Application of Magnesium Oxide for Metal Removal in Mine Water Treatment
Abstract
1. Introduction
2. Materials and Methods
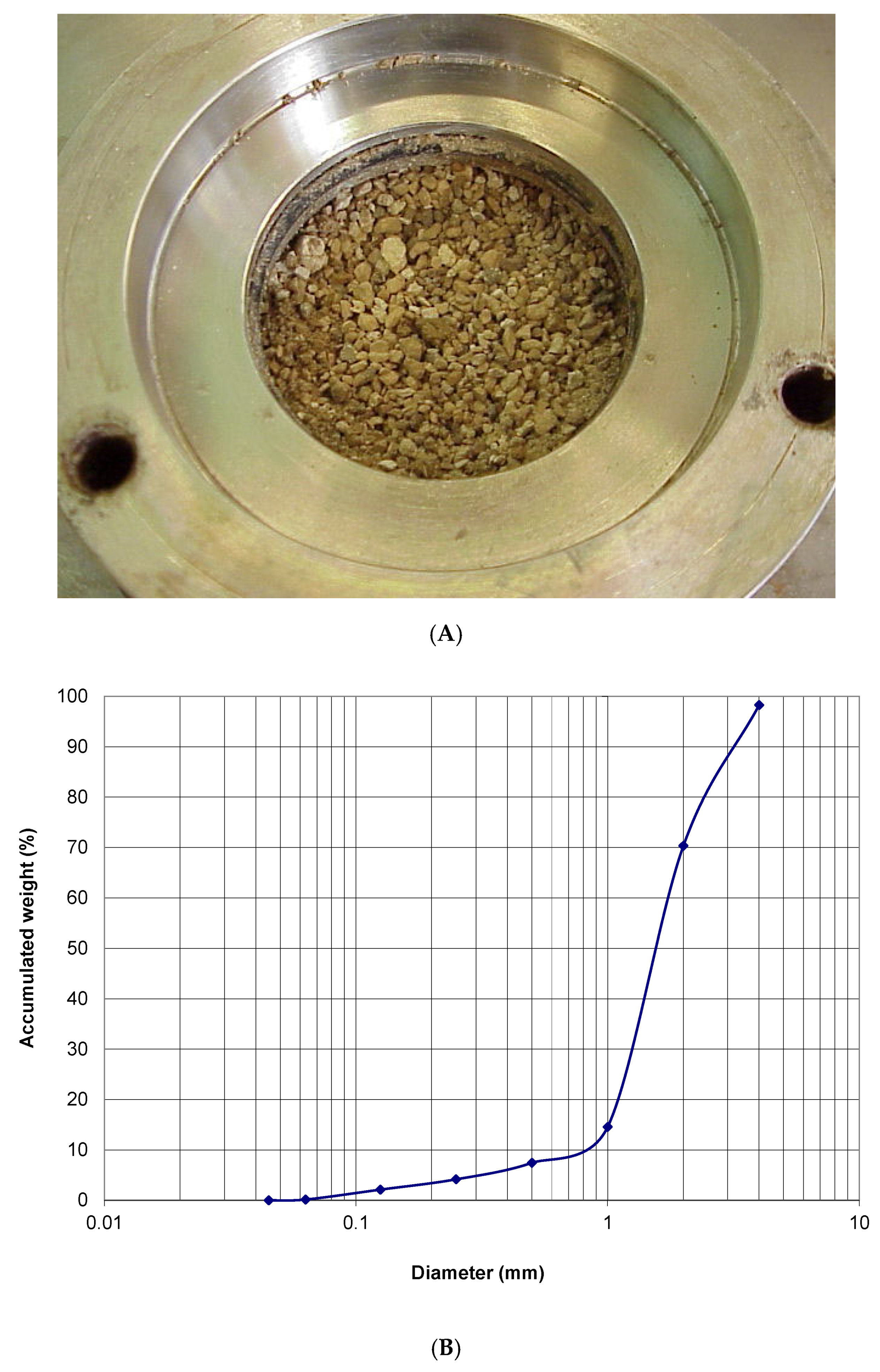
2.1. Characterization of MgO
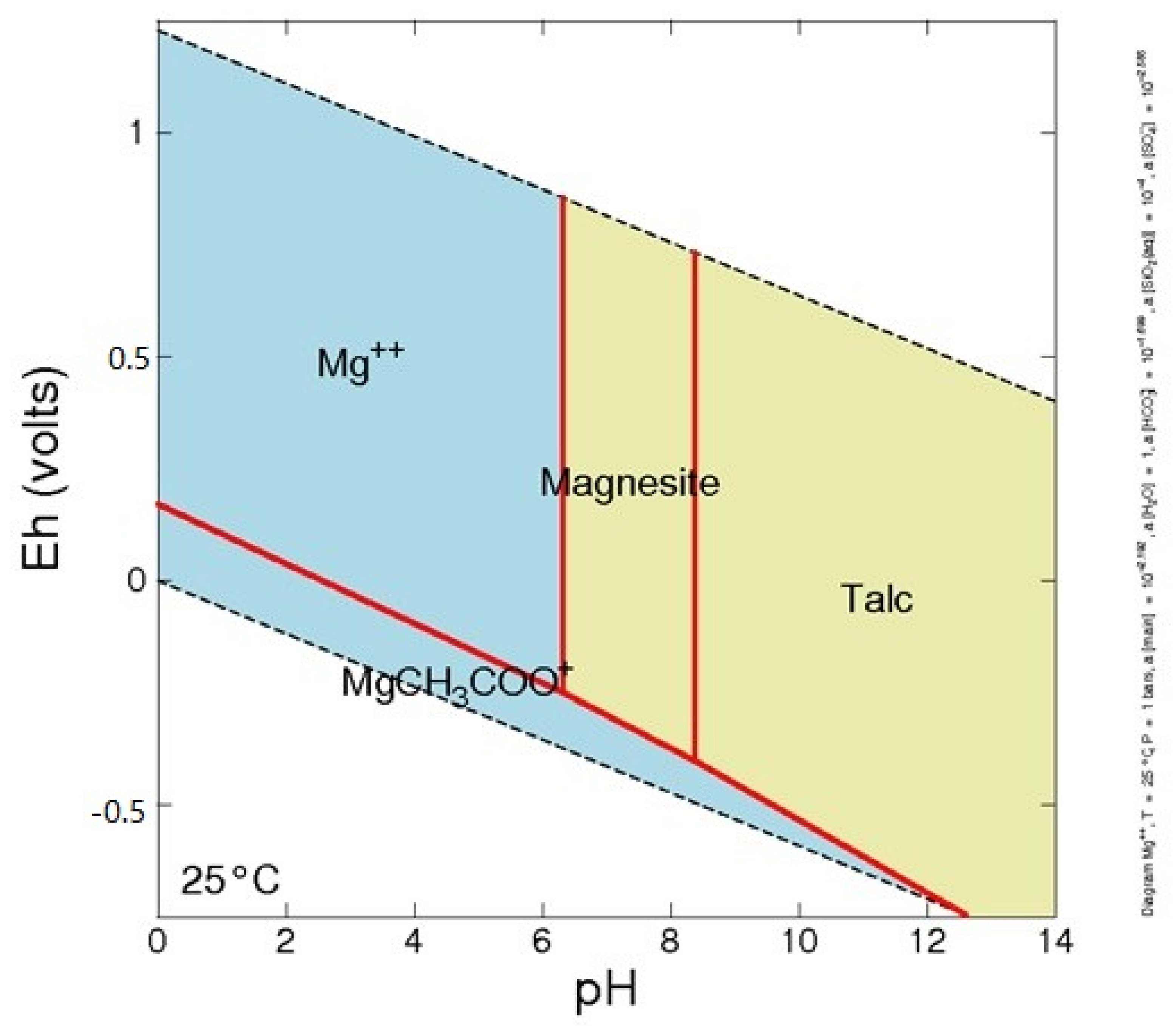
2.2. Geochemistry of MgO and Mine Wastes
2.3. Mine Water, Mine Wastes, and MgO
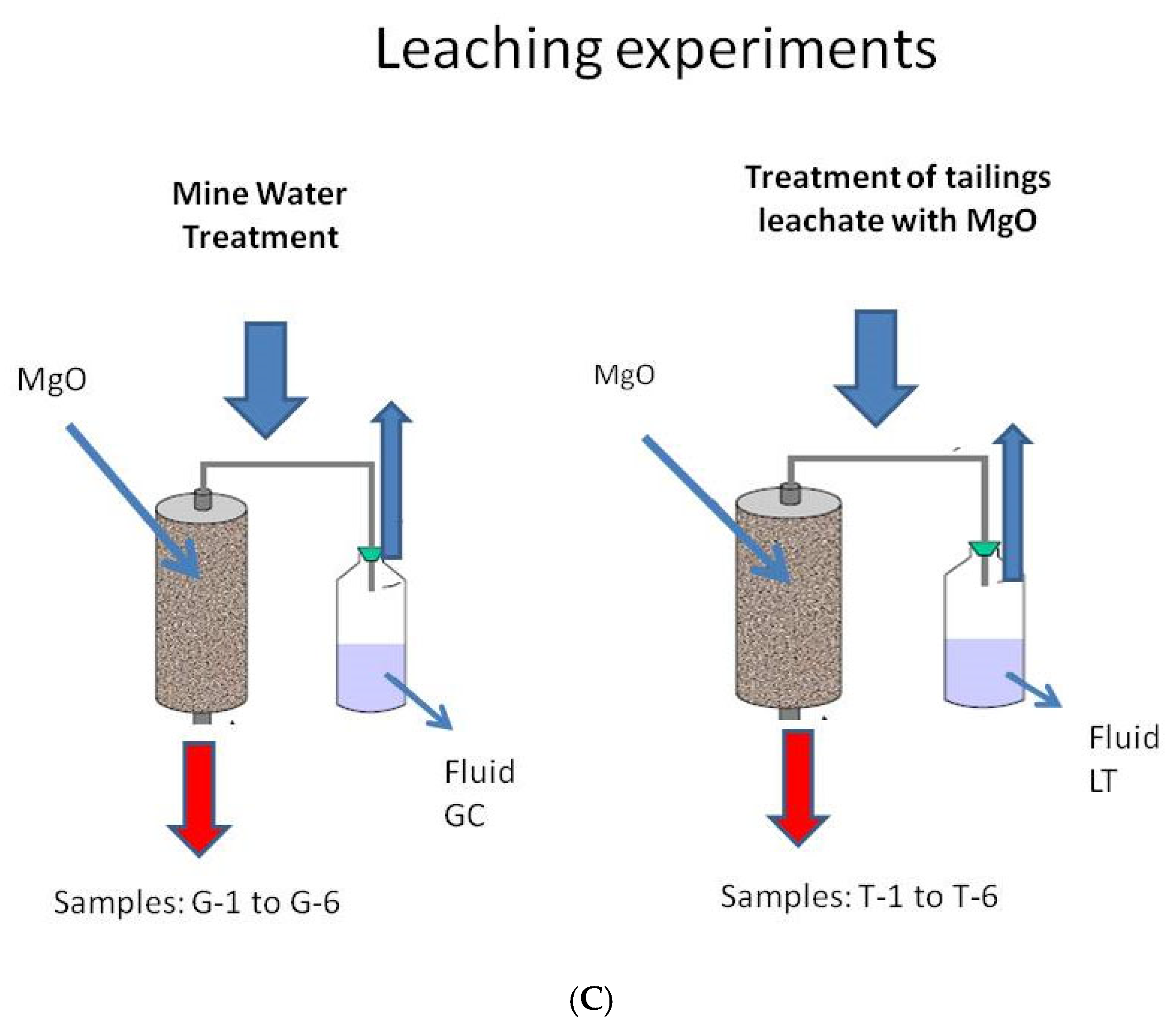
2.4. Column Leaching Experiments
2.5. Geochemical Modeling
3. Results and Discussion
3.1. Mine Wastes and MgO
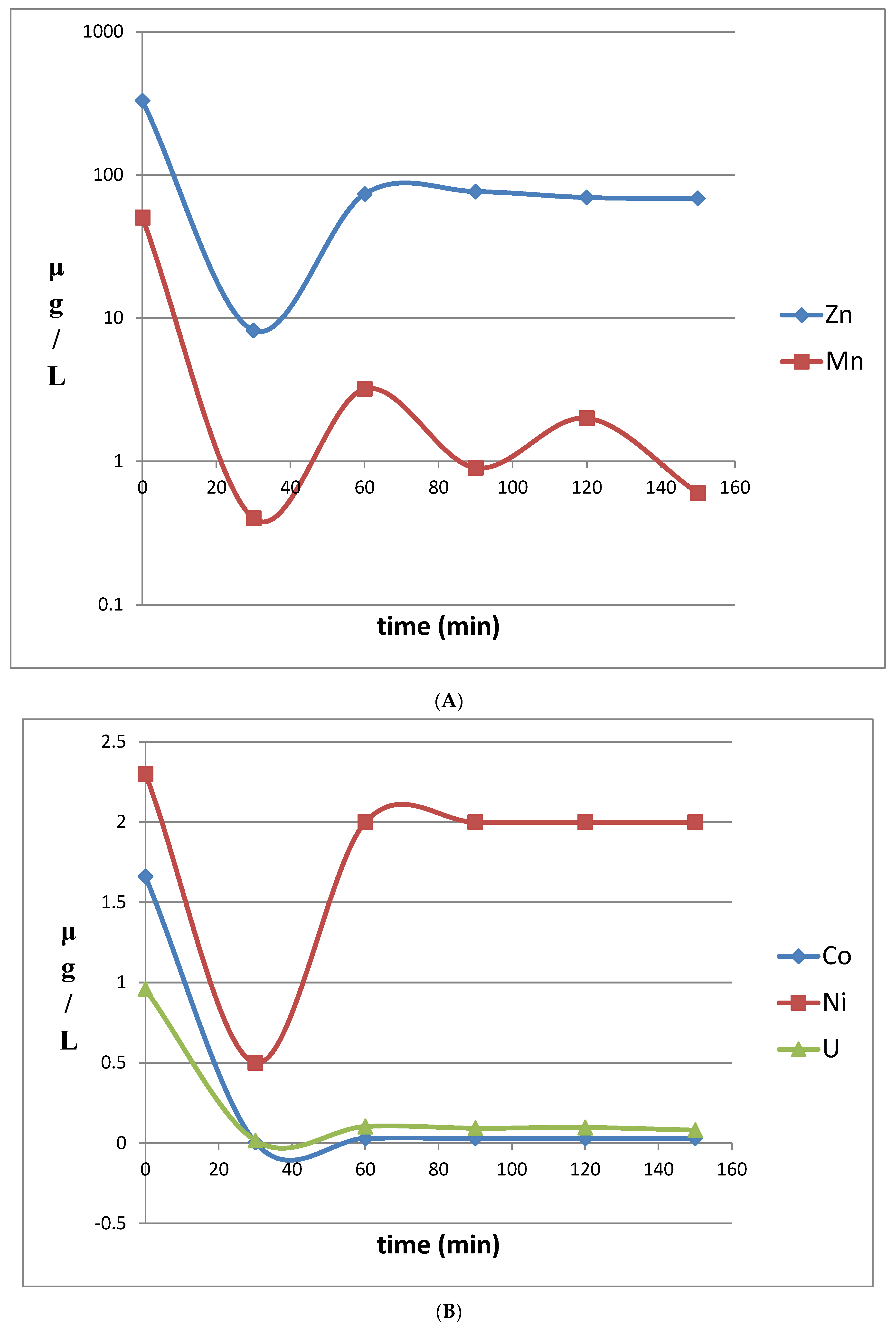
3.2. Mine Water Treatment
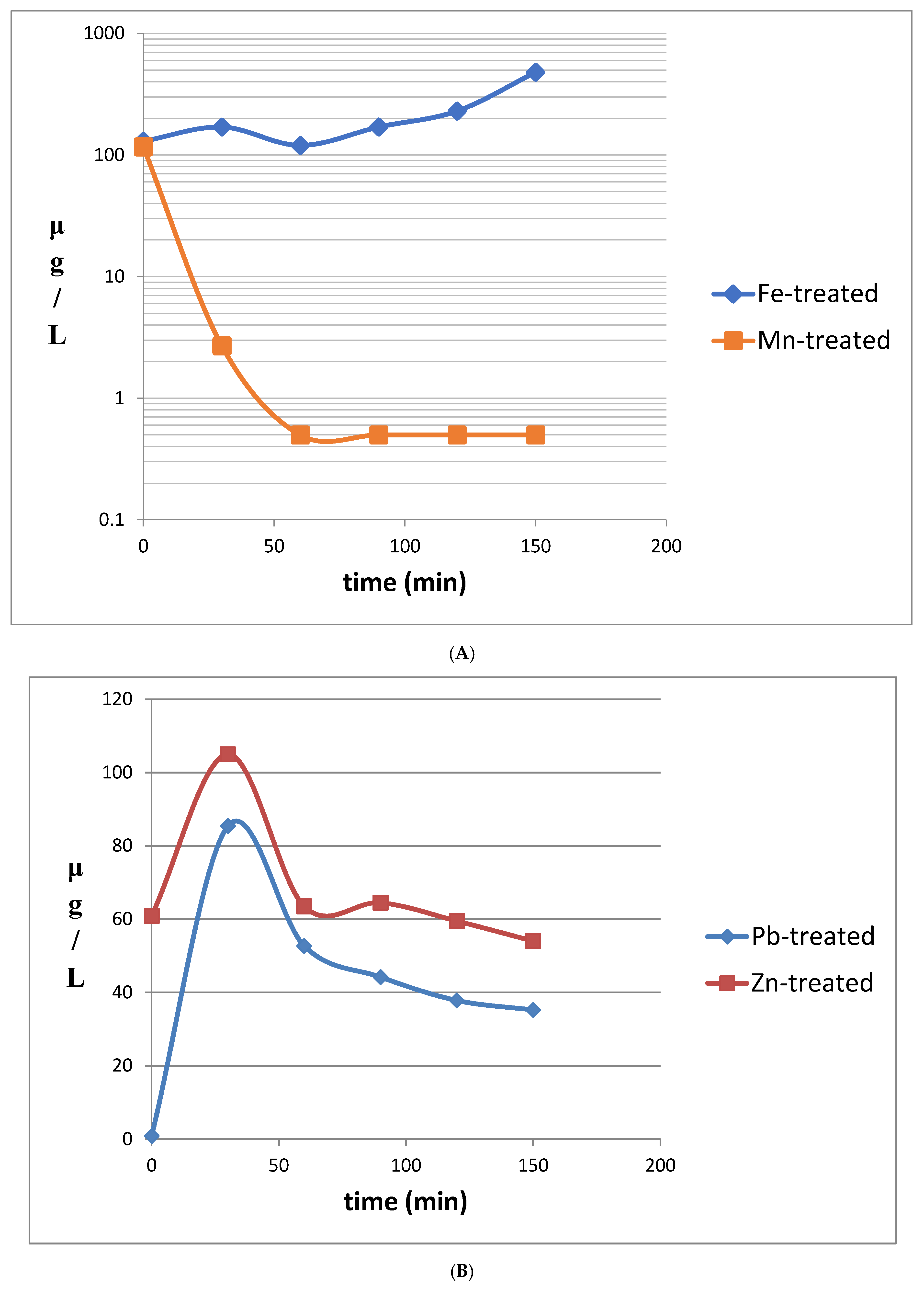
3.3. Treatment of Mine Tailings
3.4. Geochemical Modeling
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Al, T.A.; Martin, C.J.; Blowes, D.W. Carbonate-mineral/water interactions in sulfide-rich mine tailings. Geochim. Cosmochim. Acta 2000, 64, 3933–3948. [Google Scholar] [CrossRef]
- Navarro, A.; Cardellach, E. Mobilization of Ag, Heavy metals and Eu from the waste deposit of Las Herrerías mine (Almería, SE Spain). Environ. Geol. 2009, 56, 1389–1404. [Google Scholar] [CrossRef]
- Navarro, A.; Domènech, L.M. Arsenic and metal mobility from Au mine tailings in Rodalquilar (Almería, SE Spain). Environ. Earth Sci. 2010, 60, 121–138. [Google Scholar] [CrossRef]
- Plante, B.; Benzaazoua, M.; Bussière, B. Predicting Geochemical Behaviour of Waste Rock with Low Acid Generating potential Using Laboratory Kinetic Tests. Mine Water Environ. 2011, 30, 2–21. [Google Scholar] [CrossRef]
- Navarro, A.; Font, X.; Viladevall, M. Metal Mobilization and Zinc-Rich Circumneutral Mine Drainage from the Abandoned Mining Area of Osor (Girona, NE Spain). Mine Water Environ. 2015, 34, 329–342. [Google Scholar] [CrossRef]
- MEND. Review of Water Quality Issues in Neutral pH Drainage: Examples and Emerging Priorities for the Mining Industry in Canada; MEND Report 10.1; MEND Initiative: Ottawa, ON, Canada, 2004. [Google Scholar]
- Heikkinen, P.M.; Räisänen, M.L.; Johnson, R.H. Geochemical Characterization of Seepage and Drainage Water Quality from Two Sulphide Mine Tailings Impoundments: Acid Mine Drainage versus Neutral Mine Drainage. Mine Water Environ. 2009, 28, 30–49. [Google Scholar] [CrossRef]
- Heikkinen, P.M.; Räisänen, M.L. Trace metal and as solid-phase speciation in sulphide mine tailings-Indicators of spatial distribution of sulphide oxidation in active tailings impoundments. Appl. Geochem. 2009, 24, 1224–1237. [Google Scholar] [CrossRef]
- Navarro, A.; Martínez, F. Evaluation of Metal Attenuation from Mine Tailings in SE Spain (Sierra Almagrera): A Soil-Leaching Column Study. Mine Water Environ. 2010, 29, 53–67. [Google Scholar] [CrossRef]
- Lottermoser, B. Mine Wastes, 3rd ed.; Springer: Berlin, Germany, 2010. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; Balkema: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Younger, P.L.; Banwart, S.A.; Hedin, R.S. Mine Water: Hydrology, Pollution, Remediation; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Wolkersdorfer, C. Water Management at Abandoned Flooded Underground Mines; Springer: Berlin, Germany, 2008. [Google Scholar]
- Santos, S.; Machado, R.; Correia, M.J.N.; Carvalho, J.R. Treatment of acid mining waters. Miner. Eng. 2004, 17, 225–232. [Google Scholar] [CrossRef]
- Othman, A.; Sulaiman, A.; Sulaiman, S.K. Carbide lime in acid mine drainage treatment. J. Water Process Eng. 2017, 15, 31–36. [Google Scholar] [CrossRef]
- Gusek, J.J.; Figueroa, L.A. Mitigation of Metal Mining Influenced Water; Society for Mining Metallurgy, and Exploration, Inc.: Littelton, CO, USA, 2009; Volume 2. [Google Scholar]
- PIRAMID. Engineering Guidelines for the Passive Remediation of Acidic and/or Metalliferous Mine Drainage and Similar Wastewaters; Piramid Consortium, Research Project of the European Commission 5th Framework Programma; PIRAMID Consortium; University of Newcastle Upon Tyne: Newcastle upon Tyne, UK, 2003. [Google Scholar]
- Kaur, G.; Couperthwaite, S.J.; Hatton-Jones, B.W.; Millar, G.J. Alternative neutralization materials for acid mine drainage treatment. J. Water Process Eng. 2018, 22, 46–58. [Google Scholar] [CrossRef]
- Nuttall, C.A.; Younger, P.L. Zinc removal from hard, circum-neutral mine waters using a novel closed-bed limestone reactor. Water Res. 2000, 34, 1262–1268. [Google Scholar] [CrossRef]
- Mayes, W.M.; Potter, H.A.B.; Jarvis, A.P. Novel approach to zinc removal from circum-neutral mine waters using pelletized recovered hydrous ferric oxide. J. Hazard. Mater. 2009, 162, 512–520. [Google Scholar] [CrossRef] [PubMed]
- U.S. Army Corps of Engineers. Engineering and Design: Precipitation/Coagulation/Flocculation; Manual EM 1110-1-4012; U.S. Army Corps of Engineers: Washington, DC, USA, 2001. [Google Scholar]
- Navarro, A.; Chimenos, J.M.; Muntaner, D.; Fernández, I. Permeable reactive barriers for the removal of heavy metals: Lab-scale experiments with low-grade magnesium oxide. Ground Water Monitor. Remed. 2006, 26, 142–152. [Google Scholar] [CrossRef]
- Navarro, A.; Cardellach, E.; Corbella, M. Immobilization of Cu, Pb, and Zn in mine-contaminated soils using reactive materials. J. Hazard. Mater. 2011, 186, 1576–1585. [Google Scholar] [CrossRef]
- Bologo, V.; Maree, J.P.; Carlsson, F. Application of magnesium hydroxide and barium hydroxide for the removal of metals and sulphate from mine water. Water SA 2012, 38, 23–28. [Google Scholar] [CrossRef]
- Siciliano, A. Removal of Cr (VI) from Water Using a New Reactive Material: Magnesium Oxide Supported Nanoscale Zero-Valent Iron. Materials. 2016, 9, 666. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, Y.; Huang, C.; Huang, M.; Chen, J.; Rao, T.; Ran, X. Removal of the heavy metal ion nickel (II) via an adsorption method using flower globular magnesium hydroxide. J. Hazard. Mater. 2019, 373, 131–140. [Google Scholar] [CrossRef]
- Masindi, V.; Madzivire, G.; Tekere, M. Reclamation of water and the synthesis of gypsum and limestone from acid mine-drainage treatment process using a combination of pre-treated magnesite nanosheets, lime, and CO2 bubbling. Water Res. Ind. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Sulaiman, A.; Othman, A.; Ibrahim, I. The use of magnesium oxide in acid mine drainage treatment. Mater. Today Proceed. 2018, 5, 21566–21573. [Google Scholar] [CrossRef]
- Kastyvehik, A.; Karam, A.; Aïder, M. Effectiveness of alkaline amendments in acid mine drainage remediation. Environ. Technol. Innov. 2016, 6, 49–59. [Google Scholar] [CrossRef]
- Kim, T.Y.; Ahn, J.; Kim, C.; Choi, S.; Ho, T.T.; Moon, D.H.; Hwang, I. Carbonation/granulation of mine tailings using a MgO/ground-granule blast-furnace-slag binder. J. Hazard. Mater. 2019, 378, 120760. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wang, F.; Al-Tabbaa, A. Three-year performance of in-situ solidified/stabilized soil using novel MgO-bearing binders. Chemosphere 2016, 144, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ni, P.; Yi, Y. Comparison of reactive magnesia, quick lime, and ordinary Portland cement for stabilization/solidification of heavy metal-contaminated soils. Sci. Total Environ. 2019, 671, 741–753. [Google Scholar] [CrossRef]
- Hwang, K.Y.; Seo, J.Y.; Phan, H.Q.H.; Ahn, J.Y.; Hwang, I. MgO-based binder for treating contaminated sediments: Characteristics of metal stabilization and mineral carbonation. Clean Soil Air Water 2014, 42, 355–363. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Cho, D.; Tsang, D.C.W.; Yang, J.; Hou, D.; Baek, K.; Kua, H.W.; Poon, C. Novel synergy of Si-rich minerals and reactive MgO for stabilization/solidification of contaminated sediment. J. Hazard. Mater. 2019, 365, 695–706. [Google Scholar] [CrossRef]
- Al-Zoubi, H.; Rieger, A.; Steinberger, P.; Pelz, W.; Haseneder, R.; Härtel, G. Optimization Study for Treatment of Acid Mine Drainage Using Membrane Technology. Sep. Sci. Technol. 2010, 45, 2004–2016. [Google Scholar] [CrossRef]
- Wolkersdorfer, C.; Baierer, C. Improving Mine Water Quality by Low Density Sludge Storage in Flooded Underground Workings. Mine Water Environ. 2013, 32, 3–15. [Google Scholar] [CrossRef]
- Noosai, N.; Vijayan, V.; Kengskool, K. Model application for acid mine drainage treatment processes. Int. J. Energy Environ. 2014, 5, 693–700. [Google Scholar]
- Miller, A.; Figueroa, L.; Wildeman, T. Zinc and nickel removal in simulated limestone treatment of mining influenced water. Appl. Geochem. 2011, 26, 125–132. [Google Scholar] [CrossRef]
- Madzivire, G.; Gitari, W.M.; Vadapalli, V.R.K.; Ojumu, T.V.; Petrik, L.F. Fate of sulphate removed during the treatment of circumneutral mine water and acid mine drainage with coal fly ash: Modelling and experimental approach. Miner. Eng. 2011, 24, 1467–1477. [Google Scholar] [CrossRef]
- Masindi, V.; Gitari, M.W.; Tutu, H.; De Beer, M. Passive remediation of acid mine drainage using cryptocrystalline magnesite: A batch experimental and geochemical modeling approach. Water SA 2015, 41, 677–682. [Google Scholar] [CrossRef]
- Weber, A.; Kassahun, A. How Geochemical Modeling Helps Understanding Processes in Mine Water Treatment Plants—Examples from Former Uranium Minin Sites in Germany. In Proceedings of the 13th International Mine Water Association Congress—“Mine Water & Circular Economy—A Green Congress”, Lappeenranta, Finland, 25–30 June 2017. [Google Scholar]
- Johnson, B.C.; Rohal, P.; Eary, T. Coupling PHREEQC with GoldSim for a more Dynamic Water Modeling Experience. In Proceedings of the MWD Conference: “Risk to Opportunity”, Pretoria, South Africa, 10–14 September 2018. [Google Scholar]
- Dube, G.M.; Novhe, O.; Ramasenya, K.; Van Zweel, N. Passive Treatment Technologies for the Treatmemnt of AMD From Abandoned Coal Mines, eMalahleni, South Africa-Column Experiments. J. Ecol. Toxicol. 2018, 2, 2–5. [Google Scholar]
- Magagane, N.; Masindi, V.; Ramakkovhu, M.M.; Shongwe, M.B.; Muedi, K.L. Facile thermal activation of non-reactive cryptocrystalline magnesite and its application on the treatment of acid mine drainage. J. Environ. Manag. 2019, 236, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Bori, J.; Vallès, B.; Navarro, A.; Riva, M.C. Ecotoxicological risks of the abandoned F-Ba-Pb-Zn mining area of Osor (Spain). Environ. Geochem. Health 2017, 39, 665–679. [Google Scholar] [CrossRef] [PubMed]
- SAIC. An Assessment of Laboratory Leaching Tests for Predicting the Impacts of Fill Material on Ground Water and Surface Water Quality; Toxic Cleanup Program; SAIC: Shanghai, China, 2003. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; USGS: Reston, VA, USA, 1999. [Google Scholar]
- Agencia de Residus de Catalunya. Nivels Genèrics de Referencia dels Elements Traça en Sòls a Catalunya per a la Protecció de la Salut Humana; Generalitat de Catalunya: Barcelona, Spain, 2010. [Google Scholar]
- Alpaslan, B.; Yukselen, M.A. Remediation of lead contaminated soils by stabilization/solidification. Water Air Soil Pollut. 2001, 133, 253–263. [Google Scholar] [CrossRef]
- De Angelis, G.; Medici, F.; Montereali, M.R.; Pietrelli, L. Reuse of residues arising from lead batteries recycle: A feasibility study. Waste Manag. 2002, 22, 925–930. [Google Scholar] [CrossRef]
- Wehrer, M.; Totsche, K.V. Effective rates of heavy metal release from alkaline wastes-Quantified by column outflow experiments and inverse simulations. J. Contam. Hydrol. 2008, 101, 53–66. [Google Scholar] [CrossRef]
- Grathwohl, P. On equilibration of pore water in column leaching tests. Waste Manag. 2014, 34, 908–918. [Google Scholar] [CrossRef]
- Bethke, C.M.; Yeakel, S. The Geochemist´s Workbench Release 9.0, GWB Essentials Guide; Aqueous Solutions LLC: Champaign, IL, USA, 2011. [Google Scholar]
- Hay, R.; Celik, K. Accelerated carbonation of reactive magnesium oxide cement (RMC)-based composite with supercritical carbon dioxide (scCO2). J. Clean. Prod. 2020, 248, 119282. [Google Scholar] [CrossRef]
- Giro-Palma, J.; Formosa, J.; Chimenos, J.M. Stabilization Study of a Contaminated Soil with Metal(loid)s Adding Different Low-Grade MgO Degrees. Sustainability 2020, 12, 7340. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakamura, A.; Niinae, M.; Nakata, H.; Fujii, H.; Tasaka, Y. Lead immobilization in artificially contaminated kaolinite using magnesium oxide-based materials: Immobilization mechanisms and long-term evaluation. Chem. Eng. J. 2013, 232, 380–387. [Google Scholar] [CrossRef]
- Jang, S.B.; Choong, C.E.; Pichiah, S.; Choi, J.Y.; Yoon, Y.; Choi, E.H.; Jang, M. In-situ growth of manganese oxide on self-assembled 3D-magnesium hydroxide coated on polyurethane: Catalytic oxidation mechanism and application for Mn(II) removal. J. Hazard. Mater. 2022, 424, 127267. [Google Scholar] [CrossRef] [PubMed]
- Szymoniak, L.; Claveau-Mallet, D.; Haddad, M.; Barbeau, B. Application of Magnesium Oxide Media for Remineralization and Removal of Divalent Metals in Drinking Water Treatment: A Review. Water 2022, 14, 633. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Hara, J.; Zhang, M.; Kawabe, Y. Effects of Silicic Acido n Leaching Behavior of Arsenic from Spent Magnesium-Based Adsorbents Containing Arsenite. Sustainability 2022, 14, 4236. [Google Scholar] [CrossRef]
| Element | Al | Ag | As | Au | Ba | Be | Bi | Ca | Cd | Co | Cr | Cu | Fe | Hg | K | Mg | Mn | Mo | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | % | ppm | ppm | ppb | ppm | ppm | ppm | % | ppm | ppm | ppm | ppm | % | ppm | % | % | ppm | ppm | % |
| Detection limit | 0.01 | 0.3 | 0.01 | 2 | 50 | 1 | 2 | 0.01 | 0.3 | 0.1 | 0.3 | 1 | 0.01 | 0.05 | 0.01 | 0.01 | 1 | 0.05 | 0.01 |
| Osor tailings (TAL) | 4.46 | 0.6 | 12.5 | <2 | 5110 | 2 | 0.3 | 7.33 | 7.6 | 14 | 39 | 47 | 1.78 | <1 | 1.86 | 0.49 | 684 | 1 | 1.0 |
| Osor ore | 0.24 | 66.2 | 164 | 32 | 2190 | 1 | <2 | 21.4 | 68.5 | 15 | 119 | 88 | 0.96 | 5 | 0.43 | 0.08 | 109 | 1 | 0.14 |
| MgO | 0.42 | <0.3 | 17.5 | <2 | <50 | <1 | <2 | 7.07 | <0.3 | 47 | 95 | 28 | 2.16 | <1 | 0.30 | 32.6 | 965 | <1 | 0.08 |
| CAL * | --- | --- | 30 | --- | 1000 | 90 | --- | --- | 55 | 90 | 1000 | 1000 | --- | 30 | --- | --- | --- | 70 | ----- |
| Element | Ni | P | Pb | Rb | S | Sb | Se | Sr | Ta | Ti | Th | U | V | W | Zn | La | Ce | ||
| Unit | ppm | % | ppm | ppm | % | ppm | ppm | ppm | ppm | % | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ||
| Detection limit | 1 | 0.001 | 1 | 1 | 0.01 | 0.005 | 0.1 | 1 | 0.5 | 0.01 | 0.1 | 0.01 | 2 | 0.05 | 1 | 0.01 | 0.1 | ||
| Osor tailings (TAL) | 18 | 0.041 | 940 | 103 | 0.23 | 1 | <0.1 | 105 | <0.5 | 0.24 | 5.2 | 2.4 | 45 | <1 | 2370 | 27.8 | 55 | ||
| Osor ore | 31 | 0.008 | >5000 | <15 | 5.78 | 208 | <3 | 74 | <0.5 | 0.03 | 1.5 | <0.5 | 15 | <1 | 34000 | 9 | 12 | ||
| MgO | 27 | 0.030 | <3 | <15 | 0.04 | 1.4 | <3 | 83 | <0.5 | 0.02 | 0.8 | 1.5 | 47 | <1 | 6 | <0.5 | 13 | ||
| CAL * | 1000 | -- | 550 | -- | -- | 30 | 70 | -- | 45 | -- | -- | -- | 1000 | -- | 1000 | -- | -- |
| Analyte Symbol | Time | pH | Eh | EC | Mn | Fe | Co | Ni | Zn | Pb | As | Se | U | Mo | Cd | Sb | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | min | pH unit | mV | μS/cm | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L |
| Detection Limit | 0.1 | 10 | 0.005 | 0.3 | 0.5 | 0.01 | 0.03 | 0.2 | 0.001 | 0.1 | 0.01 | 0.01 | 0.2 | ||||
| GC | --- | 7.46 | 98.6 | 678 | 841 | 120 | 19.1 | 17.9 | 2900 | 2.11 | 1.59 | 4.4 | 9.16 | 1.0 | 1.98 | 0.17 | 7.3 |
| G-1 | 0 | 10.7 | −750 | 7200 | 50.4 | 50 | 1.66 | 2.3 | 329 | 118 | 1.54 | 2.6 | 0.957 | 6.4 | 1.04 | 5.87 | 29.8 |
| G-2 | 30 | 12.4 | −725 | 4530 | 0.4 | 370 | <0.005 | 0.5 | 8.2 | 1970 | 0.36 | 2.2 | 0.017 | 9.9 | 0.04 | 5.64 | 2.8 |
| G-3 | 60 | 12.6 | −776 | 6080 | 3.2 | 580 | <0.03 | <2 | 73.5 | 2470 | 0.34 | 1.4 | 0.104 | 7.1 | 0.06 | 13 | 4.9 |
| G-4 | 90 | 12.6 | −820 | 6470 | 0.9 | 550 | <0.03 | <2 | 76.5 | 1870 | 0.48 | 2.2 | 0.093 | 6.3 | <0.05 | 12.9 | 6.1 |
| G-5 | 120 | 12.7 | −810 | 6730 | 2 | 630 | <0.03 | <2 | 69.5 | 1350 | 0.31 | 2 | 0.098 | 5.7 | 0.05 | 11.6 | 5.5 |
| G-6 | 150 | 12.7 | −750 | 6780 | 0.6 | 710 | <0.03 | <2 | 68.5 | 1000 | 0.4 | 1.8 | 0.081 | 5.4 | <0.05 | 11.3 | 6 |
| Analyte Symbol | Time | F | Cl | NO3 (as N) | SO4 | HCO3− |
|---|---|---|---|---|---|---|
| Unit Symbol | min | mg/L | mg/L | mg/L | mg/L | mg/L |
| Detection Limit | --- | 0.01 | 0.03 | 0.01 | 0.03 | 1 |
| GC | --- | 2.73 | 22.4 | 0.4 | 210.7 | 408 |
| G-1 | 0 | 0.08 | 50.4 | 0.3 | 254.8 | 1222 |
| G-6 | 150 | 0.2 | 30.1 | 0.1 | 123.3 | 3013 |
| Analyte Symbol | Time | pH | Eh | EC | Mn | Fe | Co | Ni | Zn | Pb | As | Se | U | Mo | Cd | Sb | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | min | pH unit | mV | uS/cm | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L |
| Detection Limit | 0.1 | 10 | 0.005 | 0.3 | 0.5 | 0.01 | 0.03 | 0.2 | 0.001 | 0.1 | 0.01 | 0.01 | 0.2 | ||||
| LT | 0 | 7.77 | 122 | 2420 | 80.9 | 830 | 1.85 | 3.8 | 2380 | 98.1 | 2.25 | 1.5 | 1.17 | 6.4 | 8.73 | 1.59 | 10.4 |
| T-1 | 0 | 12.3 | −700 | 4570 | 117 | 130 | 2.55 | 1.4 | 60.9 | 0.89 | 1.6 | 2.1 | 0.225 | 5.4 | 0.19 | 6.42 | 5 |
| T-2 | 30 | 12.6 | −678 | 5870 | 2.7 | 170 | <0.03 | <2 | 105 | 85.4 | 0.6 | 2.2 | 0.118 | 3.8 | <0.05 | 12.1 | 3.4 |
| T-3 | 60 | 12.6 | −800 | 6400 | <0.5 | 120 | <0.03 | <2 | 63.5 | 52.7 | 0.31 | 2.3 | 0.026 | 3.5 | <0.05 | 12.8 | 1.9 |
| T-4 | 90 | 12.6 | −600 | 6700 | <0.5 | 170 | <0.03 | <2 | 64.5 | 44.2 | 0.33 | 2 | 0.088 | 3.1 | <0.05 | 12.9 | 2.8 |
| T-5 | 120 | 12.7 | −710 | 6860 | <0.5 | 230 | <0.03 | <2 | 59.5 | 37.8 | 0.3 | 2.2 | 0.07 | 2.9 | <0.05 | 13 | 2.7 |
| T-6 | 150 | 12.7 | −560 | 7030 | <0.5 | 480 | <0.03 | <2 | 54 | 35.2 | 0.26 | 1.9 | 0.06 | 2.5 | <0.05 | 14.4 | 2.6 |
| MCL * | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 120 | 1200 | 150 | 60 | 40 | ---- | 200 | 20 | 100 | 600 |
| Analyte Symbol | Time | F | Cl | NO2 (as N) | Br | NO3 (as N) | PO4 (as P) | SO4 | HCO3− |
|---|---|---|---|---|---|---|---|---|---|
| Unit Symbol | min | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L |
| Detection Limit | --- | 0.01 | 0.03 | 0.01 | 0.03 | 0.01 | 0.02 | 0.03 | 1 |
| LT | 0 | 2.3 | 36.4 | 0.33 | <0.6 | 34.3 | <0.4 | 1790 | 72 |
| T-1 | 0 | 0.25 | 78.8 | --- | --- | 0.5 | --- | 119.7 | 344 |
| T-6 | 150 | 0.38 | 8.4 | --- | --- | 0.1 | --- | 87.4 | 2950 |
| MCL * | --- | 40 | 8500 | --- | --- | --- | --- | 7000 | --- |
| Species | G-1 | G-6 | T-1 | T-6 |
|---|---|---|---|---|
| CdCO3 | 8.16 × 10−9 | 4.47 × 10−11 | 2.01 × 10−11 | 6.41 × 10−12 |
| Cd(OH)5 | 1.66 × 10−10 | 2.29 × 10−9 | 1.26 × 10−9 | 2.42 × 10−10 |
| CdOH+ | 6.92 × 10−11 | 1.10 × 10−11 | 1.28 × 10−11 | 1.18 × 10−12 |
| Cd2+ | 2.48 × 10−11 | 4.59 × 10−14 | 1.15 × 10−13 | 6.04 × 10−15 |
| MgCO3 | 3.53 × 10−3 | 1.47 × 10−6 | 4.65 × 10−4 | 3.83 × 10−7 |
| Mg2+ | 2.42 × 10−3 | 3.29 × 10−7 | 6.53 × 10−4 | 8.16 × 10−8 |
| MgSO4 | 3.13 × 10−4 | 9.68 × 10−9 | 3.44 × 10−5 | 1.75 × 10−9 |
| MgHCO3 | 1.88 × 10−5 | 1.61 × 10−6 * | 1.51 × 10−3 * | 3.58 × 10−7 * |
| Mn2+ | 4.83 × 10−7 | 7.40 × 10−12 | 1.50 × 10−8 | 8.53 × 10−12 |
| MnOH+ | 4.3 × 10−7 | 5.37 × 10−10 | 5.36 × 10−7 | 5.55 × 10−10 |
| MnSO4 | 6.26 × 10−8 | 2.05 × 10−13 | 7.87 × 10−10 | 1.74 × 10−13 |
| MnHCO3+ | 5.79 × 10−9 | 1.04 × 10−8 ** | 1.58 × 10−6 ** | 8.57 × 10−9 ** |
| Zn(CO3)2−2 | 2.81 × 10−6 | 5.94 × 10−11 | 1.68 × 10−11 | 7.68 × 10−11 |
| Zn(OH)2 | 1.83 × 10−6 | 1.52 × 10−8 | 6.72 × 10−8 | 1.46 × 10−8 |
| Zn(OH)3− | 3.52 × 10−7 | 3.57 × 10−7 | 5.51 × 10−7 | 3.04 × 10−7 |
| ZnCO3 | 2.79 × 10−8 | 6.79 × 10−7 *** | 5.13 × 10−7 *** | 5.10 × 10−7 *** |
| Species | GC | G-1 | G-6 | T-1 | T-6 |
|---|---|---|---|---|---|
| Artinite | −2.68 | 3.43 | 0.24 | 5.33 | −1.05 |
| Brucite | −2.70 | 1.80 | 1.85 | 4.45 | 1.15 |
| Cd(OH)2 | −5.63 | −3.16 | −2.01 | −2.27 | −2.99 |
| Chrysotile | 2.44 | 13.69 | 5.80 | 16.20 | 3.89 |
| Nesquehonite | −1.60 | 0.06 | −3.18 | −0.69 | −3.77 |
| Otavite | 0.53 | 0.30 | −1.98 | −2.35 | −2.84 |
| Hydrozincite | 39.79 | 35.12 | 17.90 | 20.87 | 18.04 |
| Smithsonite | −0.55 | −2.85 | −8.35 | −7.82 | −8.25 |
| Talc | 3.79 | 12.95 | −3.02 | 10.01 | −4.72 |
| ZnCO3:H2O | −0.21 | −2.64 | −8.09 | −7.56 | −7.99 |
| Portlandite | −8.06 | −4.89 | −0.09 | −0.86 | −0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, A.; Martínez da Matta, M.I. Application of Magnesium Oxide for Metal Removal in Mine Water Treatment. Sustainability 2022, 14, 15857. https://doi.org/10.3390/su142315857
Navarro A, Martínez da Matta MI. Application of Magnesium Oxide for Metal Removal in Mine Water Treatment. Sustainability. 2022; 14(23):15857. https://doi.org/10.3390/su142315857
Chicago/Turabian StyleNavarro, Andrés, and María Izabel Martínez da Matta. 2022. "Application of Magnesium Oxide for Metal Removal in Mine Water Treatment" Sustainability 14, no. 23: 15857. https://doi.org/10.3390/su142315857
APA StyleNavarro, A., & Martínez da Matta, M. I. (2022). Application of Magnesium Oxide for Metal Removal in Mine Water Treatment. Sustainability, 14(23), 15857. https://doi.org/10.3390/su142315857






