Ammonia Recovery from Organic Waste Digestate via Gas–Liquid Stripping: Application of the Factorial Design of Experiments and Comparison of the Influence of the Stripping Gas
Abstract
1. Introduction
2. Materials and Methods
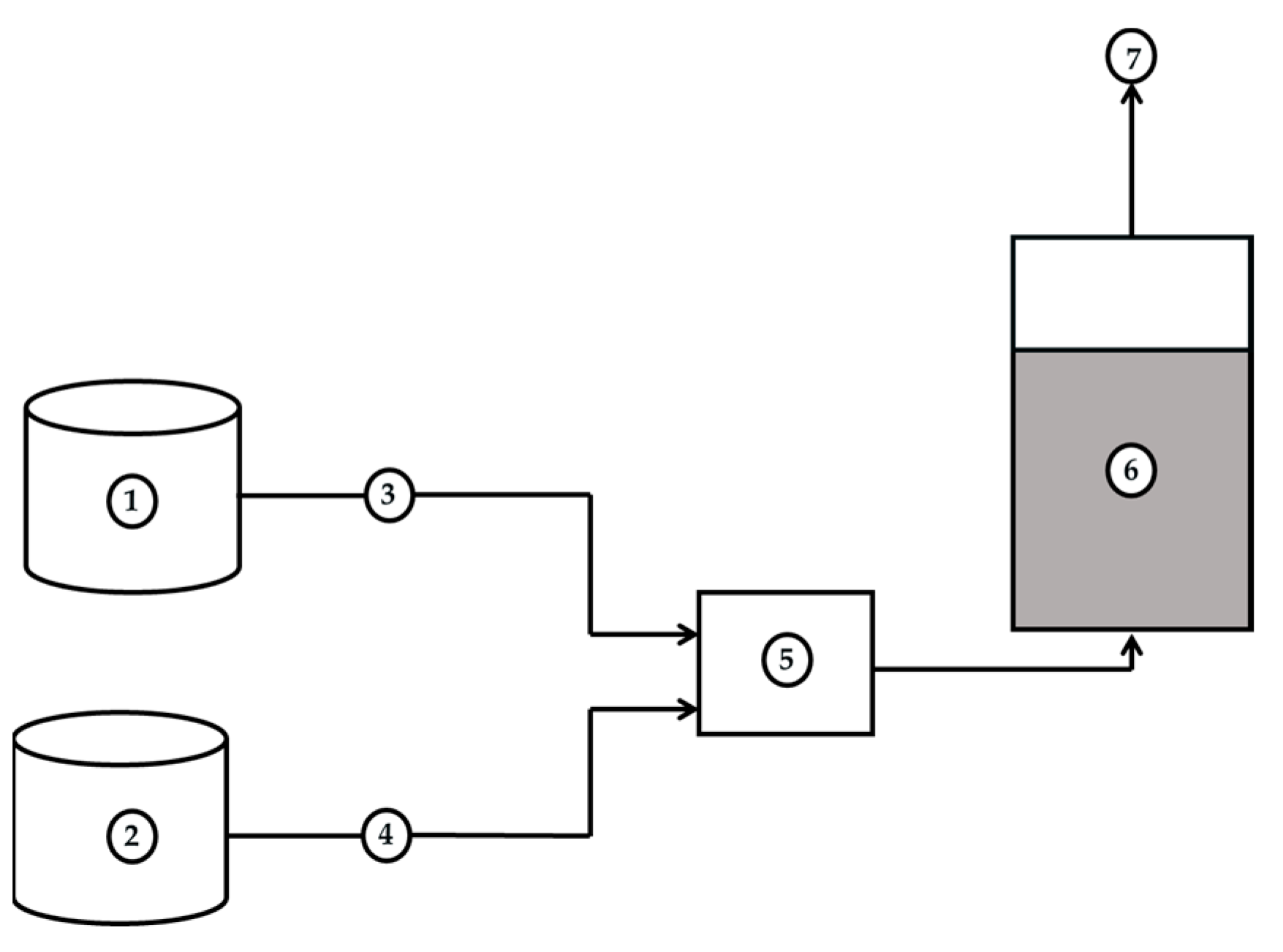
2.1. Experimental Setup and Procedure
2.2. Design of Experiments
2.3. Biochemical Parameters
2.4. Measurement of Error in the Stripping Process
2.5. Calculations
- (i)
- Ammonia Recovery
- (ii)
- Fraction of free ammonia (F)
3. Results and Discussion
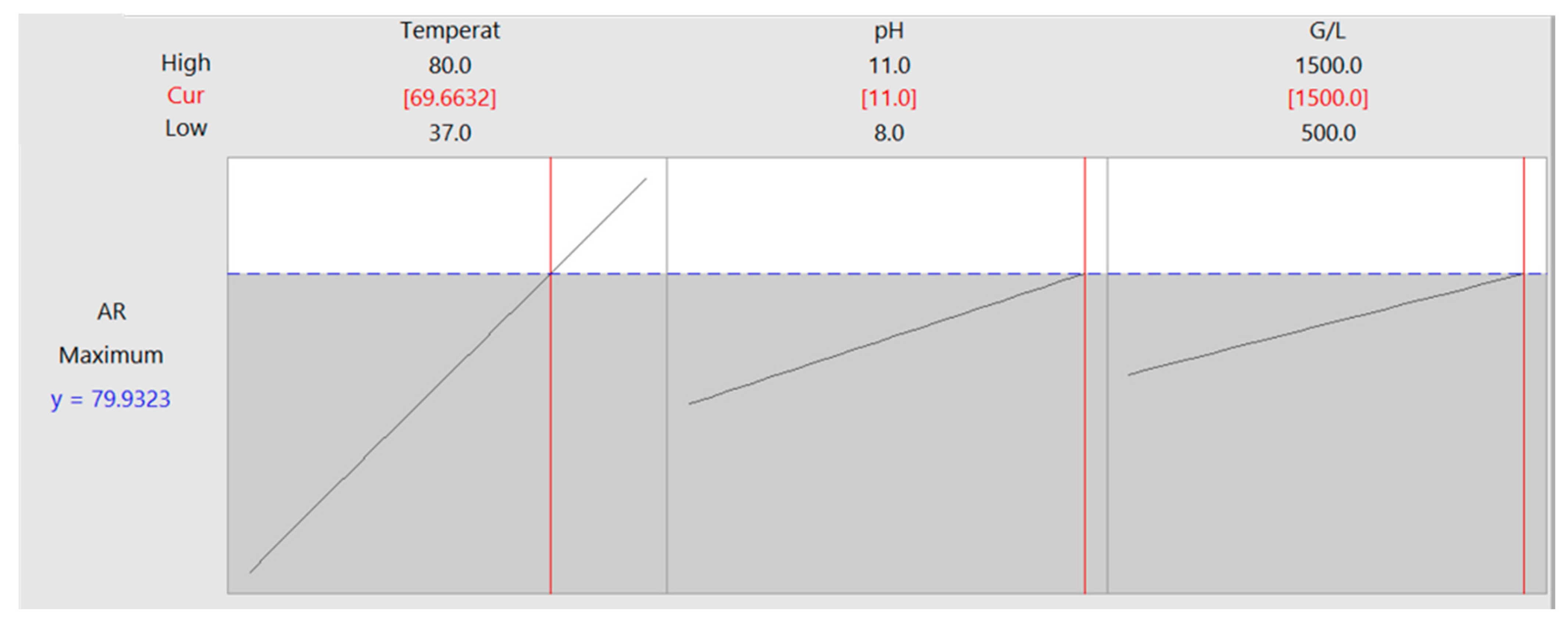
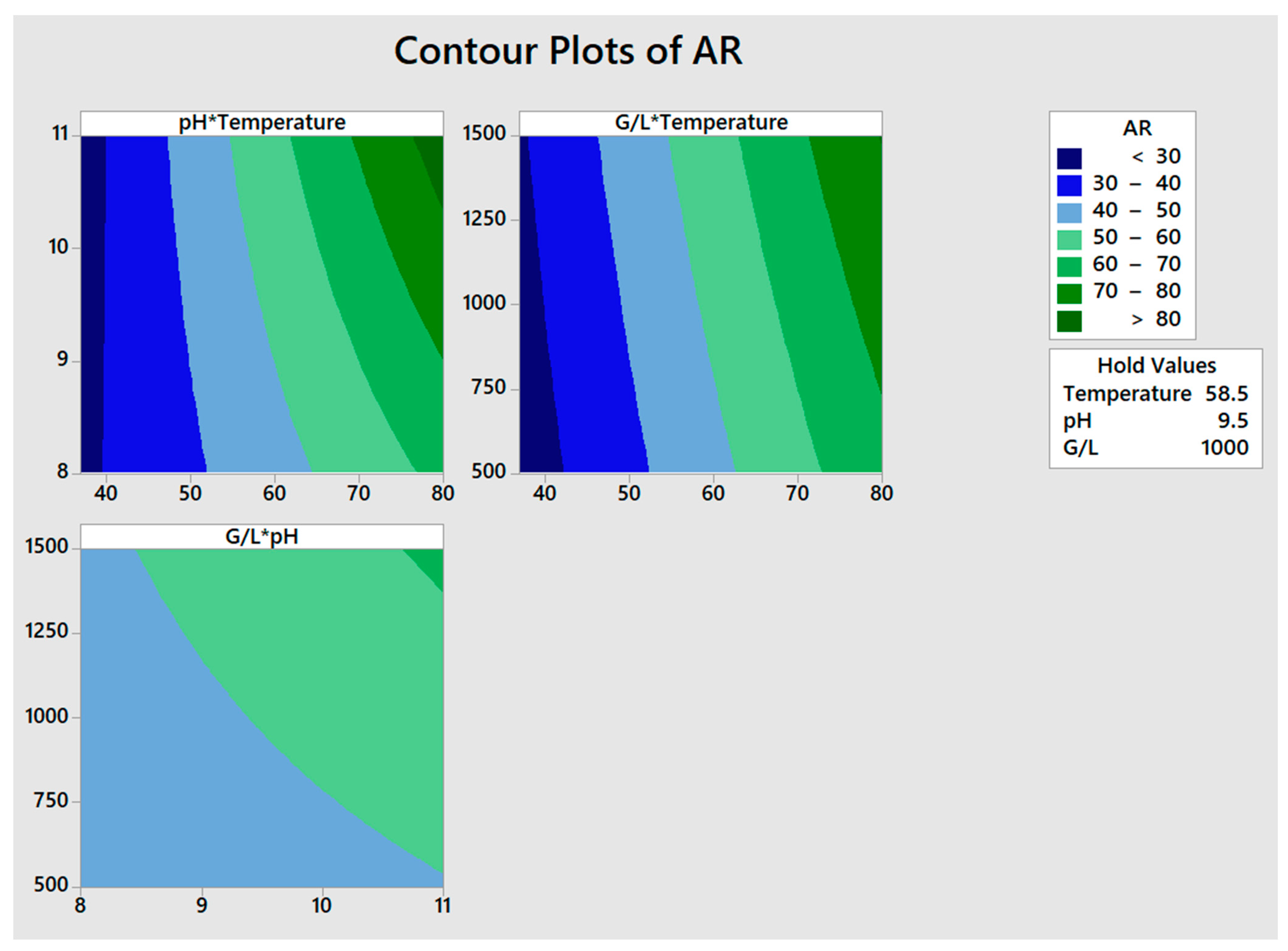
3.1. Factorial Design of Experiments
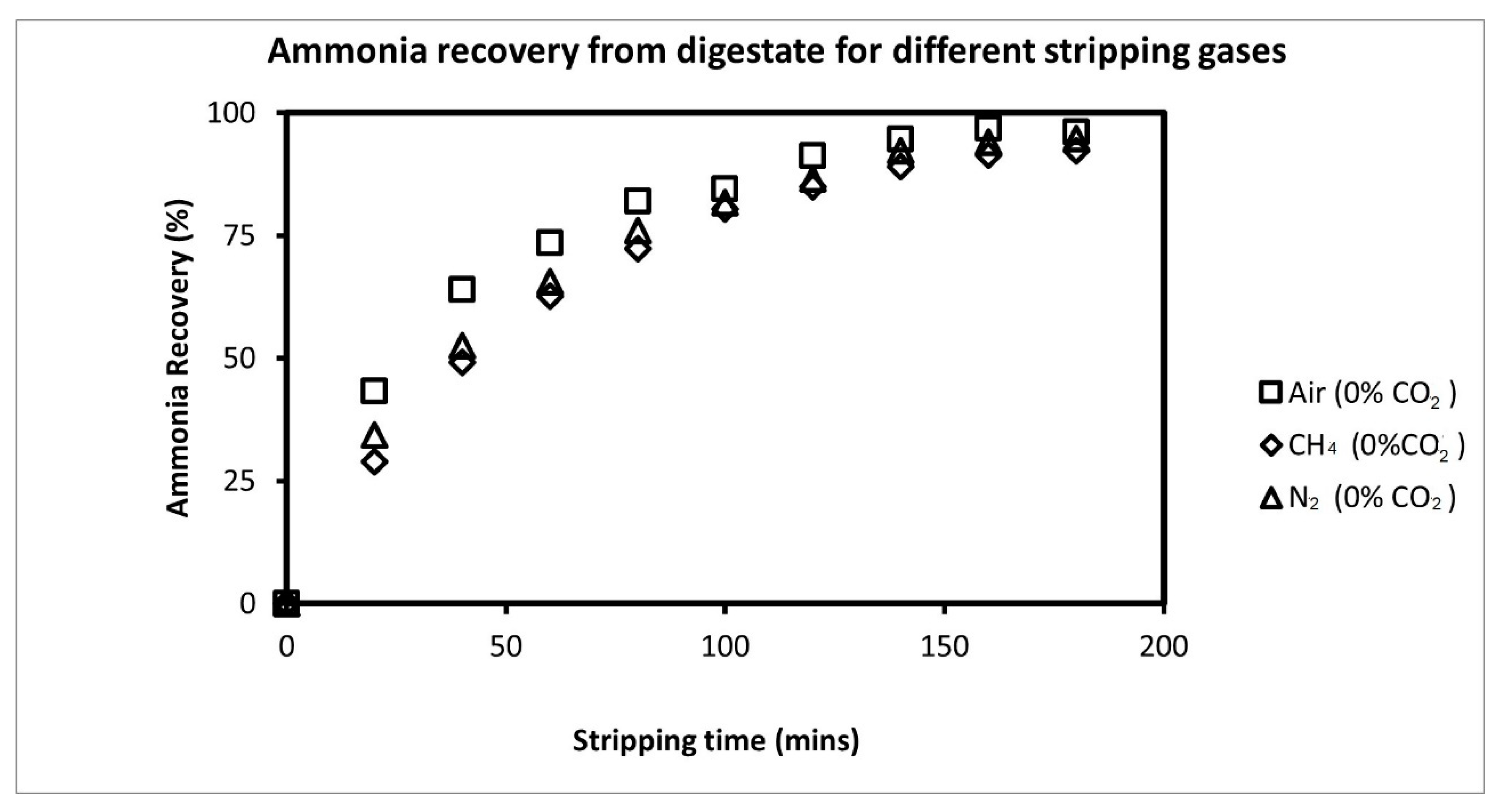
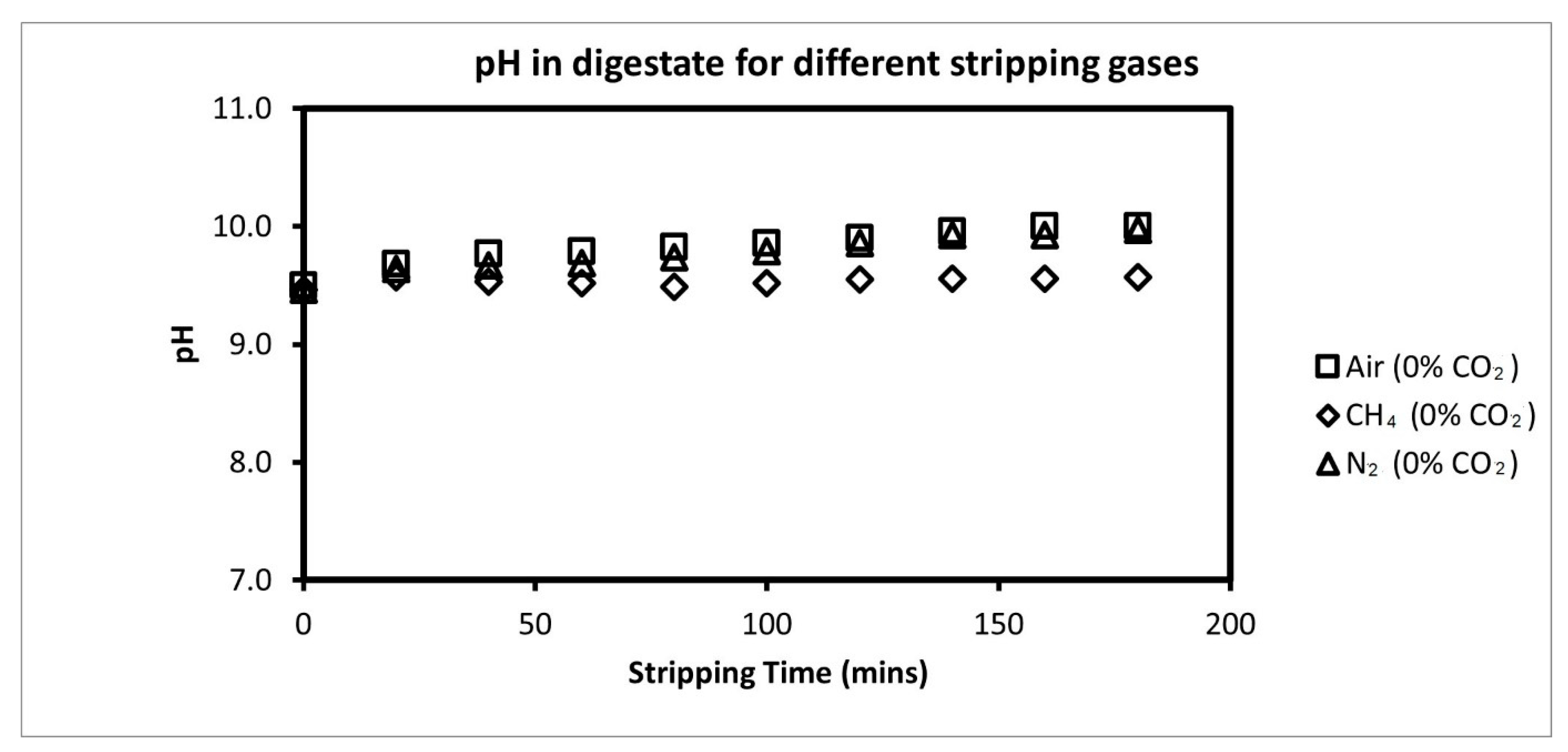
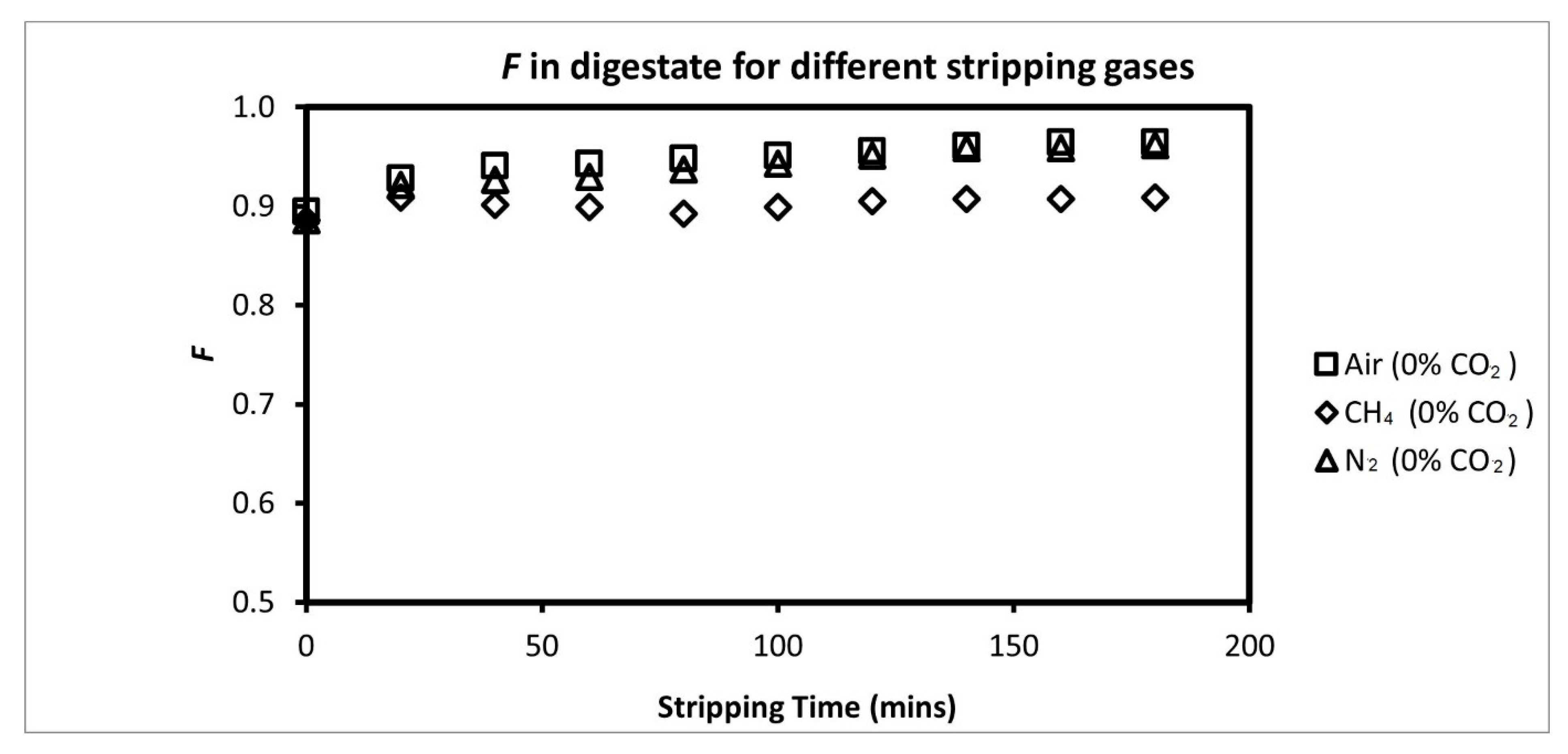
3.2. Comparison of Ammonia Recovery from Different Stripping Gases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.; Li, L.; Peng, Y.; Yang, P.; Peng, X.; Sun, Y.; Wang, X. State indicators of anaerobic digestion: A critical review on process monitoring and diagnosis. Renew. Sustain. Energy Rev. 2021, 148, 111260. [Google Scholar] [CrossRef]
- Gallert, C.; Winter, J. Mesophilic and thermophilic anaerobic digestion of source-sorted organic wastes: Effect of ammonia on glucose degradation and methane production. Appl. Microbiol. Biotechnol. 1997, 48, 405–410. [Google Scholar] [CrossRef]
- Krakat, N.; Anjum, R.; Dietz, D.; Demirel, B. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef]
- Leverenz, H.; Adams, R.; Hazard, J.; Tchobanoglous, G. Continuous thermal stripping process for ammonium removal from digestate and centrate. Sustainability 2021, 13, 2185. [Google Scholar] [CrossRef]
- Huang, H.; He, L.; Zhang, Z.; Lei, Z.; Liu, R.; Zheng, W. Enhanced biogasification from ammonia-rich swine manure pretreated by ammonia fermentation and air stripping. Int. Biodeterior. Biodegrad. 2019, 140, 84–89. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Angelidaki, I. Strategies for optimizing recovery of the biogas process following ammonia inhibition. Bioresour. Technol. 2008, 99, 7995–8001. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Backes, A.M.; Aulinger, A.; Bieser, J.; Matthias, V.; Quante, M. Ammonia emissions in Europe, part II: How ammonia emission abatement strategies affect secondary aerosols. Atmos. Environ. 2016, 126, 153–161. [Google Scholar] [CrossRef]
- Deng, Z.; Linden NVan Guillen, E.; Spanjers, H.; Van Lier, J.B. Recovery and applications of ammoniacal nitrogen from nitrogen-loaded residual streams: A review. J. Environ. Manag. 2021, 295, 113096. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Serna-Maza, A.; Heaven, S.; Banks, C.J. Biogas stripping of ammonia from fresh digestate from a food waste digester. Bioresour. Technol. 2015, 190, 66–75. [Google Scholar] [CrossRef]
- Palakodeti, A.; Azman, S.; Rossi, B.; Dewil, R.; Appels, L. A critical review of ammonia recovery from anaerobic digestate of organic wastes via stripping. Renew. Sustain. Energy Rev. 2021, 143, 110903. [Google Scholar] [CrossRef]
- Srinath, E.G.; Loehr, R.C. Ammonia desorption by diffused aeration. Water Pollut. Control Fed. 1974, 46, 1939–1957. [Google Scholar]
- Georgiou, D.; Liliopoulos, V.; Aivasidis, A. Investigation of an integrated treatment technique for anaerobically digested animal manure: Lime reaction and settling, ammonia stripping and neutralization by biogas scrubbing. Bioresour. Technol. Rep. 2019, 5, 127–133. [Google Scholar] [CrossRef]
- Baldi, M.; Collivignarelli, M.C.; Abbà, A.; Benigna, I. The valorization of ammonia in manure digestate by means of alternative stripping reactors. Sustainability 2018, 10, 3073. [Google Scholar] [CrossRef]
- Yellezuome, D.; Zhu, X.; Wang, Z.; Liu, R. Mitigation of ammonia inhibition in anaerobic digestion of nitrogen-rich substrates for biogas production by ammonia stripping: A review. Renew. Sustain. Energy Rev. 2022, 157, 112043. [Google Scholar] [CrossRef]
- Bonmatı, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion August. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Ni, J. Mechanistic models of ammonia release from liquid manure: A review. J. Agric. Eng. Res. 1999, 72, 1–17. [Google Scholar] [CrossRef]
- Jamaludin, Z.; Rollings-Scattergood, S.; Lutes, K.; Vaneeckhaute, C. Evaluation of sustainable scrubbing agents for ammonia recovery from anaerobic digestate. Bioresour. Technol. 2018, 270, 596–602. [Google Scholar] [CrossRef]
- Kinidi, L.; Tan, I.A.W.; Wahab, N.B.A.; Fikri, K.; Hipolito, C.N.; Salleh, S.F. Recent Development in Ammonia Stripping Process for Industrial Wastewater Treatment. Int. J. Chem. Eng. 2018, 2018, 3181087. [Google Scholar] [CrossRef]
- Liu, L.; Pang, C.; Wu, S.; Dong, R. Optimization and evaluation of an air-recirculated stripping for ammonia removal from the anaerobic digestate of pig manure. Process Saf. Environ. Prot. 2015, 94, 350–357. [Google Scholar] [CrossRef]
- Bi, S.; Qiao, W.; Xiong, L.; Mahdy, A.; Wandera, S.M.; Yin, D.; Dong, R. Improved high solid anaerobic digestion of chicken manure by moderate in situ ammonia stripping and its relation to metabolic pathway. Renew. Energy 2020, 146, 2380–2389. [Google Scholar] [CrossRef]
- Hafner, S.D.; Bisogni, J.J. Modeling of ammonia speciation in anaerobic digesters. Water Res. 2009, 43, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Budzianowski, W.; Koziol, A. Stripping of Ammonia from Aqueous Solutions in the Presence of Carbon Dioxide. Chem. Eng. Res. Des. 2005, 83, 196–204. [Google Scholar] [CrossRef]
- Hafner, S.D.; Montes, F.; Alan Rotz, C. The role of carbon dioxide in emission of ammonia from manure. Atmos. Environ. 2013, 66, 63–71. [Google Scholar] [CrossRef]
- Lazić, Ž.R. Design of Experiments in Chemical Engineering; Wiley VCH GmbH: Weinheim, Germany, 2005. [Google Scholar] [CrossRef]
- Douglas, C.M. Design and Analysis of Experiments, 5th ed.; John Wiley and Sons: Hoboken, NJ, USA, 1997. [Google Scholar] [CrossRef]
- Sambusiti, C.; Monlau, F.; Ficara, E.; Musatti, A.; Rollini, M.; Barakat, A.; Melpei, F. Comparison of various post-treatments for recovering methane from agricultural digestate. Fuel Process. Technol. 2015, 137, 359–365. [Google Scholar] [CrossRef]
- Jiang, A.; Zhang, T.; Zhao, Q.B.; Li, X.; Chen, S.; Frear, C.S. Evaluation of an integrated ammonia stripping, recovery, and biogas scrubbing system for use with anaerobically digested dairy manure. Biosyst. Eng. 2014, 119, 117–126. [Google Scholar] [CrossRef]
- Zhao, Q.-B.; Ma, J.; Zeb, I.; Yu, L.; Chen, S.; Zheng, Y.-M.; Frear, C. Ammonia recovery from anaerobic digester effluent through direct aeration. Chem. Eng. J. 2015, 279, 31–37. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.W.; Jahng, D. Ammonia stripping for enhanced biomethanization of piggery wastewater. J. Hazard. Mater. 2012, 199, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Provolo, G.; Perazzolo, F.; Mattachini, G.; Finzi, A.; Naldi, E.; Riva, E. Nitrogen removal from digested slurries using a simplified ammonia stripping technique. Waste Manag. 2017, 69, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Iyer, K.; Heaven, S.; Banks, C.J. Ammonia removal in anaerobic digestion by biogas stripping: An evaluation of process alternatives using a first order rate model based on experimental findings. Chem. Eng. J. 2011, 178, 138–145. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 1995. [Google Scholar] [CrossRef]
- Bousek, J.; Scroccaro, D.; Sima, J.; Weissenbacher, N.; Fuchs, W. Influence of the gas composition on the efficiency of ammonia stripping of biogas digestate. Bioresour. Technol. 2015, 203, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xin, Y.; Shi, H.; Ai, P.; Yu, L.; Li, X.; Chen, S. Closing ammonia loop in efficient biogas production: Recycling ammonia pretreatment of wheat straw. Biosyst. Eng. 2019, 180, 182–190. [Google Scholar] [CrossRef]
- Liquide, A. Air Liquide Product Catalogue 2022. Available online: https://mygas.airliquide.be/catalog-gas-products (accessed on 18 October 2022).
- Saadabadi, S.A.; Thallam Thattai, A.; Fan, L.; Lindeboom, R.E.F.; Spanjers, H.; Aravind, P.V. Solid Oxide Fuel Cells fuelled with biogas: Potential and constraints. Renew. Energy 2019, 134, 194–214. [Google Scholar] [CrossRef]
| Run | Temperature (°C) | pH | G/L | Ammonia Recovery (%) |
|---|---|---|---|---|
| 1 | 80 | 11 | 1500 | 97 |
| 2 | 80 | 8 | 500 | 61 |
| 3 | 37 | 11 | 500 | 26 |
| 4 | 37 | 8 | 1500 | 32 |
| 5 | 37 | 8 | 500 | 24 |
| 6 | 80 | 8 | 1500 | 64 |
| 7 | 80 | 11 | 500 | 73 |
| 8 | 37 | 11 | 1500 | 26 |
| 9 | 57 | 9.5 | 1000 | 47 |
| Parameter | Main Effects | Regression Coefficients |
|---|---|---|
| Temperature | 46.75 | 23.38 |
| pH | 10.25 | 5.13 |
| G/L | 8.75 | 4.38 |
| Temperature * pH | 12.25 | 6.13 |
| pH * G/L | 3.25 | 1.63 |
| Temperature * G/L | 4.75 | 2.37 |
| Ammonia Recovery (%) | |||
|---|---|---|---|
| % CO2 | Air + CO2 | CH4 + CO2 | N2 + CO2 |
| 0 | 96 | 92 | 94 |
| 20 | 66 | 64 | 70 |
| 40 | 50 | 57 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palakodeti, A.; Azman, S.; Dewil, R.; Appels, L. Ammonia Recovery from Organic Waste Digestate via Gas–Liquid Stripping: Application of the Factorial Design of Experiments and Comparison of the Influence of the Stripping Gas. Sustainability 2022, 14, 17000. https://doi.org/10.3390/su142417000
Palakodeti A, Azman S, Dewil R, Appels L. Ammonia Recovery from Organic Waste Digestate via Gas–Liquid Stripping: Application of the Factorial Design of Experiments and Comparison of the Influence of the Stripping Gas. Sustainability. 2022; 14(24):17000. https://doi.org/10.3390/su142417000
Chicago/Turabian StylePalakodeti, Advait, Samet Azman, Raf Dewil, and Lise Appels. 2022. "Ammonia Recovery from Organic Waste Digestate via Gas–Liquid Stripping: Application of the Factorial Design of Experiments and Comparison of the Influence of the Stripping Gas" Sustainability 14, no. 24: 17000. https://doi.org/10.3390/su142417000
APA StylePalakodeti, A., Azman, S., Dewil, R., & Appels, L. (2022). Ammonia Recovery from Organic Waste Digestate via Gas–Liquid Stripping: Application of the Factorial Design of Experiments and Comparison of the Influence of the Stripping Gas. Sustainability, 14(24), 17000. https://doi.org/10.3390/su142417000








