The Effect of Rearing Scale and Density on the Growth and Nutrient Composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rearing and Colony Maintenance
2.2. Experimental Setup
2.3. Measuring Growth and Biomass Parameters
2.4. Chemical Analyses
2.5. The Analysis of Free Amino Acids
2.6. Statistical Analyses
2.7. Feed Density vs. Growth, a Model-Based Approach
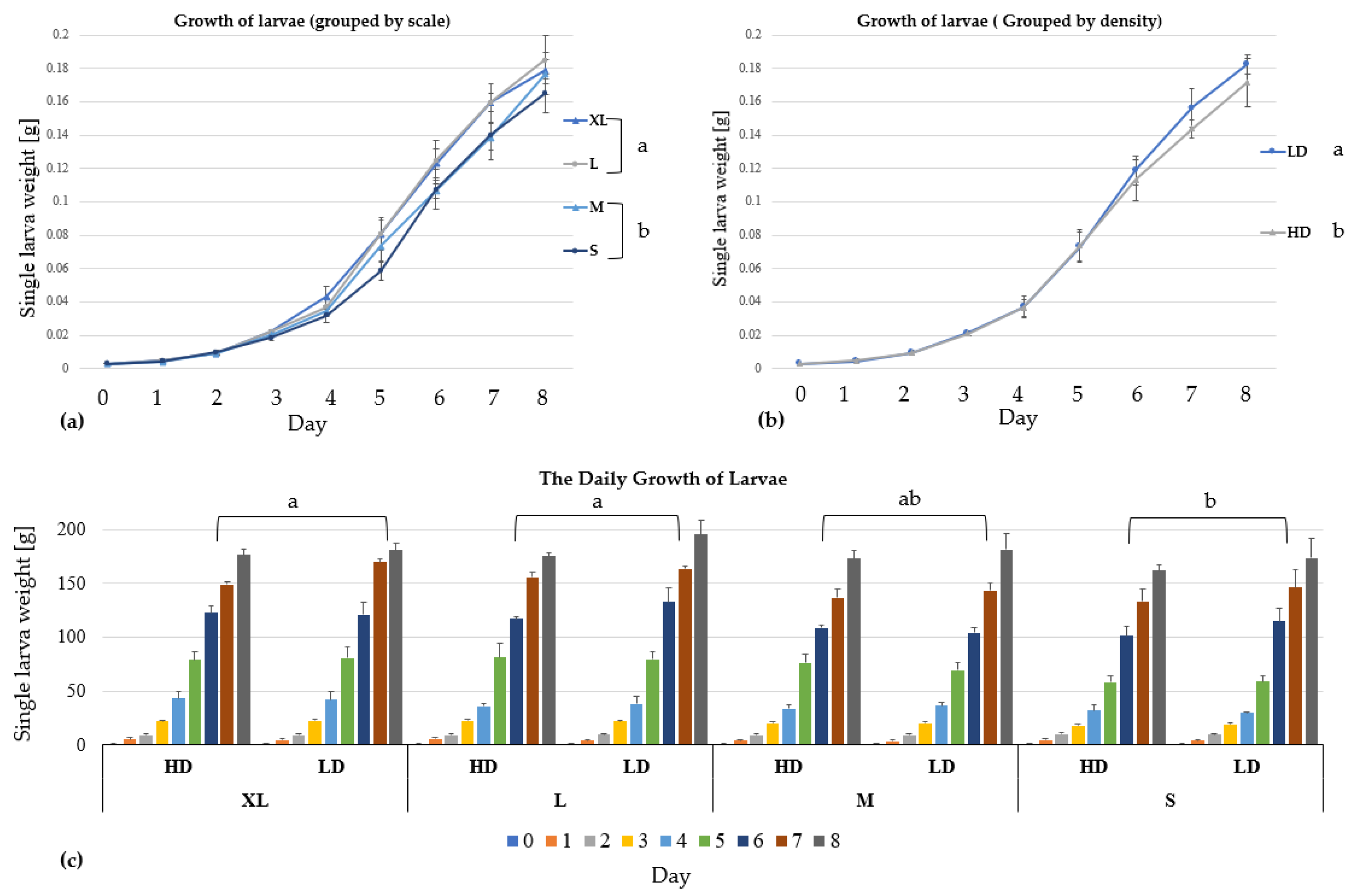
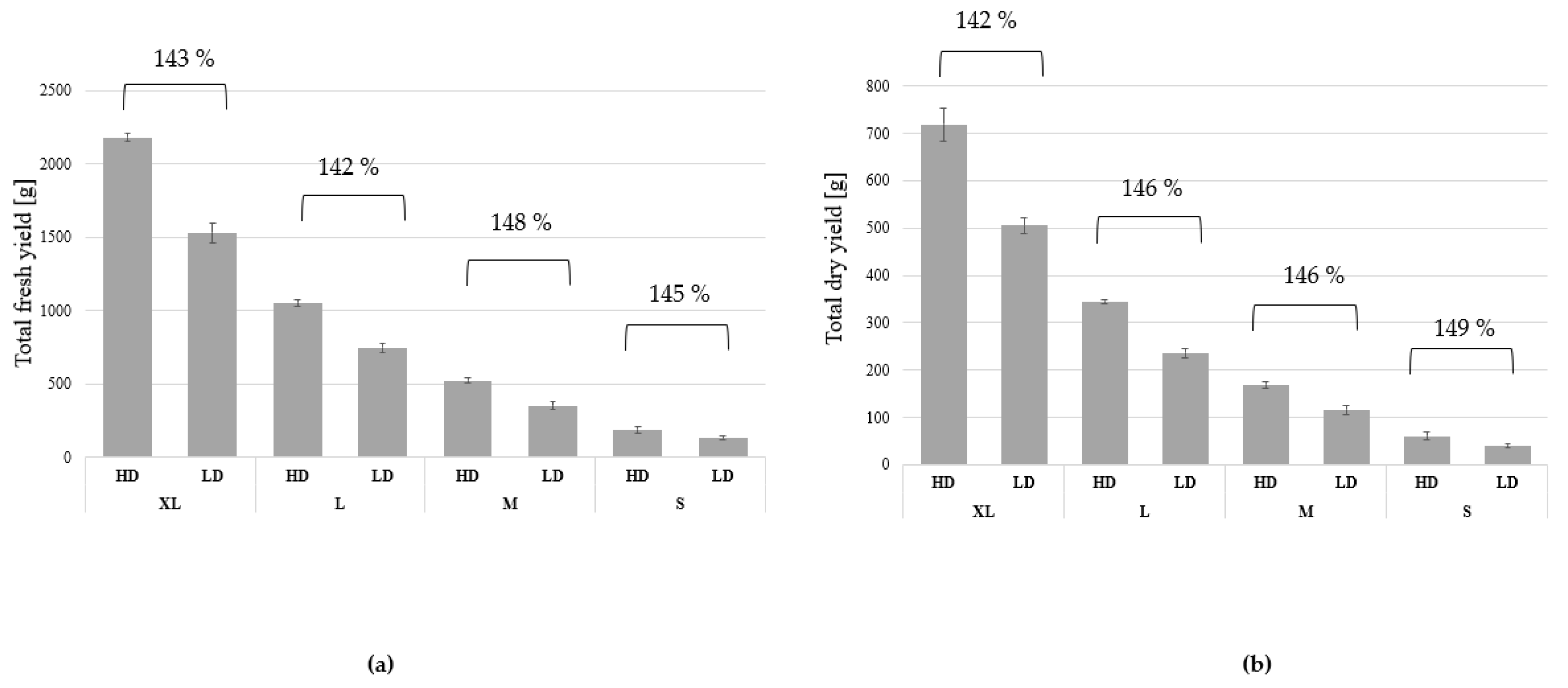
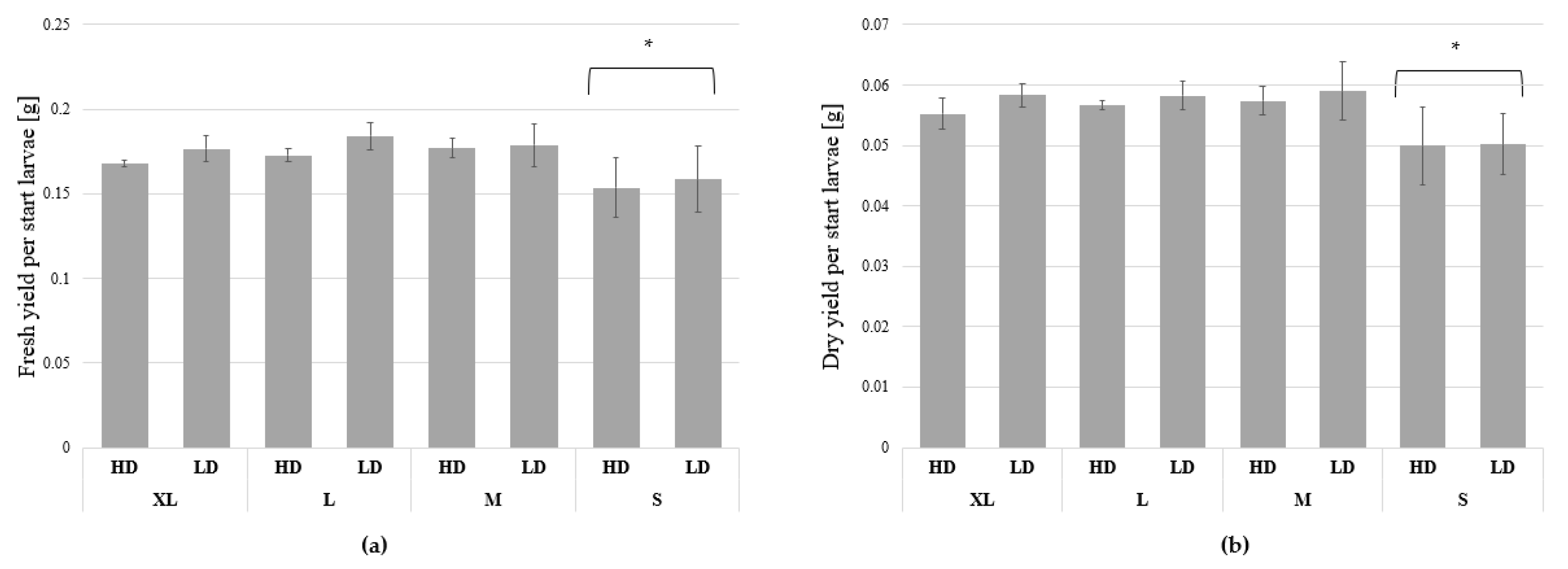
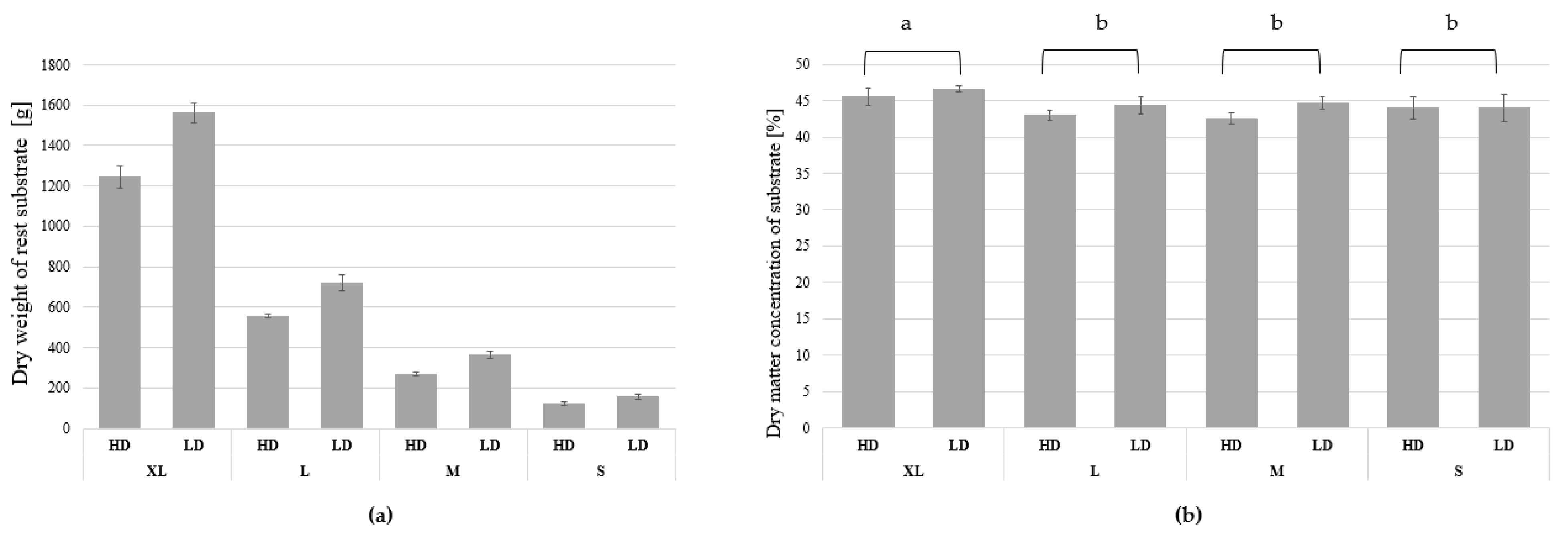
3. Results
3.1. Growth and Biomass Parameters
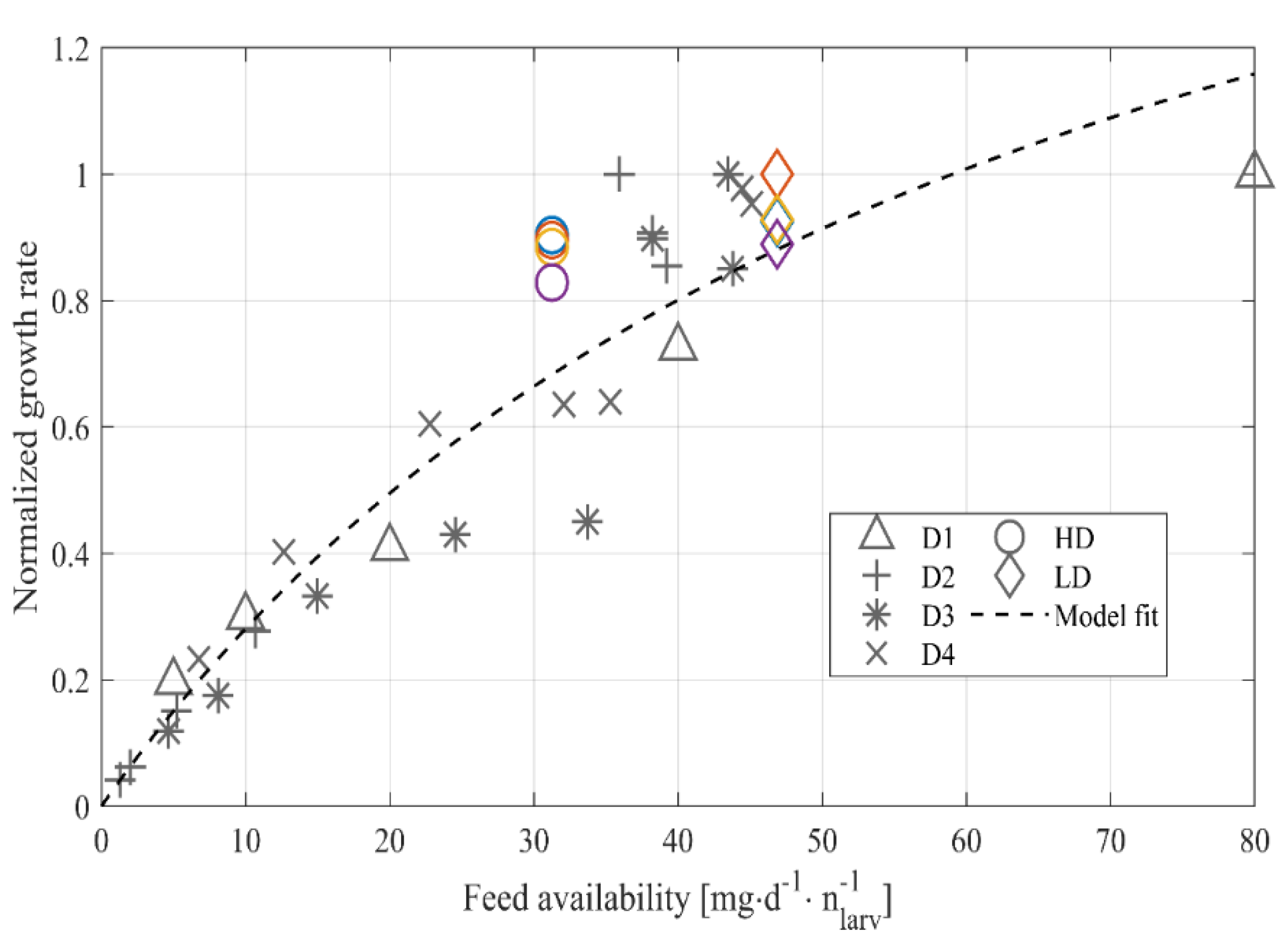
3.2. Model-Based Approach
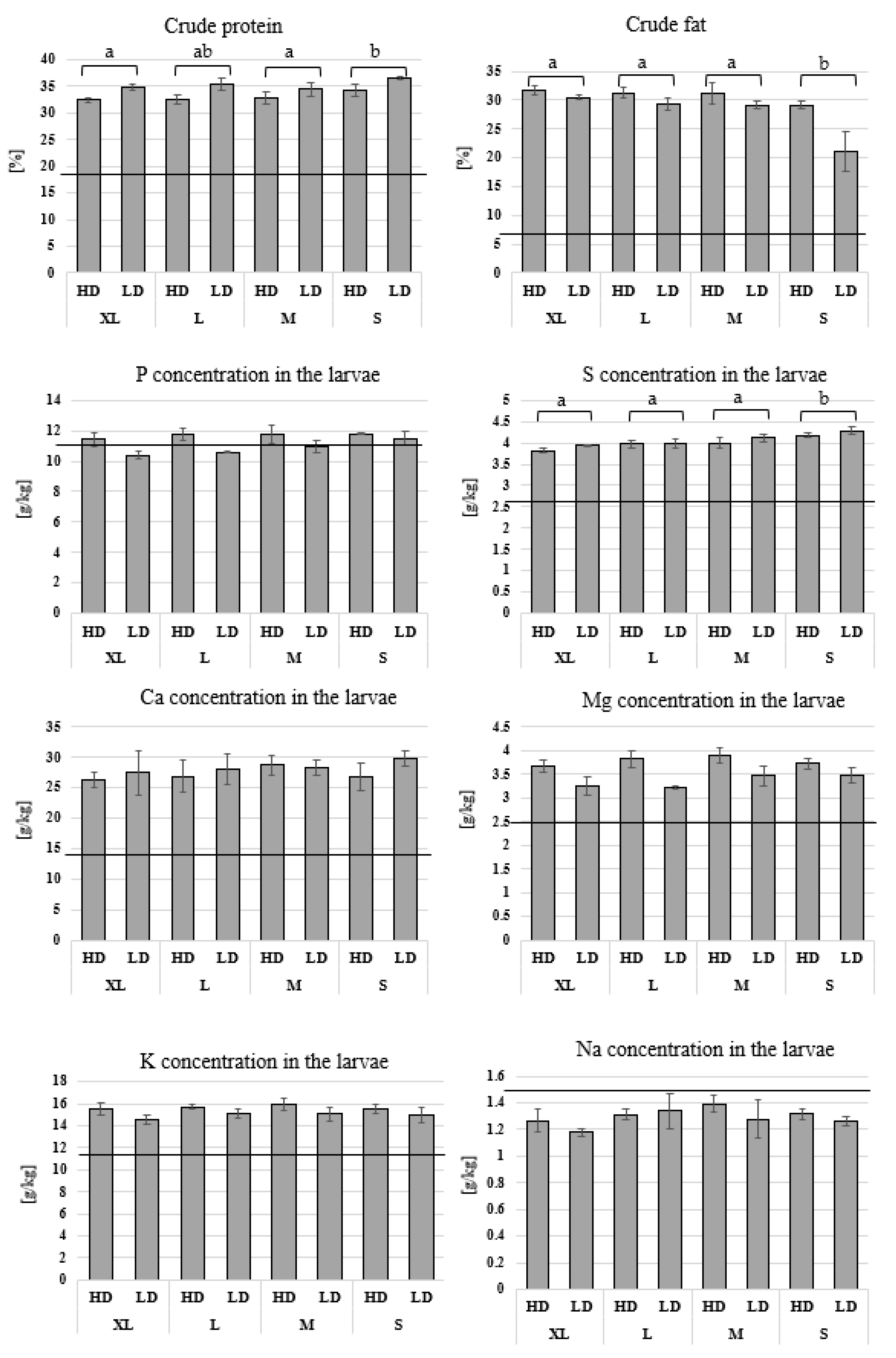
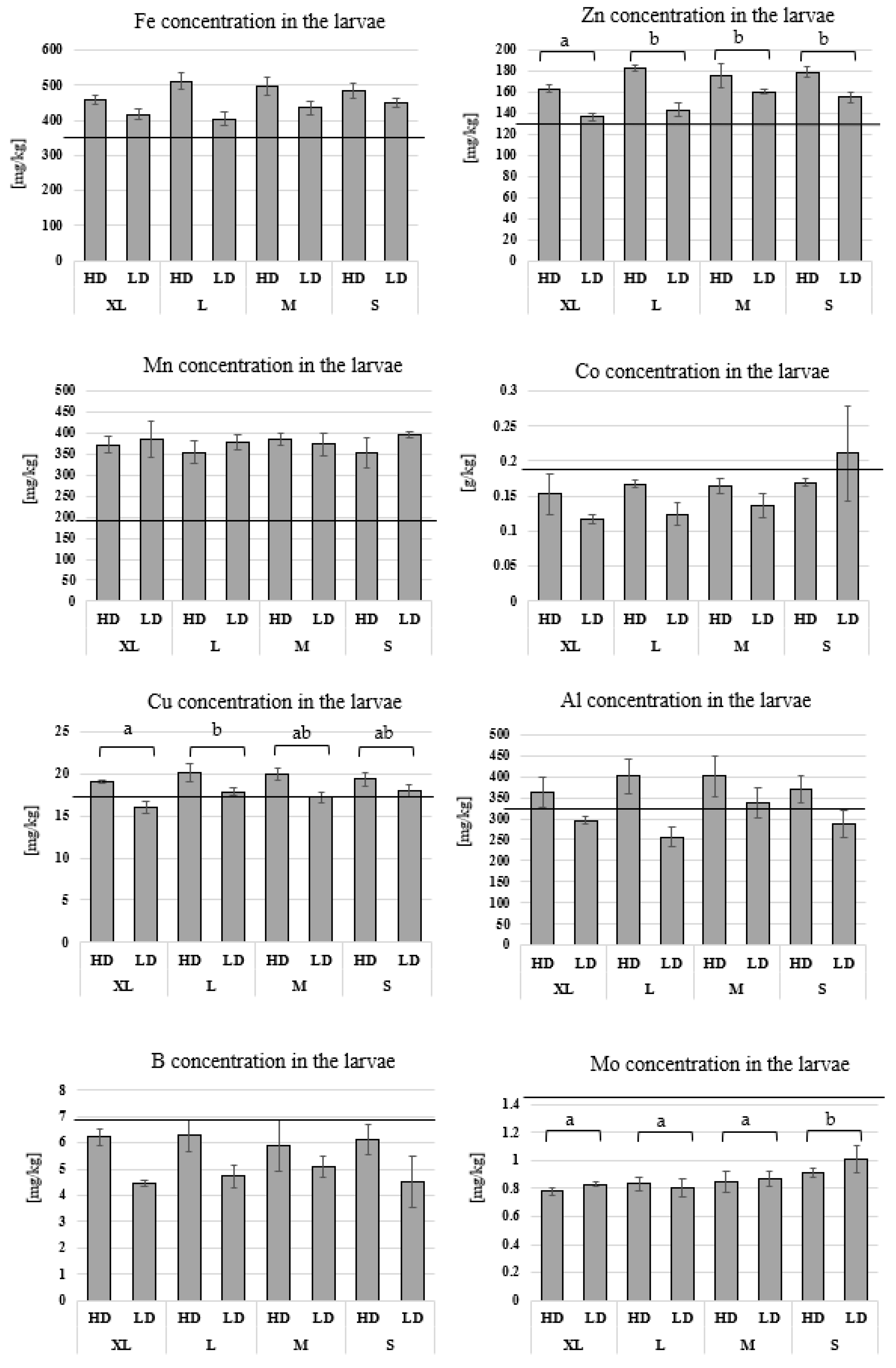
3.3. Chemical Analysis
4. Discussion
5. Conclusions, Limitations, and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Van Huis, A.; Dicke, M.; van Loon, J.J.A. Insects to feed the world. J. Insects Food Feed 2015, 1, 3–5. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.; Van Broekhoven, S.; Van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and characterisation of protein fractions from five insect species. Food Chem 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of black soldier fly (Diptera: Stratiomyidae) larvae to recycle food waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Oonincx, D.; Van Huis, A.; Van Loon, J. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed 2015, 1, 131–139. [Google Scholar] [CrossRef]
- Van Huis, A.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO Forestry Paper; FAO: Rome, Italy, 2013. [Google Scholar]
- Ermolaev, E.; Lalander, C.; Vinnerås, B. Greenhouse gas emissions from small-scale fly larvae composting with Hermetia illucens. Waste Manag. 2019, 96, 65–74. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Chen, J.; Nowar, E.E.; Li, Z.; Hu, R.; Tomberlin, J.K.; Yu, Z.; Li, Q.; Wang, S. Reducing greenhouse gas emissions and enhancing carbon and nitrogen conversion in food wastes by the black soldier fly. J. Environ. Manag. 2020, 260, 110066. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Choi, S.; Hassanzadeh, N. BSFL Frass: A Novel Biofertilizer for Improving Plant Health While Minimizing Environmental Impact. Candian Sci. Fair J. 2019, 2, 41–46. [Google Scholar]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Musyoka, M.W.; Ekesi, S. Exploring black soldier fly frass as novel fertilizer for improved growth, yield, and nitrogen use efficiency of maize under field conditions. Front. Plant Sci. 2020, 11, 1447. [Google Scholar] [CrossRef] [PubMed]
- Phl, C.P.V.; Walraven, M.; Bézagu, M.; Lefranc, M.; Ray, C. Industrial Symbiosis in Insect Production—A Sustainable Eco-Efficient and Circular Business Model. Sustainability 2020, 12, 10333. [Google Scholar]
- Walter, A.; Klammsteiner, T.; Gassner, M.; Heussler, C.D.; Kapelari, S.; Schermer, M.; Insam, H. Black Soldier Fly School Workshops as Means to Promote Circular Economy and Environmental Awareness. Sustainability 2020, 12, 9574. [Google Scholar] [CrossRef]
- Torgerson, K.; Meijering, J.; Sok, J.; Dicke, M.; Lansink, A.O. Towards circular agriculture–exploring insect waste streams as a crop and soil health promoter. J. Insects Food Feed 2021, 7, 357–368. [Google Scholar] [CrossRef]
- Ortiz, J.C.; Ruiz, A.T.; Morales-Ramos, J.; Thomas, M.; Rojas, M.; Tomberlin, J.; Yi, L.; Han, R.; Giroud, L.; Jullien, R. Insect mass production technologies. In Insects as Sustainable Food Ingredients; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–201. [Google Scholar]
- Nijhout, H. The control of body size in insects. Dev. Biol. 2003, 261, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Osuga, I.M.; Cheseto, X.; Ekesi, S.; Dicke, M.; van Loon, J.J. Nutritional composition of black soldier fly larvae feeding on agro-industrial by-products. Entomol. Exp. Appl. 2020, 168, 472–481. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Adler, P.H.; Myers, H.M. Development of the black soldier fly (Diptera: Stratiomyidae) in relation to temperature. Environ. Entomol. 2009, 38, 930–934. [Google Scholar] [CrossRef]
- Harnden, L.M.; Tomberlin, J.K. Effects of temperature and diet on black soldier fly, Hermetia illucens (L.)(Diptera: Stratiomyidae), development. Forensic Sci. Int. 2016, 266, 109–116. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.; Niassy, S.; van Loon, J.J.; Dicke, M. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS ONE 2018, 13, e0206097. [Google Scholar] [CrossRef] [Green Version]
- Charabidze, D.; Bourel, B.; Gosset, D. Larval-mass effect: Characterisation of heat emission by necrophageous blowflies (Diptera: Calliphoridae) larval aggregates. Forensic Sci. Int. 2011, 211, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Slone, D.; Gruner, S.V. Thermoregulation in larval aggregations of carrion-feeding blow flies (Diptera: Calliphoridae). J. Med. Entomol. 2007, 44, 516–523. [Google Scholar] [CrossRef]
- Paz, A.S.P.; Carrejo, N.S.; Rodríguez, C.H.G. Effects of larval density and feeding rates on the bioconversion of vegetable waste using black soldier fly larvae Hermetia illucens (L.),(Diptera: Stratiomyidae). Waste Biomass Valorization 2015, 6, 1059–1065. [Google Scholar]
- Bryant, E.H.; Sokal, R.R. The fate of immature housefly populations at low and high densities. Popul. Ecol. 1967, 9, 19–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Zhu, F.; Wang, X.; Wang, X.; Lei, C. The gut microbiota in larvae of the housefly Musca domestica and their horizontal transfer through feeding. AMB Express 2017, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Padmanabha, M.; Kobelski, A.; Hempel, A.J.; Streif, S. A comprehensive dynamic growth and development model of Hermetia illucens larvae. PLoS ONE 2020, 15, e0239084. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.; Subramanian, S.; Ekesi, S.; van Huis, A.; Borgemeister, C. The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Miranda, C.D.; Cammack, J.A.; Tomberlin, J.K. Mass production of the black soldier fly, Hermetia illucens (L.),(Diptera: Stratiomyidae) reared on three manure types. Animals 2020, 10, 1243. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.)(Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Addison, M.F.; Terblanche, J.S. Mass-rearing of insects for pest management: Challenges, synergies and advances from evolutionary physiology. J. Crop Prot. 2012, 38, 87–94. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing methods for the black soldier fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef] [Green Version]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.; Fogliano, V.; Lakemond, C.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Shek, D.T.; Ma, C. Longitudinal data analyses using linear mixed models in SPSS: Concepts, procedures and illustrations. Sci. World J. 2011, 11, 42–76. [Google Scholar] [CrossRef] [Green Version]
- Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Kossen, N.W.F. Scale-up in Biotechnology. In Recent Advances in Biotechnology; Vardar-Sukan, F., Sukan, Ş.S., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 147–182. [Google Scholar]
- Formenti, L.R.; Nørregaard, A.; Bolic, A.; Hernandez, D.Q.; Hagemann, T.; Heins, A.L.; Larsson, H.; Mears, L.; Mauricio-Iglesias, M.; Krühne, U. Challenges in industrial fermentation technology research. Biotechnol. J. 2014, 9, 727–738. [Google Scholar] [CrossRef]
- Yang, F.; Tomberlin, J.K. Comparing Selected Life-History Traits of Black Soldier Fly (Diptera: Stratiomyidae) Larvae Produced in Industrial and Bench-Top-Sized Containers. J. Insect Sci. 2020, 20, 25. [Google Scholar] [CrossRef]
- Dzepe, D.; Nana, P.; Fotso, A.; Tchuinkam, T.; Djouaka, R. Influence of larval density, substrate moisture content and feedstock ratio on life history traits of black soldier fly larvae. J. Insects Food Feed. 2020, 6, 133–140. [Google Scholar] [CrossRef]
- Ratte, H.T. Temperature and Insect Development. In Environmental Physiology and Biochemistry of Insects; Hoffmann, K.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 33–66. [Google Scholar]
- Shumo, M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.; Subramanian, S.; Ekesi, S.; Van Huis, A.; Borgemeister, C. Influence of temperature on selected life-history traits of black soldier fly (Hermetia illucens) reared on two common urban organic waste streams in Kenya. Animals 2019, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- Gligorescu, A.; Toft, S.; Hauggaard-Nielsen, H.; Axelsen, J.A.; Nielsen, S.A. Development, metabolism and nutrient composition of black soldier fly larvae (Hermetia illucens; Diptera: Stratiomyidae) in relation to temperature and diet. J. Insects Food Feed. 2018, 4, 123–133. [Google Scholar] [CrossRef]
- Bonelli, M.; Bruno, D.; Caccia, S.; Sgambetterra, G.; Cappellozza, S.; Jucker, C.; Tettamanti, G.; Casartelli, M. Structural and functional characterization of Hermetia illucens larval midgut. Front. Physiol. 2019, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Bae, S.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y. Biochemical characterization of digestive enzymes in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). J. Asia Pac. Entomol. 2011, 14, 11–14. [Google Scholar] [CrossRef]
- Gold, M.; Von Allmen, F.; Zurbrügg, C.; Zhang, J.; Mathys, A. Identification of bacteria in two food waste black soldier fly larvae rearing residues. Front. Microbiol. 2020, 11, 2897. [Google Scholar] [CrossRef] [PubMed]
- Bloukounon-Goubalan, A.; Saïdou, A.; Chrysostome, C.; Kenis, M.; Amadji, G.; Igué, A.; Mensah, G. Physical and chemical properties of the agro-processing by-products decomposed by larvae of Musca domestica and Hermetia illucens. Waste Biomass Valoriz. 2020, 11, 2735–2743. [Google Scholar] [CrossRef]
- Shishkov, O.; Hu, M.; Johnson, C.; Hu, D.L. Black soldier fly larvae feed by forming a fountain around food. J. R. Soc. Interface 2019, 16, 20180735. [Google Scholar] [CrossRef] [PubMed]
- Shishkov, O.; Hu, D.L. Synchronizing pile formation of black soldier fly larvae. Eur. Phys. J. Spec. Top. 2020, 229, 2779–2789. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Sun, J.; Hu, G.; Wang, M.; Amendt, J.; Wang, J. Temperature-dependent development of the blow fly Chrysomya pinguis and its significance in estimating postmortem interval. R. Soc. Open Sci. 2019, 6, 190003. [Google Scholar] [CrossRef] [Green Version]
- Villazana, J.; Alyokhin, A. Development of black soldier fly larvae (Diptera: Stratiomyidae) on seafood wastes. J. Insects Food Feed. 2019, 5, 313–319. [Google Scholar] [CrossRef]
- Cammack, J.A.; Tomberlin, J.K. The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly Hermetia illucens (L.)(Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villazana, J.; Alyokhin, A. Tolerance of immature black soldier flies (Diptera: Stratiomyidae) to cold temperatures above and below freezing point. J. Econ. Entomol. 2019, 112, 2632–2637. [Google Scholar] [CrossRef]
- Lalander, C.; Ermolaev, E.; Wiklicky, V.; Vinnerås, B. Process efficiency and ventilation requirement in black soldier fly larvae composting of substrates with high water content. Sci. Total Environ. 2020, 729, 138968. [Google Scholar] [CrossRef]
- Myers, H.M.; Tomberlin, J.K.; Lambert, B.D.; Kattes, D. Development of black soldier fly (Diptera: Stratiomyidae) larvae fed dairy manure. Environ. Entomol. 2014, 37, 11–15. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tomberlin, J.K.; Vanlaerhoven, S. Influence of resources on Hermetia illucens (Diptera: Stratiomyidae) larval development. J. Med. Entomol 2013, 50, 898–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diener, S.; Solano, N.M.S.; Gutiérrez, F.R.; Zurbrügg, C.; Tockner, K. Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valoriz. 2011, 2, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, D.C.; Newton, G.L.; Thompson, S.A.; Savage, S. A value added manure management system using the black soldier fly. Bioresour. Technol. 1994, 50, 275–279. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N.; Sinha, A.K. Conversion of spent coffee and donuts by black soldier fly (Hermetia illucens) larvae into potential resources for animal and plant farming. Insects 2021, 12, 332. [Google Scholar] [CrossRef]
- Bohm, K.; Hatley, G.A.; Robinson, B.H.; Gutiérrez-Ginés, M.J. Black Soldier Fly-based bioconversion of biosolids creates high-value products with low heavy metal concentrations. Resour. Conserv. Recycl. 2022, 180, 106149. [Google Scholar] [CrossRef]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Dhanani, K.; Hoffman, L.C. Food Safety of Consuming Black Soldier Fly (Hermetia illucens) Larvae: Microbial, Heavy Metal and Cross-Reactive Allergen Risks. Foods 2021, 10, 1934. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Manoni, M.; Ferrari, L.; Tretola, M.; Cazzola, R.; Givens, I. The Contribution of Dietary Magnesium in Farm Animals and Human Nutrition. Nutrients 2021, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Delezie, E.; Bierman, K.; Nollet, L.; Maertens, L. Impacts of calcium and phosphorus concentration, their ratio, and phytase supplementation level on growth performance, foot pad lesions, and hock burn of broiler chickens. J. Appl. Poult. Res. 2015, 24, 115–126. [Google Scholar] [CrossRef]
- Pratt, J.J., Jr. A qualitative analysis of the free amino acids in insect blood. Ann. Entomol. Soc. Am. 1950, 43, 573–580. [Google Scholar] [CrossRef]
- Rønnestad, I.; Conceição, L.E.; Aragão, C.; Dinis, M.T. Free amino acids are absorbed faster and assimilated more efficiently than protein in postlarval Senegal sole (Solea senegalensis). J. Nutr. 2000, 130, 2809–2812. [Google Scholar] [CrossRef] [Green Version]
- Rérat, A.A. Nutritional supply of proteins and absorption of their hydrolysis products: Consequences on metabolism. Proc. Nutr. Soc. 1993, 52, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindran, V.; Hew, L.; Ravindran, G.; Bryden, W. Apparent ileal digestibility of amino acids in dietary ingredients for broiler chickens. Anim. Sci. 2005, 81, 85–97. [Google Scholar] [CrossRef]
- León-Quinto, T.; Serna, A. Cryoprotective Response as Part of the Adaptive Strategy of the Red Palm Weevil, Rhynchophorus ferrugineus, against Low Temperatures. Insects 2022, 13, 134. [Google Scholar] [CrossRef]



| Scale | Box Area [cm2] | LPB (HD) | LPB (LD) | FPB [g] |
|---|---|---|---|---|
| Box XL | 2060 | 13,000 | 8666 | 10,000 |
| Box L | 963.60 | 6078 | 4051 | 4677 |
| Box M | 465.80 | 2939 | 1958 | 2260 |
| Box S | 193.72 | 1222 | 814 | 940 |
| Protein | [%] | 17.41 |
| Fat | [%] | 3.79 |
| Fibers | [%] | 26.06 |
| Potassium | [g/kg] | 13.68 |
| Magnesium | [g/kg] | 3.96 |
| Manganese | [mg/kg] | 155.56 |
| Molybdenum | [mg/kg] | 1.58 |
| Sodium | [g/kg] | 1.61 |
| Phosphorus | [g/kg] | 11.88 |
| Aluminum | [mg/kg] | 333.4 |
| Boron | [mg/kg] | 11.47 |
| Calcium | [g/kg] | 10.57 |
| Cobalt | [mg/kg] | 0.2 |
| Copper | [mg/kg] | 17.82 |
| Iron | [mg/kg] | 350.92 |
| Sulfur | [g/kg] | 2.89 |
| Zinc | [mg/kg] | 134.14 |
| PPS * | PEA | ASP | THR | SER | ASN | GLU | GLN | PRO | GLY | ALA * | CIT * | CYS2 | CYSTHA | MET | ILE | LEU | TYR | PHE | BAIB | GABA | HIS | 1MHIS | TRP | ANS * | HLY | ORN | LYS | ARG | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XL | HD | Mean | 3434 | 30,515 | 6675 | 7911 | 5045 | 12,036 | 24,233 | 38,971 | 29,856 | 21,380 | 39,318 | 7118 | 5092 | 1223 | 1878 | 6440 | 8395 | 8676 | 3402 | 4730 | 10,511 | 14,205 | 18,912 | 4176 | 27,394 | 55,903,797 | 13,920 | 11,205 | 4069 |
| SD | 136 | 478 | 1202 | 208 | 171 | 7126 | 7682 | 5974 | 627 | 8512 | 10,421 | 1346 | 646 | 77 | 483 | 341 | 523 | 390 | 132 | 517 | 2636 | 736 | 598 | 48 | 9542 | 7,398,661 | 1954 | 2288 | 223 | ||
| LD | Mean | 3498 | 29,463 | 9572 | 10,468 | 6447 | 12,931 | 19,727 | 42,991 | 35,828 | 22,272 | 37,537 | 4951 | 3936 | 1658 | 3479 | 6414 | 9002 | 8757 | 3721 | 9365 | 15,907 | 14,406 | 17,295 | 4503 | 25,213 | 46,419,417 | 15,199 | 13,880 | 3956 | |
| SD | 96 | 7966 | 1592 | 3306 | 1127 | 7955 | 10,052 | 7570 | 12,126 | 8606 | 7383 | 2963 | 2260 | 1328 | 1549 | 54 | 828 | 2439 | 1929 | 6192 | 9487 | 4427 | 5220 | 482 | 3935 | 23,589,743 | 5144 | 1739 | 1283 | ||
| L | HD | Mean | 3916 | 36,259 | 8872 | 9497 | 6536 | 9269 | 18,272 | 38,910 | 29,436 | 18,392 | 50,226 | 10,181 | 4595 | 1098 | 2319 | 5681 | 7883 | 10,530 | 4506 | 4125 | 11,272 | 17,739 | 18,372 | 4720 | 23,156 | 57,144,043 | 14,447 | 12,223 | 3320 |
| SD | 236 | 2067 | 667 | 1145 | 1961 | 2450 | 1643 | 6614 | 4873 | 1698 | 8260 | 3662 | 756 | 103 | 257 | 1521 | 1608 | 1186 | 1663 | 476 | 1852 | 3877 | 5304 | 602 | 8239 | 17,178,051 | 1085 | 1367 | 907 | ||
| LD | Mean | 3594 | 32,216 | 7440 | 7493 | 6080 | 11,103 | 21,361 | 42,885 | 29,606 | 29,131 | 29,838 | 7037 | 4752 | 1192 | 1724 | 6195 | 8155 | 9299 | 3375 | 4133 | 10,310 | 13,481 | 19,636 | 4443 | 16,199 | 37,313,191 | 13,899 | 10,326 | 3431 | |
| SD | 205 | 675 | 840 | 1224 | 1360 | 8089 | 8276 | 4482 | 467 | 1848 | 1219 | 506 | 1472 | 20 | 33 | 281 | 500 | 2797 | 333 | 275 | 915 | 2570 | 1463 | 593 | 13,265 | 15,763,243 | 127 | 375 | 836 | ||
| M | HD | Mean | 4099 | 30,892 | 10,277 | 8562 | 5261 | 6695 | 24,158 | 36,820 | 25,142 | 25,566 | 45,590 | 9752 | 4912 | 1079 | 2424 | 7590 | 10,208 | 11,208 | 4323 | 4293 | 9063 | 16,222 | 22,499 | 5309 | 26,614 | 73,804,290 | 12,277 | 10,052 | 3969 |
| SD | 106 | 2020 | 1502 | 731 | 369 | 1483 | 5993 | 3882 | 2083 | 10,265 | 11,846 | 2302 | 1032 | 227 | 396 | 1341 | 1728 | 339 | 782 | 857 | 3292 | 2229 | 1771 | 130 | 497 | 3,935,438 | 1096 | 1633 | 178 | ||
| LD | Mean | 3432 | 29,121 | 7241 | 5886 | 7087 | 19,825 | 20,285 | 36,957 | 28,489 | 12,826 | 33,161 | 2574 | 1083 | 724 | 1916 | 6025 | 8155 | 7193 | 3437 | 3514 | 7160 | 14,481 | 17,649 | 3857 | 15,116 | 74,447,000 | 8742 | 12,808 | 4220 | |
| SD | 211 | 1624 | 616 | 1112 | 4989 | 6181 | 16,873 | 6050 | 1838 | 5584 | 23,723 | 2986 | 41 | 12 | 470 | 119 | 327 | 609 | 43 | 780 | 780 | 2498 | 1493 | 190 | 6109 | 27,228,822 | 3014 | 1730 | 2278 | ||
| S | HD | Mean | 4331 | 33,529 | 10,380 | 9533 | 5760 | 10,460 | 25,249 | 41,344 | 24,676 | 22,656 | 57,649 | 11,425 | 4618 | 1033 | 2346 | 8159 | 10,544 | 11,153 | 4217 | 4082 | 9676 | 18,424 | 21,886 | 4935 | 40,744 | 75,551,852 | 13,295 | 11,057 | 4192 |
| SD | 210 | 1199 | 1053 | 186 | 169 | 1789 | 6074 | 5750 | 381 | 1970 | 6141 | 1010 | 309 | 253 | 275 | 324 | 1310 | 998 | 337 | 493 | 2543 | 732 | 1461 | 348 | 6481 | 4,897,885 | 615 | 1772 | 110 | ||
| LD | Mean | 3669 | 31,453 | 8022 | 8676 | 5298 | 8806 | 18,750 | 42,347 | 31,943 | 23,050 | 39,834 | 7102 | 4700 | 993 | 1631 | 6667 | 8803 | 10,827 | 3466 | 5205 | 10,085 | 15,626 | 20,238 | 5078 | 26,196 | 38,380,854 | 15,609 | 12,204 | 3823 | |
| SD | 268 | 1354 | 776 | 516 | 228 | 587 | 1728 | 2989 | 1870 | 11,049 | 13,130 | 1260 | 481 | 172 | 166 | 246 | 380 | 1234 | 173 | 158 | 531 | 841 | 1226 | 633 | 3082 | 19,813,867 | 1191 | 389 | 226 | ||
| Start feed | 311 | 2103 | 2468 | 3774 | 1570 | 3146 | 5887 | 684 | 12,038 | 2714 | 5839 | 688 | 2927 | 1628 | 1296 | 1139 | 1923 | 799 | 1159 | 4669 | 4673 | 4027 | 3834 | 673 | 27,121 | 12,108,562 | 5189 | 14,740 | 85 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakti, W.; Schulz, S.; Marten, V.; Mewis, I.; Padmanabha, M.; Hempel, A.-J.; Kobelski, A.; Streif, S.; Ulrichs, C. The Effect of Rearing Scale and Density on the Growth and Nutrient Composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae. Sustainability 2022, 14, 1772. https://doi.org/10.3390/su14031772
Yakti W, Schulz S, Marten V, Mewis I, Padmanabha M, Hempel A-J, Kobelski A, Streif S, Ulrichs C. The Effect of Rearing Scale and Density on the Growth and Nutrient Composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae. Sustainability. 2022; 14(3):1772. https://doi.org/10.3390/su14031772
Chicago/Turabian StyleYakti, Wael, Simon Schulz, Virginia Marten, Inga Mewis, Murali Padmanabha, Arne-Jens Hempel, Alexander Kobelski, Stefan Streif, and Christian Ulrichs. 2022. "The Effect of Rearing Scale and Density on the Growth and Nutrient Composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae" Sustainability 14, no. 3: 1772. https://doi.org/10.3390/su14031772
APA StyleYakti, W., Schulz, S., Marten, V., Mewis, I., Padmanabha, M., Hempel, A.-J., Kobelski, A., Streif, S., & Ulrichs, C. (2022). The Effect of Rearing Scale and Density on the Growth and Nutrient Composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) Larvae. Sustainability, 14(3), 1772. https://doi.org/10.3390/su14031772






