Incorporation of Glass and Plastic Waste into Alkali-Activated Mill Residue Bricks
Abstract
1. Introduction
2. Results and Discussion
2.1. Compressive Strength Evaluation
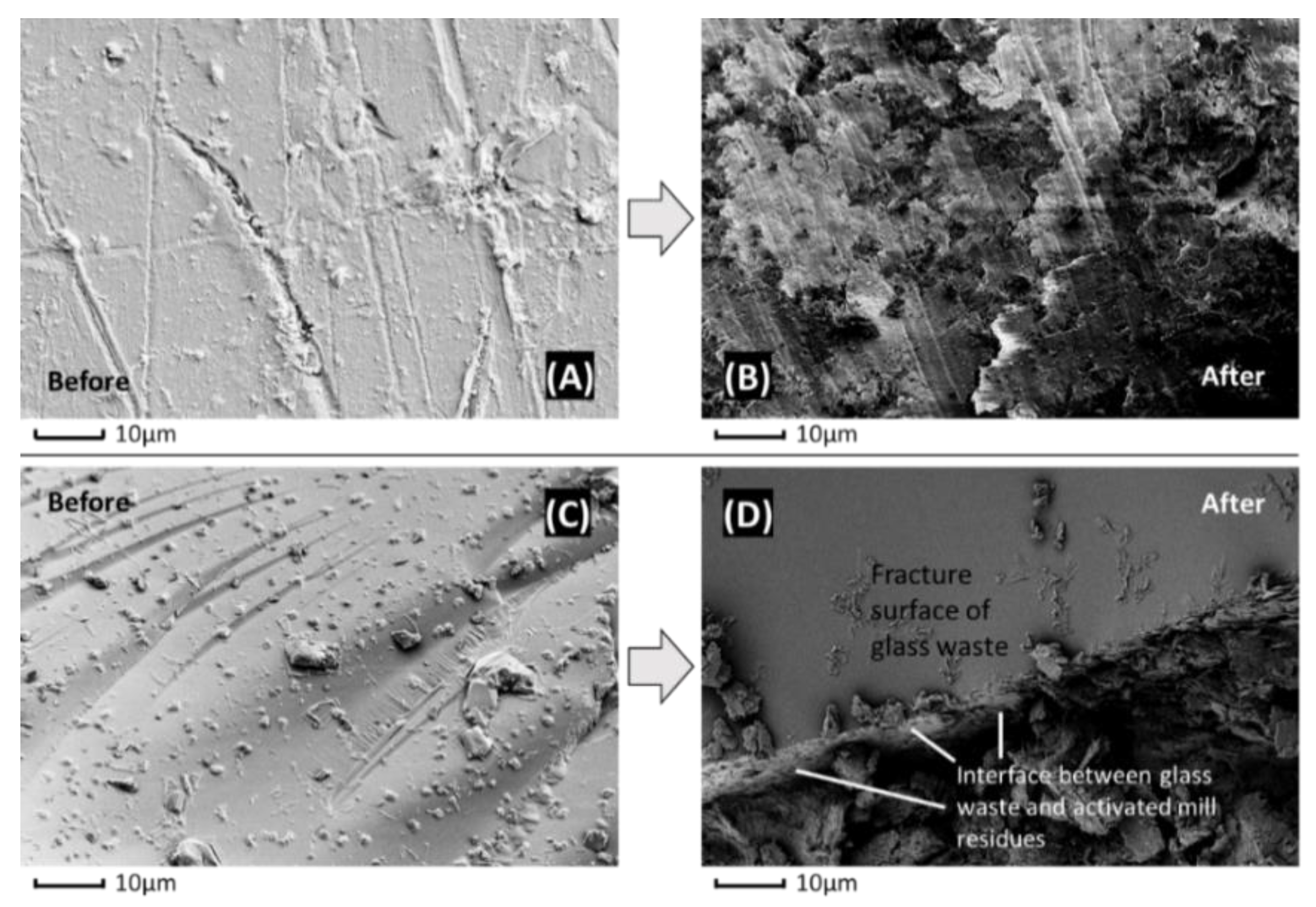
2.1.1. Glass Waste
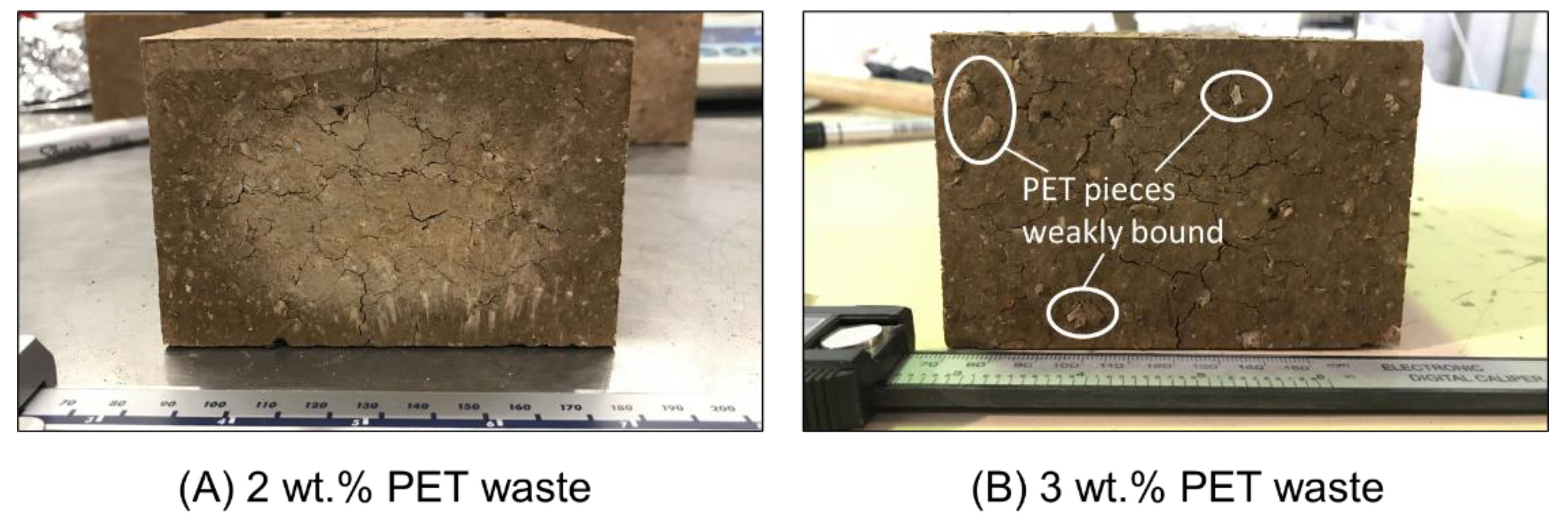
2.1.2. Plastic Waste
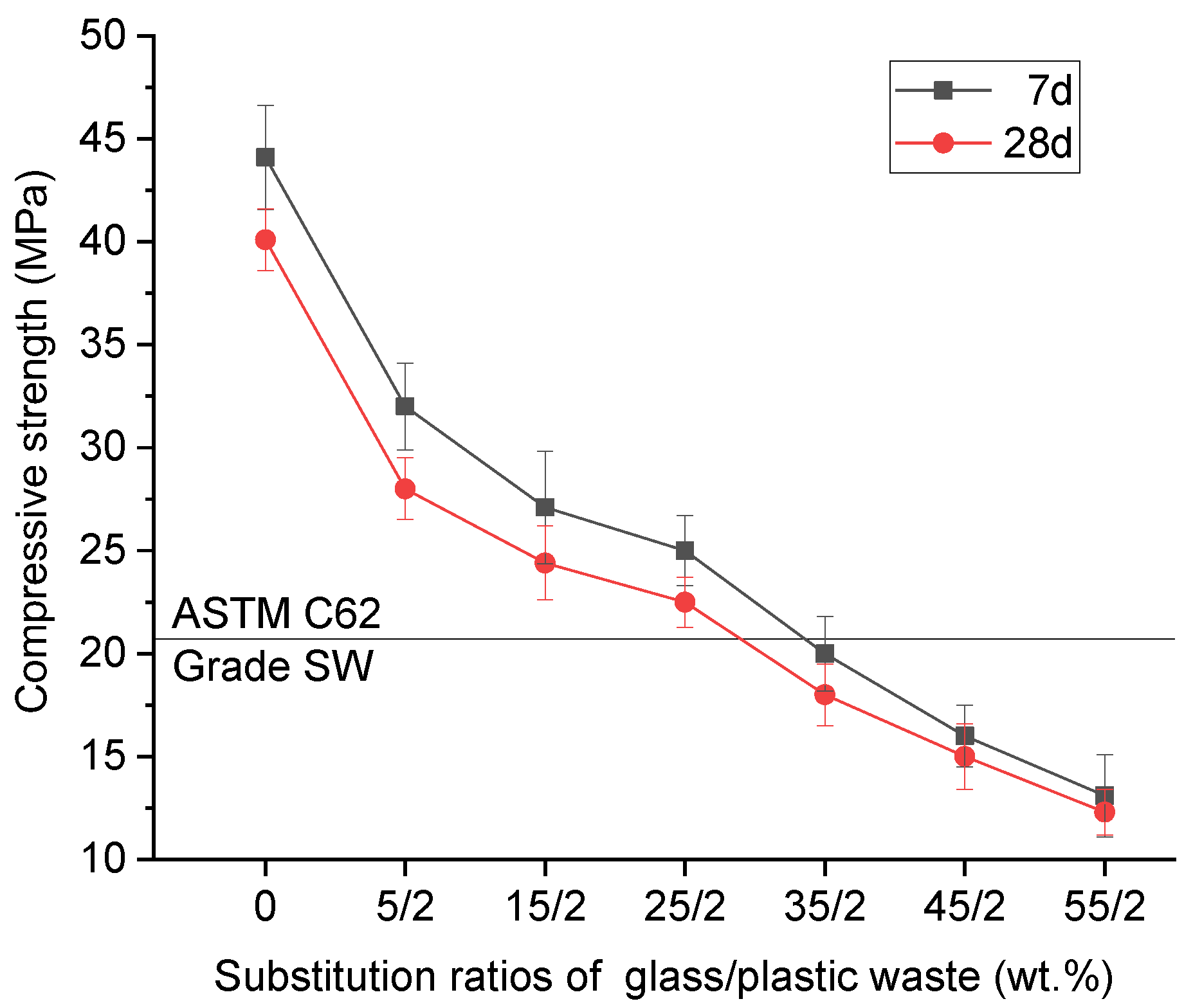
2.1.3. Combined Glass and Plastic Waste
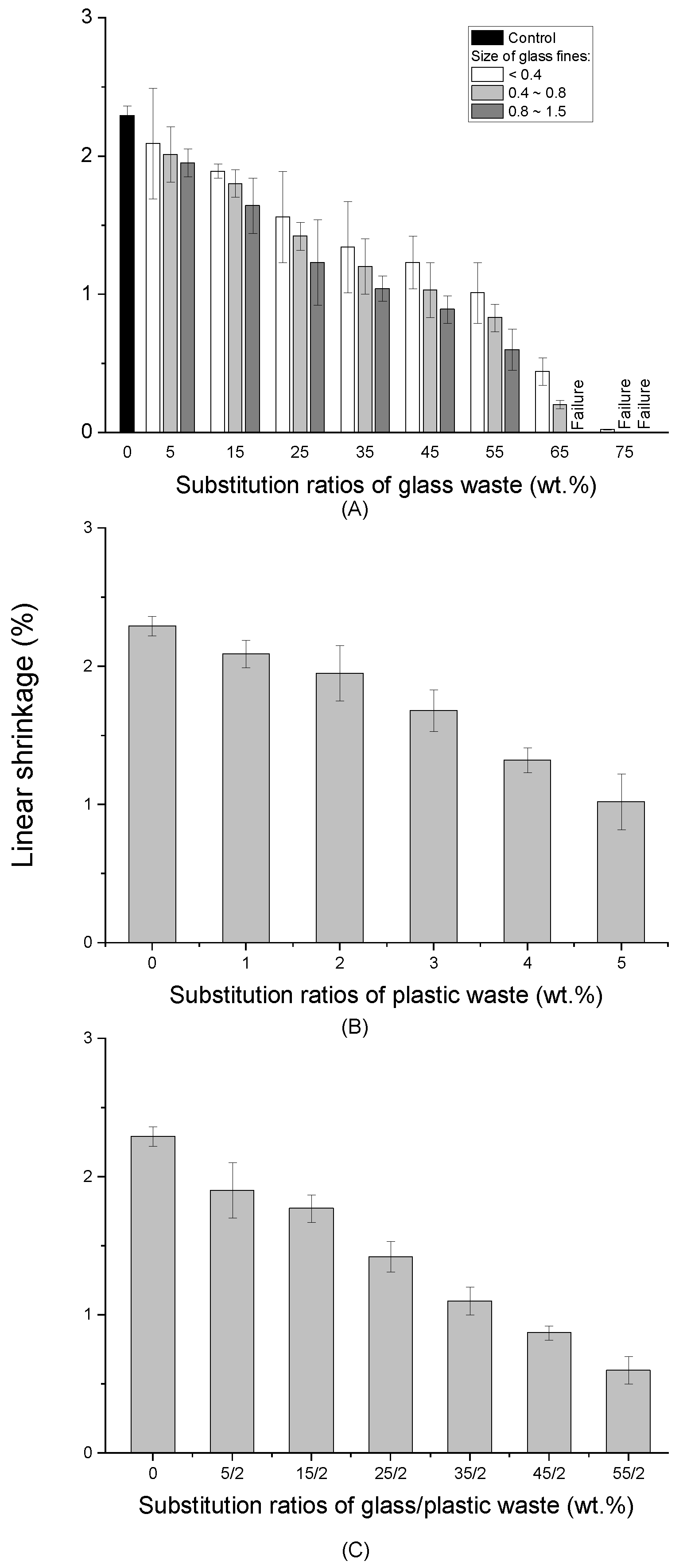
2.2. Linear Shrinkage
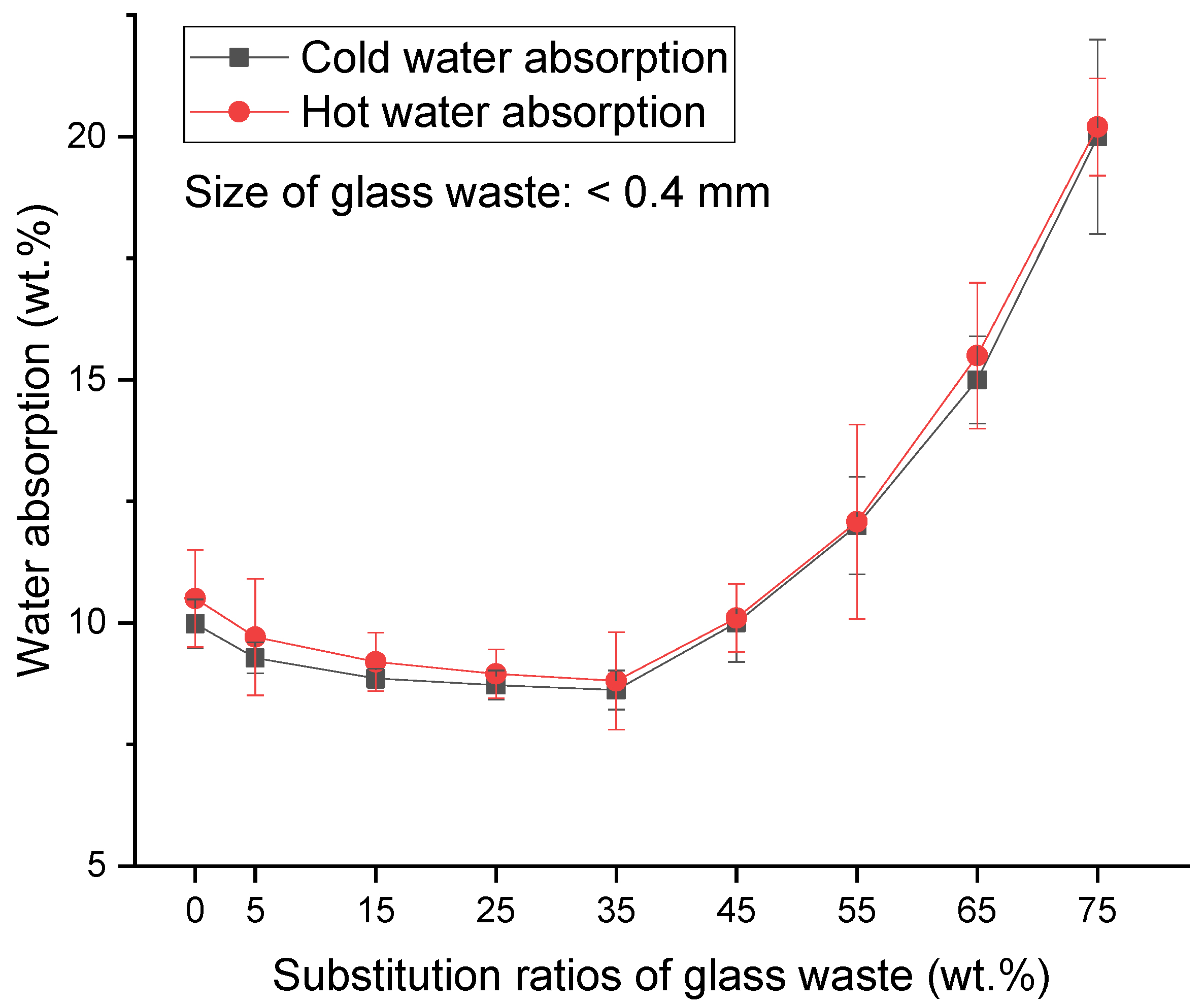
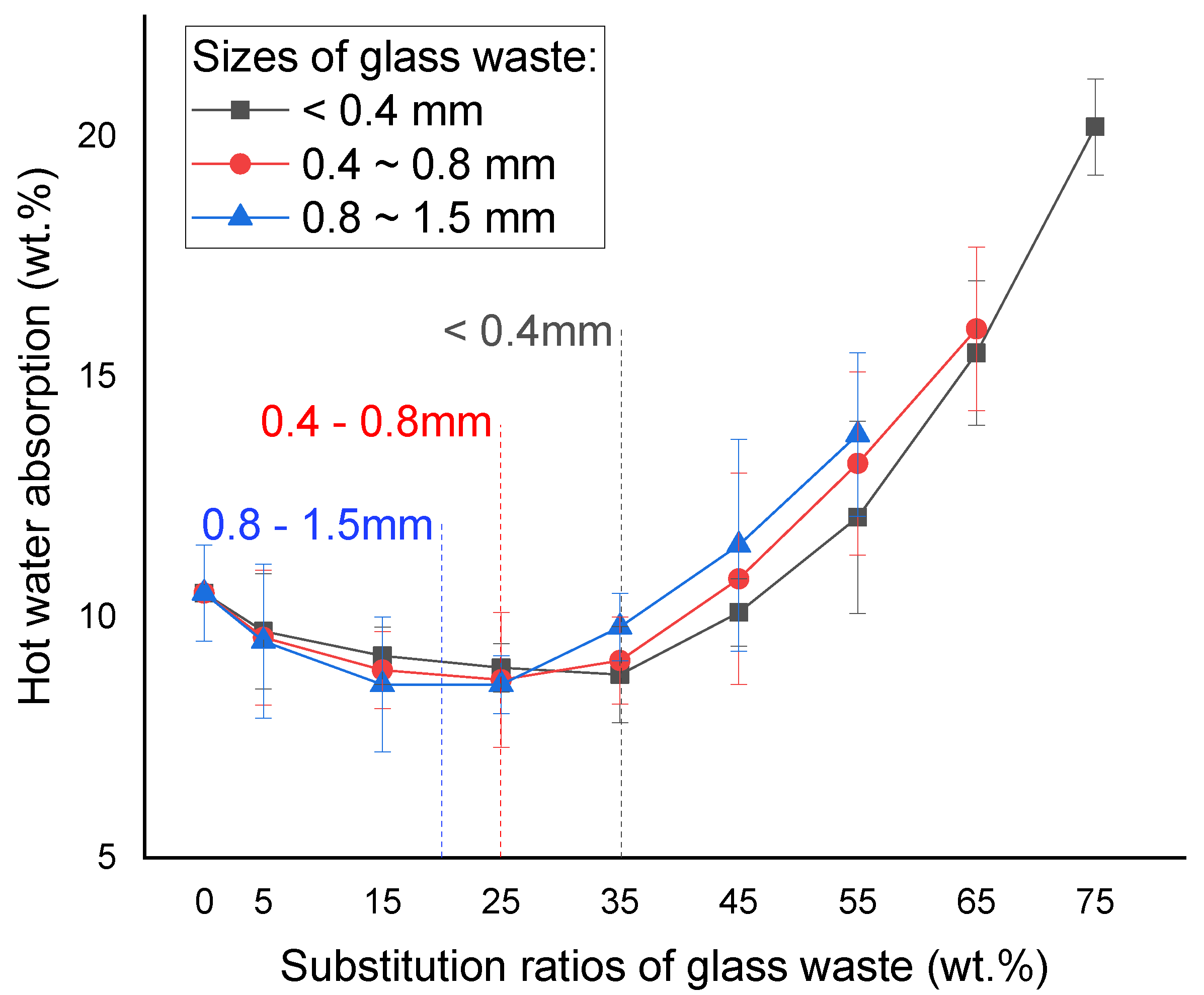
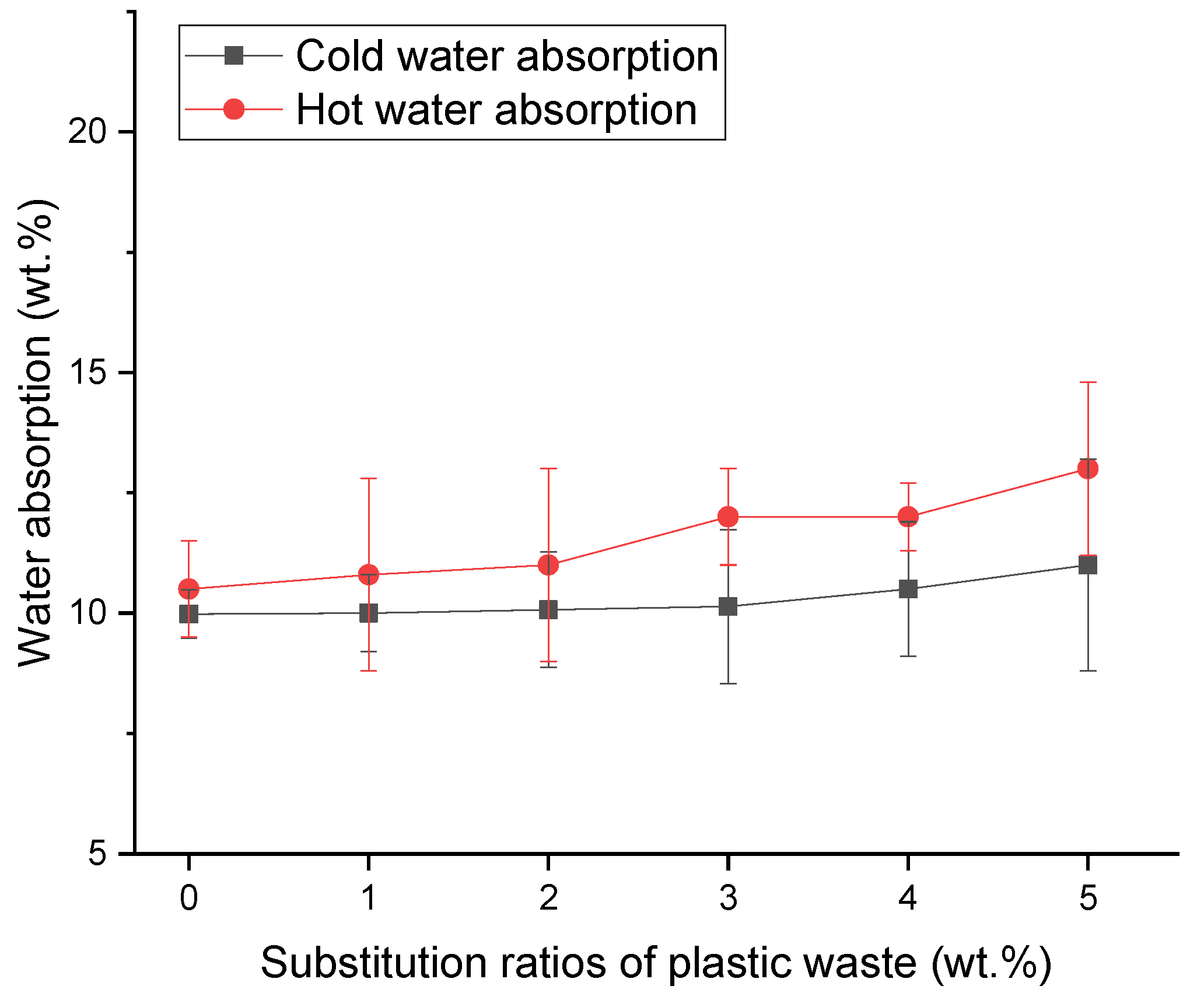
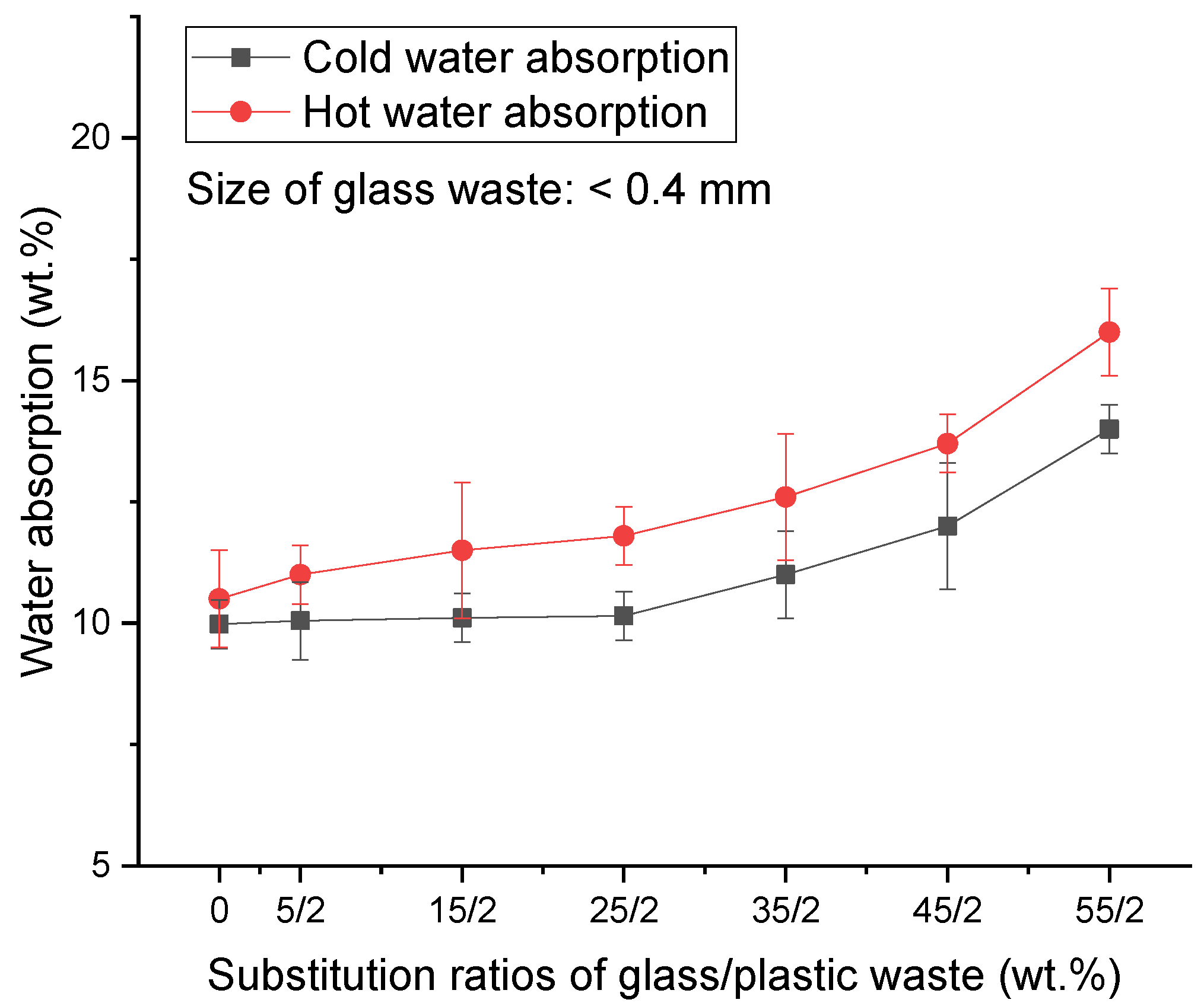
2.3. Water Absorption
2.3.1. Glass Waste
2.3.2. Plastic Waste
2.3.3. Combined Glass and Plastic Waste
2.4. Comparison of Fired Bricks and Waste-Added Alkali-Activated Bricks
3. Materials and Methods
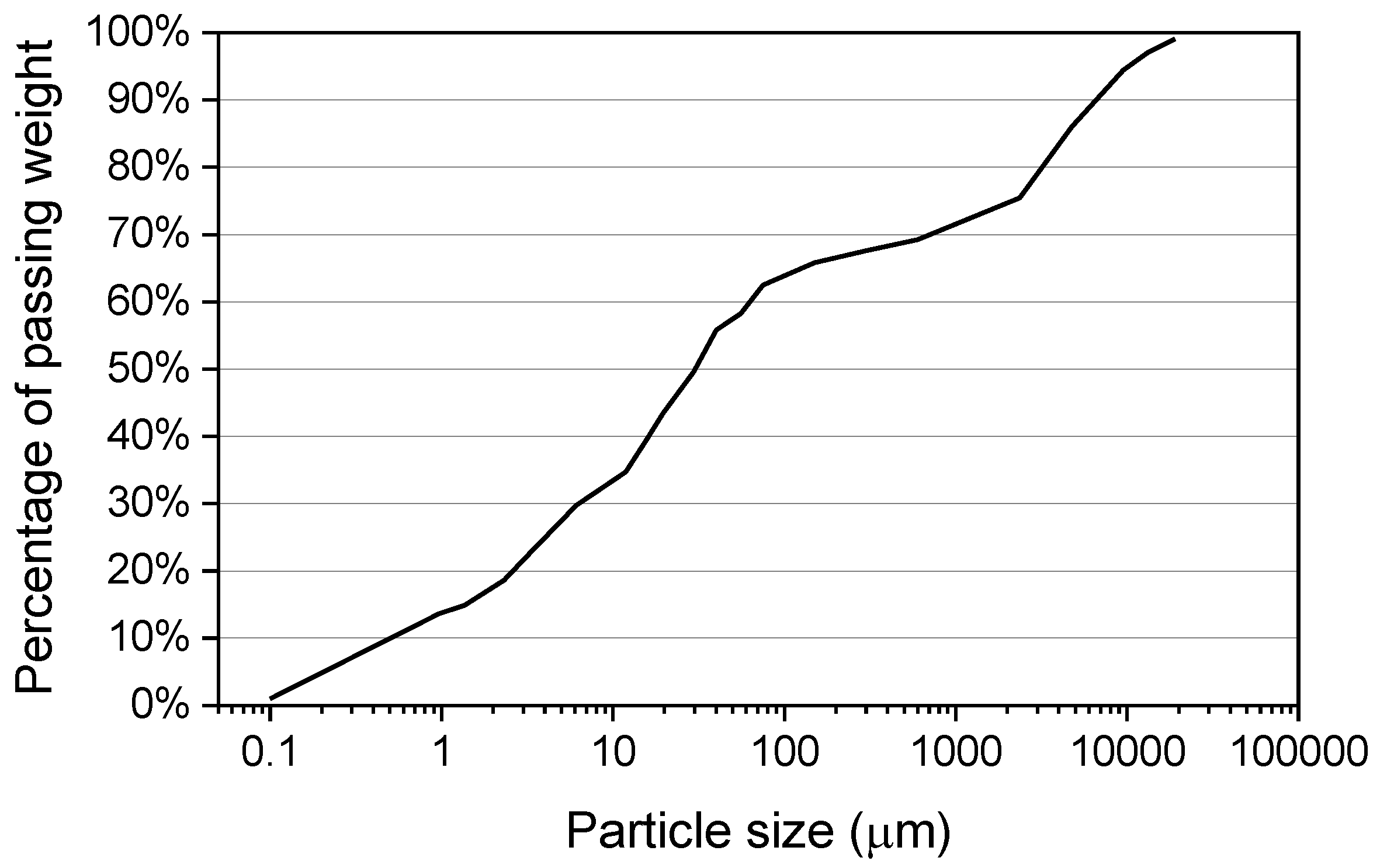
3.1. Materials
3.2. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaza, S. Overcoming the grip of consumerism. Buddh. Christ. Stud. 2000, 20, 23–42. [Google Scholar] [CrossRef]
- Lee, J.-S.; Yoo, H.-M.; Park, S.-W.; Cho, S.-J.; Seo, Y.-C. Recycling of cathode ray tube panel glasses as aggregates of concrete blocks and clay bricks. J. Mater. Cycles Waste Manag. 2016, 18, 552–562. [Google Scholar] [CrossRef]
- Morais, A.S.C.; Caldas, T.C.d.C.; Monteiro, S.N.; Vieira, C.M.F. Recycling of Fluorescent Lamp Glass into Clayey Ceramic; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1053–1060. [Google Scholar] [CrossRef]
- Pickin, J.; Randell, P.; Trinh, J.; Grant, B. National Waste Report 2018; Department of the Environment and Energy & Blue Environment Pty Ltd.: Docklands, VIC, Australia, 2018. [Google Scholar]
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic waste release caused by COVID-19 and its fate in the global ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2111530118. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and analysis of microplastics and nanoplastics in complex environmental samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.; Xu, G.; Shi, H.; Leung, J.Y.; Wang, Y. Microplastic accumulation via trophic transfer: Can a predatory crab counter the adverse effects of microplastics by body defence? Sci. Total Environ. 2021, 754, 142099. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastics the Facts 2018: An Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope: Brussels, Belgium, 2018. [Google Scholar]
- Saikia, N.; De Brito, J. Use of plastic waste as aggregate in cement mortar and concrete preparation: A review. Constr. Build. Mater. 2012, 34, 385–401. [Google Scholar] [CrossRef]
- Farzanian, K.; Vafaei, B.; Ghahremaninezhad, A.J.M. The behavior of superabsorbent polymers (SAPs) in cement mixtures with glass powders as supplementary cementitious materials. Materials 2019, 12, 3597. [Google Scholar] [CrossRef]
- Bisht, K.; Ramana, P.J.C.; Materials, B. Sustainable production of concrete containing discarded beverage glass as fine aggregate. Constr. Build. Mater. 2018, 177, 116–124. [Google Scholar] [CrossRef]
- Guo, P.; Meng, W.; Nassif, H.; Gou, H.; Bao, Y.J.C.; Materials, B. New perspectives on recycling waste glass in manufacturing concrete for sustainable civil infrastructure. Constr. Build. Mater. 2020, 257, 119579. [Google Scholar] [CrossRef]
- Kim, I.S.; Choi, S.Y.; Yang, E.I.J.C.; Materials, B. Evaluation of durability of concrete substituted heavyweight waste glass as fine aggregate. Constr. Build. Mater. 2018, 184, 269–277. [Google Scholar] [CrossRef]
- Rahma, A.; El Naber, N.; Issa Ismail, S. Effect of glass powder on the compression strength and the workability of concrete. Cogent Eng. 2017, 4, 1373415. [Google Scholar] [CrossRef]
- Abdallah, S.; Fan, M. Characteristics of concrete with waste glass as fine aggregate replacement. Int. J. Eng. Tech. Res. 2014, 2, 11–17. [Google Scholar]
- Adaway, M.; Wang, Y. Recycled glass as a partial replacement for fine aggregate in structural concrete–Effects on compressive strength. Electron. J. Struct. Eng. 2015, 14, 116–122. [Google Scholar] [CrossRef]
- Ahmad, J.; Aslam, F.; Martinez-Garcia, R.; de-Prado-Gil, J.; Qaidi, S.; Brahmia, A. Effects of waste glass and waste marble on mechanical and durability performance of concrete. Sci. Rep. 2021, 11, 21525. [Google Scholar] [CrossRef]
- Steyn, Z.; Babafemi, A.; Fataar, H.; Combrinck, R.J.C.; Materials, B. Concrete containing waste recycled glass, plastic and rubber as sand replacement. Constr. Build. Mater. 2021, 269, 121242. [Google Scholar] [CrossRef]
- Bahij, S.; Omary, S.; Feugeas, F.; Faqiri, A. Fresh and hardened properties of concrete containing different forms of plastic waste–A review. Waste Manag. 2020, 113, 157–175. [Google Scholar] [CrossRef]
- Jaivignesh, B.; Sofi, A. Study on mechanical properties of concrete using plastic waste as an aggregate. IOP Conf. Ser. Earth Environ. Sci. 2017, 80, 012016. [Google Scholar]
- Frigione, M. Recycling of PET bottles as fine aggregate in concrete. Waste Manag. 2010, 30, 1101–1106. [Google Scholar] [CrossRef]
- Tyrell, M.; Goode, A.H. Waste Glass as a Flux for Brick Clays; Bureau of Mines: Washington, DC, USA, 1972.
- Kim, K.; Hwang, J. Characterization of ceramic tiles containing LCD waste glass. Ceram. Int. 2016, 42, 7626–7631. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Panyachai, T.; Kaewsimork, K.; Siritai, C. Fabrication of Lightweight Clay Bricks from Recycled Glass Wastes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 213–219. [Google Scholar] [CrossRef]
- Akinyele, J.; Igba, U.; Ayorinde, T.; Jimoh, P. Structural efficiency of burnt clay bricks containing waste crushed glass and polypropylene granules. Case Stud. Constr. Mater. 2020, 13, e00404. [Google Scholar] [CrossRef]
- Hasan, M.; Siddika, A.; Akanda, M.; Ali, P.; Islam, M. Effects of waste glass addition on the physical and mechanical properties of brick. Innov. Infrastruct. Solut. 2021, 6, 36. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Mansori, M.; Hakkou, R.J.W.; Valorization, B. Recycling feasibility of glass wastes and calamine processing tailings in fired bricks making. Waste Biomass Valorization 2017, 8, 1479–1489. [Google Scholar] [CrossRef]
- Phonphuak, N.; Kanyakam, S.; Chindaprasirt, P. Utilization of waste glass to enhance physical–mechanical properties of fired clay brick. J. Clean. Prod. 2016, 112, 3057–3062. [Google Scholar] [CrossRef]
- Ponce Peña, P.; González Lozano, M.A.; Rodríguez Pulido, A.; Lara Castro, R.H.; Quiñones Jurado, Z.V.; Pérez Medina, J.C.; Poisot Vázquez, M.E.; Villavicencio Torres, A. Effect of Crushed Glass Cullet Sizes on Physical and Mechanical Properties of Red Clay Bricks. Adv. Mater. Sci. Eng. 2016, 2016, 2842969. [Google Scholar] [CrossRef]
- Morais, A.S.C.; Vieira, C.M.F.; Rodriguez, R.J.S.; Monteiro, S.N.; Candido, V.S.; Ferreira, C.L. Fluorescent Lamp Glass Waste Incorporation into Clay Ceramic: A Perfect Solution. J. Miner. 2016, 68, 2425–2434. [Google Scholar] [CrossRef]
- Guzman, A.D.M.; Munno, M.G.T. Design of a Brick With Sound Absorption Properties Based on Plastic Waste and Sawdust. Access IEEE 2015, 3, 1260–1271. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Boquera, L.; Chàfer, M.; Vérez, D. Embodied energy and embodied carbon of structural building materials: Worldwide progress and barriers through literature map analysis. Energy Build. 2021, 231, 110612. [Google Scholar] [CrossRef]
- Dabaieh, M.; Heinonen, J.; El-Mahdy, D.; Hassan, D.M. A comparative study of life cycle carbon emissions and embodied energy between sun-dried bricks and fired clay bricks. J. Clean. Prod. 2020, 275, 122998. [Google Scholar] [CrossRef]
- Arulrajah, A.; Kua, T.-A.; Horpibulsuk, S.; Phetchuay, C.; Suksiripattanapong, C.; Du, Y.-J. Strength and microstructure evaluation of recycled glass-fly ash geopolymer as low-carbon masonry units. Constr. Build. Mater. 2016, 114, 400–406. [Google Scholar] [CrossRef]
- Vafaei, B.; Farzanian, K.; Ghahremaninezhad, A.J.C.; Materials, B. The influence of superabsorbent polymer on the properties of alkali-activated slag pastes. Constr. Build. Mater. 2020, 236, 117525. [Google Scholar] [CrossRef]
- Suksiripattanapong, C.; Horpibulsuk, S.; Chanprasert, P.; Sukmak, P.; Arulrajah, A. Compressive strength development in fly ash geopolymer masonry units manufactured from water treatment sludge. Constr. Build. Mater. 2015, 82, 20–30. [Google Scholar] [CrossRef]
- Quijorna, N.; Coz, A.; Andres, A.; Cheeseman, C. Recycling of Waelz slag and waste foundry sand in red clay bricks. Resour. Conserv. Recycl. 2012, 65, 1–10. [Google Scholar] [CrossRef]
- Si, R.; Dai, Q.; Guo, S.; Wang, J. Mechanical property, nanopore structure and drying shrinkage of metakaolin-based geopolymer with waste glass powder. J. Clean. Prod. 2020, 242, 118502. [Google Scholar] [CrossRef]
- Tang, S.; He, Y.; Deng, X.; Cui, X. Thermal Catalytic-Cracking Low-Density Polyethylene Waste by Metakaolin-Based Geopolymer NaA Microsphere. Molecules 2022, 27, 2557. [Google Scholar] [CrossRef] [PubMed]
- Novais, R.M.; Ascensão, G.; Seabra, M.; Labrincha, J. Waste glass from end-of-life fluorescent lamps as raw material in geopolymers. Waste Manag. 2016, 52, 245–255. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.-W.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of glass waste for development of Geopolymer composites–Mechanical properties and rheological characteristics: A review. Constr. Build. Mater. 2019, 220, 547–564. [Google Scholar] [CrossRef]
- Zhang, Z.; Wong, Y.C.; Arulrajah, A. Feasibility of producing non-fired compressed masonry units from brick clay mill residues by alkali activation. J. Clean. Prod. 2021, 306, 126916. [Google Scholar] [CrossRef]
- Zhang, Z.; Wong, Y.C.; Arulrajah, A.; Sofi, M.; Sabri, Y.J.C.; Materials, B. Reaction mechanism of alkali-activated brick clay mill residues. Constr. Build. Mater. 2022, 341, 127817. [Google Scholar] [CrossRef]
- ASTM C62; Standard Specification for Building Brick (Solid Masonry Units Made From Clay or Shale). ASTM International: West Conshohocken, PA, USA, 2013.
- Tan, K.H.; Du, H.J.C.; Composites, C. Use of waste glass as sand in mortar: Part I–Fresh, mechanical and durability properties. Cem. Concr. Compos. 2013, 35, 109–117. [Google Scholar] [CrossRef]
- BIA. Manufacturing of Brick; Brick Industry Association: Reston, VA, USA, 2006. [Google Scholar]
- Koroneos, C.; Dompros, A. Environmental assessment of brick production in Greece. Build. Environ. 2007, 42, 2114–2123. [Google Scholar] [CrossRef]
- ASTM C326-09; Standard Test Method for Drying and Firing Shrinkages of Ceramic Whiteware Clays. ASTM: West Conshohocken, PA, USA, 2018.
- ASTM C67; Standard Test Methods for Sampling and Testing Brick and Structural Clay Tile. ASTM: West Conshohocken, PA, USA, 2016.

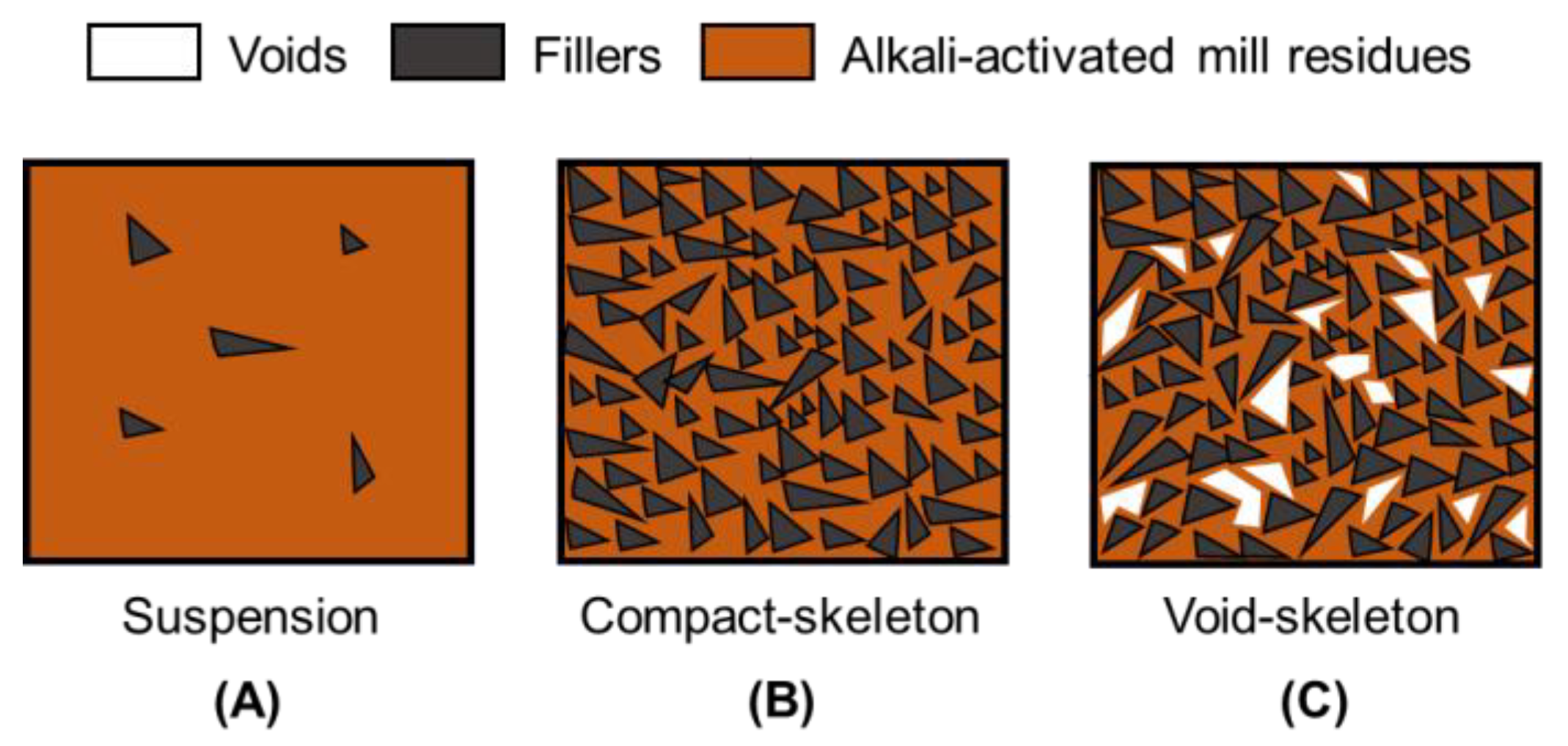
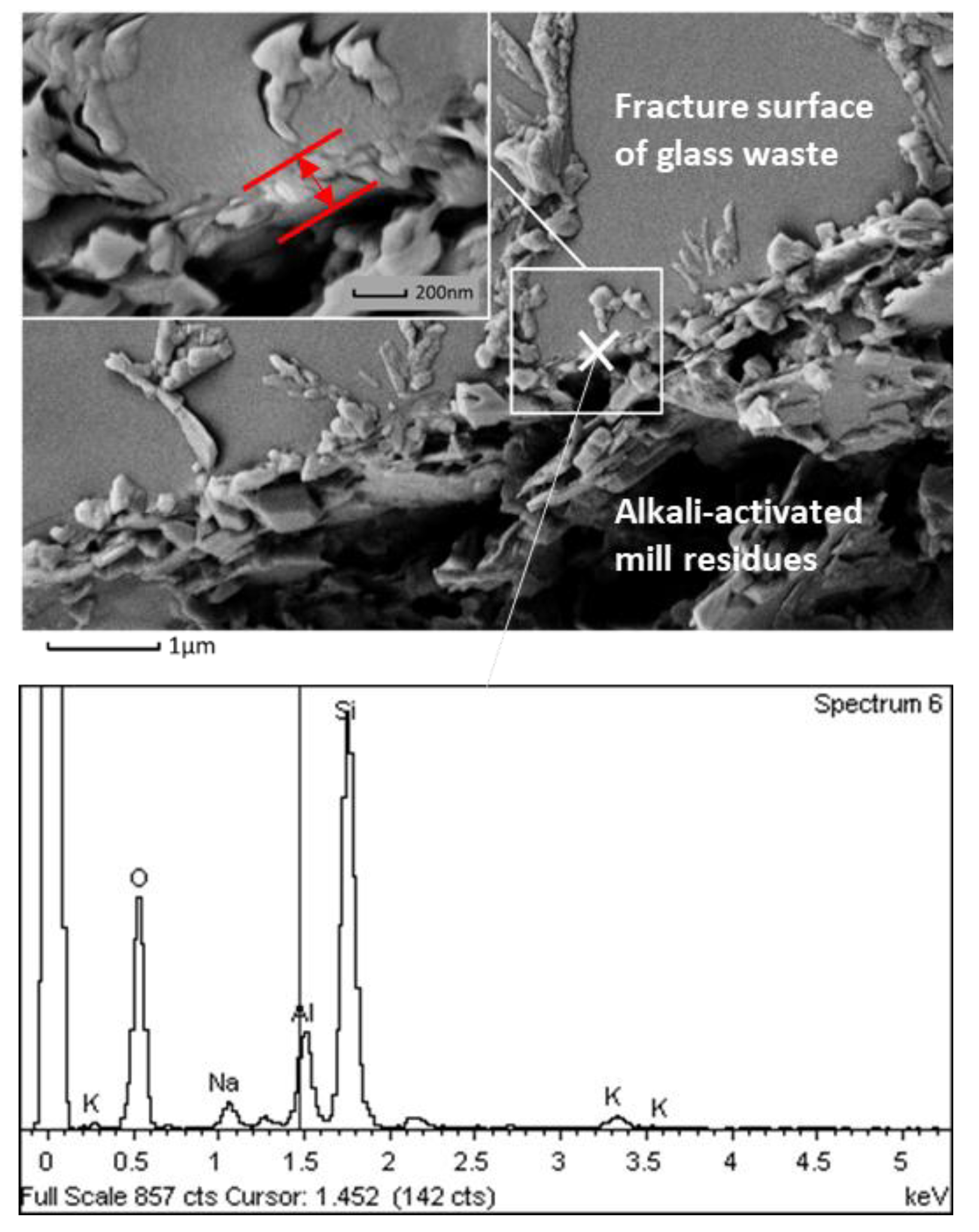
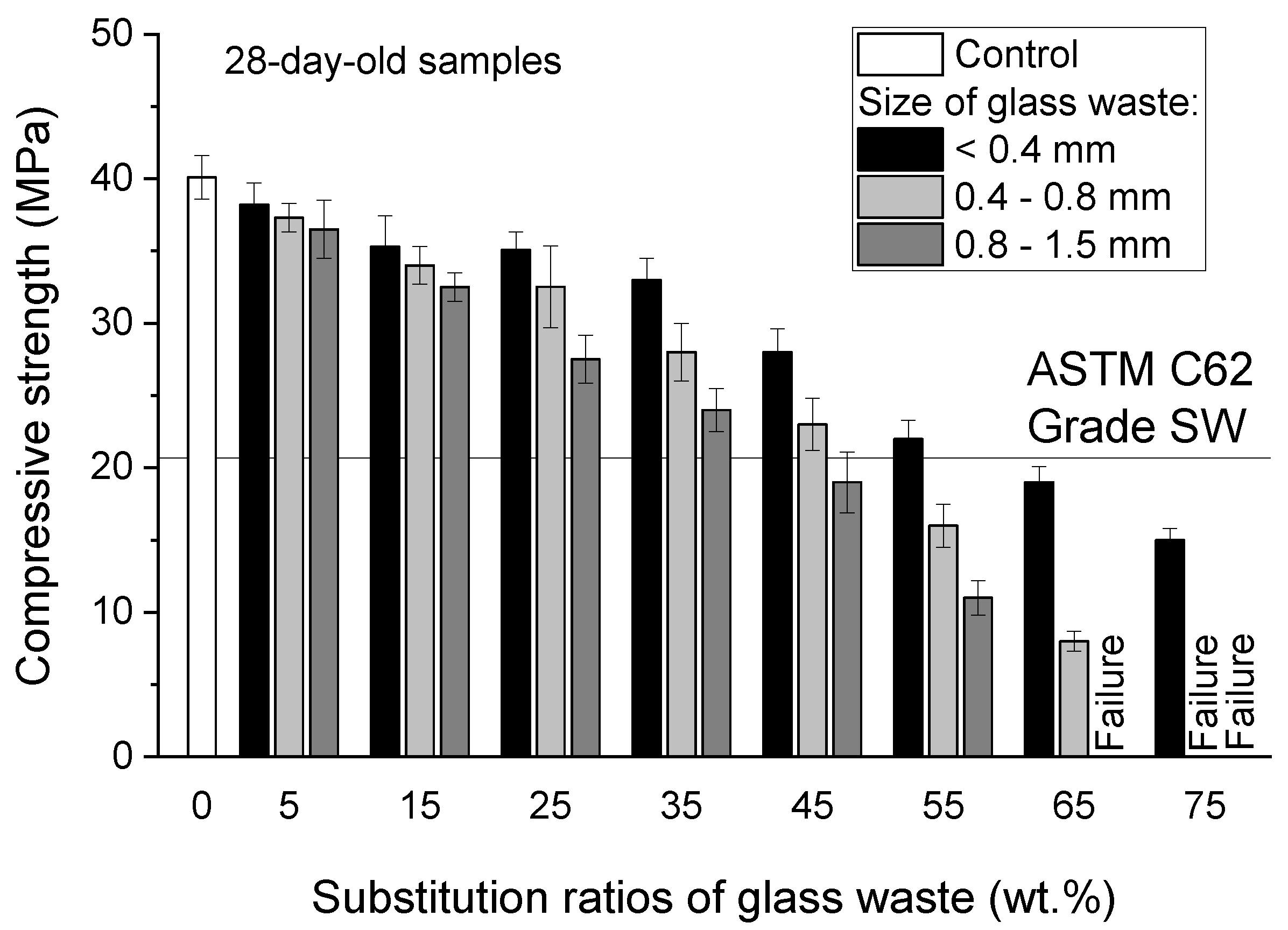
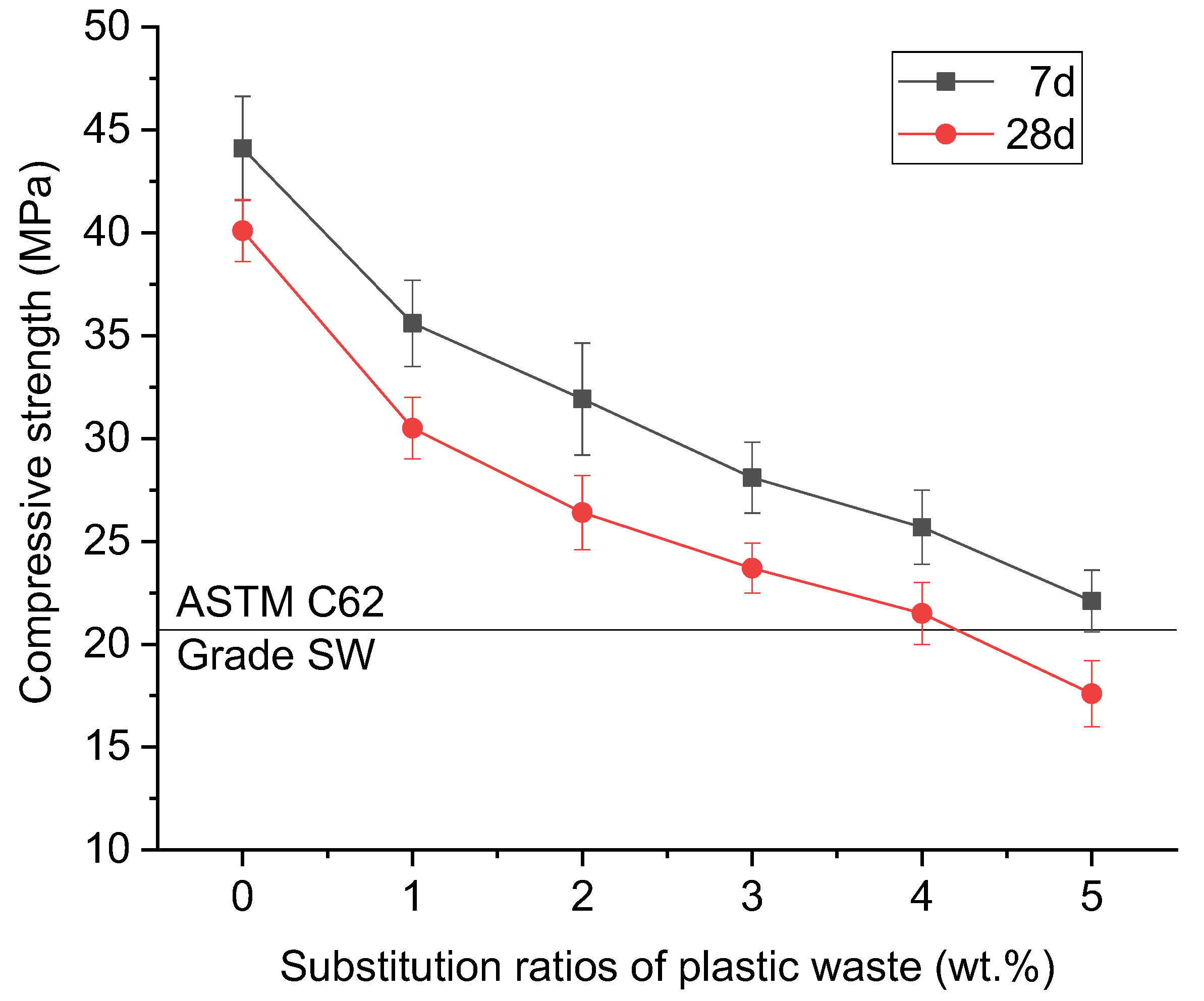

| Phases | Alkali-Activated Bricks Incorporating Glass and Plastic Waste | Conventional Fired Bricks |
|---|---|---|
| Raw materials sourcing | Mill residues Glass waste Plastic waste Sodium silicate Sodium hydroxide | Clay Shale |
| Mixing | Requiring some modifications on the ordinary mixing system | Ordinary mixing system |
| Shaping | Pressing/extruding | Pressing/extruding [47] |
| Drying/Curing | 50 °C and 90% RH for 48 h 155 °C and 80% RH for 24 h | 38–204 °C with moderate humidity for 24–48 h [47] |
| Firing | None | Up to 1316 °C for 10–40 h [47] |
| Cooling | No specification required | Gradually cooling for several hours [47] |
| Specifications | |
|---|---|
| Sodium hydroxide (SH) concentration | 8 M |
| Sodium silicate composition | 14.7 wt.% Na2O, 29.4 wt.% SiO2, 55.9 wt.% H2O |
| Alkaline activator ratio (in addition to the total dry mix) | 20 wt.% |
| SH/SS ratio by mass | 1.0 |
| Equivalent water/solid ratio | 15 wt.% |
| Sample ID | Glass Waste | Plastic Waste | Mill Residues | |
|---|---|---|---|---|
| wt.% | Sizes (mm) | wt.% | wt.% | |
| Control | -- | -- | -- | 100 |
| G5 | 5 | <0.4 0.4–0.8 0.8–1.5 | -- | 95 |
| G15 | 15 | <0.4 0.4–0.8 0.8–1.5 | -- | 85 |
| G25 | 25 | <0.4 0.4–0.8 0.8–1.5 | -- | 75 |
| G35 | 35 | <0.4 0.4–0.8 0.8–1.5 | -- | 65 |
| G45 | 45 | <0.4 0.4–0.8 0.8–1.5 | -- | 55 |
| G55 | 55 | <0.4 0.4–0.8 0.8–1.5 | -- | 45 |
| G65 | 65 | <0.4 0.4–0.8 0.8–1.5 | -- | 35 |
| G75 | 75 | <0.4 0.4–0.8 0.8–1.5 | -- | 25 |
| P1 | -- | -- | 1 | 99 |
| P2 | -- | -- | 2 | 98 |
| P3 | -- | -- | 3 | 97 |
| P4 | -- | -- | 4 | 96 |
| P5 | -- | -- | 5 | 95 |
| G5P2 | 5 | <0.4 | 2 | 93 |
| G15P2 | 15 | <0.4 | 2 | 83 |
| G25P2 | 25 | <0.4 | 2 | 73 |
| G35P2 | 35 | <0.4 | 2 | 63 |
| G45P2 | 45 | <0.4 | 2 | 53 |
| G55P2 | 55 | <0.4 | 2 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wong, Y.C.; Sofi, M.; Mendis, P. Incorporation of Glass and Plastic Waste into Alkali-Activated Mill Residue Bricks. Sustainability 2022, 14, 16533. https://doi.org/10.3390/su142416533
Zhang Z, Wong YC, Sofi M, Mendis P. Incorporation of Glass and Plastic Waste into Alkali-Activated Mill Residue Bricks. Sustainability. 2022; 14(24):16533. https://doi.org/10.3390/su142416533
Chicago/Turabian StyleZhang, Zipeng, Yat Choy Wong, Massoud Sofi, and Priyan Mendis. 2022. "Incorporation of Glass and Plastic Waste into Alkali-Activated Mill Residue Bricks" Sustainability 14, no. 24: 16533. https://doi.org/10.3390/su142416533
APA StyleZhang, Z., Wong, Y. C., Sofi, M., & Mendis, P. (2022). Incorporation of Glass and Plastic Waste into Alkali-Activated Mill Residue Bricks. Sustainability, 14(24), 16533. https://doi.org/10.3390/su142416533






