The Potential for Future Shifts in Tree Species Distribution Provided by Dispersal and Ecological Niches: A Comparison between Beech and Oak in Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Forest Inventory and Analysis of Data
2.2. Baseline Climate and Future Projections
2.3. Descriptive Analysis of Bioclimatic Envelopes
2.4. Dispersal Potential
2.5. Intersection of Climatic Suitability and Potential Dispersal
3. Results
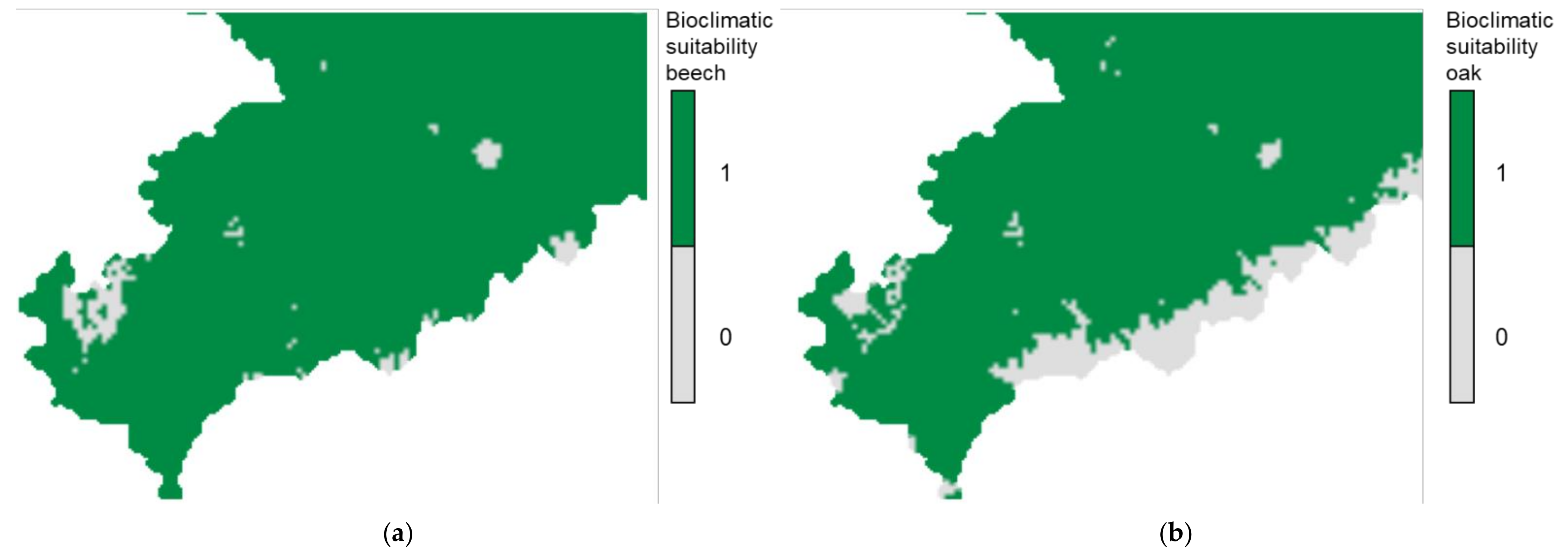
3.1. Descriptive Analysis of Bioclimatic Suitability
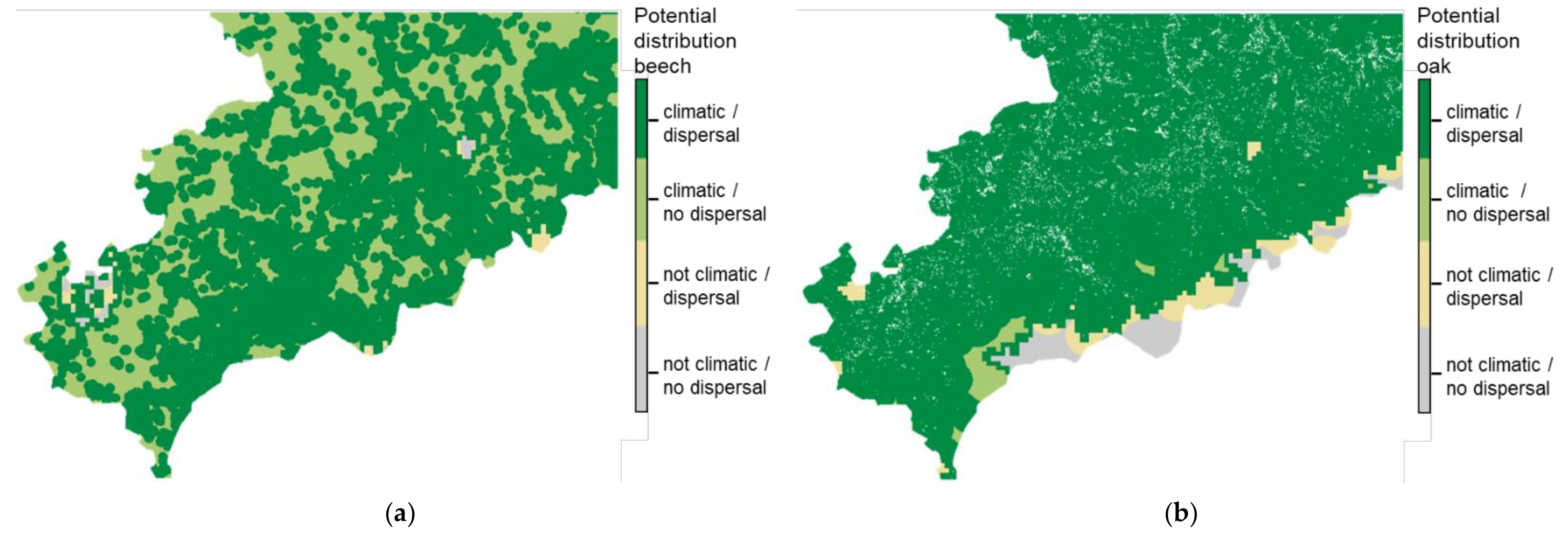
3.2. Potential Dispersal of Oak and Beech
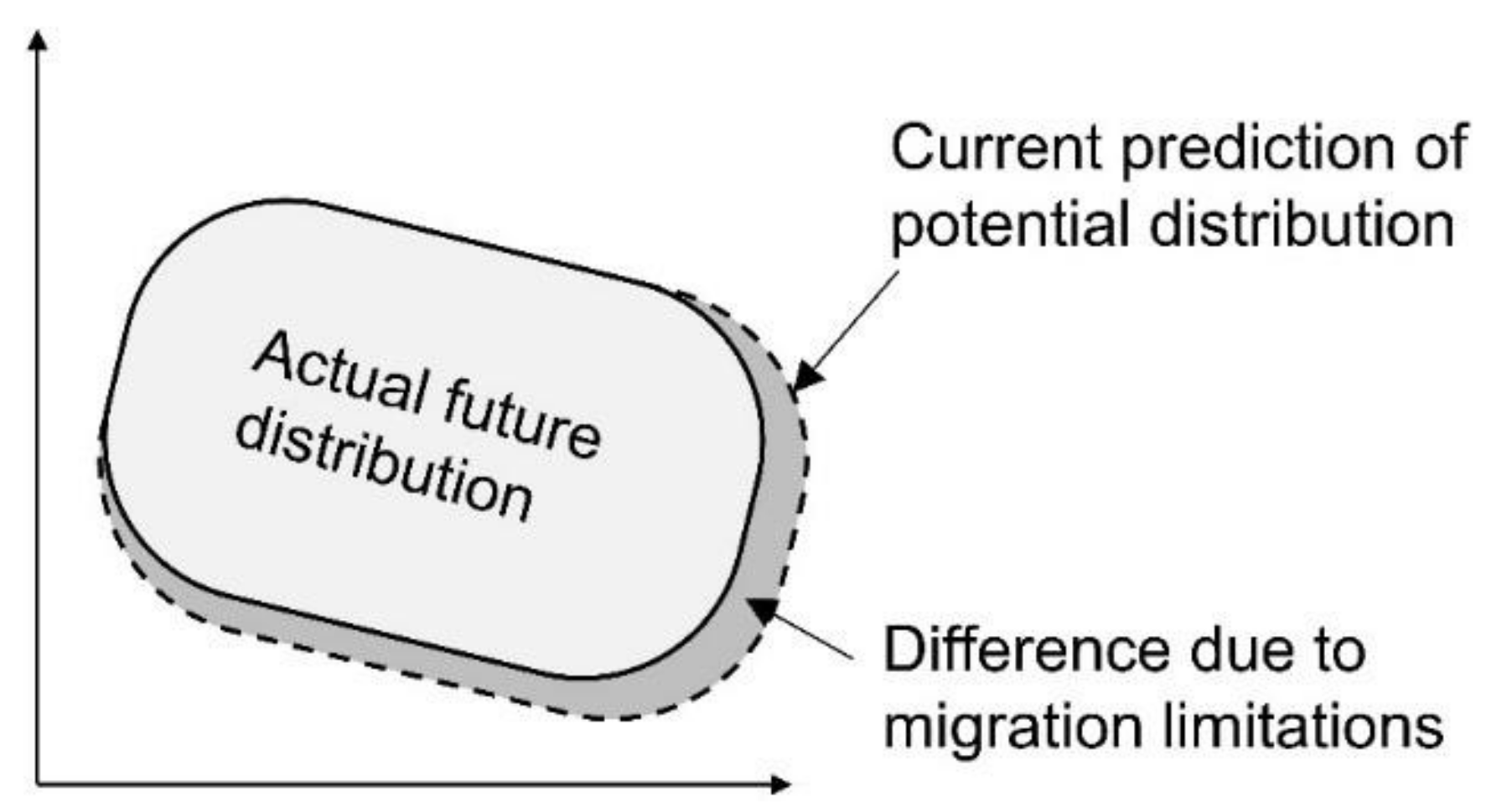
3.3. Difference Due to Migration Restrictions
4. Discussion
4.1. Methodological Discussion
4.2. Bioclimatic Suitability of Beech and Oak
4.3. Potential Dispersal and Consideration of Seed Tree Fragmentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, E.W. Quercus L. J. Ecol. 1959, 47, 169–222. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; The Publications Office of the European Union: Luxembourg, 2016; pp. 160–163. [Google Scholar]
- Thomson, J.D.; Weiblen, G.; Thomson, B.A.; Alfaro, S.; Legendre, P. Untangling multiple factors in spatial distributions: Lilies, gophers, and rocks. Ecology 1996, 77, 1698–1715. [Google Scholar] [CrossRef]
- Guthke, J.; Spethmann, W. Physiological and pathological aspects of long-term storage of acorns. Ann. For. Sci. 1993, 50, 384–387. [Google Scholar] [CrossRef]
- Savill, P.S. The Silviculture of Trees Used in British Forestry, 3rd ed.; CABI: Wallingford, UK, 2019; Available online: http://slubdd.de/katalog?TN_libero_mab2 (accessed on 18 August 2021).
- Peters, R. Beech Forests; Kluwer: Dordrecht, The Netherlands, 1997; ISBN 0169-3174. [Google Scholar]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas Mit Den Alpen in Ökologischer, Dynamischer und Historischer SICHT; UTB: Stuttgart, Germany, 2010; ISBN 3825281043. [Google Scholar]
- Bolte, A.; Czajkowski, T.; Kompa, T. The north-eastern distribution range of European beech—A review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Jönsson, A.M. Soil treatment effects on bark lesions and frost sensitivity of beech (Fagus sylvatica) in southern Sweden. For. Ecol. Manag. 2000, 129, 167–175. [Google Scholar] [CrossRef]
- Visjic, C.; Dohrenbusch, A. Frostresistenz und phänologie europäischer Buchenprovenienzen (Fagus sylvatica L.). Allg. Forst-Und Jagdztg. 2004, 175, 101–108. [Google Scholar]
- Bolte, A.; Czajkowski, T.; Cocozza, C.; Tognetti, R.; de Miguel, M.; Pšidová, E.; Ditmarová, L.; Dinca, L.; Delzon, S.; Cochard, H.; et al. Desiccation and mortality dynamics in seedlings of different European beech (Fagus sylvatica L.) populations under extreme drought conditions. Front. Plant Sci. 2016, 7, 751. [Google Scholar] [CrossRef]
- Firbas, F. Spät- und Nacheiszeitliche Waldgeschichte Mitteleuropas Nördlich der Alpen Zweiter Band Waldgeschichte der Einzelnen Landschaften; Fischer: Jena, Germany, 1952. [Google Scholar]
- Huntley, B.; Bartlein, P.J.; Prentice, I.C. Climatic control of the distribution and abundance of beech (Fagus L.) in Europe and North America. J. Biogeogr. 1989, 16, 551–560. [Google Scholar] [CrossRef]
- Penalba, M.C. The history of the Holocene vegetation in northern Spain from pollen analysis. J. Ecol. 1994, 82, 815–832. [Google Scholar] [CrossRef]
- Clark, J.S.; Fastie, C.; Hurtt, G.; Jackson, S.T.; Johnson, C.; King, G.A.; Lewis, M.; Lynch, J.; Pacala, S.; Prentice, C.; et al. Reid’s paradox of rapid plant migration: Dispersal theory and interpretation of paleoecological records. Bioscience 1998, 48, 13–24. [Google Scholar] [CrossRef]
- Brewer, S.; Cheddadi, R.; de Beaulieu, J.L.; Reille, M. The spread of deciduous Quercus throughout Europe since the last glacial period. For. Ecol. Manag. 2002, 156, 27–48. [Google Scholar] [CrossRef]
- Lowe, A.; Unsworth, C.; Gerber, S.; Davies, S.; Munro, R.; Kelleher, C.; King, A.; Brewer, S.; White, A.; Cottrell, J. Route, speed and mode of oak postglacial colonisation across the British Isles: Integrating molecular ecology, palaeoecology and modelling approaches. Bot. J. Scotl. 2005, 57, 59–81. [Google Scholar] [CrossRef]
- Le Corre, V.; Machon, N.; Petit, R.J.; Kremer, A. Colonization with long-distance seed dispersal and genetic structure of maternally inherited genes in forest trees: A simulation study. Genet. Res. 1997, 69, 117–125. [Google Scholar] [CrossRef]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests; Revised and Extended Version of the 6th German edition; Springer International Publishing AG: Cham, Switzerland, 2017; ISBN 9783319430409. [Google Scholar]
- Björkman, L. The establishment of Fagus sylvatica at the stand-scale in southern Sweden. Holocene 1999, 9, 237–245. [Google Scholar] [CrossRef]
- López-Merino, L.; López-Sáez, J.A.; Zapata, M.R.; Garcia, M.G. Reconstructing the history of beech (Fagus sylvatica L.) in the north-western Iberian Range (Spain): From Late-Glacial refugia to the Holocene anthropic-induced forests. Rev. Palaeobot. Palynol. 2008, 152, 58–65. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the INTERGOVERNMENTAL Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; ISBN 978-92-9169-143-2. [Google Scholar]
- Takolander, A.; Hickler, T.; Meller, L.; Cabeza, M. Comparing future shifts in tree species distributions across Europe projected by statistical and dynamic process-based models. Reg. Eniron. Chang. 2018, 19, 251–266. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Ruiz-Benito, P.; Ratcliffe, S.; Zavala, M.A.; Martinez-Vilalta, J.; Vilà-Cabrera, A.; Lloret, F.; Madrigal-González, J.; Wirth, C.; Greenwood, S.; Kändler, G.; et al. Climate-and successional-related changes in functional composition of European forests are strongly driven by tree mortality. Glob. Chang. Biol. 2017, 23, 4162–4176. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Anderegg, L.D.L.; Kerr, K.L.; Trugman, A.T. Widespread drought-induced tree mortality at dry range edges indicates that climate stress exceeds species’ compensating mechanisms. Glob. Chang. Biol. 2019, 25, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y.; Hoch, G.; Randin, C.F.; Lenz, A.; Kollas, C.; Körner, C. Tree recruitment of European tree species at their current upper elevational limits in the Swiss Alps. J. Biogeogr. 2012, 39, 1439–1449. [Google Scholar] [CrossRef]
- Zhu, K.; Woodall, C.W.; Clark, J.S. Failure to migrate: Lack of tree range expansion in response to climate change. Glob. Chang. Biol. 2012, 18, 1042–1052. [Google Scholar] [CrossRef]
- Bell, D.M.; Bradford, J.B.; Lauenroth, W.K. Early indicators of change: Divergent climate envelopes between tree life stages imply range shifts in the western United States. Glob. Ecol. Biogeogr. 2014, 23, 168–180. [Google Scholar] [CrossRef]
- Buras, A.; Rammig, A.; Zang, C.S. Quantifying impacts of the 2018 drought on European ecosystems in comparison to 2003. Biogeosciences 2020, 17, 1655–1672. [Google Scholar] [CrossRef]
- Büntgen, U.; Urban, O.; Krusic, P.J.; Rybníček, M.; Kolář, T.; Kyncl, T.; Trnka, M. Recent European drought extremes beyond Common Era background variability. Nat. Geosci. 2021, 14, 190–196. [Google Scholar] [CrossRef]
- Zhu, K.; Woodall, C.W.; Ghosh, S.; Gelfand, A.E.; Clark, J.S. Dual impacts of climate change: Forest migration and turnover through life history. Glob. Chang. Biol. 2014, 20, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Rigling, A.; Bigler, C.; Eilmann, B.; Feldmeyer-Christe, E.; Gimmi, U.; Ginzler, C.; Graf, U.; Mayer, P.; Vacchiano, G.; Weber, P.; et al. Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Chang. Biol. 2013, 19, 229–240. [Google Scholar] [CrossRef]
- Peñuelas, J.; Ogaya, R.; Boada, M.; Jump, A.S. Migration, invasion and decline: Changes in recruitment and forest structure in a warming-linked shift of European beech forest in Catalonia (NE Spain). Ecography 2007, 30, 829–837. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Meier, E.S.; Edwards Jr, T.C.; Kienast, F.; Dobbertin, M.; Zimmermann, N.E. Co-occurrence patterns of trees along macro-climatic gradients and their potential influence on the present and future distribution of Fagus sylvatica L. J. Biogeogr. 2011, 38, 371–382. [Google Scholar] [CrossRef]
- Saltré, F.; Saint-Amant, R.; Gritti, E.S.; Brewer, S.; Gaucherel, C.; Davis, B.A.S.; Chuine, I. Climate or migration: What limited European beech post-glacial colonization? Glob. Ecol. Biogeogr. 2013, 22, 1217–1227. [Google Scholar] [CrossRef]
- Mellert, K.H.; Fensterer, V.; Küchenhoff, H.; Reger, B.; Kölling, C.; Klemmt, H.-J.; Ewald, J. Hypothesis-driven species distribution models for tree species in the Bavarian Alps. J. Veg. Sci. 2011, 22, 635–646. [Google Scholar] [CrossRef]
- Gray, L.K.; Hamann, A. Tracking suitable habitat for tree populations under climate change in western North America. Clim. Chang. 2013, 117, 289–303. [Google Scholar] [CrossRef]
- Zimmermann, N.E.; Jandl, R.; Hanewinkel, M.; Kunstler, G.; Kölling, C.; Gasparini, P.; Breznikar, A.; Meier, E.S.; Normand, S.; Ulmer, U.; et al. Potential future ranges of tree species in the Alps. In Management Strategies to Adapt Alpine Space Forests to Climate Change Risks; IntechOpen: London, UK, 2013; pp. 37–48. ISBN 9789535142409. [Google Scholar]
- Svenning, J.-C.; Skov, F. Limited filling of the potential range in European tree species. Ecol. Lett. 2004, 7, 565–573. [Google Scholar] [CrossRef]
- Meier, E.S.; Lischke, H.; Schmatz, D.R.; Zimmermann, N.E. Climate, competition and connectivity affect future migration and ranges of European trees. Glob. Ecol. Biogeogr. 2012, 21, 164–178. [Google Scholar] [CrossRef]
- Neilson, R.P.; Pitelka, L.F.; Solomon, A.M.; Nathan, R.A.; Midgley, G.F.; Fragoso, J.M.V.; Lischke, H.; Thompson, K.E. Forecasting regional to global plant migration in response to climate change. Bioscience 2005, 55, 749–759. [Google Scholar] [CrossRef]
- Araujo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Clark, C.J.; Poulsen, J.R.; Levey, D.J.; Osenberg, C.W. Are plant populations seed limited? A critique and meta-analysis of seed addition experiments. Am. Nat. 2007, 170, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Seaborn, T.J.; Goldberg, C.S.; Crespi, E.J. Integration of dispersal data into distribution modeling: What have we done and what have we learned? Front. Biogeogr. 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Nilsson, S.G. Ecological and evolutionary interactions between reproduction of beech Fagus silvatica and seed eating animals. Oikos 1985, 44, 157–164. [Google Scholar] [CrossRef]
- Kollmann, J.; Schill, H.-P. Spatial patterns of dispersal, seed predation and germination during colonization of abandoned grassland by Quercus petraea and Corylus avellana. Vegetatio 1996, 125, 193–205. [Google Scholar] [CrossRef]
- Den Ouden, J.; Jansen, P.A.; Smit, R. Jays, mice and oaks: Predation and dispersal of Quercus robur and Q. petraea in North-western Europe. Seed Fate Predation Dispersal Seedl. Establ. 2005, 223–240. [Google Scholar] [CrossRef]
- Sagnard, F.; Pichot, C.; Dreyfus, P.; Jordano, P.; Fady, B. Modelling seed dispersal to predict seedling recruitment: Recolonization dynamics in a plantation forest. Ecol. Model. 2007, 203, 464–474. [Google Scholar] [CrossRef]
- Wagner, S.; Collet, C.; Madsen, P.; Nakashizuka, T.; Nyland, R.D.; Sagheb-Talebi, K. Beech regeneration research: From ecological to silvicultural aspects. For. Ecol. Manag. 2010, 259, 2172–2182. [Google Scholar] [CrossRef]
- Chybicki, I.J.; Burczyk, J. Realized gene flow within mixed stands of Quercus robur L. and Q. petraea (Matt.) L. revealed at the stage of naturally established seedling. Mol. Ecol. 2010, 19, 2137–2151. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.M. Spatial patterns in long-distance dispersal of Quercus ilex acorns by jays in a heterogeneous landscape. Ecography 2003, 26, 573–584. [Google Scholar] [CrossRef]
- Kunstler, G.; Thuiller, W.; Curt, T.; Bouchaud, M.; Jouvie, R.; Deruette, F.; Lepart, J. Fagus sylvatica L. recruitment across a fragmented Mediterranean Landscape, importance of long distance effective dispersal, abiotic conditions and biotic interactions. Divers. Distrib. 2007, 13, 799–807. [Google Scholar] [CrossRef]
- Kurek, P.; Dobrowolska, D.; Wiatrowska, B. Dispersal distance and burial mode of acorns in Eurasian Jays Garrulus glandarius in European temperate forests. Acta Ornithol. 2019, 53, 155–162. [Google Scholar] [CrossRef]
- Martínez-Baroja, L.; Pérez-Camacho, L.; Villar-Salvador, P.; Rebollo, S.; Quiles, P.; Gómez-Sánchez, D.; Molina-Morales, M.; Leverkus, A.B. Massive and effective acorn dispersal into agroforestry systems by an overlooked vector, the Eurasian magpie (Pica pica). Ecosphere 2019, 10, e02989. [Google Scholar] [CrossRef]
- Kleinschmit, J.R.G.; Bacilieri, R.; Kremer, A.; Roloff, A. Comparison of morphological and genetic traits of pedunculate oak (Q. robur L.) and sessile oak (Q. petraea (Matt.) Liebl.). Silvae Genet. 1995, 44, 256–268. [Google Scholar]
- Hertel, H.; Degen, B. Unterscheidung von Stiel-und Traubeneichen (Quercus robur L. und Q. petraea [Mattuschka] Liebl.) mit Hilfe von genetischen und morphologischen Merkmalen. For. Snow Landsc. Res. Birmensdorf 2000, 75, 169–183. [Google Scholar]
- Schaller, A.S.; Franke, J.; Bernhofer, C. Climate dynamics: Temporal development of the occurrence frequency of heavy precipitation in Saxony, Germany. Meteorol. Z. 2020, 29, 335–348. [Google Scholar] [CrossRef]
- Spekat, A.; Enke, W.; Franke, J. Regionale Klimaprojektionen für Sachsen WMSax2.0; Sächsische Landesbibliothek—Staats-und Universitätsbibliothek Dresden: Dresden, Germany, 2020. [Google Scholar]
- Enke, W.; Spekat, A.; Kreienkamp, F. Classification by multiple regression-a new approach towards the classification of extremes. Meteorol. Hydrol. Water Manag. Res. Oper. Appl. 2016, 4, 25–39. [Google Scholar]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F.; et al. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Eddy, W.F. A new convex hull algorithm for planar sets. ACM Trans. Math. Softw. TOMS 1977, 3, 398–403. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 July 2021).
- Bivand, R.S.; Pebesma, E.; Gomez-Rubio, V. Applied Spatial Data Analysis with R, 2nd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Hoffmann, K.; Coenradie, B.; Haag, L.; Nitzsche, V. Sachsenforst setzt auf Fernerkundung: Sachsens forstliche Praxis nutzt mit großem Erfolg Daten aus der Fernerkundung. LWF Aktuell 2017, 4, 26–29. [Google Scholar]
- Bivand, R.; Rundel, C. rgeos: Interface to Geometry Engine—Open Source (’GEOS’). 2019. Available online: https://CRAN.R-project.org/package=rgeos (accessed on 1 October 2021).
- Axer, M.; Schlicht, R.; Wagner, S. Modelling potential density of natural regeneration of European oak species (Quercus robur L., Quercus petraea (Matt.) Liebl.) depending on the distance to the potential seed source: Methodological approach for modelling dispersal from inventory data at forest enterprise level. For. Ecol. Manag. 2021, 482, 118802. [Google Scholar] [CrossRef]
- Fahrmeir, L.; Kneib, T.; Lang, S.; Marx, B. Regression Models, Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 3642343333. [Google Scholar]
- Stoyan, D.; Wagner, S. Estimating the fruit dispersion of anemochorous forest trees. Ecol. Model. 2001, 145, 35–47. [Google Scholar] [CrossRef]
- Clark, J.S.; Silman, M.; Kern, R.; Macklin, E.; HilleRisLambers, J. Seed dispersal near and far: Patterns across temperate and tropical forests. Ecology 1999, 80, 1475–1494. [Google Scholar] [CrossRef]
- Soberón, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Kölling, C. Klimahüllen für 27 Waldbaumarten. AFZ-DerWald 2007, 23, 1242–1245. [Google Scholar]
- Ammer, C.; Bickel, E.; Kölling, C. Converting Norway spruce stands with beech-a review of arguments and techniques. Austrian J. For. Sci. 2008, 125, 3–26. [Google Scholar] [CrossRef]
- Dobrowski, S.Z.; Abatzoglou, J.; Swanson, A.K.; Greenberg, J.A.; Mynsberge, A.R.; Holden, Z.A.; Schwartz, M.K. The climate velocity of the contiguous U nited S tates during the 20th century. Glob. Chang. Biol. 2013, 19, 241–251. [Google Scholar] [CrossRef]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef]
- Silva, D.; Rezende Mazzella, P.; Legay, M.; Corcket, E.; Dupouey, J.L. Does natural regeneration determine the limit of European beech distribution under climatic stress? For. Ecol. Manag. 2012, 266, 263–272. [Google Scholar] [CrossRef]
- Woodall, C.W.; Zhu, K.; Westfall, J.A.; Oswalt, C.M.; D’amato, A.W.; Walters, B.F.; Lintz, H.E. Assessing the stability of tree ranges and influence of disturbance in eastern US forests. For. Ecol. Manag. 2013, 291, 172–180. [Google Scholar] [CrossRef]
- Monsimet, J.; Devineau, O.; Petillon, J.; Lafage, D. Explicit integration of dispersal-related metrics improves predictions of SDM in predatory arthropods. Sci. Rep. 2020, 10, 16668. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Sobrino, C.; Ramil-Rego, P.; Gómez-Orellana, L.; Da Ferreiro Costa, J.; Díaz Varela, R.A. Climatic and human effects on the post-glacial dynamics of Fagus sylvatica L. in NW Iberia. Plant Ecol. 2009, 203, 317–340. [Google Scholar] [CrossRef]
- GARCÍA-VALDÉS, R.; Gotelli, N.J.; Zavala, M.A.; Purves, D.W.; Araújo, M.B. Effects of climate, species interactions, and dispersal on decadal colonization and extinction rates of Iberian tree species. Ecol. Model. 2015, 309–310, 118–127. [Google Scholar] [CrossRef]
- Liang, Y.; Duveneck, M.J.; Gustafson, E.J.; Serra-Diaz, J.M.; Thompson, J.R. How disturbance, competition, and dispersal interact to prevent tree range boundaries from keeping pace with climate change. Glob. Chang. Biol. 2018, 24, e335–e351. [Google Scholar] [CrossRef]
- Saltré, F.; Duputié, A.; Gaucherel, C.; Chuine, I. How climate, migration ability and habitat fragmentation affect the projected future distribution of European beech. Glob. Chang. Biol. 2015, 21, 897–910. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Normand, S.; Skov, F. Postglacial dispersal limitation of widespread forest plant species in nemoral Europe. Ecography 2008, 31, 316–326. [Google Scholar] [CrossRef]
- Nathan, R. Long-distance dispersal of plants. Science 2006, 313, 786–788. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions. Princeton University Press: New Jersey, NJ, USA, 2011; Volume 49. [Google Scholar] [CrossRef]
- Woodward, F.I.; Woodward, F.I. Climate and Plant Distribution; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Tranquillini, W.; Plank, A. Ecophysiological investigations on beech at various altitudes in northern and southern Tyrol. Centralblatt für das Gesamte Forstwesen 1989, 106, 225–246. [Google Scholar]
- Sykes, M.T.; Prentice, I.C.; Cramer, W. A bioclimatic model for the potential distributions of north European tree species under present and future climates. J. Biogeogr. 1996, 23, 203–233. [Google Scholar]
- Chuine, I.; Beaubien, E.G. Phenology is a major determinant of tree species range. Ecol. Lett. 2001, 4, 500–510. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; García-Valdés, R.; Ruíz-Benito, P.A.; Zavala, M. Disentangling the relative importance of climate, size and competition on tree growth in Iberian forests: Implications for forest management under global change. Glob. Chang. Biol. 2011, 17, 2400–2414. [Google Scholar] [CrossRef]
- Zimmermann, J.; Hauck, M.; Dulamsuren, C.; Leuschner, C. Climate Warming-Related Growth Decline Affects Fagus sylvatica, But Not Other Broad-Leaved Tree Species in Central European Mixed Forests. Ecosystems 2015, 18, 560–572. [Google Scholar] [CrossRef]
- Di Filippo, A.; Biondi, F.; Maugeri, M.; Schirone, B.; Piovesan, G. Bioclimate and growth history affect beech lifespan in the Italian Alps and Apennines. Glob. Chang. Biol. 2012, 18, 960–972. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Penuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef]
- Geßler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 2006, 21, 35303. [Google Scholar] [CrossRef]
- van der Maaten, E. Climate sensitivity of radial growth in European beech (Fagus sylvatica L.) at different aspects in southwestern Germany. Trees 2012, 26, 777–788. [Google Scholar] [CrossRef]
- Müller-Haubold, H.; Hertel, D.; Seidel, D.; Knutzen, F.; Leuschner, C. Climate Responses of Aboveground Productivity and Allocation in Fagus sylvatica: A Transect Study in Mature Forests. Ecosystems 2013, 16, 1498–1516. [Google Scholar] [CrossRef]
- Scharnweber, T.; Manthey, M.; Criegee, C.; Bauwe, A.; Schröder, C.; Wilmking, M. Drought matters—Declining precipitation influences growth of Fagus sylvatica L. and Quercus robur L. in north-eastern Germany. For. Ecol. Manag. 2011, 262, 947–961. [Google Scholar] [CrossRef]
- Kunz, J.; Löffler, G.; Bauhus, J. Minor European broadleaved tree species are more drought-tolerant than Fagus sylvatica but not more tolerant than Quercus petraea. For. Ecol. Manag. 2018, 414, 15–27. [Google Scholar] [CrossRef]
- Muffler, L.; Schmeddes, J.; Weigel, R.; Barbeta, A.; Beil, I.; Bolte, A.; Buhk, C.; Holm, S.; Klein, G.; Klisz, M.; et al. High plasticity in germination and establishment success in the dominant forest tree Fagus sylvatica across Europe. Glob. Ecol. Biogeogr. 2021, 30, 1583–1596. [Google Scholar] [CrossRef]
- Walentowski, H.; Falk, W.; Mette, T.; Kunz, J.; Bräuning, A.; Meinardus, C.; Zang, C.; Sutcliffe, L.M.; Leuschner, C. Assessing future suitability of tree species under climate change by multiple methods: A case study in southern Germany. Ann. For. Res. 2017, 60, 101–126. [Google Scholar] [CrossRef]
- Cheaib, A.; Badeau, V.; Boe, J.; Chuine, I.; Delire, C.; Dufrêne, E.; François, C.; Gritti, E.S.; Legay, M.; Pagé, C.; et al. Climate change impacts on tree ranges: Model intercomparison facilitates understanding and quantification of uncertainty. Ecol. Lett. 2012, 15, 533–544. [Google Scholar] [CrossRef]
- Dittmar, C.; Elling, W. Phenological phases of common beech (Fagus sylvatica L.) and their dependence on region and altitude in Southern Germany. Eur. J. For. Res. 2006, 125, 181–188. [Google Scholar] [CrossRef]
- Dittmar, C.; Fricke, W.; Elling, W. Impact of late frost events on radial growth of common beech (Fagus sylvatica L.) in Southern Germany. Eur. J. For. Res. 2006, 125, 249–259. [Google Scholar] [CrossRef]
- Kreyling, J.; Thiel, D.; Nagy, L.; Jentsch, A.; Huber, G.; Konnert, M.; Beierkuhnlein, C. Late frost sensitivity of juvenile Fagus sylvatica L. differs between southern Germany and Bulgaria and depends on preceding air temperature. Eur. J. For. Res. 2012, 131, 717–725. [Google Scholar] [CrossRef]
- Machar, I.; Vlckova, V.; Bucek, A.; Vozenilek, V.; Salek, L.; Jerabkova, L. Modelling of Climate Conditions in Forest Vegetation Zones as a Support Tool for Forest Management Strategy in European Beech Dominated Forests. Forests 2017, 8, 82. [Google Scholar] [CrossRef]
- Pavlović, L.; Stojanović, D.; Mladenović, E.; Lakićević, M.; Orlović, S. Potential Elevation Shift of the European Beech Stands (Fagus sylvatica L.) in Serbia. Front. Plant Sci. 2019, 10, 849. [Google Scholar] [CrossRef]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; de Winter, W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change—Range, abundance, genetic diversity and adaptive response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Thurm, E.A.; Hernandez, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative tree species under climate warming in managed European forests. For. Ecol. Manag. 2018, 430, 485–497. [Google Scholar] [CrossRef]
- Märkel, U.; Dolos, K. Tree Species Site Suitability as a Combination of Occurrence Probability and Growth and Derivation of Priority Regions for Climate Change Adaptation. Forests 2017, 8, 181. [Google Scholar] [CrossRef]
- Friedrichs, D.A.; Trouet, V.; Büntgen, U.; Frank, D.C.; Esper, J.; Neuwirth, B.; Löffler, J. Species-specific climate sensitivity of tree growth in Central-West Germany. Trees 2009, 23, 729–739. [Google Scholar] [CrossRef]
- Michelot, A.; Bréda, N.; Damesin, C.; Dufrêne, E. Differing growth responses to climatic variations and soil water deficits of Fagus sylvatica, Quercus petraea and Pinus sylvestris in a temperate forest. For. Ecol. Manag. 2012, 265, 161–171. [Google Scholar] [CrossRef]
- Nechita, C.; Popa, I.; Eggertsson, Ó. Climate response of oak (Quercus spp.), an evidence of a bioclimatic boundary induced by the Carpathians. Sci. Total Environ. 2017, 599-600, 1598–1607. [Google Scholar] [CrossRef]
- Árvai, M.; Morgós, A.; Kern, Z. Growth-climate relations and the enhancement of drought signals in pedunculate oak (Quercus robur L.) tree-ring chronology in Eastern Hungary. IFor. Biogeosci. For. 2018, 11, 267–274. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Mette, T.; Dolos, K.; Meinardus, C.; Bräuning, A.; Reineking, B.; Blaschke, M.; Pretzsch, H.; Beierkuhnlein, C.; Gohlke, A.; Wellstein, C. Climatic turning point for beech and oak under climate change in Central Europe. Ecosphere 2013, 4, 1–19. [Google Scholar] [CrossRef]
- Sork, V.L.; Davis, F.W.; Westfall, R.; Flint, A.; Ikegami, M.; Wang, H.; Grivet, D. Gene movement and genetic association with regional climate gradients in California valley oak (Quercus lobata Née) in the face of climate change. Mol. Ecol. 2010, 19, 3806–3823. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Klein, E.K.; Jordano, P. Dispersal processes driving plant movement: Challenges for understanding and predicting range shifts in a changing world. J. Ecol. 2017, 105, 1–5. [Google Scholar] [CrossRef]
- Oddou-Muratorio, S.; Bontemps, A.; Klein, E.K.; Chybicki, I.; Vendramin, G.G.; Suyama, Y. Comparison of direct and indirect genetic methods for estimating seed and pollen dispersal in Fagus sylvatica and Fagus crenata. For. Ecol. Manag. 2010, 259, 2151–2159. [Google Scholar] [CrossRef]
- Dobrovolny, L.; Tesař, V. Extent and distribution of beech (Fagus sylvatica L.) regeneration by adult trees individually dispersed over a spruce monoculture. J. For. Sci. 2010, 56, 589–599. [Google Scholar] [CrossRef]
- Millerón, M.; Lopez de Heredia, U.; Lorenzo, Z.; Alonso, J.; Dounavi, A.; Gil, L.; Nanos, N. Assessment of spatial discordance of primary and effective seed dispersal of European beech (Fagus sylvatica L.) by ecological and genetic methods. Mol. Ecol. 2013, 22, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.; Chadœuf, J.; Gugerli, F.; Lascoux, M.; Buiteveld, J.; Cottrell, J.; Dounavi, A.; Fineschi, S.; Forrest, L.L.; Fogelqvist, J.; et al. High rates of gene flow by pollen and seed in oak populations across Europe. PLoS ONE 2014, 9, e85130. [Google Scholar] [CrossRef]
- Gerzabek, G.; Oddou-Muratorio, S.; Hampe, A. Recruitment of a genotyped Quercus robur L. seedling cohort in an expanding oak forest stand: Diversity, dispersal, and performance across habitats. Ann. For. Sci. 2020, 77, 78. [Google Scholar] [CrossRef]
- Löf, M.; Ammer, C.; Coll, L.; Drössler, L.; Huth, F.; Madsen, P.; Wagner, S. Regeneration Patterns in Mixed-Species Stands. In Dynamics, Silviculture and Management of Mixed Forests; Springer: Berlin, Germany, 2018; pp. 103–130. [Google Scholar]
- Jensen, T.S. Seed-seed predator interactions of European beech, Fagus silvatica and forest rodents, Clethrionomys glareolus and Apodemus flavicollis. Oikos 1985, 44, 149–156. [Google Scholar] [CrossRef]
- Perea, R.; Miguel, A.S.; Gil, L. Acorn dispersal by rodents: The importance of re-dispersal and distance to shelter. Basic Appl. Ecol. 2011, 12, 432–439. [Google Scholar] [CrossRef]
- Turcek, F.J. Ökologische Beziehungen der Vögel und Gehölze; Verlag der Slowakischen Akademie der Wissenschaften: Bratislava, Slovakia, 1961. [Google Scholar]
- Moreno, J.; Lundberg, A.; Carlson, A. Hoarding of individual nuthatches Sitta europaea and march tits Parus palustris. Ecography 1981, 4, 263–269. [Google Scholar] [CrossRef]
- Amann, F. Balzfüttern, Nahrung und Samenverstecken bei der Sumpfmeise Parus palustris. Der Ornithol. Beob. 2007, 104, 91–100. [Google Scholar]
- Bossema, I. Jays and oaks: An eco-ethological study of a symbiosis. Behaviour 1979, 70, 1–116. [Google Scholar] [CrossRef]
- Mellanby, K. The effects of some mammals and birds on regeneration of oak. J. Appl. Ecol. 1968, 5, 359–366. [Google Scholar] [CrossRef]
- Pesendorfer, M.B.; Sillett, T.S.; Koenig, W.D.; Morrison, S.A. Scatter-hoarding corvids as seed dispersers for oaks and pines: A review of a widely distributed mutualism and its utility to habitat restoration. Condor Ornithol. Appl. 2016, 118, 215–237. [Google Scholar] [CrossRef]
- Perea, R.; San Miguel, A.; Gil, L. Flying vs. climbing: Factors controlling arboreal seed removal in oak-beech forests. For. Ecol. Manag. 2011, 262, 1251–1257. [Google Scholar] [CrossRef]
- Cramer, J.M.; Mesquita, R.C.; Bruce Williamson, G. Forest fragmentation differentially affects seed dispersal of large and small-seeded tropical trees. Biol. Conserv. 2007, 137, 415–423. [Google Scholar] [CrossRef]
- Krosby, M.; Tewksbury, J.; Haddad, N.M.; Hoekstra, J. Ecological connectivity for a changing climate. Conserv. Biol. 2010, 24, 1686–1689. [Google Scholar] [CrossRef]
- Vitt, P.; Havens, K.; Kramer, A.T.; Sollenberger, D.; Yates, E. Assisted migration of plants: Changes in latitudes, changes in attitudes. Biol. Conserv. 2010, 143, 18–27. [Google Scholar] [CrossRef]
- Malanson, G.P.; Armstrong, M.P. Dispersal probability and forest diversity in a fragmented landscape. Ecol. Model. 1996, 87, 91–102. [Google Scholar] [CrossRef]
- Collingham, Y.C.; Huntley, B. Impacts of habitat fragmentation and patch size upon migration rates. Ecol. Appl. 2000, 10, 131–144. [Google Scholar] [CrossRef]
- Pitelka, L.F. Plant migration and climate change: A more realistic portrait of plant migration is essential to predicting biological responses to global warming in a world drastically altered by human activity. Plant Migration Workshop Group. Am. Sci. 1997, 85, 464–473. [Google Scholar]
- Feurdean, A.; Bhagwat, S.A.; Willis, K.J.; Birks, H.J.B.; Lischke, H.; Hickler, T. Tree Migration-Rates: Narrowing the Gap between Inferred Post-Glacial Rates and Projected Rates. PLoS ONE 2013, 8, e71797. [Google Scholar] [CrossRef]
- Kremer, A.; Ronce, O.; Robledo-Arnuncio, J.J.; Guillaume, F.; Bohrer, G.; Nathan, R.; Bridle, J.R.; Gomulkiewicz, R.; Klein, E.; Ritland, K.; et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol. Lett. 2012, 15, 378–392. [Google Scholar] [CrossRef]
- Leonardi, S.; Menozzi, P. Genetic variability of Fagus sylvatica L. in Italy: The role of postglacial recolonization. Heredity 1995, 75, 35–44. [Google Scholar] [CrossRef][Green Version]
- Moracho, E.; Moreno, G.; Jordano, P.; Hampe, A. Unusually limited pollen dispersal and connectivity of Pedunculate oak (Quercus robur) refugial populations at the species’ southern range margin. Mol. Ecol. 2016, 25, 3319–3331. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Axer, M.; Schlicht, R.; Kronenberg, R.; Wagner, S. The Potential for Future Shifts in Tree Species Distribution Provided by Dispersal and Ecological Niches: A Comparison between Beech and Oak in Europe. Sustainability 2021, 13, 13067. https://doi.org/10.3390/su132313067
Axer M, Schlicht R, Kronenberg R, Wagner S. The Potential for Future Shifts in Tree Species Distribution Provided by Dispersal and Ecological Niches: A Comparison between Beech and Oak in Europe. Sustainability. 2021; 13(23):13067. https://doi.org/10.3390/su132313067
Chicago/Turabian StyleAxer, Maximilian, Robert Schlicht, Rico Kronenberg, and Sven Wagner. 2021. "The Potential for Future Shifts in Tree Species Distribution Provided by Dispersal and Ecological Niches: A Comparison between Beech and Oak in Europe" Sustainability 13, no. 23: 13067. https://doi.org/10.3390/su132313067
APA StyleAxer, M., Schlicht, R., Kronenberg, R., & Wagner, S. (2021). The Potential for Future Shifts in Tree Species Distribution Provided by Dispersal and Ecological Niches: A Comparison between Beech and Oak in Europe. Sustainability, 13(23), 13067. https://doi.org/10.3390/su132313067





