Abstract
In tropical countries, where human consumption of insects is a traditional practice, insects are mainly harvested in the natural environment. These insects are thus exposed to all forms of pollution, particularly metallic pollutants. However, an inventory of scientific knowledge on the risks related to the consumption of insects is missing in the international scientific literature. It is therefore essential to conduct research on the trace metals (TMEs) contained in insects caught in the wild. The objective of this work is to evaluate the level of accumulation of TMEs by insect species commonly consumed in Togo to estimate the health risk related to insect consumption. To do this, 12 species of insects consumed in their adult stage were collected across the whole territory of Togo. These samples were analyzed by atomic absorption spectrophotometry to determine TMEs. It appears that traces of different metallic elements exist in the samples at variable rates. The aquatic species Cybister tripunctatus is the most contaminated, with levels of cadmium (0.504 mg/kg) and lead (0.501 mg/kg) at the limit of edibility threshold. The concentrations of all TMEs in insects during this study are within acceptable limits for human consumption. The risk of human contamination with TMEs through insect consumption is therefore low.
1. Introduction
Many peoples have introduced insect consumption into their customs. Indeed, about 2.5 billion people in tropical countries (Asia, Africa and Latin America) eat insects daily [1,2] for their multiple benefits. According to global institutions and researchers interested in entomophagy, edible insects possess nutritional, economic and environmental potential [2]. As a result, insect consumption has become a practice that is gaining popularity around the world. There is now a growing craze for entomophagy [3]. In Europe and more generally in the West, although still very marginalized, entomophagy tends to spread with the emergence in various countries of restaurants specializing in insect-based menus, which is associated with the industrial production of edible insects [4,5,6]. In contrast, in tropical countries where insects are traditionally consumed, insects are still harvested in the wild [7,8]. In Togo, as elsewhere in the tropics, insects intended for human consumption are collected in the wild in various environments [9]. These environments are likely to be polluted by the discharge of human household and industrial waste and the use of pesticides. The latter, which may be rich in organic matter and fertilizing elements, also contains undesirable chemical elements, especially metallic pollutants [10]. These metal pollutants are considered very dangerous for the environment because of their tendency to bioaccumulate in organisms [11,12]. Insects do not escape this phenomenon of bioaccumulation of TMEs. Thus, insects harvested from the wild for consumption may be exposed to high concentrations of TMEs that accumulate in their bodies and pose health risks to consumers. Few data on the bioaccumulation of TMEs in edible insect species are available in the international scientific literature. For example, a study on mealworms (Tenebrio molitor) showed that these insects accumulate cadmium and lead when fed organic matter containing these TMEs [13]. In addition, another study in Indonesia showed that TMEs such as As, Pb, Cd and Hg were found in very low amounts in the sago group (Rhynchophorus bilineatus) consumed by native populations. Based on the few available studies, the levels of TMEs detected in insects for human consumption are in compliance with Regulation (EU) 1881/2006 for contaminants [14]. However, extrapolation of these data to insects consumed in general may be inaccurate, as important metabolic and physiological differences exist between insect species [15]. The presence of TMEs in insects used for human consumption therefore deserves further research. It is within this framework that this study aims to evaluate the level of accumulation of TMEs by insect species commonly consumed in Togo in order to estimate the health risk of insect consumption for the consuming populations.
2. Materials and Methods
2.1. Framework
Insect sampling was carried out throughout the Togolese territory according to the localities where these species are consumed. The geographical coordinates of all the sampling localities are shown in Figure 1.
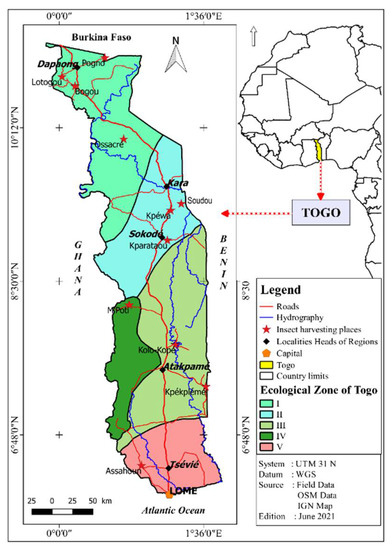
Figure 1.
Sample collection locations.
This sampling took place from September 2018 to August 2019 at the rate of one trip per month. Twelve (12) adult stage species from the order Coleoptera (8), Orthoptera (3) and Isoptera (1) were collected. Three sites were chosen for the collection of each species (Table 1).

Table 1.
Harvest locations of insect species consumed in Togo.
These species were collected in different ecosystems (crop, forest, savanna, water) of the different ecological zones of Togo according to their period of abundance identified by Badanaro [9]. Guides identified as knowledgeable about the insect species consumed in the study environments assisted in the actual recognition of insect species consumed in the field. The active method (sight hunting with swath nets) was used to capture insects except for 3 species: C. tripunctatus, M. bellicosus and B. membranaceus. C. tripunctatus is an aquatic insect that was collected with craft nets intended for fish harvesting. After rain, winged termites are usually attracted to light sources at dusk. Therefore, light traps were used to trap and collect winged adults of M. bellicosus. B. membranaceus was collected by digging out its galleries with a hoe. During the period of activity, these galleries are recognizable by a small mound of soil deposited outside by the insect as it digs its gallery. Samples collected for identification were sent to the laboratory of Applied Entomology of the Faculty of Sciences of the University of Lomé, where they were identified to the species level using determination keys. Insects destined for chemical analysis were collected alive during the same periods and placed in a cooler containing previously frozen carbo-ice [16] in order to maintain the “cold chain” throughout the transport to the laboratory. Once at the laboratory, the samples were frozen.
2.2. Treatment of Samples in the Laboratory
Frozen samples were then thawed at room temperature. Ten grams of a sample of each species from each sampling locality were weighed. Then, they were placed in previously numbered trays and introduced to the ISUZU type AS oven at 65 °C for their drying [17]. They were then crushed by species in a Moulinex of the General Electric Interlabs type and sifted to obtain the flour. The grindings were stored in airtight containers and kept in a refrigerator for TME analysis.
2.3. Chemical Analysis
The determination of TMEs was carried out at the Faculty of Engineering of Vasile Alecsandri University of Bacau. The solubilization of the insect crushers was performed by an acid attack on a sand bath using two concentrated solutions: nitric acid and hydrogen peroxide. Indeed, 1 g of each grind was introduced in a Teflon to which 1 mL of hydrogen peroxide and 8 mL of nitric acid were added. After stirring, the Teflon containers were heated on a sand bath for about 2 h at a temperature of about 150 °C. The recovery of the products obtained after heating was done with 2 mL of distilled water. After cooling, the solution obtained after digestion was transferred to a 100 mL volumetric flask and supplemented with demineralized water. After homogenization, the solution was filtered through a Wattman paper. Thus, the filtrate was collected in a closed bottle. The determination of TMEs was carried out from this filtrate by the flame atomic absorption spectrophotometer Agilent 7500 ICP-MS cu UP 213 using standard solutions. This study was therefore interested in the following 17 TMEs: Ag, As, Be, Cd, Cr, Co, Cu, Ga, Hg, Mn, Ni, Pb, Rb, Se, Sr, V, Zn. These TMEs are the most sought in food because they are the most toxic to living organisms.
The real concentrations were determined with the following formula (ISO 17294:2003):
where RC is the real concentration, CS is the analyte concentration, DV is the dilution volume, and M is mass of the test sample.
2.4. Statistical Analysis
Statistical tests were carried out with the SPSS (statistical package social sciences) software. All the tests were performed in triplicate. The results are presented as mean ± standard deviation. Analysis of variance (ANOVA-1) was used to compare the means. Observed differences between means are considered statistically significant if P is less than 0.05.
3. Results
3.1. Composition of the Insects Studied in Toxic TMEs above a Certain Threshold
The results of the analysis of toxic TMEs above a certain threshold and beneficial TMEs above that threshold are reported in Table 2.

Table 2.
Toxic TME composition above a certain threshold of the main edible insect species in Togo (mg/kg).
Analysis of the results in the table shows that all the species studied contain Ag (0.050–7.636 mg/kg), As (1.534–6.36 mg/kg), Cr (1.01–5.64 mg/kg), Co (0. 932–11.59 mg/kg), Cu (3.023–5.832 mg/kg), Mn (2.224–9.927 mg/kg), Ni (0.136–8.637 mg/kg), Se (0.410–3.094 mg/kg), V (0.895–3.778 mg/kg) and Zn (4.117–12.041 mg/kg). These results show significant differences between species for each element. C. tripunctatus contains more Ag, Cr and V. The highest concentrations of Cu and Mn were found in S. interrupta, while the highest concentration of Co and Ni were found in K. angulifera. The contents of Cu, Mg and Zn are statistically higher in 58.33% of the species studied (A. ruficornis, B. membranaceus, G. varians, P. cordata, P. marginata, R. sobrina and S. interrupta).
3.2. Composition of the Insects Studied in Toxic TMEs
The concentrations of toxic TMEs in the insect species studied, i.e., elements for which no positive role for living organisms is known to date, are shown in Table 3. This table shows that all the insect species studied do not contain Be. On the other hand, they do contain TMEs such as Cd (0.016–0.504 mg/kg), Ga (0.164–0.475 mg/kg), Hg (0.010–0.315 mg/kg), Pb (0.041–0.501 mg/kg), Rd (0.125–0.471 mg/kg), and Sr (0.074–0.493 mg/kg). The levels of these TMEs were statistically variable among species. The results revealed low to average concentrations sometimes exceeding the required standards, notably in C. tripunctatus for Cd (0.504 mg/kg) and Pb (0.501 mg/kg).

Table 3.
Toxic TME compositions of the main edible insect species in Togo.
4. Discussion
TMEs are chemical elements whose concentration in the organism’s dry matter must be below 0.01% for those involved in metabolism [18]. However, when living beings are present in an environment with high concentrations of these elements, they can accumulate in organisms and be transferred to other organisms through food chains. Ten (Ag, As, Cr, Co, Cu, Mn, Ni, Se, V, Zn) of the 17 TMEs investigated in this study are involved in metabolism. An organism’s requirements for these TMEs lie between the deficiency threshold and the toxicity threshold [19]. All the insect species studied contain all 10 toxic TMEs above a certain threshold. Since these TMEs are involved in metabolism, their presence in the studied insects is a nutritional advantage [20,21,22]. Indeed, iron, zinc, selenium, copper, and manganese intervene in the immune system as antioxidant enzyme cofactors [23]. In general, the average contents of cobalt, selenium, copper, zinc, and manganese of the studied insect species are higher than the results obtained by Ramos-Elorduy et al. [24] following the analysis of orthoptera of different species and Igwe et al. [25] on the termite Macrotermes nigeriensis (Isoptera: Termitidae). However, even when essential, these TMEs can be toxic at high concentrations. The Cu, Mg and Zn contents are the highest (58.33%) in the majority of the species studied. When comparing the values obtained in this study with the safety limits of the recommended nutritional intake of these trace elements, which are 5 mg/kg, 8 mg/kg and 15 mg/kg for Cu, Mg and Zn, respectively, the values obtained are below these thresholds [26]. Therefore, these insects do not present a risk of intoxication in these elements. Other TMEs such as Cd, Pb and Hg have no known metabolic function to date [27]. These TMEs can contaminate living organisms and be taken up by the food chain. Once transferred to humans, who are at the end of the trophic chain, via the digestive tract, they can cause serious pathologies [27]. Although the concentrations of Pb, Cd and Hg in the insects studied are higher than those found in the sago grub (Rhynchophorus bilineatus (Coleoptera: Dryophthoridae)) consumed by indigenous populations in Indonesia [28], they comply with Regulation (EU) 1881/2006 (which sets the safety limit in food at 0.5 mg/kg for Pb, Cd and Hg) except in C. tripunctatus for Cd (0.504 mg/kg) and for Pb (0.501 mg/kg). However, cadmium levels in C. tripunctatus were not statistically different from those in G. trivittata (0.497 mg/kg), and S. interrupta (0.488 mg/kg) was below the threshold. Similarly, the lead content of C. tripunctatus is not statistically higher than that of M. bellicosus (0.482 mg/kg). Other toxic TMEs such as Ga, Rb, and Sr, for which toxicological reference values are not fixed, were found in the studied insects but at low concentrations. The consumption of wild insects collected in Togo does not present a risk of intoxication by TMEs. These results are in agreement with those of Poma et al. [29]. According to these authors, the concentrations of all the TMEs tested in their study (Cd, Ar, Cr, Pb and Sn) were within acceptable limits for human consumption. These results are also consistent with those of Idowu et al. [30] and Mabossy-Mobouna et al. [31]. C. tripunctatus is the only aquatic insect species studied in this study. This species has the highest levels of Cd and Pb, exceeding the toxicity thresholds. It therefore appears to be the most contaminated. However, as only one aquatic species was studied, no relationship can be established between the living environment of this species and the level of contamination of the species, although aquatic environments are known to contain high quantities of TMEs [32].
5. Conclusions
This study made it possible to evaluate the level of accumulation of TMEs by insect species commonly consumed in Togo. Apart from Be, all the TMEs sought (16) were found in the 12 insect species studied. Their concentration in the insects differed according to the species. However, the concentrations of all the TMEs investigated in edible insects during this study are within the acceptable limits for human consumption. The aquatic species C. tripunctatus is the most contaminated with levels of 0.504 mg/kg for Cd and 0.501 mg/kg for Pb, at the limit of edibility threshold (0.5) for these elements. The risk of human contamination with TMEs through the consumption of insects is therefore low.
Author Contributions
F.B. and A.T.-B. conceived, designed the project and performed the sampling. N.B. and F.B. performed the experiments. F.B. analyzed the data. N.B., F.B. and A.T.-B. wrote the manuscript. V.N. and M.P.-L. supervised all activities. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their thanks to the International Foundation for Science (IFS) and the Agence Universitaire de la Francophonie (AUF), whose funding allowed the sampling of species throughout Togo and the analysis of samples through the Eugen Ionesco grant, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization of the United Nations (FAO). Insectes Comestibles Perspectives pour la Sécurité Alimentaire et l’Alimentation Animale 2014. Available online: https://www.fao.org/3/i3253f/I3253F.pdf (accessed on 2 November 2021).
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security. FAO Forestry Paper, Volume 171. 2013. Available online: https://www.fao.org/3/i3253e/i3253e.pdf (accessed on 10 November 2021).
- Quellet, S. Développement d’un Outil d’Aide à la Decision pour une Utilisation Durable des Insectes Comestibles. Master’s Thesis, Université de Sherbrooke, Sherbrooke, QC, Canada, 2017. [Google Scholar]
- Chen, X.; Feng, Y. Review on nutritive value of edible insects. Chin. For. Sci. Technol. 2002, 12, 54–59. [Google Scholar]
- Séré de Lanauze, G. L’adoption d’un produit alimentaire nouveau face à des freins culturels forts. Le cas de l’entomophagie en France. Décisions Mark. 2015, 79, 15–33. [Google Scholar] [CrossRef]
- Barre, A.; Velazquez, E.; Delplanque, A.; Caze-Subra, S.; Bienvenu, F.; Bienvenu, J.; Benoit, H.; Rougé, P. Les allergènes croissants des insectes comestibles. Rev. Française Allergol. 2016, 56, 522–532. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J. Energy supplied by edible insects from mexico and their nutritional and ecological importance. Ecol. Food Nutr. 2008, 47, 280–297. [Google Scholar] [CrossRef]
- Van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badanaro, F. Les Insectes Comestibles au Togo: Ethnoentomologie et Potentiel Nutritionnel. Ph.D. Thesis, Université de Lomé, Lomé, Togo, 2015. [Google Scholar]
- Coutin, F.A.; Philippe, F.; Vermande, P. Contribution à la caractérisation physico-chimique des déchets de deux marchés de la commune de Port-au-Prince (Haïti): Propositions de traitements. Déchets Sci. Tech. 2007, 47, 30–33. [Google Scholar] [CrossRef]
- Bimizi, A.; Diallo, A.; Eklu-Gadegbeku, K.; Gnandi, K.; Bakoma, B.; Aklikokou, K.; Creppy, E.E.; Gbeassor, M. Evaluation de la contamination de quelques produits maraîchers par les métaux lourds à Lomé. J. Rech. Sci. Univ. 2012, 14, 115–122. [Google Scholar]
- Ouro-Sama, K.; Solitoke, H.D.; Gnandi, K.; Afiademanyo, K.M.; Bowessidjaou, E.J. Évaluation et risques sanitaires de la bioaccumulation de métaux lourds chez des espèces halieutiques du système lagunaire togolais. Rev. Électron. Sci. Environ. 2014, 14. [Google Scholar] [CrossRef]
- Vijver, M.; Jager, T.; Posthuma, L.; Peijnenburg, W. Metal uptake from soils and soil sediment mixtures by larvae of Tenebrio molitor (L.) (Coleoptera). Ecotoxicol. Environ. Saf. 2003, 54, 277–289. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Supeanu, A.; Jansson, A.; Boqvist, S.; Vagsholm, I. Novel foods: A risk profile for the house cricket (Acheta domesticus). EFSA J. 2018, 16, e16082. [Google Scholar] [CrossRef]
- Bonneau, S. Nourrir le Monde de Demain: Avantages et Risques de l’Entomophagie. Sciences du Vivant [q-bio]. L’Université Clermont Auvergne, France, 2020. Available online: https://dumas.ccsd.cnrs.fr/dumas-03125122/document (accessed on 12 September 2021).
- Akinnawo, O.; Ketiku, A.O. Chemical composition and fatty acid profile of edible larva of Cirina forda (Westwood). Afr. J. Biomed. Res. 2000, 3, 93–96. [Google Scholar]
- Ntukuyoh, A.I.; Udiong, D.S.; Ikpe, E.; Akpakpan, A.E. Evaluation of Nutritional Value of Termites (Macrotermes bellicosus): Soldiers, Workers, and Queen in the Niger Delta Region of Nigeria. Int. J. Food Nutr. Saf. 2012, 1, 60–65. [Google Scholar]
- Mench, M.; Blaise, D. Contamination des sols et de nos aliments par les éléments traces. Courr. Environ. INRA 2004, 52, 31–56. [Google Scholar]
- Cheftel, H.; Truffert, L. Oligo-éléments et leur toxicité dans l’alimentation de l’homme. Ann. Nutr. Aliment. 1972, 26, B521–B561. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Shantibala, T.; Lokeshwari, R.K.; Debaraj, H. Nutritional and antinutritional composition of the five species of aquatic edible insects consumed in Manipur, India. J. Insect Sci. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Elorduy, J.B.; Pino Moreno, J.M.; Martínez Camacho, V.H. Could Grasshoppers Be a Nutritive Meal? Food Nutr. Sci. 2012, 3, 164–175. [Google Scholar]
- Igwe, C.U.; Ujowundu, C.O.; Nwaogu, L.A.; Okwu, G.N. Chemical Analysis of an Edible African Termite, Macrotermes nigeriensis; a Potential Antidote to Food Security Problem. Biochem. Anal. Biochem. 2011, 1, 105. [Google Scholar] [CrossRef]
- Charbit, V. Les Oligoelements: Rôle et Conseils du Pharmacien d’Officine. Ph.D. Thesis, L’Université de Marseille, Marseille, France, 2017. Available online: https://dumas.ccsd.cnrs.fr/dumas-01565830/document (accessed on 5 September 2021).
- Coic, Y.; Coppenet, M. Les Oligo-Éléments en Agriculture et Élevage; INRA: Paris, France, 1989. [Google Scholar]
- Köhler, R.; Irias-Mata, A.; Ramandey, E.; Purwestri, R.; Biesals, H.K. Nutrient composition of the Indonesian sago grub (Rhynchophorus bilineatus). Int. J. Trop. Insect Sci. 2020, 40, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, J.F.; Covaci, A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.B.; Ademolu, K.O.; Bamidele, J.A. Nutrition and Heavy Metal Levels in the Mound Termite, Macrotermes bellicosus (Smeathman) (Isoptera: Termitidae), at three Sites under Varying Land use in Abeokuta, Southwestern Nigeria. Afr. Entomol. 2013, 22, 156–162. [Google Scholar] [CrossRef]
- Mabossy-Mobouna, G.; Kinkela, T.; Lenga, A. Apports nutritifs des chenilles d’Imbrasia truncata consommées au Congo-Brazzaville. J. Anim. Plant Sci. 2017, 31, 5050–5062. [Google Scholar]
- Gbaruko, B.C.; Friday, O.U. Bioaccumulation of heavy metals in some fauna and flora. Int. J. Environ. Sci. Technol. 2007, 4, 197–202. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).