Abstract
Grape pomace is the major component in grape fruits and is mostly wasted after wine and juice making processes. To recycle the residual biomass in grape pomace, extraction conditions of polysaccharides from grape pomace (GPP) were investigated. Three parameters affecting the crude GPP extraction, material to solvent ratio, extraction time, and extraction temperature were determined through single parameter optimization and then further optimized by orthogonal test. Results showed that the optimum extraction conditions were material to solvent ratio of 1:25, extraction temperature of 75 °C, and extraction time of 40 min, with extraction time as the most significant factor among them. Crude GPP was purified by gel column chromatography and chemically characterized. UV-Vis spectra analysis indicated that the GPP fraction did not contain any proteins or nucleic acids. FT-IR analysis implied that GPP consisted of α- and β-pyranose with carboxyl groups. Monosaccharide composition analysis indicated that GPP was composed of arabinose, glucose, galactose, and mannose with a molar ratio of 18.4:14.1:10.8:3.0. These results provide a theoretic basis for the production and utilization of GPP.
1. Introduction
Grape pomace is the major component of its fruits, contributing roughly half of the total weight, and poses a severe environmental issue after winemaking and juice processing [1]. Utilization of grape pomace by bioactive components extraction has been extensively performed, and minor components such as anthocyanins, hydroxycinnamic acids, flavanols, and flavanol glycosides in grape pomace are characterized [2,3,4,5]. However, little is known about the utilization of the major macromolecular components in grape pomace. It is thus of great health and economic significance to investigate the isolation method, chemical structure, and potential utilization of the polysaccharides in grape pomace (GPP) as an important biomass reutilization.
Polysaccharides are complex macromolecules widely present in plants, animals, microorganisms, and marine lives [6]. In the past decades, numerous studies have revealed that polysaccharides exhibit a variety of biological activities including antiradiation, antioxidation, antitumor, antiviral, anticoagulant, and antiaging [7]. In particular, plant-derived bioactive polysaccharides often yield broad market demand and attract increasing attention [8,9,10]. Polysaccharides are also playing an increasingly significant role in our diet since they modulate gut microbiota via different chemical structures [11].
For the extraction of plant-derived polysaccharides from plants such as Dendrobium wardianum, Glycyrrhiza, and Psidium guajava, various methods have been developed, including hot water extraction, ultrasonic-assisted extraction, enzyme-assisted extraction, and microwave-assisted extraction [12,13,14,15]. For food grade polysaccharide production, hot water extraction has been broadly applied due to the absence of organic chemical reagents which endows its convenience and environmental friendliness [2].
In this study, single parameter optimization was applied to optimized parameters of material to solvent ratio, extraction times, and extraction temperature first. Subsequently, the orthogonal test was employed to evaluate the synergistic effect of these parameters. The isolated crude GPP was further purified with chromatography, and its chemical properties were primarily characterized. This research provides an initial chemical account of the polysaccharide from grape pomace and lays the foundation for its future utilization in food and healthcare.
2. Materials and Methods
2.1. Materials
The Beibinghong grape (Vitis vinifera × Vitis amurensis) pomace was gifted from Dr. Yibin Lan in the College of Food Science & Nutritional Engineering, China Agricultural University, which is the same as previous research [2]. Trifluoroacetic acid, D-glucose, L-arabinose, D-galactose, and D-mannose were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). Absolute ethanol (≥99.7%), methanol, and acetonitrile were purchased from Modern East Technology Development Co., Ltd. (Beijing, China). Phenol, hydrochloric acid, sulfuric acid (95–98%), aqueous ammonia, sodium hydroxide, and calcium chloride dehydrate were purchased from Sinopharm Chemical Reagents Co., Ltd. (Shanghai, China).
2.2. Extraction of GPP
The frozen grape pomace (−4 °C) was dried in a drying oven (DGH-9030, Yiheng Technology Instrument, Shanghai, China) at 65 °C for 6 h until the material was free of lumps and the surface was dry. Dry grape pomace was crushed in a pulverizer (LFP-800T, Yongkang Hongtaiyang Electromechanical, Yongkang, Zhejiang, China), and sieved to obtain powders (60 mesh). Crushed grape pomace was loaded in the filter paper, which was placed inside the Soxhlet extractor, and refluxed with absolute ethanol for 6 h to remove lipids. Grape pomace materials, after being defatted, were collected and dried in the drying oven at 65 °C for 6–8 h to provide desired polysaccharides.
A portion of pretreated dried grape pomace was incubated with 90 °C distilled water at material to solvent ratio of 1:25 for 30 min to perform preliminary extraction of GPP. The fundamental extraction conditions were modified from the earlier investigation [16]. The residue was removed with gauze to obtain a clear solution before being further concentrated to 20 mL with a rotary evaporator (RE-200, Yarong Biochemical Instrument, Shanghai, China). Then, a four-fold volume of absolute ethanol was added to precipitate the polysaccharides. After standing overnight, the extract solution was centrifuged at 6000× g rpm for 10 min at room temperature to obtain precipitants. The polysaccharide content of GPP was determined with the phenol-sulfuric acid method, as previously described [17].
2.3. Single Parameter Optimization
Parameters of material to solvent ratio, extraction time, and extraction temperature were optimized to investigate the influence of these parameters on the extraction yield. During the optimization, one parameter was changed while the other parameters were kept constant in each experiment. All experiments were repeated at least three times.
2.3.1. Material to Solvent Ratio
Grape pomace mixed with distilled water at different material to solvent ratios of 1:20, 1:30, 1:40, 1:50, and 1:60, were incubated in the water bath at 90 °C for 30 min. The GPP yield was measured as described above.
2.3.2. Extraction Time
Grape pomace mixed with distilled water at a material to solvent ratio of 1:25 were incubated in the water bath at 90 °C for 20 min, 30 min, 40 min, 50 min, and 60 min, respectively. The GPP yield was measured as described above.
2.3.3. Extraction Temperature
Grape pomace mixed with distilled water at a material to solvent ratio of 1:25, were incubated in the water bath for 30 min at different temperatures of 60 °C, 70 °C, 80 °C, 90 °C, and 100 °C, respectively. The GPP yield was measured as described above.
2.4. Orthogonal Test Design of GPP Extraction
Single parameter optimization confirmed that three parameters for GPP extraction, material to solvent ratio, extraction time, and extraction temperature, profoundly affected GPP extraction yield. An orthogonal L9 (33) test design (Table 1) was subsequently applied to further optimize the parameters of GPP extraction to investigate the optimal extraction condition [18]. A total of nine experiments were conducted, each experiment was performed in triplicate, and the yield (%) of GPP was the dependent variable.

Table 1.
Factors and levels for the orthogonal test.
2.5. Purification of GPP
First, crude GPP was treated to remove pectin and small molecule impurities. The pH of the grape pomace polysaccharide solution was adjusted to 8.5 with ammonia solution. CaCl2 solution (10%) was titrated to the GPP solution until no pectin precipitate was observed, and the pectin precipitate was removed by centrifugation. The supernatant was dialyzed (with a molecular weight cutoff of 3000 Da, 4 °C) for 48 h to remove impurities and the dialysis water was changed every 5–6 h.
Crude GPP was adjusted to a concentration of 5.0 mg/mL and applied to HiPrep™ Sephacryl™ S-400 HR gel filtration chromatography column (GE Healthcare, Boston, MA, USA) to remove impurities [17]. The polysaccharides contents were eluted at a flow rate of 1 mL/min and then every 5 mL of polysaccharides elution was collected automatically. The major polysaccharide peak was obtained for further characterization.
2.6. Chemical Analysis of Purified GPP
2.6.1. UV-Vis Spectroscopy
A total of 0.25 mg purified GPP was dissolved in 1.0 mL deionized water and examined with a DS-11 FX+ spectrophotometer (DeNovix, Wilmington, DE, USA). The light path was 1 cm, and the absorbance was recorded in the wavelength range of 190 nm to 600 nm with a step size of 1 nm.
2.6.2. FT-IR Spectroscopy
Dried GPP was ground into powder and mixed with KBr before being pressed into a pellet. Spectrum measurement was taken three times in the frequency range from 400 to 4000 cm−1 with a PerkinElmer FT-IR spectrometer (Spectrum 100, PerkinElmer, Waltham, MA, USA). The average number of scans was 16, and the spectral resolution was 4 cm−1. The collected spectra were processed with the Spectrum 10 software (PerkinElmer).
2.6.3. Monosaccharide Composition Analysis with a Pre-Column PMP-HPLC Method
GPP was hydrolyzed with an equal volume of trifluoroacetic acid (0.4 M, TFA) for 6 h at 120 °C in a sealed glass tube full of nitrogen. Subsequently, methanol was added into the hydrolysate (1:1, v/v) to remove the residual TFA for repeating three times through a rotary evaporator. The dried hydrolysate was dissolved into ultrapure water. Sample hydrolysate was mixed with an equal volume of 0.3 mol/L NaOH solution and 0.5 mol/L methanol solution of 1-phenyl-3-methyl-5-pyrazolone (PMP) and incubated in the water bath at 70 °C for 30 min. After the mixture was cooled to room temperature, hydrochloric acid was added to neutralize excess NaOH. In order to remove excess PMP, the residue was dissolved in an equal volume of trichloromethane, and the organic phase was discarded after a violent shake and centrifugation (3000× g rpm/min). This step was repeated three times to completely clear redundant reagents and obtain a derivatized GPP solution.
Each of the monosaccharide standard solutions (mannose, glucose, galactose, and arabinose) was prepared at a concentration of 1 g/mL using the same operation conducted in the GPP solution to gain derivatized monosaccharide standard solutions. Both the monosaccharide standard and GPP were filtered (0.22 µm) and analyzed on an Eclipse XDB-C18 column (250 mm × 4.6 mm, Agilent, Palo Alto, CA, USA) with a column temperature of 30 °C and detection wavelength of 254 nm. The mobile phase was a mixture of 0.1 mol/L phosphate buffer (pH 6.7) and acetonitrile with a volume ratio of 83:17, and the flow rate was set to 1.0 mL/min simultaneously. A standard monosaccharide solution of known concentration was used to determine the molar ratio of the monosaccharide component by calculating the peak area [17,19].
2.7. Statistical Analysis
Each experiment was repeated three times independently. All data were statistically analyzed using one-way ANOVA on SPSS (version 22.0, IBM Corp., Armonk, NY, USA). p < 0.05 was considered as a statistically significant difference between different groups. The data were recorded as means ± standard deviation (SD).
3. Results and Discussion
3.1. Effect of Single Parameter on the Extraction Yield of GPP
The effect of the material to solvent ratio on the extraction yield of GPP was first investigated (Figure 1A). The extraction yield of GPP increased significantly when the material to solvent ratio increased from 1:20 to 1:30 and reached the highest extraction yield of 15.3%, but slightly decreased when the material to solvent ratio was higher than 1:30. These changes might be due to the increased dissolution rate of polysaccharides with increasing solvent volume [20]. Differences in solution concentration outside and inside plant cells can influence the diffusion rate of solute particles and prompt more polysaccharide molecules to enter into the solution [21]. In view of the fact that GPP extraction yield increased little when the ratio of raw material to solvent surpassed 1:30, and the economical industry objective of reducing the waste of raw materials, the material to solvent ratio of 1:30 was chosen for further experiments.
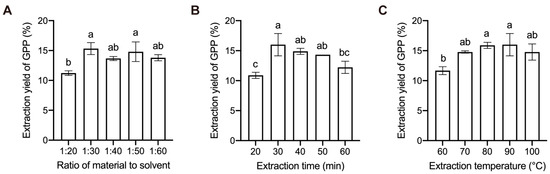
Figure 1.
Effect of single parameter on the extraction yield of grape pomace polysaccharide (GPP). (A) Material to solvent ratio; (B) extraction time; (C) extraction temperature. Data are means ± SD (n = 3). The error bars represent the standard deviation. Values marked by the same letter are not significantly different (p < 0.05).
Extraction time was another factor that significantly impacted the extraction yield. As shown in Figure 1B, in the extraction system with a material to solvent ratio of 1:25 and incubation temperature of 90 °C, the extraction yield increased rapidly from 10.9% to 16.0% under the change in the extraction time from 20 to 30 min. As the extraction time continued to extend, the extraction yield changed indistinctively. This may be related to the fact that polysaccharides have been sufficiently extracted [22,23]. Considering that a longer extraction time accompanies with lengthening production cycle, an extraction time of 30 min was identified as the optimal extraction time.
As shown in Figure 1C, the extraction yield increased and gradually reached the maximal value with the increase in extraction temperature, and then decreased lightly during the extraction temperature ranging from 90 °C to 100 °C. This phenomenon can be attributed to polysaccharide hydrolysis under high temperature and long extraction time [24]. Regarding energy saving, 70 °C was an appropriate extraction time for crude GPP.
3.2. Optimization of the Polysaccharide Extraction Parameters Condition
According to single parameter optimization, the parameters of material to solvent ratio, extraction temperature, and extraction time can affect the extraction yield of GPP. Their combinational effects on the extraction of polysaccharides were evaluated through an orthogonal L9 (33) test design (Table 1) to detect the optimal extraction conditions. The yields of GPP obtained from the orthogonal test were shown in Table 2. Based on the orthogonal analysis, we employed statistical software SPSS v. 22.0 to calculate the values of K (range analysis) and R (variance analysis).

Table 2.
Analysis of orthogonal test results.
According to R values, it can be found that the extraction time played the most crucial role in the extraction of GPP. Moreover, the importance of parameters affecting GPP extraction yield was: extraction time > material to solvent ratio > extraction temperature. Combining the K values, the optimal combination of variables would be the A1B3C2, that is, the raw material to solvent ratio was 1:25, extraction temperature was 75 °C, and extraction time was 40 min.
3.3. Purification of GPP
The crude polysaccharide was further purified with gel filtration chromatography and the polysaccharide content in each elution fraction was determined. As shown in Figure 2A, one major polysaccharide peak was obtained. Purified GPP was approximately 80.0% of the total polysaccharides in content. In addition, the purified GPP was examined with a UV-Vis spectrophotometer at 190 to 600 nm band. As shown in Figure 2B, there is no absorption peak at 260 nm or 280 nm, indicating all proteins and nucleic acids were removed during the purification process.
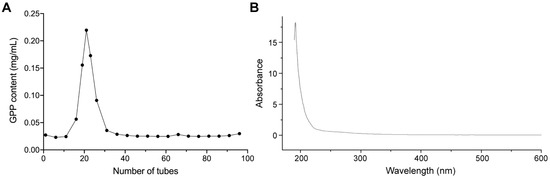
Figure 2.
Purification and UV-Vis characterization of grape pomace polysaccharide (GPP). (A) The GPP content in each elution fraction. The extracted GPP was purified with an S-400 HR gel filtration chromatography column and collected. Each elution fraction was obtained continuously for 1 min, for a total of around 100 min. The polysaccharide content of each tube was determined with the phenol-sulfuric acid method. (B) The UV-Vis spectra of GPP. The purified GPP was collected and concentrated. The absorbance of the product between 190 and 600 nm was recorded on a Hitachi U-3900H spectrophotometer.
3.4. Monosaccharide Composition of Purified GPP
The purified GPP was acid-hydrolyzed and PMP-derivatized. Its monosaccharide composition was analyzed by comparing the retention time of hydrolysate with monosaccharide standards. The content of monosaccharides was calculated by each their peak area. As indicated in Figure 3, the monosaccharide composition of GPP was mainly composed of mannose and glucose with small quantities of galactose and arabinose. Monosaccharide standards of mannose, glucose, galactose, and arabinose were analyzed and establish the curve for calculating each mole fraction simultaneously [17]. The peak area of the chromatogram for each of the above monosaccharides was linear to their concentration, which allowed us to calculate the mole ratios of the APP monosaccharide composition in detail. The molar ratios of monosaccharides were: 3.0:14.1:10.8:18.4 of mannose: glucose: galactose: arabinose in the purified GPP. The most crucial step in identifying the polysaccharide’s physicochemical characteristics, structure, and structure-bioactivity relationship is to measure its monosaccharide composition [25]. This finding provides a significant metric for determining the quality standard of GPP. Additionally, we found that GPP had a simple and uniform monosaccharide composition, which might contribute to its high water solubility and benefit subsequent processing and usage [26].
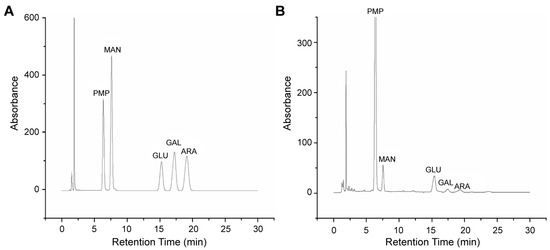
Figure 3.
Monosaccharide composition of grape pomace polysaccharide (GPP). (A) Chromatogram of standard monosaccharides: PMP: 1-phenyl-3-methyl-5-pyrazolone; MAN: mannose; GLU: glucose; GAL: galactose; ARA: arabinose. (B) Chromatogram of GPP with peak identity: PMP: 1-phenyl-3-methyl-5-pyrazolone; MAN: mannose; GLU: glucose; GAL: galactose; ARA: arabinose.
3.5. FT-IR Spectroscopy Analysis
In order to illuminate the major functional groups of GPP, FT-IR was performed and the result was shown in Figure 4. The strong and broad absorption bands at ~3320 cm−1 were attributed to the glycosyl hydroxy (O−H) stretching vibrations, and the absorption peak at 2970 cm−1 was attributed to asymmetric C–H stretching vibrations, which were the typical bands of polysaccharide [27]. The absorption bands from 1800 cm−1 to 600 cm−1, the “fingerprint” region, were related to the conformation and surface structure of molecules. Signals at 1720 cm−1 were attributed to C=O stretching from the carboxyl group [28]. The absorption bands at 1580 cm−1 corresponded to C–C stretch of polysaccharides [29]. The signals at 1408 cm−1 were attributed to C–O stretching of the carboxylate anion group [28]. These signals implied the existence of carboxyl groups. The absorption bands at 1136 cm−1 and 1068 cm−1 corresponded to ring vibrations overlapped by C–O–C and C–O–H vibrations, indicating GPP contains pyranose ring [28,30,31]. In addition, characteristic peaks between 904 cm−1 and 788 cm−1 were observed in the spectra, implying the presence of both β-pyranose and α-pyranose rings [18,28,31]. Based on these findings, it could be inferred that GPP consisted of pyranose with both α-conformation and β-conformation, containing carboxyl groups simultaneously.
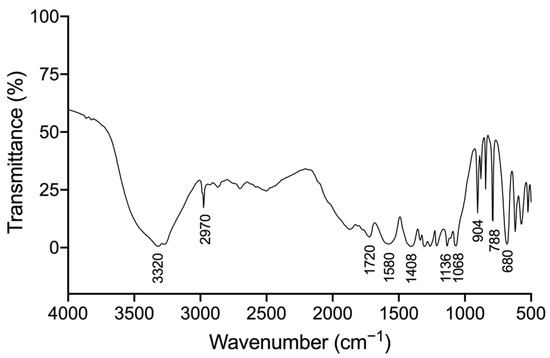
Figure 4.
FT-IR spectra of grape pomace polysaccharide (GPP). The transmittance of GPP mixed with KBr on a FT-IR spectrometer of 400–4000 cm−1.
4. Conclusions
In this study, crude GPP was extracted by orthogonal test design optimized hot water extraction method and the optimum extraction conditions of GPP from grape pomace were 75 °C with a 1:25 raw material to solvent ratio for 40 min while extraction time is the most significant factor among them. The crude polysaccharide was further purified with gel filtration chromatography and chemically characterized. Structural characterization showed that the GPP polysaccharide was composed of arabinose, glucose, galactose, and mannose at a molar ratio of 18.4:14.1:10.8:3.0. In addition, FT-IR analysis suggested that GPP consisted of α-pyranose and β-pyranose, containing carboxyl groups simultaneously. These investigations can contribute to the application of GPP in future research and healthcare applications.
Author Contributions
Conceptualization, D.Y.; Data curation, X.M., Y.N., W.Y. and D.Y.; Formal analysis, X.M., Y.N. and D.Y.; Investigation, X.M., Y.N., W.Y. and D.Y.; Project administration, X.M., Y.N. and D.Y.; Resources, D.Y.; Supervision, D.Y.; Visualization, X.M., Y.N. and D.Y.; Writing—original draft, X.M., Y.N. and D.Y.; Writing—review and editing, D.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Laboratory of Biomacromolecules, grant number 2022kf05.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to Chih-chen Wang in Institute of Biophysics, Chinese Academy of Sciences for her support and encouragement in our research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Canalejo, D.; Guadalupe, Z.; Martínez-Lapuente, L.; Ayestarán, B.; Pérez-Magariño, S. Optimization of a method to extract polysaccharides from white grape pomace by-products. Food Chem. 2021, 365, 130445. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, D. Economical and eco-friendly isolation of anthocyanins from grape pomace with higher efficiency. Food Chem. X 2022, 15, 100419. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Z.; Song, T.; Lv, G. Optimization of enzyme-assisted extraction of antitumor polysaccharides from Hericium erinaceus mycelia. Food Sci. Technol. Res. 2017, 23, 31–39. [Google Scholar] [CrossRef]
- Fan, T.; Hu, J.; Fu, L.; Zhang, L. Optimization of enzymolysis-ultrasonic assisted extraction of polysaccharides from Momordica charabtia L. by response surface methodology. Carbohydr. Polym. 2015, 115, 701–706. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Wang, T.; Cai, Z.; Cao, H.; Zhang, H.; Cao, Y.; Chen, B.; Yang, D. Statistics on the bioactive anthocyanin/proanthocyanin products in China online sales. Food Sci. Nutr. 2021, 9, 5428–5434. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; Wen, P.; Shen, M.; Li, H.; Ren, Y.; Xiao, Y.; Song, Q.; Chen, Y.; Yu, Q.; et al. Two water-soluble polysaccharides from mung bean skin: Physicochemical characterization, antioxidant and antibacterial activities. Food Hydrocoll. 2020, 100, 105412. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Cano-Mozo, E.; Bueno-Herrera, M.; Canalejo, D.; Doco, T.; Ayestarán, B.; Guadalupe, Z. The effects of grape polysaccharides extracted from grape by-products on the chemical composition and sensory characteristics of white wines. Molecules 2022, 27, 4815. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Chen, T.; Chen, X. A review of the antibacterial activity and mechanisms of plant polysaccharides. Trends Food Sci. Technol. 2022, 123, 264–280. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef]
- Meng, X.; Zheng, J.; Wang, F.; Zheng, J.; Yang, D. Dietary fiber chemical structure determined gut microbiota dynamics. iMeta 2022, e64. [Google Scholar] [CrossRef]
- Ye, G.; Li, J.; Zhang, J.; Liu, H.; Ye, Q.; Wang, Z. Structural characterization and antitumor activity of a polysaccharide from Dendrobium wardianum. Carbohydr. Polym. 2021, 269, 118253. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ma, X.; Zhang, K.; Li, S.; Wang, X.; Liu, X.; Liu, J.; Fan, W.; Li, Y.; et al. Study on the kinetic model, thermodynamic and physicochemical properties of Glycyrrhiza polysaccharide by ultrasonic assisted extraction. Ultrason. Sonochem. 2019, 51, 249–257. [Google Scholar] [CrossRef]
- Amutha Gnana Arasi, M.A.; Gopal Rao, M.; Bagyalakshmi, J. Optimization of microwave-assisted extraction of polysaccharide from Psidium guajava L. fruits. Int. J. Biol. Macromol. 2016, 91, 227–232. [Google Scholar] [CrossRef]
- Rondeau, P.; Gambier, F.; Jolibert, F.; Brosse, N. Compositions and chemical variability of grape pomaces from French vineyard. Ind. Crops Prod. 2013, 43, 251–254. [Google Scholar] [CrossRef]
- Li, Q.; Ju, H.; Zhai, C. Extraction technology of crude polysaccharide from Schisandra chinensis. J. Food Sci. 2004, 25, 5. [Google Scholar]
- Zhang, F.; Zheng, J.; Li, Z.; Cai, Z.; Wang, F.; Yang, D. Purification, characterization, and self-assembly of the polysaccharide from Allium Schoenoprasum. Foods 2021, 10, 1352. [Google Scholar] [CrossRef]
- Wu, G.-H.; Hu, T.; Huang, Z.-L.; Jiang, J.-G. Characterization of water and alkali-soluble polysaccharides from Pleurotus tuberregium sclerotia. Carbohydr. Polym. 2013, 96, 284–290. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Amid, B.T. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res. Int. 2012, 46, 387–398. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Zheng, W.; Gao, Y.; Wang, M.; Zhang, Y.; Gao, Q. Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 2016, 92, 30–36. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.-G.; Zhu, H.-J. An efficient enzymatic modification of lily polysaccharide in ionic liquid under ultrasonic irradiation. Biochem. Eng. J. 2013, 79, 25–28. [Google Scholar] [CrossRef]
- Cheung, Y.C.; Wu, J.Y. Kinetic models and process parameters for ultrasound-assisted extraction of water-soluble components and polysaccharides from a medicinal fungus. Biochem. Eng. J. 2013, 79, 214–220. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, N.; Xiong, S.; Li, S.; Yang, B. Extraction of BaChu mushroom polysaccharides and preparation of a compound beverage. Carbohydr. Polym. 2008, 73, 289–294. [Google Scholar]
- Liu, X.; Zhou, B.; Lin, R.; Jia, L.; Deng, P.; Fan, K.; Wang, G.; Wang, L.; Zhang, J. Extraction and antioxidant activities of intracellular polysaccharide from Pleurotus sp. mycelium. Int. J. Biol. Macromol. 2010, 47, 116–119. [Google Scholar] [CrossRef]
- Li, P.; Yan, Z.; Chen, Y.; He, P.; Yang, W. Analysis of monosaccharide composition of water-soluble polysaccharides from Codium fragile by ultra-performance liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2021, 44, 1452–1460. [Google Scholar] [CrossRef]
- Zhang, R.; Edgar, K.J. Properties, chemistry, and applications of the bioactive polysaccharide curdlan. Biomacromolecules 2014, 15, 1079–1096. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Li, S.; Li, Q.; Fan, W.; Kiatoukosin, L.; Chen, J. Extracellular polysaccharides of endophytic fungus Alternaria tenuissima F1 from Angelica sinensis: Production conditions, purification, and antioxidant properties. Int. J. Biol. Macromol. 2019, 133, 172–183. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, Y.; Jia, J.; Ren, X.; Wang, Y. Extraction, purification and properties of water-soluble polysaccharides from mushroom Lepista nuda. Int. J. Biol. Macromol. 2019, 128, 858–869. [Google Scholar] [CrossRef]
- Ji, P.; Wei, Y.; Xue, W.; Hua, Y.; Zhang, M.; Sun, H.; Song, Z.; Zhang, L.; Li, J.; Zhao, H.; et al. Characterization and antioxidative activities of polysaccharide in Chinese angelica and its processed products. Int. J. Biol. Macromol. 2014, 67, 195–200. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef]
- Sardari, R.R.R.; Kulcinskaja, E.; Ron, E.Y.C.; Björnsdóttir, S.; Friðjónsson, Ó.H.; Hreggviðsson, G.Ó.; Karlsson, E.N. Evaluation of the production of exopolysaccharides by two strains of the thermophilic bacterium Rhodothermus marinus. Carbohydr. Polym. 2017, 156, 1–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).