Abstract
Rice ranks second among cereals in dietary uses around the world. Rice is deficient in iron (Fe), and these are important micronutrients for infants, men, and women. Fortification of rice with iron would help to minimize nutrient deficiency disorders among humans. The current study aims to introduce nutrient-rich rice. The effects of iron on germination, growth, photosynthetic pigment, antioxidant activity, and reduction of oxidative stress were investigated in four Oryza sativa L. cultivars. O. sativa of four different cultivars (Basmati-515, PK-386, KSK-133, and Basmati-198) were grown under five treatments (100, 200, 300, 400, and 500 mM) of iron sulphate (FeSO4) in soil of pH 7.5, along with control, by using six replicates. The result revealed that Fe treatment significantly affected seed germination percentage, plant growth parameters, biomass, photosynthetic pigments (chl a, chl b, total chlorophyll, and carotenoids), antioxidant enzymatic and non-enzymatic activity, and reduced oxidative stress. The findings also showed that Fe application reduced the oxidative stress including malondialdehyde content and hydrogen peroxide, by increasing the antioxidant enzymatic activity, i.e., catalase, ascorbate peroxidase, superoxide dismutase, peroxidase, glutathione peroxidase, 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH), and non-enzymatic antioxidant compounds (proline, amino acid, total soluble protein, phenolics, flavonoids, reducing-non-reducing sugar, and carbohydrates) in all cultivars of O. sativa. Furthermore, FeSO4 induced a significant increase in proline, free amino acid, and total carbohydrates in the leaves of all O. sativa cultivars, but Basmati-198 showed the significantly highest content by 169, 88, and 110%, respectively, at concentration of 500 mM. The present research work showed that soil application of FeSO4 improved the seed germination, plant growth, and antioxidants enzymatic and non-enzymatic activity, denatured the ROS (reactive oxygen species) in alkaline soil. In order to understand the underlying mechanisms, long-term field investigations should be carried out at the molecular level to examine patterns of iron uptake and plant growth.
1. Introduction
Rice (Oryza sativa L.) is the best targeted and recommended crop for biofortification because it is one of the most consumed crops. Most countries commonly use rice as a source of nutrition [1]. According to a global synthesis, rice paddy soils are a quantitatively important carbon store. Additionally, the management of nutrients and crop residues had an effect on soil chemical and microbiological activity, which directly affects soil organic carbon and interacts with nutrient cycling [2]. For optimum growth, plants require a variety of vital micronutrients, such as iron (Fe), manganese (Mn), boron (B), chloride (Cl), copper (Cu), molybdenum (Mo), nickel (Ni), and zinc (Zn) [3]. Malnutrition is a serious issue in developing countries, especially in Asia and Africa, where it affects millions of people [4]. Because they are fully dependent on plant-based diets that are high in carbohydrates but low in micronutrients [5]. Malnutrition was made worse by the consumption of high-yielding but low-micronutrient crops [6]. Micronutrients such as Zn, Fe, and Se are essential for many critical processes, including cognition, development, the immune system, and maintaining antioxidant activity [7]. Therefore, it is important to provide micronutrients to crops in order to reduce micronutrient malnutrition globally [8]. According to some research, iron insufficiency is the most prevalent micronutrient imbalance in human populations [9]. Fe is important for a variety of processes, including DNA synthesis, respiration, photosynthesis, the electron transport chain, and nitrogen reduction but is a scarce nutrient for plants in alkaline soil [10]. These nutrients are very important for development and plant growth, as well as for stress tolerance and innate immunity because they are involved in many metabolic processes [11]. Alkaline soils account for 30% of the Earth’s surface and have low levels of micronutrients that are readily available to plants, such as iron, because the iron absorption genes and gene products are ineffective at an alkaline pH [12]. Iron deficiency affects the crop yield and productivity [13]; plants’ ability to withstand biotic [14] and abiotic stresses has been linked to the amount of micronutrients they can absorb [15].
People who are unable to buy additional micronutrient-rich products for a balanced diet are particularly prone to micronutrient deficits [16]. To cope with this problem, many studies offer particular recommendations that could contribute to the management of micronutrient application optimization in contemporary crop production [8,17]. Iron is primarily obtained from plants, either directly as staple crops or indirectly as animal feed. A long-term solution to iron deficiency is biofortification, which involves raising the iron content of edible plant parts and is recommended due to its sustainability and cost effectiveness [18,19].
In rice plants, the significantly more evidence of the strategy of using fertilizer and its impact on the micronutrients of grain was found [20]. The growth of rice plants was markedly increased by the application of iron conditions [21]. The positive effect of iron (Fe) applied to the leaves on morphological and biochemical activities of plants (leaf length, leaf fresh, dry weights, root fresh, and dry weights), while total soluble solids (TSS) and essential oil contents) were significantly affected [22]. The application of Fe to the leaves increased grain yield by 7.2%, increased peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT), and increased net photosynthesis by 19.3% [23]. Previous studies showed that maize leaves and bean roots under iron-deficient conditions showed an increased in lipid peroxidation maize leaves (iron-deficient) [24,25]. Iron deficiency decreased hydroxyl radical (•OH) but increased superoxide anion [26].
Due to present issues in agriculture imposed by the present situation of population increase and undernourishment, to ensure food and nutritional security [27], it is essential to find techniques that can increase the concentration of Fe in cereal crops. The purpose of the study was to investigate the relative effectiveness of method of applying iron, treat iron deficiency, and increase iron content. The long-term objective of this research is to provide innovative approaches for increasing agricultural yield on alkaline soils that are prone to Fe deficiency. This information might potentially enable the manipulation of these systems to raise the Fe concentrations in edible parts of the plants for food biofortification. Therefore, the goal of the current study is to reduce the iron deficit in four cultivars of O. sativa Basmati-198, Basmati-515, PK-386, and KSK-133. We designed the current experiment to assess how different iron treatments affect rice growth, photosynthetic activity, antioxidant responses, oxidative stress, amino acids, and iron uptake.
2. Material and Methods
2.1. Experimental Design
Certified rice seeds (Oryza sativa L.) of four different cultivars; (Basmati-198, Basmati-515, PK-386, and KSK-133) were obtained from the Rice Research Centre, Kala Shah Kaku. Two fine Basmati varieties were chosen for this study based on their iron content. According to our previous study, two Basmati varieties, Basmati-515 and Basmati-198, have the highest and lowest iron contents of 22.0 and 14.1 ppm, respectively, while PK-386 had 19.0 ppm [28]. KSK-133, a coarse variety with high yields and extra-long grains, was chosen. Pot experiments were carried out in Botanical Garden of the University of the Punjab, Lahore, in 2021 and 5–6 seeds were planted in each pot. The experimental area of Punjab University stands between 31°29′57.78″ N latitude and 74°17′58.60″ E longitude and has a moderate climate. A freshly prepared 5% sodium hypochlorite solution was used to surface sterilize the seeds. All seeds were soaked in it for 30 min, followed by three rinses with distilled water. Seeds were grown in plastic pots under natural environmental conditions after radicles emerged, and the germination period for the soil experiment was 2 July 2021 (day temperature: 36 °C and night temperature: 27 °C). A randomized complete block design (RCBD) was used in this experiment with six replications and contained five treatments (100, 200, 300, 400, and 500 mM) of iron sulfate (FeSO4) along with control as shown in Figure 1. The soil used in this study had the following physio-chemical properties: EC (0.60 dS m−1), pH 7.5, organic matter content 0.2%, available phosphorus (3.1 mg kg−1) 3.5, available potassium (62 mg kg−1), iron (3.16 mg kg−1), saturation 24%, and a silty loam texture. After the application of FeSO4. The pH of the soil decreased slightly after FeSO4 application but remained constant at pH 7.5 throughout the experiment when sodium carbonate was added. Potassium and phosphorus decreased when the pH was changed to acidic, but iron content increased to 3.9 mg kg−1.
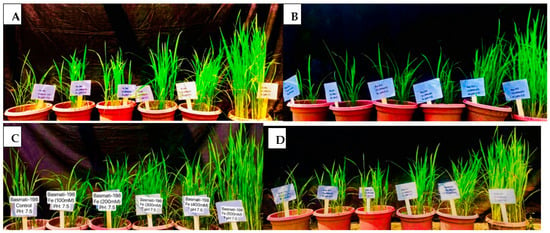
Figure 1.
Assignment of pots according to the randomized complete block design (RCBD). In all cases, a design placed with 5 treatments (100, 200, 300, 400 and 500 mM) of FeSO4 along with control in alkaline soil (pH 7.5) for four cultivars of O. sativa, including PK-386 (A), Basmati-515 (B), Basmati-198 (C) and KSK-133 (D).
2.2. Determination of Morphological Data
Plants were harvested 25 days following sowing in order to collect morphological data. To accomplish this, distilled water is used to clean the harvested plants of dust and dirt. Plant height (cm), root and shoot length (cm), root and shoot fresh weight (g), root and shoot dry weight (g) were measured as morphological parameters. Seed germination percentage was determined by using the method of [29].
2.3. Determination of Relative Water Contents (RWC%)
Using the procedure of [30], the relative water contents were measured. Fresh leaves were weighed and they were subsequently immersed in 10 mL distilled water for 24 h, then turgid weight was observed. After oven drying at 70 °C, dried weight was measured by formula:
RWC (%) = [Wf (fresh weight) − Wd (dry weight)/(Wt (turgid weight) − Wd (dry weight)] × 100
2.4. Determination of Photosynthetic Pigments (Chlorophylls and Carotenoids)
Fresh leaf from each plant in each treatment was used to estimate the total chlorophyll and carotenoids content. Each plant’s fresh leaf mass, weighing 0.1 g, was submerged in falcon tubes filled with 95% ethanol. The leaves were kept at room temperature and incubated for 48 h in the dark. The absorbance of the extracts was then measured using a spectrophotometer at 663, 645, and 480 nm. The procedures described by [31,32], respectively, were used to measure the content of chlorophyll and carotenoids.
2.5. Determination of Antioxidant Enzymatic Activities
Fresh plant tissue (root and leaf) weighing 0.2 g was obtained, and extraction was done in liquid nitrogen and phosphate buffer (pH 7.0). Then, extract was centrifuged for 20 min at 4 °C at 12,000 rpm. Supernatants were discarded, and enzyme extracts were stored in additional Eppendorf tubes and kept in a −20 °C refrigerator for biochemical and antioxidant analyses. The rate of H2O2 degradation at 240 nm was used to measure catalase activity. A measure of 25 µL of enzyme extracts was added to 50 mM PBS reaction mixture (pH 7.4), and 15 mM H2O2 by method of [33]. According to [34], the activity of ascorbate peroxidase (APX) was evaluated. For assessing APX activity, a solution comprising enzyme extract of 100 μL, 7.5 mM ascorbate, 300 Mm of H2O2, and 25 mM potassium phosphate buffer was used. The fluctuations in wavelength at 290 nm were used to predict the ascorbate oxidation pattern.
The method [35] was used to measure the POD activity in O. sativa by measuring absorbance at 470 nm. SOD activity in the root and leaf of O. sativa was determined by the method of [36]. GPX was determined by using the [37] method, which involved the oxidation of guaiacol. Using a spectrophotometer, variations in absorbance at 460 nm were measured every 10 s for 60 s. In 70 mL of ethanol, 2 mg of total DPPH was dissolved, and the mixture was agitated for 24 h [38]. Next, combine 3 mL of DPPH and 1 mL of water extract in a tube, and then use a spectrophotometer to record the reading (517 nm). DPPH activity was determined using [39].
2.6. Determination of Oxidative Stress
To evaluate the H2O2 content, 3 mL of sample extract, 1 mL of H2SO4, and 0.1% titanium sulphate were mixed, then centrifuged (6000× g) for 15 min. At 410 nm, the absorbance was measured. The procedure of [40] was used to measure the amount of H2O2.
The amount of malondialdehyde (MDA) was used to measure the extent of lipid peroxidation. For this method, phosphate buffer (50 mM) of pH 7.8 with 1% polyethylene pyrrole was used to grind 0.1 g of frozen roots and leaves to centrifuging the mixture at 10,000× g for 15 min at 4 °C. According to [41], the amount of MDA was determined using corrected absorbance and an extinction value of 155 nM−1 cm−1.
2.7. Statistical Analysis
SPSS 22.0 was used for the statistical analysis of the data, and the analysis of variance (ANOVA) was used for multiple treatment comparisons, the least significant difference test (p < 0.05) was used. Origin-Pro 2017 was used to create the graphical presentation.
3. Results
3.1. Effect of FeSO4 on Morphological Parameters in Four O. sativa Cultivars
In the present research work, we elucidated various growth parameters under various levels (100, 200, 300, 400, and 500 mM) of FeSO4 in alkaline soil of pH 7.5 in four cultivars. We illustrate the different morphological characteristics of each rice cultivar in Figure 2 and leaf area in Figure 3A. Present results showed that all morphological parameters (plant height, shoot and root length, root and shoot dry weight, root and shoot fresh weight, and germination rate) in all cultivars were increased with the increase in the levels of FeSO4 in comparison to control. Plant height reached its maximum at 500 mM, with 45.5, 39, 44, and 47.2% in Basmati-515, KSK-133, PK-386, and Basmati-198, respectively, Figure 2A.
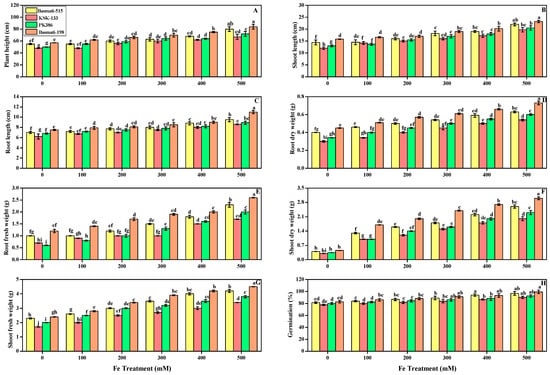
Figure 2.
Effect of different FeSO4 concentrations (0, 100, 200, 300, 400, and 500 mM) on the morphological characteristics of Oryza sativa (cultivars), namely total plant height (A), shoot and root length (B,C), root dry weight and fresh weight (D,E), shoot dry and shoot fresh weight (F,G), and germination percentage (H). Different small letters above the bars denote significant differences, determined by Duncan’s multiple test. Data presented are the average of six replicates (n = 6). Error bars represent the standard deviation (SD) of six replicates.
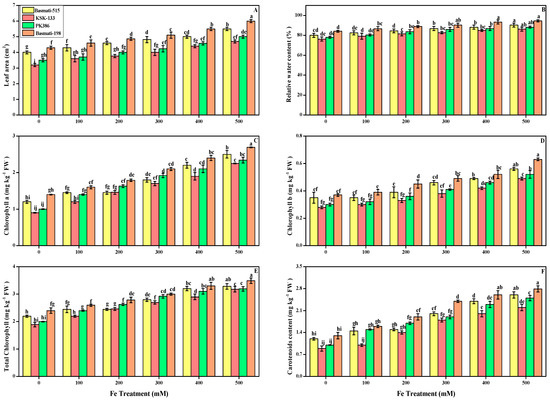
Figure 3.
Effect of different FeSO4 concentrations (0, 100, 200, 300, 400, and 500 mM) on the leaf area, relative water content, and photosynthetic pigment of O. sativa (cultivars), namely leaf area (A), relative water content (B), chlorophyll a (C), chlorophyll b (D), total chlorophyll (E), and carotenoids (F). Different small letters above the bars denote significant differences, determined Duncan’s multiple test. Data presented are the average of six replicates (n = 6). Error bars represent the standard deviation (SD) of six replicates.
Similarly, among all FeSO4 levels, the highest level of 500 mM produced significant high results in all other parameters (shoot and root length, root and shoot dry weight, root and fresh weight, and germination rate). Furthermore, results showed that Basmati-198 showed more significant results for the iron treatment in comparison to the other varieties Basmati-515, KSK-133, and PK-386 at all levels of FeSO4 in the alkaline soil. However, the germination percentage was not significantly different in Basmati-198 and Basmati-515 at all levels. Both showed the highest percentage, with 99 and 98%, respectively, in Figure 2H.
3.2. Effect of FeSO4 on Leaf Area, Relative Water Content, and Photosynthetic Contents
In this study, we also elucidated relative water content and photosynthetic pigments under various levels of FeSO4 in cultivars of O. sativa in Figure 3. Our results showed that increasing concertation of FeSO4 induced the increase (p < 0.05) in the photosynthetic pigments. Relative water content peaked at 500 mM, with 12.85, 13, 13, and 12.5% in Basmati-515, KSK-133, PK-386, and Basmati-198, respectively, in comparison to their relative control in Figure 3B. No significant difference was observed between Basmati-198 and Basmati-515 at the levels of 400 and 500 mM.
Similarly, among all FeSO4 levels, the highest levels of 300, 400, and 500 mM produced significant high chlorophyll content in all O. sativa. Furthermore, carotenoids in KSK-133 were significantly low in comparison to all other cultivars while significantly high when compared with their relative control plants at all levels of FeSO4. At the level of 100 mM, carotenoids were increased by 12.3% in iron-treated plants, while 57, 102, 176, and 226% increases were observed at the other levels of 200, 300, 400, and 500 mM, respectively, in KSK-133 Figure 3F.
3.3. Influence of FeSO4 on Antioxidant Enzymes in Four O. sativa Cultivars
In the current work, we also assessed the antioxidant enzymes from leaves and roots of all cultivars grown in alkaline soil with the application of FeSO4. Figure 4 illustrates the findings of the antioxidants including catalase (CAT), ascorbate peroxidase (APX), peroxidase (POD), and superoxide dismutase (SOD) from the roots and leaves of O. sativa cultivars and glutathione peroxidase (GPX) in Figure 5. The findings also revealed that increasing the concentration of FeSO4 caused a significant (p < 0.05) increase in CAT activity even at a small concentration of 100 mM by 98 and 152% in leaves and roots, respectively. At all FeSO4 levels in the alkaline soil, Basmati-198 outperformed the other cultivars, Basmati-515, KSK-133, and PK-386. As CAT activity increased in Basmati-198, increase in all other antioxidant activities, including APX (69 and 55%), POD (86 and 166%), and SOD (108 and 133%), GPX (143 and 97%) was observed in leaves and roots at a level of 500 mM (Figure 4 and Figure 5A).
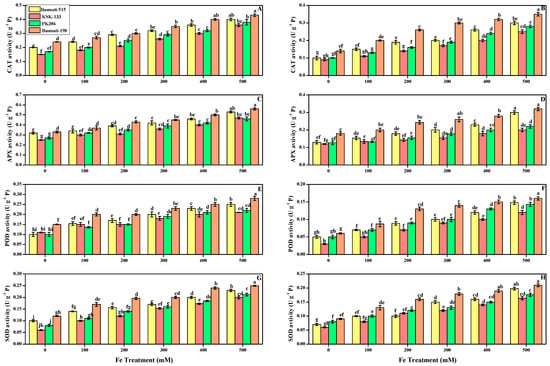
Figure 4.
Effect of different FeSO4 concentrations (0, 100, 200, 300, 400, and 500 mM) on the antioxidant enzymes in the leaves and roots of O. sativa (cultivars), namely CAT activity in leaf (A), and root (B), APX activity in leaf (C) and root (D), POD activity in leaf (E) and root (F), SOD activity in leaf (G) and root (H). Different small letters above the bars denote significant differences, by Duncan’s multiple test. Data presented are the average of six replicates (n = 6). Error bars represent the standard deviation (SD) of six replicates.
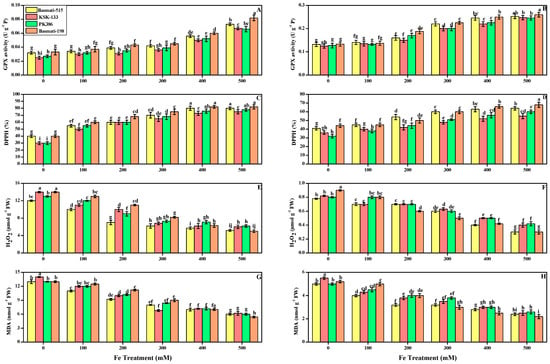
Figure 5.
Effect of different FeSO4 concentrations (0, 100, 200, 300, 400, and 500 mM) on the antioxidant and oxidative stress markers in leaves and roots of O. sativa cultivars), namely GPX in leaf (A), and in root (B), DPPH in leaf (C), and root (D), H2O2 in leaf (E), and root (F), MDA in leaf (G), and root (H). Different small letters above the bars denote significant differences, determined by Duncan’s multiple test. Data presented are the average of six replicates (n = 6). Error bars represent the standard deviation (SD) of six replicates.
3.4. Effect of FeSO4 on DPPH and Oxidative Stress in Roots and Leaves of Four O. sativa Cultivars
Oxidative stress, such as malondialdehyde (MDA) content, hydrogen peroxide (H2O2), and total antioxidants (DPPH) were also measured in the present research work. The results about DPPH, MDA, and H2O2 in O. sativa cultivars grown in alkaline soil with different iron treatments are shown in Figure 5. We also elucidated that the increase in the concentration of FeSO4 from 100 to 500 mM significantly increased the DPPH by 45, 50, 42, and 36.6% in the leaves of Basmati-515, KSK-133, PK-386, and Basmati-198 in Figure 5C, and at the level of 400 and 500 mM with the value of 45 and 36.6% in Basmati-515 and Basmati-198, respectively. Similarly, among all O. sativa cultivars, DPPH activity in roots was high in Basmati-198 by 54% in Figure 5D.
A decrease in oxidative stress in O. sativa cultivars is shown in Figure 5E–H. From the given results, we also elucidated that H2O2 and MDA content decreased significantly by increasing in the concentration of FeSO4 in plant tissue of all cultivars in comparison to control or treated with low concentration of iron. H2O2 significantly reduced by 56, 60, 54, and 66% in the leaves of Basmati-515, KSK-133, PK-386, and Basmati-198 at the concentration of 500 Mm in Figure 5E. FeSO4 in the roots reduced significantly by 61, 51, 47, and 66% in the leaves of Basmati-515, KSK-133, PK-386, and Basmati-198 in Figure 5F at highest concentration (500 mM) of FeSO4. Similarly, the highest concentration (500 mM) of FeSO4 reduced MDA in the leaves and roots of O. sativa cultivar Basmati-198 by 62 and 57%, respectively.
3.5. Effect of FeSO4 on Non-Enzymatic Antioxidants in Leaves of Four O. sativa Cultivars
In the current study, we also identified certain non-enzymatic activities of O. sativa cultivars when different iron concentrations were applied, containing total carbohydrates, free amino acids, proline, soluble sugar, reducing and non-reducing sugar, phenolic, and flavonoids. The data related to all antioxidants are presented in Figure 6. The findings demonstrated that all non-antioxidant activities significantly increased with increasing FeSO4 concentration. We further determined from the results that the increasing FeSO4 concentration caused a significant increase in soluble sugars by 160, 206, 172, and 120% in the leaves of Basmati-515, KSK-133, PK-386, and Basmati-198 at the level of 500 mM in Figure 6A. Reducing and non-reducing sugar also showed the same pattern in increasing sugar level by increasing iron treatment. Among all the varieties, Basmati-198 showed the highest average of reducing and non-reducing sugar by 246 and 134%, respectively, Figure 6B,C.

Figure 6.
Effect of different FeSO4 concentrations (0, 100, 200, 300, 400, and 500 mM) on the non-antioxidant activities in the leaves of O. sativa (cultivars), namely soluble sugar (A), reducing sugar (B), non-reducing sugar (C), flavonoids (D), phenolics (E), proline (F), free amino acid (G), total carbohydrates (H). Different small letters above the bars denote significant differences, investigated by Duncan’s multiple test. Data presented are the average of six replicates (n = 6). Error bars represent the standard deviation (SD) of six replicates.
Flavonoid content increased significantly in all O. sativa cultivars at the level of 400 and 500 mM, Figure 6D. Furthermore, phenolic content increased by 14, 200, 266, and 166% in Basmati-515, KSK-133, PK-386, and Basmati-198, respectively, Figure 6E. Similarly, the highest concentration of FeSO4 induced a significant increase in proline, free amino acid, and total carbohydrates in the leaves of all O. sativa cultivars, but Basmati-198 showed the highest by 169, 88, and 110%, respectively, when compared with those plants which are iron deficient or treated with low concentration of iron Figure 6F–H.
4. Discussion
At least one of the following micronutrients—iron, zinc, selenium, calcium, and vitamins—is deficient in people. Undernutrition and micronutrient deficiencies make a significant contribution in burden of diseases around the world [42]. Fe and Zn, which are two necessary minerals for health, are the most common micronutrient deficiencies globally [43]. Globally, alkaline soil, as well as those with anaerobic conditions, exhibit micronutrient shortages [44]. Depending on the soil type and soil pH, different micronutrient amounts are required [45,46]. According to [47], soils in humid climates are typically acidic with low pH, whereas soils in arid climates are typically alkaline with high soil pH. One of the key elements influencing plant nutrient availability [48], microbial activity [49,50], and crop growth [51,52] is soil pH [53]. Several of the responses associated with Fe deficiency are increased by alkalinity, even when plants have a regular supply of iron [54] and Fe deficiency was the main abiotic stresses [55,56] that reduced the yield and quality of crops [57].
Our current findings revealed that plants with low levels of Fe, significantly decreased in plant height, shoot and root fresh and dry weight, germination percentage, leaf chlorophyll content, carotenoid content, antioxidants, amino acids and carbohydrates, while oxidative stress was significantly increased in all O. sativa cultivars (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). Numerous studies have also identified a decline in growth-related characteristics of O. sativa cultivars [23], which our current investigation also revealed in Figure 2 and Figure 3. Another study reported that plants were grown under the application of zinc and iron enhanced the plant growth parameters by decreasing the oxidative stress. With NP, especially at higher NP rates, plant height, panicle length, shoot, root, panicle, and grain dry weight all rose [58]. Under Fe deficient conditions, chl a, chl b, total chlorophyll, and carotene content significantly decreased as in Figure 3. Plants contain Fe in their photosynthetic cells. Therefore, by an iron shortage, the constituent proteins of chloroplasts are adversely affected, resulting in chlorotic plant leaves and decreased photosynthetic efficiency in photosynthetic cells [59]. By applying various treatments of FeSO4, the morphological and photosynthetic characteristics primarily demonstrated positive results (Figure 3). This is because plants need iron for optimal growth and development, as well as because their metabolism depends on it for both enzymatic and metabolic processes [60]. Seed germination percentage was high at the highest level of FeSO4 (400 and 500 mM) in all rice cultivars. In rice and maize, 500 ppm of nanoscale Fe2O3 treatment resulted in the highest seed germination percentage (100%) and seedling vigor index values [61].
As shown by [62], there was an increase in total chlorophyll and carotenoid content. Another study reported a similar finding that the application of micronutrients such as Zn, Fe, Mn, and Cu in wheat varieties shows a significant increase in crop growth rate, plant height, and yield. This result also suggests that during the growth, the application of FeSO4 improved photosynthesis pigments (chlorophyll and carotenoid contents) [63].
At the highest concentration (500 mM), chlorophyll content was significantly high in all O. sativa cultivars. Iron is involved in the synthesis of chlorophyll, and it is essential for the maintenance of chloroplast structure and function [10,64,65,66]. According to [67], plant height, productive tillers, and yield were increased significantly with the treatment of Zn and Fe levels compared to control. In contrast to our findings, another study reported that protein content increased but fiber and water content significantly decreased with an increase in the iron and phosphorus fertility rates in wheat [68].
Plants in stressful conditions display an imbalance of free radicals and a dysregulated reactive oxygen metabolism, which leads to the buildup of ROS, which in turn causes and accelerates lipid peroxidation and culminates in the breakdown of membrane integrity [69,70]. In the present study, an integrative overview of antioxidant enzymatic (CAT, APX, POD, SOD, GPX, DPPH) and non-enzymatic activity (proline content, free amino acids, soluble sugar, reducing and non-reducing sugar, flavonoids, total soluble protein, phenolics, and total carbohydrates) showed that iron deficiency causes an increase in oxidative damage. To deal with this situation, the use of FeSO4 increased antioxidant enzymatic and non-enzymatic activity (Figure 4, Figure 5 and Figure 6). The signaling molecules for oxidative stress were proposed to have the potential to stimulate the antioxidant machinery [71,72,73,74], which could play a significant role in the Fe efficiency characteristic [75]. According to reports for many crops, excessive reactive oxygen species (ROS) synthesis results in oxidative stress [76,77,78,79,80,81]. Both electron transfer to O2 and the production of free radicals such as O−2 and OH− to produce singlet oxygen can contribute to this stress [82,83,84]. The species of the plant also affects how it responds to oxidative stress. A number of antioxidant enzymes, including as SOD, POD, CAT, and APX, help to reduce ROS [85]. The present study revealed that all antioxidants (CAT, APX, POD, SOD, GPX, DPPH), proline content, free amino acids, soluble sugar, reducing and non-reducing sugar, flavonoids, total soluble protein, phenolics, and total carbohydrates were also increased at an increasing level. The significant increase was observed at the highest level (500 mM) of FeSO4 as compared to normal (iron-deficient plants). Antioxidant activity rose in wheat leaves compared to control when treated with micronutrients [85]. Additionally, SOD, POD, and CAT all contain Fe, which has an impact on their biological functions [86,87,88]. SOD activity in the leaves and roots of Fe-deficient plants somewhat increased, whereas POD and CAT and other activity in the plants dropped by 52% and 35%, respectively, in comparison to the control. In comparison to non-treated plants, MDA concentration rose in leaves and roots under iron deficiency. However, Ref. [89] investigated that under high Fe deficiency condition, antioxidant enzymatic activities were decreased in plant tissue. As a result, antioxidant enzyme activity of different plants varied towards Fe deficiency. Our current study revealed that the reactive oxygen was disrupted as a result of Fe deficiency, consequential high accumulation of MDA. These findings demonstrated that severe Fe deficiency reduced ability to scavenge reactive oxygen species by increasing oxidative stress, which interfered with the normal growth of O. sativa. Furthermore, according to our study, application of iron increased the antioxidant enzymatic and non-enzymatic activity. All plants must have micronutrients such as Fe in order to grow and develop. According to [90], iron is a crucial component of most redox reactions, and it is essential for numerous cellular processes, proteins, and structural and catalytic enzymes.
In addition to antioxidant enzymes non-enzymatic activity, we determined antioxidant non-enzymatic activity (reducing and non-reducing sugar, soluble sugar, phenolic, flavonoids, proline, free amino acid, and total carbohydrates) from the leaves of O. sativa cultivars when different iron concentrations were applied (Figure 6). Common plant natural compounds such as flavonoids have the ability to reduce oxidative stress and inhibit ROS-related damage [91,92,93,94]. There is evidence that the content of total phenolics and total flavonoids was raised by applying nano-Fe and Zn treatments to the leaves of Rosmarinus officinalis [21]. In our study, the phenolic content increased by 14, 200, 266, and 166% in Basmati-515, KSK-133, PK-386, and Basmati-198, respectively (Figure 6E). The phenolic compound content of pea plants was increased by foliar spraying them with micronutrients, which are key phytochemical components with substantial antioxidant potential [95]. Other non-enzymatic proteins, free amino acids, and carbohydrates were also increased, and the significantly high results was noted at the high concentration of 500 mM. These findings are consistent with earlier research by [96], particularly at high concentrations (3 g/L), which revealed an increase in total soluble protein and total soluble carbohydrates in bean plants compared to controls with Zn and Fe. Our study showed that Fe has a positive effect on plant growth, antioxidant enzymatic, and non-enzymatic activity by decreasing oxidative stress because iron is essential for both plant productivity and nutritional quality.
5. Conclusions
The above-mentioned findings lead to the conclusion that FeSO4 can be applied externally to soil to counteract the deleterious effects of alkaline soil on soil iron content. The present research work showed that soil application of FeSO4 improved seed germination, plant growth, antioxidant enzymatic and non-enzymatic activity, and denatured the ROS in alkaline soil. In comparison to all O. sativa cultivars, Basmati-198 showed significantly higher results than others at a high concentration of 500 mM. It was found that rice plants treated with 500 mM FeSO4 showed better results than plants treated with other concentrations such as 100, 200, 300, and 400 mM. Iron sulfate, which likewise reduced ROS, caused changes in the plants’ ultrastructure, and increased the activity of their antioxidants Therefore, in order to understand the underlying mechanisms, long-term field investigations should be carried out at the molecular level to examine patterns of iron uptake and plant growth.
Author Contributions
Conceptualization, A.S.; data curation, B.A. and S.H.; formal analysis, A.Z. and B.A.; funding acquisition, M.A.N., A.S.A. and S.H.; investigation, A.S.; methodology, A.Z.; software, A.S.A.; validation, A.Z.; visualization, M.A.N.; writing—original draft, A.S.; writing—review and editing, A.Z., B.A., M.A.N., A.S.A. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Institutional Fund projects under grant no. (IFPDP-91-22). Therefore, the authors gratefully acknowledge technical and financial support from the Ministry of Education and King Abdulaziz University, Deanship of Scientific Research, Jeddah, Saudi Arabia, for technical and financial support.
Institutional Review Board Statement
None of the authors of this article have conducted any experimental investigations using humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, Q.; Zhang, C.; Chan, M.; Zhao, D.; Chen, J.; Wang, Q.; Li, Q.; Yu, H.; Gu, M.; Sun, S.S. Biofortification of rice with the essential amino acid lysine: Molecular characterization, nutritional evaluation, and field performance. J. Exp. Bot. 2016, 67, 4285–4296. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ge, T.; van Groenigen, K.J.; Yang, Y.; Wang, P.; Cheng, K.; Zhu, Z.; Wang, J.; Li, Y.; Guggenberger, G. Rice paddy soils are a quantitatively important carbon store according to a global synthesis. Commun. Earth Environ. 2021, 2, 154. [Google Scholar] [CrossRef]
- Marschner, P.; Rengel, Z. Nutrient availability in soils. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 315–330. [Google Scholar]
- Thakur, A.; Singh, S.; Singh, N.; Ali, B.; Hafeez, A.; Vodnar, D.C.; Marc, R.A. Nutritional evaluation, phytochemical makeup, antibacterial and antioxidant properties of wild plants utilized as food by the Gaddis—A tribal tribe in the Western Himalayas. Front. Agron. 2022, 114. [Google Scholar] [CrossRef]
- Wakeel, A.; Farooq, M.; Bashir, K.; Ozturk, L. Micronutrient malnutrition and biofortification: Recent advances and future perspectives. Plant Micronutr. Use Effic. 2018, 225–243. [Google Scholar] [CrossRef]
- Kumar, D.; Rao, S.K.; Kumar, A.; Singh, T.B. Risk factors of mortality in hospitalized children with severe acute malnutrition. Indian J. Pediatr. 2019, 86, 1069. [Google Scholar] [CrossRef]
- Afridi, M.S.; Ali, S.; Salam, A.; César Terra, W.; Hafeez, A.; Sumaira; Ali, B.; S. AlTami, M.; Ameen, F.; Ercisli, S.; et al. Plant Microbiome Engineering: Hopes or Hypes. Biology 2022, 11, 1782. [Google Scholar] [CrossRef]
- Bana, R.S.; Jat, G.S.; Grover, M.; Bamboriya, S.D.; Singh, D.; Bansal, R.; Choudhary, A.K.; Kumar, V.; Laing, A.M.; Godara, S. Foliar Nutrient Supplementation with Micronutrient-Embedded Fertilizer Increases Biofortification in Eggplant Fruit and Soil Biological Activity While Enhancing Plant Productivity. Sci. Rep. 2022, 12, 5146. [Google Scholar] [CrossRef]
- Svenson, N.J.; Patmore, R.; Cox, H.J.; Bailey, J.R.; Holding, S. Iron Age or New Age: Ironing out the Diagnosis of Anaemia of Inflammation from Iron Deficiency Anaemia. Blood 2015, 126, 3354. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Yasmeen, S.; Wahab, A.; Saleem, M.H.; Ali, B.; Qureshi, K.A.; Jaremko, M. Melatonin as a Foliar Application and Adaptation in Lentil (Lens culinaris Medik.) Crops under Drought Stress. Sustainability 2022, 14, 16345. [Google Scholar] [CrossRef]
- Waters, B.M.; Amundsen, K.; Graef, G. Gene expression profiling of iron deficiency chlorosis sensitive and tolerant soybean indicates key roles for phenylpropanoids under alkalinity stress. Front. Plant Sci. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, S.; Gaur, S.; Singh, S.; Yadav, V.; Liu, S.; Singh, V.P.; Sharma, S.; Srivastava, P.; Prasad, S.M. Acquisition and homeostasis of iron in higher plants and their probable role in abiotic stress tolerance. Front. Environ. Sci. 2018, 5, 86. [Google Scholar] [CrossRef]
- Metayi, M.H.; Abd El-Naby, S.S.; El-Habal, N.A.; Fahmy, H.H.; Abdou, M.S.; Ali, B.; Abdel-Rheim, K.H.; Abdel-Megeed, A. Omani Frankincense nanoemulsion formulation efficacy and its latent effects on biological aspects of the spiny bollworm Earias insulana (Boisd.). Front. Physiol. 2022, 13, 2129. [Google Scholar] [CrossRef] [PubMed]
- Cesco, S.; Nikolic, M.; Römheld, V.; Varanini, Z.; Pinton, R. Uptake of 5 9Fe from soluble 5 9Fe-humate complexes by cucumber and barley plants. Plant Soil 2002, 241, 121–128. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Shukla, A.K.; Behera, S.K.; Tripathi, R.; Prakash, C.; Nayak, A.K.; Kumar, P.S.; Chitdeshwari, T.; Kumar, D.; Nayak, R.K.; Babu, P.S. Evaluation of spatial spreading of phyto-available sulphur and micronutrients in cultivated coastal soils. PLoS ONE 2021, 16, e0258166. [Google Scholar] [CrossRef]
- Connorton, J.M.; Balk, J. Iron biofortification of staple crops: Lessons and challenges in plant genetics. Plant Cell Physiol. 2019, 60, 1447–1456. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and iron agronomic biofortification of Brassicaceae microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef]
- Saha, S.; Chakraborty, M.; Padhan, D.; Saha, B.; Murmu, S.; Batabyal, K.; Seth, A.; Hazra, G.C.; Mandal, B.; Bell, R.W. Agronomic biofortification of zinc in rice: Influence of cultivars and zinc application methods on grain yield and zinc bioavailability. Front. Crop. Res. 2017, 210, 52–60. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Mehrabani, L.V.; Tzortzakis, N. Foliar application of nano-zinc and iron affects physiological attributes of Rosmarinus officinalis and quietens NaCl salinity depression. J. Soil Sci. Plant Nutr. 2020, 20, 335–345. [Google Scholar] [CrossRef]
- Valizadeh-Kamran, R.; Toorchi, M.; Mogadam, M.; Mohammadi, H.; Pessarakli, M. Effects of freeze and cold stress on certain physiological and biochemical traits in sensitive and tolerant barley (Hordeum vulgare) genotypes. J. Plant Nutr. 2018, 41, 102–111. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Qiu, J.; Wang, H.; Wang, S.; Tang, L.; Tong, X.; Zhang, J. Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’ pathway to synergistically inhibit seed germination in rice (Oryza sativa). New Phytol. 2020, 228, 1336–1353. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Tanumihardjo, S.A. An integrated approach to evaluate food antioxidant capacity. J. Food Sci. 2007, 72, R159–R165. [Google Scholar] [CrossRef] [PubMed]
- Abdelmajid, K.; Karim, B.H.; Chedly, A. Symbiotic response of common bean (Phaseolus vulgaris L.) to iron deficiency. Acta Physiol. Plant. 2008, 30, 27–34. [Google Scholar] [CrossRef]
- Tewari, R.K.; Hadacek, F.; Sassmann, S.; Lang, I. Iron deprivation-induced reactive oxygen species generation leads to non-autolytic PCD in Brassica napus leaves. Environ. Exp. Bot. 2013, 91, 74–83. [Google Scholar] [CrossRef]
- Haider, M.W.; Nafees, M.; Ahmad, I.; Ali, B.; Maryam, B.; Iqbal, R.; Vodnar, D.C.; Marc, R.A.; Kamran, M.; Saleem, M.H.; et al. Postharvest dormancy-related changes of endogenous hormones in relation to different dormancy-breaking methods of potato (Solanum tuberosum L.) tubers. Front. Plant Sci. 2022, 13, 945256. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Naseer, S.; Saleem, A.; Sabar, M.; Ahmed, S.; Sardar, R.; Shahzadi, F.; Raza, Q. Genetic diversity studies for grain iron and zinc content analysis for Elite rice (Oryza sativa L.) genotype by using SSR markers. J. Food Compos. Anal. 2023, 115, 104816. [Google Scholar] [CrossRef]
- Djavanshir, K.; Pourbeik, H. Germination value—A new formula. Silvae Genet. 1976, 25, 79–83. [Google Scholar]
- Sade, N.; Galkin, E.; Moshelion, M. Measuring Arabidopsis, tomato and barley leaf relative water content (RWC). Bio-Protocol 2015, 5, e1451. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. ISBN 0076-6879. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Sakharov, I.Y.; Ardila, G.B. Variations of peroxidase activity in cocoa (Theobroma cacao L.) beans during their ripening, fermentation and drying. Food Chem. 1999, 65, 51–54. [Google Scholar] [CrossRef]
- Chen, C.-N.; Pan, S.-M. Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot. Bull. Acad. Sin. 1996, 37, 107–111. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar]
- Cervato, G.; Carabelli, M.; Gervasio, S.; Cittera, A.; Cazzola, R.; Cestaro, B. Antioxbdant properties of oregano (Origanum vulgare) leaf extracts. J. Food Biochem. 2000, 24, 453–465. [Google Scholar] [CrossRef]
- Marinova, G.; Batchvarov, V. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms: Effect of heavy metals. Aquat. Bot. 1981, 11, 67–77. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Beal, T.; Massiot, E.; Arsenault, J.E.; Smith, M.R.; Hijmans, R.J. Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS ONE 2017, 12, e0175554. [Google Scholar] [CrossRef]
- Shekari, F.; Mohammadi, H.; Pourmohammad, A.; Avanes, A.; Benam, M.B.K. Spring wheat yielding and the content of protein and zinc in its grain depending on zinc fertilisation. Electron. J. Polish Agric. Univ. 2015, 18, 1–12. [Google Scholar]
- Kalra, T.; Tomar, P.C.; Arora, K. Micronutrient encapsulation using nanotechnology: Nanofertilizers. Plant Arch. 2020, 20, 1748–1753. [Google Scholar]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K.; et al. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- Umar, U.d.; Ahmed, N.; Zafar, M.Z.; Rehman, A.; Naqvi, S.A.H.; Zulfiqar, M.A.; Malik, M.T.; Ali, B.; Saleem, M.H.; Marc, R.A. Micronutrients Foliar and Drench Application Mitigate Mango Sudden Decline Disorder and Impact Fruit Yield. Agronomy 2022, 12, 2449. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008; Volume 13. [Google Scholar]
- Adnan, M.; Fahad, S.; Saleem, M.H.; Ali, B.; Mussart, M.; Ullah, R.; Arif, M.; Ahmad, M.; Shah, W.A.; Romman, M. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 2022, 12, 11997. [Google Scholar] [CrossRef]
- Al-Zaban, M.I.; Alhag, S.K.; Dablool, A.S.; Ahmed, A.E.; Alghamdi, S.; Ali, B.; Al-Saeed, F.A.; Saleem, M.H.; Poczai, P. Manufactured Nano-Objects Confer Viral Protection against Cucurbit Chlorotic Yellows Virus (CCYV) Infecting Nicotiana benthamiana. Microorganisms 2022, 10, 1837. [Google Scholar] [CrossRef]
- Solanki, M.K.; Solanki, A.C.; Rai, S.; Srivastava, S.; Kashyap, B.K.; Divvela, P.K.; Kumar, S.; Yandigeri, M.S.; Kashyap, P.L.; Shrivastava, A.K.; et al. Functional interplay between antagonistic bacteria and Rhizoctonia solani in the tomato plant rhizosphere. Front. Microbiol. 2022, 13, 990850. [Google Scholar] [CrossRef]
- Saini, A.; Manuja, S.; Kumar, S.; Hafeez, A.; Ali, B.; Poczai, P. Impact of Cultivation Practices and Varieties on Productivity, Profitability, and Nutrient Uptake of Rice (Oryza sativa L.) and Wheat (Triticum aestivum L.) Cropping System in India. Agriculture 2022, 12, 1678. [Google Scholar] [CrossRef]
- Hussain, S.S.; Rasheed, M.; Hamzah Saleem, M.; Ahmed, Z.I.; Hafeez, A.; Jilani, G.; Alamri, S.; Hashem, M.; Ali, S. Salt tolerance in maize with melatonin priming to achieve sustainability in yield on salt affected soils. Pak. J. Bot. 2023, 55, 1. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Wu, W.; Liu, H. Factors affecting variations of soil pH in different horizons in hilly regions. PLoS ONE 2019, 14, e0218563. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, H.; Wang, N.; Li, J.; Zhao, W.; Du, J.; Wang, D.; Ling, H.-Q. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 2008, 18, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Khan, M.S.; Hafeez, A.; Fazil, M.; Khan, M.N.; Ali, B.; Javed, M.A.; Imran, M.; Shati, A.A.; Alfaifi, M.Y. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted industrial soils. Braz. J. Biol. 2022, 84, e264473. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Ullah, S.; Hafeez, A.; Khan, M.N.; Javed, M.A.; Ali, B.; Din, I.U.; Bangash, S.A.K.; Wahab, S.; Wahid, N. Exogenous Ca/Mg quotient reduces the inhibitory effects of PEG induced osmotic stress on Avena sativa L. Braz. J. Biol. 2022, 84, e264642. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Adnan, M.; Basir, A.; Fahad, S.; Hafeez, A.; Saleem, M.H.; Ahmad, M.; Gul, F.; Durrishahwar, F.; Subhan, F. Impact of tillage and potassium levels and sources on growth, yield and yield attributes of wheat. Pak. J. Bot. 2022, 55. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Ur Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Roncel, M.; González-Rodríguez, A.A.; Naranjo, B.; Bernal-Bayard, P.; Lindahl, A.M.; Hervás, M.; Navarro, J.A.; Ortega, J.M. Iron deficiency induces a partial inhibition of the photosynthetic electron transport and a high sensitivity to light in the diatom Phaeodactylum tricornutum. Front. Plant Sci. 2016, 7, 1050. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Imran, M.; Alnusairi, G.S.H.; Alharbi, B.M.; Riaz, M.; Abbas, Z.; Rizwan, M.; Soliman, M.H. Role of iron–lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol. Biochem. 2020, 155, 70–84. [Google Scholar] [CrossRef]
- Kasivelu, G.; Selvaraj, T.; Malaichamy, K.; Kathickeyan, D.; Shkolnik, D.; Chaturvedi, S. Nano-micronutrients [γ-Fe2O3 (iron) and ZnO (zinc)]: Green preparation, characterization, agro-morphological characteristics and crop productivity studies in two crops (rice and maize). New J. Chem. 2020, 44, 11373–11383. [Google Scholar] [CrossRef]
- Ghafari, H.; Razmjoo, J. Response of Durum Wheat to Foliar Application of Var ied Sources and Rates of Iron Fertilizers. J. Agric. Sci. Technol. 2018, 17, 321–331. [Google Scholar]
- Aziz, A.; Basheer, F.; Sengar, A.; Khan, S.U.; Farooqi, I.H. Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci. Total Environ. 2019, 686, 681–708. [Google Scholar] [CrossRef]
- Ahmad, M.; Ishaq, M.; Shah, W.A.; Adnan, M.; Fahad, S.; Saleem, M.H.; Khan, F.U.; Mussarat, M.; Khan, S.; Ali, B.; et al. Managing Phosphorus Availability from Organic and Inorganic Sources for Optimum Wheat Production in Calcareous Soils. Sustainability 2022, 14, 7669. [Google Scholar] [CrossRef]
- Saeed, S.; Ullah, A.; Ullah, S.; Noor, J.; Ali, B.; Khan, M.N.; Hashem, M.; Mostafa, Y.S.; Alamri, S. Validating the Impact of Water Potential and Temperature on Seed Germination of Wheat (Triticum aestivum L.) via Hydrothermal Time Model. Life 2022, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Saleem, K.; Asghar, M.A.; Saleem, M.H.; Raza, A.; Kocsy, G.; Iqbal, N.; Ali, B.; Albeshr, M.F.; Bhat, E.A. Chrysotile-Asbestos-Induced Damage in Panicum virgatum and Phleum pretense Species and Its Alleviation by Organic-Soil Amendment. Sustainability 2022, 14, 10824. [Google Scholar] [CrossRef]
- Jalal, A.; Shah, S.; Teixeira Filho, M.; Carvalho, M.; Khan, A.; Shah, T.; Hussain, Z.; Younis, M.; Ilyas, M. Yield and phenological indices of wheat as affected by exogenous fertilization of zinc and iron. Braz. J. Agric. Sci. Bras. Ciências Agrárias 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Shahbazi, F.; Nematollahi, A. Influences of phosphorus and foliar iron fertilization rate on the quality parameters of whole wheat grain. Food Sci. Nutr. 2019, 7, 442–448. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Shabala, S. Linking ploidy level with salinity tolerance: NADPH-dependent ‘ROS–Ca2+ hub’in the spotlight. J. Exp. Bot. 2019, 70, 1063–1067. [Google Scholar] [CrossRef]
- Ma, J.; Ali, S.; Saleem, M.H.; Mumtaz, S.; Yasin, G.; Ali, B.; Al-Ghamdi, A.A.; Elshikh, M.S.; Vodnar, D.C.; Marc, R.A.; et al. Short-term responses of Spinach (Spinacia oleracea L.) to the individual and combinatorial effects of Nitrogen, Phosphorus and Potassium and silicon in the soil contaminated by boron. Front. Plant Sci. 2022, 13, 983156. [Google Scholar] [CrossRef]
- Ma, J.; Saleem, M.H.; Ali, B.; Rasheed, R.; Ashraf, M.A.; Aziz, H.; Ercisli, S.; Riaz, S.; Elsharkawy, M.M.; Hussain, I.; et al. Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress-insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Front. Plant Sci. 2022, 13, 950120. [Google Scholar] [CrossRef]
- Ma, J.; Saleem, M.H.; Yasin, G.; Mumtaz, S.; Qureshi, F.F.; Ali, B.; Ercisli, S.; Alhag, S.K.; Ahmed, A.E.; Vodnar, D.C.; et al. Individual and combinatorial effects of SNP and NaHS on morpho-physio-biochemical attributes and phytoextraction of chromium through Cr-stressed spinach (Spinacia oleracea L.). Front. Plant Sci. 2022, 13, 973740. [Google Scholar] [CrossRef]
- Nawaz, H.; Ali, A.; Saleem, M.H.; Ameer, A.; Hafeez, A.; Alharbi, K.; Ezzat, A.; Khan, A.; Jamil, M.; Farid, G. Comparative effectiveness of EDTA and citric acid assisted phytoremediation of Ni contaminated soil by using canola (Brassica napus). Braz. J. Biol. 2022, 82, e261785. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.W.U.; Montgomery, B.L. Interdependence of tetrapyrrole metabolism, the generation of oxidative stress and the mitigative oxidative stress response. Redox Biol. 2015, 4, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Hafeez, A.; Ahmad, S.; Javed, M.A.; Afridi, M.S.; Dawoud, T.M.; Almaary, K.S.; Muresan, C.C.; Marc, R.A.; Alkhalifah, D.H.M. Bacillus thuringiensis PM25 ameliorates oxidative damage of salinity stress in maize via regulating growth, leaf pigments, antioxidant defense system, and stress responsive gene expression. Front. Plant Sci. 2022, 13, 921668. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Hafeez, A.; Afridi, M.S.; Khan, S.; Ullah, I.; Amaral Júnior, A.T.d.; Alatawi, A.; Ali, S. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Javed, M.A.; Afridi, M.S.; Abbasi, H.A.; Qayyum, A.; Batool, T.; Ullah, A.; Marc, R.A.; Al Jaouni, S.K.; et al. Role of endophytic bacteria in salinity stress amelioration by physiological and molecular mechanisms of defense: A comprehensive review. S. Afr. J. Bot. 2022, 151, 33–46. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A. Bacillus mycoides PM35 Reinforces Photosynthetic Efficiency, Antioxidant Defense, Expression of Stress-Responsive Genes, and Ameliorates the Effects of Salinity Stress in Maize. Life 2022, 12, 219. [Google Scholar] [CrossRef]
- Farooq, T.H.; Rafy, M.; Basit, H.; Shakoor, A.; Shabbir, R.; Riaz, M.U.; Ali, B.; Kumar, U.; Qureshi, K.A.; Jaremko, M. Morpho-Physiological Growth Performance and Phytoremediation Capabilities of Selected Xerophyte Grass Species Towards Cr and Pb Stress. Front. Plant Sci. 2022, 13, 997120. [Google Scholar] [CrossRef]
- Dola, D.B.; Mannan, M.A.; Sarker, U.; Mamun, M.A.A.; Islam, T.; Ercisli, S.; Saleem, M.H.; Ali, B.; Pop, O.L.; Marc, R.A. Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci 2022, 13, 992535. [Google Scholar] [CrossRef]
- Afridi, M.S.; Javed, M.A.; Ali, S.; De Medeiros, F.H.V.; Ali, B.; Salam, A.; Sumaira; Marc, R.A.; Alkhalifah, D.H.M.; Selim, S.; et al. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 2022, 13, 899464. [Google Scholar] [CrossRef]
- Akram, N.A.; Saleem, M.H.; Shafiq, S.; Naz, H.; Farid-ul-Haq, M.; Ali, B.; Shafiq, F.; Iqbal, M.; Jaremko, M.; Qureshi, K.A. Phytoextracts as Crop Biostimulants and Natural Protective Agents—A Critical Review. Sustainability 2022, 14, 14498. [Google Scholar] [CrossRef]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Anjum, R.M.A.; Wang, B. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef] [PubMed]
- Afzal, J.; Hu, C.; Imtiaz, M.; Elyamine, A.M.; Rana, M.S.; Imran, M.; Farag, M.A. Cadmium tolerance in rice cultivars associated with antioxidant enzymes activities and Fe/Zn concentrations. Int. J. Environ. Sci. Technol. 2019, 16, 4241–4252. [Google Scholar] [CrossRef]
- Amna; Ali, B.; Azeem, M.A.; Qayyum, A.; Mustafa, G.; Ahmad, M.A.; Javed, M.T.; Chaudhary, H.J. Bio-Fabricated Silver Nanoparticles: A Sustainable Approach for Augmentation of Plant Growth and Pathogen Control. In Sustainable Agriculture Reviews 53; Springer: Berlin/Heidelberg, Germany, 2021; pp. 345–371. [Google Scholar]
- Mehmood, S.; Khatoon, Z.; Amna; Ahmad, I.; Muneer, M.A.; Kamran, M.A.; Ali, J.; Ali, B.; Chaudhary, H.J.; Munis, M.F.H. Bacillus sp. PM31 harboring various plant growth-promoting activities regulates Fusarium dry rot and wilt tolerance in potato. Arch. Agron. Soil Sci. 2021, 1–15. [Google Scholar] [CrossRef]
- Zainab, N.; Amna; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Munis, M.F.H.; Hashem, M.; et al. Pgpr-mediated plant growth attributes and metal extraction ability of sesbania sesban l. In industrially contaminated soils. Agronomy 2021, 11, 11. [Google Scholar] [CrossRef]
- Jia, N.; Qiao, H.; Zhu, W.; Zhu, M.; Meng, Q.; Lu, Q.; Zu, Y. Antioxidant, immunomodulatory, oxidative stress inhibitory and iron supplementation effect of Astragalus membranaceus polysaccharide-iron (III) complex on iron-deficiency anemia mouse model. Int. J. Biol. Macromol. 2019, 132, 213–221. [Google Scholar] [CrossRef]
- Hall, J.L.; Williams, L.E. Transition metal transporters in plants. J. Exp. Bot. 2003, 54, 2601–2613. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, S.; Khan, M.N.; Khan, W.M.; Razak, S.A.; Wahab, S.; Hafeez, A.; Khan Bangash, S.A.; Poczai, P. The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L. Sustainability 2022, 14, 12924. [Google Scholar] [CrossRef]
- Faryal, S.; Ullah, R.; Khan, M.N.; Ali, B.; Hafeez, A.; Jaremko, M.; Qureshi, K.A. Thiourea-Capped Nanoapatites Amplify Osmotic Stress Tolerance in Zea mays L. by Conserving Photosynthetic Pigments, Osmolytes Biosynthesis and Antioxidant Biosystems. Molecules 2022, 27, 5744. [Google Scholar] [CrossRef]
- Salam, A.; Afridi, M.S.; Javed, M.A.; Saleem, A.; Hafeez, A.; Khan, A.R.; Zeeshan, M.; Ali, B.; Azhar, W.; Sumaira; et al. Nano-Priming against Abiotic Stress: A Way Forward towards Sustainable Agriculture. Sustainability 2022, 14, 14880. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P. Iron complexes of flavonoids-antioxidant capacity and beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Lingyun, Y.; Jian, W.; Chenggang, W.; Shan, L.; Shidong, Z. Effect of zinc enrichment on growth and nutritional quality in pea sprouts. J. Food Nutr. Res. 2016, 4, 100–107. [Google Scholar]
- Naghavi, F. Effect of zinc and lead toxicity on some physiological parameters of Glycine max L. J. Biomed. Environ. Sci. 2014, 4, 192–201. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).