Microcosm Study on the Potential of Aquatic Macrophytes for Phytoremediation of Phosphorus-Induced Eutrophication
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Protocol and Plant Culture Environment
Cultural Conditions and Experimental Set-Up
2.2. Plant Biomass and Phosphorous Assay
2.3. Phosphorous Analysis and Working Solution
2.3.1. Amount of P Removed
2.3.2. Removal Rate of P
2.3.3. Phosphorous Uptake
2.3.4. Removal Efficiency of P
2.3.5. Bioconcentration Factor (BCF)
2.4. Statistical Analysis
3. Results and Discussion
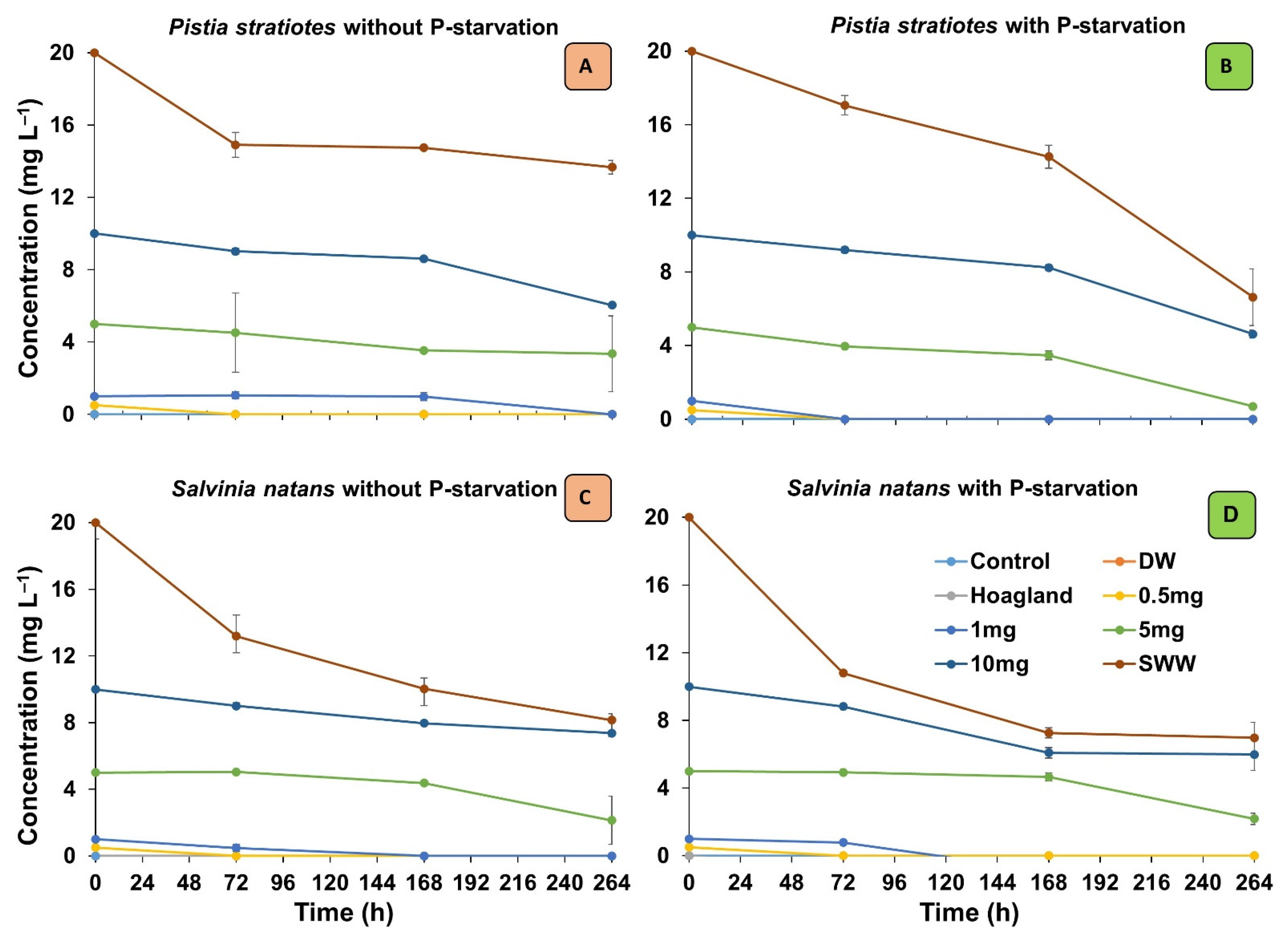
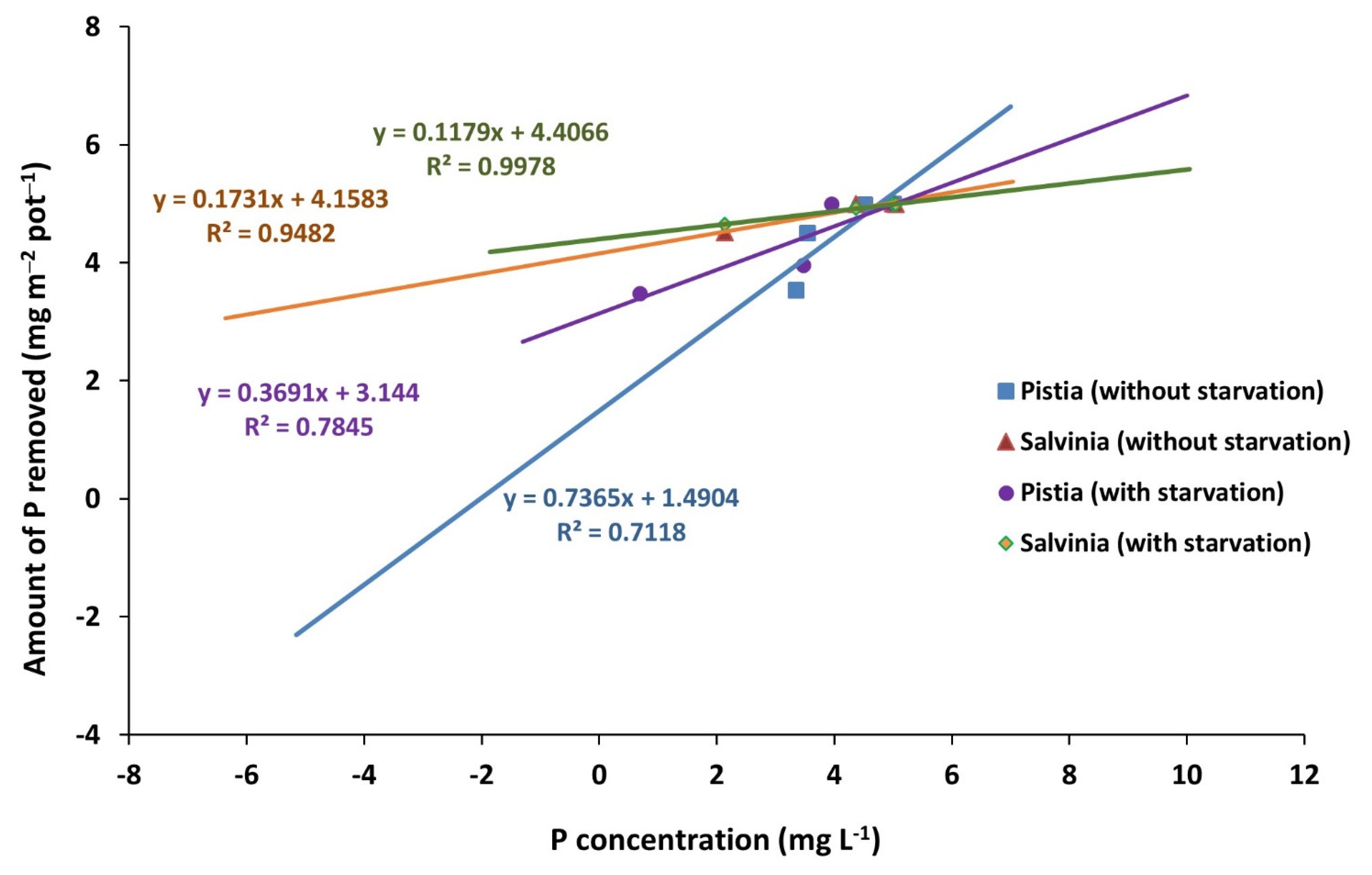
3.1. Phosphorous Removal from Solutions
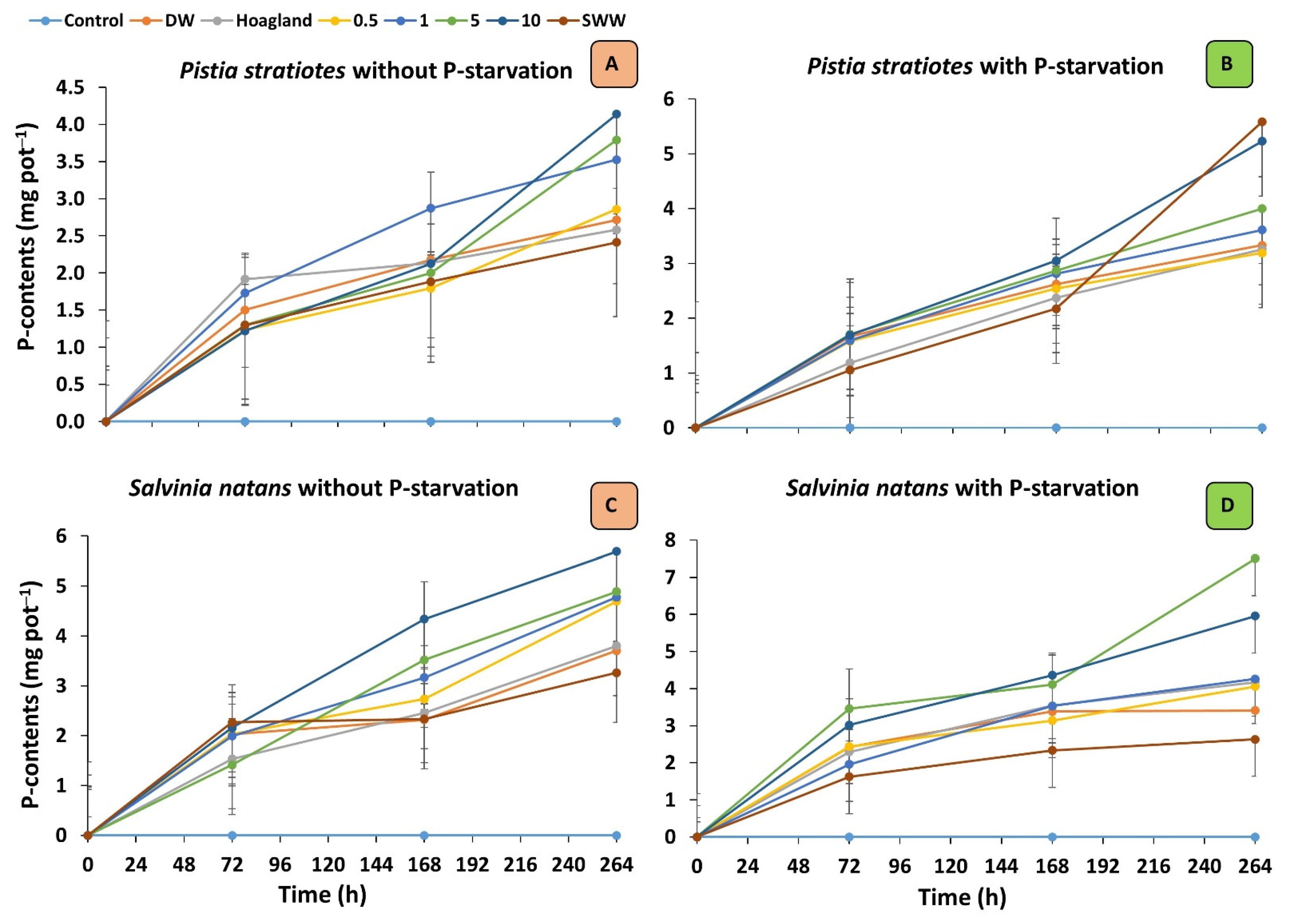
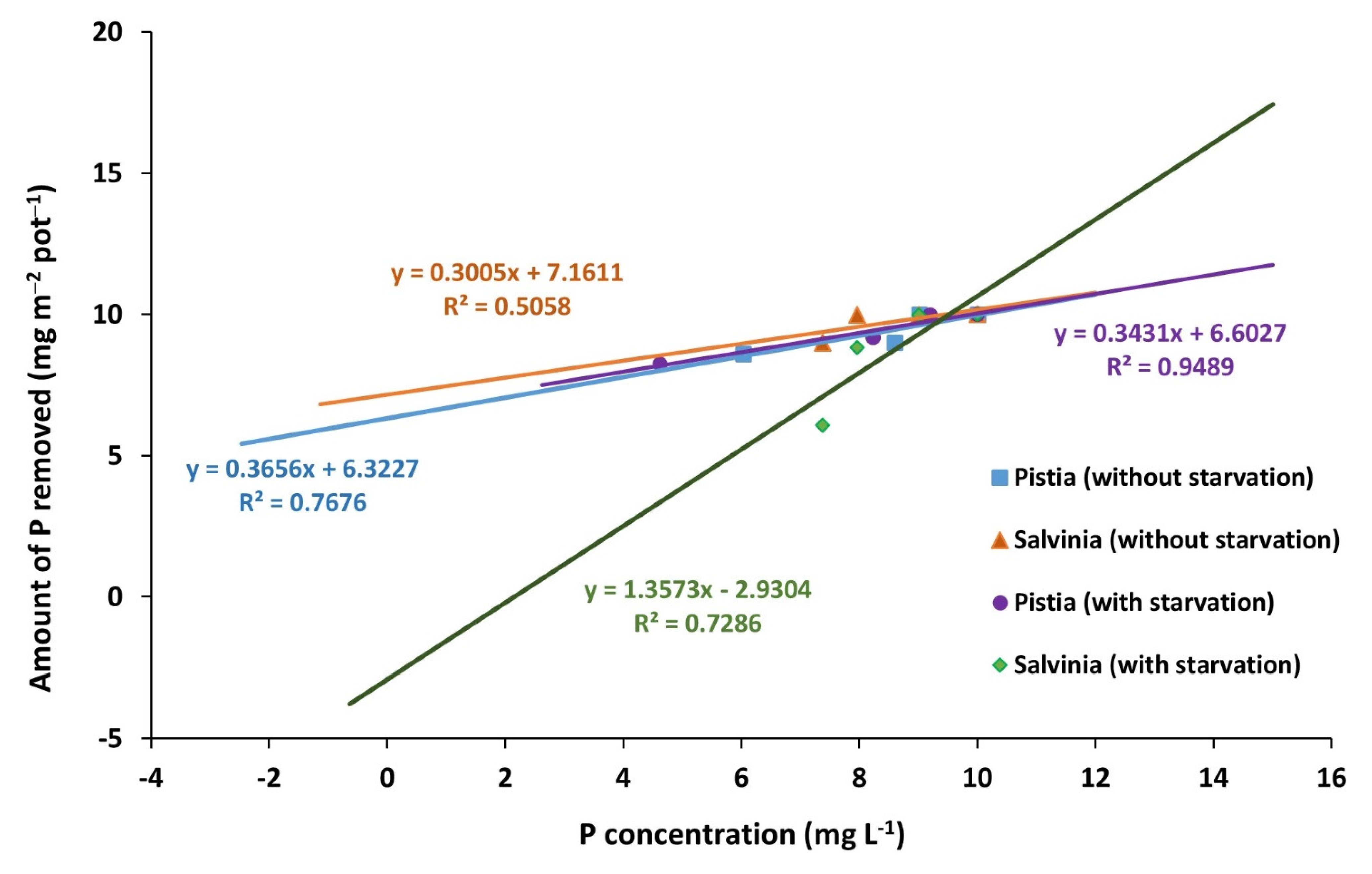
3.2. Phosphorous Content and Removal Rate of Plants
3.3. Removal Efficiency of Plants
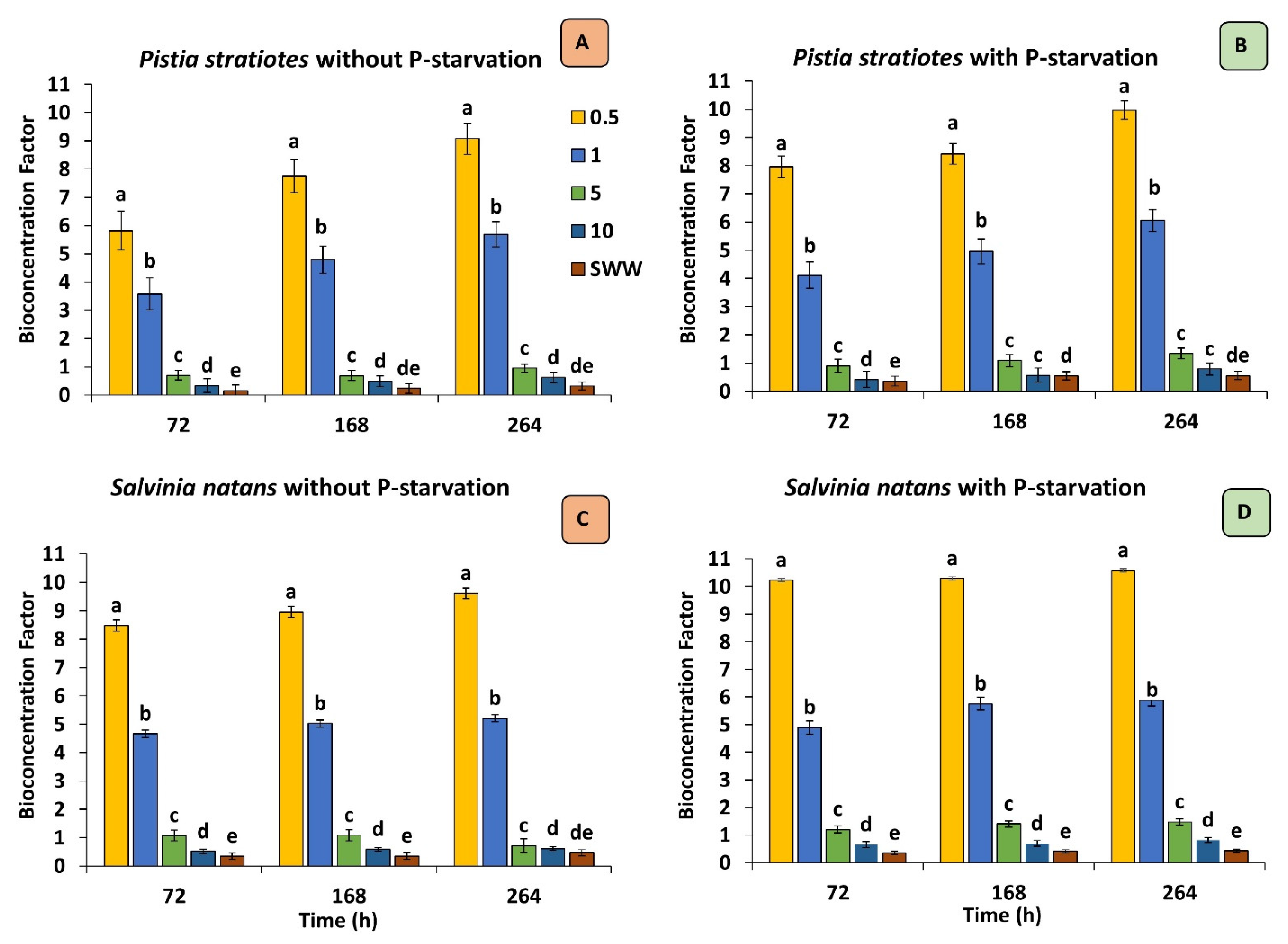
3.4. Bioconcentration Factor (BCF)
3.5. Biomass of Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhtar, M.S.; Oki, Y.; Nakashima, Y.; Nishigaki, M.; Adachi, T.; Kamigaki, T. Microcosm Investigation on Differential Potential of Free Floating Azolla Macrophytes for Phytoremediation of P-Controlled Water Eutrophication. Int. J. Agric. Biol. 2016, 18, 204–212. [Google Scholar] [CrossRef]
- Rauchfuss, T.B. Phosphorus: An Outline of Its Chemistry, Biochemistry and Technology, Fifth Edition, Studies in Inorganic Chemistry, #20 By D. E. C. Corbridge (University of Leeds). Elsevier: Amsterdam. 1995. 1220 pp. $514.75. ISBN 0-444-89307-5. J. Am. Chem. Soc. 1996, 118, 7871. [Google Scholar]
- Cordell, D.; White, S. Peak Phosphorus: Clarifying the Key Issues of a Vigorous Debate about Long-Term Phosphorus Security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Sarvajayakesavalu, S.; Lu, Y.; Withers, P.J.A.; Pavinato, P.S.; Pan, G.; Chareonsudjai, P. Phosphorus Recovery: A Need for an Integrated Approach. Ecosyst. Health Sustain. 2018, 4, 48–57. [Google Scholar] [CrossRef]
- Jain, C.K.; Singh, S. Best Management Practices for Agricultural Nonpoint Source Pollution: Policy Interventions and Way Forward. World Water Policy 2019, 5, 207–228. [Google Scholar] [CrossRef]
- Lu, Q.; He, Z.L.; Graetz, D.A.; Stoffella, P.J.; Yang, X. Phytoremediation to Remove Nutrients and Improve Eutrophic Stormwaters Using Water Lettuce (Pistia stratiotes L.). Environ. Sci. Pollut. Res. 2010, 17, 84–96. [Google Scholar] [CrossRef]
- Khan, M.N.; Mohammad, F. Eutrophication: Challenges and Solutions BT-Eutrophication: Causes, Consequences, and Control; Ansari, A.A., Gill, S.S., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 1–15. [Google Scholar]
- Xiang, W.; Xiao, -E.Y.; Rengel, Z. Phytoremediation Facilitates Removal of Nitrogen and Phosphorus from Eutrophicated Water and Release from Sediment. Environ. Monit. Assess. 2009, 157, 277–285. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Dhir, B. Introduction BT-Phytoremediation: Role of Aquatic Plants in Environmental Clean Up; Dhir, B., Ed.; Springer: Delhi, India, 2013; pp. 1–20. [Google Scholar]
- Victor, K.K.; Séka, Y.; Norbert, K.K.; Sanogo, T.A.; Celestin, A.B. Phytoremediation of Wastewater Toxicity Using Water Hyacinth (Eichhornia Crassipes) and Water Lettuce (Pistia stratiotes). Int. J. Phytoremediation 2016, 18, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nizam, N.U.; Mohd Hanafiah, M.; Mohd Noor, I.; Abd Karim, H.I. Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater. Appl. Sci. 2020, 10, 2712. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Shah, A.; Ditta, A.; Nawaz, M.A.; Tariq, M. Plant growth regulators, and EDTA improve phytoremediation potential and antioxidant response of Dysphania ambrosioides (L.) Mosyakin & Clemants in a Cd-spiked soil. Environ. Sci. Pollut. Res. 2021, 28, 43417–43430. [Google Scholar]
- Daraz, U.; Li, Y.; Ahmad, I.; Iqbal, R.; Ditta, A. Remediation technologies for acid mine drainage: Recent trends and future perspectives. Chemosphere 2023, 311, 137089. [Google Scholar] [CrossRef] [PubMed]
- Irshad, S.; Xie, Z.; Mehmood, S.; Nawaz, A.; Ditta, A.; Mahmood, Q. Insights into conventional and recent technologies for arsenic bioremediation: A systematic review. Environ. Sci. Pollut. Res. 2021, 28, 18870–18892. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; De Busk, W.F. Nutrient Removal Potential of Selected Aquatic Macrophytes. J. Environ. Qual. 1985, 14, 459–462. [Google Scholar] [CrossRef]
- Mahadevaiah; Kumar, M.S.Y.; Galil, M.S.A.; Suresha, M.S.; Sathish, M.A.; Nagendrappa, G. A Simple Spectrophotometric Determination of Phosphate in Sugarcane Juices, Water and Detergent Samples. E-J. Chem. 2007, 4, 576560. [Google Scholar] [CrossRef]
- Gaballah, M.S.; Ismail, K.; Beltagy, A.; Zein Eldin, A.M.; Ismail, M.M. Wastewater Treatment Potential of Water Lettuce (Pistia stratiotes) with Modified Engineering Design. J. Water Chem. Technol. 2019, 41, 197–205. [Google Scholar] [CrossRef]
- Zutshi, D.P.; Vass, K.K. Ecology and Production of Salvinia natans Hoffim; in Kashmir. Hydrobiologia 1971, 38, 303–320. [Google Scholar] [CrossRef]
- Su, F.; Li, Z.; Li, Y.; Xu, L.; Li, Y.; Li, S.; Chen, H.; Zhuang, P.; Wang, F. Removal of Total Nitrogen and Phosphorus Using Single or Combinations of Aquatic Plants. Int. J. Environ. Res. Public Health 2019, 16, 4663. [Google Scholar] [CrossRef]
- Hamid, A.; Zeb, M.; Mehmood, A.; Akhtar, S.; Saif, S. Assessment of Wastewater Quality of Drains for Irrigation. J. Environ. Prot. 2013, 4, 937–945. [Google Scholar] [CrossRef]
- Shardendu, S.; Sayantan, D.; Sharma, D.; Irfan, S. Luxury Uptake and Removal of Phosphorus from Water Column by Representative Aquatic Plants and Its Implication for Wetland Management. ISRN Soil Sci. 2012, 2012, 516947. [Google Scholar] [CrossRef]
- Kurniawan, A.; Khasanah, K.; Jayatri, F.N.M. Study on the Application of Phytoremediation of Phosphate Content to Eutrophication in Cengklik Reservoir, Boyolali Regency. IOP Conf. Ser. Earth Environ. Sci. 2022, 986, 12075. [Google Scholar] [CrossRef]
- Nivetha, C.; Subraja, S.; Sowmya, R.; Induja, N.M. Water Lettuce for Removal of Nitrogen and Phosphate from Sewage. J. Mech. Civ. Eng. 2016, 13, 104–107. [Google Scholar]
- Qin, H.; Zhang, Z.; Liu, M.; Liu, H.; Wang, Y.; Wen, X.; Zhang, Y.; Yan, S. Site Test of Phytoremediation of an Open Pond Contaminated with Domestic Sewage Using Water Hyacinth and Water Lettuce. Ecol. Eng. 2016, 95, 753–762. [Google Scholar] [CrossRef]
- Yu, S.; Miao, C.; Song, H.; Huang, Y.; Chen, W.; He, X. Efficiency of Nitrogen and Phosphorus Removal by Six Macrophytes from Eutrophic Water. Int. J. Phytoremediat. 2019, 21, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.R.A.; Alkredaa, R.S.; Al-Wabel, M.I. Heavy Metal Contamination in Sediments and Mangroves from the Coast of Red Sea: Avicennia Marina as Potential Metal Bioaccumulator. Ecotoxicol. Environ. Saf. 2013, 97, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Nabi, M. Heavy Metals Accumulation in Aquatic Macrophytes from an Urban Lake in Kashmir Himalaya, India. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100509. [Google Scholar] [CrossRef]
- Prado, C.; Chocobar-Ponce, S.; Pagano, E.; Prado, F.; Rosa, M. Differential Effects of Zn Concentrations on Cr(VI) Uptake by Two Salvinia Species: Involvement of Thiol Compounds. Int. J. Phytoremediation 2021, 23, 10–17. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, İ.; Ünay, A.; Abdel-DAIM, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of Floating Aquatic Plants in Phytoremediation of Heavy Metals Polluted Water: A Review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Al-Hamdani, S.H.; Sirna, C.B. Physiological Responses of Salvinia Minima to Different Phosphorus and Nitrogen Concentrations. Am. Fern J. 2008, 98, 71–82. [Google Scholar] [CrossRef]
- Room, P.M. Equations Relating Growth and Uptake of Nitrogen by Salvinia Molesta to Temperature and the Availability of Nitrogen. Aquat. Bot. 1986, 24, 43–59. [Google Scholar] [CrossRef]
- Ayache, L.; Boudehane, A.S.M.A. Wastewater treatment by floating macrophytes (Salvinia natans) under Algerian semi-Arid climate. Eur. J. Eng. Nat. Sci. 2019, 3, 103–110. [Google Scholar]
- Das, S.; Goswami, S.; Talukdar, A.D. A study on cadmium phytoremediation potential of water lettuce, Pistia stratiotes L. Bull. Environ. Contam. Toxicol. 2014, 92, 169–174. [Google Scholar] [CrossRef] [PubMed]


| Salts | Salt Names | Quantity (g 5 L−1) |
|---|---|---|
| C6H12O6 | Glucose | 3.60 |
| C13H24O4 | Peptone | 2.70 |
| C85H124N14O16S | Yeast | 0.36 |
| (NH4)2SO4 | Ammonium Sulfate | 2.88 |
| KH2PO4 | Potassium dihydrogen phosphate | 0.50 |
| MgSO4·7H2O | Magnesium sulfate heptahydrate | 0.72 |
| MnSO4·7H2O | Manganese sulfate heptahydrate | 0.0648 |
| FeCl3·6H2O | Ferric chloride hexahydrate | 0.0036 |
| NaHCO3 | Sodium Bicarbonate | 9.00 |
| CaCl2·2H2O | Calcium chloride dihydrate | 0.072 |
| Salts | Salt Names | Quantity (mg L–1) |
|---|---|---|
| NH4NO3 | Ammonium Nitrate | 2.00 |
| CaCl2·2H2O | Calcium chloride dihydrate | 2.06 |
| KCl | Potassium Chloride | 2.00 |
| MgSO4·7H2O | Magnesium sulfate heptahydrate | 0.48 |
| MnSO4·5H2O | Manganese sulfate pentahydrate | 0.005 |
| EDTA-Na-Fe·H2O | Ethylenediaminetetraacetic acid disodium | 0.062 |
| ZnSO4·6H2O | Zinc sulfate hexahydrate. | 0.007 |
| H2MoO4·H2O | Molybdic acid monohydrate | 0.005 |
| CuSO4·5H2O | Copper Sulfate pentahydrate | 0.006 |
| H3BO3 | Boric Acid | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dean, S.; Akhtar, M.S.; Ditta, A.; Valipour, M.; Aslam, S. Microcosm Study on the Potential of Aquatic Macrophytes for Phytoremediation of Phosphorus-Induced Eutrophication. Sustainability 2022, 14, 16415. https://doi.org/10.3390/su142416415
Dean S, Akhtar MS, Ditta A, Valipour M, Aslam S. Microcosm Study on the Potential of Aquatic Macrophytes for Phytoremediation of Phosphorus-Induced Eutrophication. Sustainability. 2022; 14(24):16415. https://doi.org/10.3390/su142416415
Chicago/Turabian StyleDean, Sarah, Muhammad Shahbaz Akhtar, Allah Ditta, Mohammad Valipour, and Sohaib Aslam. 2022. "Microcosm Study on the Potential of Aquatic Macrophytes for Phytoremediation of Phosphorus-Induced Eutrophication" Sustainability 14, no. 24: 16415. https://doi.org/10.3390/su142416415
APA StyleDean, S., Akhtar, M. S., Ditta, A., Valipour, M., & Aslam, S. (2022). Microcosm Study on the Potential of Aquatic Macrophytes for Phytoremediation of Phosphorus-Induced Eutrophication. Sustainability, 14(24), 16415. https://doi.org/10.3390/su142416415








