Effect of Acorn Thermotherapy and Short-Term Storage on Morphological Characteristics of Related Quercus Robur L. Seedlings
Abstract
1. Introduction
2. Materials and Methods
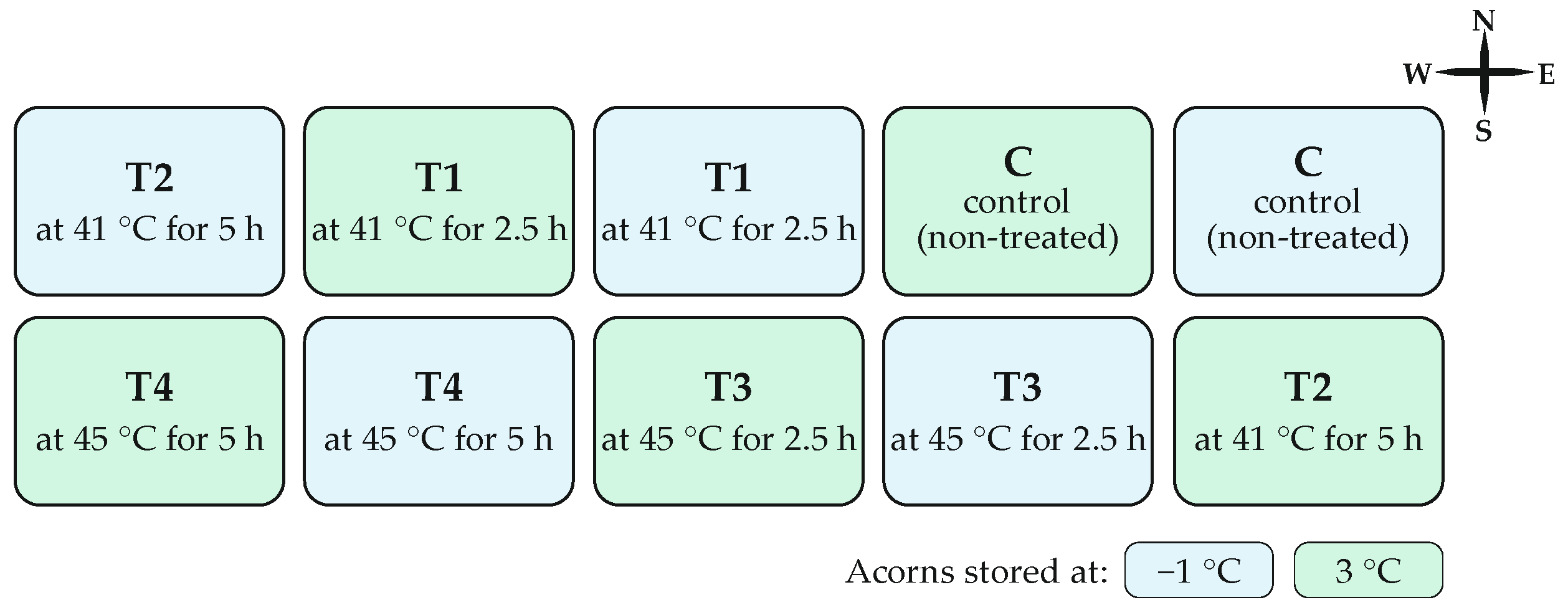
2.1. Acorn Treatment and Storage
2.2. Morphological Characteristics of Seedlings
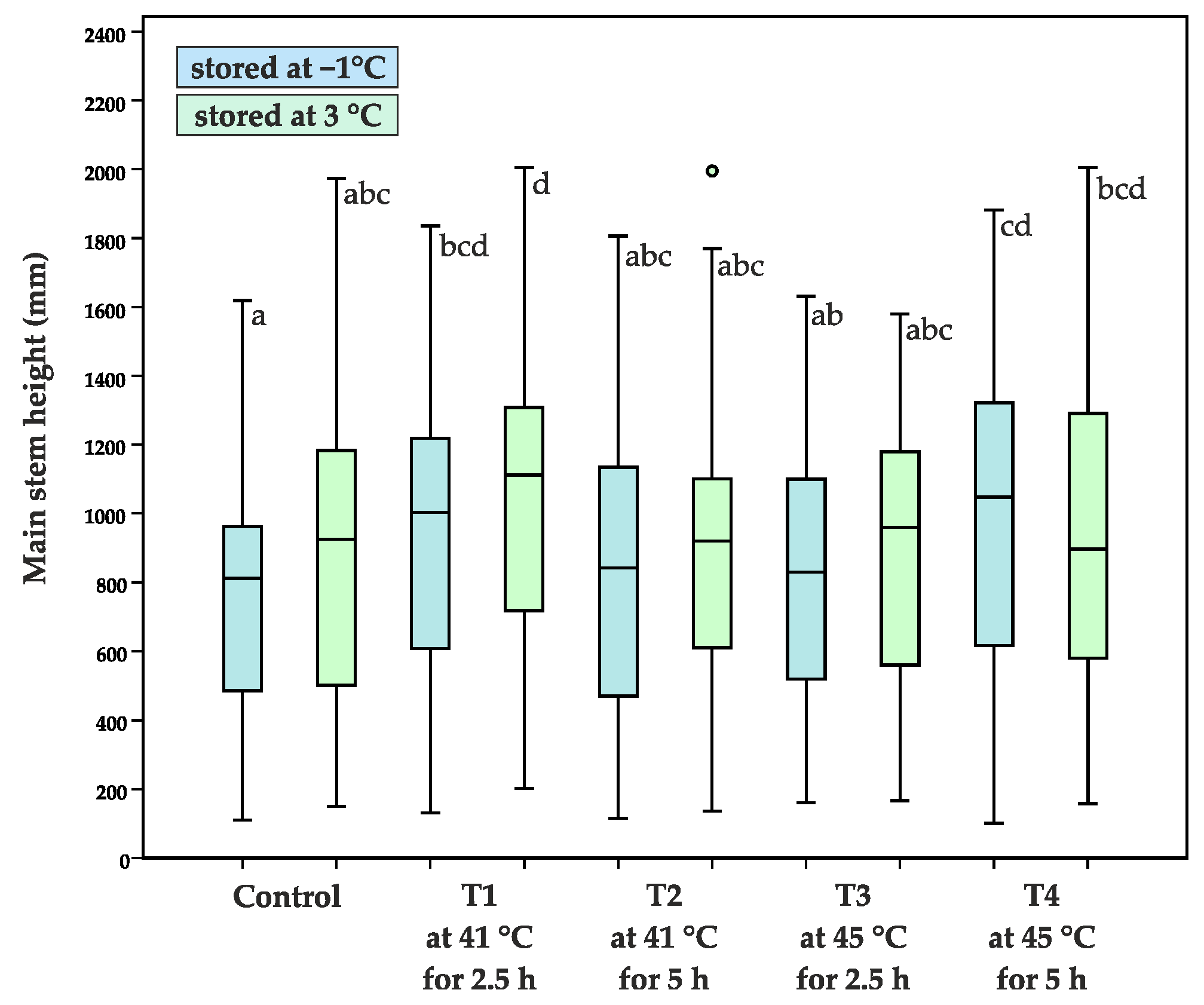
- height of the main stem (H, mm), measured from the ground level to the base of the terminal bud
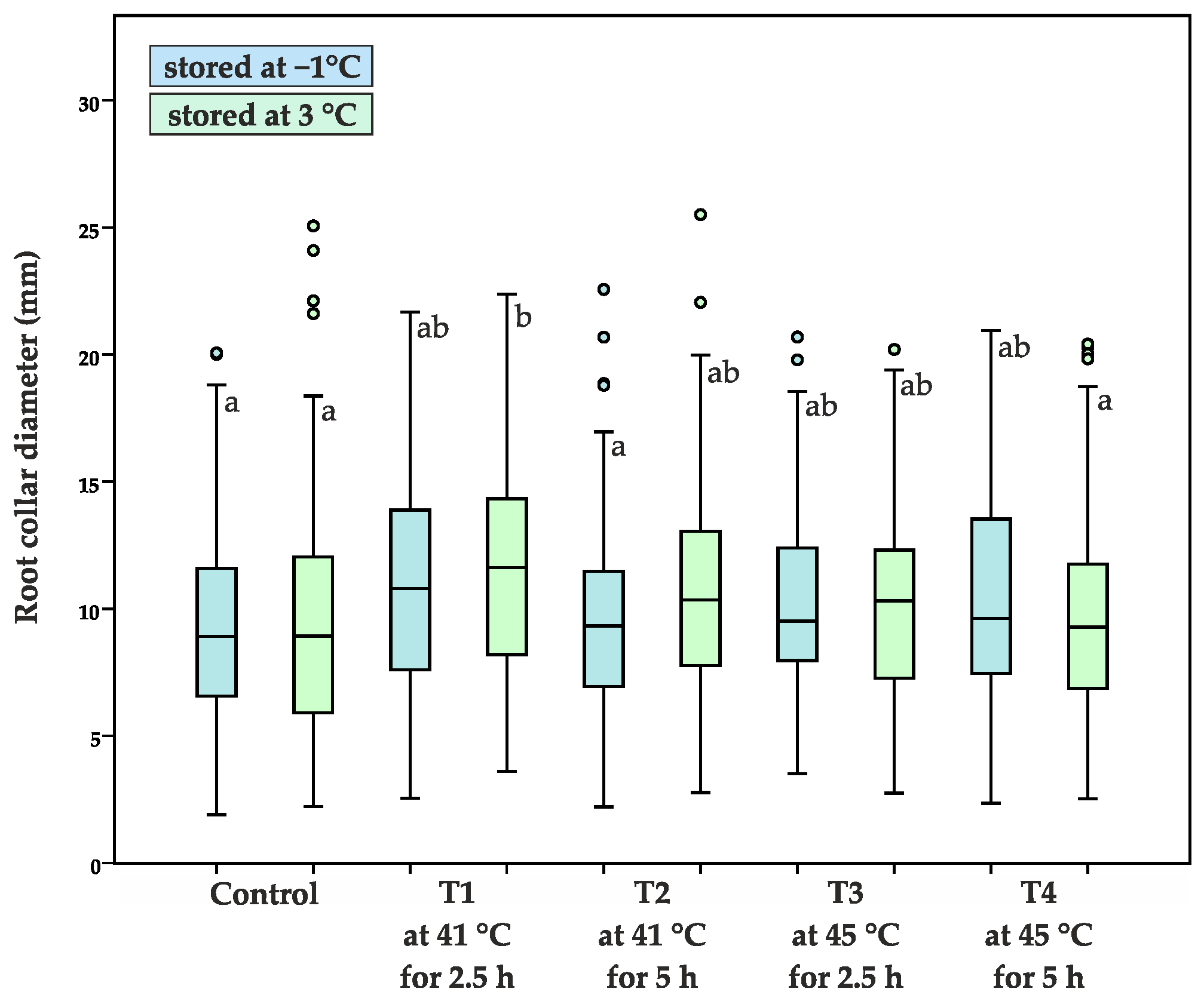
- root collar diameter (D, mm), measured at the ground level
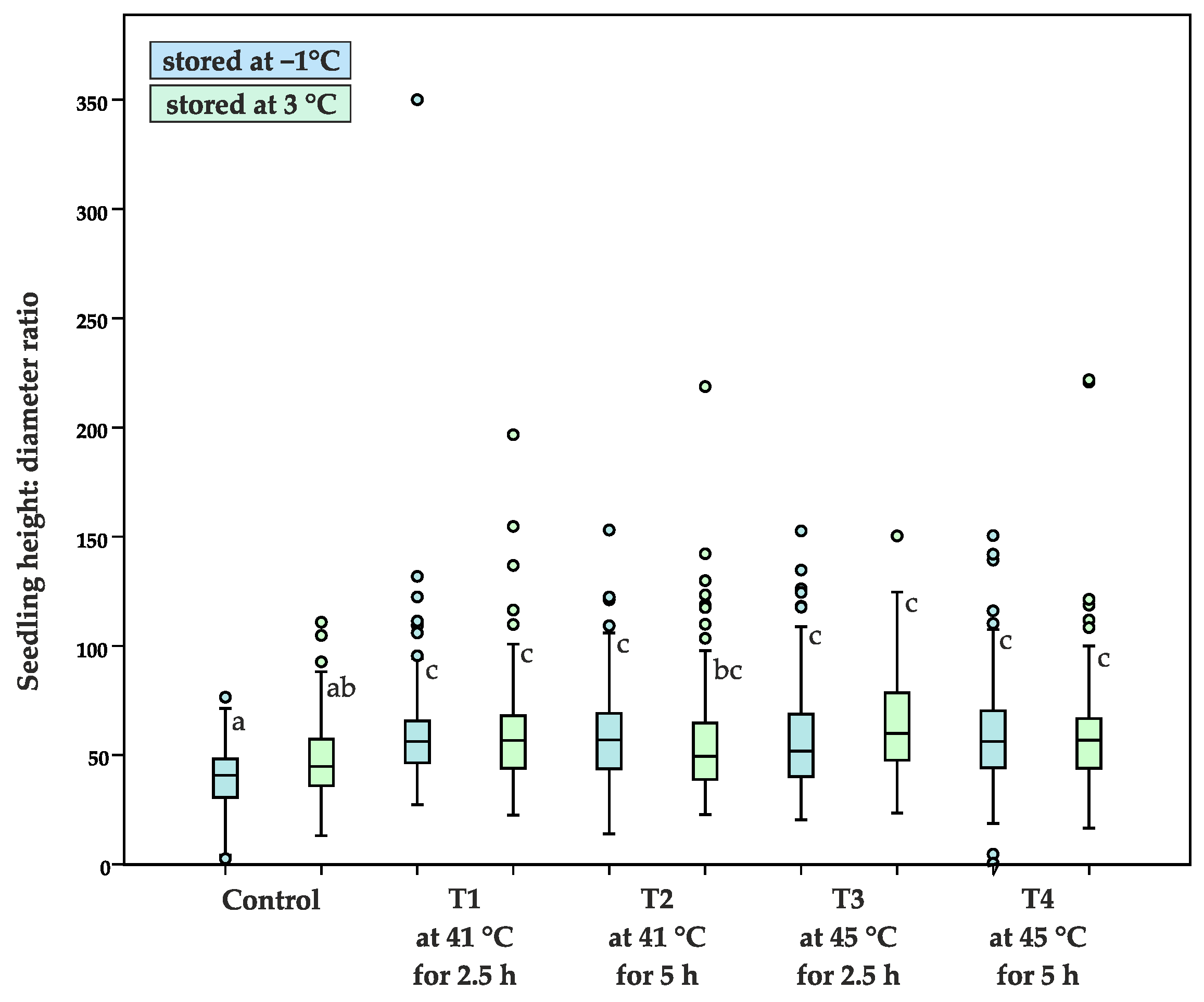
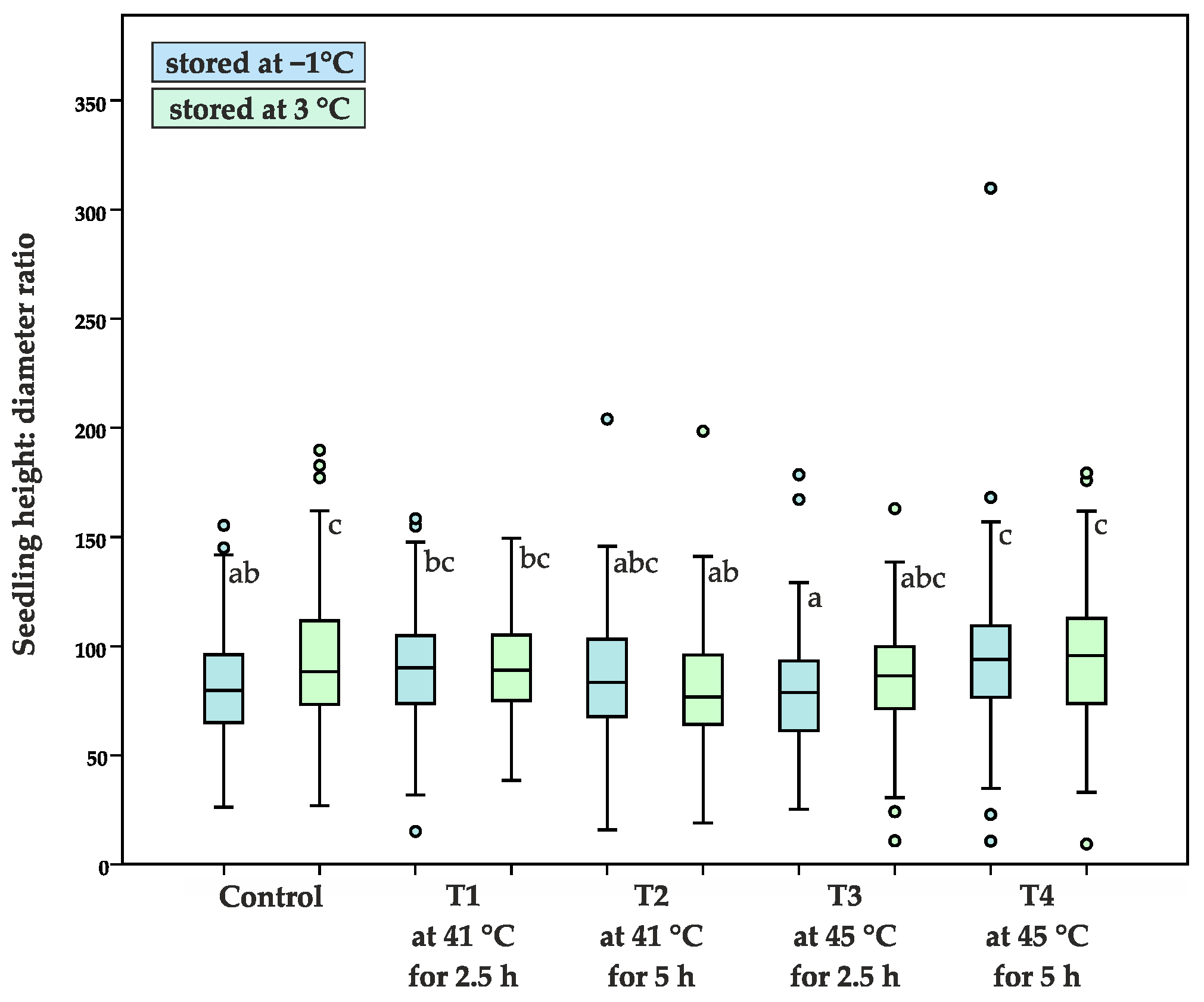
- height: diameter ratio (H/D), the ratio of the main stem height (H) and the root collar diameter (D)
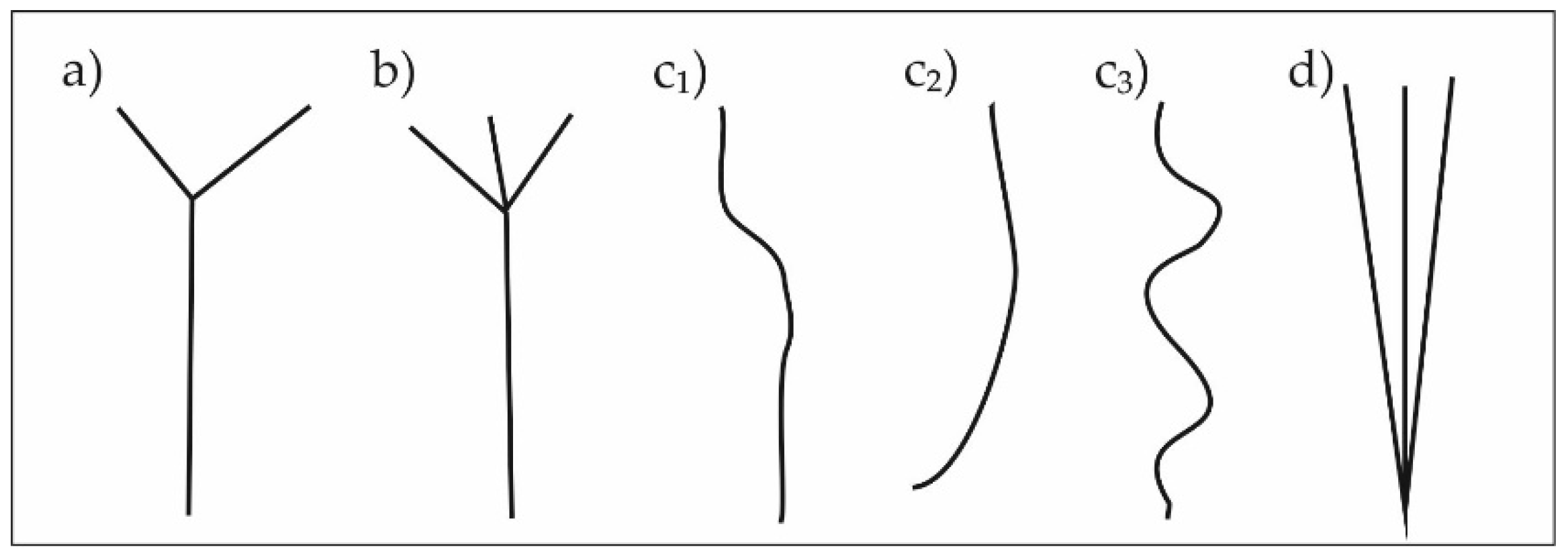
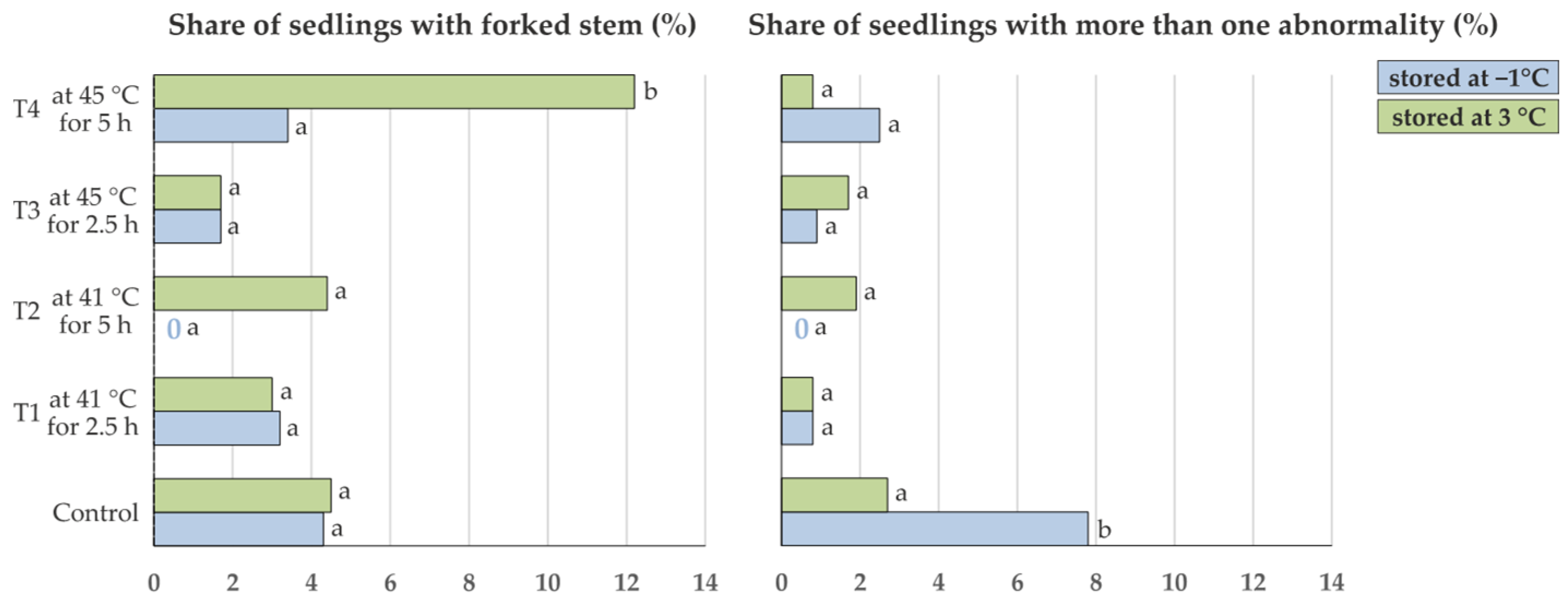
- presence of a forked stem (separation of the main stem in two stems above ground level, FS, yes/no) (Figure 2)
- presence of a brushy stem (separation of the main stem in three or more stems above ground level, BS, yes/no) (Figure 2)
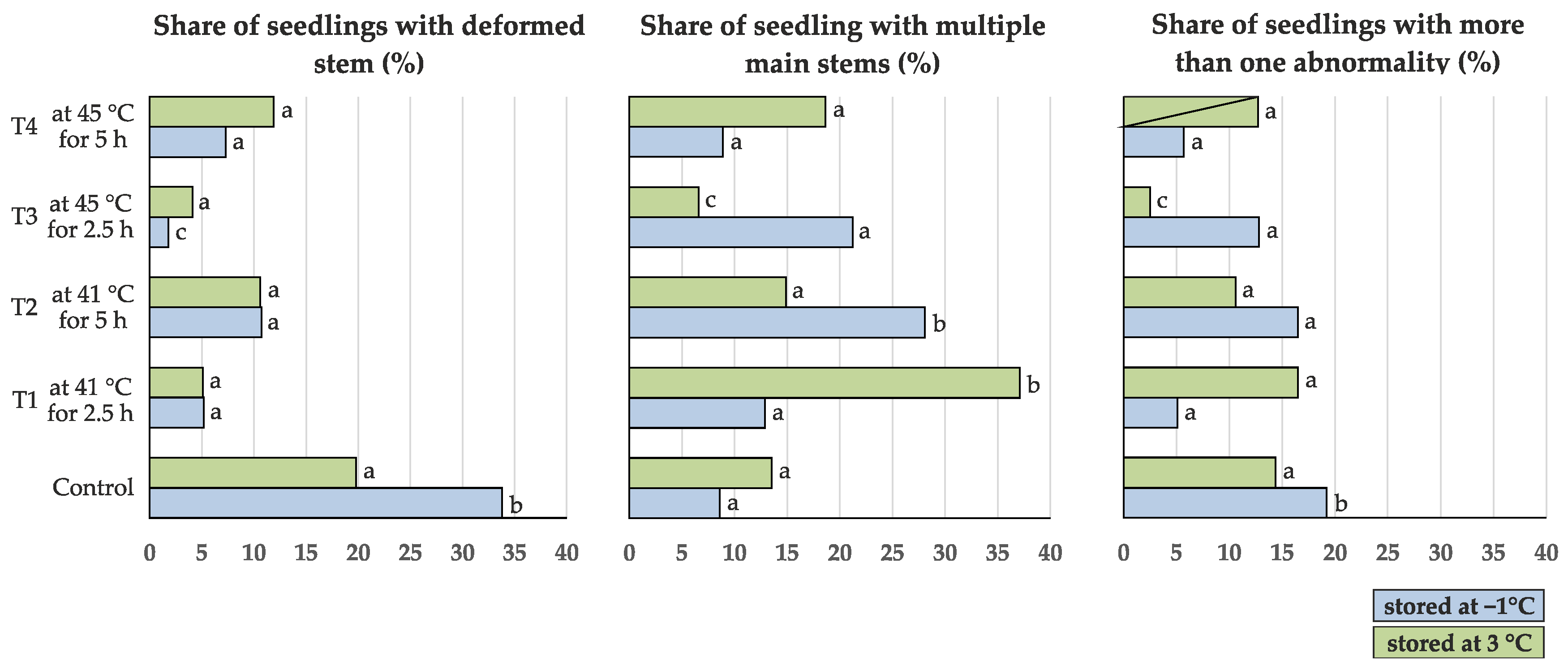
- presence of a deformed stem (DS, yes/no) (Figure 2)
- presence of multiple (more than one) main stems growing from the ground level (MS, yes/no) (Figure 2)
- occurrence of more than one stem abnormality (forked, brushy, deformed or multiple main stems) on a single seedling (ABN, yes/no)
3. Results
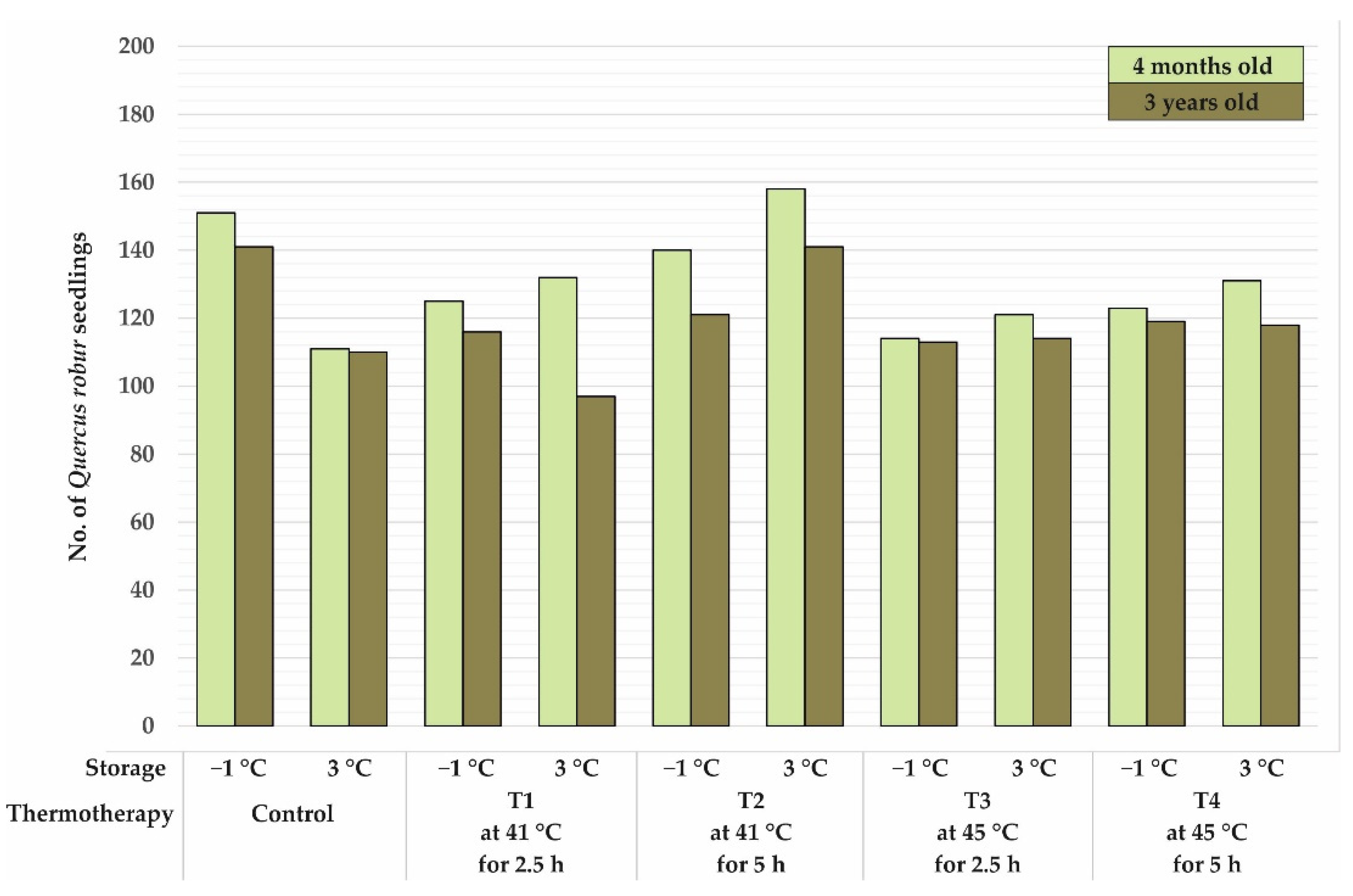
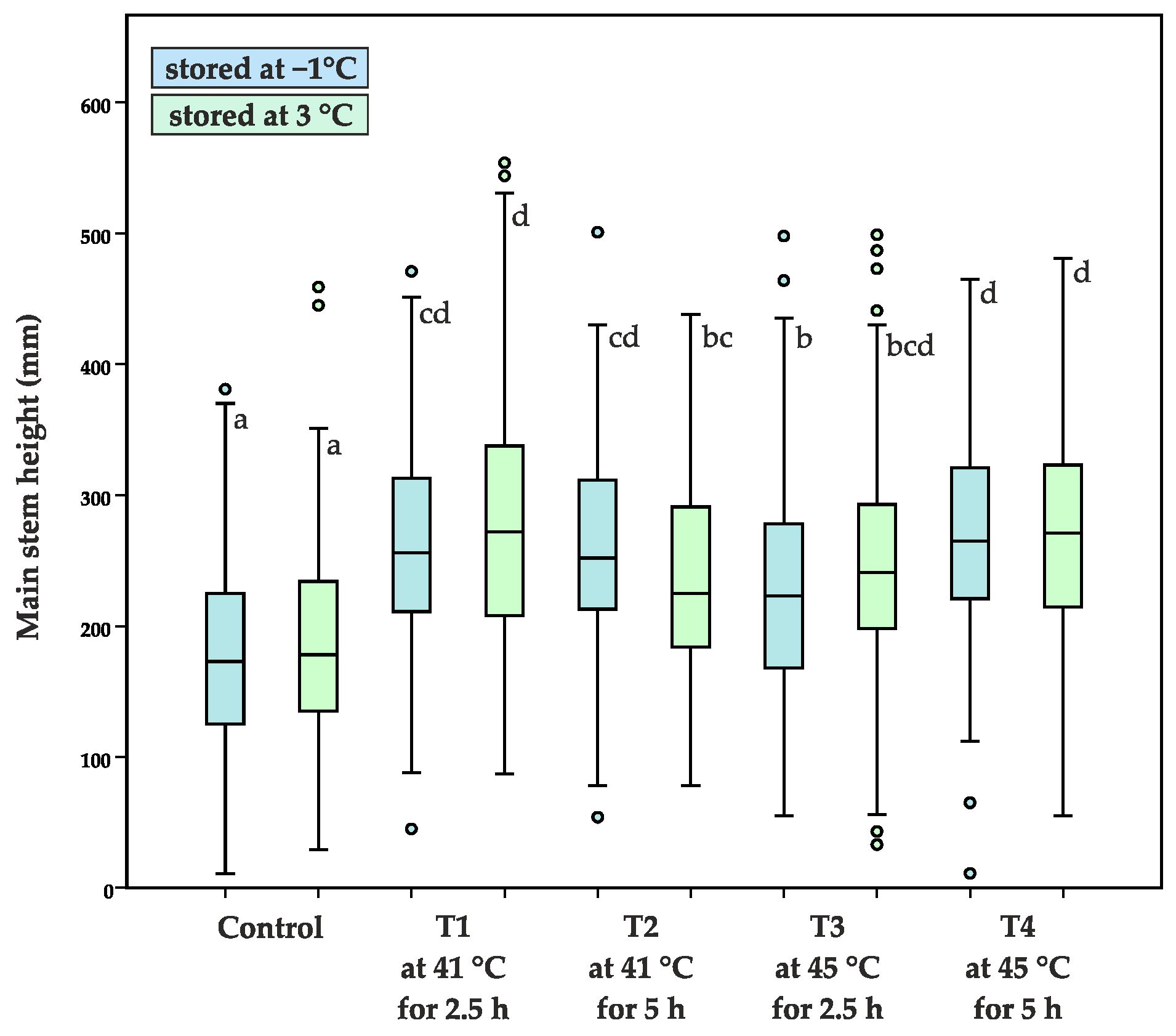
3.1. Morphological Characteristics of Four Months Old Seedlings
3.2. Morphological Characteristics of Three Year Old Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Thermotherapy Treatment and Storage Temperature | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | T4 | ||||||
| −1 °C | 3 °C | −1 °C | 3 °C | −1 °C | 3 °C | −1 °C | 3 °C | −1 °C | 3 °C | |
| Average main stem height (mm) | 176 ± 72 a | 186 ± 78 a | 263 ± 81 cd | 276 ± 91 d | 258 ± 73 cd | 235 ± 72 bc | 226 ± 77 b | 247 ± 88 bcd | 269 ± 87 d | 271 ± 89 d |
| 748 ± 332 a | 870 ± 424 abc | 936 ± 404 bcd | 1042 ± 421 d | 822 ± 394 abc | 859 ± 359 abc | 809 ± 365 ab | 875 ± 384 abc | 973 ± 458 cd | 916 ± 433 bcd | |
| Average root collar diameter (mm) | 4.5 ± 1.5 | 4.1 ± 1.5 | 4.7 ± 1.4 | 5.0 ± 1.8 | 4.6 ± 1.4 | 4.6 ± 1.6 | 4.2 ± 1.3 | 4.1 ± 1.5 | 4.7 ± 1.6 | 4.9 ± 1.7 |
| 9.4 ± 3.9 a | 9.4 ± 4.6 a | 10.6 ± 4.2 ab | 11.6 ± 4.5 b | 9.6 ± 4.0 a | 10.7 ± 4.1 ab | 10.3 ± 3.9 ab | 10.2 ± 3.7 ab | 10.4 ± 4.5 ab | 9.7 ± 3.8 a | |
| Average height: diameter ratio | 39.8 ± 13.1 a | 47.7 ± 17.9 ab | 60.7 ± 32.3 c | 60.1 ± 23.8 c | 59.9 ± 21.8 c | 55.4 ± 25.5 bc | 58.1 ± 25.4 c | 64.2 ± 23.4 c | 60.8 ± 26.0 c | 60.0 ± 28.2 c |
| 80.9 ± 24.0 ab | 93.8 ± 29.8 c | 89.5 ± 26.4 bc | 90.7 ± 22.1 bc | 85.3 ± 25.5 abc | 81.8 ± 28.0 ab | 78.3 ± 25.4 a | 85.1 ± 23.4 abc | 93.9 ± 33.3 c | 94.6 ± 28.8 c | |
| Share of seedlings with forked stem (%) | 4.3 a | 4.5 a | 3.2 a | 3.0 a | 0.0 a | 4.4 a | 1.7 a | 1.7 a | 3.4 a | 12.2 b |
| 20.5 | 27.0 | 25.9 | 25.8 | 20.7 | 29.0 | 15.9 | 15.7 | 26.8 | 27.3 | |
| Share of seedlings with brushy stem (%) | 5.0 | 2.7 | 0.8 | 3.8 | 2.1 | 4.4 | 3.5 | 1.7 | 3.4 | 6.9 |
| 17.2 | 10.8 | 11.2 | 11.3 | 13.2 | 15.6 | 12.4 | 9.0 | 12.2 | 15.2 | |
| Share of seedlings with deformed stem (%) | 10.6 | 10.9 | 8.0 | 10.6 | 9.3 | 7.0 | 5.3 | 7.9 | 5.0 | 4.6 |
| 33.8 b | 19.8 a | 5.2 a | 5.1 a | 10.7 a | 10.6 a | 1.8 c | 4.1 a | 7.3 a | 11.9 a | |
| Share of seedlings with multiple main stems (%) | 24.1 | 20.0 | 12.0 | 17.4 | 12.9 | 13.3 | 14.0 | 17.5 | 14.3 | 16.0 |
| 8.6 a | 13.5 a | 12.9 a | 37.1 b | 28.1 b | 14.9 a | 21.2 a | 6.6 c | 8.9 a | 18.6 a | |
| Share of seedlings with more than one abnormality (%) | 7.8 b | 2.7 a | 0.8 a | 0.8 a | 0.0 a | 1.9 a | 0.9 a | 1.7 a | 2.5 a | 0.8 a |
| 19.2 b | 14.4 a | 5.1 a | 16.5 a | 16.5 a | 10.6 a | 12.8 a | 2.5 c | 5.7 a | 12.7 a | |
References
- Mölder, A.; Meyer, P.; Nagel, R.-V. Integrative management to sustain biodiversity and ecological continuity in central european temperate oak (Quercus robur, Q. petraea) forests: An overview. For. Ecol. Manag. 2019, 437, 324–339. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; De Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publications Office of the EU: Luxembourg, 2016; pp. 160–163. [Google Scholar]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Bobiec, A.; Reif, A.; Öllerer, K. Seeing the oakscape beyond the forest: A landscape approach to the oak regeneration in Europe. Landsc. Ecol. 2018, 33, 513–528. [Google Scholar] [CrossRef]
- Demeter, L.; Molnár, Á.P.; Öllerer, K.; Csóka, G.; Kiš, A.; Vadász, C.; Horváth, F.; Molnár, Z. Rethinking the natural regeneration failure of pedunculate oak: The pathogen mildew hypothesis. Biol. Conserv. 2021, 253, 108928. [Google Scholar] [CrossRef]
- Gibbs, J.; Greig, B. Biotic and abiotic factors affecting the dying back of pedunculate oak Quercus robur L. For. Int. J. For. Res. 1997, 70, 399–406. [Google Scholar] [CrossRef]
- Oršanić, M.; Drvodelić, D. Natural regeneration of pedunculate oak. In Proceedings of the Forest Management Systems and Regeneration of Floodplain Forest Sites, Mendel University of Agriculture and Forestry, Brno, Czech Republic, 9 October 2007; pp. 99–106. [Google Scholar]
- Vasić, V.; Konstantinović, B.; Orlović, S. Application of post-emergence herbicides in the regeneration of pedunculate oak (Quercus robur L.) forests. For. Int. J. For. Res. 2014, 87, 407–415. [Google Scholar] [CrossRef]
- Novak Agbaba, S. Mycoflora of Pedunculate Oak (Quercus robur L.) in Croatia. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2006. [Google Scholar]
- Mattsson, A. Predicting field performance using seedling quality assessment. New For. 1997, 13, 227–252. [Google Scholar] [CrossRef]
- Colombo, S.J. How to Improve the quality of broadleaved seedlings produced in tree nurseries. In Proceedings of the Nursery Production and Stand Establishment of Broad-Leaves to Promote Sustainable Forest Management, Agency for the Environmental Protection and for Technical Services, Rome, Italy, 7 May 2001; pp. 41–53. [Google Scholar]
- Haase, D.L. Morphological and physiological evaluations of seedling quality. In Proceedings of the Forest and Conservation Nursery Association, U.S. Department of Agriculture, Forestry Service, Fort Collins, CO, USA, 17–19 September 2007; pp. 3–8. [Google Scholar]
- Haase, D.L. Understanding forest seedling quality: Measurements and interpretation. Tree Plant. Notes 2008, 52, 24–30. [Google Scholar]
- Kuehne, C.; Kublin, E.; Pyttel, P.; Bauhus, J. Growth and form of Quercus robur and Fraxinus excelsior respond distinctly different to initial growing space: Results from 24-year-old nelder experiments. J. For. Res. 2013, 24, 1–14. [Google Scholar] [CrossRef]
- Aniszewska, M.; Błuszkowska, U.; Zychowicz, W.; Brzózko, J. Impact of mechanical treatment of pedunculate oak (Quercus robur L.) seeds on germination time and seedling quality. J. For. Res. 2020, 25, 420–425. [Google Scholar] [CrossRef]
- Devetaković, J.R.; Nonić, M.; Prokić, B.; Šijačić-Nikolić, M.; Popović, V. Acorn size influence on the quality of pedunculate oak (Quercus robur L.) one-year old seedlings. Reforesta 2019, 8, 17–24. [Google Scholar] [CrossRef]
- Giertych, M.J.; Chmielarz, P. Size variability in embryonic axes, cotyledons, acorns and seedlings in fifteen species of the genus Quercus. Trees 2020, 34, 593–601. [Google Scholar] [CrossRef]
- Drvodelić, D.; Oršanić, M. Izbor kvalitetne šumske sadnice poljskog jasena (Fraxinus angustifolia vahl) za umjetnu obnovu i pošumljavanje. Šumarski List 2019, 143, 577–585. [Google Scholar] [CrossRef]
- Nikolić, N.; Orlović, S.; Krstić, B.; Kevrešan, Ž. Variability of acorn nutrient concentrations in pedunculate oak (Quercus robur L.) genotypes. J. For. Sci. 2006, 52, 51–60. [Google Scholar] [CrossRef]
- Welander, N.T.; Ottosson, B. The influence of shading on growth and morphology in seedlings of Quercus robur L. and Fagus sylvatica L. For. Ecol. Manag. 1998, 107, 117–126. [Google Scholar] [CrossRef]
- Nilsson, U.; Gemmel, P.; Löf, M.; Welander, T. Germination and early growth of sown Quercus robur L. in relation to soil preparation, sowing depths and prevention against predation. New For. 1996, 12, 69–86. [Google Scholar] [CrossRef]
- Phillips, R.L. Environmental factors contribute to acorn quality: Elevation, on- or off-tree collection influence the viability of blue oak acorns. Calif. Agric. 1992, 46, 30–32. [Google Scholar] [CrossRef]
- Hendry, G.A.; Finch-Savage, W.E.; Thorpe, P.C.; Atherton, N.M.; Buckland, S.M.; Nilsson, K.A.; Seel, W.E. Free radical processes and loss of seed viability during desiccation in the recalcitrant species Quercus robur L. New Phytol. 1992, 122, 273–279. [Google Scholar] [CrossRef]
- Gordon, A. Seed dormancy, seed treatment and seed sowing. For. Comm. Bull. 1992, 83, 116–121. [Google Scholar]
- Özbingöl, N.; O’reilly, C. Increasing acorn moisture content followed by freezing-storage enhances germination in pedunculate oak. Forestry 2005, 78, 73–81. [Google Scholar] [CrossRef]
- Gosling, P.G. The effect of drying Quercus robur acorns to different moisture contents, followed by storage, either with or without imbibition. For. Int. J. For. Res. 1989, 62, 41–50. [Google Scholar] [CrossRef]
- Doody, C.N.; O’Reilly, C. Drying and soaking pretreatments affect germination in pedunculate oak. Ann. For. Sci. 2008, 65, 1. [Google Scholar] [CrossRef]
- Finch-Savage, W.; Clay, H.; Budge, S.; Dent, K.; Clarkson, J.; Whipps, J. Biological control of Sclerotinia pseudotuberosa and other fungi during moist storage of Quercus robur seeds. Eur. J. Plant Pathol. 2003, 109, 615–624. [Google Scholar] [CrossRef]
- Vasinauskienė, R.; Silingiene, G.; Sinkevičienė, J. Surface sterilization of english oak (Quercus robur L.) acorns using wet water steam. Balt. For. 2020, 26, 435. [Google Scholar] [CrossRef]
- Knudsen, I.; Thomsen, K.; Jensen, B.; Poulsen, K. Effects of hot water treatment, biocontrol agents, disinfectants and a fungicide on storability of english oak acorns and control of the pathogen, Ciboria batschiana. For. Pathol. 2004, 34, 47–64. [Google Scholar] [CrossRef]
- Delatour, C. A curative control method for Ciboria batschiana on acorns. Eur. J. For. Pathol. 1978, 8, 193–200. [Google Scholar] [CrossRef]
- Suszka, B.; Muller, C.; Bonnet-Masimbert, M. Seeds of Forest Broadleaves: From Harvest to Sowing; INRA: Paris, France, 1996; ISBN 2-7380-0659-0. [Google Scholar]
- Kranjec Orlović, J.; Drvodelić, D.; Vukelić, M.; Rukavina, M.; Diminić, D.; Oršanić, M. Impact of thermotherapy and short-term storage on Quercus robur L. acorn mycobiota and germination. Forests 2021, 12, 528. [Google Scholar] [CrossRef]
- Schröder, T. Über die Eignung Verschiedener Physikalisch-Technischer Verfahren zur Phytosanitären Behandlung und zur Lagerung von Forstsaatgut Unter Besonderer Berücksichtigung der Stiel-und Traubeneiche; Parey Buchverlag: Berlin, Germany, 1999; ISBN 3-8263-3244-X. [Google Scholar]
- Kehr, R.D.; Schröder, T. Long-term storage of oak seeds-new methods and mycological aspects. In Proceedings of the Tree Seed Pathology Meeting, Opocno, Czech Republic, 9–11 October 1996; pp. 50–61. [Google Scholar]
- Kalemba, E.M.; Wawrzyniak, M.K.; Suszka, J.; Chmielarz, P. Thermotherapy and storage temperature manipulations limit the production of reactive oxygen species in stored pedunculate oak acorns. Forests 2021, 12, 1338. [Google Scholar] [CrossRef]
- Bauer Živković, A.; Pap, P. The importance of thermotherapy on pedunculate oak acorn germination in field conditions. In Proceedings of the Challenges and Threats to Contemporary Forestry, University of Belgrade, Faculty of Forestry, Belgrade, Serbia, 1 November 2015; p. 19. [Google Scholar]
- Tylkowski, T. Height increment of 1-year shoots of the english oak (Quercus robur L.) and the northern red oak (Q. borealis Michx. = Q. rubra L.) on 4-year-old roots of seedlings raised from acorns stored over 1–5 winters. Arbor. Korn. 1982, 27, 357–365. [Google Scholar]
- Merouani, H.; Branco, C.; Almeida, M.H.; Pereira, J.S. Effects of acorn storage duration and parental tree on emergence and physiological status of cork oak (Quercus suber L.) seedlings. Ann. For. Sci. 2001, 58, 543–554. [Google Scholar] [CrossRef]
- Benamirouche, S.; Chouial, M.; Messaoudene, M. Storage of cork oak (Quercus suber L., 1753) acorns and effect of storage duration on seedlings vigour: Artificial regeneration implications. Rev. Décologie 2018, 73, 80–95. [Google Scholar]
- Garcia-Perez, M.A.; Nunez-Anton, V. Cellwise residual analysis in two-way contingency tables. Educ. Psychol. Meas. 2003, 63, 825–839. [Google Scholar] [CrossRef]
- Thompson, B.E. Seedling morphological evaluation: What you can tell by looking. In Proceedings of the Workshop on Evaluating Seedling Quality: Principles, Procedures, and Predictive Abilities of Major Tests, Forest Research Laboratory, Oregon State University, Corvallis, OR, USA, 16 October 1984; pp. 59–71. [Google Scholar]
- Velásquez-Valle, R.; Reveles Hernández, M. Effect of thermotherapy on emergence and vegetative characteristics of genotypes of garlic. Rev. Mex. Cienc. Agrícolas 2019, 10, 447–452. [Google Scholar] [CrossRef][Green Version]
- Kleinschmit, J. Intraspecific variation of growth and adaptive traits in european oak species. Ann. For. Sci. 1993, 50, 166–185. [Google Scholar] [CrossRef]
- Kormanik, P.P.; Sung, S.; Kormanik, T.; Schlarbaum, S.; Zarnoch, S.J. Effect of acorn size on development of northern red oak 1–0 seedlings. Can. J. For. Res. 1998, 28, 1805–1813. [Google Scholar] [CrossRef]
- Branco, M.; Branco, C.; Merouani, H.; Almeida, M.H. Germination success, survival and seedling vigour of Quercus suber acorns in relation to insect damage. For. Ecol. Manag. 2002, 166, 159–164. [Google Scholar] [CrossRef]
- Wallraf, A.; Wagner, S. Effects of initial plant density, interspecific competition, tending and age on the survival and quality of oak (Quercus robur L.) in young mixed stands in European Russia. For. Ecol. Manag. 2019, 446, 272–284. [Google Scholar] [CrossRef]
- Hautsalo, J.; Mathieu, P.; Elshibli, S.; Vakkari, P.; Raisio, J.; Pulkkinen, P. Variation in height and survival among northern populations of pedunculate oak (Quercus robur L.): Results of a 13-year field study. Silva Fenn. 2015, 49, 1274. [Google Scholar] [CrossRef][Green Version]
- Buras, A.; Sass-Klaassen, U.; Verbeek, I.; Copini, P. Provenance selection and site conditions determine growth performance of pedunculate oak. Dendrochronologia 2020, 61, 125705. [Google Scholar] [CrossRef]
- Johnson, J.D.; Cline, M.L. Seedling quality of southern pines. In Forest Regeneration Manual; Springer: Dordrecht, The Netherlands, 1991; pp. 143–159. [Google Scholar]
- Roller, K.J. Suggested Minimum Standards for Containerized Seedlings in Nova Scotia; Fisheries and Environment Canada, Canadian Forestry Service, Maritimes Forest Research Centre: Ottawa, ON, Canada, 1977; p. 18. [Google Scholar]
- Gregory, F.; Hancock, C. The rate of transport of natural auxin in woody shoots. Ann. Bot. 1955, 19, 451–465. [Google Scholar] [CrossRef]
- Tworkoski, T. Morphological and hormonal relationships in shoots of pillar and standard peach trees. In Proceedings of the 30th Annual Meeting of the Plant Growth Regulator Society of America, Vancouver, BC, Canada, 3 August 2003; p. 190. [Google Scholar]
- Sander, I.L.; Clark, F.B. Reproduction of Upland Hardwood Forests in the Central States; US Forest Service: Washington, DC, USA, 1971; p. 25.
- Weaver, T.; Jacobs, J. Occurrence of multiple stems in whitebark pine. In Proceedings of the Symposium on Whitebark Pine Ecosystems: Ecology and Management of a High-Mountain Resource, Intermountain Research Station, Forest Service U.S. Department of Agriculture, Bozeman, MT, USA, 29 March 1989; pp. 156–159. [Google Scholar]
- Assmann, E. The Principles of Forest Yield Study: Studies in the Organic Production, Structure, Increment and Yield of Forest Stands; Pergamon Press: Oxford, UK, 1970; ISBN 1-4831-5093-3. [Google Scholar]
- Werger, M.; Guo, K.; Li, R. Effect of acorn burying depth on germination, seedling emergence and development of Quercus aliena var. acuteserrata. Acta Bot. Sin. 2001, 43, 974–978. [Google Scholar]
- Rock, J.; Puettmann, K.; Gockel, H.; Schulte, A. Spatial aspects of the influence of silver birch (Betula pendula L.) on growth and quality of young oaks (Quercus spp.) in Central Germany. Forestry 2004, 77, 235–247. [Google Scholar] [CrossRef]
| Thermotherapy Treatment Conditions | Group Label |
|---|---|
| at 41 °C for 2.5 h | T1 |
| at 41 °C for 5 h | T2 |
| at 45 °C for 2.5 h | T3 |
| at 45 °C for 5 h | T4 |
| no treatment (control) | C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kranjec Orlović, J.; Drvodelić, D.; Španjol, E.; Bogunović, S.; Diminić, D.; Oršanić, M. Effect of Acorn Thermotherapy and Short-Term Storage on Morphological Characteristics of Related Quercus Robur L. Seedlings. Sustainability 2022, 14, 1307. https://doi.org/10.3390/su14031307
Kranjec Orlović J, Drvodelić D, Španjol E, Bogunović S, Diminić D, Oršanić M. Effect of Acorn Thermotherapy and Short-Term Storage on Morphological Characteristics of Related Quercus Robur L. Seedlings. Sustainability. 2022; 14(3):1307. https://doi.org/10.3390/su14031307
Chicago/Turabian StyleKranjec Orlović, Jelena, Damir Drvodelić, Ela Španjol, Sanja Bogunović, Danko Diminić, and Milan Oršanić. 2022. "Effect of Acorn Thermotherapy and Short-Term Storage on Morphological Characteristics of Related Quercus Robur L. Seedlings" Sustainability 14, no. 3: 1307. https://doi.org/10.3390/su14031307
APA StyleKranjec Orlović, J., Drvodelić, D., Španjol, E., Bogunović, S., Diminić, D., & Oršanić, M. (2022). Effect of Acorn Thermotherapy and Short-Term Storage on Morphological Characteristics of Related Quercus Robur L. Seedlings. Sustainability, 14(3), 1307. https://doi.org/10.3390/su14031307






