Biochar-Assisted Bioengineered Strategies for Metal Removal: Mechanisms, Key Considerations, and Perspectives for the Treatment of Solid and Liquid Matrixes
Abstract
1. Introduction
2. Critical Properties of Biochar in Its Interaction with Microorganisms
2.1. Adsorption Capacity
2.2. Surface Area and Porosity
2.3. Electron-Exchange Capacity
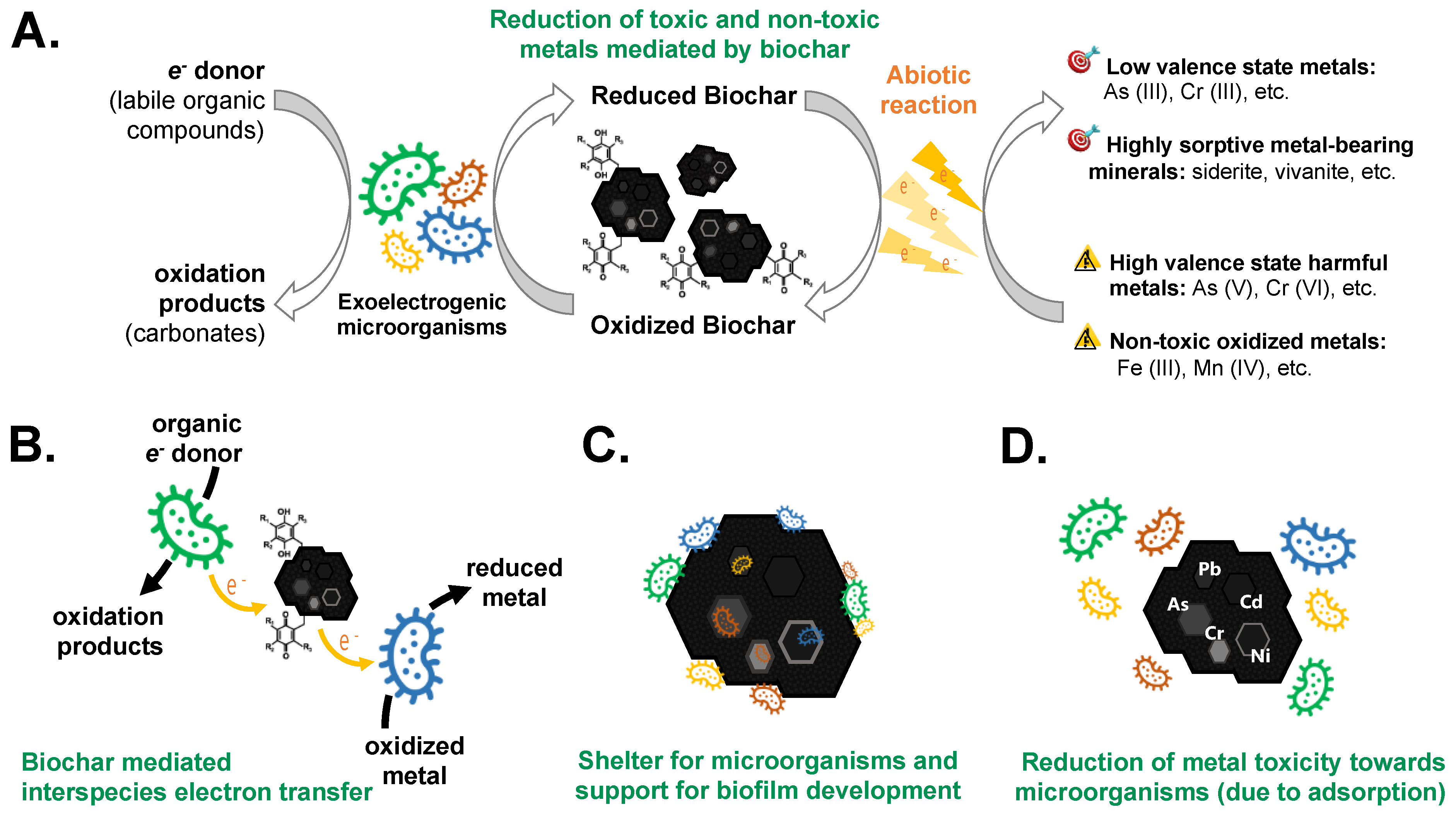
3. Biochar-Microorganism Interactions Promoting Heavy Metal Removal
3.1. Biochar-Mediated Microbial Redox Reactions
3.2. Reduction in Non-Toxic Metals Mediated by Biochar
3.3. Reduction in Toxic Metals Mediated by Biochar
3.4. Biochar-Mediated Interspecies Electron Transfer
3.5. Biochar as a Microbial Shelter and Biofilm Carrier
4. Applications for Solid Matrixes Treatment
4.1. Mine Tailing Bioremediation/Metal-Containing Solid Waste
4.2. Tailing Stabilization
4.3. Leaching from Metal-Containing Solid Waste
4.4. Polluted Soil Bioremediation
4.5. Biochar Application in Composting/Vermicomposting Processes
5. Applications for Liquid Matrixes Treatment
5.1. Bioreactors
5.2. Constructed Wetlands
5.2.1. Use of Biochar in Constructed Wetlands for Metal Removal
5.2.2. Scenario of Beneficial Microorganism—Biochar Interactions in Constructed Wetlands
6. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015; ISBN 978-1-134-48953-4. [Google Scholar]
- Hu, Q.; Jung, J.; Chen, D.; Leong, K.; Song, S.; Li, F.; Mohan, B.C.; Yao, Z.; Prabhakar, A.K.; Lin, X.H.; et al. Biochar Industry to Circular Economy. Sci. Total Environ. 2021, 757, 143820. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of Biochar for the Removal of Pollutants from Aqueous Solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, Present, and Future of Biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest Trends in Heavy Metal Removal from Wastewater by Biochar Based Sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Zhao, J.J.; Shen, X.J.; Domene, X.; Alcañiz, J.M.; Liao, X.; Palet, C. Comparison of Biochars Derived from Different Types of Feedstock and Their Potential for Heavy Metal Removal in Multiple-Metal Solutions. Sci. Rep. 2019, 9, 9869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of Biochar Properties as a Function of Feedstock Sources and Production Temperatures. J. Hazard. Mater. 2013, 256, 1–9. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Ding, W.; Pullammanappallil, P.; Zimmerman, A.R.; Cao, X. Enhanced Lead Sorption by Biochar Derived from Anaerobically Digested Sugarcane Bagasse. Sep. Sci. Technol. 2011, 46, 1950–1956. [Google Scholar] [CrossRef]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Zhang, X.; Qi, H.; Ma, J.; Xiao, Y.; et al. Adsorption Characteristics of Pb(II) Using Biochar Derived from Spent Mushroom Substrate. Sci. Rep. 2019, 9, 15999. [Google Scholar] [CrossRef]
- Li, A.Y.; Deng, H.; Jiang, Y.H.; Ye, C.H.; Yu, B.G.; Zhou, X.L.; Ma, A.Y. Superefficient Removal of Heavy Metals from Wastewater by Mg-Loaded Biochars: Adsorption Characteristics and Removal Mechanisms. Langmuir 2020, 36, 9160–9174. [Google Scholar] [CrossRef]
- Li, Y.; Gao, L.; Lu, Z.; Wang, Y.; Wang, Y.; Wan, S. Enhanced Removal of Heavy Metals from Water by Hydrous Ferric Oxide-Modified Biochar. ACS Omega 2020, 5, 28702–28711. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, L.; Wang, C.Q.; Zhang, Q.P.; Liu, Q.C.; Li, Y.D.; Xiao, R. Adsorption of Cd(II) from Aqueous Solutions by Rape Straw Biochar Derived from Different Modification Processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.Q.; Chen, C.; Cui, H.J.; Fu, M.L. Enhanced Removal of Cd(Ii) from Aqueous Solution Using CaCO3 Nanoparticle Modified Sewage Sludge Biochar. RSC Adv. 2017, 7, 16238–16243. [Google Scholar] [CrossRef]
- Luo, L.; Xu, C.; Chen, Z.; Zhang, S. Properties of Biomass-Derived Biochars: Combined Effects of Operating Conditions and Biomass Types. Bioresour. Technol. 2015, 192, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, P.; Nanda, S.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Evaluation of the Physiochemical Development of Biochars Obtained from Pyrolysis of Wheat Straw, Timothy Grass and Pinewood: Effects of Heating Rate. J. Anal. Appl. Pyrolysis 2013, 104, 485–493. [Google Scholar] [CrossRef]
- Sun, J.; Lian, F.; Liu, Z.; Zhu, L.; Song, Z. Biochars Derived from Various Crop Straws: Characterization and Cd(II) Removal Potential. Ecotoxicol. Environ. Saf. 2014, 106, 226–231. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E. Effect of Feedstock and Pyrolysis Temperature on Properties of Biochar Governing End Use Efficacy. Biomass Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]
- Chen, W.; Ding, S.; Lin, Z.; Peng, Y.; Ni, J. Different Effects of N2-Flow and Air-Limited Pyrolysis on Bamboo-Derived Biochars’ Nitrogen and Phosphorus Release and Sorption Characteristics. Sci. Total Environ. 2020, 711, 134828. [Google Scholar] [CrossRef]
- Ding, W.; Dong, X.; Ime, I.M.; Gao, B.; Ma, L.Q. Pyrolytic Temperatures Impact Lead Sorption Mechanisms by Bagasse Biochars. Chemosphere 2014, 105, 68–74. [Google Scholar] [CrossRef]
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q. Effects of Heating Rate on Slow Pyrolysis Behavior, Kinetic Parameters and Products Properties of Moso Bamboo. Bioresour. Technol. 2014, 169, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, C.; Greco, G.; Canevesi, R.L.S.; Izquierdo, M.T.; Fierro, V.; Celzard, A.; González, B.; Manyà, J.J. Influence of Activation Conditions on Textural Properties and Performance of Activated Biochars for Pyrolysis Vapors Upgrading. Fuel 2021, 289, 119759. [Google Scholar] [CrossRef]
- Liu, H.; Xu, G.; Li, G. Preparation of Porous Biochar Based on Pharmaceutical Sludge Activated by NaOH and Its Application in the Adsorption of Tetracycline. J. Colloid Interface Sci. 2021, 587, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, X.; Liu, H.; Cheng, W.; Bao, Y. Modification of Calcium-Rich Biochar by Loading Si/Mn Binary Oxide after NaOH Activation and Its Adsorption Mechanisms for Removal of Cu(II) from Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124960. [Google Scholar] [CrossRef]
- He, M.; Xu, Z.; Sun, Y.; Chan, P.S.; Lui, I.; Tsang, D.C.W. Critical Impacts of Pyrolysis Conditions and Activation Methods on Application-Oriented Production of Wood Waste-Derived Biochar. Bioresour. Technol. 2021, 341, 125811. [Google Scholar] [CrossRef]
- Encinas-Vázquez, A.; Quezada-Renteria, J.A.; Cervantes, F.J.; Pérez-Rábago, C.A.; Molina-Freaner, F.E.; Pat-Espadas, A.M.; Estrada, C.A. Unraveling the Mechanisms of Lead Adsorption and Ageing Process on High-Temperature Biochar. J. Chem. Technol. Biotechnol. 2021, 96, 775–784. [Google Scholar] [CrossRef]
- Siatecka, A.; Oleszczuk, P. Mechanism of Aging of Biochars Obtained at Different Temperatures from Sewage Sludges with Different Composition and Character. Chemosphere 2022, 287, 132258. [Google Scholar] [CrossRef]
- Lovley, D.R. Syntrophy Goes Electric: Direct Interspecies Electron Transfer. Annu. Rev. Microbiol. 2017, 71, 643–664. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Cervantes, F.J. The Role of Humic Substances in Mitigating Greenhouse Gases Emissions: Current Knowledge and Research Gaps. Sci. Total Environ. 2021, 750, 141677. [Google Scholar] [CrossRef]
- Graber, E.R.; Tsechansky, L.; Lew, B.; Cohen, E. Reducing Capacity of Water Extracts of Biochars and Their Solubilization of Soil Mn and Fe. Eur. J. Soil Sci. 2014, 65, 162–172. [Google Scholar] [CrossRef]
- Joseph, S.; Husson, O.; Graber, E.R.; Van Zwieten, L.; Taherymoosavi, S.; Thomas, T.; Nielsen, S.; Ye, J.; Pan, G.; Chia, C.; et al. The Electrochemical Properties of Biochars and How They Affect Soil Redox Properties and Processes. Agronomy 2015, 5, 322. [Google Scholar] [CrossRef]
- Li, S.; Shao, L.; Zhang, H.; He, P.; Lü, F. Quantifying the Contributions of Surface Area and Redox-Active Moieties to Electron Exchange Capacities of Biochar. J. Hazard. Mater. 2020, 394, 122541. [Google Scholar] [CrossRef] [PubMed]
- Saquing, J.M.; Yu, Y.H.; Chiu, P.C. Wood-Derived Black Carbon (Biochar) as a Microbial Electron Donor and Acceptor. Environ. Sci. Technol. Lett. 2016, 3, 62–66. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, Y.; Obadamudalige, N.L.; Ok, Y.S.; Bolan, N.; Li, Q. Redox-Mediated Biochar-Contaminant Interactions in Soil; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 978-0-12811-729-3. [Google Scholar]
- Yuan, Y.; Bolan, N.; Prévoteau, A.; Vithanage, M.; Biswas, J.K.; Ok, Y.S.; Wang, H. Applications of Biochar in Redox-Mediated Reactions. Bioresour. Technol. 2017, 246, 271–281. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, Y.; Tang, L.; Xie, Q.; Liu, Q.; Zhong, L.; Fu, L.; Fan, C. Effects and Mechanisms of Modified Biochars on Microbial Iron Reduction of Geobacter Sulfurreducens. Chemosphere 2021, 283, 130983. [Google Scholar] [CrossRef]
- Xu, S.; Adhikari, D.; Huang, R.; Zhang, H.; Tang, Y.; Roden, E.; Yang, Y. Biochar-Facilitated Microbial Reduction of Hematite. Environ. Sci. Technol. 2016, 50, 2389–2395. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, T.; Subdiaga, E.; Obst, M.; Haderlein, S.B.; Maisch, M.; Kretzschmar, R.; Angenent, L.T.; Kappler, A. Aggregation-Dependent Electron Transfer via Redox-Active Biochar Particles Stimulate Microbial Ferrihydrite Reduction. Sci. Total Environ. 2020, 703, 135515. [Google Scholar] [CrossRef]
- Kappler, A.; Wuestner, M.L.; Ruecker, A.; Harter, J.; Halama, M.; Behrens, S. Biochar as an Electron Shuttle between Bacteria and Fe(III) Minerals. Environ. Sci. Technol. Lett. 2014, 1, 339–344. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Liu, J.; Liu, J.; Xia, F.; Wang, C.; Dahlgren, R.A.; Liu, W. Mechanism of Cr(VI) Removal by Magnetic Greigite/Biochar Composites. Sci. Total Environ. 2020, 700, 134414. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and Mechanisms of Biochar-Microbe Interactions in Soil Improvement and Pollution Remediation: A Review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Hunter, H.A.; Ling, F.T.; Peters, C.A. Coprecipitation of Heavy Metals in Calcium Carbonate from Coal Fly Ash Leachate. ACS EST Water 2021, 1, 339–345. [Google Scholar] [CrossRef]
- Bibi, I.; Niazi, N.K.; Choppala, G.; Burton, E.D. Chromium(VI) Removal by Siderite (FeCO3) in Anoxic Aqueous Solutions: An X-ray Absorption Spectroscopy Investigation. Sci. Total Environ. 2018, 640, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Erdem, M.; Özverdi, A. Lead Adsorption from Aqueous Solution onto Siderite. Sep. Purif. Technol. 2005, 42, 259–264. [Google Scholar] [CrossRef]
- Guo, H.; Stüben, D.; Berner, Z. Adsorption of Arsenic(III) and Arsenic(V) from Groundwater Using Natural Siderite as the Adsorbent. J. Colloid Interface Sci. 2007, 315, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Muehe, E.M.; Morin, G.; Scheer, L.; Le Pape, P.; Esteve, I.; Daus, B.; Kappler, A. Arsenic(V) Incorporation in Vivianite during Microbial Reduction of Arsenic(V)-Bearing Biogenic Fe(III) (Oxyhydr)Oxides. Environ. Sci. Technol. 2016, 50, 2281–2291. [Google Scholar] [CrossRef]
- Wu, S.; Fang, G.; Wang, D.; Jaisi, D.P.; Cui, P.; Wang, R.; Wang, Y.; Wang, L.; Sherman, D.M.; Zhou, D. Fate of As(III) and As(V) during Microbial Reduction of Arsenic-Bearing Ferrihydrite Facilitated by Activated Carbon. ACS Earth Space Chem. 2018, 2, 878–887. [Google Scholar] [CrossRef]
- Bae, S.; Sihn, Y.; Kyung, D.; Yoon, S.; Eom, T.; Kaplan, U.; Kim, H.; Schäfer, T.; Han, S.; Lee, W. Molecular Identification of Cr(VI) Removal Mechanism on Vivianite Surface. Environ. Sci. Technol. 2018, 52, 10647–10656. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of Heavy Metals from Aqueous Solution by Biochars Derived from Anaerobically Digested Biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.F.; Liu, Y.G.; Gu, Y.L.; Xu, Y.; Zeng, G.M.; Hu, X.J.; Liu, S.B.; Wang, X.; Liu, S.M.; Li, J. Biochar-Based Nano-Composites for the Decontamination of Wastewater: A Review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef]
- Michelson, K.; Sanford, R.A.; Valocchi, A.J.; Werth, C.J. Nanowires of Geobacter Sulfurreducens Require Redox Cofactors to Reduce Metals in Pore Spaces Too Small for Cell Passage. Environ. Sci. Technol. 2017, 51, 11660–11668. [Google Scholar] [CrossRef]
- Choppala, G.; Bolan, N.; Kunhikrishnan, A.; Bush, R. Differential Effect of Biochar upon Reduction-Induced Mobility and Bioavailability of Arsenate and Chromate. Chemosphere 2016, 144, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, H.; Zhang, Y.; Xu, Z.; Cao, X. Biochar as Both Electron Donor and Electron Shuttle for the Reduction Transformation of Cr(VI) during Its Sorption. Environ. Pollut. 2019, 244, 423–430. [Google Scholar] [CrossRef]
- Qin, J.; Li, Q.; Liu, Y.; Niu, A.; Lin, C. Biochar-Driven Reduction of As(V) and Cr(VI): Effects of Pyrolysis Temperature and Low-Molecular-Weight Organic Acids. Ecotoxicol. Environ. Saf. 2020, 201, 110873. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yang, Z.; Si, M.; Zhu, F.; Yang, W.; Zhao, F.; Shi, Y. Application of Biochars in the Remediation of Chromium Contamination: Fabrication, Mechanisms, and Interfering Species. J. Hazard. Mater. 2021, 407, 124376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y.; et al. Investigation of the Adsorption-Reduction Mechanisms of Hexavalent Chromium by Ramie Biochars of Different Pyrolytic Temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, Y.; Yuan, Y.; Tang, J.; Zhou, S. Biochar as Electron Acceptor for Microbial Extracellular Respiration. Geomicrobiol. J. 2016, 33, 530–536. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar Application for the Remediation of Heavy Metal Polluted Land: A Review of In Situ Field Trials. Sci. Total Environ. 2018, 619, 815–826. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.M.; Klasson, K.T.; Wartelle, L.H. Contaminant Immobilization and Nutrient Release by Biochar Soil Amendment: Roles of Natural Organic Matter. Chemosphere 2010, 80, 935–940. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Kim, B.Y.; Ahn, J.H.; Lee, Y.H.; Zhang, M.; Moon, D.H.; Al-Wabel, M.I.; Lee, S.S. Impact of Soybean Stover- and Pine Needle-Derived Biochars on Pb and As Mobility, Microbial Community, and Carbon Stability in a Contaminated Agricultural Soil. J. Environ. Manag. 2016, 166, 131–139. [Google Scholar] [CrossRef]
- Lindsay, M.B.J.; Wakeman, K.D.; Rowe, O.F.; Grail, B.M.; Ptacek, C.J.; Blowes, D.W.; Johnson, D.B. Microbiology and Geochemistry of Mine Tailings Amended with Organic Carbon for Passive Treatment of Pore Water. Geomicrobiol. J. 2011, 28, 229–241. [Google Scholar] [CrossRef]
- Bandara, T.; Franks, A.; Xu, J.; Bolan, N.; Wang, H.; Tang, C. Chemical and Biological Immobilization Mechanisms of Potentially Toxic Elements in Biochar-Amended Soils. Crit. Rev. Environ. Sci. Technol. 2020, 50, 903–978. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, T.; Kleindienst, S.; Straub, D.; Kretzschmar, R.; Angenent, L.T.; Kappler, A. A Coupled Function of Biochar as Geobattery and Geoconductor Leads to Stimulation of Microbial Fe(III) Reduction and Methanogenesis in a Paddy Soil Enrichment Culture. Soil Biol. Biochem. 2021, 163, 108446. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and Potential of Direct Interspecies Electron Transfer in Anaerobic Digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Holmes, D.E.; Dang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Potential Enhancement of Direct Interspecies Electron Transfer for Syntrophic Metabolism of Propionate and Butyrate with Biochar in Up-Flow Anaerobic Sludge Blanket Reactors. Bioresour. Technol. 2016, 209, 148–156. [Google Scholar] [CrossRef]
- Cooney, M.J.; Lewis, K.; Harris, K.; Zhang, Q.; Yan, T. Start Up Performance of Biochar Packed Bed Anaerobic Digesters. J. Water Process Eng. 2016, 9, e7–e13. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, D.; Liu, Y.; Xu, H.; Nan, H.; Li, D.; Kan, Y.; Cao, X. Biochar as Simultaneous Shelter, Adsorbent, PH Buffer, and Substrate of Pseudomonas Citronellolis to Promote Biodegradation of High Concentrations of Phenol in Wastewater. Water Res. 2020, 172, 115494. [Google Scholar] [CrossRef] [PubMed]
- Kołtowski, M.; Charmas, B.; Skubiszewska-Zięba, J.; Oleszczuk, P. Effect of Biochar Activation by Different Methods on Toxicity of Soil Contaminated by Industrial Activity. Ecotoxicol. Environ. Saf. 2017, 136, 119–125. [Google Scholar] [CrossRef]
- Jayakumar, A.; Wurzer, C.; Soldatou, S.; Edwards, C.; Lawton, L.A.; Mašek, O. New Directions and Challenges in Engineering Biologically-Enhanced Biochar for Biological Water Treatment. Sci. Total Environ. 2021, 796, 148977. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the “charosphere”—Does Biochar in Agricultural Soil Provide a Significant Habitat for Microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Rodríguez-Vila, A.; Forján, R.; Guedes, R.S.; Covelo, E.F. Changes on the Phytoavailability of Nutrients in a Mine Soil Reclaimed with Compost and Biochar. Water Air Soil Pollut. 2016, 227, 453. [Google Scholar] [CrossRef]
- Xing, Y.; Luo, X.; Liu, S.; Wan, W.; Huang, Q.; Chen, W. A Novel Eco-Friendly Recycling of Food Waste for Preparing Biofilm-Attached Biochar to Remove Cd and Pb in Wastewater. J. Clean. Prod. 2021, 311, 127514. [Google Scholar] [CrossRef]
- Frankel, M.L.; Bhuiyan, T.I.; Veksha, A.; Demeter, M.A.; Layzell, D.B.; Helleur, R.J.; Hill, J.M.; Turner, R.J. Removal and Biodegradation of Naphthenic Acids by Biochar and Attached Environmental Biofilms in the Presence of Co-Contaminating Metals. Bioresour. Technol. 2016, 216, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Tian, C.; Li, L.; Liang, Y.; Yan, S.; Hu, M.; Xu, W.; Lin, Z.; Chai, L. Microinteraction Analysis between Heavy Metals and Coexisting Phases in Heavy Metal Containing Solid Wastes. ACS EST Eng. 2022, 2, 547–563. [Google Scholar] [CrossRef]
- Lee, J.; Pandey, B.D. Bio-Processing of Solid Wastes and Secondary Resources for Metal Extraction—A Review. Waste Manag. 2012, 32, 3–18. [Google Scholar] [CrossRef]
- Gómez-Ramírez, M.; Tenorio-Sánchez, S.A. Treatment of Solid Waste Containing Metals by Biological Methods; IntechOpen: London, UK, 2020; ISBN 978-1-83880-465-7. [Google Scholar]
- United Nations. University Discarded Kitchen, Laundry, Bathroom Equipment Comprises over Half of World E-Waste: UNU Report 2015; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Ioannidis, T.A.; Zouboulis, A.I. Solidification/Stabilization of Hazardous Solid Wastes. In Water Encyclopedia; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 835–840. ISBN 9780471478447. [Google Scholar]
- Robinson, B.H. E-Waste: An Assessment of Global Production and Environmental Impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Z.; Bhatnagar, A.; Jeyakumar, P.; Wang, H.; Wang, Y.; Li, X. Microorganisms-Carbonaceous Materials Immobilized Complexes: Synthesis, Adaptability and Environmental Applications. J. Hazard. Mater. 2021, 416, 125915. [Google Scholar] [CrossRef]
- Li, J.; Xia, C.; Cheng, R.; Lan, J.; Chen, F.; Li, X.; Li, S.; Chen, J.; Zeng, T.; Hou, H. Passivation of Multiple Heavy Metals in Lead–Zinc Tailings Facilitated by Straw Biochar-Loaded N-Doped Carbon Aerogel Nanoparticles: Mechanisms and Microbial Community Evolution. Sci. Total Environ. 2022, 803, 149866. [Google Scholar] [CrossRef]
- Frewert, A.; Trippe, K.; Cheeke, T.E. Can Locally Sourced Inoculum and Biochar Synergistically Improve the Establishment of Mycorrhizal Fungi in Mine Tailings? Restor. Ecol. 2022, 30, 13518. [Google Scholar] [CrossRef]
- Risueño, Y.; Petri, C.; Conesa, H.M. A Critical Assessment on the Short-Term Response of Microbial Relative Composition in a Mine Tailings Soil Amended with Biochar and Manure Compost. J. Hazard. Mater. 2021, 417, 126080. [Google Scholar] [CrossRef]
- Chen, H.; Tang, L.; Wang, Z.; Su, M.; Tian, D.; Zhang, L.; Li, Z. Evaluating the Protection of Bacteria from Extreme Cd (II) Stress by P-Enriched Biochar. Environ. Pollut. 2020, 263, 114483. [Google Scholar] [CrossRef]
- Fellet, G.; Marchiol, L.; Delle Vedove, G.; Peressotti, A. Application of Biochar on Mine Tailings: Effects and Perspectives for Land Reclamation. Chemosphere 2011, 83, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Yao, J.; Jordan, G.; Roha, B.; Min, N.; Li, H.; Lu, C.; Arundo Donax, L. Stem-Derived Biochar Increases As and Sb Toxicities from Nonferrous Metal Mine Tailings. Environ. Sci. Pollut. Res. 2020, 27, 2433–2443. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Majumder, C.B.; Mohanty, B. Treatment of Arsenic Contaminated Water in a Batch Reactor by Using Ralstonia Eutropha MTCC 2487 and Granular Activated Carbon. J. Hazard. Mater. 2008, 153, 588–599. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; García-Figueroa, A.C.; Amábilis-Sosa, L.E.; Molina-Freaner, F.E.; Pat-Espadas, A.M. Stabilization of Potentially Toxic Elements Contained in Mine Waste: A Microbiological Approach for the Environmental Management of Mine Tailings. J. Environ. Manag. 2020, 270, 110873. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, Y.; Yan, W.; Chen, L.; Dummi Mahadevan, G.; Zhao, F. Enhanced Bioleaching Efficiency of Metals from E-Wastes Driven by Biochar. J. Hazard. Mater. 2016, 320, 393–400. [Google Scholar] [CrossRef]
- Kadivar, S.; Pourhossein, F.; Mousavi, S.M. Recovery of Valuable Metals from Spent Mobile Phone Printed Circuit Boards Using Biochar in Indirect Bioleaching. J. Environ. Manag. 2021, 280, 111642. [Google Scholar] [CrossRef]
- Dong, Y.; Chong, S.; Lin, H. Enhanced Effect of Biochar on Leaching Vanadium and Copper from Stone Coal Tailings by Thiobacillus Ferrooxidans. Environ. Sci. Pollut. Res. 2022, 29, 20398–20408. [Google Scholar] [CrossRef]
- Tirry, N.; Tahri Joutey, N.; Sayel, H.; Kouchou, A.; Bahafid, W.; Asri, M.; El Ghachtouli, N. Screening of Plant Growth Promoting Traits in Heavy Metals Resistant Bacteria: Prospects in Phytoremediation. J. Genet. Eng. Biotechnol. 2018, 16, 613–619. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the Molecular Mechanisms for the Enhanced Phytoremediation of Heavy Metals through Plant Growth Promoting Rhizobacteria: A Review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Cai, Y.; Fu, S.; Luo, Y.; Wang, H.; Liang, C.; Lin, Z.; Hu, S.; Li, Y.; et al. Biochar Decreases Soil N2O Emissions in Moso Bamboo Plantations through Decreasing Labile N Concentrations, N-Cycling Enzyme Activities and Nitrification/Denitrification Rates. Geoderma 2019, 348, 135–145. [Google Scholar] [CrossRef]
- Anil, K.; Lakshmi, T. Phosphate Solubilization Potential and Phosphatase Activity of Rhizospheric Trichoderma spp. Braz. J. Microbiol. 2010, 41, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Gupta, G.; Jha, P.N. Assessment of Mineral Phosphate-Solubilizing Properties and Molecular Characterization of Zinc-Tolerant Bacteria. J. Basic Microbiol. 2012, 52, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Signes-Pastor, A.; Burló, F.; Mitra, K.; Carbonell-Barrachina, A.A. Arsenic Biogeochemistry as Affected by Phosphorus Fertilizer Addition, Redox Potential and PH in a West Bengal (India) Soil. Geoderma 2007, 137, 504–510. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Curtin, D. Soil Acidification and Liming Interactions with Nutrientand Heavy Metal Transformationand Bioavailability. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2003; Volume 78, pp. 215–272. ISBN 978-0-12-000796-7. [Google Scholar]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestier, C. Heavy Metal Toxicity: Cadmium Permeates through Calcium Channels and Disturbs the Plant Water Status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xue, C.; Nie, X.; Liu, Y.; Chen, F. Effects of Biochar Application on Soil Potassium Dynamics and Crop Uptake. J. Plant Nutr. Soil Sci. 2018, 181, 635–643. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Z.; Zhao, Y.; Gu, Y.; Wang, Y.; Yu, J.; Xu, H. The Performance of Biochar-Microbe Multiple Biochemical Material on Bioremediation and Soil Micro-Ecology in the Cadmium Aged Soil. Sci. Total Environ. 2019, 686, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Awasthi, S.K.; Liu, T.; Verma, S.; Zhang, Z.; Pandey, A.; Varjani, S.; Li, R.; Taherzadeh, M.J.; Awasthi, M.K. Patterns of Heavy Metal Resistant Bacterial Community Succession Influenced by Biochar Amendment during Poultry Manure Composting. J. Hazard. Mater. 2021, 420, 126562. [Google Scholar] [CrossRef]
- Khan, M.B.; Cui, X.; Jilani, G.; Tang, L.; Lu, M.; Cao, X.; Sahito, Z.A.; Hamid, Y.; Hussain, B.; Yang, X.; et al. New Insight into the Impact of Biochar during Vermi-Stabilization of Divergent Biowastes: Literature Synthesis and Research Pursuits. Chemosphere 2020, 238, 124679. [Google Scholar] [CrossRef]
- Li, R.; Wang, Q.; Zhang, Z.; Zhang, G.; Li, Z.; Wang, L.; Zheng, J. Nutrient Transformation during Aerobic Composting of Pig Manure with Biochar Prepared at Different Temperatures. Environ. Technol. 2015, 36, 815–826. [Google Scholar] [CrossRef]
- Cui, E.; Wu, Y.; Zuo, Y.; Chen, H. Effect of Different Biochars on Antibiotic Resistance Genes and Bacterial Community during Chicken Manure Composting. Bioresour. Technol. 2016, 203, 11–17. [Google Scholar] [CrossRef]
- Song, X.; Liu, M.; Wu, D.; Qi, L.; Ye, C.; Jiao, J.; Hu, F. Heavy Metal and Nutrient Changes during Vermicomposting Animal Manure Spiked with Mushroom Residues. Waste Manag. 2014, 34, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Cui, X.; Jilani, G.; Lazzat, U.; Zehra, A.; Hamid, Y.; Hussain, B.; Tang, L.; Yang, X.; He, Z. Eisenia Fetida and Biochar Synergistically Alleviate the Heavy Metals Content during Valorization of Biosolids via Enhancing Vermicompost Quality. Sci. Total Environ. 2019, 684, 597–609. [Google Scholar] [CrossRef]
- Hu, B.; Song, Y.; Wu, S.; Zhu, Y.; Sheng, G. Slow Released Nutrient-Immobilized Biochar: A Novel Permeable Reactive Barrier Filler for Cr(VI) Removal. J. Mol. Liq. 2019, 286, 110876. [Google Scholar] [CrossRef]
- Kumarasinghe, U.; Kawamoto, K.; Saito, T.; Sakamoto, Y.; Mowjood, M.I.M. Evaluation of Applicability of Filling Materials in Permeable Reactive Barrier (PRB) System to Remediate Groundwater Contaminated with Cd and Pb at Open Solid Waste Dump Sites. Process Saf. Environ. Prot. 2018, 120, 118–127. [Google Scholar] [CrossRef]
- Roé-Sosa, A.; Rangel-Peraza, J.G.; Rodríguez-Mata, A.E.; Pat-Espadas, A.; Bustos-Terrones, Y.; Diaz-Peña, I.; Vu, C.M.; Amabilis-Sosa, L.E. Emulating Natural Wetlands Oxygen Conditions for the Removal of N and P in Agricultural Wastewaters. J. Environ. Manag. 2019, 236, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Pat-Espadas, A.; Loredo Portales, R.; Amabilis-Sosa, L.; Gómez, G.; Vidal, G. Review of Constructed Wetlands for Acid Mine Drainage Treatment. Water 2018, 10, 1685. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Behera, S.S.; Patra, J.K.; Thatoi, H.; Parhi, P.K. Chapter 17—Potential Application of Bacterial Biofilm for Bioremediation of Toxic Heavy Metals and Dye-Contaminated Environments. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms; Yadav, M.K., Singh, B.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–281. ISBN 9780444642790. [Google Scholar]
- Kasak, K.; Truu, J.; Ostonen, I.; Sarjas, J.; Oopkaup, K.; Paiste, P.; Kõiv-Vainik, M.; Mander, Ü.; Truu, M. Biochar Enhances Plant Growth and Nutrient Removal in Horizontal Subsurface Flow Constructed Wetlands. Sci. Total Environ. 2018, 639, 67–74. [Google Scholar] [CrossRef]
- Chen, J.; Deng, S.; Jia, W.; Li, X.; Chang, J. Removal of Multiple Heavy Metals from Mining-Impacted Water by Biochar-Filled Constructed Wetlands: Adsorption and Biotic Removal Routes. Bioresour. Technol. 2021, 331, 125061. [Google Scholar] [CrossRef]
- Kataki, S.; Chatterjee, S.; Vairale, M.G.; Dwivedi, S.K.; Gupta, D.K. Constructed Wetland, an Eco-Technology for Wastewater Treatment: A Review on Types of Wastewater Treated and Components of the Technology (Macrophyte, Biolfilm and Substrate). J. Environ. Manag. 2021, 283, 111986. [Google Scholar] [CrossRef]
- Austin, G.; Yu, K. Constructed Wetlands and Sustainable Development; Routledge: London, UK, 2016; ISBN 9781315694221. [Google Scholar]
- Stefanakis, A. Constructed Wetlands for Industrial Wastewater Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; ISBN 9781119268321. [Google Scholar]
- Huang, J.; Wang, J.; Wang, S.; Guo, S. Different Biochars as Microbial Immobilization Substrates for Efficient Copper II Removal. Spectrosc. Lett. 2020, 53, 712–725. [Google Scholar] [CrossRef]
- Yu, G.; Li, P.; Wang, G.; Wang, J.; Zhang, Y.; Wang, S.; Yang, K.; Du, C.; Chen, H. A Review on the Removal of Heavy Metals and Metalloids by Constructed Wetlands: Bibliometric, Removal Pathways, and Key Factors. World J. Microbiol. Biotechnol. 2021, 37, 157. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and Molecular Mechanisms of Plant-Microbe-Metal Interactions: Relevance for Phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, W.; Zhang, Z.; Qin, F.; Jiang, J.; He, A.; Sheng, G.D. Enhanced Removal of Cadmium from Wastewater with Coupled Biochar and Bacillus subtilis. Water Sci. Technol. 2021, 83, 2075–2086. [Google Scholar] [CrossRef]
- Huang, F.; Li, K.; Wu, R.-R.; Yan, Y.-J.; Xiao, R.-B. Insight into the Cd2+ Biosorption by Viable Bacillus Cereus RC-1 Immobilized on Different Biochars: Roles of Bacterial Cell and Biochar Matrix. J. Clean. Prod. 2020, 272, 122743. [Google Scholar] [CrossRef]
- Amabilis-Sosa, L.E.; Siebe, C.; Moeller-Chávez, G.; Durán-Domínguez-de-Bazúa, M. del C. Accumulation and Distribution of Lead and Chromium in Laboratory-Scale Constructed Wetlands Inoculated with Metal-Tolerant Bacteria. Int. J. Phytoremediat. 2015, 17, 1090–1096. [Google Scholar] [CrossRef]
- Yu, G.; Wang, G.; Li, J.; Chi, T.; Wang, S.; Peng, H.; Chen, H.; Du, C.; Jiang, C.; Liu, Y.; et al. Enhanced Cd2+ and Zn2+ Removal from Heavy Metal Wastewater in Constructed Wetlands with Resistant Microorganisms. Bioresour. Technol. 2020, 316, 123898. [Google Scholar] [CrossRef]
- Mota, R.; Rossi, F.; Andrenelli, L.; Pereira, S.B.; De Philippis, R.; Tamagnini, P. Released Polysaccharides (RPS) from Cyanothece Sp. CCY 0110 as Biosorbent for Heavy Metals Bioremediation: Interactions between Metals and RPS Binding Sites. Appl. Microbiol. Biotechnol. 2016, 100, 7765–7775. [Google Scholar] [CrossRef]


| Biochar–Microorganism Interaction | Mechanism of Metal (Loid) Stabilization | Key Biochar Properties | Factors Determining Key Biochar Properties |
|---|---|---|---|
| Electron-shuttling-mediated reactions. Biochar-mediated interspecies electron transfer. |
|
|
|
| Microbial shelter. | Growth stimulation of key microorganisms catalyzing metal transformations. |
|
|
| Biofilm development on biochar’s surface. | Serving as an attachment surface for the development of biofilms of key microorganisms catalyzing metal transformations. |
|
|
| Decrease in heavy metal toxicity towards microorganisms. | Facilitation of microbial growth and metabolic activity by diminishing metal/metalloid toxicity. |
|
|
| Indirectly improving key microbial activity by improving the soil quality. | Facilitation of microbial growth and metabolic activity by improving soil quality. |
|
|
| Parameter | Biochar | Constructed Wetland | Constructed Wetland Filled with Biochar (10% v/v) | Observations and References | |
|---|---|---|---|---|---|
| Whole System | Package Media | ||||
| Porosity | 0.8–0.11 | 0.3–0.55 | 0.27–0.39 | n = 10 [52,61,75,85,89,91,110,111,119,120] | |
| Hydraulic conductivity, mm3/(mm2 d) | 0.9–5.7 | 38–87 | 2.6–43 | n = 10 [52,61,75,85,89,91,110,111,119,120] | |
| Pb removal | 2.4–12.5 mg/g | 44.2–68% | 47–52% 1.2–5.4 mg/g | 67–76% | n = 6 Biochar < 500 °C [10,11,21,29,52,61] |
| Pb removal | 6.1–42.5 mg/g | 26–52% | 12–21% 1.2–5.4 mg/g | 56–71% | n = 6 Biochar > 500 °C [10,11,21,29,52,75] |
| Cr removal | 3–27 mg/g | 35–55.8% | 14–19% 1.9–6 mg/g | 41–65% | n = 8 [46,55,56,57,58,59,61,107] |
| Cu removal | 0.5–14 mg/g | 8–36% | 5–28% 0.7–10.3 mg/g | 22–45% | n = 7 [27,61,75,85,91,104,110] |
| Hg removal | 0.5–2.3 mg/g | 5–75% | 0.5–53% 0.2–1.3 mg/g | 12–83% | n = 3 [110,111,120] |
| Cd removal | 0.6–40 mg/g | 35–67% | 18.6–57.6% 0.5–11.5 mg/g | 45–76% | n = 9 [14,20,72,101,105,106,119,120] |
| Zn removal | 4.5–38 mg/g | 57–100% | 1.2–63% 4.2–13 mg/g | 67–100% | n = 4 [52,75,110,119] |
| As removal | 1.8–3.2 mg/g | 75–99% | 1.4–43% 0.2–1.7 mg/g | 79–99% | n = 4 [55,57,110,119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amabilis-Sosa, L.E.; Valenzuela, E.I.; Quezada-Renteria, J.A.; Pat-Espadas, A.M. Biochar-Assisted Bioengineered Strategies for Metal Removal: Mechanisms, Key Considerations, and Perspectives for the Treatment of Solid and Liquid Matrixes. Sustainability 2022, 14, 17049. https://doi.org/10.3390/su142417049
Amabilis-Sosa LE, Valenzuela EI, Quezada-Renteria JA, Pat-Espadas AM. Biochar-Assisted Bioengineered Strategies for Metal Removal: Mechanisms, Key Considerations, and Perspectives for the Treatment of Solid and Liquid Matrixes. Sustainability. 2022; 14(24):17049. https://doi.org/10.3390/su142417049
Chicago/Turabian StyleAmabilis-Sosa, Leonel E., Edgardo I. Valenzuela, Javier A. Quezada-Renteria, and Aurora M. Pat-Espadas. 2022. "Biochar-Assisted Bioengineered Strategies for Metal Removal: Mechanisms, Key Considerations, and Perspectives for the Treatment of Solid and Liquid Matrixes" Sustainability 14, no. 24: 17049. https://doi.org/10.3390/su142417049
APA StyleAmabilis-Sosa, L. E., Valenzuela, E. I., Quezada-Renteria, J. A., & Pat-Espadas, A. M. (2022). Biochar-Assisted Bioengineered Strategies for Metal Removal: Mechanisms, Key Considerations, and Perspectives for the Treatment of Solid and Liquid Matrixes. Sustainability, 14(24), 17049. https://doi.org/10.3390/su142417049








