Abstract
At higher ammonium concentrations, nitritation can be easily attained by picking out the inhibitor. In low-concentrated reactors, nitrite accumulation without using any chemical inhibitor is a challenging process. In this study, two continuous stirred-tank reactors (CSTR) with biofilm and without biofilm were operated with total ammonium nitrogen feed concentrations of ~50 mg/L and ~30 mg/L and effluent concentrations of ~1 mg/L. A CSTR without biofilm was operated in three phases. In phase 1, a substrate-shock concentration of 1 to 2000 mg total ammonium nitrogen (TAN)/L was tested. It was found that the shock concentration was not successful in long-term operations because nitrite-oxidizing bacteria (NOB) recovered rapidly. In phases 2 and 3, the sludge-treatment method was applied, and a high nitrite accumulation efficiency was achieved (~98%). In a CSTR with biofilm, the free ammonia shock concentration was ~91.7 mg/L, and a nitrite accumulation efficiency of ~90% was achieved.
1. Introduction
A shortcut for the biological nitrogen removal process utilizes the direct conversion of ammonium to nitrite to nitrogen gas [1]. Nitrite-oxidizing bacteria (NOB) are believed to be rate-limiting under conventional operating conditions [2,3]. For example, at higher ammonium concentrations, nitritation can be easily attained by picking out the inhibitor, such as free ammonia (FA), free nitrous acid (FNA), and dissolved oxygen (DO) [4,5,6], and temperatures greater than 25 °C, because ammonium-oxidizing bacteria (AOB) exhibit higher growth rates at higher temperatures [7].
As NOB are sensitive to FA concentrations [8], the inhibition of NOB begins at 0.1–1 mg FA/L. For NOB inhibition, the FA concentration range should be 1–10 mg FA/L [1]. The free nitrous acid (FNA) concentration threshold value to suppress NOB is ~0.02 mg HNO2-N/L [4,9]. NOB suppression can also be achieved using an intermittent supply of DO combined with an anaerobic ammonium oxidation process [10], although selective chemicals such as hydroxylamine (NH2OH) [11], sodium azide (NaN3) [8,12], sulfides, salts, heavy metals, chlorate, cyanate, halide, hydrazine, and organic chemicals [13,14] have also been used. Tests with toxic compounds found that NOB were more affected than AOB at an azide concentration of 0.3µm/L, and nickel and copper were equal or more toxic to Nitrosomonas sp. than to Nitrobacter sp. However, the inhibitors also reduced the performance of AOB. The uptake rate of AOB reportedly decreased from 90% to 75% due to NH2OH [15]. The addition of chemicals for NOB suppression required a costly supplementary treatment method to eliminate chemicals hazardous to organisms from wastewater. Inhibitors such as NH2OH [15], copper(II) and arsenic(III) [16], zinc oxide nanoparticles (ZnO NPs) [17], chromium(III) and Cr(IV) [18], and FA and FNA concentrations [19] not only suppressed NOB but also reduced the performance of AOB to some extent.
In a sludge-treatment method introduced by Wang et al. (2017) in which an FA concentration of 210 mg FA/L was used, 22% of activated sludge from a sequencing batch reactor (SBR) was taken and treated on a daily basis on the sidestream [20]. However, Wang et al.’s method has two drawbacks. First, daily sludge treatment may not be required until and unless NOB is activated in the reactor. Second, sludge treatment requires an additional batch reactor to suppress NOB.
In this study, two reactors were operated: a continuous stirrer-tank reactor (CSTR) and a continuous stirrer-tank biofilm reactor (CSTBR). The CSTR was used to determine how long NOB can be suppressed after a single treatment and shock concentration. The CSTBR was used to reduce operational costs because sludge in a CSTR is treated in a separate batch reactor, while NOB in a CSTBR is suppressed within the reactor and no additional batch reactor is required.
2. Material and Methods
2.1. Cells Cultures and Mineral Media
The AOB species for the continuous stirred-tank reactor model (CSTR) were obtained from a reactor operated in our laboratory (R1) [21,22]. The inoculum contained both AOB (0.67%) and NOB (0.64%). A total of 2 L of liquid from R1 was fed directly into the model CSTR. The influent feed was initiated instantly, pH was kept at 8 ± 0.1 throughout the process, the temperature was kept at ~30 °C, and enough DO was supplied through an air diffusor. The feed was composed of NH4HCO3, MgSO47H2O, FeSO47H2O, CaCl2, KCl, MnSO4H2O, and KH2PO4 minerals. For the CSTBR, 2.5 L of inoculum was obtained from the model CSTR and the same operational parameters (influent feed, effluent, and mineral medium) were used.
2.2. Reactor Operation
The model CSTR cell recycled 5 L of liquid with ~50 mg of total ammonium nitrogen (TAN)/L and ~30 mg TAN/L of influent feed and 1 mg TAN/L of effluent for 205 days. Nitritation was achieved within a few days of operation. The substrate shocks were performed during the first of three phases of operation, and sludge treatment was tested in phases 2 and 3. The CSTBR chemostat handled 2.5 L with ~50 mg TAN/L influent feed and 1 mg TAN/L effluent concentration for 105 days, with nitritation achieved within a few days of operation. Substrate shocks were tested within the reactor.
2.3. Experimental Details
2.3.1. Model Continuous-Stirred Tank Reactor
The model CSTR with cell recycling was fabricated in the laboratory. The influent feed was pumped and the cells were recycled continuously. After successful nitritation, the cells were subjected to a substrate shock within the reactor, and sludge was treated in separate batch reactors. After sludge treatment in the batch reactor, the cells were rinsed three times and retained in the model CSTR at the same operational conditions used to achieve nitrification (Figure 1).
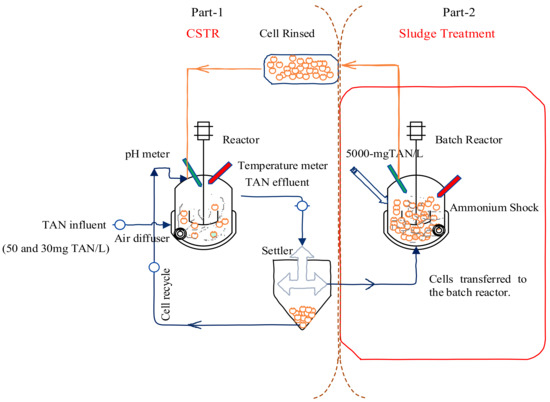
Figure 1.
Schematic diagram of CSTR. Part 1: CSTR operation in which cells are recycled. Part 2: settled cells are pumped into a batch reactor where they are treated using TAN concentrations of 5000 mg/L, with substrate-shock cells rinsed and returned to the CSTR.
2.3.2. Continuous Stirrer-Tank Biofilm Reactor
The CSTBR chemostat with a 2.5 L volume was fabricated in the laboratory. Square-shaped spunches were put in the reactor to develop a biofilm, and DO was supplied through an air diffuser. Influent was pumped in continuously and complete nitrititation was achieved within a few days of operation. After complete nitritation, the substrate shock was tested within the reactor for nitrite accumulation (Figure 2).
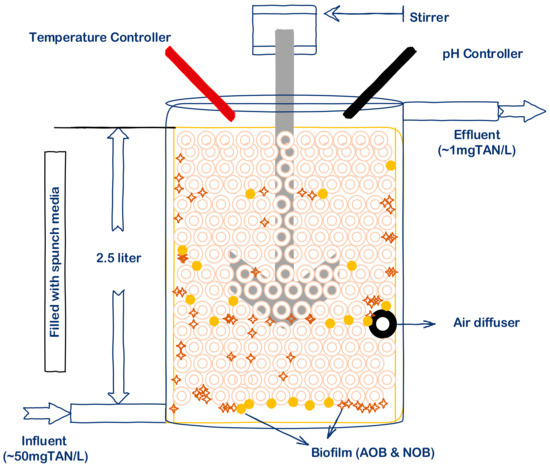
Figure 2.
Schematic diagram of CSTBR. Spunches were added in the reactor for the development of biofilm and sufficient DO was supplied via an air diffuser.
2.4. Substrate Shocks for NOB Suppression
2.4.1. Substrate Shocks during Steady-State Operation
A known quantity of ammonium bicarbonate (NH4HCO3) in powder form was injected into the CSTR for 65 operational days to verify the inhibition of NOB (Table 1). The operating conditions remained constant throughout the process. In the CSTBR, the liquid was drawn from the bottom of the reactor, a known concentration of ammonium was diluted in the liquid, and the resulting mixtures were returned to the reactor for 24 h. The shock liquid was then drawn through the bottom of the reactor, the influent feed was initiated, and nitrite, nitrate, and ammonium concentrations were measured to determine the effect of the shock on NOB.

Table 1.
Substrate shocks and nitrite accumulation.
2.4.2. Sludge Treatment
The influent feed concentration was stopped, and the cell suspension was transferred into 5 L jugs and centrifuged to separate the cells from the suspension. After centrifuging, the cells were rinsed three times and resuspended in a batch reactor. A known concentration of ammonia bicarbonate (NH4 HCO3) was added to make the required ammonia concentrations (3000 and 5000 mgTAN/L). Operational parameters such as temperature were controlled at ~30 °C, DO was ≥4 mg/L, and the pH was set to ~8. The substrate shock continued for 12 h in treatment 1 and for 24 h in the remaining treatments. Water with a pH < 4 was circulated with a flow rate of 10 mL/min in all treatments (except treatment 1) in the CSTR to remove any NOB attached to silicon tubes or walls of the reactor to improve the efficiency of nitrite accumulation. After the 24 h shock, the CSTR was washed and the cells were rinsed three times and resuspended in 10 L of mineral medium to maintain the environmental conditions for the AOB at those before sludge treatment before being transferred to the CSTR. The influent feed was initiated and TAN, nitrite, nitrate, and mixed liquor-suspended solids concentrations were recorded once a day. However, due to substrate shock during the sludge treatment, the ammonium concentration rose only by 6–10% above the steady-state concentration.
2.4.3. Sampling and Analysis
The TAN effluent and influent concentration, nitrite, and nitrate concentrations were measured through a standard method [17]. The FA and FNA concentrations were measured by Equations (1) and (2) [13,18].
3. Results and Discussion
3.1. Substrate Shocks and Nitrite Accumulation
The stability of biological reactors can be changed temporarily by substrate shock and returned to an original steady-state condition within a few days of operation [11,23,24,25,26,27]. The experimental results showed that NOB are more sensitive to FA concentrations [19].
We investigated the effect of substrate shock on NOB performance at lower influent and effluent concentrations. The CSTR began operating at an influent feed concentration of ~50 mg TAN/L and the reactor was stabilized after 33 operational days. Furthermore, the reactor was allowed to remain in a steady state for 65 days to achieve nitritation and sufficient sludge accumulation for the substrate shocks. The FA (0.45 to 181.4 mg/L) was initially added several times during the steady-state operation (Table 1). Substrate shocks ranging from 0.45 to 4.54 mg/L did not affect NOB performance, possibly due to a lower hydraulic retention time and shock concentrations diluted with influent feed. The NOB suppression began at 9.07 mg FA/L and increased as the FA shock rose. Similar results have been reported by Chung et al. (2012) [25]. At a higher shock concentration (181.4 mg FA/L), complete NOB suppression was achieved, but NOB recovered to the original steady-state position, and the mass balance was disturbed due to the conversion of some shock concentrations to nitrite (Figure 3). A concentration of 210 mg FAN/L was used in the batch tests to suppress NOB [15]. Suppression of NOB with a substrate shock was not as effective as long-term nitrification due to dilution of the shock concentration and mass-balance disturbance.
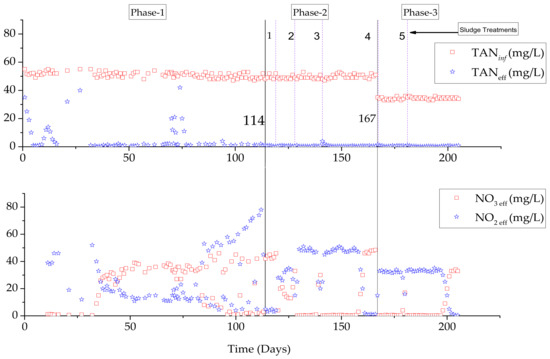
Figure 3.
CSTR performance at different substrate shocks and after sludge treatment (Sludge treatment stages: 1, 2, 3, 4, and 5).
In the CSTR, the substrate shock disturbed the mass balance, and an additional batch reactor was required to treat the sludge. The CSTBR was operated to reduce the operational cost and avoid mass imbalance, and an FA concentration of ~90.7 mg/L was used to suppress NOB for 24 h. After the shock, AOB performance was reduced, but it recovered rapidly without the need to alter the operational conditions, and a nitrite accumulation of 90% was achieved. After 16 days of nitrite accumulation, the same shock was repeated three times, and a nitrite concentration of 90% accumulated in the main stream (Figure 4).
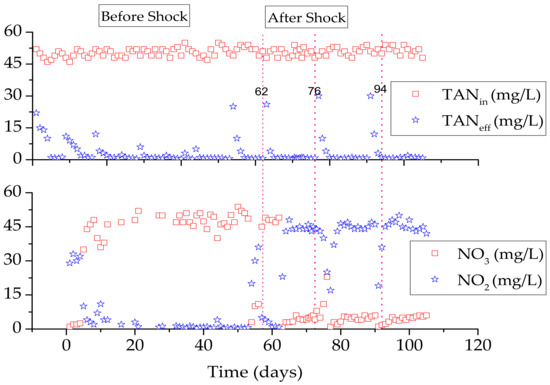
Figure 4.
CSTBR performance before and after shocks (substrate shock during operational days 62, 76 and 94).
3.2. Sludge Treatment and Nitrite Accumulation
A separate batch reactor was prepared for the substrate shocks. The cells were extracted from the CSTR on day 119 and resuspended in a batch reactor: 272 mg/L of FAN was added in treatment 1, and 453 mg/L was added in treatments 3, 4, and 5, with the same operational conditions maintained for 12 h (as in the CSTR). Next, the cells were resuspended in the CSTR, the influent feed was initiated, and nitrite accumulation reached 61% ± 7% over eight days. On day 128, the procedure was repeated and cells were shocked for 24 h to enhance the efficiency of the pH < 4 process water circulated in the CSTR to remove cells attached to silicon tubes or walls of the reactors. These measures increased the process efficiency to ~98% ± 2%, and the conditions were maintained for 10 days. On day 11 of the substrate shock, efficiency decreased to ~50%, and on day 12 it rose to 60%. On day 141, a similar procedure with a similar shock concentration was applied and an efficiency of ~98% ± 2% was achieved. This was maintained for 18 days and again nitrite accumulation decreased by ~40% on day 19 and 60% on day 20. On day 167, the cells were subjected to the same substrate shocks, but the influent concentration decreased to 35 mg TAN/L. As a result, the nitrite accumulation rate was maintained at ~98% ± 2% for 14 days (Figure 5).
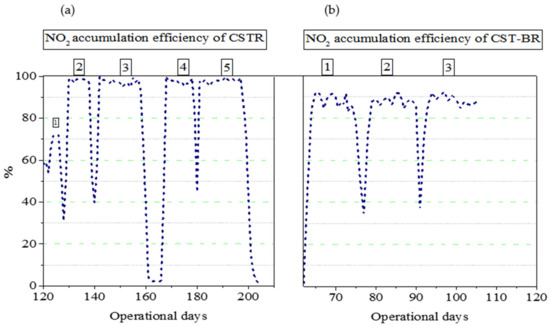
Figure 5.
Nitrite accumulation efficiency (%). (a) The NO2 accumulation efficiency of the CSTR after sludge treatment. The numbers from 1 to 5 show the number of sludge treatments tested after NOB recovery. (b) The NO2 accumulation efficiency after substrate shocks. The numbers from 1 to 3 show the number of shocks tested after NOB recovery.
The first time sludge treatment using FA shock was investigated [20], a nitrite accumulation efficiency greater than 90% was achieved. In that study, a portion of the activated sludge from the mainstream reactor or the sludge return line was transferred daily to the FA treatment unit, requiring additional cost and labor. In this study, the sludge was not treated on a daily schedule but at eight-day intervals in the first shock (sludge treatment 1), 10-day intervals in the second shock (sludge treatment 2), 18-day intervals in the third shock (sludge treatment 3), 14-day intervals in the fourth shock (sludge treatment 4), and 19-day intervals in the fifth shock (sludge treatment 5). Compared with Wang et al. (2014, 2017) in this study, the system was more efficient, did not require sludge treatment on a daily basis, and no external nitrogen source was required, indicating that the system can reduce WWTP costs. Compared with the CSTR, no batch reactor was required in the CSTBR and 90% efficiency was achieved in the mainstream, further reducing the cost of operation.
Wang et al. (2014) used an FNA shock to increase nitrite accumulation efficiency in the SBR [28]. However, for the FNA shock experiment, nitrogen proved to be an important source to shock the NOB. This was only possible in a high-concentration reactor or two separate reactors, one high-concentration and one low-concentration (sidestream treatment), which allowed NO2 to be recycled. Nitrite accumulation efficiency was low with FNA treatment (80%) [28]. In contrast to FNA treatment, the efficiency of NOB suppression with FA treatment was high, and nitrite accumulation reached 98% ± 2% (Table 2).

Table 2.
Comparison of reactor configurations: NOB suppression techniques, nitrite accumulation ratio, AOB%, NOB%, SRT, temperature, effluent ammonium concentrations, and influent concentration.
4. Conclusions
Ammonia removal through the nitrite pathway can reduce DO demand by 25%, decrease COD by 40%, and reduce sludge production. Different techniques have been used to remove ammonia through the nitrite pathway. In this research, substrate-shock and sludge-treatment techniques were applied. A substrate shock of 90.7 mg FA/L concentration was tested in a CSTBR, with ~90% nitrite accumulating in the mainstream. The shock concentration was repeated three times after 16 days, achieving the same efficiency. Sludge treatment proved successful for long-term nitrite accumulation in the mainstream, with nitrite accumulation reaching ~98% in a CSTR. In sludge treatment 1, 61% ± 7% nitrite accumulated, and in treatments 2, 3, 4, and 5, ~98% ± 2% efficiency was achieved. The efficiency for sludge treatments 1, 2, and 4 was sustained for 8–12 days, and for sludge treatments 3 and 4, efficiency was sustained for 18 days. The CSTBR offered advantages over the CSTR; a separate batch reactor was required for CSTR sludge treatment but an extra batch reactor was not required when sludge was shocked in the same reactor.
Author Contributions
Conceptualization, H.A.K. and W.B.; Methodology, H.A.K. and W.B.; Software, S.P.; Formal analysis, W.B. and S.P.; Investigation, H.A.K.; Resources, W.B.; Data curation, H.A.K. and S.P.; Writing–original draft, H.A.K.; Writing–review & editing, H.A.K.; Visualization, S.P.; Supervision, W.B.; Project administration, W.B.; Funding acquisition, W.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program funded by the Ministry of Education (NRF-2019R1D1A1A02046973) through the National Research Foundation of South Korea. Hareef Ahmed Keerio is grateful to the Higher Education Commission of Pakistan for granting a Ph.D. scholarship and then placement as a Assistant Professor in Quaid e Awam University of Engineering, Science and Technolgy (QUEST) Nawabshah.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CSTR | continuous stirred-tank reactor |
| CSTBR | continuous stirred-tank biofilm reactor |
| SBR | sequencing batch reactor |
| WWTPs | wastewater treatment plants |
| SRT | sludge retention time |
| NAR | nitrite accumulation ratio |
| AOB | ammonium-oxidizing bacteria |
| NOB | nitrite-oxidizing bacteria |
| TAN | total ammonium nitrogen |
| TANin | tan influent concentration |
| TANeff | tan effluent concentration |
| FA | free ammonia |
| FNA | free nitrous acid |
| DO | dissolved oxygen |
| BOD | biological oxygen demand |
References
- Turk, O.; Mavinic, D. Preliminary assessment of a shortcut in nitrogen removal from wastewater. Can. J. Civ. Eng. 1986, 13, 600–605. [Google Scholar] [CrossRef]
- Zhou, Y.; Oehmen, A.; Lim, M.; Vadivelu, V.; Ng, W.J. The role of nitrite and free nitrous acid (FNA) in wastewater treatment plants. Water Res. 2011, 45, 4672–4682. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.-C.; Zheng, P.; Mahmood, Q.; Zhang, L. Performance of a nitrifying airlift reactor using granular sludge. Sep. Purif. Technol. 2008, 63, 670–675. [Google Scholar] [CrossRef]
- Ahn, Y.-H. Sustainable nitrogen elimination biotechnologies: A review. Process Biochem. 2006, 41, 1709–1721. [Google Scholar] [CrossRef]
- Rostron, W.M.; Stuckey, D.C.; Young, A.A. Nitrification of high strength ammonia wastewaters: Comparative study of immobilisation media. Water Res. 2001, 35, 1169–1178. [Google Scholar] [CrossRef]
- Khan, H.; Bae, W. Optimized operational strategies based on maximum nitritation, stability, and nitrite accumulation potential in a continuous partial nitritation reactor. Process Biochem. 2016, 51, 1058–1068. [Google Scholar] [CrossRef]
- Hellinga, C.; Schellen, A.; Mulder, J.W.; van Loosdrecht, M.v.; Heijnen, J. The SHARON process: An innovative method for nitrogen removal from ammonium-rich waste water. Water Sci. Technol. 1998, 37, 135–142. [Google Scholar] [CrossRef]
- Anthonisen, A.; Loehr, R.; Prakasam, T.; Srinath, E. Inhibition of nitrification by ammonia and nitrous acid. J. (Water Pollut. Control Fed.) 1976, 48, 835–852. [Google Scholar]
- Pedrouso, A.; del Rio, A.V.; Morales, N.; Vazquez-Padin, J.R.; Campos, J.L.; Méndez, R.; Mosquera-Corral, A. Nitrite oxidizing bacteria suppression based on in-situ free nitrous acid production at mainstream conditions. Sep. Purif. Technol. 2017, 186, 55–62. [Google Scholar] [CrossRef]
- Ma, B.; Bao, P.; Wei, Y.; Zhu, G.; Yuan, Z.; Peng, Y. Suppressing nitrite-oxidizing bacteria growth to achieve nitrogen removal from domestic wastewater via anammox using intermittent aeration with low dissolved oxygen. Sci. Rep. 2015, 5, 13048. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wei, Y.; Chen, M. In-situ restoring nitrogen removal for the combined partial nitritation-anammox process deteriorated by nitrate build-up. Biochem. Eng. J. 2015, 98, 127–136. [Google Scholar] [CrossRef]
- Pedrouso, A.; del Rio, A.V.; Campos, J.; Méndez, R.; Mosquera-Corral, A. Biomass aggregation influences NaN3 short-term effects on anammox bacteria activity. Water Sci. Technol. 2016, 75, 1007–1013. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Nitrification inhibition by silver nanoparticles. Water Sci. Technol. 2009, 59, 1699–1702. [Google Scholar] [CrossRef]
- Harper, W.F., Jr.; Terada, A.; Poly, F.; le Roux, X.; Kristensen, K.; Mazher, M.; Smets, B.F. The effect of hydroxylamine on the activity and aggregate structure of autotrophic nitrifying bioreactor cultures. Biotechnol. Bioeng. 2009, 102, 714–724. [Google Scholar] [CrossRef]
- Tang, C.-J.; Duan, C.-S.; Liu, P.; Chai, X.; Min, X.; Wang, S.; Xiao, R.; Wei, Z. Inhibition kinetics of ammonium oxidizing bacteria under Cu(II) and As(III) stresses during the nitritation process. Chem. Eng. J. 2018, 352, 811–817. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Fu, H.; Chen, T.; Liu, S.; Zheng, S.; Zhang, J. Effect of zinc oxide nanoparticles on nitrogen removal, microbial activity and microbial community of CANON process in a membrane bioreactor. Bioresour. Technol. 2017, 243, 93–99. [Google Scholar] [CrossRef]
- Kapoor, V.; Elk, M.; Li, X.; Impellitteri, C.A.; Domingo, J.W.S. Effects of Cr(III) and Cr(VI) on nitrification inhibition as determined by SOUR, function-specific gene expression and 16S rRNA sequence analysis of wastewater nitrifying enrichments. Chemosphere 2016, 147, 361–367. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, H.; Wang, J.; Liu, Z.; Guan, Q. Effect of free ammonia inhibition on NOB activity in high nitrifying performance of sludge. RSC Adv. 2018, 8, 31987–31995. [Google Scholar] [CrossRef]
- Wang, Q.; Duan, H.; Wei, W.; Ni, B.-J.; Laloo, A.; Yuan, Z. Achieving stable mainstream nitrogen removal via the nitrite pathway by sludge treatment using free ammonia. Environ. Sci. Technol. 2017, 51, 9800–9807. [Google Scholar] [CrossRef]
- Keerio, H.A.; Bae, W.; Park, J.; Kim, M. Substrate uptake, loss, and reserve in ammonia-oxidizing bacteria (AOB) under different substrate availabilities. Process Biochem. 2020, 91, 303–310. [Google Scholar] [CrossRef]
- Federation, W.E.; Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Briggs, G.E.; Haldane, J.B.S. A note on the kinetics of enzyme action. Biochem. J. 1925, 19, 338. [Google Scholar] [CrossRef] [PubMed]
- Keerio, H.A. Substrate Uptake Mechanisms, Self-Inhibition, and Nitrite Accumulation by Ammonium Oxidizing Bacteria (AOB). Ph.D. Thesis, Hanyang University, Seoul, Republic of Korea, 2020. [Google Scholar]
- Chung, J.; Kim, S.; Baek, S.; Lee, N.-H.; Park, S.; Lee, J.; Lee, H.; Bae, W. Acceleration of aged-landfill stabilization by combining partial nitrification and leachate recirculation: A field-scale study. J. Hazard. Mater. 2015, 285, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Zhang, M.; Li, W.; Zhang, J.; Zheng, P. Performance stability of a lab-scale internal-loop airlift bio-particle reactor under substrate concentration shocks for simultaneous partial nitrification and anaerobic ammonia oxidation. Sep. Purif. Technol. 2015, 141, 322–330. [Google Scholar] [CrossRef]
- Keerio, H.A.; Bae, W. Experimental Investigation of Substrate Shock and Environmental Ammonium Concentration on the Stability of Ammonia-Oxidizing Bacteria (AOB). Water 2020, 12, 223. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, L.; Jiang, G.; Hu, S.; Yuan, Z. Side-stream sludge treatment using free nitrous acid selectively eliminates nitrite oxidizing bacteria and achieves the nitrite pathway. Water Res. 2014, 55, 245–255. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Wang, S.; Zheng, Y.; Huang, H.; Ge, S. Effective and robust partial nitrification to nitrite by real-time aeration duration control in an SBR treating domestic wastewater. Process Biochem. 2009, 44, 979–985. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Li, B.; Zhang, Q.; Wu, L.; Li, X.; Peng, Y. Enhanced nitrogen and phosphorus removal from municipal wastewater in an anaerobic-aerobic-anoxic sequencing batch reactor with sludge fermentation products as carbon source. Bioresour. Technol. 2017, 244, 1158–1165. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Q.; Ma, B.; Li, J.; Ma, L.; Wang, S.; Peng, Y. Rapid achievement of nitritation using aerobic starvation. Environ. Sci. Technol. 2017, 51, 4001–4008. [Google Scholar] [CrossRef]
- Ji, Z.; Chen, Y. Using sludge fermentation liquid to improve wastewater short-cut nitrification-denitrification and denitrifying phosphorus removal via nitrite. Environ. Sci. Technol. 2010, 44, 8957–8963. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, K.; Han, X.; Du, B.; Wei, Q.; Wei, D. Achievement, performance and characteristics of microbial products in a partial nitrification sequencing batch reactor as a pretreatment for anaerobic ammonium oxidation. Chemosphere 2017, 183, 212–218. [Google Scholar] [CrossRef]
- Yin, J.; Xu, H.; Shen, D. Partial nitrification in sequencing batch reactors treating low ammonia strength synthetic wastewater. Water Environ. Res. 2014, 86, 606–614. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, L.; Li, B.; Zhang, Q.; Wang, S.; Peng, Y. Enhancing ammonium oxidizing bacteria activity was key to single-stage partial nitrification-anammox system treating low-strength sewage under intermittent aeration condition. Bioresour. Technol. 2017, 231, 36–44. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, M.; Guo, Y.; Peng, Y.; Yuan, Y. Long-term partial nitritation and microbial characteristics in treating low C/N ratio domestic wastewater. Environ. Sci. Water Res. Technol. 2018, 4, 820–827. [Google Scholar] [CrossRef]
- Qian, F.; Wang, J.; Shen, Y.; Wang, Y.; Wang, S.; Chen, X. Achieving high performance completely autotrophic nitrogen removal in a continuous granular sludge reactor. Biochem. Eng. J. 2017, 118, 97–104. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, J.; Horn, H.; Yang, X.; Wang, S. Achieving nitrite accumulation in a continuous system treating low-strength domestic wastewater: Switchover from batch start-up to continuous operation with process control. Appl. Microbiol. Biotechnol. 2012, 94, 517–526. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).