfully deliver this wide range of impacts. This then should be supported by a longer-term upstream, centralized programme of tree breeding and biotechnology to diversify and further enhance the quality of tree products for new African industries based on expanded ideotypes. The role of diverse inter-disciplinary, public–private and socially responsible agriculture-related collaborations will be more crucial than ever. With increasing cooperation between regional and trans-African governments, there is much wider scope for scholarly and technological exchanges to enable a sustainable agricultural revolution. In this way, agriculture can be more dynamic and address the barriers constraining both the productivity of agriculture and the lives of rural communities. This will require an enabling policy environment based on a new mindset at the level of international agribusiness and trade, development agencies, donors and policy/decision makers, one that recognizes the need for a holistic and integrated set of environmental, social and economic benefit flows in Africa and around the world to address climate change, hunger, poverty, social injustice and loss of wildlife habitat. This new mindset would represent recognition of ‘political will’ as the missing sixth form of capital for sustainable rural development. Encouragingly, Africa’s “Agenda 2063” and the recent FAO/UNDP/UNEP Report “A multi-billion-dollar opportunity: Repurposing agricultural support to transform food systems” [523] offer hope of a positive change in the policy agenda.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su14042355/s1: Supplementary Materials Table S1. Research Institutes/Organizations/Universities/Departments engaged in Tree Domestication in Africa (based on affiliations of coauthors of 759 papers published 2012–2021). Supplementary Materials Table S2. Science Journals and Books publishing ‘Tree Domestication’ papers.
Author Contributions
This review paper was conceptualized by R.R.B.L. who also wrote Section 1, Section 2, Section 3, Section 4, Section 9, Section 10, Section 11 and Section 12; M.-L.T.A. wrote Section 5 with A.D. and N.P.A.; A.E.A. wrote Section 6 with L.M.; P.S.H. wrote Section 7; T.M. wrote Section 8 with S.H.; S.H. collated the References. All authors revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leakey, R.R.B.; Last, F.T.; Longman, K.A. Domestication of forest trees: A process to secure the productivity and future diversity of tropical ecosystems. Commonw. For. Rev. 1982, 61, 33–42. [Google Scholar]
- Leakey, R.R.B.; Newton, A.C. Tropical Trees: The Potential for Domestication and the Rebuilding of Forest Resources; HMSO: London, UK, 1994; Volume 29.
- Leakey, R.R.B. Living with the Trees of Life: Towards the Transformation of Tropical Agriculture; CABI: Wallingford, UK, 2012. [Google Scholar]
- Jamnadass, R.H.; McMullin, S.; Iiyama, M.; Dawson, I.K.; Powell, B.; Termote, C.; Ickowitz, A.; Kehlenbeck, K.; Vinceti, B.; Vliet, N.v. Understanding the roles of forests and tree-based systems in food provision. In Forest, Trees and Landscapes for Food Security and Nutrition: A Global Assessment Report; IUFRO World Series 33; IUFRO: Vienna, Austria, 2015; pp. 25–49. [Google Scholar]
- Jamnadass, R.; Ofori, D.; Dawson, I.; Tchoundjeu, Z.; McMullin, S.; Hendre, P.; Graudal, L. Enhancing Agric. Syst. through tree domestication. In Sustainable Development through Trees on Farms: Agroforestry in Its Fifth Decade; World Agroforestry (ICRAF) Southeast Asia Regional Program: Bogor, Indonesia, 2019; pp. 45–59. [Google Scholar]
- Awodoyin, R.O.; Olubode, O.S.; Ogbu, J.U.; Balogun, R.B.; Nwawuisi, J.U.; Orji, K.O. Indigenous fruit trees of tropical Africa: Status, opportunity for development and biodiversity management. Agric. Sci. 2015, 6, 31. [Google Scholar] [CrossRef]
- Dawson, I.K.; Guariguata, M.R.; Loo, J.; Weber, J.C.; Lengkeek, A.; Bush, D.; Cornelius, J.; Guarino, L.; Kindt, R.; Orwa, C. What is the relevance of smallholders’ Agric. Syst. for conserving tropical tree species and genetic diversity in circa situm, in situ and ex situ settings? A review. Biodivers. Conserv. 2013, 22, 301–324. [Google Scholar] [CrossRef]
- Dawson, I.K.; Leakey, R.; Clement, C.R.; Weber, J.C.; Cornelius, J.P.; Roshetko, J.M.; Vinceti, B.; Kalinganire, A.; Tchoundjeu, Z.; Masters, E. The management of tree genetic resources and the livelihoods of rural communities in the tropics: Non-timber forest products, smallholder agroforestry practices and tree commodity crops. For. Ecol. Manag. 2014, 333, 9–21. [Google Scholar] [CrossRef]
- Dawson, I.; Leakey, R.; Place, F.; Clement, C.; Weber, J.; Cornelius, J.; Roshetko, J.; Tchoundjeu, Z.; Kalinganire, A.; Masters, E. Trees, tree genetic resources and the livelihoods of rural communities in the tropics. In Thematic Study for the State of the World’s Forest Genetic Resources; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Van Damme, P. The Role of Tree Domestication in Green Market Product Value Chain Development in Africa. Afrika Focus 2018, 31, 115–128. [Google Scholar] [CrossRef]
- Leakey, R.R.B. Trees: Delivering productive and sustainable farming systems: An update. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Leakey, R.R.B., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 389–391. [Google Scholar]
- Leakey, R.R.B. Trees: Meeting the social, economic and environmental needs of poor farmers-scoring sustainable development goals: An update. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Academic Press: San Diego, CA, USA, 2017; pp. 417–420. [Google Scholar]
- Shackleton, C.M.; de Vos, A. How many people globally actually use non-timber forest products? For. Policy Econ. 2022, 135, 102659. [Google Scholar] [CrossRef]
- Leakey, R.R.B. From ethnobotany to mainstream agriculture: Socially modified Cinderella species capturing ‘trade-ons’ for ‘land maxing’. Planta 2019, 250, 949–970. [Google Scholar] [CrossRef]
- Leakey, R.R.B. Converting ‘trade-offs’ to ‘trade-ons’ for greatly enhanced food security in Africa: Multiple environmental, economic and social benefits from ‘socially modified crops’. Food Secur. 2018, 10, 505–524. [Google Scholar] [CrossRef]
- Leakey, R.R.B.; Weber, J.C.; Page, T.; Cornelius, J.P.; Akinnifesi, F.K.; Roshetko, J.M.; Tchoundjeu, Z.; Jamnadass, R. Tree domestication in agroforestry: Progress in the second decade (2003–2012). In Agroforestry—The Future of Global Land Use; Nair, P.K., Garrity, D., Eds.; Springer: New York, NY, USA, 2012; pp. 145–173. [Google Scholar]
- Mariod, A.A. Wild fruits: Medicinal value and health benefits. In Wild Fruits: Composition, Nutritional Value and Products; Springer: Chem, Switzerland, 2019; pp. 125–131. [Google Scholar]
- Leakey, R.R.B. Agroforestry: Participatory domestication of trees. In Encyclopedia of Agriculture and Food Systems; van Alfen, N.K., Ed.; Elsevier Publishers: San Diego, CA, USA, 2014; Volume 1, pp. 253–269. [Google Scholar]
- Degrande, A.; Tchoundjeu, Z.; Kwidja, R.; Fouepe, G.F. Rural Resource Centres: A Community Approach to Extension. In What Works in Rural Advisory Services? Global Good Practice Notes; Global Forum for Rural Advisory Services (GFRAS): Lausanne, Switzerland, 2015; pp. 69–72. [Google Scholar]
- Degrande, A.; Tchoundjeu, Z.; Kwidja, R. Rural resource centres: Farmer centred approach to extension. In CTA Top 20 Innovations That Benefit Smallholder Farmers; Media, W., Ed.; The Technical Centre for Agricultural and Rural Cooperation (CTA): Wageningen, The Netherland, 2015; pp. 33–35. [Google Scholar]
- Leakey, R.R.B.; Prabhu, R. Towards Multifunctional Agriculture–An African Initiative. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Leakey, R.R.B., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 393–414. [Google Scholar]
- Leakey, R.R.B. A re-boot of tropical agriculture benefits food production, rural economies, health, social justice and the environment. Nat. Food 2020, 1, 260–265. [Google Scholar] [CrossRef]
- Ofori, D.A.; Gyau, A.; Dawson, I.K.; Asaah, E.; Tchoundjeu, Z.; Jamnadass, R. Developing more productive African Agric. Syst. and improving food and nutritional security through tree domestication. Curr. Opin. Environ. Sustain. 2014, 6, 123–127. [Google Scholar] [CrossRef]
- Hendre, P.S.; Muthemba, S.; Kariba, R.; Muchugi, A.; Fu, Y.; Chang, Y.; Song, B.; Liu, H.; Liu, M.; Liao, X. African Orphan Crops Consortium (AOCC): Status of developing genomic resources for African orphan crops. Planta 2019, 250, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Leakey, R.R.B.; Mabhaudhi, T.; Gurib-Fakim, A. African Lives Matter: Wild Food Plants Matter for Livelihoods, Justice, and the Environment—A Policy Brief for Agricultural Reform and New Crops. Sustainability 2021, 13, 7252. [Google Scholar] [CrossRef]
- Dawson, I.K.; Hendre, P.; Powell, W.; Sila, D.; McMullin, S.; Simons, T.; Revoredo-Giha, C.; Odeny, D.A.; Barnes, A.P.; Graudal, L. Supporting Human Nutrition in Africa through the Integration of New and Orphan Crops into Food Systems; World Agroforestry Centre: Nairobi, Kenya, 2018. [Google Scholar]
- McMullin, S.; Stadlmayr, B.; Mausch, K.; Revoredo-Giha, C.; Burnett, F.; Guarino, L.; Brouwer, I.D.; Jamnadass, R.; Graudal, L.; Powell, W. Determining appropriate interventions to mainstream nutritious orphan crops into African food systems. Glob. Food Secur. 2021, 28, 100465. [Google Scholar] [CrossRef]
- Graudal, L.; Dawson, I.K.; Hale, I.; Powell, W.; Hendre, P.; Jamnadass, R. Systems approach’plant breeding illustrated by trees. Trends Plant Sci. 2021, 27, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Leakey, R.R.B. Socially modified organisms in multifunctional agriculture-addressing the needs of smallholder farmers in Africa. Arch. Crop Sci. 2017, 1, 20–29. [Google Scholar]
- Dawson, I.K.; Powell, W.; Hendre, P.; Bančič, J.; Hickey, J.M.; Kindt, R.; Hoad, S.; Hale, I.; Jamnadass, R. The role of genetics in mainstreaming the production of new and orphan crops to diversify food systems and support human nutrition. New Phytol. 2019, 224, 37–54. [Google Scholar] [CrossRef]
- Takoutsing, B.; Tsobeng, A.; Tchoundjeu, Z.; Degrande, A.; Asaah, E. Vegetative propagation of Garcinia lucida Vesque (Clusiaceae) using leafy stem cuttings and grafting. Afrika Focus 2014, 27, 57–71. [Google Scholar] [CrossRef]
- Essougong, U.P.K.; Fouepe, G.H.F.; Degrande, A. Can community-based organisations deliver adequate agricultural information to farmers? Evidence from rural resources centres in Cameroon. Inf. Dev. 2019, 35, 435–446. [Google Scholar] [CrossRef]
- Jamnadass, R.; Mumm, R.H.; Hale, I.; Hendre, P.; Muchugi, A.; Dawson, I.K.; Powell, W.; Graudal, L.; Yana-Shapiro, H.; Simons, A.J.; et al. Enhancing African orphan crops with genomics. Nat. Genet. 2020, 52, 356–360. [Google Scholar] [CrossRef]
- Wouapi, H.A.N.; Tchomkachue, C.A.; Degrande, A. Contribution of networking to the sustainability of community-based relay organisations in Western Cameroon: Case study of CIEFAD and KUGWE rural resource centres. Net J. Agric. Sci. 2019, 7, 99–111. [Google Scholar]
- Leakey, R.R.B.; Akinnifesi, F.K. Towards a domestication strategy for indigenous fruit trees in the tropics. In Indigenous Fruit Trees in the Tropics: Domestication, Utilization and Commercialization; Akinnifesi, F.K., Leakey, R.R.B., Ajayi, O.C., Sileshi, G., Tchoundjeu, Z., Matakala, P., Kwesiga, F., Eds.; CAB International: Wallingford, UK, 2008; pp. 28–49. [Google Scholar]
- Leakey, R.R.B.; Pate, K.; Lombard, C. Domestication potential of Marula (Sclerocarya birrea subsp caffra) in South Africa and Namibia: 2. Phenotypic variation in nut and kernel traits. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Leakey, R.R.B., Ed.; Academic Press: San Diego, CA, USA, 2017; Volume 64, pp. 245–256. [Google Scholar]
- Leakey, R.R.B. Plant cloning: Macro-propagation. In Encyclopedia of Agriculture and Food Systems; van Alfen, N., Ed.; Elsevier Publishers: San Diego, CA, USA, 2014; Volume 4, pp. 349–359. [Google Scholar]
- Leakey, R.R.B. Trees: An important source of food and non-food products for farmers—An update. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Leakey, R.R.B., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 99–100. [Google Scholar]
- Meinhold, K.; Darr, D. Using a multi-stakeholder approach to increase value for traditional Agric. Syst.: The case of baobab (Adansonia digitata L.) in Kilifi, Kenya. Agrofor. Syst. 2021, 95, 1343–1358. [Google Scholar] [CrossRef]
- Jenya, H.; Munthali, C.R.; Mhango, J. Amenability of African baobab (Adansonia digitata L.) to vegetative propagation techniques. J. Sustain. For. 2018, 37, 632–644. [Google Scholar] [CrossRef]
- Venter, S.M.; Witkowski, E.T.F. Where are the young baobabs? Factors affecting regeneration of Adansonia digitata L. in a communally managed region of southern Africa. J. Arid. Environ. 2013, 92, 1–13. [Google Scholar] [CrossRef]
- Venter, S.M.; Witkowski, E.T.F. Fruits of our labour: Contribution of commercial baobab (Adansonia digitata L.) fruit harvesting to the livelihoods of marginalized people in northern Venda, South Africa. Agrofor. Syst. 2013, 87, 159–172. [Google Scholar] [CrossRef]
- Rashford, J. The use of Baobab leaves (Adansonia digitata L.) for food in Africa: A review. Econ. Bot. 2018, 72, 478–495. [Google Scholar] [CrossRef]
- Sogodogo, H.N.; Sanogo, K.; Sylvestre, D.S.; Traoré, S.S.; Ipou, J. Farmers’ perceptions of the impacts of Adansonia digitata L. leaves exploitation on its conservation and on livelihoods of local communities in Mali, West Africa. Eur. Sci. J. 2021, 17, 41. [Google Scholar] [CrossRef]
- Schumann, K.; Wittig, R.; Thiombiano, A.; Becker, U.; Hahn, K. Uses, management, and population status of the baobab in eastern Burkina Faso. Agrofor. Syst. 2012, 85, 263–278. [Google Scholar] [CrossRef]
- Gurashi, N.; Kordofani, M.; Adam, Y. Ethnobotany of wild baobab (Adansonia digitata L.): A way forward for species domestication and conservation in Sudan. J. For. Environ. Sci. 2017, 33, 270–280. [Google Scholar]
- Agúndez, D.; Lawali, S.; Mahamane, A.; Alía, R.; Soliño, M. Consumer preferences for baobab products and implication for conservation and improvement policies of forest food resources in Niger (West Africa). Econ. Bot. 2018, 72, 396–410. [Google Scholar] [CrossRef]
- Wanjeri, N.; Owino, W.; Kyallo, F.; Habte, T.; Krawinkel, M. Accessibility, availability and consumption of baobab during food emergencies in Kitui and Kilifi counties in Kenya. Afr. J. Food Agric. Nutr. Dev. 2020, 20, 16403–16419. [Google Scholar] [CrossRef]
- Adam, Y.O.; Pretzsch, J.; Pettenella, D. Contribution of Non-Timber Forest Products livelihood strategies to rural development in drylands of Sudan: Potentials and failures. Agric. Syst. 2013, 117, 90–97. [Google Scholar] [CrossRef]
- Adam, Y.O. An analytical framework for assessing the effect of income from non-timber forest products on poverty alleviation in Savannah region, Sudan. Univ. Khartoum J. Agric. Sci. 2013, 21, 1–25. [Google Scholar]
- Gebauer, J.; Adam, Y.O.; Sanchez, A.C.; Darr, D.; Eltahir, M.E.; Fadl, K.E.; Fernsebner, G.; Frei, M.; Habte, T.-Y.; Hammer, K. Africa’s wooden elephant: The baobab tree (Adansonia digitata L.) in Sudan and Kenya: A review. Genet. Resour. Crop Evol. 2016, 63, 377–399. [Google Scholar] [CrossRef]
- Parkouda, C.; Sanou, H.; Tougiani, A.; Korbo, A.; Nielsen, D.S.; Tano-Debrah, K.; Ræbild, A.; Diawara, B.; Jensen, J.S. Variability of Baobab (Adansonia digitata L.) fruits’ physical characteristics and nutrient content in the West African Sahel. Agric. Syst. 2012, 85, 455–463. [Google Scholar] [CrossRef]
- Simbo, D.J.; De Smedt, S.; Van den Bilcke, N.; De Meulenaer, B.; Van Camp, J.; Uytterhoeven, V.; Tack, F.; Samson, R. Opportunities for domesticating the African baobab (Adansonia digitata L.): Multi-trait fruit selection. Agric. Syst. 2013, 87, 493–505. [Google Scholar] [CrossRef]
- Muthai, K.U.; Karori, M.S.; Muchugi, A.; Indieka, A.S.; Dembele, C.; Mng’omba, S.; Jamnadass, R. Nutritional variation in baobab (Adansonia digitata L.) fruit pulp and seeds based on Africa geographical regions. Food Sci. Nutr. 2017, 5, 1116–1129. [Google Scholar] [CrossRef]
- Omondi, M.; Rimberia, F.K.; Wainaina, C.M.; Mukundi, J.B.N.; Orina, J.; Gebauer, J.; Kehlenbeck, K. Fruit morphological diversity and productivity of baobab (Adansonia digitata L.) in coastal and lower eastern Kenya. For. Trees Livelihoods 2019, 28, 266–280. [Google Scholar] [CrossRef]
- Stadlmayr, B.; Wanangwe, J.; Waruhiu, C.G.; Jamnadass, R.; Kehlenbeck, K. Nutritional composition of baobab (Adansonia digitata L.) fruit pulp sampled at different geographical locations in Kenya. J. Food Compos. Anal. 2020, 94, 103617. [Google Scholar] [CrossRef]
- Egbadzor, K.F. Studies on baobab diversity, seed germination and early growth. S. Afr. J. Bot. 2020, 133, 178–183. [Google Scholar] [CrossRef]
- Muthai, U.; Indieka, A.; Muchugi, A.; Karori, S.; Mng’omba, S.; Ky-Dembele, C.; Jamnadass, R. Quantitative variation of fatty acid composition in seed oil from baobab (Adansonia digitata L.) wild populations in sub-Sahara Africa. S. Afr. J. Bot. 2019, 123, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, A.; Usman, A.; Yahaya, A.; Umar, M.; Halilu, A.; Abubakar, H.; Kwanashie, A.; Mahadi, M.; Ibrahim, B. Genetic diversity for nutritional traits in the leaves of baobab, Adansonia digitata. Afr. J. Biotechnol. 2014, 13, 301–306. [Google Scholar] [CrossRef][Green Version]
- Kehlenbeck, K.; Padulosi, S.; Alercia, A. Descriptors for baobab (Adansonia digitata L.); Bioversity International: Rome, Italy, 2015. [Google Scholar]
- Pettigrew FRS, J.D.; Bell, K.L.; Bhagwandin, A.; Grinan, E.; Jillani, N.; Meyer, J.; Wabuyele, E.; Vickers, C.E. Morphology, ploidy and molecular phylogenetics reveal a new diploid species from Africa in the baobab genus Adansonia (Malvaceae: Bombacoideae). Taxon 2012, 61, 1240–1250. [Google Scholar] [CrossRef]
- Cron, G.V.; Karimi, N.; Glennon, K.L.; Udeh, C.A.; Witkowski, E.T.; Venter, S.M.; Assogbadjo, A.E.; Baum, D.A. One African baobab species or two? Synonymy of Adansonia kilima and A. digitata. Taxon 2016, 65, 1037–1049. [Google Scholar] [CrossRef]
- Korbo, A.; Kjær, E.D.; Sanou, H.; Ræbild, A.; Jensen, J.S.; Hansen, J.K. Breeding for high production of leaves of baobab (Adansonia digitata L.) in an irrigated hedge system. Tree Genet. Genomes 2013, 9, 779–793. [Google Scholar] [CrossRef]
- Korbo, A.; Sanou, H.; Ræbild, A.; Jensen, J.S.; Hansen, J.K.; Kjær, E.D. Comparison of East and West African populations of baobab (Adansonia digitata L.). Agric. Syst. 2012, 85, 505–518. [Google Scholar] [CrossRef]
- Munthali, C.R.Y.; Chirwa, P.W.; Akinnifesi, F.K. Phenotypic variation in fruit and seed morphology of Adansonia digitata L.(baobab) in five selected wild populations in Malawi. Agric. Syst. 2012, 85, 279–290. [Google Scholar] [CrossRef]
- Munthali, C.R.Y.; Chirwa, P.W.; Akinnifesi, F.K. Genetic variation among and within provenances of Adansonia digitata L.(Baobab) in seed germination and seedling growth from selected natural populations in Malawi. Agric. Syst. 2012, 86, 419–431. [Google Scholar] [CrossRef]
- Munthali, C.R.Y.; Chirwa, P.W.; Changadeya, W.; Akinnifesi, F.K. Genetic differentiation and diversity of Adansonia digitata L. (baobab) in Malawi using microsatellite markers. Agric. Syst. 2013, 87, 117–130. [Google Scholar] [CrossRef][Green Version]
- Chládová, A.; Kalousová, M.; Mandák, B.; Kehlenbeck, K.; Prinz, K.; Šmíd, J.; Van Damme, P.; Lojka, B. Genetic diversity and structure of baobab (Adansonia digitata L.) in southeastern Kenya. R. Soc. Open Sci. 2019, 6, 190854. [Google Scholar] [CrossRef]
- Wiehle, M.; Goenster, S.; Gebauer, J.; Mohamed, S.A.; Buerkert, A.; Kehlenbeck, K. Effects of transformation processes on plant species richness and diversity in homegardens of the Nuba Mountains, Sudan. Agric. Syst. 2014, 88, 539–562. [Google Scholar] [CrossRef]
- Gebauer, J.; Luedeling, E. A note on baobab (Adansonia digitata L.) in Kordofan, Sudan. Genet. Resour. Crop Evol. 2013, 60, 1587–1596. [Google Scholar] [CrossRef]
- Gurashi, N.; Kordofani, M.A. Morphological variation in fruit shapes of Adansonia digitata L. from Blue Nile and North Kordofan States, Sudan. J. For. Prod. Ind. 2014, 3, 106–111. [Google Scholar]
- Wiehle, M.; Prinz, K.; Kehlenbeck, K.; Goenster, S.; Mohamed, S.A.; Buerkert, A.; Gebauer, J. The role of homegardens and forest ecosystems for domestication and conservation of Ziziphus spina-christi (L.) Willd. in the Nuba Mountains, Sudan. Genet. Resour. Crop Evol. 2014, 61, 1491–1506. [Google Scholar] [CrossRef]
- De Smedt, S.; Sanchez, A.C.; Van den Bilcke, N.; Simbo, D.; Potters, G.; Samson, R. Functional responses of baobab (Adansonia digitata L.) seedlings to drought conditions: Differences between western and south-eastern Africa. Environ. Exp. Bot. 2012, 75, 181–187. [Google Scholar] [CrossRef]
- Agúndez, D.; Lawali, S.; Mahamane, A.; Alía, R.; Soliño, M. Farmers’ Preferences for Conservation and Breeding Programs of Forestry Food Resources in Niger. Forests 2020, 11, 697. [Google Scholar] [CrossRef]
- Falemara, B.; Chomini, M.; Thlama, D.; Udenkwere, M. Pre-germination and dormancy response of Adansonia digitata L seeds to pre-treatment techniques and growth media. Eur. J. Bot. Plant Sci. Pathol. 2014, 2, 13–23. [Google Scholar]
- Agbohessou, M.; Salako, K.; Idohou, R.; Gbedomon, R.; Hounkpèvi, A.; Chadare, F.; Kakaï, R.G.; Assogbadjo, A. Status of vegetative propagation of baobab: A review. Afr. Crop Sci. J. 2020, 28, 215–224. [Google Scholar] [CrossRef]
- Anjarwalla, P.; Ofori, D.; Owino, A.; Matuku, D.; Adika, W.; Njogu, K.; Kehlenbeck, K. Testing different grafting methods for vegetative propagation of baobab (Adansonia digitata L.) in Kenya to assist its domestication and promote cultivation. For. Trees Livelihoods 2017, 26, 85–95. [Google Scholar] [CrossRef]
- Darr, D.; Chopi-Msadala, C.; Namakhwa, C.D.; Meinhold, K.; Munthali, C. Processed baobab (Adansonia digitata L.) food products in malawi: From poor men’s to premium-priced specialty food? Forests 2020, 11, 698. [Google Scholar] [CrossRef]
- Jäckering, L.; Fischer, S.; Kehlenbeck, K. A value chain analysis of baobab (Adansonia digitata L.) products in Eastern and Coastal Kenya. J. Agric. Rural. Dev. Trop. Subtrop. (JARTS) 2019, 120, 91–104. [Google Scholar]
- Modiba, E.; Osifo, P.; Rutto, H. Biodiesel production from baobab (Adansonia digitata L.) seed kernel oil and its fuel properties. Ind. Crops Prod. 2014, 59, 50–54. [Google Scholar] [CrossRef]
- Wynberg, R.; Laird, S.; Van Niekerk, J.; Kozanayi, W. Formalization of the natural product trade in southern Africa: Unintended consequences and policy blurring in biotrade and bioprospecting. Soc. Nat. Resour. 2015, 28, 559–574. [Google Scholar] [CrossRef]
- Ahmed, M.F.e.M.; Adam, Y.O.; Chimuleke, M.; Darr, D.; Elwasila, E.M.M.; Gebauer, J.; Habte, T.-Y.; Johnson, H.; Kehlenbeck, K.; Krawinkel, M.; et al. Promoting the use of Baobab (Adansonia digitata L.) in rural communities in Eastern Africa. In Proceedings of the Bridging the Gap between Increasing Knowledge and Decreasing Resources, Tropentag, Prague, 7–19 September 2014. [Google Scholar]
- Chivandi, E.; Mukonowenzou, N.; Nyakudya, T.; Erlwanger, K.H. Potential of indigenous fruit-bearing trees to curb malnutrition, improve household food security, income and community health in Sub-Saharan Africa: A review. Food Res. Int. 2015, 76, 980–985. [Google Scholar] [CrossRef]
- Venter, S.M.; Witkowski, E.T.F. Using a deterministic population model to evaluate population stability and the effects of fruit harvesting and livestock on baobab (Adansonia digitata L.) populations in five land-use types. For. Ecol. Manag. 2013, 303, 113–120. [Google Scholar] [CrossRef]
- Mkwezalamba, I.; Munthali, C.R.; Missanjo, E. Phenotypic variation in fruit morphology among provenances of Sclerocarya birrea (A. Rich.) Hochst. Int. J. For. Res. 2015, 2015, 735418. [Google Scholar]
- Blair, A.M.; Thompson, D.I.; Twine, W.C.; Grab, S. The social-ecological drivers across land-use intersects driving marula tree population dynamics in north-eastern South Africa. For. Ecol. Manag. 2021, 492, 119209. [Google Scholar] [CrossRef]
- Sinthumule, N.I.; Mashau, M.L. Attitudes of Local Communities towards Marula Tree (Sclerocarya birrea subsp. caffra) Conservation at the Villages of Ha-Mashau and Ha-Mashamba in Limpopo Province, South Africa. Resources 2019, 8, 22. [Google Scholar] [CrossRef]
- Sinthumule, N.I.; Mzamani, L.C.M. Communities and conservation: Marula trees (Sclerocarya birrea subsp. caffra) under communal management at Matiyane Village, Limpopo Province, South Africa. Trop. Conserv. Sci. 2019, 12, 1940082919828969. [Google Scholar] [CrossRef]
- Cook, R.; Witkowski, E.; Helm, C.; Henley, M.; Parrini, F. Recent exposure to African elephants after a century of exclusion: Rapid accumulation of marula tree impact and mortality, and poor regeneration. For. Ecol. Manag. 2017, 401, 107–116. [Google Scholar] [CrossRef]
- Cheikhyoussef, A.; Embashu, W. Ethnobotanical knowledge on indigenous fruits in Ohangwena and Oshikoto regions in Northern Namibia. J. Ethnobiol. Ethnomedicine 2013, 9, 1–13. [Google Scholar] [CrossRef]
- Kugedera, A.T. Assessing the distribution and stem densities of Sclerocarya birrea in arable and non-arable lands: A case of Zimbabwe. Acta Sci. Microbiol. 2019, 2, 59–66. [Google Scholar]
- Kugedera, A.T. Harvesting and utilization of Marula (Sclerocarya birrea) by Smallholder farmers: A review. JOJ Wildl. Biodivers. 2019, 1, 76–79. [Google Scholar]
- Kugedera, A.T. Assessing the utilisation of Marula (Sclerocarya birrea) by smallholder farmers in Vuravhi Communal Lands of Chivi District, Masvingo, Zimbabwe. Agric. Sci. Vet. Med. 2020, 8, 11–15. [Google Scholar]
- Nyoka, B.; Chanyenga, T.; Mng’omba, S.; Akinnifesi, F.; Sagona, W. Variation in growth and fruit yield of populations of Sclerocarya birrea (A. Rich.) Hochst. Agrofor. Syst. 2015, 89, 397–407. [Google Scholar] [CrossRef]
- Leakey, R.R.B.; Shackleton, S.; Du Plessis, P. Domestication potential of Marula (Sclerocarya birrea subsp caffra) in South Africa and Namibia: 1. Phenotypic variation in fruit traits. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Leakey, R., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 235–244. [Google Scholar]
- Msukwa, V.J.; Missanjo, E.; Chilima, C.Z.; Mng’omba, S.A.; Sagona, W.; Mkwezalamba, I.; Munthali, C.R.Y. Growth performance and fruit production of Sclerocarya birrea (a. rich.) Hochst. Provenances in Malawi. Int. J. Sci. Res. Agric. Sci. 2016, 3, 42–49. [Google Scholar]
- Msukwa, V.J.; Munthali, C.; Nyoka, B.; Missanjo, E.; Kamanga, M.; Graziosi, I.; Msiska, V. Foliage pests of Marula (Sclerocarya birrea) in Malawi: Susceptibility of different provenances. Agrofor. Syst. 2021, 95, 383–393. [Google Scholar] [CrossRef]
- Leakey, R.R.B. Domestication potential of Marula (Sclerocarya birrea subsp caffra) in South Africa and Namibia: 3. Multi-trait selection. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Leakey, R., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 257–264. [Google Scholar]
- Msukwa, V.J.; Munthali, C.R.Y.; Nyoka, B.I.; Missanjo, E. Phenology of Sclerocarya birrea (A. Rich.) Hochst. Provenances. Emerg. Sci. J. 2019, 3, 10–22. [Google Scholar] [CrossRef]
- Sène, A.L.; Diallo, A.; Diallo, M.D.; Sagna, M.B.; Ndiaye, O.; Guisse, A. Influence of temperature and rainfall on the phenology of Sclerocarya birrea (A. Rich) Hoscht in the Ferlo Zone (Senegal). Am. J. Agric. For. 2020, 8, 181–189. [Google Scholar]
- Bationo-Kando, P.; Sawadogo, B.; Kiebre, Z.; Kientega, P.; Sawadogo, N.; Nanema, K.R.; Traore, E.R.; Sawadogo, M.; Zongo, J.D. Productivity Characteristics and Development Strategies of Sclerocarya birrea in Burkina Faso. Afr. Crop Sci. J. 2016, 24, 35–47. [Google Scholar] [CrossRef]
- Leakey, R.R.B. Trees: Capturing Useful Traits in Elite Cultivars: An Update. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Leakey, R.R.B., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 155–158. [Google Scholar] [CrossRef]
- Kugedera, A.T. Assessing cultivation practices used for the regeneration of Sclerocarya birrea: A case of Zimbabwe. Octa J. Biosci. Int. 2019, 7, 37–43. [Google Scholar]
- Stadlmayr, B.; Charrondiere, U.R.; Eisenwagen, S.; Jamnadass, R.; Kehlenbeck, K. Nutrient composition of selected indigenous fruits from sub-Saharan Africa. J. Sci. Food Agric. 2013, 93, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Muok, B.O. Potentials and utilization of indigenous fruit trees for food and nutrition security in East Africa. Glob. Adv. Res. J. Agric. Sci. 2019, 8, 40–49. [Google Scholar]
- Muhammad, N.; Omogbai, I.; Maigandi, S.; Abubakar, I.; Shamaki, S. Quantification of Sclerocarya birrea (Marula) fruits as feed supplement for ruminants in dry sub-humid zone of Nigeria. World Sci. News 2016, 46, 88–99. [Google Scholar]
- Todou, G.; Nnanga, J.F.; Bayé-Niwah, C.; Kamblaba, P.; Froumsia, M.; A, I. Ethnobotanical study of indigenous woody plants in traditional agroforestry of the Sudano-Sahelian zone of Cameroon: Case of Mandara Mountains. Int. J. Agric. Environ. Sci. 2019, 6, 1–8. [Google Scholar]
- Fujioka, Y. Diversification of Marula Uses in South Africa How local people involved in the use of non-timber forest products by company/cooperative? In Proceedings of the General Meeting of the Association of Japanese Geographers Annual Meeting of the Association of Japanese Geographers, Spring, Tokyo, Japan, 26–28 March 2014; p. 100257. [Google Scholar]
- Enweremadu, C.C.; Rutto, H.L. Optimization and modeling of process variables of biodiesel production from marula oil using response surface methodology. J. Chem. Soc. Pak. 2015, 37, 256–265. [Google Scholar]
- Molelekoa, T.B.; Regnier, T.; da Silva, L.S.; Augustyn, W.A. Potential of marula (Sclerocarya birrea subsp. caffra) waste for the production of vinegar through surface and submerged fermentation. S. Afr. J. Sci. 2018, 114, 1–6. [Google Scholar] [CrossRef]
- Bille, P.; Shikongo-Nambabi, M.; Cheikhyoussef, A. Value addition and processed products of three indigenous fruits in Namibia. Afr. J. Food Agric. Nutr. Dev. 2013, 13, 7192–7212. [Google Scholar]
- Ngemakwe, P.N.; Remize, F.; Thaoge, M.; Sivakumar, D. Phytochemical and nutritional properties of underutilised fruits in the southern African region. S. Afr. J. Bot. 2017, 113, 137–149. [Google Scholar] [CrossRef]
- Sivakumar, D.; Remize, F.; Garcia, C. Bioactive compounds in Southern African fruits. In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy, H.N., Bapat, V.A., Eds.; Springer: Cham, Switzerland, 2020; pp. 607–623. [Google Scholar] [CrossRef]
- Louis, H.; Akakuru, O.; Linus, M.; Innocent, J.; Amos, P. Qualitative and Quantitative Phytochemical Analyses of Sclerocarya birrea and Sterculia setigera in Kem and Yola, Adamawa State, Nigeria. Am. J. Biomed. Res. 2018, 6, 1–10. [Google Scholar]
- Leakey, R.R.B.; Page, T. The ‘ideotype concept’ and its application to the selection of ‘AFTP’ cultivars. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Leakey, R.R.B., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 267–272. [Google Scholar]
- Bello-Bravo, J.; Lovett, P.N.; Pittendrigh, B.R. The Evolution of Shea Butter’s “Paradox of paradoxa” and the Potential Opportunity for Information and Communication Technology (ICT) to improve Quality, Market Access and Women’s Livelihoods across Rural Africa. Sustainability 2015, 7, 5752–5772. [Google Scholar] [CrossRef]
- Aleza, K.; Villamor, G.B.; Wala, K.; Dourma, M.; Atakpama, W.; Batawila, K.; Akpagana, K. Woody species diversity of Vitellaria paradoxa CF Gaertn traditional agroforests under different land management regimes in Atacora district (Benin, West Africa). Int. J. Biodivers. Conserv. 2015, 7, 245–253. [Google Scholar]
- Zizka, A.; Thiombiano, A.; Dressler, S.; Nacoulma, B.M.; Ouédraogo, A.; Ouédraogo, I.; Ouédraogo, O.; Zizka, G.; Hahn, K.; Schmidt, M. Traditional plant use in Burkina Faso (West Africa): A national-scale analysis with focus on traditional medicine. J. Ethnobiol. Ethnomed. 2015, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guissou, K.M.L.; Kristiansen, T.; Lykke, A.M. Local perceptions of food plants in eastern Burkina Faso. Ethnobot. Res. Appl. 2015, 14, 199–209. [Google Scholar] [CrossRef][Green Version]
- Aleza, K.; Villamor, G.B.; Nyarko, B.K.; Wala, K.; Akpagana, K. Shea (Vitellaria paradoxa Gaertn CF) fruit yield assessment and management by farm households in the Atacora district of Benin. PLoS ONE 2018, 13, e0190234. [Google Scholar] [CrossRef] [PubMed]
- Aleza, K.; Wala, K.; Bayala, J.; Villamor, G.B.; Dourma, M.; Atakpama, W.; Akpagana, K. Population structure and regeneration status of Vitellaria paradoxa (CF Gaertner) under different land management regimes in Atacora department, Benin. Agrofor. Syst. 2015, 89, 511–523. [Google Scholar] [CrossRef]
- Gnonlonfoun, I.; Assogbadjo, A.E.; Gnanglè, C.P.; Kakaï, R.L.G. New indicators of vulnerability and resilience of Agric. Syst. to climate change in West Africa. Agron. Sustain. Dev. 2019, 39, 1–12. [Google Scholar] [CrossRef]
- Dimobe, K.; Tondoh, J.E.; Weber, J.C.; Bayala, J.; Ouédraogo, K.; Greenough, K. Farmers’ preferred tree species and their potential carbon stocks in southern Burkina Faso: Implications for biocarbon initiatives. PLoS ONE 2018, 13, e0199488. [Google Scholar] [CrossRef]
- Dimobe, K.; Ouédraogo, A.; Ouédraogo, K.; Goetze, D.; Stein, K.; Schmidt, M.; Nacoulma, B.M.I.; Gnoumou, A.; Traoré, L.; Porembski, S. Climate change reduces the distribution area of the shea tree (Vitellaria paradoxa CF Gaertn.) in Burkina Faso. J. Arid. Environ. 2020, 181, 104237. [Google Scholar] [CrossRef]
- Elias, M. Gender, knowledge-sharing and management of shea (Vitellaria paradoxa) parklands in central-west Burkina Faso. J. Rural. Stud. 2015, 38, 27–38. [Google Scholar] [CrossRef]
- Avaligbé, Y.J.F.; Chabi, F.O.; Gnanglè, C.P.; Bello, O.D.; Yabi, I.; Ahoton, L.; Saïdou, A. Modelling the Current and Future Spatial Distribution Area of Shea Tree (Vittelaria paradoxa CF Gaertn) in the Context of Climate Change in Benin. Am. J. Clim. Chang. 2021, 10, 263–281. [Google Scholar] [CrossRef]
- Djekonbe, P.; Avana, T.; Womeni, M. Influence des pressions parasitaires (Loranthaceae) et anthropiques sur la dynamique des peuplements du karité (Vitellaria paradoxa Gaertn. CF) au Tchad. Rev. Sci. Tech. Environ. Du Bassin Du Congo-RIFFEAC 2018, 11, 39–48. [Google Scholar]
- Sanou, J.; Bazié, H.R.; Bayala, J. Treating shea trees as crops improves women’s livelihoods in Burkina Faso. In Multifunctional Land Uses in Africa; Simelton, E., Ostwald, M., Eds.; Routledge: New York, NY, USA, 2019; pp. 47–60. [Google Scholar]
- Karambiri, M.; Elias, M.; Vinceti, B.; Grosse, A. Exploring local knowledge and preferences for shea (Vitellaria paradoxa) ethnovarieties in Southwest Burkina Faso through a gender and ethnic lens. For. Trees Livelihoods 2017, 26, 13–28. [Google Scholar] [CrossRef]
- Gwali, S.; Vaillant, A.; Nakabonge, G.; Okullo, J.B.L.; Eilu, G.; Muchugi, A.; Bouvet, J.-M. Genetic diversity in shea tree (Vitellaria paradoxa subspecies nilotica) ethno-varieties in Uganda assessed with microsatellite markers. For. Trees Livelihoods 2015, 24, 163–175. [Google Scholar] [CrossRef]
- Yao, S.D.M.; DIarrassouba, N.; Attikora, A.; Fofana, I.J.; Dago, D.N.; Silue, S.; Vanderschuren, H. Morphological diversity patterns among selected elite Shea trees (Vitellaria paradoxa CF Gaertn.) from Tchologo and Bagoué districts in Northern Côte d’Ivoire. Int. J. Genet. Mol. Biol. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Merinosy, M.F.F.; Achigan-Dako, E.G.; Gnanglè, P.C.; Kassa, E.; Boffa, J.-M. Morphotype Classification Criteria, Nomenclature of Shea Varieties [Vitellaria paradoxa (CF Gaertn)], and Influence of Sociocultural Factors on Perceived Shea Natural Variation Across Parklands in Benin. Res. Sq. 2021, 1–26. [Google Scholar] [CrossRef]
- Bondé, L.; Ouédraogo, O.; Ouédraogo, I.; Thiombiano, A.; Boussim, J.I. Variability and estimating in fruiting of shea tree (Vitellaria paradoxa CF Gaertn) associated to climatic conditions in West Africa: Implications for sustainable management and development. Plant Prod. Sci. 2019, 22, 143–158. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Ibrahim, G.; Mohammed, Z.; Oyi, R.A. Studies on the Physico-Chemical Variation of Shea Nuts/Butter. Spec. J. Chem. 2019, 4, 1–7. [Google Scholar]
- Zhang, J.; Kurita, M.; Shinozaki, T.; Ukiya, M.; Yasukawa, K.; Shimizu, N.; Tokuda, H.; Masters, E.T.; Akihisa, M.; Akihisa, T. Triterpene glycosides and other polar constituents of shea (Vitellaria paradoxa) kernels and their bioactivities. Phytochemistry 2014, 108, 157–170. [Google Scholar] [CrossRef]
- Yao, S.D.M.; Diarrassouba, N.; Alui, K.A.; Fofana, V.P.; Ble, P.A.; Diallo, R.; Kone, B. Effect of the Grafting Method on the Recovery and Growth of Juvenile Shea (Vitellaria paradoxa Gaertn CF) Plants Grafted in Nursery. East Afr. Sch. J. Agric. Life Sci. 2020, 3, 406–414. [Google Scholar] [CrossRef]
- Delaney, A.; Dembele, A.; Nombré, I.; Gnane Lirasse, F.; Marshall, E.; Nana, A.; Vickery, J.; Tayleur, C.; Stout, J.C. Local-scale tree and shrub diversity improves pollination services to shea trees in tropical West African parklands. J. Appl. Ecol. 2020, 57, 1504–1513. [Google Scholar] [CrossRef]
- Ingram, V.; Haverhals, M.; Petersen, S.; Elias, M.; Basnett, B.S.; Phosiso, S. Gender and forest, tree and agroforestry value chains: Evidence from literature. In Gender and Forests; Routledge: London, UK, 2016; pp. 251–272. [Google Scholar]
- Aneni, T.I.; Adaigbe, V.C.; Eziashi, E.I.; Esiegbuya, O.D. Insect Pest Management of Post Harvest Shea Fruits in Storage. Int. J. Sci. Food Agric. 2020, 4, 330–337. [Google Scholar] [CrossRef]
- Ibrahim, M.G.; Shehu, A.A.; Dauda, S.M.; Ahmad, D. Design, fabrication and testing of shea nut shelling machine. Int. Food Res. J. 2016, 23, S71–S79. [Google Scholar]
- Lovett, P.N. Shea butter: Properties and processing for use in food. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Specialty Oils and Fats in Food and Nutrition; Talbot, G., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 125–158. [Google Scholar] [CrossRef]
- Seghieri, J. Shea tree (Vitellaria paradoxa Gaertn. f.): From local constraints to multi-scale improvement of economic, agronomic and environmental performance in an endemic Sudanian multipurpose agroforestry species. Agrofor. Syst. 2019, 93, 2313–2330. [Google Scholar] [CrossRef]
- Schmidt, L.; Matunda, B.I.; Ndangalasi, H.J.; Kjær, E.D. Sex ratio of dioecious Allanblackia stuhlmannii (Engl.) Engl. in Tanzanian Usambara forests and farmlands. Agrofor. Syst. 2020, 94, 597–606. [Google Scholar] [CrossRef]
- Crockett, S.L. Allanblackia oil: Phytochemistry and use as a functional food. Int. J. Mol. Sci 2015, 16, 22333–22349. [Google Scholar] [CrossRef] [PubMed]
- Sefah, W.; Sefah, L.; Ofori, H. A preliminary development of an optimised method for analyzing Allanblackia parviflora kernel and seed oils using high-performance thin layer chromatography (HPTLC). J. Chromatogr. Sep. Tech. 2019, 10, 2. [Google Scholar]
- Sefah, W.; Horwitz, P.; Boyce, M.C.; Sefah, L. Oil yields for Allanblackia parviflora (tallow tree) in Ghana: The effects of oil extraction methods, tree morphology and environmental characteristics. Afr. J. Food Sci. 2019, 13, 163–181. [Google Scholar]
- Okonkwo, S.O.; Babalola, O.T.; Oyediran, R. Domestication potential of indigenous fruit trees: Viability and vitality of Cola lepidota (K. Schum) seeds. In Proceedings of the 2nd Commonwealth Forestry Association of Nigeria Chapter Conference, Akure, Nigeria, 2–3 December 2019; O, A.B., Adetogun, A.C., Adejoba, O.R., Osunsina, I.O., Eds.; pp. 364–369. [Google Scholar]
- Mpanda, M.; Munjuga, M.; Reyes, T.; Said, A.; Rutatina, F.; Kimaro, A.; van Noordwijk, M. Allanblackia, butterflies and cardamom: Sustaining livelihoods alongside biodiversity conservation on the forest–agroforestry interface in the East Usambara Mountains, Tanzania. For. Trees Livelihoods 2014, 23, 127–142. [Google Scholar] [CrossRef]
- Ofori, D.; Asomaning, J.; Peprah, T.; Agyeman, V.; Anjarwalla, P.; Tchoundjeu, Z.; Mowo, J.; Jamnadass, R. Addressing constraints in propagation of Allanblackia spp. through seed sectioning and air layering. Curr. Opin. Environ. Sustain. 2015, 6, 123–127. [Google Scholar] [CrossRef]
- Tsobeng, A.; Ofori, D.; Tchoundjeu, Z.; Asaah, E.; Van Damme, P. Improving growth of stockplants and rooting ability of leafy stem cuttings of Allanblackia floribunda Oliver (Clusiaceae) using different NPK fertilizers and periods of application. New For. 2016, 47, 179–194. [Google Scholar] [CrossRef]
- Tsobeng, A.; Asaah, E.; Leakey, R.R.B.; Tchoundjeu, Z.; van Damme, P.; Ofori, D.; Jamnadass, R. Effects of pre-severance irradiance on the growth of Allanblackia floribunda stockplants and on the subsequent rooting capacity of leafy stem cuttings. New For. 2019, 50, 505–517. [Google Scholar] [CrossRef]
- Tsobeng, A.; Asaah, E.; Tchoundjeu, Z.; Van Damme, P.; Ofori, D.; Jamnadass, R. Growth, flowering and fruiting of stecklings, grafts and seedlings of Allanblackia floribunda Oliver (Clusiaceae). Agric. Syst. 2017, 91, 259–270. [Google Scholar] [CrossRef]
- Leakey, R.R.B.; Asaah, E.K. Underutilised species as the backbone of multifunctional agriculture-the next wave of crop domestication. Acta Horticult. 2011, 979, 293–310. [Google Scholar]
- Anjarwalla, P.; Musili, P.M.; Gachuiri, A.; Ofori, D.A. Allanblackia Meeting: Workshop Report; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2015. [Google Scholar]
- Awono, A.; Ingram, V.; Schure, J.; Levang, P. Guide for Small and Medium Enterprises in the Sustainable Non-Timber Forest Product Trade in Central Africa; CIFOR: Bogor, Indonesia, 2013. [Google Scholar]
- Bourou, S.; Bowe, C.; Diouf, M.; Van Damme, P. Ecological and human impacts on stand density and distribution of tamarind (Tamarindus indica L.) in Senegal. Afr. J. Ecol. 2012, 50, 253–265. [Google Scholar] [CrossRef]
- Ebifa-Othieno, E.; Mugisha, A.; Nyeko, P.; Kabasa, J.D. Knowledge, attitudes and practices in tamarind (Tamarindus indica L.) use and conservation in Eastern Uganda. J. Ethnobiol. Ethnomedicine 2017, 13, 1–13. [Google Scholar] [CrossRef]
- Aworh, O.C. Promoting food security and enhancing Nigeria’s small farmers’ income through value-added processing of lesser-known and under-utilized indigenous fruits and vegetables. Food Res. Int. 2015, 76, 986–991. [Google Scholar] [CrossRef]
- Fandohan, A.B.; Salako, V.K.; Assogbadjo, A.E.; Diallo, B.O.; Van Damme, P.; Sinsin, B. Effect of climatic conditions on flowering and fruiting of Tamarindus indica (Fabaceae). J. Hortic. For. 2015, 7, 186–192. [Google Scholar]
- Fandohan, B.; Gouwakinnou, G.N.; Fonton, N.H.; Sinsin, B.; Liu, J. Impact des changements climatiques sur la répartition géographique des aires favorables à la culture et à la conservation des fruitiers sous-utilisés: Cas du tamarinier au Bénin. Biotechnol. Agron. Soc. Environ. (BASE) 2013, 17, 450–462. [Google Scholar]
- Van den Bilcke, N.; Alaerts, K.; Ghaffaripour, S.; Simbo, D.; Samson, R. Physico-chemical properties of tamarind (Tamarindus indica L.) fruits from Mali: Selection of elite trees for domestication. Genet. Resour. Crop Evol. 2014, 61, 537–553. [Google Scholar] [CrossRef]
- Chimsah, F.; Nyarko, G.; Abubakari, A. A review of explored uses and study of nutritional potential of tamarind (Tamarindus indica L.) in Northern Ghana. Afr. J. Food Sci. 2020, 14, 285–294. [Google Scholar] [CrossRef]
- Maruza, I.; Musemwa, L.; Mapurazi, S.; Matsika, P.; Munyati, V.; Ndhleve, S. Future prospects of Ziziphus mauritiana in alleviating household food insecurity and illnesses in arid and semi-arid areas: A review. World Dev. Perspect. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Todou, G.; Doudou, K.; Vroumsia, T. Diversity and local transformation of indigenous edible fruits in Sahelian domain of Cameroon. J. Anim. Plant Sci. 2017, 26, 5289–5300. [Google Scholar]
- Sotelo, M.C.; Weber, J.C.; Silva, D.A.; Andrade, C.; Muniz, G.I.; Garcia, R.A.; Kalinganire, A. Growth and fuelwood properties of five tree and shrub species in the Sahelian and Sudanian ecozones of Mali: Relationships with mean annual rainfall and geographical coordinates. New For. 2014, 45, 179–197. [Google Scholar] [CrossRef]
- Kalinganire, A.; Weber, J.C.; Coulibaly, S. Improved Ziziphus mauritiana germplasm for Sahelian smallholder farmers: First steps toward a domestication programme. For. Trees Livelihoods 2012, 21, 128–137. [Google Scholar] [CrossRef]
- Wiehle, M.; Prinz, K.; Kehlenbeck, K.; Goenster, S.; Mohamed, S.A.; Finkeldey, R.; Buerkert, A.; Gebauer, J. The African baobab (Adansonia digitata, Malvaceae): Genetic resources in neglected populations of the Nuba Mountains, Sudan. Am. J. Bot. 2014, 101, 1498–1507. [Google Scholar] [CrossRef]
- Rousseau, M.; Delvaux, C.; Mwakalukwa, E.E.; Mbwambo, L.; Solefack, M.C.M.; Ravaomanalina, H.B.; Geldenhuys, C.J.; Seintsheng, N.N.; Bourland, N.; Beeckman, H. Prunus africana (Hook. f.) Kalkman (the African Cherry). Med. Aromat. Plants World-Afr. 2017, 3, 127–142. [Google Scholar]
- Nzweundji, J.G.; Konan, K.; Nyochembeng, L.M.; Tchinda, N.D.; Niemenak, N. Improved germination of threatened medicinal Prunus africana for better domestication: Effects of temperature, growth regulators and salts. J. For. Res. 2020, 31, 2403–2411. [Google Scholar] [CrossRef]
- Nzweundji, J.G.; Awasom, I.; Dongfasiteli, N.T.; Niemenak, N.H. A Review of the Classification, Propagation Techniques and Toxicity of Endangered Prunus africana (Hook f.) Kalkman for Its Improved Domestication and Conservation. In Prunus—Classification, Cultivation and Toxicity; Schneider, W., Ed.; Nova Publications: Delhi, India, 2020; pp. 163–185. [Google Scholar]
- Kamko, J.; Tchiechoua, Y.; Ngonkeu, E.; Nzweundji, J.; Tchatat, M.; Eloumou, D.; Mam, C.; Chamedjeu, R.; Tekeu, H.; Lessa, F. Effect of Arbuscular Mycorrhizal Fungi Used as Biofertilizer for the Vegetative Propagation of Prunus africana (Hook. f.) Kalkman. Int. J. Plant Res. 2020, 10, 53–60. [Google Scholar] [CrossRef]
- Komakech, R.; Kim, Y.-G.; Kim, W.J.; Omujal, F.; Yang, S.; Moon, B.C.; Okello, D.; Rahmat, E.; Nambatya Kyeyune, G.; Matsabisa, M.G. A Micropropagation Protocol for the Endangered Medicinal Tree Prunus africana (Hook f.) Kalkman: Genetic Fidelity and Physiological Parameter Assessment. Front. Plant Sci. 2020, 11, 1871. [Google Scholar] [CrossRef]
- Nzweundji, J.G.; Ngwe, M.F.S.N.; Siljak-Yakovlev, S. Insights on cytogenetic of the only strict African representative of genus Prunus (P. africana): First genome size assessment, heterochromatin and rDNA chromosome pattern. Caryol. Int. J. Cytol. Cytosyst. Cytogenet. 2019, 72, 9–19. [Google Scholar]
- Ingram, V.; Loo, J.; Vinceti, B.; Dawson, I.; Muchugi, A.; Duminil, J.; Awono, A.; Asaah, E.; Tchoundjeu, Z. Ensuring the Future of the Pygeum Tree (Prunus africana)-Briefing on Prunus africana Cultivation and Harvesting; LEI Wageningen UR: The Hague, The Netherlands, 2015. [Google Scholar]
- Tchiechoua, Y.H.; Kinyua, J.; Ngumi, V.W.; Odee, D.W. Effect of indigenous and introduced arbuscular mycorrhizal fungi on growth and phytochemical content of vegetatively propagated Prunus africana (Hook. f.) Kalkman provenances. Plants 2020, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Sosef, M.S.; Dauby, G.; Blach-Overgaard, A.; van der Burgt, X.; Catarino, L.; Damen, T.; Deblauwe, V.; Dessein, S.; Dransfield, J.; Droissart, V. Exploring the floristic diversity of tropical Africa. BMC Biol. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, I.N. Biotechnology Application for the Improvement of Nigeria’s Indigenous Tree Species: The Challenges of Micropropagation. Int. J. Sci. Res. Environ. Sci. 2013, 1, 107–114. [Google Scholar] [CrossRef]
- Djeugap, F.; Bernier, L.; Dostaler, D.; Khasa, D.; Fontem, D.; Nwaga, D. Opportunités et contraintes agroforestières de Ricinodendron heudelotii au Cameroun. Int. J. Biol. Chem. Sci. 2013, 7, 344–355. [Google Scholar] [CrossRef]
- Degrande, A.; Gyau, A.; Foundjem-Tita, D.; Tollens, E. Improving smallholders’ participation in tree product value chains: Experiences from the Congo Basin. For. Trees Livelihoods 2014, 23, 102–115. [Google Scholar] [CrossRef]
- Egbe, E.; Kuchambi, I.; Tchoundjeu, Z. Phenotypic variation in fruits and nuts of Cola acuminata in three populations of the centre region of Cameroon. Int. Res. J. Plant Sci. 2013, 4, 236–247. [Google Scholar]
- Ingram, V.; Ewane, M.; Ndumbe, L.N.; Awono, A. Challenges to governing sustainable forest food: Irvingia spp. from southern Cameroon. For. Policy Econ. 2017, 84, 29–37. [Google Scholar] [CrossRef]
- Ingram, V.; Vinceti, B.; van Vliet, N. Wild plant and animal genetic resources. In Handbook of Agricultural Biodiversity; Hunter, D., Guarino, L., Spillane, C., McKeown, P.C., Eds.; Routledge: London, UK, 2017; pp. 65–85. [Google Scholar]
- Tsobeng, A.; Tchoundjeu, Z.; Degrande, A.; Asaah, E.; Takoutsing, B.; Sado, T. Contribution de la domestication participative à la culture des PFNL: Le cas des groupements paysans des zones de forêts et savanes humides au Cameroun. In Vivre et se Nourrir de la Forêt en Afrique Centrale; FAO: Rome, Italy, 2016; pp. 147–157. [Google Scholar]
- Caspa, R.G.; Tchouamo, I.R.; Mate Mweru, J.P.; Amang, M.J. Marketing Ricinodendron heudelotii kernels and Gnetum spp. leaves around Lobeke National Park, East Cameroon. Tropicultura 2018, 36, 565–577. [Google Scholar]
- Njoh, G.E.; Wanie, C.M. Spatial distribution and species abundance area of Non Timber Forest Products in the Mount Cameroon National Park and adjoining forest zones. Int. J. For. Anim. Fish. Res. 2018, 2, 113–122. [Google Scholar] [CrossRef]
- Roques, E.; Lachaux, C.; Tournebize, T.; Epanda, A.M.; Akongongol, M.M. A social and economic model to protect biodiversity: Sustainable oilseeds value chains from Non-Timber Forest Products (NTFP) to protect the Dja Faunal Reserve (DFR) in Cameroon. OCL-Oilseeds Fats Crops Lipids 2019, 26, 1–8. [Google Scholar]
- Olufunmilayo, E.D.; Jimoh, K.A.; Oyewole, S.O.; Ayeni, A.E. Non-Timber Forest Products (NTFPs) as a means of Livelihood and Safety Net among the Rurals in Nigeria: A Review. Am. J. Sci. Manag. 2019, 6, 27–31. [Google Scholar]
- Adelani, D.; Oni, B.; Ariyo, O. Effect of leaflitters of nitrogen fixing acacia trees on the growth of African star apple (Chrysophyllum albidum g. don): A step towards enhancing the growth of an endangered species. J. Res. For. Wildl. Environ. 2020, 12, 130–140. [Google Scholar]
- Caspa, R.; Myambi, G.; Amang, M.J.; Mabe, M.; Nwegueh, A.; Foahom, B. Socio-economic Benefits of Non-timber Forest Products to the AFCOE2M Communities of Southern Cameroon. Sustain. Agric. Res. 2020, 9, 30–38. [Google Scholar] [CrossRef]
- Hirai, M.; Yasuoka, H. It’s Not the Availability, But the Accessibility that Matters: Ecological and Economic Potential of Non-Timber Forest Products in Southeast Cameroon. Afr. Study Monogr. 2020, 60, 59–83. [Google Scholar]
- Ingram, V. Forest to farm to market interfaces for non-timber forest products in Central Africa. Nat. Faune 2012, 26, 43–48. [Google Scholar]
- Fongnzossie, E.; Nkongmeneck, B.-A. Sustainability Assessment of Non Timber Forest Products in South-Eastern Cameroon Rainforests. Sci. Educ. 2016, 4, 66–74. [Google Scholar]
- Momo, S.M.C.; Nguetsop, V.F.; Dongmo, N.; Kenguem Kinjouo Ghislain, J.; Avana-Tientcheu, M.L. The effects of some ecological and anthropic factors on the survival and bark recovery rate of Prunus africana (Rosaceae) in South-West Cameroon. Int. J. Curr. Res. Biosci. Plant Biol. 2016, 3, 25–31. [Google Scholar]
- Momo, S.M.C.; Temgoua, L.F.; Ngueguim, J.R.; Nkongmeneck, B. Comparison of plant communities between primary and secondary tropical forests of Mount Oku, Cameroon. J. Ecol. Nat. Environ. 2016, 8, 163–174. [Google Scholar] [CrossRef]
- Guedje, N.M.; Tadjouteu, F.; Onana, J.M.; Nga, E.N.; Ndoye, O. Garcinia lucida Vesque (Clusiaceae): From traditional uses to pharmacopeic monograph for an emerging local plant-based drug development. J. Appl. Biosci. 2017, 109, 10594–10608. [Google Scholar] [CrossRef]
- Yogom, B.T.; Avana-Tientcheu, M.-L.; Mboujda, M.F.M.; Momo, S.T.; Fonkou, T.; Tsobeng, A.; Barnaud, A.; Duminil, J. Ethnicity Differences in Uses and Management Practices of Bitter Kola Trees (Garcinia kola) in Cameroon. Econ. Bot. 2020, 74, 429–444. [Google Scholar] [CrossRef]
- Kamga, Y.B.; Nguetsop, V.F.; Caroline, M.S.M.; Riera, B. Diversité floristique des ligneux et structure des formations à Garcinia kola Heckel dans les régions du Centre et de l’Est, Cameroun. Eur. Sci. J. 2018, 14, 451–484. [Google Scholar] [CrossRef]
- Avana-Tientcheu, M.L.; Momo, S.M.C.; Kamga, Y.B.; Nguetsop, V.F. Vulnerability assessment of Gnidia glauca (Thymelaeaceae) exploitation, traditional uses and domestication potential in the community forest of Kilum-Ijim, North Western Cameroon. J. Ecol. Nat. Environ. 2018, 10, 182–191. [Google Scholar]
- Ngansop, T.M.; Biye, E.H.; Fongnzossie, F.E.; Forbi, P.F.; Chimi, D.C. Using transect sampling to determine the distribution of some key non-timber forest products across habitat types near Boumba-Bek National Park, Southeast Cameroon. BMC Ecol. 2019, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Gallois, S.; van Andel, T.; Heger, T.; Sonké, B.; Henry, A.G. Comparing apples and pears: The hidden diversity of central african bush mangoes (Irvingiaceae). Econ. Bot. 2020, 74, 178–194. [Google Scholar] [CrossRef]
- Foundjem-Tita, D.; Speelman, S.; D’Haese, M.; Degrande, A.; Van Huylenbroeck, G.; Van Damme, P.; Tchoundjeu, Z. A tale of transaction costs and forest law compliance: Trade permits for Non Timber Forests Products in Cameroon. For. Policy Econ. 2014, 38, 132–142. [Google Scholar] [CrossRef]
- Foundjem-Tita, D.; D’Haese, M.; Speelman, S.; Degrande, A.; Gyau, A.; Van Damme, P.; Tchoundjeu, Z.; Van Huylenbroeck, G. Would strictly enforced forestry regulations affect farmers’ stated intentions to plant indigenous fruits trees? Insights from Cameroon. Food Policy 2014, 49, 95–106. [Google Scholar] [CrossRef]
- Gyau, A.; Faith, A.N.; Fondjem-Tita, D.; Ajaga, N.; Catacutan, D. Small-holder farmers’ access and rights to land: The case of Njombé in the littoral region of Cameroon. Afr. Focus 2014, 27, 23–39. [Google Scholar] [CrossRef]
- Laird, S.; Wynberg, R. Diversity and change in the commercial use of genetic resources: Implications for access and benefit-sharing policy. Int. J. Ecol. Econ. Stat. 2012, 26, 1–14. [Google Scholar]
- Wiersum, K.; Ingram, V.; Ros-Tonen, M. Governing access to resources and markets in non-timber forest product chains. For. Trees Livelihoods 2014, 23, 6–18. [Google Scholar] [CrossRef]
- Ingram, V.J. Win-Wins in Forest Product Value Chains? How Governance Impacts the Sustainability of Livelihoods Based on Non-Timber Forest Products from Cameroon; African Studies Centre: Leiden, The Netherlands, 2014. [Google Scholar]
- Leakey, R.R.B. Non-Timber Forest Products—A misnomer. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Leakey, R.R.B., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 97–98. [Google Scholar]
- Ene-Obong, H.N.; Sanusi, R.A.; Udenta, E.A.; Williams, I.O.; Anigo, K.M.; Chibuzo, E.C.; Aliyu, H.M.; Ekpe, O.O.; Davidson, G.I. Data collection and assessment of commonly consumed foods and recipes in six geo-political zones in Nigeria: Important for the development of a National Food Composition Database and Dietary Assessment. Food Chem. 2013, 140, 539–546. [Google Scholar] [CrossRef]
- Sneyd, L.Q. Wild food, prices, diets and development: Sustainability and food security in urban Cameroon. Sustainability 2013, 5, 4728–4759. [Google Scholar] [CrossRef]
- Sneyd, L.Q. Zoning in: The contributions of buyam–sellams to constructing Cameroon’s wild food zone. Geoforum 2015, 59, 73–86. [Google Scholar] [CrossRef]
- Bolanle-Ojo, O.; Onyekwelu, J. Socio-economic importance of Chrysophyllum albidum in rainforest and derived savanna ecosystems of Ondo State, Nigeria. Eur. J. Agric. For. Res. 2014, 2, 43–51. [Google Scholar]
- Iroko, O.; Bolanle-Ojo, O.; Williams, O. Participatory domestication of indigenous fruit trees: Contribution to sustainable livelihood in West Africa. In Proceedings of Forests and Forest Products: Key To Sustainable Livelihood, Proceedings of the 4th Biennial National Conference of the Forest and Forest Products Society (FFPS), Ibadan, Nigeria, 22–26 April 2014; FFPS: Akure, Nigeria, 2014; pp. 475–480. [Google Scholar]
- Malleson, R.; Asaha, S.; Egot, M.; Kshatriya, M.; Marshall, E.; Obeng-Okrah, K.; Sunderland, T. Non-timber forest products income from forest landscapes of Cameroon, Ghana and Nigeria–an incidental or integral contribution to sustaining rural livelihoods? Int. For. Rev. 2014, 16, 261–277. [Google Scholar] [CrossRef]
- Onyekwelu, J.; Stimm, B. Conservation and socio-economic importance of some agroforestry fruit tree species: Farmers’ participation, tree growth characteristics and market assessment. Int. For. Rev. 2014, 16, 22–109. [Google Scholar]
- Angoni, H. Non-timber forest products and their contributions on the income of local residents in the Douala-Edéa Wildlife Reserve of Cameroon. J. Ecol. Nat. Environ. 2015, 7, 263–270. [Google Scholar]
- Ntoko, V.N. Evaluation of Ecotourism Potentials and Sustainable Commercialisation of Non-Timber Forest Products as Alternatives to the Over Exploitation of Natural Resources by Populations Living at the Periphery of the Lobeke National Park. Master’s Thesis, Université Joseph Fourier, Grenoble, France, 2015. [Google Scholar]
- Degrande, A.; Foundjem-Tita, D.; Mbosso, C.; Godwill, N. Augmenter les bénéfices générés par les PFNL à travers le développement de l’entrepreneuriat paysan: L’exemple du njansang (Centre Cameroun). In Vivre et se Nourrir de la Forêt en Afrique Centrale; FAO: Rome, Italy, 2016; pp. 192–201. [Google Scholar]
- Awono, A.; Eba’a Atyi, R.; Foundjem-Tita, D.; Levang, P. Vegetal non-timber forest products in Cameroon, contribution to the national economy. Int. For. Rev. 2016, 18, 66–77. [Google Scholar]
- Nfornkah, B.N.; Martin, T.; Cedric, C.D.; Walter, G.; Frederick, M. Indigenous knowledge on Irvingia gabonensis (bush mango) sustainability in the Takamanda National Park (TNP) communities, South West Cameroon. For. Trees Livelihoods 2018, 27, 257–263. [Google Scholar] [CrossRef]
- Akuailou, E.-N.; Assanvo, B.J.; Chatigre, K.O.; Monsan, V.; Coxam, V.; Kati-Coulibaly, S. Survey on the consumption and study of physicochemical characteristics of Irvingia wombolu vermoesen seeds in Ivory Coast. Int. J. Adv. Res. 2018, 6, 379–392. [Google Scholar]
- Fungo, R.; Tieguhong, J.; Muyonga, J.; Odjo, S.; Tchingsabe, O.; Tchatat, M. Perceived nutrition benefits and socio-demographic factors affecting consumption of forest foods in eastern and southern Cameroon. Afr. Crop Sci. J. 2018, 26, 203–217. [Google Scholar] [CrossRef]
- Zima, G.G.; Mialoundama, F.; Yangakola, J.M.; Kossa, I. Importance Des Produits Forestiers Non Ligneux Medicinaux D’origine Vegetale Et Impacts Des Activites Anthropiques Sur Leur Durabilite Dans Le Sud-Ouest De La Republique Centrafricaine. Eur. Sci. J. 2018, 14, 202–220. [Google Scholar] [CrossRef]
- Forje, G.W.; Martin, T.; Nfornkah, B.N.; Djomo, C.C.; Fokeng, R.M. Bush Mango (Irvingia spp.) As an Important Alternative Livelihood Source for the Indigenes of the Korup National Park Communities, South West Cameroon Bush Mango (Irvingia spp.). Environ. Earth Sci. Res. J. 2019, 6, 141–148. [Google Scholar]
- Fungo, R.; Tieguhong, J.C.; Iponga, D.; Tchatat, M.; Kahindo, J.; Muyonga, J.H.; Donn, P.; Tchingsabe, O.; Kaaya, A.; Ngondi, J.L.; et al. Can wild forest foods contribute to food security and dietary diversity of rural populations adjoining forest concessions? Insights from Gabon, DR Congo and Cameroon. Int. For. Rev. 2021, 22, 1–16. [Google Scholar]
- Iponga, D.; Yobo, C.M.; Ingram, V.; Bengone, N.N.; Ngoye, A. Livelihoods, economic contribution and sustainability of the bush mango (Irvingia gabonensis) value chain from three provinces of Gabon. Int. For. Rev. 2018, 20, 115–129. [Google Scholar] [CrossRef]
- Mahonya, S.; Shackleton, C.M.; Schreckenberg, K. Non-timber forest product use and market chains along a deforestation gradient in southwest Malawi. Front. For. Glob. Change 2019, 71, 1–12. [Google Scholar] [CrossRef]
- Avana-Tientcheu, M.L.; Tiambo, C.K. Breeding and Productivity in Ending Hunger and Achieving Food Security and Nutrition. In Zero Hunger. Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A., Brandli, L., Özuyar, P., Wall, T., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–18. [Google Scholar]
- Takoutsing, B.; Tchoundjeu, Z.; Degrande, A.; Asaah, E.; Gyau, A.; Nkeumoe, F.; Tsobeng, A. Assessing the quality of seedlings in small-scale nurseries in the highlands of Cameroon: The use of growth characteristics and quality thresholds as indicators. Small-Scale For. 2014, 13, 65–77. [Google Scholar] [CrossRef]
- Essien, B.; Essien, J.; Nwite, J.; Ogbu, J.; Okereke, S.; Agunannah, M. Contribution of plant species in homestead farms, to food security and sustainability in Ebonyi state South eastern Nigeria. Afr. J. Plant Sci. 2013, 7, 317–324. [Google Scholar] [CrossRef][Green Version]
- Degrande, A.; Tadjo, P.; Takoutsing, B.; Asaah, E.; Tsobeng, A.; Tchoundjeu, Z. Getting trees into farmers’ fields: Success of rural nurseries in distributing high quality planting material in Cameroon. Small-Scale For. 2013, 12, 403–420. [Google Scholar] [CrossRef]
- Onyekwelu, J.C.; Stimm, B.; Mosandl, R.; Olusola, J. Domestication of Socio-economically Important Forest Food Tree Species: Effects of Light Intensities on Germination and Early Growth of Chrysophyllum albidum and Irvingia gabonensis. In Proceedings of the Agricultural Development within the Rural-Urban Continuum, Tropentag, Stuttgart-Hohenheim, 17–19 September 2013. [Google Scholar]
- Kanmegne, G.; Mbouobda, H.D.; Mbakop, C.N.; Omokolo, D.N. The influence of stockplant fertilization on tissue concentrations of nitrogen, carbohydrates and amino acids and on the rooting of leafy stem cuttings of Cola anomala K. Schum (Malvaceae). New For. 2017, 48, 17–31. [Google Scholar] [CrossRef]
- Padonou, E.; Tovissodé, F.; Idohou, R.; Salako, V.; Fantondji, L.; Vihotogbé, R.; Fandohan, B.; Assogbadjo, A. Pilot assessment of locally acknowledged morphotypes of Irvingia gabonensis (Aubry-Lecomte) Baill. in southwestern Benin (West Africa). Fruits 2017, 72, 306–316. [Google Scholar] [CrossRef]
- Tsobeng, A.; Akem, M.; Avana, M.-L.; Muchugi, A.; Degrande, A.; Tchoundjeu, Z.; Jamnadass, R.; Na’a, F. Tree-to-tree variation in fruits of three populations of Trichoscypha acuminata (Engl.) in Cameroon. Sci. Afr. 2020, 7, e00235. [Google Scholar] [CrossRef]
- Vihotogbé, R.; Idohou, R.; Gebauer, J.; Sinsin, B.; Peterson, A.T. Estimation of cultivable areas for Irvingia gabonensis and I. wombolu (Irvingiaceae) in Dahomey-Gap (West Africa). Agrofor. Syst. 2019, 93, 937–946. [Google Scholar] [CrossRef]
- Jagoret, P.; Kwesseu, J.; Messie, C.; Michel-Dounias, I.; Malezieux, E. Farmers’ assessment of the use value of agrobiodiversity in multispecies systems. An application to cocoa agroforests in Central Cameroon. Agrofor. Syst. 2014, 88, 983–1000. [Google Scholar] [CrossRef]
- Gyau, A.; Franzel, S.; Chiatoh, M.; Nimino, G.; Owusu, K. Collective action to improve market access for smallholder producers of agroforestry products: Key lessons learned with insights from Cameroon’s experience. Curr. Opin. Environ. Sustain. 2014, 6, 68–72. [Google Scholar] [CrossRef]
- Mbolo, M.M.A.; Zekeng, J.C.; Mala, W.A.; Fobane, J.L.; Chimi, C.D.; Ngavounsia, T.; Nyako, C.M.; Menyene, L.F.E.; Tamanjong, Y.V. The role of cocoa Agric. Syst. in conserving forest tree diversity in the Central region of Cameroon. Agric. Syst. 2016, 90, 577–590. [Google Scholar]
- Saj, S.; Torquebiau, E.; Hainzelin, E.; Pages, J.; Maraux, F. The way forward: An agroecological perspective for Climate-Smart Agriculture. Agric. Ecosyst. Environ. 2017, 250, 20–24. [Google Scholar] [CrossRef]
- Awazi, N.P.; Avana-Tientcheu, M.-L. Agroforestry as a sustainable means to farmer–grazier conflict mitigation in Cameroon. Agrofor. Syst. 2020, 94, 2147–2165. [Google Scholar] [CrossRef]
- Atangana, A.R.; Gnangoh, J.Z.; Yao, A.K.; Kouakou, T.d.A.; Mian Ndri Nda, A.; Kouamé, C. Rebuilding Tree Cover in Deforested Cocoa Landscapes in Côte d’Ivoire: Factors Affecting the Choice of Species Planted. Forests 2021, 12, 198–211. [Google Scholar] [CrossRef]
- Rimlinger, A.; Carrière, S.M.; Avana, M.-L.; Nguegang, A.; Duminil, J. The influence of farmers’ strategies on local practices, knowledge, and varietal diversity of the safou tree (Dacryodes edulis) in Western Cameroon. Econ. Bot. 2019, 73, 249–264. [Google Scholar] [CrossRef]
- Maňourová, A.; Leuner, O.; Tchoundjeu, Z.; Van Damme, P.; Verner, V.; Přibyl, O.; Lojka, B. Medicinal potential, utilization and domestication status of bitter kola (Garcinia kola Heckel) in West and Central Africa. Forests 2019, 10, 124–141. [Google Scholar] [CrossRef]
- Dadjo, C.; Nyende, A.B.; Salako, K.V.; Hounkpevi, A.; Assogbadjo, A.E. Socio–economic factors determining conservation and cultivation of Garcinia kola Heckel—a medicinal plant extinct in the wild in Benin. Econ. Bot. 2020, 74, 115–125. [Google Scholar] [CrossRef]
- Rimlinger, A.; Avana, M.-L.; Awono, A.; Chakocha, A.; Gakwavu, A.; Lemoine, T.; Marie, L.; Mboujda, F.; Vigouroux, Y.; Johnson, V. Trees and their seed networks: The social dynamics of urban fruit trees and implications for genetic diversity. PLoS ONE 2021, 16, e0243017. [Google Scholar] [CrossRef] [PubMed]
- Rimlinger, A.; Duminil, J.; Lemoine, T.; Avana, M.-L.; Chakocha, A.; Gakwavu, A.; Mboujda, F.; Tsogo, M.; Elias, M.; Carrière, S.M. Shifting perceptions, preferences and practices in the African fruit trade: The case of African plum (Dacryodes edulis) in different cultural and urbanization contexts in Cameroon. J. Ethnobiol. Ethnomed. 2021, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Makueti, J.; Tchoundjeu, Z.; Tsobeng, A.; Numbisi, F.; Tsafack, S. Local communities’ perception and willingness on sustainable management of a natural threatened resource: Case study of Baillonella toxisperma Pierre in eastern Cameroon. J. Biodivers. Environ. Sci. (JBES) 2015, 6, 74–92. [Google Scholar]
- Guedje, N.M.; Tchamou, N.; Lejoly, J. Tree response to bark harvest: The case of a medicinal species, Garcinia lucida, as source of raw materials for plant-based drug development. J. Appl. Biosci. 2016, 99, 9476–9491. [Google Scholar] [CrossRef]
- Ganglo, C.; Dan, C.; Aoudji, A.K.; Gbetoho, A.J.; Ganglo, J.C. Importance Socio-Économique De Xylopia Aethiopica (Dun) A. Rich. Pour Les Populations Du Sud-Bénin. Eur. Sci. J. 2017, 13, 187–201. [Google Scholar] [CrossRef][Green Version]
- Savi, M.K.; Noumonvi, R.; Chadaré, F.J.; Daïnou, K.; Salako, V.K.; Idohou, R.; Assogbadjo, A.E.; Kakaï, R.G. Synergy between traditional knowledge of use and tree population structure for sustainability of Cola nitida (Vent.) Schott. & Endl in Benin (West Africa). Environ. Dev. Sustain. 2019, 21, 1357–1368. [Google Scholar]
- Lawin, I.F.; Houètchégnon, T.; Fandohan, A.B.; Salako, V.K.; Assogbadjo, A.E.; Ouinsavi, C.A.I.N. Connaissances et usages de Cola millenii K. Schum.(Malvaceae) en zones guinéenne et soudano-guinéenne au Bénin. Bois et Forêts des Tropiques 2019, 339, 61–74. [Google Scholar] [CrossRef]
- Tcheghebe, O.T.; Seukep, A.J.; Tatong, F.N. A review on traditional uses, phytochemical composition and pharmacological profile of Canarium schweinfurthii Eng. Nat. Sci. 2016, 14, 17–22. [Google Scholar]
- Kuete, V. Medicinal Spices and Vegetables from Africa: Therapeutic Potential against Metabolic, Inflammatory, Infectious and Systemic Diseases; Academic Press: San Diego, CA, USA, 2017. [Google Scholar]
- Tsewoue, M.R.; Avana-Tientcheu, M.L.; Tchoundjeu, Z. Étude ethnobotanique et contribution de Canarium schweinfurthii (Engl)(Burseraceae) aux services écosystémiques des agroforêts à base de caféiers dans le Département de Bamboutos (Ouest, Cameroun). J. Appl. Biosci. 2019, 135, 13808–13820. [Google Scholar] [CrossRef]
- Traoré, L.; Hien, M.; Ouédraogo, I. Usages, disponibilité et stratégies endogènes de préservation de Canarium schweinfurthii (Engl.)(Burseraceae) dans la région des Cascades (Burkina Faso)-Uses, availability and endogenous conservation strategies of Canarium schweinfurthii. Ethnobot. Res. Appl. 2021, 21, 1–17. [Google Scholar]
- Zanh, G.G.; Barima, Y.S.S.; Kouakou, K.A.; Sangne, Y.C. Usages des produits forestiers non-ligneux selon les communautés riveraines de la forêt classée du Haut-Sassandra (Centre-Ouest de la Côte d’Ivoire). Int. J. Pure App. Biosci. 2016, 4, 212–225. [Google Scholar]
- Omotayo, A.O.; Aremu, A.O. Underutilized African indigenous fruit trees and food–nutrition security: Opportunities, challenges, and prospects. Food Energy Secur. 2020, 9, e220. [Google Scholar] [CrossRef]
- Kouebou, C.; Achu, M.; Nzali, S.; Chelea, M.; Bonglaisin, J.; Kamda, A.; Djiele, P.; Yadang, G.; Ponka, R.; Newilah, G.N. A review of composition studies of Cameroon traditional dishes: Macronutrients and minerals. Food Chem. 2013, 140, 483–494. [Google Scholar] [CrossRef]
- Fasogbon, B.M.; Taiwo, K.A.; Adeniran, H.A. Sensory profiling of ogbono soup mix and its nutritional characterisation. J. Appl. Res. Child. 2017, 8, 552–563. [Google Scholar]
- Tata-Ngome, P.I.; Shackleton, C.; Degrande, A.; Tieguhong, J.C. Addressing constraints in promoting wild edible plants’ utilization in household nutrition: Case of the Congo Basin forest area. Agric. Food Secur. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Tata-Ngome, P.I.; Shackleton, C.; Degrande, A.; Nossi, E.J.; Ngome, F. Assessing household food insecurity experience in the context of deforestation in Cameroon. Food Policy 2019, 84, 57–65. [Google Scholar] [CrossRef]
- Ijang, T.; Shackleton, C.; Degrande, A. Knowledge and knowledge sources on the importance of fruits for nutritional security are unaffected by deforestation status in Cameroon. Fruits Int. J. Trop. Subtrop. Hortic. 2019, 74, 272–281. [Google Scholar] [CrossRef]
- Ingram, V.; Ndoye, O.; Iponga, D.M.; Tieguhong, J.C.; Nasi, R. Non-timber forest products: Contribution to national economy and strategies for sustainable management. In The Forests of the Congo Basin: State of the Forest 2010; Wasseige, C.D., Marcken, P.D., Bayol, N., Hiol, F.H., Mayaux, P., Desclée, B., Nasi, R., Billand, A., Defourny, P., Eba’a, R., Eds.; Publications Office of the European Union: Luxembourg, 2010; pp. 137–154. [Google Scholar]
- Etowa, E.; Ojogu, O.E.; Odunlami, S.S. Leveraging rural livelihoods with forest conservation in Nigeria: The role of Non-Timber Forest Products. Rev. Agric. Appl. Econ. 2015, 18, 33–42. [Google Scholar]
- Kimengsi, J.N.; Mukong, A.K.; Balgah, R.A. Livelihood diversification and household well-being: Insights and policy implications for forest-based communities in Cameroon. Soc. Nat. Resour. 2020, 33, 876–895. [Google Scholar] [CrossRef]
- Ike, P.C. Original Research Articles Structure, Causality and Price Transmission Tests in Marketing of Irvingia Seed (Ogbono) in Enugu State, Nigeria. Agric. Trop. Et Subtrop. 2014, 47, 29–35. [Google Scholar] [CrossRef]
- Ojogho, O.; Egware, R. Determinants of Irvingia species kernel (ogbono) supply and demand in Delta State, Nigeria. J. Agric. Sci. 2014, 9, 118–128. [Google Scholar] [CrossRef]
- Ingram, V.; Schure, J.; Awono, A.; Ndoye, O. Gender and forest product value chains in the Congo Basin. For. Trees Livelihoods 2013, 23, 67–88. [Google Scholar] [CrossRef]
- Tieguhong, J.C.; Ingram, V.; Mala, W.A.; Ndoye, O.; Grouwels, S. How governance impacts non-timber forest product value chains in Cameroon. For. Policy Econ. 2015, 61, 1–10. [Google Scholar] [CrossRef]
- Termote, C.; Everaert, G.; Meyi, M.B.; Djailo, B.D.A.; Van Damme, P. Wild edible plant markets in Kisangani, Democratic Republic of Congo. Hum. Ecol. 2012, 40, 269–285. [Google Scholar] [CrossRef]
- Iponga, D.M.; Mikolo-Yobo, C.; Lescuyer, G.; Assoumou, F.M.; Levang, P.; Tieguhong, J.C.; Ngoye, A. The contribution of NTFP-gathering to rural people’s livelihoods around two timber concessions in Gabon. Agric. Syst. 2018, 92, 157–168. [Google Scholar]
- Cunningham, A.; Anoncho, V.; Sunderland, T. Power, policy and the Prunus africana bark trade, 1972–2015. J. Ethnopharmacol. 2016, 178, 323–333. [Google Scholar] [CrossRef]
- Ferket, B.; Degrande, A.; Van Damme, P. Improving commercialization of Cola spp. in Cameroon–applying lessons from NTFPs in a context of domestication. Int. For. Rev. 2015, 17, 112–123. [Google Scholar]
- Gyau, A.; Takoutsing, B.; Degrande, A.; Franzel, S. Producers’ motivation for collective action for kola production and marketing in Cameroon. J. Agric. Rural. Dev. Trop. Subtrop. (JARTS) 2012, 113, 43–50. [Google Scholar]
- Cosyns, H.; Van Damme, P.; De Wulf, R.; Degrande, A. Can rural development projects generate social capital? A case study of Ricinodendron heudelotii kernel marketing in Cameroon. Small-Scale For. 2014, 13, 163–182. [Google Scholar] [CrossRef]
- Facheux, C.; Gyau, A.; Russell, D.; Foundjem-Tita, D.; Mbosso, C.; Franzel, S.; Tchoundjeu, Z. Comparison of three modes of improving benefits to farmers within agroforestry product market chains in Cameroon. Afr. J. Agric. Res. 2012, 7, 2336–2343. [Google Scholar] [CrossRef]
- Foundjem-Tita, D.; Haese, M.; Van Damme, P.; Tchoundjeu, Z.; Gyau, A.; Facheux, C.; Mbosso, C. Building long-term relationships between producers and trader groups in the non-timber forest product sector in Cameroon. Afr. J. Agric. Res. 2012, 7, 230–239. [Google Scholar] [CrossRef][Green Version]
- Mbosso, C.; Tabougue, P.; Franzel, S.; Tchoundjeu, Z.; Foundjem-Tita, D. No appropriate technology so far for Ricinodendron heudelotii (Baill. Pierre ex Pax) processing in Cameroon: Performance of mechanized kernel extraction. Afr. J. Agric. Res. 2013, 8, 5741–5751. [Google Scholar]
- Awono, A.; Tchindjang, M.; Levang, P. Will the proposed forest policy and regulatory reforms boost the NTFP sector in Cameroon? Int. For. Rev. 2016, 18, 78–92. [Google Scholar] [CrossRef]
- Ofundem, T.; Ndip, N.R.; Abdon, A.; Patrice, L. Bush mango (Irvingia spp.): Forest and on-farm resource availability and market chains in the Southwest Region of Cameroon. For. Trees Livelihoods 2017, 26, 170–182. [Google Scholar] [CrossRef]
- Degrande, A.; Siohdjie, Y.Y.; Franzel, S. Disseminating agroforestry innovations in Cameroon: Are relay organizations effective? In Agro-Ecological Intensification of Agriculture Systems in the African Highlands; Routledge: New York, NY, USA, 2013; pp. 241–250. [Google Scholar]
- Pichop, G.; Abukutsa-Onyango, M.; Noorani, A.; Nono-Womdim, R. Importance of indigenous food crops in tropical Africa: Case study. Acta Hortic. 2014, 1128, 315–322. [Google Scholar] [CrossRef]
- Ingram, V. Changing governance arrangements: NTFP value chains in the Congo Basin. Int. For. Rev. 2017, 19, 152–169. [Google Scholar] [CrossRef]
- Leakey, R.; Van Damme, P. The role of tree domestication in green market product value chain development. For. Trees Livelihoods 2014, 23, 116–126. [Google Scholar] [CrossRef]
- Ogbu, J.; Umeokechukwu, C. Aspects of fruit biology of three wild edible monkey kola species fruits (Cola spp: Malvaceae). Annu. Res. Rev. Biol. 2014, 4, 2007–2014. [Google Scholar] [CrossRef]
- Jusu, A.; Cuni-Sanchez, A. Priority indigenous fruit trees in the African rainforest zone: Insights from Sierra Leone. Genet. Resour. Crop Evol. 2017, 64, 745–760. [Google Scholar] [CrossRef]
- Lawin, I.F.; Fandohan, A.B.; Salako, K.V.; Assogbadjo, A.E.; Ouinsavi, C.A.I.N. Morphological variability of fruits of Cola millenii K. Schum. from seven phytogeographical districts in Benin: Opportunity for domestication. Genet. Resour. Crop Evol. 2021, 68, 1225–1242. [Google Scholar] [CrossRef]
- Akpovo, A.H.; Fandohan, A.B. Usages, distribution des connaissances traditionnelles et valeur économique de Ricinodendron heudelotii au Bénin. Rev. Maroc. Des Sci. Agron. Vétérinaires 2021, 9, 276–287. [Google Scholar]
- Leakey, R.R.B. A holistic approach to sustainable agriculture: Trees, science and global society. In Agroforestry for Sustainable Agriculture; Mosquera-Losada, M.R., Prabhu, R., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 275–299. [Google Scholar]
- Kengni, E.; Mbofung, C.; Tchoundjeu, Z.; Tchouanguep, F. Sensory evaluation of tropical bush mango (Irvingia gabonensis) fruits. Pak. J. Nutr. 2017, 16, 562–570. [Google Scholar] [CrossRef][Green Version]
- Makueti, J.T.; Tchoundjeu, Z.; Van Damme, P.; Kalinganire, A.; Asaah, E.; Tsobeng, A.C. Methodological approach to indigenous fruit trees breeding: Case of Dacryodes edulis (G. Don) HJ Lam.(Burseraceae) in Cameroon. Int. J. Agron. Agric. Res. 2015, 7, 142–162. [Google Scholar]
- Ondo-Azi, A.; Missang, C.E.; Ndoutoumou, P.N.; Silou, T. Classification of Safou (Dacryodes edulis) fruit size and shape on mass and outer dimensions. IOSR J. Agric. Vet. Sci. 2017, 10, 64–67. [Google Scholar] [CrossRef]
- Ene-Obong, H.; Igile, G.; Ekpo, A.; Egbung, E.; Agbo, M. Variations in the nutrients and bioactive compounds of different accessions of the West African pear (Dacryodes edulis): Implications for dietary intake assessment and health. J. Food Compos. Anal. 2019, 79, 80–86. [Google Scholar] [CrossRef]
- Okonkwo, H.O.; Ubaekwe, R.E.; Okeke, A.N.; Malizu, L.C. Phenotypic (fruit and seed characters) selection of Dacryodes edulis (Don. G. Lam H. J.) tree for vegetative propagation. Niger. J. Environ. Sci. Technol. 2021, 5, 347–353. [Google Scholar] [CrossRef]
- Vihotogbé, R.; van den Berg, R.G.; Sosef, M.S. Morphological characterization of African bush mango trees (Irvingia species) in West Africa. Genet. Resour. Crop Evol. 2013, 60, 1597–1614. [Google Scholar] [CrossRef]
- Vihotogbé, R.; Missihoun, A.; Sinsin, B.; Sosef, M. Genetic diversity of bitter and sweet African bush mango trees (Irvingia spp., Irvingiaceae) in West and Central Africa. Afr. J. Biotechnol. 2015, 14, 3062–3074. [Google Scholar] [CrossRef]
- Adebo, U.; Matthew, J. Multiple Sequence Alignment Reveals Diversity among Eight African Bush Mango (Irvingia gabonensis Aubry-Lecomte ex O’Rorke) Cultivars. J. Exp. Agric. Int. 2021, 91–96. [Google Scholar] [CrossRef]
- Lawal, A.; Rotimi, O.P. Exploring the morphological traits for plus trees domestication of Garcinia kola (Heckel) in Ondo State, Nigeria. In Proceedings of the Nigeria Tropical Biology Association 5th Annual Biodiversity Conference, Akure, Nigeria, 19–20 May 2015; Federal University of Technology: Akure, Nigeria, 2015; pp. 76–81. [Google Scholar]
- Ashiru, M.; Sifau, M.O.; Ogunkanmi, L.A.; Adekoya, K.O.; Oboh, B.O. Morphological Characterization of Garcinia Kola Hackel (Bitter Kola) from Southern Nigeria. Ann. West Univ. Timis. Ser. Biol. 2018, 21, 199–206. [Google Scholar]
- Olawuyi, O.J.; Azeez, A.A. Molecular Evaluation of Garcinia kola Heckel Accessions Using RAPD Markers. Am. J. Mol. Biol. 2019, 9, 41–51. [Google Scholar] [CrossRef]
- Azeez, S.; Matthew, J.; Olaoluwa, E.; Adegboyega, G.; Akinyoola, I. Morphological and molecular studies of three species of Boerhavia L. from Ile-Ife, Nigeria. Ife J. Sci. 2020, 22, 225–236. [Google Scholar] [CrossRef]
- Okonkwo, H.O.; Eric, E.E.; Ejizu, A.N. Rooting failure and flowering induction in marcotted Garcinia kola (Heckel) trees. J. For. Environ. Sustain. Dev. 2020, 6, 100–104. [Google Scholar]
- Komenan, A.O.; Koffi, K.K.; Gbotto, A.A.; Kone, M.; Akaffou, D.S.; Kouakou, K.L.; Kouassi, K.I.; Zoro, B.I.A. Morphological Diversity and Distribution of Garcinia kola Heckel (Clusiaceae) in Two Agro-Ecological Areas of Côte d’Ivoire. Am. J. Plant Sci. 2019, 10, 1871–1887. [Google Scholar] [CrossRef][Green Version]
- Dah-Nouvlessounon, D.; Adoukonou-Sagbadja, H.; Nafan, D.; Adjanohoun, A.; Noumavo, P.A.; Sina, H.; Daouda, B.O.; Baba-Moussa, L. Morpho-agronomic variability of three kola trees accessions [Cola nitida (Vent.) Schott et Endl., Cola acuminata (P. Beauv.) Schott et Endl., and Garcinia kola Heckel] from Southern Benin. Genet. Resour. Crop Evol. 2016, 63, 561–579. [Google Scholar] [CrossRef]
- Dadjo, C.; Toyi, M.; Nyende, A.B.; Salako, K.V.; Assogbadjo, A.E. Impact of land-use on tree and fruit morphometric variation of the bitter kola (Garcinia kola Heckel) in Benin: Insight for domestication and production. J. Hortic. For. 2018, 10, 127–134. [Google Scholar]
- Wood, T.T.; Ohimain, E.I. Molecular and Morphological Diversity Studies of Five Cola (Schott and Endl.) Species in Ibadan, Nigeria. Annu. Res. Rev. Biol. 2020, 35, 1–13. [Google Scholar] [CrossRef]
- Dadegnon, S.; Gbemavo, C.; Ouinsavi, C.; Sokpon, N. Morphological variation and ecological structure of Chrysophyllum albidum G. Don. Int. J. Plant Soil Sci. 2014, 5, 25–39. [Google Scholar] [CrossRef]
- Boboye, O.; Lawal, A.; Oyerinde, O. Assessment of the Genetic Variation in Chrysophyllum albidum G. Don Using CP SSR Marker. Assessment 2018, 23, 35–40. [Google Scholar]
- Tsobeng, A.; Tchoundjeu, Z.; Degrande, A.; Asaah, E.; Bertin, T.; Van Damme, P. Phenotypic variation in Pentaclethra macrophylla Benth (Fabaceae) from the humid lowlands of Cameroon. Afr. Focus 2015, 28, 47–61. [Google Scholar] [CrossRef]
- Avakoudjo, H.G.G.; Idohou, R.; Salako, K.V.; Hounkpèvi, A.; Koné, M.W.; Assogbadjo, A.E. Diversity in tree and fruit traits of Strychnos spinosa Lam. along a climatic gradient in Benin: A step towards domestication. Genet. Resour. Crop Evol. 2021, 68, 2423–2440. [Google Scholar] [CrossRef]
- Todou, G.; Coppens D’Eeckenbrugge, G.; Joly, H.; Akoa, A.; Onana, J.-M.; Achoundong, G. Climatic niche of Dacryodes edulis (G. Don) HJ Lam (Burseraceae), a semi-domesticated fruit tree native to Central Africa. J. Ecol. Nat. Environ. 2013, 5, 231–240. [Google Scholar] [CrossRef][Green Version]
- Tchatchoua, D.T.; Tchoundjeu, Z.; Caspa, R.G. Application of biotechnology for the domestication of Dacryodes edulis (G. Don) HJ Lam in Cameroon: A review. Afr. J. Biotechnol. 2016, 15, 1177–1183. [Google Scholar] [CrossRef]
- Tchinda, N.D.; Wanjala, B.W.; Muchugi, A.; Fotso, F.; Nzweundjl, G.; Ndoumou, D.O.; Skilton, R. Genetic diversity and gene flow revealed by microsatellite DNA markers in some accessions of African Plum (Dacryodes edulis) in Cameroon. Afr. J. Biotechnol. 2016, 15, 511–517. [Google Scholar]
- Rimlinger, A.; Marie, L.; Avana, M.-L.; Bouka, G.; Zekraoui, L.; Mariac, C.; Carrière, S.M.; Duminil, J. New microsatellite markers for Dacryodes edulis (Burseraceae), an indigenous fruit tree species from Central Africa. Mol. Biol. Rep. 2020, 47, 2391–2396. [Google Scholar] [CrossRef]
- Vihotogbé, R.; Houessou, L.; Assogbadjo, A.; Sinsin, B. Germination of seeds from earlier fruits of bitter and sweet african bush mango trees. Afr. Crop Sci. J. 2014, 22, 291–301. [Google Scholar]
- Daudu, S.M.; Adeniji, A.; Nuhu, J.O. Epidermal Studies of the Leaves and Phytochemical Analysis of the Seeds of Irvingia gabonensis and Irvingia wombolu in Anyigba Nigeria. Int. J. Innov. Biol. Chem. Sci. 2020, 13, 59–70. [Google Scholar]
- Doungous, O.; Kalendar, R.; Filippova, N.; Ngane, B.K. Utility of iPBS retrotransposons markers for molecular characterization of African Gnetum species. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020, 154, 587–592. [Google Scholar] [CrossRef]
- Biye, E.; Balkwill, K.; Cron, G. Taste versus shelf life: Intended use should guide selection of indigenous strains of Gnetum L. (Gnetaceae) for domestication in Africa. S. Afr. J. Bot. 2017, 113, 170–181. [Google Scholar] [CrossRef]
- Nyadanu, D.; Lowor, S.T.; Akpertey, A.; Tchokponhoué, D.A.; Pobee, P.; Dogbatse, J.A.; Okyere, D.; Amon-Armah, F.; Brako-Marfo, M. Genetic variability of bioactive compounds and selection for nutraceutical quality in kola [Cola nitida (Vent) Schott. and Endl.]. PLoS ONE 2020, 15, e0242972. [Google Scholar] [CrossRef] [PubMed]
- Atoyebi, J.; Adeyemo, A.; Ajiboye, T.; Tellez-Isaias, G.; Garcia-Casillas, C. Comparison of important micronutrients profile among collections of Irvingia gabonensis and Irvingia wombolu at NACGRAB, Nigeria. Food Sci. Nutr. Res. 2020, 3, 1–4. [Google Scholar] [CrossRef]
- Aniedi, U.E.; Ita, E.E.; Nwofia, G.E. Variability in nutritional traits in Tetrapleura tetraptera (schum and thonn.) taub. From cross river state, Nigeria. Pak. J. Nutr. 2013, 12, 701. [Google Scholar] [CrossRef]
- Kadji, B.R.L.; Kone, F.M.T.; Sika, A.E.; Dabonne, S. Physico-chemical properties of Safou (Dacryodes edulis) fruits grown in Côte d’Ivoire. J. Appl. Biosci. 2016, 105, 10103–10110. [Google Scholar] [CrossRef]
- Oduntan, A.; Babalola, S.; Kenneth-Obosi, O.; Awe, O.; Olabode, I.; Egbekunle, K.; Igwe, H.; Fajinmi, O.; Oduntan, O.; Afolayan, S. Evaluation of proximate, amino acid profile and oil characterisation of Irvingia wombolu fruit pulp and peel. Int. Food Res. J. 2019, 26, 1371–1377. [Google Scholar]
- Omoniyi, S.A.; Idowu, M.A.; Adeola, A.A.; Folorunso, A.A. Chemical composition and industrial benefits of dikanut (Irvingia gabonensis) kernel oil: A review. Nutr. Food Sci. 2017, 47, 741–751. [Google Scholar] [CrossRef]
- Mateus-Reguengo, L.; Barbosa-Pereira, L.; Rembangouet, W.; Bertolino, M.; Giordano, M.; Rojo-Poveda, O.; Zeppa, G. Food applications of Irvingia gabonensis (Aubry-Lecomte ex. O’Rorke) Baill., the ‘bush mango’: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2446–2459. [Google Scholar] [CrossRef]
- Mahunu, G.K.; Quansah, L.; Tahir, H.E.; Mariod, A.A. Irvingia gabonensis: Phytochemical constituents, bioactive compounds, traditional and medicinal uses. In Wild Fruits: Composition, Nutritional Value and Products; Springer: Cham, Switzerland, 2019; pp. 253–270. [Google Scholar]
- Yamoneka, J.; Malumba, P.; Blecker, C.; Gindo, M.; Richard, G.; Fauconnier, M.-L.; Lognay, G.; Danthine, S. Physicochemical properties and thermal behaviour of African wild mango (Irvingia gabonensis) seed fat. LWT-Food Sci. Technol. 2015, 64, 989–996. [Google Scholar] [CrossRef]
- Yamoneka, J.; Malumba, P.; Lognay, G.; Béra, F.; Blecker, C.; Danthine, S. Enzymatic inter-esterification of binary blends containing Irvingia gabonensis seed fat to produce cocoa butter substitute. Eur. J. Lipid Sci. Technol. 2018, 120, 1700423. [Google Scholar] [CrossRef]
- Yamoneka, J.; Malumba, P.; Lognay, G.; Blecker, C.; Danthine, S. Irvingia gabonensis seed fat as hard stock to formulate blends for trans free margarines. LWT 2019, 101, 747–756. [Google Scholar] [CrossRef]
- Mgbemena, N.M.; Ilechukwu, I.; Okwunodolu, F.U.; Chukwurah, J.-V.O.; Lucky, I.B. Chemical composition, proximate and phytochemical analysis of Irvingia gabonensis and Irvingia wombolu peels, seed coat, leaves and seeds. Ovidius Univ. Ann. Chem. 2019, 30, 65–69. [Google Scholar] [CrossRef]
- Adegbehingbe, K.T.; Adeleke, B.S.; Fakoya, S. Solid substrate fermentation of African Bush Mango (Irvingia gabonensis) seeds. J. Adv. Microbiol. 2017, 3, 1–9. [Google Scholar] [CrossRef][Green Version]
- Igwe, O.U.; Obiukwu, U.C. GC/MS Characterization of volatile components of hydrocolloids from Irvingia gabonensis and Mucuna sloanei seeds. Chem. Int. 2017, 3, 410–415. [Google Scholar]
- Ano, A.A.R.R.; Koffi, E.N.d.; Adima, A.A.; N’da, P.K.; Anin, L.A. Composition biochimique et phytochimique des tourteaux des fruits du safoutier (Dacryodes edulis) de Côte d’Ivoire. Int. J. Biol. Chem. Sci. 2018, 12, 2535–2546. [Google Scholar] [CrossRef]
- Eyenga, E.F.; Tang, E.N.; Achu, M.B.L.; Boulanger, R.; Mbacham, W.F.; Ndindeng, S.A. Physical, nutritional, and sensory quality of rice-based biscuits fortified with safou (Dacryodes edulis) fruit powder. Food Sci. Nutr. 2020, 8, 3413–3424. [Google Scholar] [CrossRef]
- Fungo, R.; Muyonga, J.; Kaaya, A.; Okia, C.; Tieguhong, J.C.; Baidu-Forson, J.J. Nutrients and bioactive compounds content of Baillonella toxisperma, Trichoscypha abut and Pentaclethra macrophylla from Cameroon. Food Sci. Nutr. 2015, 3, 292–301. [Google Scholar] [CrossRef]
- Rafiu, B.O.; Agboadediran, O.A.; Babalola, Y.O.; Lawal, I.O. Comparative Studies on Phytochemicals and Physicochemical Compositions of Chrysophyllum albidum G: Don Seeds Oil and Edible Commercial Oil. Eur. J. Med. Plants 2021, 32, 22–33. [Google Scholar] [CrossRef]
- Darfour, B.; Ofosu, D.O.; Asare, K.I.; Ofori, H.; Agbemafle, E.; Atter, A. Antibacterial Activity of Irradiated Powdered Tetrapleura tetraptera Fruit and the Moisture Sorption Isotherm of the Whole Fruit. Eur. J. Med. Plants 2015, 5, 229–236. [Google Scholar]
- Nwidu, L.L.; Alikwe, P.C.N.; Elmorsy, E.; Carter, W.G. An Investigation of potential sources of nutraceuticals from the Niger Delta Areas, Nigeria for attenuating oxidative stress. Medicines 2019, 6, 15. [Google Scholar] [CrossRef]
- Lennox, J.; Agbo, B. Medicinal Properties of Dacryodes edulis against Selected Clinical Bacterial Isolates. Curr. Res. Agric. Hortic. 2020, 1, 27–35. [Google Scholar]
- Ewere, E.G.; Okolie, N.P.; Ndem, J.I.; Oyebadejo, S.A. Immunological and hematological effects of Irvingia gabonensis stem bark in sodium arsenite-exposed rats. GSC Biol. Pharm. Sci. 2021, 15, 027–037. [Google Scholar] [CrossRef]
- Sunday, E.A.; Nnedimma, N.C.; Peter, W.G.; Orlando, G.B. Phytochemistry and Antioxidant Activity of Irvingiagabonensis (Bush mango) Seed Sample. Asian J. Biochem. Genet. Mol. Biol. 2021, 7, 25–34. [Google Scholar] [CrossRef]
- Adepoju, A.J.; Abdul-Hammed, M.; Falade, V.A.; Adedotun, I.O.; Jolaoso, F.O.; Olatunde, S.; Adebayo; Azeez, S.O. Phytochemical screening and antimicrobial activity study of Cucumeropsis mannii and Irvingia gabonensis ethanolic seeds extracts. World J. Pharm. Res. 2021, 10, 176–186. [Google Scholar]
- Azantsa, B.; Kuate, D.; Chakokam, R.; Paka, G.; Bartholomew, B.; Nash, R. The effect of extracts of Irvingia gabonensis (IGOB131) and Dichrostachys glomerata (Dyglomera™) on body weight and lipid parameters of healthy overweight participants. Funct. Foods Health Dis. 2015, 5, 200–208. [Google Scholar] [CrossRef]
- Ofoelo, L.I.; Okpashi, V.E.; Nwodo, O.F.C.; Adaora, N. Assessment of lipid profile indices of alloxan-induced diabetic rats using Irvingia gabonensis seed extracts. Transl. Biomed. 2017, 8, 128–133. [Google Scholar]
- Bassey, S.O.; Ekpe, O.O.; Udefa, A.L.; Essien, N.M.; Eteng, M.U. Ethanol extract of Irvingia gabonensis (Bush Mango) seed improves renal and hepatic functions in wistar rats. Eur. J. Pharm. Med. Res. 2018, 13, 46–50. [Google Scholar]
- Arogba, S.S.; Omada, A.A. Effects of diet substitution with defatted kernels of mango (Mangifera indica) and wild mango varieties (Irvingia gabonensis and Irvingia wombolu) on weight and plasma lipid profile of Wistar rats. Afr. J. Food Sci. 2020, 14, 10–15. [Google Scholar]
- Lee, J.; Chung, M.; Fu, Z.; Choi, J.; Lee, H.-J. The effects of Irvingia gabonensis seed extract supplementation on anthropometric and cardiovascular outcomes: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2020, 39, 388–396. [Google Scholar] [CrossRef]
- Sunday, Z.B.; Madinat, H.; Alhassan, M.W.; Abdullah, N. Nephrocurative Effect of Flavonoids-Rich Fraction of Irvingia gabonensis Seed Extract against Acetaminophen-induced Kidney Damage in Mice. West. J. Med. Biomed. Sci. 2021, 2, 131–141. [Google Scholar]
- Fajinmi, O.; Oduntan, O.; Oduntan, O.; Babalola, O.; Igwe, H.; Egbekunle, K.; Awe, F.; Olabode, I.; Afolayan, S.; Kenneth-obosi, O. Antifungal Activity of Fruit Peels (exocarp) and Pulp (mesocarp) of African Bush Mango (Irvingia wombolu). Int. J. Innov. Sci. Res. Technol. 2020, 5, 510–514. [Google Scholar]
- Sanni, O.; Erukainure, O.L.; Islam, M.S. Dacryodes edulis: Protective antioxidant effects on diabetes pathology. In Pathology: Oxidative Stress and Dietary Antioxidants; Preedy, V.R., Ed.; Elsevier Publishers: London, UK, 2020; pp. 205–212. [Google Scholar]
- Fapohunda, S.; Arinola, A.; Otuneme, O.; Adaramola, B.; Jegede, D.; Adewumi, A.; Aleshinloye, A.; Onigbinde, A. Evaluation of the nutraceutical potential of Garcinia kola Seed Oil. J. Pharmacogn. Phytochem. 2017, 6, 1894–1901. [Google Scholar]
- Leakey, R.R.B.; Tchoundjeu, Z.; Smith, R.I.; Munro, R.C.; Fondoun, J.-M.; Kengue, J.; Anegbeh, P.O.; Atangana, A.R.; Waruhiu, A.N.; Asaah, E.; et al. Evidence that subsistence farmers have domesticated indigenous fruits (Dacryodes edulis and Irvingia gabonensis) in Cameroon and Nigeria. In Multifunctional Agriculture: Achieving Sustainable Development in Africa; Leakey, R.R.B., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 87–96. [Google Scholar]
- Tchokponhoué, D.A.; Achigan-Dako, E.G.; N’Danikou, S.; Nyadanu, D.; Kahane, R.; Houéto, J.; Hotegni, N.V.F.; Odindo, A.O.; Sibiya, J. Phenotypic variation, functional traits repeatability and core collection inference in Synsepalum dulcificum (Schumach & Thonn.) Daniell reveals the Dahomey Gap as a centre of diversity. Sci. Rep. 2020, 10, 1–17. [Google Scholar]
- Achigan-Dako, E.G.; Avohou, E.S.; Linsoussi, C.; Ahanchede, A.; Vodouhe, R.S.; Blattner, F.R. Phenetic characterization of Citrullus spp.(Cucurbitaceae) and differentiation of egusi-type (C. mucosospermus). Genet. Resour. Crop Evol. 2015, 62, 1159–1179. [Google Scholar] [CrossRef]
- Tchokponhoué, D.A.; Achigan-Dako, E.G.; N’Danikou, S.; Nyadanu, D.; Kahane, R.; Odindo, A.O.; Sibiya, J. Comparative analysis of management practices and end-users’ desired breeding traits in the miracle plant [Synsepalum dulcificum (Schumach & Thonn.) Daniell] across ecological zones and sociolinguistic groups in West Africa. J. Ethnobiol. Ethnomed. 2021, 17, 1–20. [Google Scholar]
- Onyekwelu, J.C.; Stimm, B.; Mosandl, R. Seed Germination and Early Growth of Chrysophyllum albidum Seedlings under Different Light Intensities. In Proceedings of the Tropentag 2012: Resilience of Agriculture Systems Against Crises, Göttingen, Germany, 19–21 September 2012. [Google Scholar]
- Anozie, E.; Oboho, E. The Effect of Seed Source and Pre-sowing Treatment on Germination of Canarium schweinfurthii [Engl] Seeds. Asian J. Res. Agric. For. 2019, 4, 1–11. [Google Scholar] [CrossRef]
- Kouakou, K.L.; Kouakou, C.; Koffi, K.K.; Dao, J.; Manahon Martine, B.; Baudoin, J.-P.; Bi, I.A.Z. Effect of mechanical scarification and gibberellins (GA3) on seed germination and growth of Garcinia kola (Heckel). J. Appl. Biosci. 2016, 103, 9811–9818. [Google Scholar]
- Takoutsing, B.; Degrande, A.; Tchoundjeu, Z.; Asaah, E.; Tsobeng, A. Improving the Availability of Quality Planting Materials Through Community-Based Seed and Seedling Systems: The Case of Rural Resource Centres in Cameroon. In Challenges and Opportunities for Agricultural Intensification of the Humid Highland Systems of Sub-Saharan Africa; Vanlauwe, B., van Asten, P., Blomme, G., Eds.; Springer: London, UK, 2014; pp. 307–321. [Google Scholar]
- Mapon, N.I.; Mveyo, N.Y.P.; Dzokou, V.J.; Yana, W.; Tamesse, J.L. Biology of Pseudophacopteron pusillum and Pseudophacopteron eastopi (Hemiptera-Psylloidea: Phacopteronidae), pest of Dacryodes edulis (Burseraceae) in Yaounde, Cameroon. J. Entomol. Zool. Stud. 2021, 9, 407–414. [Google Scholar]
- Kanmegne, G.; Anouma’a, M.; Mbibong, D.; Mbouobda, H.; Fotso; Omokolo, D. Domestication of Cola anomala K. Schum. (Sterculiaceae): Amenability to vegetative propagation. For. Trees Livelihoods 2015, 24, 18–26. [Google Scholar] [CrossRef]
- Kanmegne, G.; Mbouobda, H.; Kamtat, G.; Omokolo, D. Interaction of stockplants shading and exogenous auxin on the propagation of Cola anomala K. Schum (Malvaceae) by cuttings. S. Afr. J. Bot. 2017, 112, 246–252. [Google Scholar] [CrossRef]
- Kouakou, K.L.; Dao, J.P.; Kouassi, K.L.; Beugré, M.M.; Koné, M.; Baudoin, J.-P.; Zoro Bi, I.A. Propagation of Garcinia kola (Heckel) by stem and root cutting. Silva Fenn. 2016, 50, 1–17. [Google Scholar] [CrossRef]
- Dao, J.P.; Kouakou, K.L.; Kouakou, C.; Cherif, M.; Ouedraogo, M.H.; Koffi, K.K.; Bi, I.A.Z. Effect of Leafy and Leafless Greenwood, Softwood and Hardwood Cuttings Success of Garcinia kola (Heckel). Agric. Sci. 2020, 11, 897–911. [Google Scholar]
- Yakubu, F.; Adejoh, O.; Ogunade, J.; Igboanugo, A. Vegetative propagation of Garcinia kola (Heckel). World J. Agric. Sci. 2014, 10, 85–90. [Google Scholar]
- Paluku, A.; Bwama, M.; Okungo, A.; Van Damme, P. Multiplication végétative de Cola acuminata (Pal. de Beauv.) Schott & Endlicher par marcottage à Kisangani, République Démocratique du Congo. Int. J. Biol. Chem. Sci. 2018, 12, 1141–1150. [Google Scholar]
- Oboho, E.; Iyadi, J. Rooting potential of mature stem cuttings of some forest tree species for vegetative propagation. J. Appl. Nat. Sci. 2013, 5, 442–446. [Google Scholar] [CrossRef]
- Paluku, A.; Bwama, M.; Okungo, A.; Tchoundjeu, Z.; van Damme, P. Production de plants d’Anonidium mannii (Oliver) Engler & Diels par la multiplication végétative. Rev. Maroc. Des Sci. Agron. Vétérinaires 2019, 7, 129–136. [Google Scholar]
- Tchinda, N.D.; Messi, H.J.C.M.; Nzweundji, G.; Tsabang, N.; Dongmo, B.; Oumar, D.; Tarkang, P.A.; Caver, A.; Ndoumou, D.O. Improving propagation methods of Ricinodendron heudelotti Baill. from cuttings. S. Afr. J. Bot. 2013, 88, 3–9. [Google Scholar] [CrossRef]
- Caspa, R.G.; Biloso, A.; Akalakou, C.; Mafolo, J.; Tsobeng, A.; Kouodiekong, L.; Tchoundjeu, Z. Nursery substrates and provenances influence rooting performance of juvenile, single-node vine cuttings of Gnetum africanum Welw.(Gnetaceae). Afr. Focus 2014, 27, 7–21. [Google Scholar] [CrossRef]
- Doungous, O.; Minyaka, E.; Medza-Mve, S.D.; Medueghue, A.F.; Ngone, M.A.; Simo, C.; Nsimi, A.M. Improving propagation methods of Gnetum africanum and G. buchholzianum from cuttings for rapid multiplication, domestication and conservation. Agrofor. Syst. 2019, 93, 1557–1565. [Google Scholar] [CrossRef]
- Minyaka, E.; Simo, C.; Medueghue, F.A.; Doungous, O.; Njukeng, J.N.; Niemenak, N.; Omokolo, N.D. Influence of Exogenous Auxins on Phenolic Compounds Contents and Polyphenol oxidasic and Peroxidasic Activities in Root Differentiation in Gnetum spp. Agricult. Sci. 2019, 10, 1073–1089. [Google Scholar]
- Elomo, C.; Nguénayé, B.; Tchoundjeu, Z.; Assah, E.; Tsobeng, A.; Avana, M.-L.; Bell, M.J.; Nkeumoe, F. Multiplication végétative de Dacryodes edulis (G. Don) HJ Lam. par marcottage aérien. Afr. focus 2014, 27, 41–56. [Google Scholar]
- Paluku, A.; Okungo, A.; Bwama, M. Bouturage de Cola acuminata (P. Beauv.) Schott & Endl.: Influence du substrat, de la longueur et de la surface foliaire sur l’enracinement de boutures à Kisangani, RD Congo. J. Appl. Biosci. 2018, 123, 12354–12362. [Google Scholar]
- Ogbu, J.U.; Awodoyin, R.O.; Isienyi, N.C. Effect of phytohormone and phenology on domestication of Pentaclethra macrophylla Benth. by marcotting in derived Savanna Zone of Southeast Nigeria. Niger. Agric. J. 2019, 50, 18–23. [Google Scholar]
- Okonkwo, H.; Olubunmi-Koyejo, A.; Akpan, U. Influence of rooting hormone on the vegetative propagation of Cola lepidota (K. Schum) by marcotting. Niger. J. Agric. Food Environ. 2020, 16, 115–121. [Google Scholar]
- Moupela, C.; Ikabanga, D.U.; Mokea-Niaty, A.; Moussavon Bikoukou, L.h.C.; Lepengue, A.N. Vegetative multiplication test of Santiria trimera (Oliv.) H.J. Lam ex Aubrév., Burseraceae by air layering, Franceville (south east of Gabon). J. Interdiscip. Rech. Sci. 2021, 2, 1–9. [Google Scholar]
- Tsobeng, A.; Asaah, E.; Kang’ethe, S.; Avana-Tientcheu, M.L.; Tchoundjeu, Z.; Muchugi, A.; Jamnadass, R. Amenability of priority indigenous fruit trees of West and Central Africa to grafting. For. Trees Livelihoods 2021, 30, 186–194. [Google Scholar] [CrossRef]
- Asaah, E.K.; Tchoundjeu, Z.; Van Damme, P. Beyond vegetative propagation of indigenous fruit trees: Case of Dacryodes edulis (G. Don) HJ Lam and Allanblackia floribunda Oliv. Afr. Focus 2012, 25, 61–72. [Google Scholar] [CrossRef]
- Atiojio, E.N.; Avana, L.; Makueti, J.; Tchoundjeu, Z.; Asaah, E. Carbon sequestration by Dacryodes edulis: Implication for the opportunities of Payment for Environmental Services (PES). Int. J. Res. 2014, 1, 1006–1020. [Google Scholar]
- Missang, C.E.; Ondo-Azi, A.; Le Quéré, J.-M.; Baron, A. Heterogeneity in safou (Dacryodes edulis (G. Don) HJ Lam) fruit softening behaviour during storage. Int. J. Agron. Plant Prod. 2013, 4, 3205–3215. [Google Scholar]
- Dossou, B.R.; Missang, C.E.; Karou, S.D.; Ameyapoh, Y. Relationship between texture and cell-wall components of safou (Dacryodes edulis (G. Don) HJ Lam) fruits at different storage conditions. J. Appl. Biosci. 2018, 125, 12566–12580. [Google Scholar] [CrossRef]
- Ajayi, S.; Eyong, O. Use of local materials In the preservation of Garcinia kola (Bitter Kola) seeds. J. Res. For. Wildl. Environ. 2016, 8, 1–13. [Google Scholar]
- Mbosso, C.; Degrande, A.; Van Damme, P.; Tsafack, S.; Nimino, G.; Tchoundjeu, Z. Gender differences in knowledge, perception and use of the Ricinodendron heudelotii (Baill. Pierre ex pax) kernel extraction machine. Int. For. Rev. 2015, 17, 124–134. [Google Scholar]
- Mbosso, C.; Degrande, A.; Villamor, G.B.; Van Damme, P.; Tchoundjeu, Z.; Tsafack, S. Factors affecting the adoption of agricultural innovation: The case of a Ricinodendron heudelotii kernel extraction machine in southern Cameroon. Agrofor. Syst. 2015, 89, 799–811. [Google Scholar] [CrossRef]
- Busari, R.; Akpenpuun, T.; Iyanda, M. Development and performance evaluation of a modified Africa Bush Mango (Irvingia gabomensis) cracker. J. Appl. Sci. Environ. Manag. 2019, 23, 291–297. [Google Scholar] [CrossRef]
- Agidi, G.; Liberty, J.; Akingbala, A. Design, Construction and Performance Evaluation of A Bush Mango Juice And Seed Extractor. Int. J. Eng. Res. Technol. (IJERT) 2013, 2, 2601–2608. [Google Scholar]
- Bamidele, O.P.; Ojedokun, O.S.; Fasogbon, B.M. Physico-chemical properties of instant ogbono (Irvingia gabonensis) mix powder. Food Sci. Nutr. 2015, 3, 313–318. [Google Scholar] [CrossRef]
- Tieguhong, J.; Ndoye, O.; Grouwels, S.; Mala, W.; Betti, J. Rural enterprise development for poverty alleviation based on non-wood forest products in Central Africa. Int. For. Rev. 2012, 14, 363–379. [Google Scholar] [CrossRef]
- Bannor, R.K.; Ros-Tonen, M.A.; Mensah, P.O.; Derkyi, M.; Nassah, V.F. Entrepreneurial behaviour among non-timber forest product-growing farmers in Ghana: An analysis in support of a reforestation policy. For. Policy Econ. 2021, 122, 102331. [Google Scholar] [CrossRef]
- Tsafack, S.; Degrande, A.; Franzel, S.; Simpson, B. Farmer-to-Farmer Extension in Cameroon: A Survey of Extension Organisations; ICRAF Working Paper No. 182; ICRAF: Nairobi, Kenya, 2014. [Google Scholar]
- Takoutsing, B.; Tchoundjeu, Z.; Degrande, A.; Asaah, E.; Tsobeng, A. Scaling-up sustainable land management practices through the concept of the rural resource centre: Reconciling farmers’ interests with research agendas. J. Agric. Educ. Ext. 2014, 20, 463–483. [Google Scholar]
- Bayala, J.; Sanou, J.; Teklehaimanot, Z.; Ouedraogo, S.; Kalinganire, A.; Coe, R.; Van Noordwijk, M. Advances in knowledge of processes in soil–tree–crop interactions in parkland systems in the West African Sahel: A review. Agric. Ecosyst. Environ. 2015, 205, 25–35. [Google Scholar] [CrossRef]
- Leßmeister, A.; Heubach, K.; Lykke, A.M.; Thiombiano, A.; Wittig, R.; Hahn, K. The contribution of non-timber forest products (NTFPs) to rural household revenues in two villages in south-eastern Burkina Faso. Agric. Syst. 2018, 92, 139–155. [Google Scholar] [CrossRef]
- Papa, C.; Nzokou, P.; Mbow, C. Farmer Livelihood Strategies and Attitudes in Response to Climate Change in Agrofor. Syst. in Kedougou, Senegal. Environ. Manag. 2020, 66, 218–231. [Google Scholar] [CrossRef]
- Kiptot, E.; Franzel, S.; Degrande, A. Gender, agroforestry and food security in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 104–109. [Google Scholar] [CrossRef]
- Kiptot, E.; Franzel, S. Gender and agroforestry in Africa: A review of women’s participation. Agric. Syst. 2012, 84, 35–58. [Google Scholar] [CrossRef]
- Segnon, A.C.; Achigan-Dako, E.G.; Gaoue, O.G.; Ahanchédé, A. Farmer’s knowledge and perception of diversified farming systems in sub-humid and semi-arid areas in Benin. Sustainability 2015, 7, 6573–6592. [Google Scholar] [CrossRef]
- Agbo, R.I.; Idohou, R.; Vihotogbé, R.; Missihoun, A.A.; Dagba, R.A.; Assogbadjo, A.E.; Agbangla, C. Spatio-temporal dynamics of suitable habitats for Detarium microcarpum Guill. & Perr.(Caesalpiniaceae), a priority food tree species in Benin (West Africa). Modeling Earth Syst. Environ. 2019, 5, 595–604. [Google Scholar]
- Lompo, D.; Vinceti, B.; Konrad, H.; Duminil, J.; Geburek, T. Fine-scale spatial genetic structure, mating, and gene dispersal patterns in Parkia biglobosa populations with different levels of habitat fragmentation. Am. J. Bot. 2020, 107, 1041–1053. [Google Scholar] [CrossRef]
- Agoyi, E.E.; Assogbadjo, A.E.; Gouwakinnou, G.; Okou, F.A.; Sinsin, B. Ethnobotanical Assessment of Moringa oleifera Lam. in Southern Benin (West Africa). Ethnobot. Res. Appl. 2014, 12, 551–560. [Google Scholar] [CrossRef]
- Dembélé, U.; Lykke, A.M.; Koné, Y.; Témé, B.; Kouyaté, A.M. Use-value and importance of socio-cultural knowledge on Carapa procera trees in the Sudanian zone in Mali. J. Ethnobiol. Ethnomed. 2015, 11, 19–23. [Google Scholar] [CrossRef]
- Agbodjento, E.; Klotoé, J.R.; Sacramento, T.I.; Dougnon, V.; Tchabi, F.L.; Déguénon, E.; Atègbo, J.-M. Ethnobotanical knowledge of medicinal plants used in the treatment of male infertility in southern Benin. Adv. Tradit. Med. 2021, 21, 655–673. [Google Scholar] [CrossRef]
- Houndonougbo, J.; Kassa, B.; Salako, V.; Idohou, R.; Assogbadjo, A.; Kakaï, R.G. Perceived variation of fruit traits, and preferences in African locust bean [Parkia biglobosa (Jacq.) Benth.] in Benin: Implications for domestication. Genet. Resour. Crop Evol. 2020, 67, 1315–1329. [Google Scholar] [CrossRef]
- Tiétiambou, F.R.S.; Salako, K.V.; Tohoun, J.R.; Ouédraogo, A. Local preferences for three indigenous oil-seed plants and attitudes towards their conservation in the Kénédougou province of Burkina Faso, West-Africa. J. Ethnobiol. Ethnomed. 2020, 16, 1–16. [Google Scholar] [CrossRef]
- Avakoudjo, H.G.G.; Hounkpèvi, A.; Idohou, R.; Koné, M.W.; Assogbadjo, A.E. Local knowledge, uses, and factors determining the use of Strychnos spinosa organs in Benin (West Africa). Econ. Bot. 2020, 74, 15–31. [Google Scholar] [CrossRef]
- Boafo, Y.A.; Saito, O.; Kato, S.; Kamiyama, C.; Takeuchi, K.; Nakahara, M. The role of traditional ecological knowledge in ecosystem services management: The case of four rural communities in Northern Ghana. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2016, 12, 24–38. [Google Scholar] [CrossRef]
- Onyekwelu, J.C.; Olusola, J.A.; Stimm, B.; Mosandl, R.; Agbelade, A.D. Farm-level tree growth characteristics, fruit phenotypic variation and market potential assessment of three socio-economically important forest fruit tree species. For. Trees Livelihoods 2015, 24, 27–42. [Google Scholar] [CrossRef]
- Sanou, L.; Savadogo, P.; Ezebilo, E.E.; Thiombiano, A. Drivers of farmers’ decisions to adopt agroforestry: Evidence from the Sudanian savanna zone, Burkina Faso. Renew. Agric. Food Syst. 2019, 34, 116–133. [Google Scholar] [CrossRef]
- Dembélé, U.; Koné, Y.; Lykke, A.M.; Témé, B.; Kouyaté, A.M. Market value chain of Carapa procera oil and its contribution to income and poverty alleviation in Mali. For. Trees Livelihoods 2019, 28, 253–265. [Google Scholar] [CrossRef]
- Salako, K.V.; Moreira, F.; Gbedomon, R.C.; Tovissodé, F.; Assogbadjo, A.E.; Kakaï, R.L.G. Traditional knowledge and cultural importance of Borassus aethiopum Mart. in Benin: Interacting effects of socio-demographic attributes and multi-scale abundance. J. Ethnobiol. Ethnomed. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Mohamed, F.; Salama, H.; El-Sayed, S.; El-Sayed, H.S.; Zahran, H. Utilization of natural antimicrobial and antioxidant of Moringa oleifera leaves extract in manufacture of cream cheese. J. Biol. Sci. 2018, 18, 92–106. [Google Scholar]
- Hounkpèvi, A.; Tosso, F.; Gbèmavo, D.; Kouassi, E.; Koné, D.; Glèlè Kakaï, R. Climate and potential habitat suitability for cultivation and in situ conservation of the black plum (Vitex doniana Sweet) in Benin, West Africa. Int. J. Agron. Agric. Res. 2016, 8, 67–80. [Google Scholar]
- Agoyi, E.E.; Padonou, E.A.; Amoussa, W.; Assogbadjo, A.E.; Kakai, R.G.; Sinsin, B. Morphological variation, cultivation techniques and management practices of Moringa oleifera in Southern Benin (West Africa). Int. J. Agron. Agric. Res. 2015, 6, 97–105. [Google Scholar]
- Djihounouck, Y.; Diop, D.; Dieng, S.; Sane, S.; Bassène, C.; Mbaye, S.; Noba, K. Diversité et importance socioeconomique des espèces fruitières sauvages comestibles en zone Kasa (Sud-Ouest du Sénégal). Eur. Sci. J. 2018, 14, 352–376. [Google Scholar]
- Assogbadjo, A.; Idohou, R.; Chadare, F.; Salako, V.; Djagoun, C.; Akouehou, G.; Mbairamadji, J. Diversity and prioritization of non timber forest products for economic valuation in Benin (West Africa). Afr. J. Rural. Dev. (AFJRD) 2017, 2, 105–115. [Google Scholar]
- Oyerinde, O.V.; Olusola, J.A.; Adebo, A.A. Variation in morphometric traits of trees, pods and seeds of Parkia biglobosa (Jacq) G. in Southwestern, Nigeria. Int. J. Conserv. Sci. 2018, 9, 185–192. [Google Scholar]
- Hounkpèvi, A.; Salako, V.K.; Donhouédé, J.C.F.; Daï, E.H.; Tovissodé, F.; Glèlè Kakaï, R.; Assogbadjo, A.E. Natural intraspecific trait variation patterns of the wild soursop Annona senegalensis (Annonaceae) along a climatic gradient in Benin, West Africa. Plant Ecol. Evol. 2020, 153, 455–465. [Google Scholar] [CrossRef]
- Salako, V.; Kegbe, A.; Chadare, F.; Kafoutchoni, K.; Amagnidé, A.; Gbedomon, R.; Assogbadjo, A.; Agbangla, C.; Kakaï, R.G. Potential for domestication of Borassus aethiopum Mart., a wild multipurpose palm species in Sub-Saharan Africa. Genet. Resour. Crop Evol. 2019, 66, 1129–1144. [Google Scholar] [CrossRef]
- Houehanou, T.D.; Prinz, K.; Hellwig, F.; Assogbadjo, A.E.; Gebauer, J.; Kakaï, R.L.G.; Sinsin, B. Morphological trait variation and relationships of Afzelia africana Sm. caused by climatic conditions and anthropogenic disturbance in Benin (West Africa). Genet. Resour. Crop Evol. 2019, 66, 1091–1105. [Google Scholar] [CrossRef]
- Akin-Idowu, P.E.; Aduloju, A.O.; Akinyoola, O.I.; Ibitoye, D.O.; Orkpeh, U.; Adebo, U.G.; Olagunju, Y.O. Biodiversity assessment of African locust bean (Parkia biglobosa) accessions from Savanna and Forest zones of Nigeria as revealed by seed storage proteins and RAPD markers. Genet. Resour. 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Weber, J.C.; Montes, C.S.; Kalinganire, A.; Abasse, T.; Larwanou, M. Genetic variation and clines in growth and survival of Prosopis africana from Burkina Faso and Niger: Comparing results and conclusions from a nursery test and a long-term field test in Niger. Euphytica 2015, 205, 809–821. [Google Scholar] [CrossRef]
- Metougui, M.L.; Mokhtari, M.; Benlhabib, O. AMMI Analysis of Yield Performances of Argan Genotypes (Argania spinosa L. Skeels) over Disparate Years and Preselection of Promising Trees. Int. J. Agric. For. 2017, 7, 103–110. [Google Scholar]
- Sarr, M.S.; Seiler, J.R.; Sullivan, J.; Diallo, A.M.; Strahm, B.D. Drought resistance and gum yield performances in a Senegalia senegal (L.) Britton progeny trial in Senegal. New For. 2021, 52, 943–957. [Google Scholar] [CrossRef]
- Kettle, C.; Atkinson, R.; Boshier, D.; Ducci, F.; Dawson, I.; Ekué, M.; Elias, M.; Graudal, L.; Jalonen, R.; Koskela, J. Priorities, challenges and opportunities for supplying tree genetic resources. In Restoring the Earth—The Next Decade; FAO Unasylva: Rome, Italy, 2020; Volume 71, pp. 51–60. [Google Scholar]
- Lompo, D.; Vinceti, B.; Gaisberger, H.; Konrad, H.; Duminil, J.; Ouedraogo, M.; Sina, S.; Geburek, T. Genetic conservation in Parkia biglobosa (Fabaceae: Mimosoideae)-what do we know? Silvae Genet. 2017, 66, 1–8. [Google Scholar] [CrossRef]
- Lykke, A.M.; Padonou, E.A. Carbohydrates, proteins, fats and other essential components of food from native trees in West Africa. Heliyon 2019, 5, e01744. [Google Scholar] [CrossRef]
- Musara, C.; Aladejana, E.B.; Mudyiwa, S.M.; Karavina, C. Parkia biglobosa (Mimosaceae): Botany, Uses, Phytochemical Properties and Pharmacological Potential. J. Pharm. Nutr. Sci. 2020, 10, 101–115. [Google Scholar] [CrossRef]
- Tittikpina, N.K.; Atakpama, W.; Hoekou, Y.; Diop, Y.M.; Batawila, K.; Akapagana, K. Strychnos spinosa Lam: Comprehensive review on its medicinal and nutritional uses. Afr. J. Complementary Altern. Med. 2020, 17, 8–28. [Google Scholar] [CrossRef]
- Klotoé, J.; Dougnon, T.V.; Ategbo, J.; Koudokpon, H.; Dramane, K. Ethnopharmacological survey on antihemorrhagic medicinal plants in south of Benin. Eur. J. Med. Plants 2013, 3, 40–51. [Google Scholar] [CrossRef]
- Fetse, J.P.; Kofie, W.; Adosraku, R.K. Ethnopharmacological Importance of Xylopia aethiopica (Dunal) A. Rich (Annonaceae)—A Review. Br. J. Pharm. Res. 2016, 11, 1–21. [Google Scholar] [CrossRef]
- Metougui, M.L.; Mokhtari, M.; Maughan, P.J.; Jellen, E.N.; Benlhabib, O. Morphological variability, heritability and correlation studies within an Argan tree population (Argania spinosa (L.) Skeels) preserved in situ. Int. J. Agric. For. 2017, 7, 42–51. [Google Scholar]
- Padonou, E.A.; Ahossou, O.D.; Okou, F.O.; Assogbadjo, A.E.; Kakaï, R.G.; Lykke, A.M.; Sinsin, B. Impact of climate on seed morphology and plant growth of Caesalpinia bonduc L. in West Africa. Int. J. Agron. Agric. Res. 2015, 6, 86–96. [Google Scholar]
- Hounsou-Dindin, G. Developing Best Agro-Ecological Practices for African Baobab Tree Adansonia digitata L. Leaves Production in Smallholders Farming Systems in Benin. Ph.D. Dissertation, University of Abomey-Calavi, Abomey-Calavi, Benin, 2018. [Google Scholar]
- Benbya, A.; Alaoui, M.M.; Gaboun, F.; Delporte, F.; Cherkaoui, S. Vegetative propagation of Argania spinosa (L.) Skeels cuttings: Effects of nutrient solution. J. Environ. Agric. Biotechnol. 2018, 3, 1369–1381. [Google Scholar] [CrossRef]
- Benbya, A.; Alaoui, M.M.; Gaboun, F.; Delporte, F.; Chlyah, O.; Cherkaoui, S. Vegetative propagation of Argania spinosa (L.) Skeels cuttings: Effects of auxins and genotype. Adv. Hortic. Sci. 2019, 33, 519–527. [Google Scholar]
- Habou, M.K.A.; Rabiou, H.; Abdou, L.; Mamoudou, B.M.; Yandoù, I.B.; Mahamane, A. Vegetative propagation of Balanites aegyptiaca (L.) Del. by air layering under Sahelian climate in Niger. Asian J. Res. Agric. For. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Mukhtar, R.B. Effect of rooting media and hormone concentrations on vegetative propagation of Balanites aegyptiaca. J. For. Res. 2019, 30, 73–76. [Google Scholar] [CrossRef]
- Abdou, L.; Karim, S.; Habou, R.; Mahamane, A. Vegetative propagation trial of Prosopis africana (Guill. et Perr.) Taub. by air layering under Sudano-Sahelian climate in the South-Central Niger. J. Bot. 2015, 10, 1–6. [Google Scholar] [CrossRef][Green Version]
- Babiker, E.E.; Juhaimi, F.A.L.; Ghafoor, K.; Abdoun, K.A. Effect of drying methods on nutritional quality of young shoots and leaves of two Moringa species as non-conventional fodders. Agric. Syst. 2018, 92, 717–729. [Google Scholar] [CrossRef]
- Jansen, M.; Guariguata, M.R.; Raneri, J.E.; Ickowitz, A.; Chiriboga-Arroyo, F.; Quaedvlieg, J.; Kettle, C.J. Food for thought: The underutilized potential of tropical tree-sourced foods for 21st century sustainable food systems. People Nat. 2020, 2, 1006–1020. [Google Scholar] [CrossRef]
- Binam, J.N.; Place, F.; Djalal, A.A.; Kalinganire, A. Effects of local institutions on the adoption of agroforestry innovations: Evidence of farmer managed natural regeneration and its implications for rural livelihoods in the Sahel. Agric. Food Econ. 2017, 5, 1–28. [Google Scholar] [CrossRef]
- Owombo, P.; Idumah, F.; Adepoju, A. Analysis of farmers’ perception and adoption of agroforestry technology as climate change mitigation strategy in Edo State, Nigeria. World News Nat. Sci. 2018, 21, 16–27. [Google Scholar]
- Franzel, S.; Degrande, A.; Kiptot, E.; Kirui, J.; Kugonza, J.; Preissing, J.; Simpson, B. Farmer-to-farmer extension. In What Works in Rural Advisory Services? Global Good Practice Notes; Global Forum for Rural Advisory Services (GFRAS): Lausanne, Switzerland, 2015; pp. 53–56. [Google Scholar]
- Teshome, M.; Dalle, G.; Asfaw, Z. Comparative Analysis of Woody Species Diversity and Abundance in Mount Duro Natural Forest and Adjacent Agricultural Landscape, Nagelle Arsi, Oromia, Ethiopia. J. Nat. Sci. Res. 2019, 9, 7–16. [Google Scholar]
- Tadesse, S. Views and attitudes of local farmers towards planting, growing, and managing trees in agroforestry system in Basona Worena District, Ethiopia. J. Agric. Sci. Food Res. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Buyinza, J.; Agaba, H.; Ongodia, G.; Eryau, K.; Sekatuba, J.; Kalanzi, F.; Kwaga, P.; Mudondo, S.; Nansereko, S. On-farm conservation and use values of indigenous trees species in Uganda. Res. J. Agric. For. Sci. 2015, 3, 19–25. [Google Scholar]
- FAO. Trees, Forests and Land Use in Drylands: The First Global Assessment-Full Report; FAO Forestry Paper No. 184; Food and Agriculture Organization (FAO): Rome, Italy, 2019. [Google Scholar]
- Zeleke, G.; Dejene, T.; Tadesse, W.; Martín-Pinto, P. Land-Use Impact on Stand Structure and Fruit Yield of Tamarindus indica L. in the Drylands of Southeastern Ethiopia. Life 2021, 11, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Endale, Y.; Derero, A.; Argaw, M.; Muthuri, C. Farmland tree species diversity and spatial distribution pattern in semi-arid East Shewa, Ethiopia. For. Trees Livelihoods 2017, 26, 199–214. [Google Scholar] [CrossRef]
- Abebe, T.; Sterck, F.; Wiersum, K.; Bongers, F. Diversity, composition and density of trees and shrubs in agroforestry homegardens in Southern Ethiopia. Agric. Syst. 2013, 87, 1283–1293. [Google Scholar] [CrossRef]
- Melaku, A.; Ebrahim, M.A. Critical Review on Wild-Edible Fruit Species in Ethiopia. Int. J. For. Res. 2021, 2021, 8538188. [Google Scholar] [CrossRef]
- Latamo, L.L. A review on the indigenous multipurpose agroforestry tree species in Ethiopia: Management, their productive and service roles and constraints. Heliyon 2021, 7, 1–8. [Google Scholar]
- Latamo, L.; Wondmagegn, B. Farmers Local Knowledge on Niche Selection, Management Strategies and Uses of Cordia africana Tree in Agroforestry Practices of Sidama Zone, Southern Ethiopia. Am. J. Agric. For. 2020, 8, 258–264. [Google Scholar]
- Kehlenbeck, K.; Asaah, E.; Jamnadass, R. Diversity of indigenous fruit trees and their contribution to nutrition and livelihoods in sub-Saharan Africa: Examples from Kenya and Cameroon. In Diversifying Food and Diets: Using Agricultural Biodiversity to Improve Nutrition and Health; Fanzo, J., Hunter, D., Borelli, T., Mattei, F., Eds.; Routledge: New York, NY, USA, 2013; pp. 257–269. [Google Scholar]
- Dejene, T.; Agamy, M.S.; Agúndez, D.; Martin-Pinto, P. Ethnobotanical survey of wild edible fruit tree species in lowland areas of Ethiopia. Forests 2020, 11, 177–193. [Google Scholar] [CrossRef]
- Adam, Y.O. Local knowledge on trees utilization and their existing threats in Rashad District of Nuba Mountains, Sudan. J. For. Environ. Sci. 2014, 30, 342–350. [Google Scholar] [CrossRef]
- Ngezahayo, J.; Havyarimana, F.; Hari, L.; Stévigny, C.; Duez, P. Medicinal plants used by Burundian traditional healers for the treatment of microbial diseases. J. Ethnopharmacol. 2015, 173, 338–351. [Google Scholar] [CrossRef]
- d’Avigdor, E.; Wohlmuth, H.; Asfaw, Z.; Awas, T. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. J. Ethnobiol. Ethnomed. 2014, 10, 1–33. [Google Scholar] [CrossRef]
- Tugume, P.; Kakudidi, E.K.; Buyinza, M.; Namaalwa, J.; Kamatenesi, M.; Mucunguzi, P.; Kalema, J. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J. Ethnobiol. Ethnomed. 2016, 12, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.A. Trees for Sustainable Livelihoods in the Horn of Africa: Studies on Aromatic Resins and Other Non-Wood Forest Products in Somalia and Kenya; University of Helsinki: Helsinki, Finland, 2020. [Google Scholar]
- Muisu, F.; Muthike, G.; Mburu, F.; Sirmah, P.; Mulongo, L. Properties of potential wood carving species in Kenya. Adv. Plants Agric. Res. 2019, 9, 1–4. [Google Scholar]
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The global hidden hunger indices and maps: An advocacy tool for action. PLoS ONE 2013, 8, e67860. [Google Scholar] [CrossRef] [PubMed]
- Omujal, F.; Bigirimana, C.; Isubikalu, P.; Malinga, M.; Bizuru, E.; Namutebi, A.; Okullo, J. Morphological and physico-chemical characteristics of Saba comorensis: A highly preferred Lake Victoria basin indigenous fruit tree in Busia District, Eastern Uganda. J. Med. Plants Stud. 2014, 2, 127–136. [Google Scholar]
- Nabatanzi, A.; Nakalembe, I. Wild food plants used by people living with HIV/AIDS in Nakisunga sub-county, Uganda. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11310–11330. [Google Scholar] [CrossRef]
- Shayo, P.F.; Mbega, E.R.; Treydte, A.C. The Potential of Oyster Nuts (Telfairia pedata) for Environmental Conservation and Food Security in Tanzania: A Review. Hum. Ecol. 2021, 49, 495–504. [Google Scholar] [CrossRef]
- Ombati, K.R.; Sirimah, P.K.; Matonyei, T.K. Towards domestication and adoption of Eswata (Markhamia lutea) in Teso North Sub County, Kenya. Res. J. J. For. 2019, 4, 1–13. [Google Scholar]
- Mathew, M.M.; Munjuga, M.R.; Cordeiro, N.J.; Coe, R.; Ofori, D.A.; Simons, A.J.; Sawe, C.T.; Jamnadass, R. Early survival and growth of A llanblackia stuhlmannii (C. lusiaceae): A threatened tropical rainforest tree of high economic value in Tanzania. Afr. J. Ecol. 2015, 53, 572–580. [Google Scholar] [CrossRef]
- Shumsky, S.; Hickey, G.M.; Johns, T.; Pelletier, B.; Galaty, J. Institutional factors affecting wild edible plant (WEP) harvest and consumption in semi-arid Kenya. Land Use Policy 2014, 38, 48–69. [Google Scholar] [CrossRef]
- Chisika, S.N.; Park, J.; Yeom, C. The Impact of Legislation on Sustainability of Farm Forests in Kenya: The Case of Lugari Sub-County in Kakamega County, Kenya. Sustainability 2020, 12, 27–41. [Google Scholar] [CrossRef]
- Abass, A.A.; Ahmed, G.A. Assessment of the Suitability of Using Jakjak Fruits (Azanza garcheana) for Production of Lokum Candy; Sudan University of Science and Technology: Khartoum, Sudan, 2017. [Google Scholar]
- Kunene, E.N.; Nxumalo, K.A.; Ngwenya, M.P.; Masarirambi, M.T. Domesticating and commercialisation of indigenous fruit and nut tree crops for food security and income generation in the Kingdom of Eswatini. Curr. J. Appl. Sci. Technol. 2020, 39, 37–52. [Google Scholar] [CrossRef]
- Mng’omba, S.A.; Akinnifesi, F.K.; Mkonda, A.; Mhango, J.; Chilanga, T.; Sileshi, G.; Ajayi, O.C. Ethnoecological knowledge for identifying Elite phenotypes of the indigenous fruit tree, Uapaca kirkiana in the Miombo woodlands of Southern Africa. Agroecol. Sustain. Food Syst. 2015, 39, 399–415. [Google Scholar] [CrossRef]
- Hall, C.; Macdiarmid, J.I.; Matthews, R.B.; Smith, P.; Hubbard, S.F.; Dawson, T.P. The relationship between forest cover and diet quality: A case study of rural southern Malawi. Food Secur. 2019, 11, 635–650. [Google Scholar] [CrossRef]
- Kucich, D.A.; Wicht, M.M. South African indigenous fruits–Underutilized resource for boosting daily antioxidant intake among local indigent populations? S. Afr. J. Clin. Nutr. 2016, 29, 150–156. [Google Scholar] [CrossRef]
- Ngadze, R.T.; Linnemann, A.R.; Nyanga, L.K.; Fogliano, V.; Verkerk, R. Local processing and nutritional composition of indigenous fruits: The case of monkey orange (Strychnos spp.) from Southern Africa. Food Rev. Int. 2017, 33, 123–142. [Google Scholar] [CrossRef]
- Sardeshpande, M.; Shackleton, C. Wild edible fruits: A systematic review of an under-researched multifunctional NTFP (non-timber forest product). Forests 2019, 10, 467–490. [Google Scholar] [CrossRef]
- Marunda, C.; Doris, M.; Gapare, W.; Alves, T.; Mmkwame, M.; Mduduzi, T. Situational analysis of trends in tree improvement and germplasm production and impacts on sustainable forest management in Southern Africa. Afr. J. Rural. Dev. 2020, 4, 1–31. [Google Scholar]
- Pokwana, S.; Tshidzumba, R.; Chirwa, P. Evaluating the potential of introducing multipurpose tree species in the rural landscapes of Weza, Ugu District municipality, KwaZulu-Natal, South Africa. Trees For. People 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Thondhlana, G.; Ruwanza, S. Homestead tree holdings: Composition, uses and challenges in Checheche Growth Point, South East Lowveld, Zimbabwe. Afr. J. Ecol. 2020, 58, 260–271. [Google Scholar] [CrossRef]
- Nkosi, N.N.; Mostert, T.H.C.; Dzikiti, S.; Ntuli, N.R. Prioritization of indigenous fruit tree species with domestication and commercialization potential in KwaZulu-Natal, South Africa. Genet. Resour. Crop Evol. 2020, 67, 1567–1575. [Google Scholar]
- Jenya, H.; Munthali, C.R.; Missanjo, E.; Chirwa, M.F.; Sagona, W. Variations in Phenology and Morphology of Uapaca kirkiana Müll. Arg. Provenances at Nauko in Liwonde Forest Reserve, Malawi. J. Sci. Res. Rep. 2016, 10, 1–13. [Google Scholar] [CrossRef]
- Mogale, M.M.P.; Raimondo, D.; VanWyk, B.-E. The ethnobotany of Central Sekhukhuneland, South Africa. S. Afr. J. Bot. 2019, 122, 90–119. [Google Scholar] [CrossRef]
- Tshikalange, T.E.; Mophuting, B.C.; Mahore, J.; Winterboer, S.; Lall, N. An ethnobotanical study of medicinal plants used in villages under Jongilanga tribal council, Mpumalanga, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 83–89. [Google Scholar] [CrossRef]
- Adamu, H.; Ushie, O.; Lawal, D.; Oga, I. Phytochemical screening of fruit of Azanza garckeana and root of Acacia macrothyrsa. Int. J. Tradit. Nat. Med. 2013, 3, 19–25. [Google Scholar]
- Dikko, Y.; Khan, M.; Tor-Anyiin, T.; Anyam, J.; Linus, U. In vitro antimicrobial activity of fruit pulp extracts of Azanza garckeana (F. hoffm.) Exell & Hillc. and isolation of one of its active principles, betulinic acid. J. Pharm. Res. Int. 2016, 14, 1–10. [Google Scholar]
- Jacob, C.; Shehu, Z.; Danbature, W.; Karu, E. Proximate analysis of the fruit Azanza garckeana (“Goron Tula”). Bayero J. Pure Appl. Sci. 2016, 9, 221–224. [Google Scholar] [CrossRef]
- Joel, J.M.; Barminas, J.T.; Riki, E.Y.; Yelwa, J.M.; Edeh, F. Extraction and characterization of hydrocolloid pectin from goron tula (Azanza garckeana) fruit. World Sci. News 2018, 101, 157–171. [Google Scholar]
- Maroyi, A. Azanza garckeana fruit tree: Phytochemistry, pharmacology, nutritional and primary healthcare applications as herbal medicine: A review. Res. J. Med. Plants 2017, 11, 115–123. [Google Scholar] [CrossRef]
- Mwove, J. Potential for development of novel food products from Azanza garckeana tree fruit: A review. Int. J. Food Stud. 2021, 10, SI121–SI132. [Google Scholar] [CrossRef]
- Ochokwu, I.; Dasuki, A.; Oshoke, J. Azanza garckeana (Goron Tula) as an edible indigenous fruit in Northeastern part of Nigeria. J. Biol. Agric. Healthc. 2015, 5, 26–31. [Google Scholar]
- Suliman, A.M.E.; Difa, I.Y.; Salih, Z.A. The nutritive value of jakjak (Azanza garckeana l.) fruit and its utilization in juice production. Asian J. Biol. Sci. 2012, 5, 209–215. [Google Scholar] [CrossRef][Green Version]
- Aremu, A.O.; Ncama, K.; Omotayo, A.O. Ethnobotanical uses, biological activities and chemical properties of Kei-apple [Dovyalis caffra (Hook.f. & Harv.) Sim]: An indigenous fruit tree of southern Africa. J. Ethnopharmacol. 2019, 241, 1–24. [Google Scholar] [CrossRef]
- Mothapo, M.J. Physico-Chemical Properties and Selected Nutritional Components of Wild Medlar (Vangueria infausta) Fruit Harvested at Two Harvesting Times; University of Limpopo: Limpopo, South Africa, 2014. [Google Scholar]
- Bvenura, C.; Afolayan, A.J. The role of wild vegetables in household food security in South Africa: A review. Food Res. Int. 2015, 76, 1001–1011. [Google Scholar] [CrossRef]
- Akweni, A.L.; Sibanda, S.; Zharare, G.E.; Zimudzi, C. Fruit-based allometry of Strychnos madagascariensis and S. spinosa (Loganiaceae) in the savannah woodlands of the Umhlabuyalingana municipality, KwaZulu-Natal, South Africa. Trees For. People 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Chawafambira, A.; Sedibe, M.M.; Mpofu, A.; Achilonu, M.C. Probiotic potential, iron and zinc bioaccessibility, and sensory quality of Uapaca kirkiana fruit jam fermented with Lactobacillus rhamnosus Yoba. Int. J. Food Sci. 2020, 2020, 8831694. [Google Scholar] [CrossRef]
- Chawafambira, A.; Sedibe, M.M.; Mpofu, A.; Achilonu, M.C. Uapaca kirkiana, an indigenous fruit tree in sub-Saharan Africa: A comprehensive review. Cogent Food Agric. 2020, 6, 1–20. [Google Scholar] [CrossRef]
- Chawafambira, A.; Sedibe, M.M.; Mpofu, A.; Achilonu, M.C. Bioactive compounds and functional potential of Uapaca kirkiana (muell. Arg.) fruits. Afr. J. Food Agric. Nutr. Dev. 2020, 20, 16490–16508. [Google Scholar] [CrossRef]
- Aremu, A.O.; Moyo, M. Health benefits and biological activities of spiny monkey orange (Strychnos spinosa Lam.): An African indigenous fruit tree. J. Ethnopharmacol. 2022, 283, 1–17. [Google Scholar] [CrossRef]
- Dhliwayo-Chiunzi, P.D.; Matimati, I.; Kachigunda, B. Identification and selection of superior phenotypes of Uapaca kirkiana Muell. Arg. (Euphorbiaceae), a priority indigenous fruit tree of Zimbabwe. Int. Sci. Technol. J. Namib. 2014, 4, 7–22. [Google Scholar]
- Tiawoun, M.A.P.; Tshisikhawe, M.P.; Gwata, E.T. Propagation potential for the conservation of Brackenridgea zanguebarica Oliv., a critically endangered plant species endemic to Vhembe District in Limpopo Province (South Africa). J. Appl. Bot. Food Qual. 2020, 93, 59–65. [Google Scholar] [CrossRef]
- Mabizela, G.S.; Slabbert, M.M.; Bester, C.C. The effect of rooting media, plant growth regulators and clone on rooting potential of honeybush (Cyclopia subternata) stem cuttings at different planting dates. S. Afr. J. Bot. 2017, 110, 75–79. [Google Scholar] [CrossRef]
- Mng’omba, S.A.; Sileshi, G.W. Effect of propagule type and fruiting time on Uapaca kirkiana fruit and seed size, seed germination and seedling growth. Trees 2015, 29, 655–662. [Google Scholar] [CrossRef]
- Meijer, S.S.; Catacutan, D.; Sileshi, G.W.; Nieuwenhuis, M. Tree planting by smallholder farmers in Malawi: Using the theory of planned behaviour to examine the relationship between attitudes and behaviour. J. Environ. Psychol. 2015, 43, 1–12. [Google Scholar] [CrossRef]
- Sheppard, J.P.; Bohn Reckziegel, R.; Borrass, L.; Chirwa, P.W.; Cuaranhua, C.J.; Hassler, S.K.; Hoffmeister, S.; Kestel, F.; Maier, R.; Mälicke, M. Agroforestry: An Appropriate and Sustainable Response to a Changing Climate in Southern Africa? Sustainability 2020, 12, 6796–6826. [Google Scholar] [CrossRef]
- Mbhenyane, X.G. Indigenous foods and their contribution to nutrient requirements. South Afr. J. Clin. Nutr. 2017, 30, 5–7. [Google Scholar]
- Moombe, K.B.; Ham, C.; Clarke, J.; Franzel, S.; Ackerman, P. Consumer preferences for Uapaca kirkiana fruits in Zambia. For. Trees Livelihoods 2014, 23, 248–260. [Google Scholar] [CrossRef]
- Ramashia, S.E.; Mamadisa, F.M.; Mashau, M.E. Effect of Parinari curatellifolia peel flour on the nutritional, physical and antioxidant properties of biscuits. Processes 2021, 9, 1262–1277. [Google Scholar] [CrossRef]
- Leakey, R.R.B. The role of trees in agroecology and sustainable agriculture in the tropics. Annu. Rev. Phytopathol. 2014, 52, 113–133. [Google Scholar] [CrossRef]
- Mabhaudhi, T.; Chimonyo, V.G.P.; Hlahla, S.; Massawe, F.; Mayes, S.; Nhamo, L.; Modi, A.T. Prospects of orphan crops in climate change. Planta 2019, 250, 695–708. [Google Scholar] [CrossRef]
- Mabhaudhi, T.; O’Reilly, P.; Walker, S.; Mwale, S. Opportunities for underutilised crops in southern Africa’s post–2015 development agenda. Sustainability 2016, 8, 302. [Google Scholar] [CrossRef]
- Chivenge, P.; Mabhaudhi, T.; Modi, A.T.; Mafongoya, P. The potential role of neglected and underutilised crop species as future crops under water scarce conditions in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2015, 12, 5685–5711. [Google Scholar] [CrossRef] [PubMed]
- McMullin, S.; Njogu, K.; Wekesa, B.; Gachuiri, A.; Ngethe, E.; Stadlmayr, B.; Jamnadass, R.; Kehlenbeck, K. Developing fruit tree portfolios that link agriculture more effectively with nutrition and health: A new approach for providing year-round micronutrients to smallholder farmers. Food Secur. 2019, 11, 1355–1372. [Google Scholar] [CrossRef]
- Razafindratsima, O.H.; Kamoto, J.F.; Sills, E.O.; Mutta, D.N.; Song, C.; Kabwe, G.; Castle, S.E.; Kristjanson, P.M.; Ryan, C.M.; Brockhaus, M. Reviewing the evidence on the roles of forests and tree-based systems in poverty dynamics. For. Policy Econ. 2021, 131, 1–14. [Google Scholar] [CrossRef]
- Imoro, Z.A.; Imoro, A.Z.; Duwiejuah, A.B.; Abukari, A. Harnessing indigenous technologies for sustainable management of land, water, and food resources amidst climate change. Front. Sustain. Food Syst. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Capitalize on African biodiversity. Nat. News 2017, 548, 1. [Google Scholar] [CrossRef]
- Yirankinyuki, F.F.; Danbature, W.L.; Silas, T.V.; Ojochenemi, Y.E.; Kudi, E.J. Assesment of Larvicidal Activities of Essential Oil Extracted from Country Onion (Afrostyrax lepidophyllus) Seed on Anopheles Mosquito Larvae. Int. J. Public Health Health Syst. 2018, 3, 102–107. [Google Scholar]
- Yirankinyuki, F.F.; Lamayi, D.W.; Muhammad, U.A.; Musa, B. Assessing the suitability of Ricinodendron heudelotii seed oil for paint formulation. IOSR J. Appl. Chem. 2018, 11, 37–42. [Google Scholar]
- Ubani, C.O.L.; Nwadire, F.C.; Igwe, O.U.; Bilar, A.; Agunanne, S.A. Halideion and methanolic extract of Irvingia gabonesis as an enhanced corrosion inhibitory agent on aluminium in HCl. J. Chem. Soc. Niger. 2021, 46, 11–15. [Google Scholar]
- Atangana, A.R.; Khasa, D.; Chang, S.; Degrande, A. Tropical Agroforestry; Springer Science: Dordrecht, The Netherland, 2014. [Google Scholar]
- Leakey, R.R.B. Addressing the causes of land degradation, food/nutritional insecurity and poverty: A new approach to agricultural intensification in the tropics and sub-tropics. In Wake up before It Is too Late: Make Agriculture Truly Sustainable Now for Food Security in a Changing Climate. UNCTAD Trade and Environment Review; Hoffman, U., Ed.; UN Publications: Geneva, Switzerland, 2013; pp. 192–198. [Google Scholar]
- A Multi-Billion-Dollar Opportunity: Repurposing Agricultural Support to Transform Food Systems. Available online: https://www.unep.org/resources/repurposing-agricultural-support-transform-food-systems (accessed on 1 January 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).