The Impact of the Neophyte Tree Fraxinus pennsylvanica [Marshall] on Beetle Diversity under Climate Change
Abstract
:1. Introduction
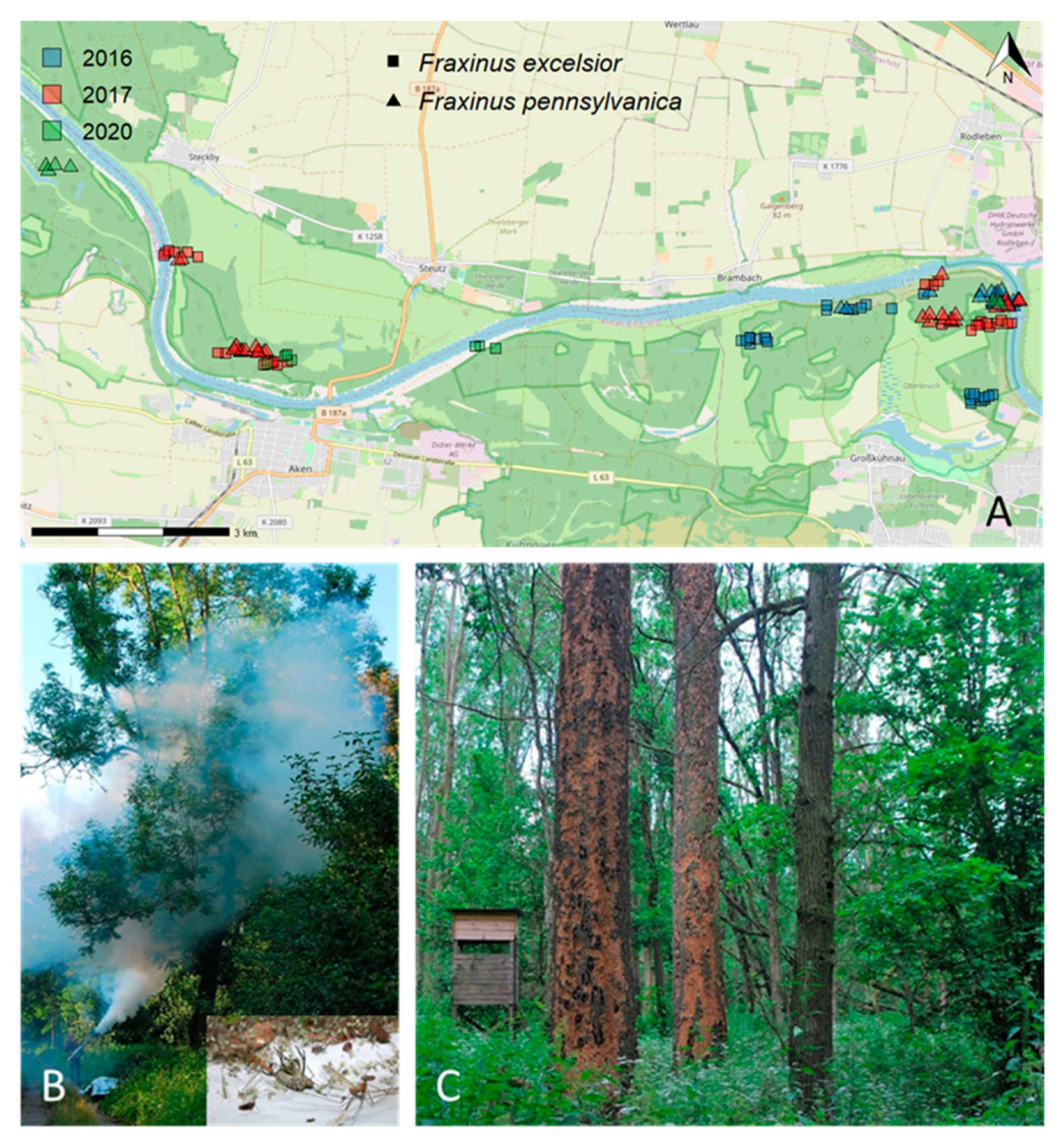
2. Materials and Methods
2.1. Insecticidal Knock-Down (Fogging)
2.2. Beetle Identification and Guild Assignment
2.3. Statistics
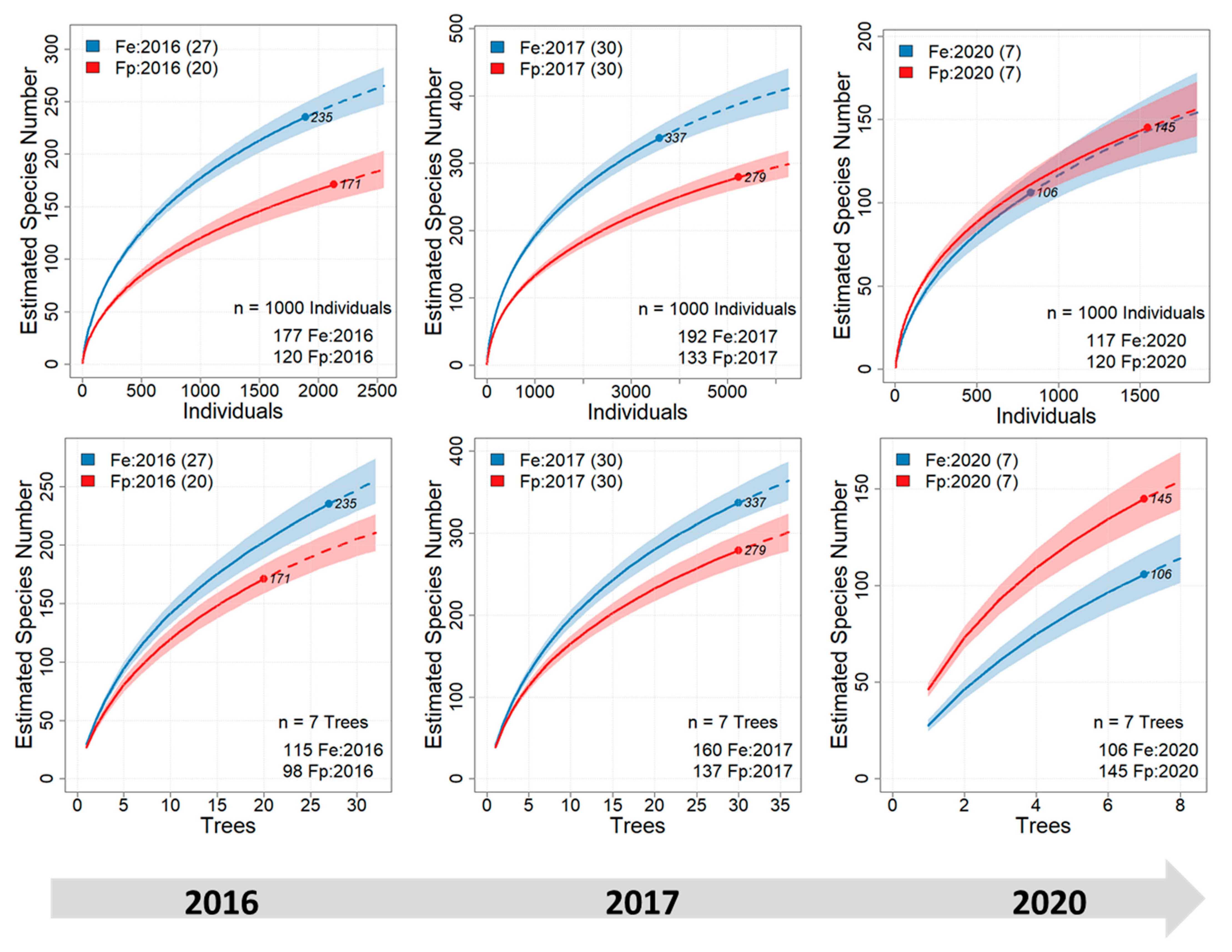
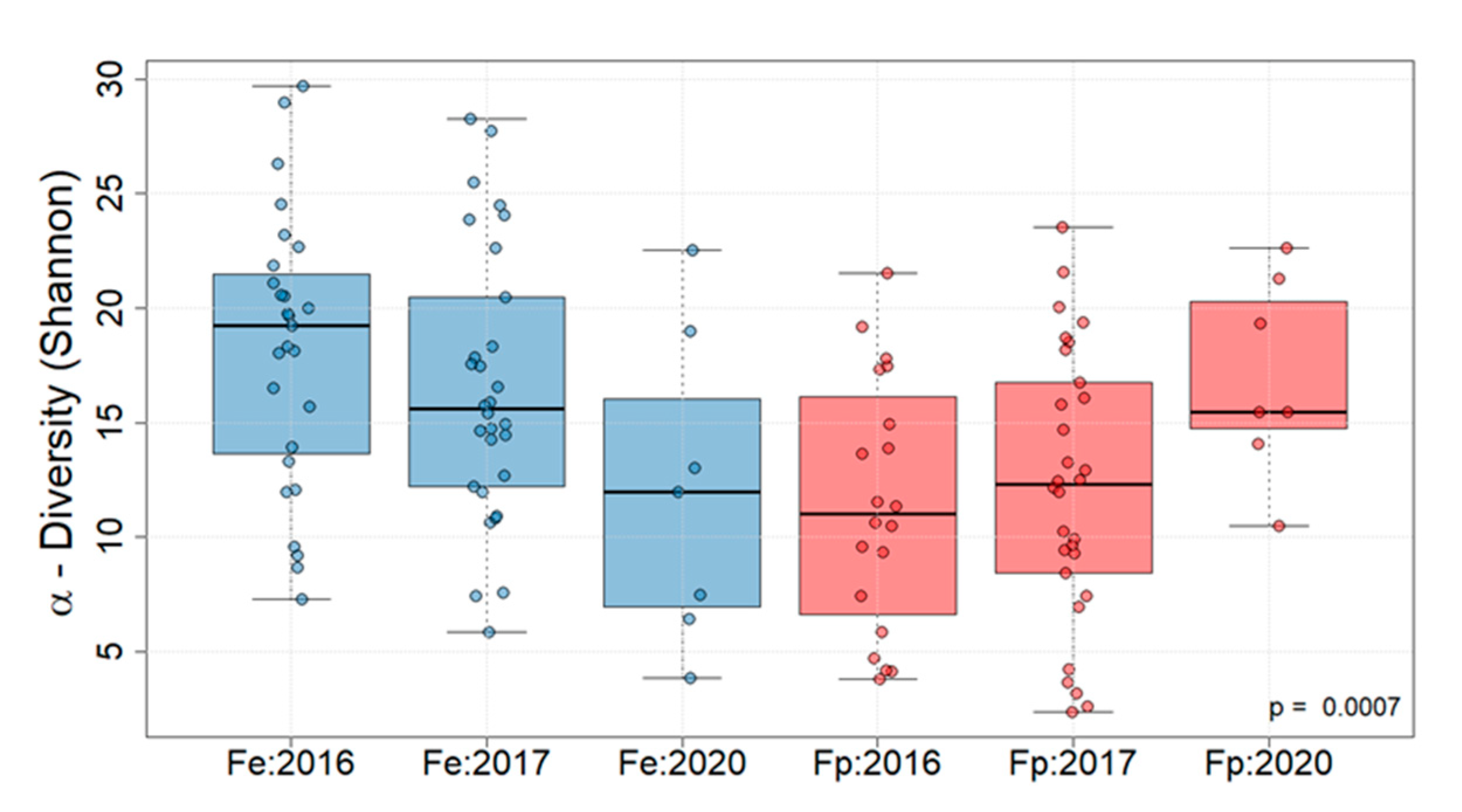
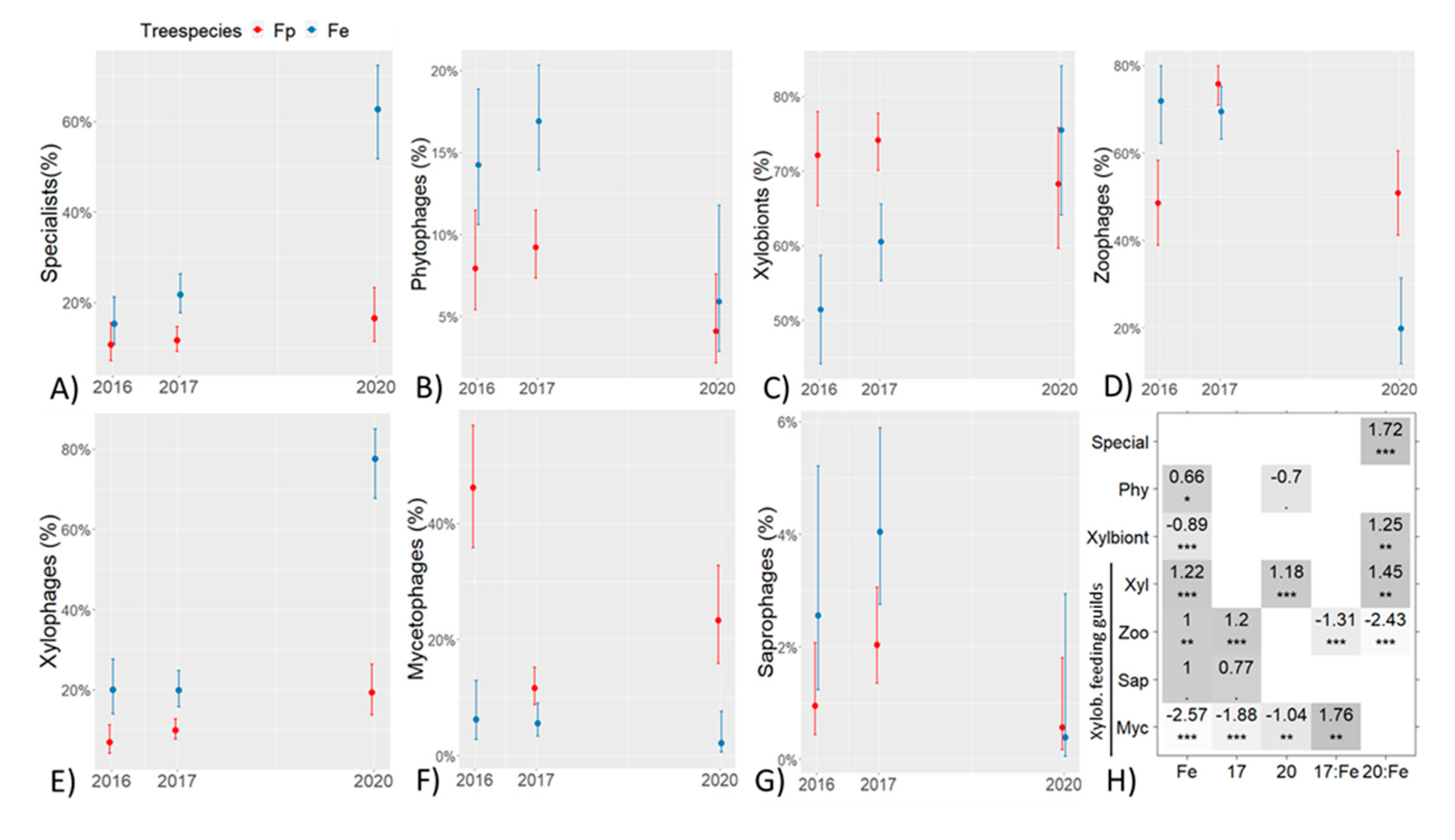
3. Results
4. Discussion
4.1. How Do Beetle Communities Differ between F. excelsior and F. pennsylvanica?
4.2. How Does Ash Dieback Affect Beetle Communities?
4.3. Fraxinus pennsylvanica—Invasive Species or Rescue Species?
4.4. What Ecological Role Do Canopy Beetle Communities Play during Forest Adaptation to Climate Change?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broome, A.; Ray, D.; Mitchell, R.; Harmer, R. Responding to ash dieback (Hymenoscyphus fraxineus) in the UK: Woodland composition and replacement tree species. Forestry 2018, 92, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Pureswaran, D.S.; Roques, A.; Battisti, A. Forest Insects and Climate Change. Curr. For. Rep. 2018, 4, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Bauhus, J.; Seeling, U.; Dieter, M.; Farwig, N.; Haffner, A.; Kätzel, R.; Kleinschmit, B.; Lang, F.; Linder, M.; Möhring, B.; et al. Wissenschaftlicher Beirat für Waldpolitik: Die Anpassung von Wäldern und Waldwirtschaft an den Klimawandel; Federal Ministry of Food and Agriculture (Germany): Berlin, Germany, 2021; p. 192.
- Pötzelsberger, E.; Spiecker, H.; Neophytou, C.; Mohren, F.; Gazda, A.; Hasenauer, H. Growing Non-native Trees in European Forests Brings Benefits and Opportunities but Also Has Its Risks and Limits. Curr. For. Rep. 2020, 6, 339–353. [Google Scholar] [CrossRef]
- Jactel, H.; Moreira, X.; Castagneyrol, B. Tree Diversity and Forest Resistance to Insect Pests: Patterns, Mechanisms, and Prospects. Annu. Rev. Entomol. 2021, 66, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Klapwijk, M.J.; Björkman, C. Mixed forests to mitigate risk of insect outbreaks. Scand. J. For. Res. 2018, 33, 772–780. [Google Scholar] [CrossRef]
- Heinrichs, S.; Öder, V.; Indreica, A.; Bergmeier, E.; Leuschner, C.; Walentowski, H. The Influence of Tilia tomentosa Moench on Plant Species Diversity and Composition in Mesophilic Forests of Western Romania–A Potential Tree Species for Warming Forests in Central Europe? Sustainability 2021, 13, 7996. [Google Scholar] [CrossRef]
- Bindewald, A.; Michiels, H.-G.; Bauhus, J. Risk is in the eye of the assessor: Comparing risk assessments of four non-native tree species in Germany. Forestry 2019, 93, 519–534. [Google Scholar] [CrossRef]
- Bindewald, A.; Miocic, S.; Wedler, A.; Bauhus, J. Forest inventory-based assessments of the invasion risk of Pseudotsuga menziesii (Mirb.) Franco and Quercus rubra L. in Germany. Eur. J. For. Res. 2021, 140, 883–899. [Google Scholar] [CrossRef]
- Goßner, M.; Ammer, U. The effects of Douglas-fir on tree-specific arthropod communities in mixed species stands with European beech and Norway spruce. Eur. J. For. Res. 2006, 125, 221–235. [Google Scholar] [CrossRef]
- Gossner, M.M.; Chao, A.; Bailey, R.; Prinzing, A. Native Fauna on Exotic Trees: Phylogenetic Conservatism and Geographic Contingency in Two Lineages of Phytophages on Two Lineages of Trees. Am. Nat. 2009, 173, 599–614. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Pautasso, M.; Holdenrieder, O. Ecological consequences of Douglas fir [Pseudotsuga menziesii] cultivation in Europe. Eur. J. For. Res. 2014, 133, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Dyderski, M.K.; Jagodziński, A.M. Impacts of invasive trees on alpha and beta diversity of temperate forest understories. Biol. Invasions 2021, 23, 235–252. [Google Scholar] [CrossRef]
- Floren, A.; Schmidl, J. Canopy Arthropod Research in Central Europe-Basic and Applied Studies from the High Frontier; Bioform: Nuremberg, Germany, 2008; p. 576. [Google Scholar]
- Noriega, J.A.; Hortal, J.; Azcarate, F.; Berg, M.P.; Bonada, N.; Briones, M.J.; Del Toro, I.; Goulson, D.; Ibanez, S.; Landis, D.A.; et al. Research trends in ecosystem services provided by insects. Basic Appl. Ecol. 2018, 26, 8–23. [Google Scholar] [CrossRef] [Green Version]
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; González-Olabarria, J.R.; Lyver, P.O.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef] [Green Version]
- Garland, G.; Banerjee, S.; Edlinger, A.; Oliveira, E.M.; Herzog, C.; Wittwer, R.; Philippot, L.; Maestre, F.T.; van der Heijden, M.G. A closer look at the functions behind ecosystem multifunctionality: A review. J. Ecol. 2021, 109, 600–613. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Bellamy, P.E.; Broome, A.; Ellis, C.J.; Hewison, R.L.; Iason, G.R.; Littlewood, N.A.; Newey, S.; Pozsgai, G.; Ray, D.; et al. Cumulative impact assessments of multiple host species loss from plant diseases show disproportionate reductions in associated biodiversity. J. Ecol. 2022, 110, 221–231. [Google Scholar] [CrossRef]
- Hultberg, T.; Sandström, J.; Felton, A.; Öhman, K.; Rönnberg, J.; Witzell, J.; Cleary, M. Ash dieback risks an extinction cascade. Biol. Conserv. 2020, 244, 108516. [Google Scholar] [CrossRef]
- Nielsen, L.R.; McKinney, L.V.; Hietala, A.M.; Kjær, E.D. The susceptibility of Asian, European and North American Fraxinus species to the ash dieback pathogen Hymenoscyphus fraxineus reflects their phylogenetic history. Eur. J. For. Res. 2017, 136, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Zacharias, D.; Breucker, A. Die Nordamerikanische Rot-Esche (Fraxinus pennsylvanica MARSH.)–zur Biologie eines in den Auenwäldern der Mittelelbe Eingebürgerten Neophyten. Braunschweiger Geobot. Arb. 2008, 9, 499–529. [Google Scholar]
- Nehring, S.; Kowarik, I.; Rabitsch, W.; Essl, F. Naturschutzfachliche Invasivitätsbewertungen für in Deutschland wild lebende gebietsfremde Gefäßpflanzen. BfN-Skripten 2013, 352, 1–202. [Google Scholar]
- Floren, A. Sampling Arthropods from the Canopy by Insecticidal Knock-Down. In Manual on Field Recording Techniques and Protocols for All Taxa Biodiversity Inventories; Eymann, J., Degreff, J., Häuser, C., Eds.; ABC Taxa: Bruselles, Belgium, 2010; Volume 8, pp. 158–172. [Google Scholar]
- Köhler, F. Totholzkäfer in Naturwaldzellen des nördlichen Rheinlandes. In Landesanstalt für Ökologie; LÖBF-Schriftenreihe, Ed.; Bodenordnung und Forsten/Landsamt für Agrarordnung NRW: Recklinghausen, Germany, 2000; Volume 18, p. 352. [Google Scholar]
- Möller, G. Struktur und Substratbindung Holzbewohnender Insekten, Schwerpunkt Coleoptera–Käfer. Master’s Thesis, Institut für Zoologie der Freien Universität Berlin, Berlin, Germany, 2009. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology. R Package Version 1.18-28/r1569. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 December 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Dunnington, D. Rosm: Plot Rater Map Tiles from Open Street Map and Other Sources. R-Package Version 0.2.5. 2019. Available online: https://rdrr.io/cran/rosm/man/rosm.html (accessed on 1 December 2021).
- Dunnington, D. Prettymapr: Scale Bar, North Arrow, and Pretty Margins in R. R Package Version 0.2.2. 2017. Available online: https://github.com/paleolimbot/prettymapr (accessed on 1 December 2021).
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Venables, W.V.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Lüdecke, D. sjPlot: Data Visualization for Statistics in Social Science. R Package Version 2.8.10. 2020. Available online: https://strengejacke.github.io/sjPlot/ (accessed on 1 December 2021).
- Senf, C.; Buras, A.; Zang, C.S.; Rammig, A.; Seidl, R. Excess forest mortality is consistently linked to drought across Europe. Nat. Commun. 2020, 11, 6200. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.E.; Hauck, M.; Hajek, P.; et al. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Trogisch, S.; Schuldt, A.; Bauhus, J.; Blum, J.A.; Both, S.; Buscot, F.; Castro-Izaguirre, N.; Chesters, D.; Durka, W.; Eichenberg, D.; et al. Toward a methodical framework for comprehensively assessing forest multifunctionality. Ecol. Evol. 2017, 7, 10652–10674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuldt, A.; Assmann, T.; Brezzi, M.; Buscot, F.; Eichenberg, D.; Gutknecht, J.; Härdtle, W.; He, J.-S.; Klein, A.; Kühn, P.; et al. Biodiversity across trophic levels drives multifunctionality in highly diverse forests. Nat. Commun. 2018, 9, 2989. [Google Scholar] [CrossRef] [Green Version]
- Thurm, E.A.; Hernández, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative tree species under climate warming in managed European forests. For. Ecol. Manag. 2018, 430, 485–497. [Google Scholar] [CrossRef]
- Canelles, Q.; Aquilué, N.; James, P.M.A.; Lawler, J.; Brotons, L. Global review on interactions between insect pests and other forest disturbances. Landsc. Ecol. 2021, 36, 945–972. [Google Scholar] [CrossRef]
- Sheil, D.; Bongers, F. Interpreting forest diversity-productivity relationships: Volume values, disturbance histories and alternative inferences. For. Ecosyst. 2020, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Langmaier, M.; Lapin, K. A Systematic Review of the Impact of Invasive Alien Plants on Forest Regeneration in European Temperate Forests. Front. Plant Sci. 2020, 11, 524969. [Google Scholar] [CrossRef]
- Campagnaro, T.; Brundu, G.; Sitzia, T. Five major invasive alien tree species in European Union forest habitat types of the Alpine and Continental biogeographical regions. J. Nat. Conserv. 2018, 43, 227–238. [Google Scholar] [CrossRef]
- Brändle, M.; Brandl, R. Is the composition of phytophagous insects and parasitic fungi among trees predictable? Oikos 2006, 113, 296–304. [Google Scholar] [CrossRef]
- Sprick, P.; Floren, A. Species richness and historical relations in arboreal phytophagous beetels: A study based on fogging samples from primeval forests of Poland, Romania and Slovenia (Coleoptera: Chrysomelidae, Curculionoidea). In Canopy Arthropod Research in Central Europe; Floren, A., Schmidl, J., Eds.; Bioform: Nuremberg, Germany, 2008; pp. 225–259. [Google Scholar]
- Drekic, M.; Poljakovic Pajnik, L.; Vasic, V.; Pap, P.; Pilipovic, A. Contribution to the study of biology of ash weevil (Stereonychus fraxini De Geer). Šumarski List 2014, 138, 387–395. [Google Scholar]
- Jansen, E. Massenauftreten von Lytta vesicatoria (LINNAEUS, 1758) (Coleoptera, Meloidae) in Leipzig. Entomol. Nachr. Ber. 2009, 53, 131. [Google Scholar]
- Krebs, C.; Schulze, H.; Fiedler, K. Comparison of herbivore communities on the native Field Maple Acer cam pestre (L.) and the invasive neophyte Box Elder Acer negundo (L.) in the NP Donau-Auen, Lower Austria. In Proceedings of the 5th Symposium for Research in Protected Areas, Mitterisill, Austria, 10–12 June 2013. [Google Scholar]
- Orlova-Bienkowskaja, J.M. Cascading ecological effects caused by the establishment of the emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae) in European Russia. Eur. J. Entomol. 2012, 112, 778–789. [Google Scholar] [CrossRef]
- Stocks, J.J.; Metheringham, C.L.; Plumb, W.J.; Lee, S.J.; Kelly, L.J.; Nichols, R.A.; Buggs, R.J.A. Genomic basis of European ash tree resistance to ash dieback fungus. Nat. Ecol. Evol. 2019, 3, 1686–1696. [Google Scholar] [CrossRef]
- Staab, M.; Liu, X.; Assmann, T.; Bruelheide, H.; Buscot, F.; Durka, W.; Erfmeier, A.; Klein, A.; Ma, K.; Michalski, S.; et al. Tree phylogenetic diversity structures multitrophic communities. Funct. Ecol. 2021, 35, 521–534. [Google Scholar] [CrossRef]
- Schuldt, A.; Ebeling, A.; Kunz, M.; Staab, M.; Guimarães-Steinicke, C.; Bachmann, D.; Buchmann, N.; Durka, W.; Fichtner, A.; Fornoff, F.; et al. Multiple plant diversity components drive consumer communities across ecosystems. Nat. Commun. 2010, 10, 1460. [Google Scholar] [CrossRef] [Green Version]
- Floren, A.; Sprick, P.; Horchler, P.J.; Müller, T. Baumkronen als Habitat gefährdeter Käfer am Beispiel von Hartholzauwäldern in Sachsen-Anhalt, Region Mittelelbe. Nat. Lanschaft 2021, 11, 509–516. [Google Scholar]
- Gandhi, K.; Herms, D.A. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol. Invasions 2010, 12, 389–405. [Google Scholar] [CrossRef]
- Branco, M.; Brockerhoff, E.G.; Castagneyrol, B.; Orazio, C.; Jactel, H. Host range expansion of native insects to exotic trees increases with area of introduction and the presence of congeneric native trees. J. Appl. Ecol. 2015, 52, 69–77. [Google Scholar] [CrossRef]
- Rodman, K.; Andrus, R.; Butkiewicz, C.; Chapman, T.; Gill, N.; Harvey, B.; Kulakowski, D.; Tutland, N.; Veblen, T.; Hart, S. Effects of Bark Beetle Outbreaks on Forest Landscape Pattern in the Southern Rocky Mountains, USA. Remote Sens. 2015, 13, 1089. [Google Scholar] [CrossRef]
- Floren, A.; Krüger, D.; Müller, T.; Dittrich, M.; Rudloff, R.; Hoppe, B.; Linsenmair, K.E. Diversity and Interactions of Wood-Inhabiting Fungi and Beetles after Deadwood Enrichment. PLoS ONE 2015, 10, e0143566. [Google Scholar]
- Castaño, C.; Camarero, J.J.; Zas, R.; Sampedro, L.; Bonet, J.A.; Alday, J.G.; Oliva, J. Insect defoliation is linked to a decrease in soil ectomycorrhizal biomass and shifts in needle endophytic communities. Tree Physiol. 2020, 40, 1712–1725. [Google Scholar] [CrossRef]
| Fe: 2016 | Fe: 2017 | Fe: 2020 | Fp: 2016 | Fp: 2017 | Fp: 2020 | |
|---|---|---|---|---|---|---|
| Samples | 27 | 30 | 7 | 20 | 30 | 7 |
| Individuals | 1892 | 3584 | 829 | 2135 | 5228 | 1546 |
| Species | 235 | 337 | 106 | 171 | 279 | 145 |
| Singletons | 98 | 134 | 54 | 77 | 109 | 61 |
| Singletons (%) | 41.7 | 39.8 | 50.9 | 45.0 | 39.1 | 42.1 |
| Mean Exp Shannon | 19 | 22 | 14 | 13 | 16 | 23 |
| Nr. of phytophages | 269 | 605 | 49 | 169 | 481 | 63 |
| % phy of all beetles | 14.2 | 16.9 | 5.9 | 7.9 | 9.2 | 4.1 |
| Nr. of xylobiont beetles | 950 | 2177 | 646 | 1492 | 3859 | 1101 |
| % xyl. of all beetles | 50.2 | 60.7 | 77.9 | 69.9 | 73.8 | 71.2 |
| Feeding guilds of xylobiont beetles | ||||||
| Xylophages | 189 | 431 | 501 | 102 | 383 | 212 |
| - (%) | 19.9 | 19.8 | 77.6 | 6.8 | 9.9 | 19.3 |
| Zoophages | 682 | 1494 | 122 | 577 | 2997 | 617 |
| (%) | 71.8 | 68.6 | 18.9 | 38.7 | 77.7 | 56.0 |
| Mycetophages | 53 | 145 | 20 | 790 | 398 | 262 |
| (%) | 5.6 | 6.7 | 3.1 | 52.9 | 10.3 | 23.8 |
| Saprophages | 26 | 107 | 3 | 23 | 81 | 10 |
| (%) | 2.7 | 4.9 | 0.5 | 1.5 | 2.1 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floren, A.; Horchler, P.J.; Müller, T. The Impact of the Neophyte Tree Fraxinus pennsylvanica [Marshall] on Beetle Diversity under Climate Change. Sustainability 2022, 14, 1914. https://doi.org/10.3390/su14031914
Floren A, Horchler PJ, Müller T. The Impact of the Neophyte Tree Fraxinus pennsylvanica [Marshall] on Beetle Diversity under Climate Change. Sustainability. 2022; 14(3):1914. https://doi.org/10.3390/su14031914
Chicago/Turabian StyleFloren, Andreas, Peter J. Horchler, and Tobias Müller. 2022. "The Impact of the Neophyte Tree Fraxinus pennsylvanica [Marshall] on Beetle Diversity under Climate Change" Sustainability 14, no. 3: 1914. https://doi.org/10.3390/su14031914
APA StyleFloren, A., Horchler, P. J., & Müller, T. (2022). The Impact of the Neophyte Tree Fraxinus pennsylvanica [Marshall] on Beetle Diversity under Climate Change. Sustainability, 14(3), 1914. https://doi.org/10.3390/su14031914





