Abstract
The fluctuations in Meloidogyne densities and fungal egg parasitism were determined from February 2015 to July 2016 in four vegetable production sites conducted under organic production and two sides conducted under integrated standards. At each site, the soil nematode densities at transplanting and at the end of the crops, the galling index, the number of eggs in roots, and the percentage of fungal egg parasitism were determined, and the fungal species were identified. In addition, two pot experiments were conducted with soil taken from each site in February 2015 and 2016 to assess the fungal egg parasitism comparing non-sterile and sterile soil from each site. In field conditions, the nematode population densities in the soil decreased along the crop rotations. The maximum number of eggs per plant was recorded in the spring–summer crops. Egg parasitism ranged from 11.2 to 55% in the organic sites and from 0.8 to 16.5% in the integrated production sites. Pochonia chlamydosporia was the only fungal species isolated in five of the six sites. In both pot experiments, the number of eggs per plant was lower in non-sterile than in sterile soils, except for the M10.45 site, where fungal egg parasites were not recovered. P. chlamydosporia was the only fungal species isolated, ranging between 11 and 74%. Therefore, P. chlamidosporia was the most prevalent fungal species related to Meloidogyne suppression.
1. Introduction
Root-knot nematodes (RKN), Meloidogyne spp., are the most damaging plant-parasitic nematodes for crops worldwide [1]. The majority of the horticultural crops are hosts of the most widespread and damaging RKN species: M. arenaria, M. incognita, and M. javanica [2], reaching estimated maximum yield losses of 88% and 98% in tomato and melon, respectively, in Spain [3,4]. These RKN species reproduce by parthenogenesis. Their life cycle consists of six biological stages: the egg, four juvenile stages (J1–J4), and the adult. The infective second-stage juveniles (J2) enter the root near the elongation zone and migrate intercellularly to circumvent the endodermal barriers and establish a permanent feeding site in the vascular cylinder. The J2 induces the formation of giant cells and root galls by affecting plant physiology and metabolism [5]. Inside the roots, the J2 becomes sedentary and molts three times to achieve the mature adult female stage, which produces eggs within a gelatinous matrix located mainly outside the root galls at low levels of soil infestation or inside the roots at mid/high levels of soil infestation due to the large volume of root galls.
RKN management strategies tend to reduce the dependence on chemical nematicides and soil fumigants by encouraging alternative control methods and promoting the conservation and enhancement of beneficial organisms, improving the antagonistic potential of soil, to achieve high levels of soil suppressiveness. The antagonistic potential of a soil is defined as “the capacity to reduce the spread of deleterious agents to plants through biotic factors” [6]. Several nematode antagonists can occur naturally in agricultural soils, providing some extent of suppressiveness to RKN [7]. High levels of soil suppressiveness can be achieved under favorable plant–RKN–antagonist interactions, cultural practices, and abiotic factors [8]. Suppressive soils, described as “soils in which soil-borne pathogens do not establish or persist, establish but cause little or no damage, or establish and cause disease for a limited period of time” [9], can exercise a general or specific suppression if it is conferred by several or a narrow number of antagonists, respectively [10]. In contrast, non-suppressive or conducive soils are those where pathogens readily cause diseases [11,12]. A few RKN-suppressive soils conferred by fungal antagonists have been described [8,13,14]. Recently, Topalović et al. [15] demonstrated M. hapla suppression by the total microbiome soil community, but the responsible microbial species were not elucidated. The majority of studies that demonstrate the level of soil suppressiveness were conducted in pot tests with soil samples taken at a single time [13,14,15]. However, only a few of them studied the level of soil suppressiveness over time, determining the fluctuations in nematode densities in field conditions and contrasting the suppressiveness in pot tests using soil samples taken in different years. Giné et al. [8] studied the RKN soil suppressiveness at two organic vegetable growing sites conducted under protected cultivation. At one site, the nematode densities decreased progressively until they reached undetectable levels, and at the other site disease severity was low, although the nematode densities at transplanting were high. At both sites, Pochonia chlamydosporia was the only fungus isolated and identified from RKN eggs. Other studies determined the presence of fungal RKN antagonists in different agricultural production areas in Spain, with Fusarium spp. and P. chlamydosporia being the most frequent out of more than 20 isolated fungal species [16,17,18,19,20]. In 2010, a survey conducted at 40 vegetable growing sites showed fungal egg parasitism at all organic production sites at percentages ranging from 4 to 80% and at 73.3% of the integrated production sites, with percentages ranging from 2 to 69% [20].
The aim of the present study was to characterize the soil suppressiveness by determining the fluctuations in RKN densities in fields over two years at 6 of the 40 sites previously studied in Giné et al. [20]: 4 sites conducted under organic production, with percentages of fungal egg parasitism between 9% and 80%, and 2 sites conducted under integrated production, with percentages of fungal egg parasitism of 6% and 69%. Moreover, the level of soil suppressiveness was also determined in pot tests.
2. Materials and Methods
2.1. Fluctuations in RKN Densities and Egg Parasitism in Field Conditions
This study was performed from winter–spring 2015 to summer 2016 in six vegetable production sites located in the Tarragona and Barcelona provinces in northeastern Spain. Four sites (M10.16, M10.41, M10.55, and M10.56) were conducted under organic production standards, and two sites (M10.43 and M10.45) were conducted under integrated production (Table 1). The crops at sites M10.16, M10.43, and M10.45 were grown in open fields, and the rest were grown inside plastic greenhouses. These sites were selected because fungal egg parasites of Meloidogyne were detected, isolated, and identified, and the microbiome associated to the J2 cuticle was determined in previous studies [20,21]. Moreover, site M10.55 was also characterized as suppressive to RKN [8]. The physicochemical properties of these soils are shown in Table 1. In sites conducted under organic production, crop residues were incorporated as green manure at the end of each crop, and crops were fertilized before transplant with composted sheep or cow manure alone or combined with chicken manure at a total rate of 1.7–2 kg m−2. The cropping sequences included crops belonging to the Brassicaceae, Chenopodiaceae, Compositae, Cucurbitaceae, and Solanaceae families. In sites conducted under integrated production, fertilization was performed with humus combined with chemical fertilizers, and at site M10.45 crop residues were occasionally incorporated into the soil. The included rotation sequences were less diverse than at the organic sites, with crops belonging to the Cucurbitaceae and Fabaceae families.

Table 1.
Physicochemical properties of four soils cultivated with vegetables under organic standards (M10.16, M10.41, M10.55, and M10.56) and two soils cultivated under integrated production (M10.43 and M10.45).
At the beginning and at the end of the crops, the RKN population densities were determined. Composite soil samples were taken from four plots of 50–85 m2 each, depending on the site. Each sample was composed of ten soil subsamples taken from the first 30 cm of soil of each plot with a soil auger with a 2.5 cm diameter. The soil subsamples were carefully mixed and sieved through a 4 mm sieve screen to remove debris and stones. Nematodes were extracted from two 250 cm3 soil subsamples by the Jenkins’s method [22], counted, and expressed as juveniles in 250 cm3 of soil. At the end of the crop, the 10 plants closest to the sampled soil were uprooted, and the galling index was estimated using the Zeck index scale from 0 to 10, where 0 = a complete and healthy root system and 10 = dead plants and roots [23]. The soil temperature and soil water content from each site were recorded at a 15 cm depth at 1 h intervals with temperature probes (5TM, Decagon devices, Inc., Pullman, WA, USA). After that, roots were washed with tap water, and 2 egg masses per plant (20 egg masses per plot) were handpicked to evaluate the percentage of fungal egg parasites and to identify the fungal species according to the method described in Giné et al. [8]. Briefly, the outer parts of the gelatinous matrix of the egg masses were removed with tweezers and placed in a 1.5 mL microcentrifuge tube (Eppendorf Ibérica SLU, San Sebastián de los Reyes, Spain) containing 1 mL of sterile distilled water. The eggs were scattered from the egg masses with a sterile pestle, and three 0.33 mL aliquots of the egg suspension were spread on three Petri dishes (9 cm diameter) containing a restrictive growing medium (streptomycin, 50 mg L−1; chloramphenicol, 50 mg L−1; chlortetracycline, 50 mg L−1; Rose Bengal, 50 mg L−1; triton x-100, 1 mL l−1; and 1% agar) [24]. The plates were incubated at 25 °C ± 0.5 °C. The number of parasitized eggs was counted at 24 and 48 h using a dissecting microscope (Leica Microsistemas, SLU, Barcelona, Spain). The eggs were considered parasitized when the hyphae grew from the inside. The percentage of parasitism in each Petri dish was calculated as the number of parasitized eggs in relation to the total number of eggs per plate. The parasitized eggs (maximum 20 per plate) were individually transferred to corn meal agar to establish pure cultures of the fungi and were incubated at 25 °C ± 0.5 °C. A single-spore culture was established and identified by cultural and morphological characteristics. Fungal isolates were stored in 1% (w/v) water–agar slants as well as lyophilized, and in both cases they were stored at 4 °C.
Finally, the plant roots from each plot were chopped and mixed, and the eggs were extracted from three 10 g subsamples by blender maceration in a 10% commercial bleach solution (40 g L−1 NaOCl) for 10 min [25], sieved through a 74 µm aperture sieve to retain root debris, collected in a 25 µm aperture sieve, counted, and expressed as the number of eggs per g of root.
2.2. Soil Suppressiveness to RKN in Pot Experiments
Two experiments were carried out using soil taken from each site in February 2015 and February 2016. The soil samples consisted of 12 soil subsamples per plot and site, and they were taken from the first 30 cm with a hoe. For each site, the soil samples were mixed and sieved through a 4 mm sieve screen to remove stones and roots. One half of the soil collected at each site was sterilized at 121 °C for 1 h, and the procedure was repeated after 1 day. Soil samples were then conserved at 4 °C until their use. The experiments were conducted following the same procedure described in Giné et al. [8]. Sterilized and non-sterilized soils were mixed with sterile sand (1:1 dry w/dry w) to avoid soil compaction and improve root development. Afterwards, the RKN density in both the sterilized and non-sterilized soil mixtures were determined to adjust the level of inoculum at 1 J2 cm−3 of soil. RKN juveniles were extracted from two 500 cm3 subsamples using Baermann trays [26] maintained at 27 ± 2 °C for a week and then counted. After that, soil was placed in 3 L pots, and the susceptible tomato cv. Durinta was transplanted into each pot at the three true developed leaves stage. The nematode inoculum consisted of the M. incognita J2 population isolate Agropolis, which emerged from eggs extracted from tomato cv. Durinta roots by blender maceration in a 5% commercial bleach (40 g L−1 NaOCl) solution for 5 min by the Hussey and Barker [25] procedure, and was placed in Baermann trays [26] for a week at 27 ± 2 °C. The nematode inoculum was added to the soil in two opposite holes (3 cm deep) at 2 cm apart from the stems of the plants. The experiments consisted of 12 and 10 replications for each soil mixture and site in experiments 1 and 2, respectively. The plants were placed randomly on the greenhouse benches and were irrigated as needed and fertilized with a slow-release fertilizer (Osmocote (R), 15N + 10P + 12K + 2MgO + microelements). The soil temperatures and soil water contents were recorded at an 8 cm depth at 1 h intervals during the experiments with temperature probes. The first experiment was conducted from 15 May to 20 July 2015 (1085 degree-day (DD); 10 °C basal temperature; [27]), and the second experiment was conducted from 19 April to 14 July 2016 (1050 DD). At the end of the experiments, the roots were washed with tap water and gently dried, and the fresh weight was determined. Afterwards, two egg masses were handpicked from each plant from both the sterilized and non-sterilized soils to determine the percentage of fungal egg parasitism, according to the method described previously, and to identify the fungal species by their morphological characteristics. Finally, the number of eggs per plant was determined by extracting them from the roots with the Hussey and Barker [25] method.
2.3. Statistical Analyses
Statistical analyses were performed using the JMP software v8 (SAS institute Inc., Cary, NC, USA). The normal distribution of the data and the homogeneity of variances were assessed. Paired comparisons between the sterilized and non-sterilized mixed soils were conducted for each site using Student’s t-test or the non-parametric Wilcoxon test (p ≤ 0.05).
3. Results
3.1. Fluctuation in RKN Densities and Egg Parasitism in Field Conditions
At the organic production sites cultivated under protection, the nematode population densities in the soil decreased at M10.41 and M10.56 but did not change at M10.55 at the end of the study. The nematodes were able to infect all crops (galling index > 0) except spinach at M10.41. The maximum numbers of eggs in the roots were registered at the end of the spring–summer crops of zucchini (1490 and 5613 eggs g−1 root, site M10.41), eggplant (310 eggs g−1 root, site M10.55), and tomato (4140 eggs g−1 root, site M10.55; 3100 eggs g−1 root, site M10.56). At these sites, the percentages of fungal egg parasitism ranged from 11.2 to 35.9% (Table 2), and P. chlamydosporia was the only fungal species isolated. The lowest values of the galling index and the number of eggs in the roots were registered in the summer–autumn chard, spinach, and radish and in the autumn–winter lettuce. In those cases, the percentage of fungal egg parasitism was not assessed because few or not egg masses were observed. At M10.56, the watermelon crop transplanted in March 2016 died two months after being transplanted due to technical problems, and consequently the nematode densities in the soil decreased from March to July 2016 (Table 2). In the organic open field, M10.16, the nematode density in the soil when transplanting tomato in May 2015 was 34 J2 250 cm−3 of soil, and the nematode reached undetectable levels six months later. Few galls in roots were observed, and few eggs in roots were registered (830 eggs g−1 root). The percentage of fungal egg parasitism was 55%, and the only fungal species isolated was P. chlamydosporia (Table 2).

Table 2.
Meloidogyne population densities in soil at planting (Pi) and at the end of the crop (Pf), galling index, number of eggs on roots, and percentage of fungal egg parasitism at four organic vegetable production sites (M10.16, M10.41, M10.55, and M10.56) and two integrated vegetable production sites (M10.43 and M10.45).
In the integrated open fields, M10.43 and M10.45, the crop rotation sequence was less diverse. At site M10.43, two French beans crops were grown during 2015. The nematode densities in the soil increased at the end of the first spring–summer crop, and a high number of eggs were detected in the roots (25,470 eggs g−1 root), although 16.5% of them were parasitized by P. chlamydosporia. At site M10.45, a low nematode density was registered when transplanting zucchini in April 2015 (6 J2 250 cm−3 soil), and the density reached undetectable levels at the end of the crop in August 2015. Few galls in roots were observed and few eggs in roots were found (250 eggs g−1 root), of which a 0.8% were parasitized by Fusarium spp. The nematode density when sowing the following faba bean crop in November 2015 was 54 J2 250 cm−3 of soil. No information related to the galling index, the density of eggs in the roots, or the percentage of fungal egg parasitism was obtained because the crop was incorporated into the ground before taking the samples in June 2016 (Table 2).
3.2. Soil Suppressiveness against RKN in Pot Tests
In both experiments, the fresh root weight of tomato plants cultivated in non-sterilized soils was lower compared to the plants cultivated in sterilized soils, irrespective of the site (p < 0.05). Moreover, no fungal egg parasites were recovered from nematode eggs produced in plants cultivated in sterilized soils (Figure 1).
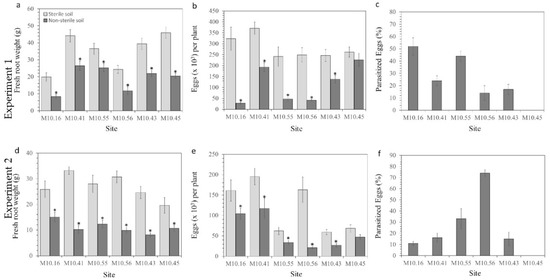
Figure 1.
Fresh root weight (a,d), Meloidogyne reproduction (b,e), and percentage of fungal egg parasitism (c,f) in the susceptible tomato cv. Durinta cultivated in sterilized or non-sterilized soils from organic and integrated vegetable production sites in pot experiments of inoculation with nematode juveniles to achieve 1 J2 cm−3 of soil after 66 days (experiment 1) or 86 days (experiment 2), respectively. Data are means ± standard errors of 12 and 10 replications in experiments 1 and 2, respectively. * indicates a significant difference between soil treatments at p < 0.05, according to Student’s t-test or the non-parametric Wilcoxon test.
In experiment 1, conducted with the soil samples taken in February 2015, fungal egg parasites were recovered from Meloidogyne eggs produced in tomato roots cultivated in almost all non-sterilized soils, except in those plants grown in the M10.45 soil. P. chlamydosporia was the only fungal species that was isolated and identified. The percentages of fungal egg parasitism ranged from 14% (M10.56) to 52% (M10.16). Smaller numbers of eggs per plant cultivated in non-sterilized compared to sterilized soils were recorded in all soils (p < 0.05), except for the M10.45 soil, where no differences were found (Figure 1a–c). Similar results were obtained in experiment 2, conducted with soil samples taken in February 2016; fungal egg parasites were recovered from roots grown in all non-sterilized soils, except from site M10.45. P. chlamydosporia was the only fungal species identified. The percentages of fungal egg parasitism ranged from 11% (M10.16) to 74% (M10.56). The number of eggs per plant was lower (p < 0.05) in non-sterilized soils than in sterilized soils, except in the M10.45 soil (Figure 1d–f).
4. Discussion
The results obtained in this study bring to light the variable antagonistic potential of six agricultural soils: four conducted under organic conditions and two conducted under integrated vegetable production standards in northeastern Spain. Fungal parasites were recovered from Meloidogyne eggs at almost all sites, with P. chlamydosporia being the most frequent and abundant fungal species isolated in different crops and field conditions. This result was confirmed in pot experiments with soil from the production sites, where P. chlamydosporia was the only fungal species isolated from nematode eggs produced in tomato. Giné et al. [20] reported a great diversity of fungal egg parasites in a previous study conducted in 2010 at 40 vegetable growing sites. Regarding the six sites included in this study, Giné et al. [20] isolated 11 fungal RKN egg parasites species, including Chaetomium spp., Colletotrichum coccodes, Cylindrocarpon olidum, Dactylella oviparasitica, Fusarium equiseti, F. oxysporum, F. solani, Fusarium spp., Penicillium citrinum, P. chlamydosporia, and Purpureocillium lilacinum, at percentages of parasitism ranging from 6.5% (M10.45) to 80.2% (M10.41). Five years later, in the present study, less diversity of fungal egg parasites was observed, with P. chlamydosporia being the only fungal species isolated from RKN eggs in 5 of the 6 sampled sites. Therefore, as proposed by Giné et al. [8,20], this fungal species has a key role in the RKN-antagonistic potential of these soils, and it is greatly resilient in agricultural systems.
Moreover, the pathogenicity of the different P. chlamydosporia isolates could vary, and therefore its suppressive potential could vary as well. For instance, Dos Santos et al. [28] reported a pathogenicity variation between 38 and 65% in M. incognita eggs, and a strong relationship was found between root colonization and parasitism. In addition to P. chlamydosporia, other components of the soil microbiome could affect RKN infection and/or reproduction and consequently the soil suppressiveness. Recently, some bacterial isolates from the cuticle of M. hapla J2 have been reported to affect nematode invasion into tomato roots [29,30]. Elhady et al. [21] used a fraction of the soil samples taken in February 2015 from the same six sites of the present study in order to determine if the soil microbiome was able to affect the RKN J2 cuticle. It was found that the most common microbial J2-attached species had minor relative abundances in the soil and were not related to the vegetable production system. Interestingly, Topalović et al. [15] also showed that the microbiome extracted from a suppressive soil to several species of plant-parasitic nematodes affected the capability of M. hapla to invade and to reproduce in tomato plants by inducing plant resistance. However, there is no information about the ability of the RKN J2-attached microbial species identified by Elhady et al. [21] to induce plant resistance. Nonetheless, according to our results, egg parasitism seems to be the main factor responsible for reducing nematode reproduction since none of the identified attached microorganisms have been reported to induce plant resistance against RKN. In fact, P. chlamydosporia and other microorganisms such as Trichoderma spp. and Bacillus firmus were able to induce resistance in tomato when applied 7 days before nematode inoculation [31,32], but Trichoderma asperllum did not have this effect when applied at transplanting [33]. In our experimental conditions, in which the infective RKN juveniles were inoculated just after transplanting, the induction of plant resistance could not occur due to the short exposition time that elapsed for the fungal–plant interaction against the nematode.
Other factors influencing the antagonistic potential of soils, such as crop rotation, can improve soil-borne disease suppression. Peralta et al. [34] reported a positive relationship between crop diversity in a rotation sequence and disease suppression in agricultural soils by bacterial species. The soil suppressiveness increased when cover crops were included in a three-crop rotation sequence, but it did not when only one crop was cultivated. In addition, fallow decreased disease suppressiveness [34]. Moreover, cover crops indirectly impact soil microbial communities by increasing some soil properties such as the total C, the total N, and the aggregation formation and stability of the soil [35,36]. For example, providing more carbon to the soil through residues and flow from cover crop root exudates can stimulate soil microbial activity and higher rates of biomass production [34,35].
The effect that crop rotation and the included plant species could have on the microbiome responsible for RKN suppression is still unknown. In fact, the rhizosphere microbial community is more affected by the plant species than the soil practices [37]. Even more, the endosphere microbiome is affected by nematode infection, as pointed out by Wolfgang et al. [38] in tomato roots infected by RKN, irrespective of the disease severity, compared to healthy roots. Previous studies conducted with the same soils as this study showed that several components of the soil microbiome could affect nematode behavior and viability [8,21]. Therefore, more field studies complemented with pot experiments are needed to understand the main components of soil suppressiveness to RKN at the agroecosystem, microcosm, and molecular levels in order to improve the sustainability of vegetable production systems.
5. Conclusions
The present study demonstrated that P. chlamydosporia is the most prevalent fungal species related to Meloidogyne suppression in sustainable vegetable production systems. This fungal species seems to be well adapted to the agroenvironmental conditions of the studied vegetable production area, as it is a resilient fungal species that is able to parasitize nematode eggs in any vegetable crop in which the nematode is able to reproduce. Moreover, the level of fungal egg parasitism can be estimated in pot conditions, which can be an indicator of the antagonistic potential of soils against root-knot nematodes.
Author Contributions
N.E., A.G. and F.J.S. conceived and designed the experiments. Z.G., N.E., I.M., A.S., S.G., A.E., A.G. and F.J.S. performed the pot and field experiments; Z.G. analyzed the data and wrote the draft of the manuscript. N.E., A.G. and F.J.S. supervised the experiments, data collection, and analyses. N.E., A.E., A.G. and F.J.S. wrote the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project AGL2013-49040-C2-1-R of MINECO and FEDER.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data from this experiment will be made available upon request to F.J.S.
Acknowledgments
The authors are grateful to Sheila Alcalá, Maria Julià, Aïda Magdalena Fullana, and Helio García for technical laboratory, greenhouse, and field support and to the growers and the field advisors for their collaboration in the present work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-Lopez, R.; Palomares-Rius, E.; Wesemael, W.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, J.; Meressa, B.H. Nematode Parasites of Vegetables. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 3rd ed.; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CABI: Boston, MA, USA, 2018; pp. 346–410. [Google Scholar]
- Expósito, A.; Pujolà, M.; Achaerandio, I.; Giné, A.; Escudero, N.; Fullana, A.M.; Cunquero, M.; Loza-Alvarez, P.; Sorribas, F.J. Tomato and melon Meloidogyne resistant rootstocks improve crop yield but melon fruit quality is influenced by the cropping season. Front. Plant Sci. 2020, 11, 560024. [Google Scholar] [CrossRef] [PubMed]
- Giné, A.; Sorribas, F.J. Quantitative approach for the early detection of selection for virulence of Meloidogyne incognita on resistant tomato in plastic greenhouses. Plant Pathol. 2017, 66, 1338–1344. [Google Scholar] [CrossRef]
- Shukla, N.; Yadav, R.; Kaur, P.; Rasmussen, S.; Goel, S.; Agarwal, M.; Jannath, A.; Gupta, R.; Kumar, A. Transcriptome analysis of root-knot nematode (Meloidogyne incognita)-infected tomato (Solanum lycopersicum) roots reveals complex gene expression profiles and metabolic networks of both host and nematode during susceptible and resistance responses. Mol. Plant Pathol. 2018, 19, 615–633. [Google Scholar] [CrossRef]
- Sikora, R.A. Management of the antagonistic potential in agricultural ecosystems for the biological control of plant parasitic nematodes. Annu. Rev. Phytopathol. 1992, 30, 245–270. [Google Scholar] [CrossRef]
- Stirling, G.R. Biological Control of Plant Parasitic Nematodes: Progress, Problems and Prospects; CABI: Wallingford, UK, 1991. [Google Scholar]
- Giné, A.; Carrasquilla, M.; Martínez-Alonso, M.; Gaju, N.; Sorribas, F.J. Characterization of soil suppressiveness to root-knot nematodes in organic horticulture in plastic greenhouse. Front. Plant Sci. 2016, 7, 164. [Google Scholar] [CrossRef]
- Baker, K.F.; Cook, R.J. Biological Control of Plant Pathogens; W.H. Freeman and Company: San Francisco, CA, USA, 1974. [Google Scholar]
- Stirling, G.R. Biological Control of Plant-Parasitic Nematodes: An Ecological Perspective, a Review of Progress and Opportunities for Further Research. In Biological Control of Plant-Parasitic Nematodes, 1st ed.; Davies, K., Spiegel, Y., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 11, pp. 1–38. [Google Scholar] [CrossRef]
- Weller, D.M.; Raaijmakers, J.M.; Gardener, B.B.M.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef]
- Garbeva, P.V.; Van Veen, J.A.; Van Elsas, J.D. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef]
- Pyrowolakis, A.; Westphal, A.; Sikora, R.A.; Becker, J.O. Identification of root-knot nematode suppressive soils. Appl. Soil Ecol. 2002, 19, 51–56. [Google Scholar] [CrossRef]
- Adam, M.; Westphal, A.; Hallmann, J.; Heuer, H. Specific microbial attachment to root knot nematodes in suppressive soil. Appl. Environ. Microbiol. 2014, 80, 2679–2686. [Google Scholar] [CrossRef]
- Topalović, O.; Heuer, H.; Reineke, A.; Zinkernagel, J.; Hallmann, J. Antagonistic role of the microbiome from a Meloidogyne hapla-suppressive soil against species of plant-parasitic nematodes with different life strategies. Nematology 2020, 22, 75–86. [Google Scholar] [CrossRef]
- Verdejo Lucas, S.; Español, M.; Ornat, C.; Sorribas, F.J. Occurrence of Pasteuria spp. in the northeastern Spain. Nematol. Mediterr. 1997, 25, 109–112. [Google Scholar]
- Verdejo-Lucas, S.; Ornat, C.; Sorribas, F.J.; Stchiegel, A. Species of root-knot nematodes and fungal egg parasites recovered from vegetables in Almería and Barcelona, Spain. J. Nematol. 2002, 34, 405–408. [Google Scholar]
- Verdejo-Lucas, S.; Blanco, M.; Talavera, M.; Stchigel, A.M.; Sorribas, F.J. Fungi recovered from root-knot nematodes infecting vegetables under protected cultivation. Biocontrol Sci. Technol. 2013, 23, 277–287. [Google Scholar] [CrossRef]
- Olivares-Bernabeu, C.M.; López-Llorca, L.V. Fungal egg-parasites of plant-parasitic nematodes from Spanish soils. Rev. Iberoam. Micol. 2002, 19, 104–110. [Google Scholar]
- Giné, A.; Bonmatí, M.; Sarro, A.; Stchiegel, A.; Valero, J.; Ornat, C.; Fernández, C.; Sorribas, F.J. Natural occurrence of fungal egg parasites of root-knot nematodes, Meloidogyne spp. in organic and integrated vegetable production systems in Spain. Biocontrol 2013, 58, 407–416. [Google Scholar] [CrossRef]
- Elhady, A.; Giné, A.; Topalovic, O.; Jacquiod, S.; Sørensen, S.J.; Sorribas, F.J.; Heuer, H. Microbiomes associated with infective stages of root-knot and lesion nematodes in soil. PLoS ONE 2017, 12, e0177145. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, W. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Zeck, W.M. Rating scheme for field evaluation of root-knot nematode infestations. Pflanzenschutz Nachr. 1971, 24, 141–144. [Google Scholar]
- Lopez-Llorca, L.V.; Duncan, J.M. New media for the estimation of fungal infection in eggs of the cereal cyst nematode, Heterodera avenae Woll. Nematologica 1986, 32, 486–489. [Google Scholar] [CrossRef]
- Hussey, R.; Barker, K. Comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Whitehead, A.G.; Hemming, J.R. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Ferris, H.; Roberts, P.A.; Thomason, I.J. Nematodes. In Integrated Pest Management for Tomatoes; University of California. Statewide Integrated Pest Management Project, Ed.; Division of Agriculture and Natural Resources, University of California: Oakland, CA, USA, 1985; pp. 60–65. [Google Scholar]
- Dos Santos, M.C.V.; Horta, J.; Moura, L.; Pires, D.V.; Conceicao, I.; Abrantes, I.; Costa, S.R. An integrative approach for the selection of Pochonia chlamydosporia isolates for biocontrol of potato cyst and root knot nematodes. Phytopathol. Mediterr. 2019, 58, 1–217. [Google Scholar]
- Topalović, O.; Elhady, A.; Hallmann, J.; Richert-Pöggeler, K.R.; Heuer, H. Bacteria isolated from the cuticle of plant-parasitic nematodes attached to and antagonized the root-knot nematode Meloidogyne hapla. Sci. Rep. 2019, 9, 11477. [Google Scholar] [CrossRef] [PubMed]
- Topalović, O.; Santos, S.S.; Heuer, H.; Nesme, J.; Kanfra, X.; Hallmann, J.; Sørensen, S.; Vestergård, M. Deciphering bacteria associated with a pre-parasitic stage of the root-knot nematode Meloidogyne hapla in nemato-suppressive and nemato-conducive soils. Appl. Soil Ecol. 2022, 172, 104344. [Google Scholar] [CrossRef]
- Ghahremani, Z.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Pochonia chlamydosporia induces plant-dependent systemic resistance to Meloidogyne incognita. Front. Plant Sci. 2019, 10, 945. [Google Scholar] [CrossRef]
- Pocurull, M.; Fullana, A.M.; Ferro, M.; Valero, P.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Commercial formulates of Trichoderma induce systemic plant resistance to Meloidogyne incognita in tomato and the effect is additive to that of the Mi-1.2 resistance gene. Front. Microbiol. 2020, 10, 3042. [Google Scholar] [CrossRef]
- Expósito, A.; García, S.; Giné, A.; Escudero, N.; Herranz, S.; Pocurull, M.; Lacunza, A.; Sorribas, F.J. Effect of molasses application alone or combined with Trichoderma asperellum T-34 on Meloidogyne spp. management and soil microbial activity in organic production systems. Agronomy 2022, 12, 1508. [Google Scholar] [CrossRef]
- Peralta, A.L.; Sun, Y.; McDaniel, M.D.; Lennon, J.T. Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 2018, 9, e02235. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Tiemann, L.K.; Grandy, A.S. Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta-analysis. Ecol. Appl. 2014 24, 560–570. [CrossRef]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; McDaniel, M.D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Harkes, P.; Van Steenbrugge, J.J.M.; Van Den Elsen, S.J.J.; Suleiman, A.K.A.; De Haan, J.J.; Holterman, M.H.M.; Helder, J. Shifts in the active rhizobiome paralleling low Meloidogyne chitwoodi densities in fields under prolonged organic soil management. Front. Plant Sci 2020, 10, 1697. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, A.; Taffner, J.; Guimarães, R.A.; Coyne, D.; Berg, G. Novel strategies for soil-borne diseases: Exploiting the microbiome and volatile-based mechanisms towards controlling Meloidogyne-based disease complexes. Front. Microbiol. 2019, 10, 1296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).