Sustainable Electroporator for Continuous Pasteurisation: Design and Performance Evaluation with Orange Juice
Abstract
:1. Introduction
2. Materials and Methods
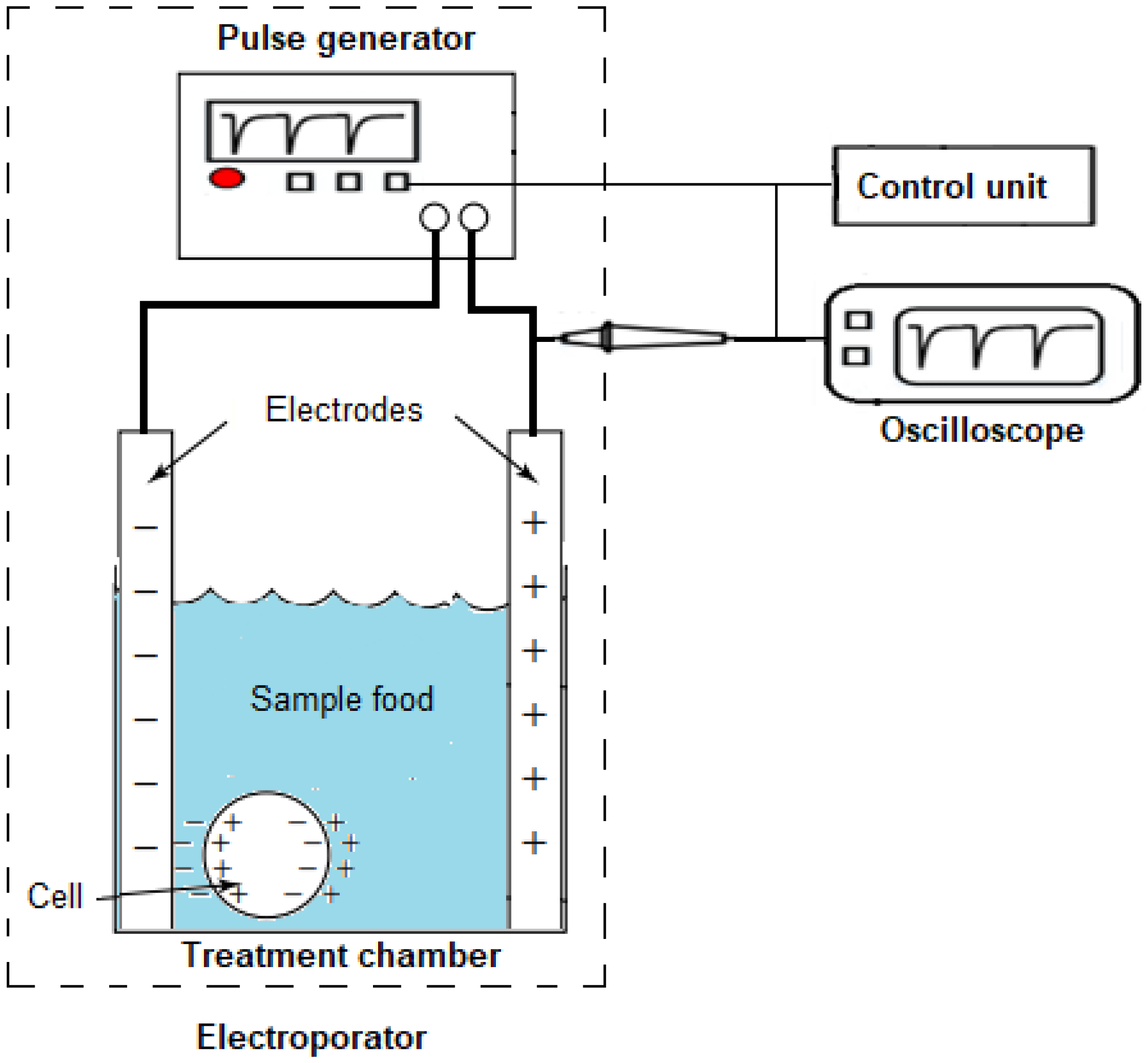
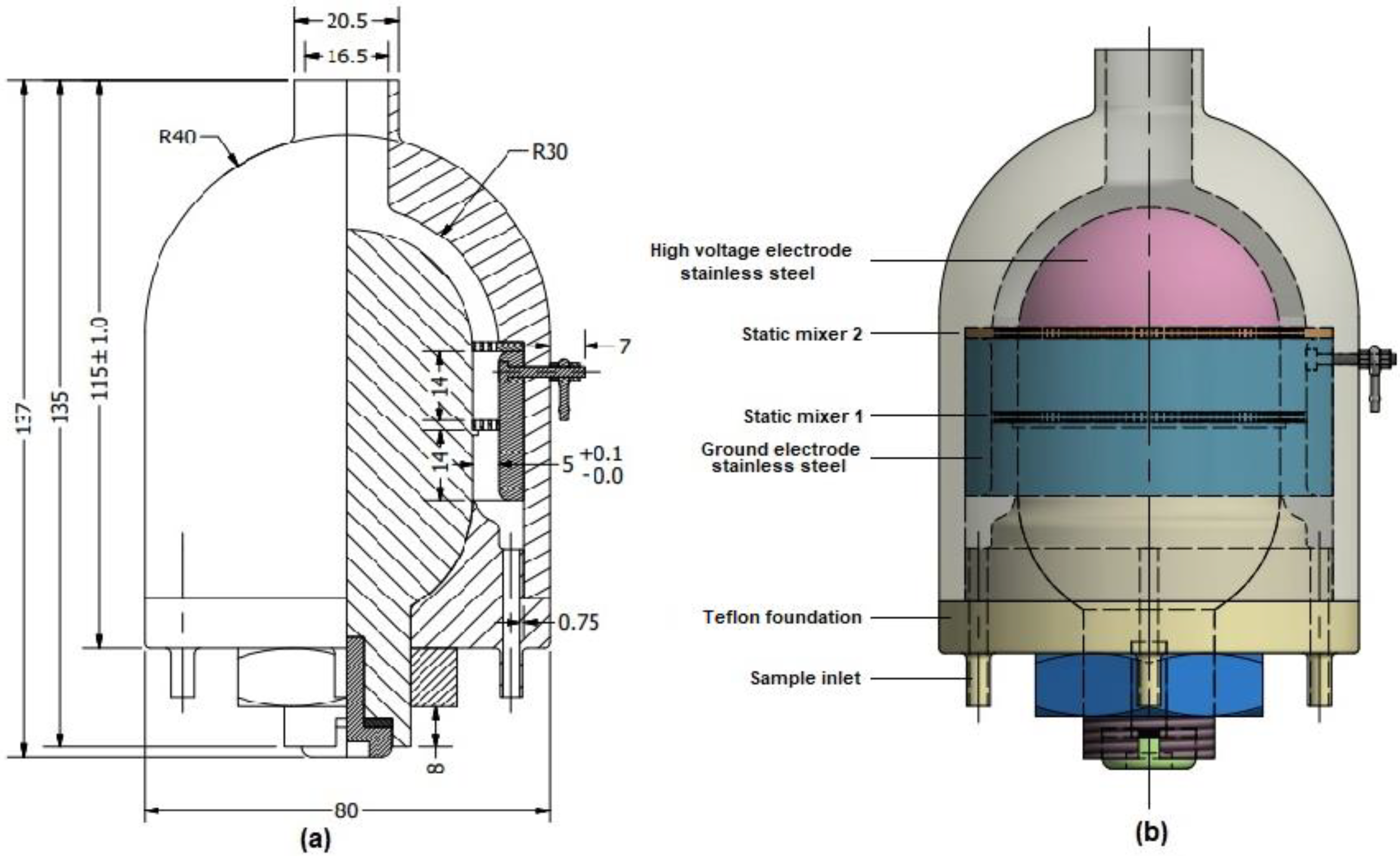
2.1. Analysis and Fabrication of a Coaxial Treatment Chamber Prototype
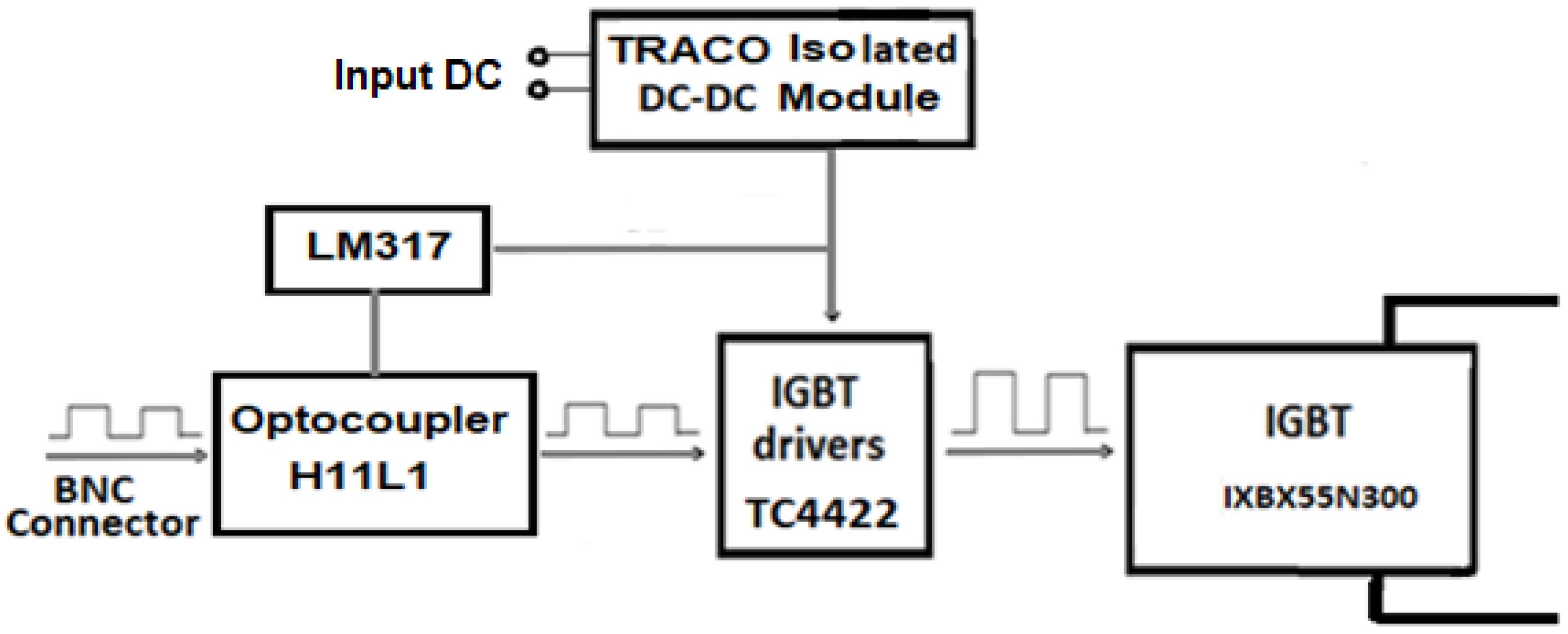
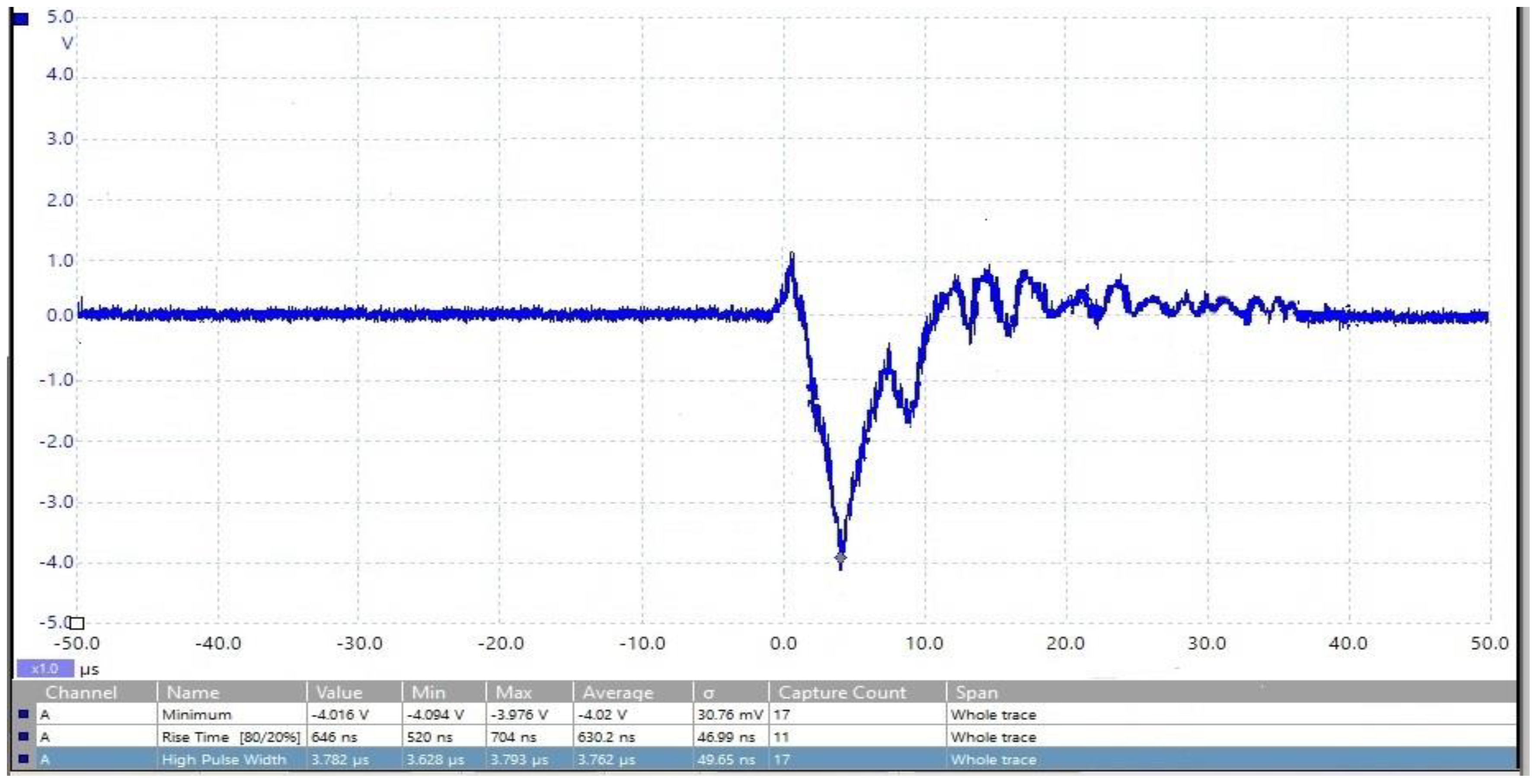
2.2. Designing a Marx Generator for Food Electroporation
2.3. Sterilizing Washable Apparatus
2.4. Preparation of the Raw Food Sample
2.5. Low-Temperature Long-Time Thermal Treated Food Sample
2.6. Food Sample Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microbial Analysis
3.2. Chemical Analysis
3.3. Energy Consumption Estimation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricci, A.; Parpinello, G.P.; Versari, A. Recent advances and applications of pulsed electric fields (PEF) to improve polyphenol extraction and color release during red winemaking. Beverages 2018, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Aadil, R.M.; Zeng, X.A.; Ali, A.; Zeng, F.; Farooq, M.A.; Han, Z.; Khalid, S.; Jabbar, S. Influence of different pulsed electric field strengths on the quality of the grapefruit juice. Int. J. Food Sci. Technol. 2015, 50, 2290–2296. [Google Scholar] [CrossRef]
- Saulis, G. Electroporation of cell membranes: The fundamental effects of pulsed electric fields in food processing. Food Eng. Rev. 2010, 2, 52–73. [Google Scholar] [CrossRef]
- Elgenedy, M.A.; Darwish, A.; Ahmed, S.; Williams, B.W. A modular multilevel generic pulse-waveform generator for pulsed electric field applications. IEEE Trans. Plasma Sci. 2017, 45, 2527–2535. [Google Scholar] [CrossRef] [Green Version]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.; Bekhit, A.E.-D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical systems for pulsed electric field applications in the food industry: An engineering perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Kempkes, M.A. Industrial Pulsed Electric Field Systems. In Handbook Electroporation; 2017; pp. 1–21. Available online: https://www.researchgate.net/profile/Michael-Kempkes/publication/315829658_Industrial_Pulsed_Electric_Field_Systems/links/59cd29ad0f7e9b454f9f8f8c/Industrial-Pulsed-Electric-Field-Systems.pdf (accessed on 22 January 2022).
- Chen, X.; Yu, L.; Jiang, T.; Tian, H.; Huang, K.; Wang, J. A High-Voltage Solid-State Switch Based on Series Connection of IGBTs for PEF Applications. IEEE Trans. Plasma Sci. 2017, 45, 2328–2334. [Google Scholar] [CrossRef]
- Huang, K.; Wang, J. Designs of pulsed electric fields treatment chambers for liquid foods pasteurization process: A review. J. Food Eng. 2009, 95, 227–239. [Google Scholar] [CrossRef]
- Kandušer, M.; Belič, A.; Čorović, S.; Škrjanc, I. Modular Serial Flow Through device for pulsed electric field treatment of the liquid samples. Sci. Rep. 2017, 7, 8115. [Google Scholar] [CrossRef] [Green Version]
- McHugh, T.; Toepfl, S. Pulsed electric field processing for fruits and vegetables. Food Technol. 2016, 70, 73–75. [Google Scholar]
- Jayaram, S.H. Sterilization of liquid foods by pulsed electric fields. Electr. Insul. Mag. IEEE 2000, 16, 17–25. [Google Scholar] [CrossRef]
- Hodgins, A.; Mittal, G.; Griffiths, M. Pasteurization of fresh orange juice using low-energy pulsed electrical field. J. Food Sci. 2002, 67, 2294–2299. [Google Scholar] [CrossRef]
- Jaeger, H.; Meneses, N.; Knorr, D. Impact of PEF treatment inhomogeneity such as electric field distribution, flow characteristics and temperature effects on the inactivation of E. coli and milk alkaline phosphatase. Innov. Food Sci. Emerg. Technol. 2009, 10, 470–480. [Google Scholar] [CrossRef]
- Buckow, R.; Semrau, J.; Sui, Q.; Wan, J.; Knoerzer, K. Numerical evaluation of lactoperoxidase inactivation during continuous pulsed electric field processing. Biotechnol. Prog. 2012, 28, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Knoerzer, K.; Baumann, P.; Buckow, R. An iterative modelling approach for improving the performance of a pulsed electric field (PEF) treatment chamber. Comput. Chem. Eng. 2012, 37, 48–63. [Google Scholar] [CrossRef]
- Schottroff, F.; Knappert, J.; Eppmann, P.; Krottenthaler, A.; Horneber, T.; McHardy, C.; Rauh, C.; Jaeger, H. Development of a continuous pulsed electric field (PEF) vortex-flow chamber for improved treatment homogeneity based on hydrodynamic optimization. Front. Bioeng. Biotechnol. 2020, 8, 340. [Google Scholar] [CrossRef]
- Araujo, E.J.; Lopes, I.J.; Ramirez, J.A. Numerical study of treatment chambers for single and multi-stage pulsed electric field systems. IET Sci. Meas. Technol. 2021, 15, 385–397. [Google Scholar] [CrossRef]
- Arshad, R.N.; Buntat, Z.B.; Dastgheib, A.M.; Jusoh, Y.M.; Munir, A.; Aadil, R.M.; Ahmad, M.H. Continuous Flow Treatment Chamber for Liquid Food Processing through Pulsed Electric Field. J. Comput. Theor. Nanosci. 2020, 17, 1492–1498. [Google Scholar]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Impact of pulsed electric fields and high voltage electrical discharges on extraction of high-added value compounds from papaya peels. Food Res. Int. 2014, 65, 337–343. [Google Scholar] [CrossRef]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Ahmad, M.H.; Sidik, M.A.B.; Nawawi, Z. Air Bubble in Liquid Food under Pulsed Electric Field Pasteurization using Coaxial Chamber. In Proceedings of the 2021 IEEE International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Johor Bahru, Malaysia, 12–14 July 2021; pp. 250–253. [Google Scholar]
- Bhadekar, R.; Bhola, J. Nonconventional preservation techniques: Current trends and future prospects. In Preservatives and Preservation Approaches in Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–147. [Google Scholar]
- Liu, G.; Carøe, C.; Qin, Z.; Munk, D.M.; Crafack, M.; Petersen, M.A.; Ahrné, L. Comparative study on quality of whole milk processed by high hydrostatic pressure or thermal pasteurization treatment. LWT 2020, 127, 109370. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshtiaghi, M.; Nakthong, N. Application of Pulsed Electric Field for Inactivation of Yeast S. cerevisiae in Apple Juice. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021; p. 012008. [Google Scholar]
- Timmermans, R.; Mastwijk, H.; Berendsen, L.; Nederhoff, A.; Matser, A.; Van Boekel, M.; Groot, M.N. Moderate intensity Pulsed Electric Fields (PEF) as alternative mild preservation technology for fruit juice. Int. J. Food Microbiol. 2019, 298, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Cortés, C.; Esteve, M.J.; Frigola, A. Color of orange juice treated by high intensity pulsed electric fields during refrigerated storage and comparison with pasteurized juice. Food Control 2008, 19, 151–158. [Google Scholar] [CrossRef]
- Lasekan, O.; Ng, S.; Azeez, S.; Shittu, R.; Teoh, L.; Gholivand, S. Effect of pulsed electric field processing on flavor and color of liquid foods. J. Food Processing Preserv. 2017, 41, e12940. [Google Scholar] [CrossRef]
- Wibowo, S.; Vervoort, L.; Tomic, J.; Santiago, J.S.; Lemmens, L.; Panozzo, A.; Grauwet, T.; Hendrickx, M.; Van Loey, A. Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 2015, 171, 330–340. [Google Scholar] [CrossRef]
- Cserhalmi, Z.; Sass-Kiss, A.; Tóth-Markus, M.; Lechner, N. Study of pulsed electric field treated citrus juices. Innov. Food Sci. Emerg. Technol. 2006, 7, 49–54. [Google Scholar] [CrossRef]
- Elez-Martínez, P.; Soliva-Fortuny, R.C.; Martín-Belloso, O. Comparative study on shelf life of orange juice processed by high intensity pulsed electric fields or heat treatment. Eur. Food Res. Technol. 2006, 222, 321–329. [Google Scholar] [CrossRef]
- Agcam, E.; Akyildiz, A.; Evrendilek, G.A. A comparative assessment of long-term storage stability and quality attributes of orange juice in response to pulsed electric fields and heat treatments. Food Bioprod. Processing 2016, 99, 90–98. [Google Scholar] [CrossRef]
- Kayalvizhi, V.; Pushpa, A.; Sangeetha, G.; Antony, U. Effect of pulsed electric field (PEF) treatment on sugarcane juice. J. Food Sci. Technol. 2016, 53, 1371–1379. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Ahmad, M.H.; Nawawi, Z.; Sidik, M.A.; Jumani, T.A.; Khan, I.; Alotaibi, H.; Khan, A. An improved electroporator with continuous liquid flow and double-exponential waveform for liquid food pasteurization. IEEE Access 2021, 9, 147732–147742. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; Bekhit, A.E.; Liu, Z.W.; Aadil, R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]


| Parameter | Value | Units |
|---|---|---|
| Required electric field | 10 | kV·cm−1 |
| Pulse width | 3 | µsec |
| Maximum flow rate | 100 | mL·sec−1 |
| Required energy per volume (specific energy) | 200 | J·mL−1 |
| Chamber volume 3.14 (32–2.52) * 3 | 25.8 | cm3 |
| 25.8 | mL |
| No. of Pulses | PEF Treatment with Sieves | PEF Treatment without Sieves | ||
|---|---|---|---|---|
| Log10 Reduction | Temperature Rise °C | Log10 Reduction | Temperature Rise °C | |
| 500 | 2.2 | 25 | 1.9 | 27.6 |
| 1000 | 1.8 | 29.8 | 1.8 | 32.4 |
| 1500 | 1.4 | 31.6 | 1.2 | 34.7 |
| Day 0 | Day 9 | p Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | S0 | S1 | S2 | S0 | S1 | S2 | T | D | T × D |
| L* | 40.31 ± 0.06 e | 40.46 ± 0.03 f | 40.12 ± 0.04 d | 36.41 ± 0.04 a | 37.94 ± 0.03 b | 39.40 ± 0.04 c | <0.001 | <0.001 | <0.001 |
| a* | 2.04 ± 0.03 a | 2.13 ± 0.07 a | 2.18 ± 0.03 a | 2.63 ± 0.18 c | 2.54 ± 0.02 c | 2.36 ± 0.09 b | 0.375 | <0.001 | 0.006 |
| b* | 27.04 ± 0.05 bc | 27.17 ± 0.05 c | 27.07 ± 0.04 bc | 26.48 ± 0.29 a | 26.61 ± 0.60 ab | 26.95 ± 0.07 abc | 0.345 | 0.008 | 0.318 |
| °Brix | 12.40 ± 0.02 f | 11.93 ± 0.04 c | 12.34 ± 0.03 e | 11.07 ± 0.03 a | 11.67 ± 0.02 b | 12.27 ± 0.02 d | <0.001 | <0.001 | <0.001 |
| pH | 3.38 ± 0.02 | 3.36 ± 0.04 | 3.37 ± 0.03 | 3.41 ± 0.03 | 3.37 ± 0.03 | 3.38 ± 0.02 | 0.156 | 0.112 | 0.665 |
| Vitamin C 1 | 47.32 ± 0.58 c | 47.76 ± 0.80 c | 46.71 ± 0.85 c | 39.62 ± 0.69 a | 40.74 ± 0.75 a | 44.21 ± 0.66 b | 0.002 | <0.001 | <0.001 |
| Conductivity 2 | 3.17 ± 0.02 b | 3.13 ± 0.01 b | 3.16 ± 0.03 b | 2.97 ± 0.02 a | 3.13 ± 0.02 b | 3.13 ± 0.02 b | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, R.N.; Abdul-Malek, Z.; Jusoh, Y.M.M.; Radicetti, E.; Tedeschi, P.; Mancinelli, R.; Lorenzo, J.M.; Aadil, R.M. Sustainable Electroporator for Continuous Pasteurisation: Design and Performance Evaluation with Orange Juice. Sustainability 2022, 14, 1896. https://doi.org/10.3390/su14031896
Arshad RN, Abdul-Malek Z, Jusoh YMM, Radicetti E, Tedeschi P, Mancinelli R, Lorenzo JM, Aadil RM. Sustainable Electroporator for Continuous Pasteurisation: Design and Performance Evaluation with Orange Juice. Sustainability. 2022; 14(3):1896. https://doi.org/10.3390/su14031896
Chicago/Turabian StyleArshad, Rai Naveed, Zulkurnain Abdul-Malek, Yanti M. M. Jusoh, Emanuele Radicetti, Paola Tedeschi, Roberto Mancinelli, Jose M. Lorenzo, and Rana Muhammad Aadil. 2022. "Sustainable Electroporator for Continuous Pasteurisation: Design and Performance Evaluation with Orange Juice" Sustainability 14, no. 3: 1896. https://doi.org/10.3390/su14031896
APA StyleArshad, R. N., Abdul-Malek, Z., Jusoh, Y. M. M., Radicetti, E., Tedeschi, P., Mancinelli, R., Lorenzo, J. M., & Aadil, R. M. (2022). Sustainable Electroporator for Continuous Pasteurisation: Design and Performance Evaluation with Orange Juice. Sustainability, 14(3), 1896. https://doi.org/10.3390/su14031896











