Multielement Analysis of Fresh and Salt Surface Water from Different Continents
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Laboratory Analysis
2.3. Quality Control and Assurance
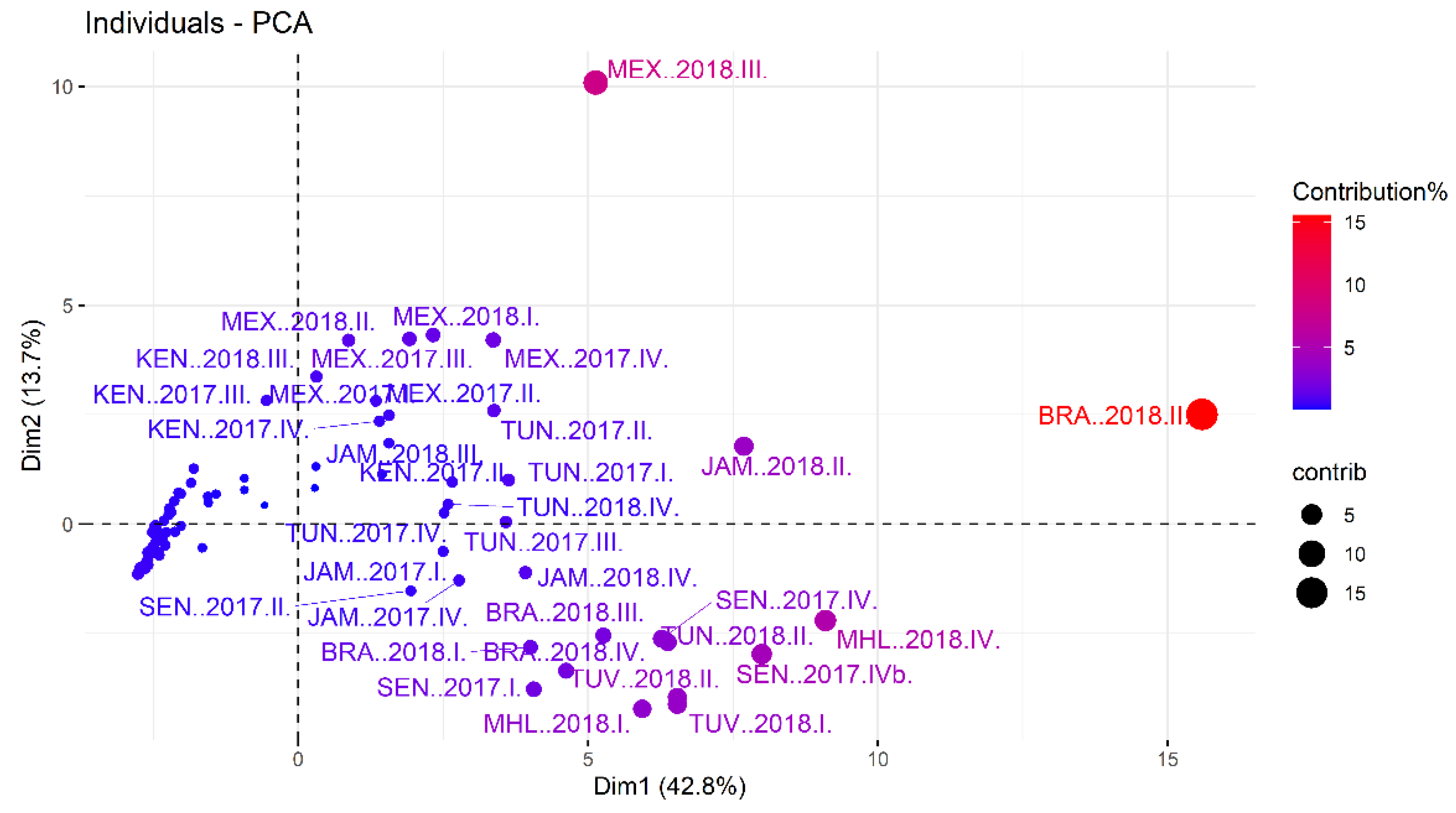
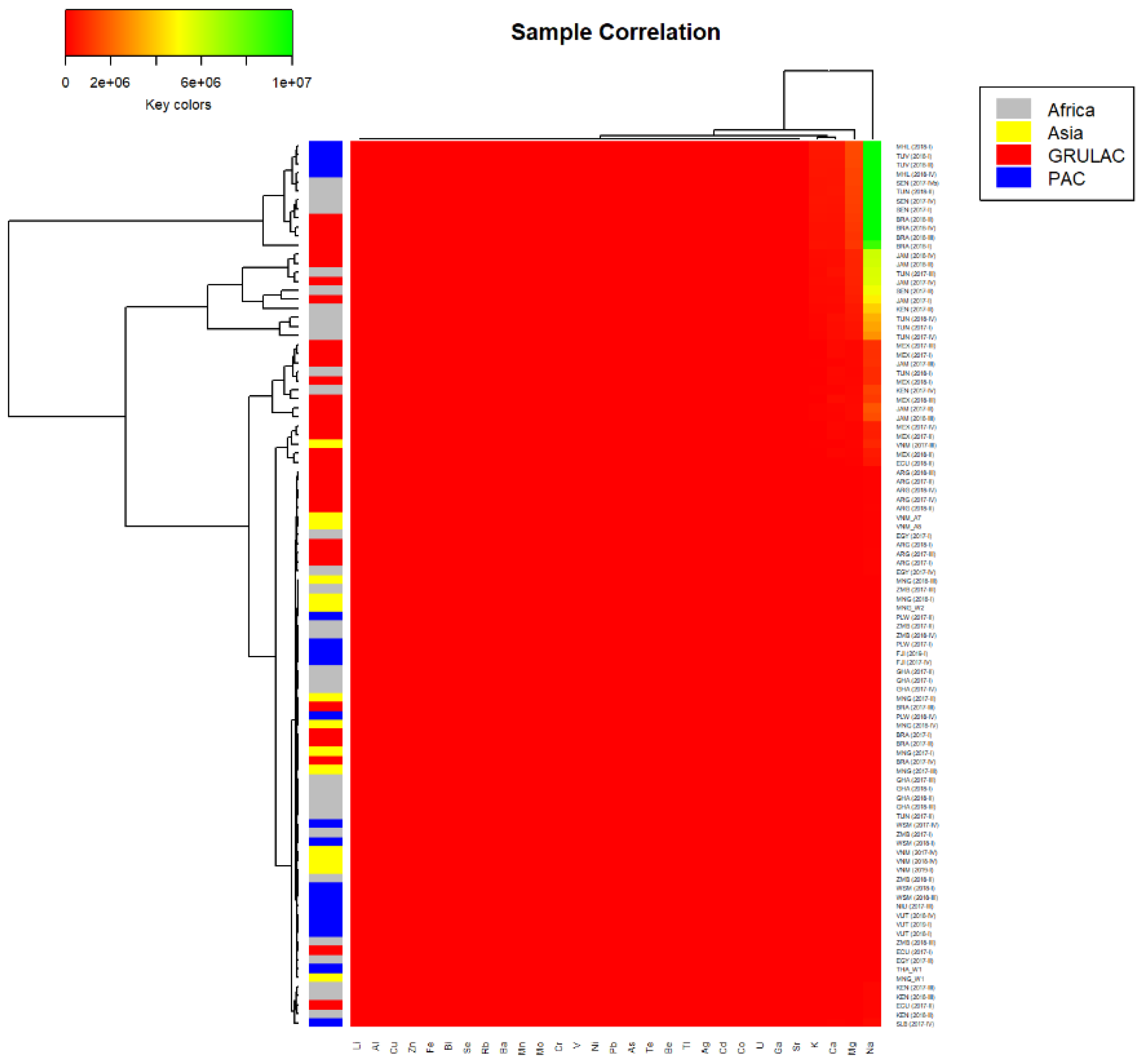
2.4. Statistics
3. Results
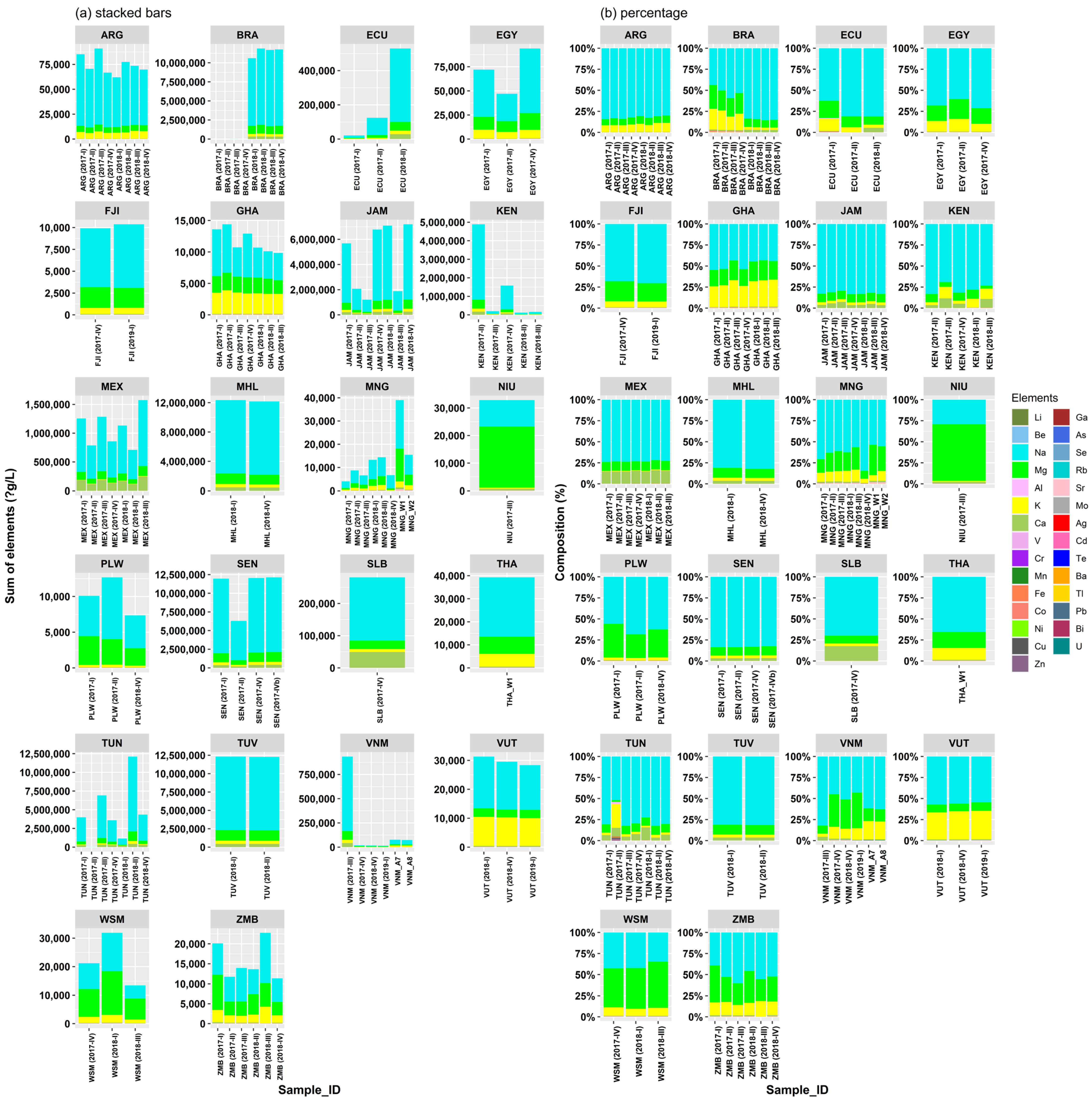
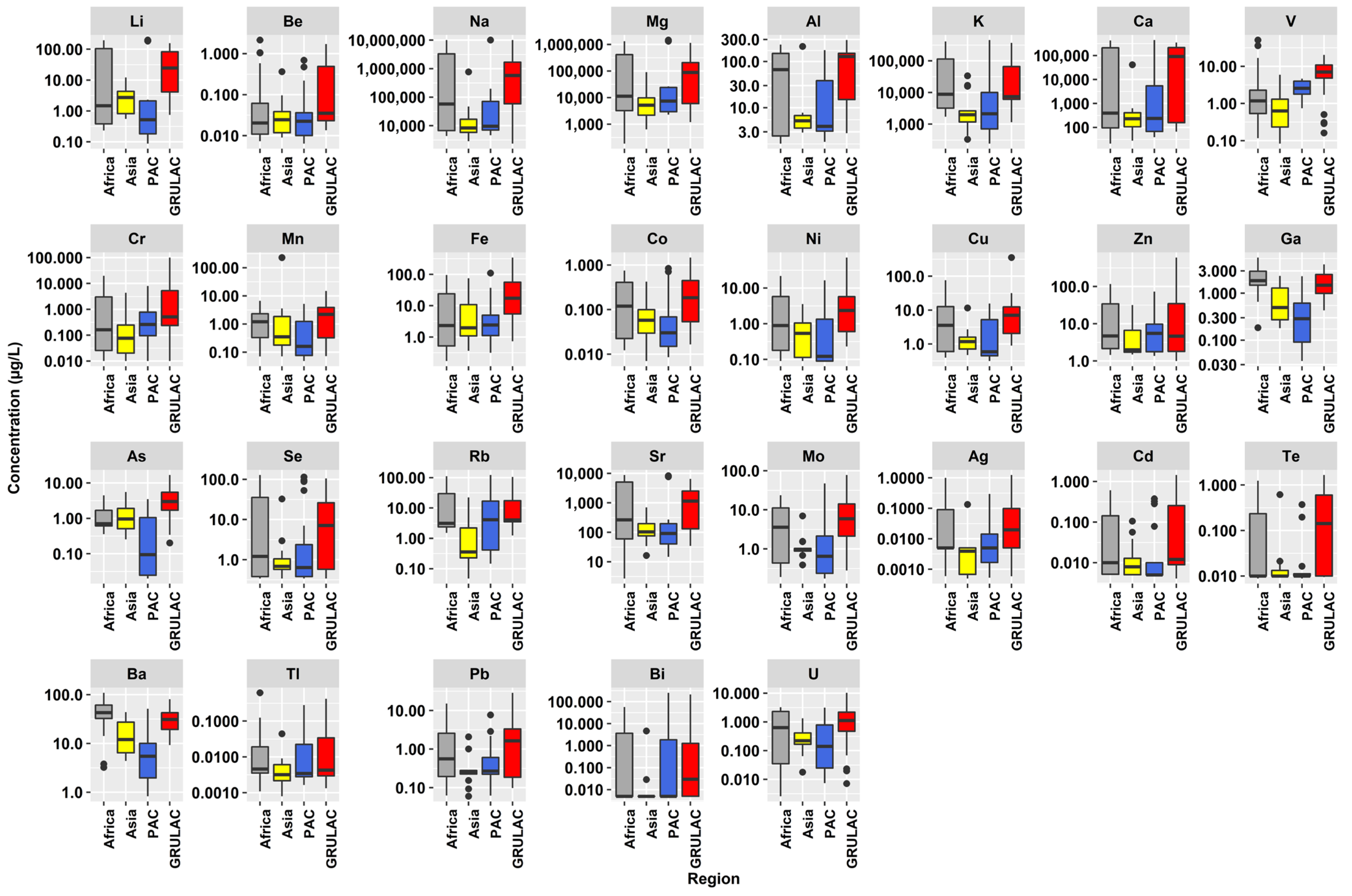
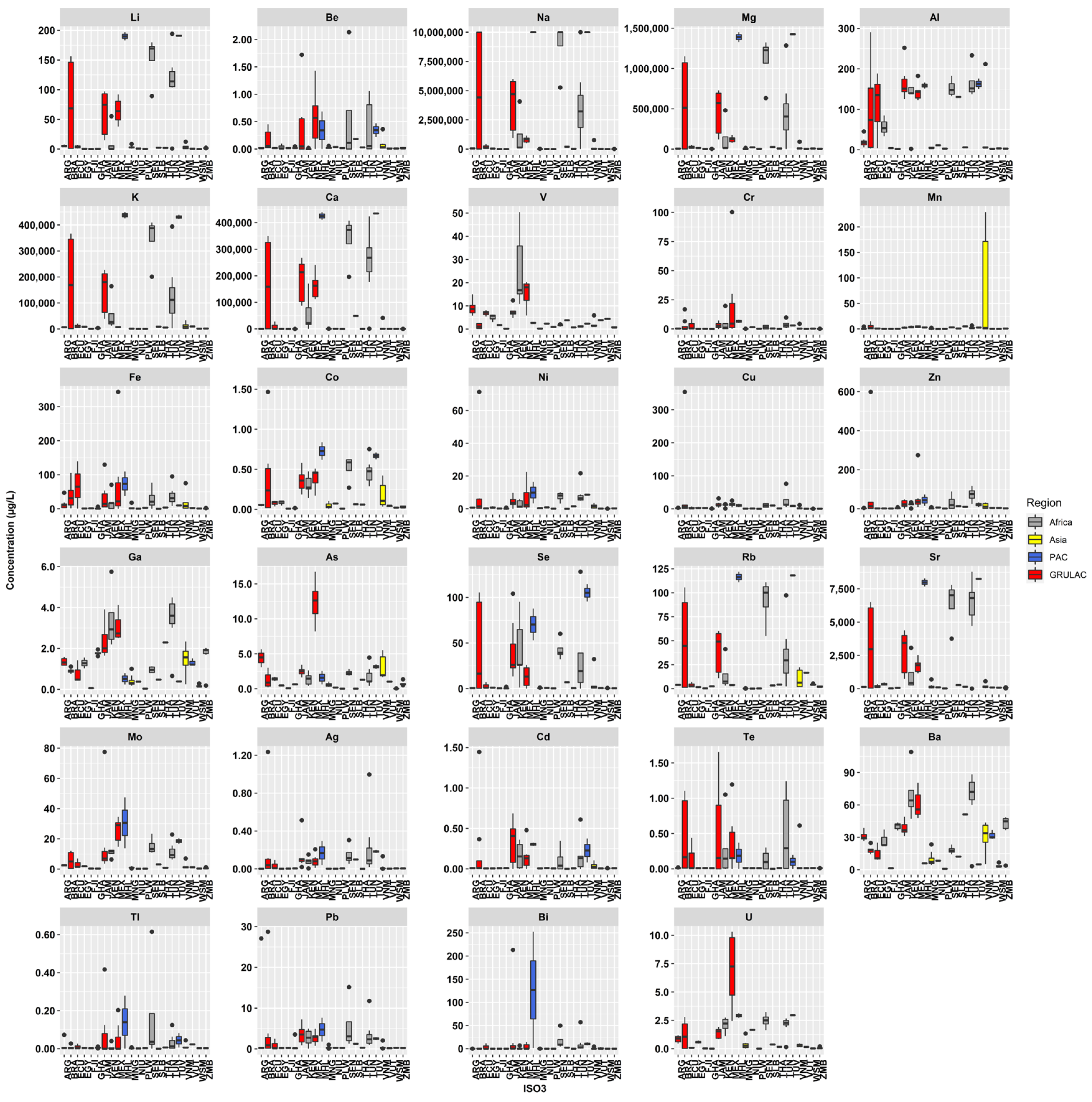
3.1. Elemental Concentrations in the Water Samples
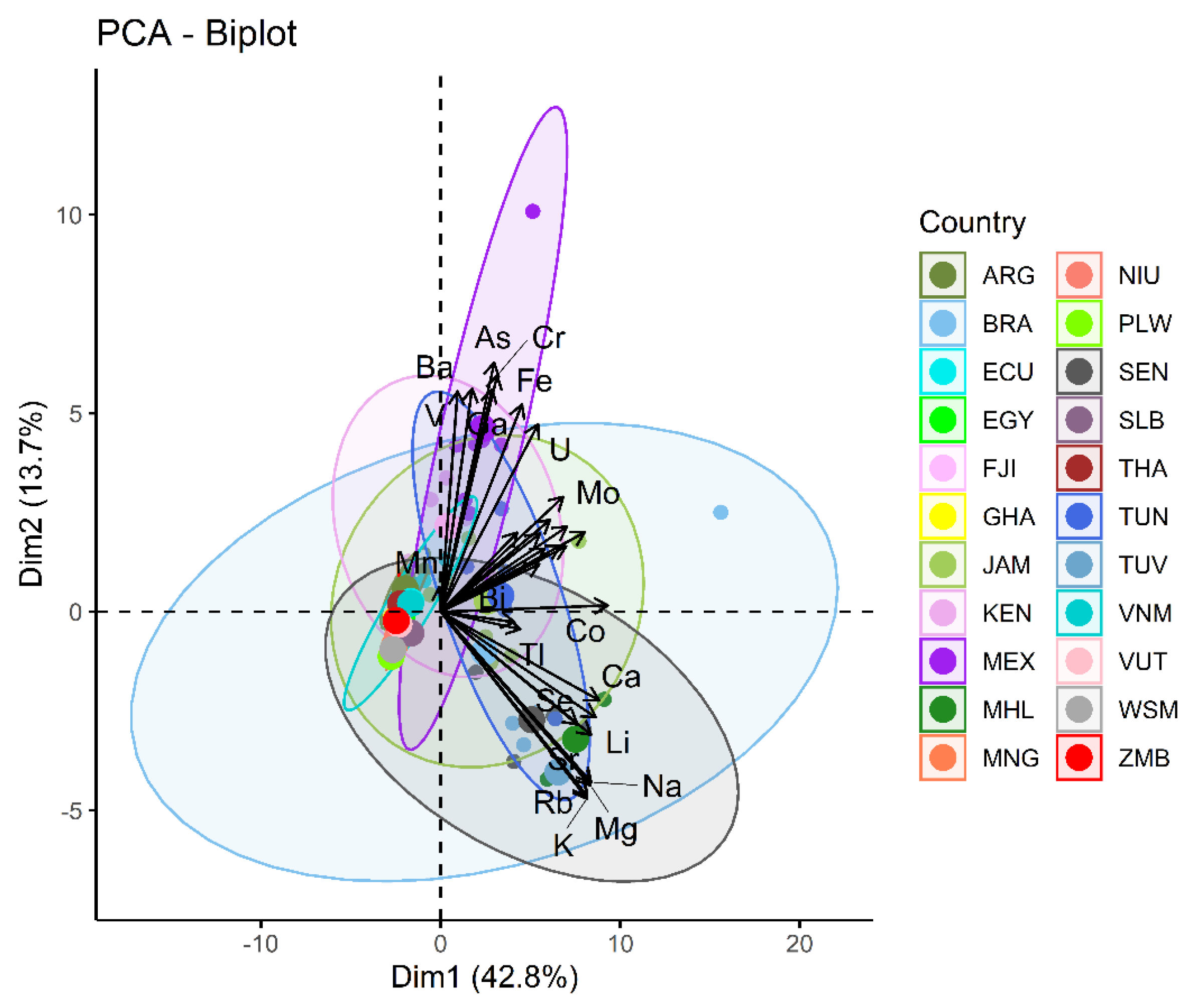
3.2. Regional Results
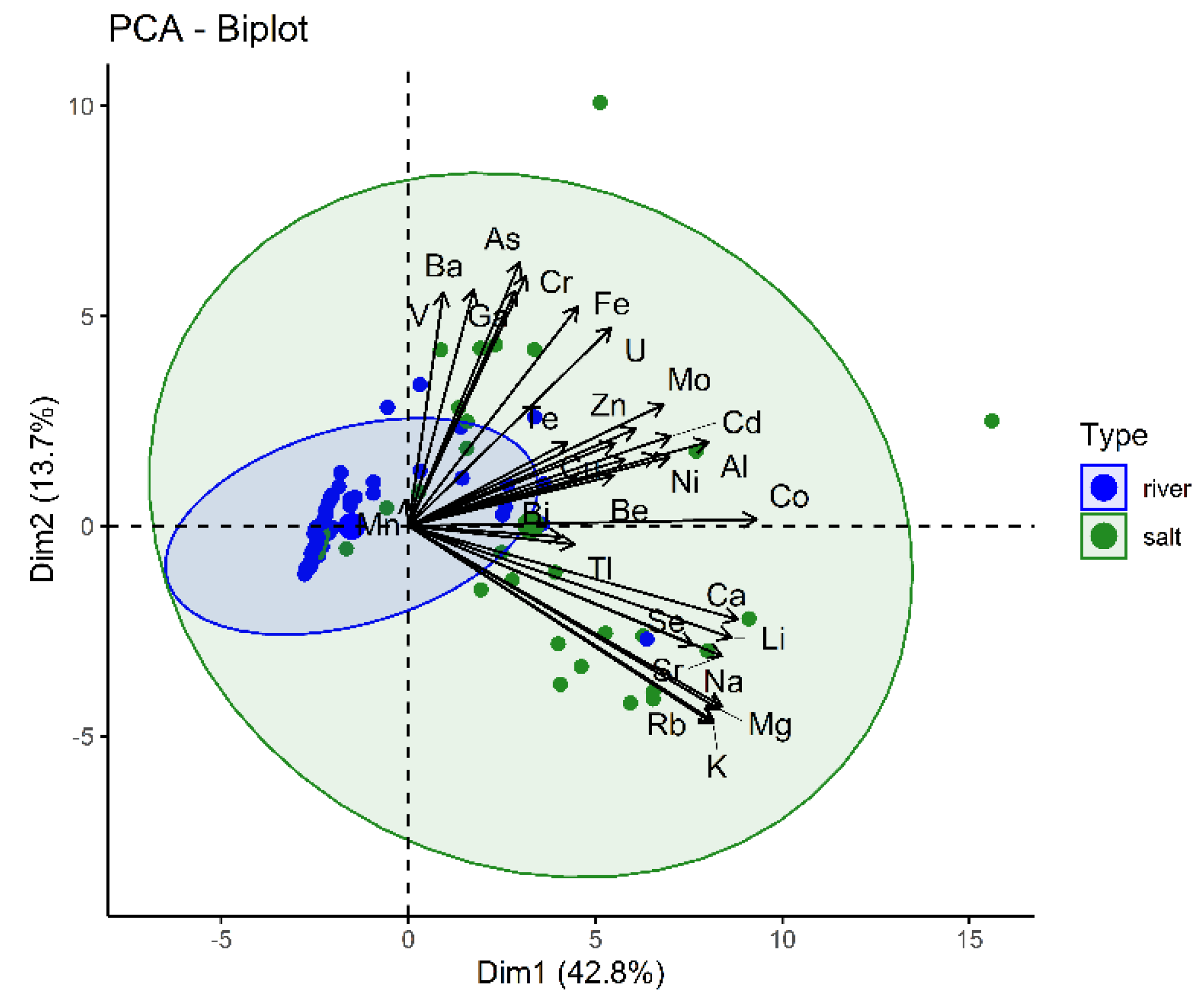
3.3. Results Based on Water Type
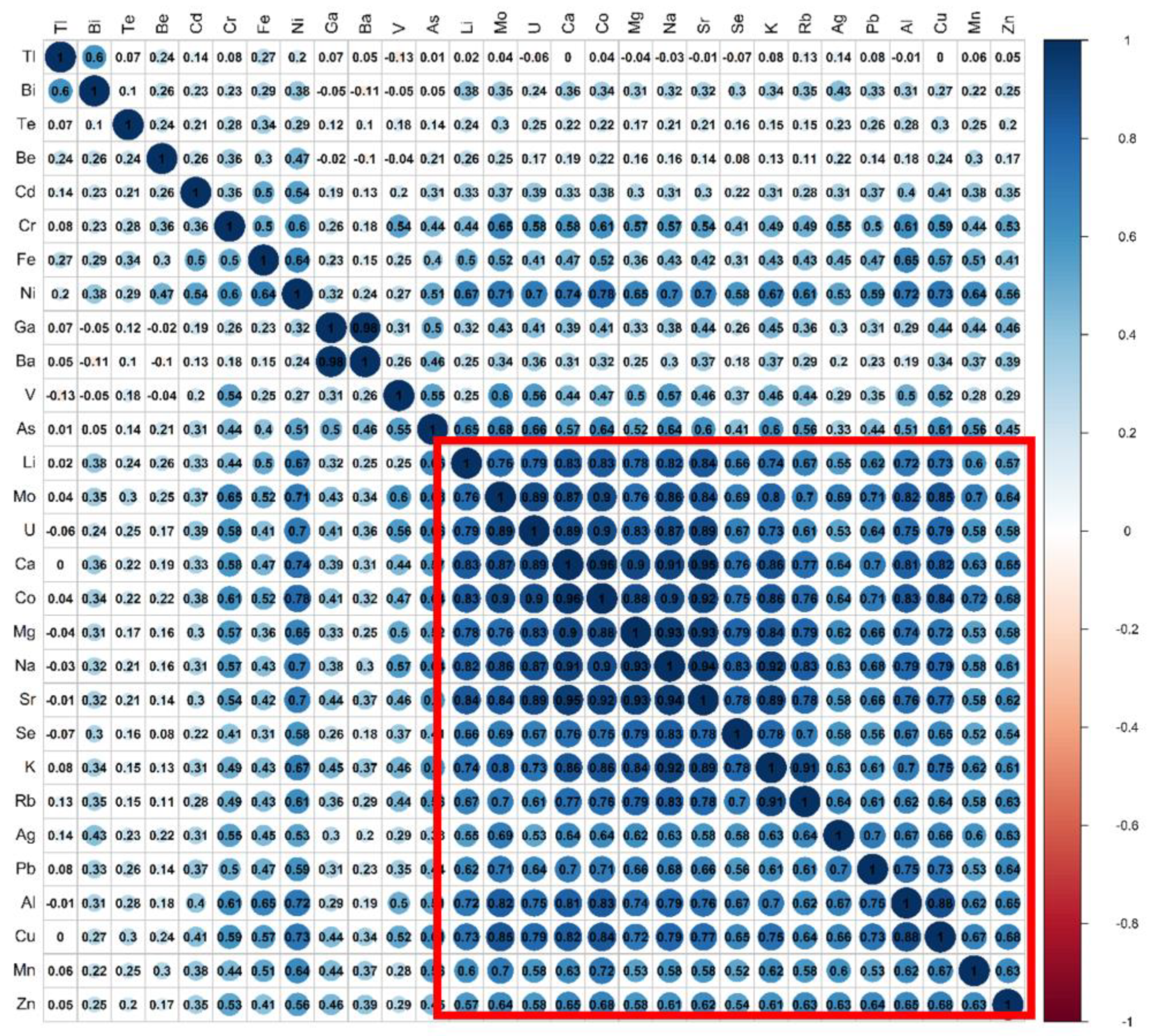
3.4. Correlation of Analytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Heinze, I.; Gross, R.; Stehle, P.; Dillon, D. Assessment of lead exposure in schoolchildren from Jakarta. Environ. Health Perspect. 1998, 106, 499–501. [Google Scholar] [CrossRef]
- Rodriguez Martin, J.A.; De Arana, C.; Ramos-Miras, J.J.; Gil, C.; Boluda, R. Impact of 70 years urban growth associated with heavy metal pollution. Environ. Pollut. 2015, 196, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Záray, G.; Óvári, M.; Salma, I.; Steffan, I.; Zeiner, M.; Caroli, S. Determination of platinum in urine and airborne particulate matter from Budapest and Vienna. Microchem. J. 2004, 76, 31–34. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Islam, K.N.; Ibrahim, M.; Masunaga, S. Arsenic and lead in foods: A potential threat to human health in Bangladesh. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess 2014, 31, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.S.; Bakar, M.A.; Akhtar, A.; Hossain, M.B.; Ali, M.M.; Islam, M.S. Heavy metal contamination in surface water and sediment of the Meghna River, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2017, 8, 273–279. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer Nature: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Hadzi, G.Y.; Essumang, D.K.; Adjei, J.K. Distribution and Risk Assessment of Heavy Metals in Surface Water from Pristine Environments and Major Mining Areas in Ghana. J. Health Pollut. 2015, 5, 86–99. [Google Scholar] [CrossRef]
- Hee, Y.Y.; Suratman, S.; Aziz, A.A. Water Quality and Heavy Metals Distribution in Surface Water of the Kelantan River basin of Malaysia. Orient. J. Chem. 2019, 35, 1254–1264. [Google Scholar] [CrossRef]
- Khelfaoui, M.; Benaissa, A.; Kherraf, S.; Madjram, M.S.; Bouras, I.; Mehri, k. Assessment of groundwater and surface water pollution by hazardous metals, using multivariate analysis and metal pollution index around the old Sidi Kamber mine, NE Algeria. Pollution 2022, 8, 889–903. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Sun, Y.; Chen, J. Novel assessment method of heavy metal pollution in surface water: A case study of Yangping River in Lingbao City, China. Environ. Eng. Res. 2016, 22, 31–39. [Google Scholar] [CrossRef]
- Luo, P.; Xu, C.; Kang, S.; Huo, A.; Lyu, J.; Zhou, M.; Nover, D. Heavy metals in water and surface sediments of the Fenghe River Basin, China: Assessment and source analysis. Water Sci. Technol. 2021, 84, 3072–3090. [Google Scholar] [CrossRef]
- Luo, Y.; Rao, J.; Jia, Q. Heavy metal pollution and environmental risks in the water of Rongna River caused by natural AMD around Tiegelongnan copper deposit, Northern Tibet, China. PLoS ONE 2022, 17, e0266700. [Google Scholar] [CrossRef]
- Radeva, K.; Seymenov, K. Surface water pollution with nutrient components, trace metals and metalloidsin agricultural and mining-affected river catchments: A case study for three tributaries of the Maritsa River, Southern Bulgaria. Geogr. Pannonica 2021, 25, 214–225. [Google Scholar] [CrossRef]
- Rima, S.A.; Rahman, M.; Saha, S.K.; Saima, J.; Hossain, M.S.; Tanni, T.N.; Bakar, M.A.; Siddique, M.A.M. Pollution Evaluation and Health Risk Assessment of Heavy Metals in The Surface Water of A Remote Island Nijhum Dweep, Northern Bay of Benga. Environ. Nanotechnol. Monit. Manag. 2021, 18, 100706. [Google Scholar] [CrossRef]
- Saha, N.; Rahman, M.S. Multivariate statistical analysis of metal contamination in surface water around Dhaka export processing industrial zone, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2018, 10, 206–211. [Google Scholar] [CrossRef]
- Shah, M.H.; Iqbal, J.; Shaheen, N.; Khan, N.; Choudhary, M.A.; Akhter, G. Assessment of background levels of trace metals in water and soil from a remote region of Himalaya. Environ. Monit. Assess. 2012, 184, 1243–1252. [Google Scholar] [CrossRef]
- Turhan, Ş.; Duran, C.; Kurnaz, A.; Hançerlioğulları, A.; Metin, O.; Altıkulaç, A. Impact of toxic metal pollution on surface water pollution: A case study of Tohma stream in Sivas, Turkey. Int. J. Environ. Anal. Chem. 2021, 1–11. [Google Scholar] [CrossRef]
- Qu, L.; Huang, H.; Xia, F.; Liu, Y.; Dahlgren, R.A.; Zhang, M.; Mei, K. Risk analysis of heavy metal concentration in surface waters across the rural-urban interface of the Wen-Rui Tang River, China. Environ. Pollut. 2018, 237, 639–649. [Google Scholar] [CrossRef]
- Withanachchi, S.S.; Ghambashidze, G.; Kunchulia, I.; Urushadze, T.; Ploeger, A. Water quality in surface water: A preliminary assessment of heavy metal contamination of the Mashavera River, Georgia. Int. J. Environ. Res. Public Health 2018, 15, 621. [Google Scholar] [CrossRef]
- Pan, H.; Zhou, G.; Yang, R.; Cheng, Z.; Sun, B. Heavy Metals and As in Ground Water, Surface Water, and Sediments of Dexing Giant Cu-Polymetallic Ore Cluster, East China. Water 2022, 14, 352. [Google Scholar] [CrossRef]
- ISO 3166; Country Codes. ISO: Geneva, Switzerland, 2022.
- United Nations (UN). Regional Groups of Member States. UN, Department for General Assembly and Conference Management. Available online: https://www.un.org/dgacm/en/content/regional-groups (accessed on 24 October 2022).
- Roșca, O.M.; Dippong, T.; Marian, M.; Mihali, C.; Mihalescu, L.; Hoaghia, M.-A.; Jelea, M. Impact of anthropogenic activities on water quality parameters of glacial lakes from Rodnei mountains, Romania. Environ. Res. 2020, 182, 109136. [Google Scholar] [CrossRef]
- GB 3838-2002; China (MEE). Environmental Quality Standards for Surface Water. MEE: Beijing, China, 2002.
- Al Obaidy, A.; Al Mashhady, A.A.; Awad, E.S.; Kadhem, A.J. Heavy metals pollution in surface water of Mahrut River, Diyala, Iraq. Int. J. Adv. Res. 2014, 2, 1039–1044. [Google Scholar]
- Mohanakavitha, T.; Divahar, R.; Meenambal, T.; Shankar, K.; Rawat, V.S.; Haile, T.D.; Gadafa, C. Dataset on the assessment of water quality of surface water in Kalingarayan Canal for heavy metal pollution, Tamil Nadu. Data Brief 2019, 22, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Baabish, A.; Sobhanei, S.; Fiedler, H. Priority perfluoroalkyl substances in surface waters—A snapshot survey from 22 developing countries. Chemosphere 2021, 273, 129612. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, H.; Sadia, M.; Baabish, A.; Sobhanei, S. Perfluoroalkane substances in national samples from global monitoring plan projects (2017–2019). Chemosphere 2022, 307, 136038. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global biogeochemical cycle of vanadium. Proc. Natl. Acad. Sci. USA 2017, 114, E11092–E11100. [Google Scholar] [CrossRef]
- Viers, J.; Dupré, B.; Gaillardet, J. Chemical composition of suspended sediments in World Rivers: New insights from a new database. Sci. Total Environ. 2009, 407, 853–868. [Google Scholar] [CrossRef]

| UN Region/Country (ISO3) | Water Type River (n = 67) | Water Type Salt (n = 31) | Subtotal (n = 98) |
|---|---|---|---|
| Africa | 28 (41.8%) | 4 (12.9%) | 32 (32.7%) |
| Egypt (EGY) | 3 (4.5%) | 3 (3.1%) | |
| Ghana (GHA) | 7 (10.4%) | 7 (7.1%) | |
| Kenya (KEN) | 5 (7.5%) | 5 (5.1%) | |
| Senegal (SEN) | 4 (12.9%) | 4 (4.1%) | |
| Tunisia (TUN) | 7 (10.4%) | 7 (7.1%) | |
| Zambia (ZMB) | 6 (9.0%) | 6 (6.1%) | |
| Asia | 14 (20.9%) | 14 (14.3%) | |
| Mongolia (MNG) | 8 (11.9%) | 8 (8.2%) | |
| Thailand (THA) | 1 (1.5%) | 1 (1.0%) | |
| Vietnam (VNM) | 6 (9.0%) | 6 (6.1%) | |
| PAC | 10 (14.9%) | 9 (29.0%) | 19 (19.4%) |
| Fiji (FJI) | 2 (3.0%) | 2 (2.0%) | |
| Marshall Islands (MHL) | 2 (6.5%) | 2 (2.0%) | |
| Niue (NIU) | 1 (3.2%) | 1 (1.0%) | |
| Palau (PLW) | 3 (4.5%) | 3 (3.1%) | |
| Solomon Islands (SLB) | 1 (3.2%) | 1 (1.0%) | |
| Tuvalu (TUV) | 2 (6.5%) | 2 (2.0%) | |
| Vanuatu (VUT) | 3 (9.7%) | 3 (3.1%) | |
| Samoa (WSM) | 4 (6.0%) | 4 (4.1%) | |
| GRULAC | 15 (22.4%) | 18 (58.1%) | 33 (33.7%) |
| Argentina (ARG) | 8 (11.9%) | 8 (8.2%) | |
| Brazil (BRA) | 4 (6.0%) | 4 (12.9%) | 8 (8.2%) |
| Ecuador (ECU) | 3 (4.5%) | 3 (3.1%) | |
| Jamaica (JAM) | 7 (22.6%) | 7 (7.1%) | |
| Mexico (MEX) | 7 (22.6%) | 7 (7.1%) | |
| Grand total | 67 (68.4%) | 31 (31.6%) | 98 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeiner, M.; Sjöberg, V.; Fiedler, H. Multielement Analysis of Fresh and Salt Surface Water from Different Continents. Sustainability 2022, 14, 16934. https://doi.org/10.3390/su142416934
Zeiner M, Sjöberg V, Fiedler H. Multielement Analysis of Fresh and Salt Surface Water from Different Continents. Sustainability. 2022; 14(24):16934. https://doi.org/10.3390/su142416934
Chicago/Turabian StyleZeiner, Michaela, Viktor Sjöberg, and Heidelore Fiedler. 2022. "Multielement Analysis of Fresh and Salt Surface Water from Different Continents" Sustainability 14, no. 24: 16934. https://doi.org/10.3390/su142416934
APA StyleZeiner, M., Sjöberg, V., & Fiedler, H. (2022). Multielement Analysis of Fresh and Salt Surface Water from Different Continents. Sustainability, 14(24), 16934. https://doi.org/10.3390/su142416934







